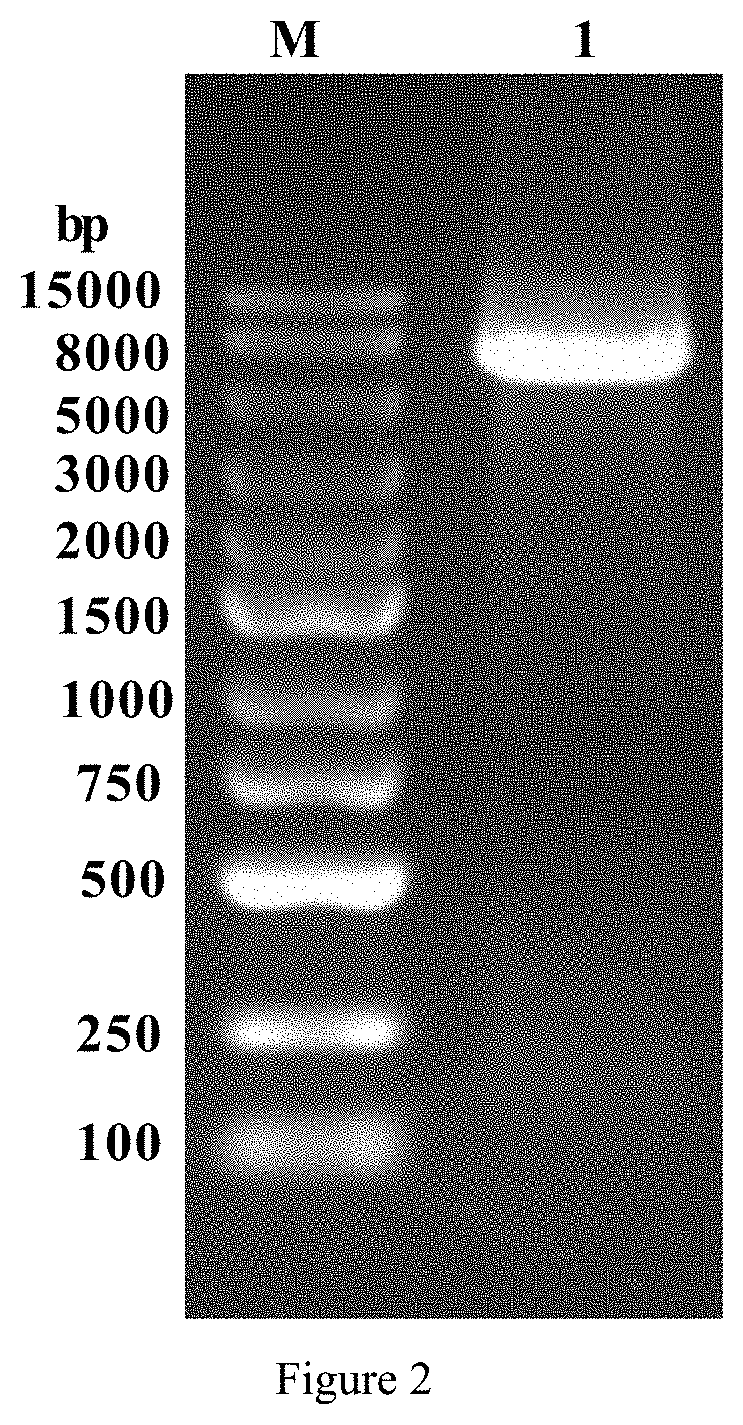
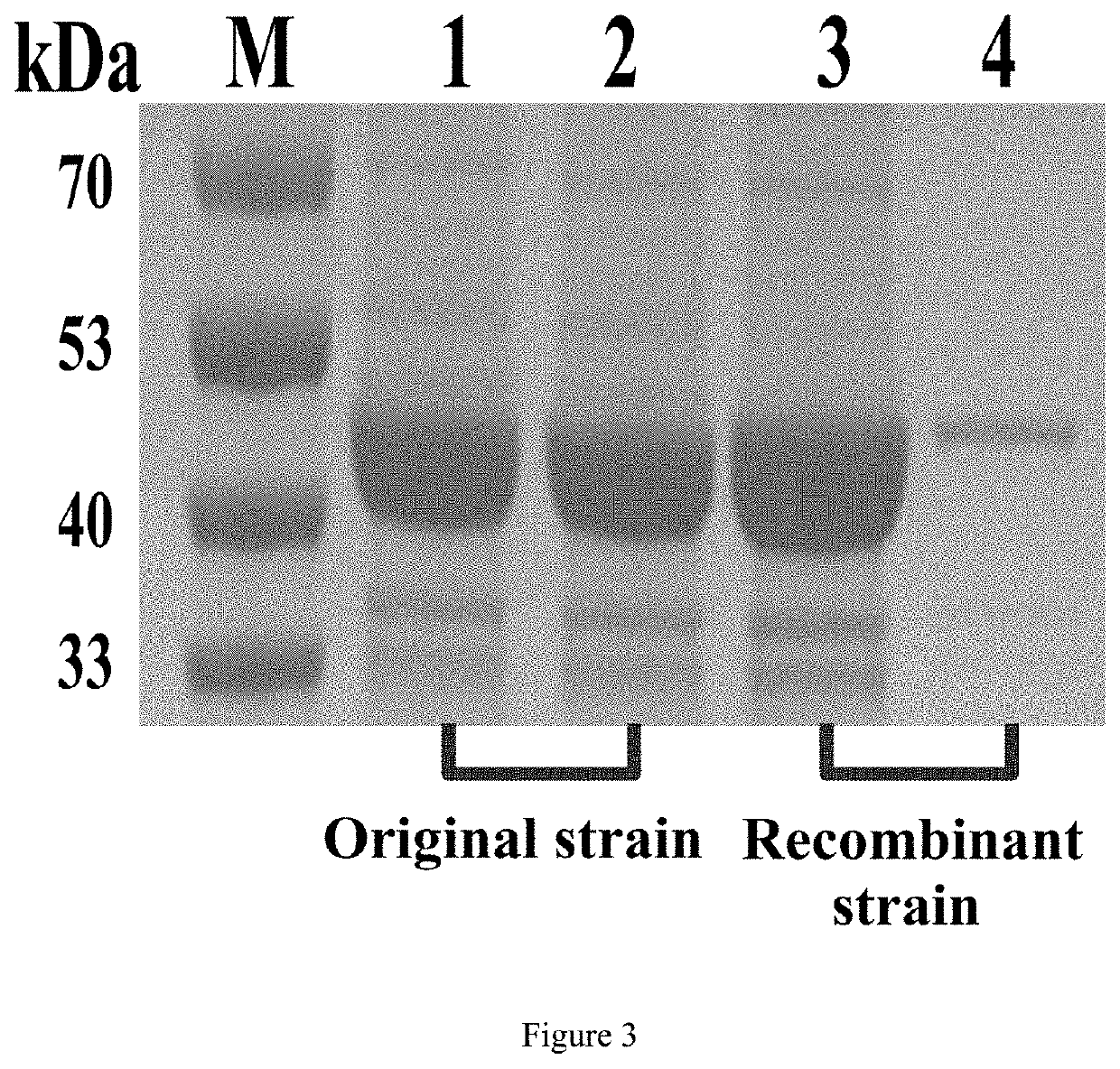
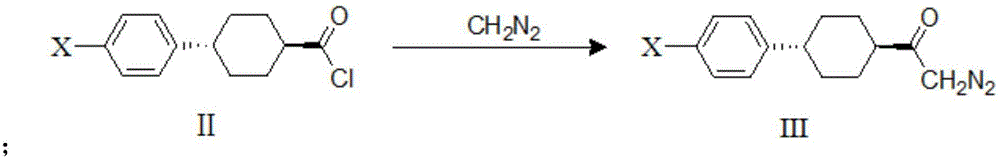Patents
Literature
32 results about "Cyclohexylacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
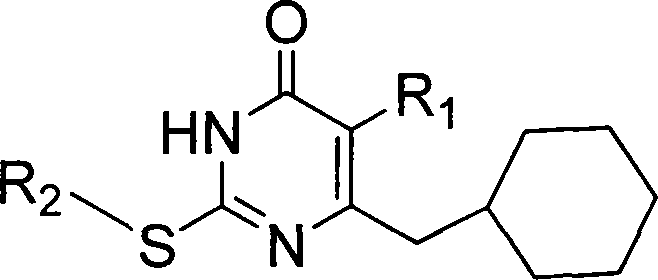
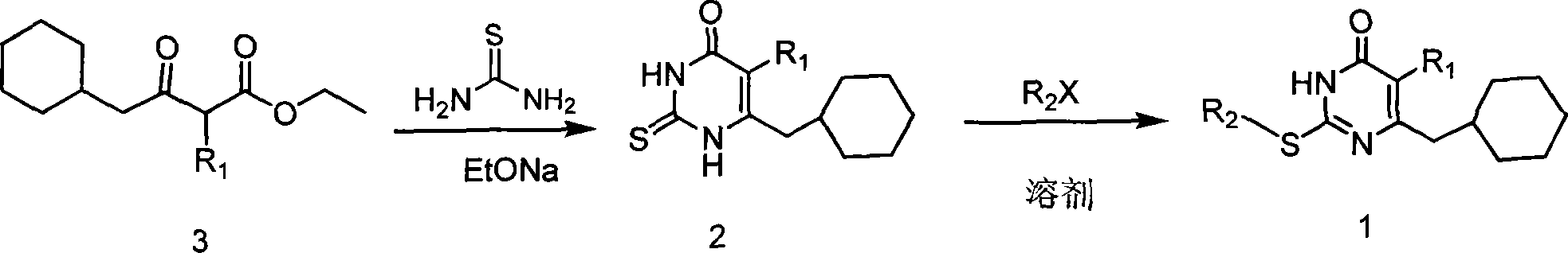
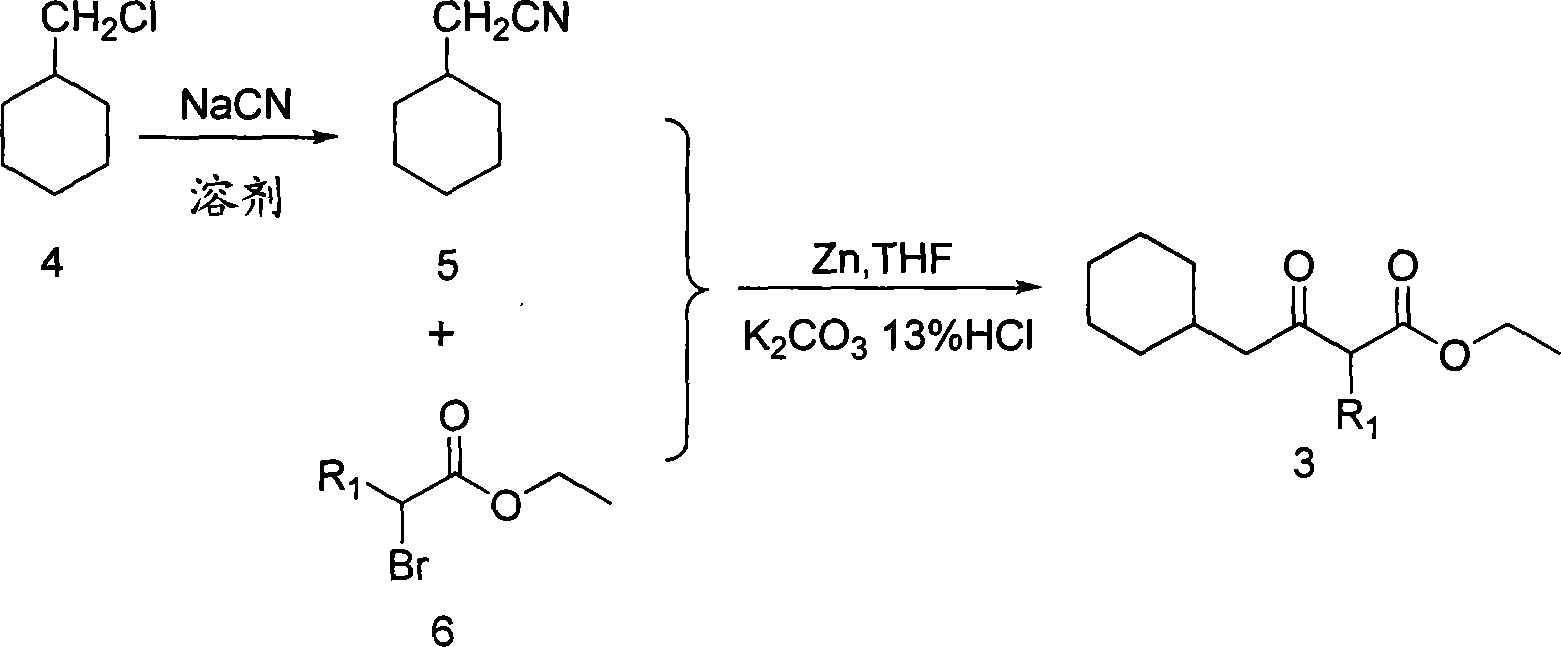
6-cyclohexyl methyl substituted s-DABO compound, method for synthesizing same and uses thereof
InactiveCN101177413AEasy to synthesizeEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryThioureaSide chain
The invention discloses 6-cyclohexyl methyl substituted S-DABO type compound, the synthesis method and the application, belonging to the medical technical field. The invention relates to a 5-alkyl-6-cyclohexyl methyl-2-(alkyl, naphthenic base, naphthenic base methyl, substituted phenylethanone) thiouracil type compound, which has general formula as shown in (I): wherein, R1 is alkyl of C1-3; alkyl, naphthenic base and naphthenic base methyl of R2=C1-8 are as shown in (II); wherein, X=OCH3, H, OH, halogen. Chloromethyl cyclohexane or cyclohexyl acetic acid is respectively used as raw material to prepare Beta- keto ester which is made into a key intermediate 5-alkyl-6-cyclohexyl methyl thiouracil together with thiourea through close loop condensation under the catalyzing of sodium alkoxide; target molecule is prepared through S-alkylation guiding C2-side chain. The invention has the advantages of simple and easy synthesis method, obvious anti-HIV virus activity and anti-resistance for the drugs and ability to be used as an alternative for anti-HIV drug.
Owner:YUNNAN UNIV +1
Pineapple flavor essence
InactiveCN101760317AConvenient sourceAnti-mildewEssential-oils/perfumesAllyl acetateCinnamyl acetate
The invention discloses a pineapple flavor essence. The raw materials of the pineapple flavor essence comprise 300.0 weight permillage of allyl caproate, 200.0 weight permillage of cyclohexyl allyl propionate, 30.0 weight permillage of ethyl acetate, 50.0 weight permillage of cyclohexyl allyl acetate, 30.0 weight permillage of geranyl hexanoate, 10.0 weight permillage of benzyl propionate, 5.0 weight permillage of petit grain oil, 2.0 weight permillage of acetic leaf alcohol ester, 10.0 weight permillage of vanillic aldehyde, 3.5 weight permillage of cinnamyl acetate, 100.0 weight permillage of ethyl butyrate, 20.0 weight permillage of ethyl oenanthate, 30.0 weight permillage of pentyl acetate, 30.0 weight permillage of cyclohexyl allyl butyrate,10.0 weight permillage of geranyl butyrate, 10.0 weight permillage of linalool, 10.0 weight permillage of citral dimethyl acetal, 0.5 weight permillage of dimethyl hydroxyl furanone, and 150.0 weight permillage of diethyl phthalate. The pineapple flavor essence of the invention is fit for perfuming products such as grease, emulsion, shampoo, and the like, and has the advantages of pure flavor, strong endurance, less dosage, and the like.
Owner:刘新光
Nitrilase mutant and application thereof in preparation of anti-epileptic drug intermediate
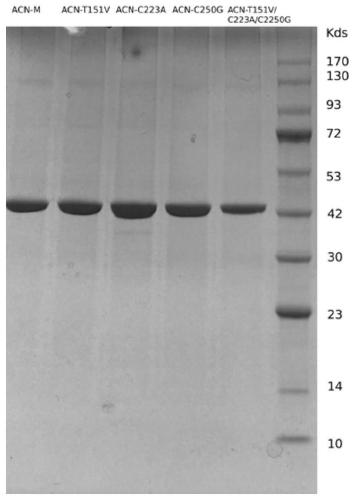
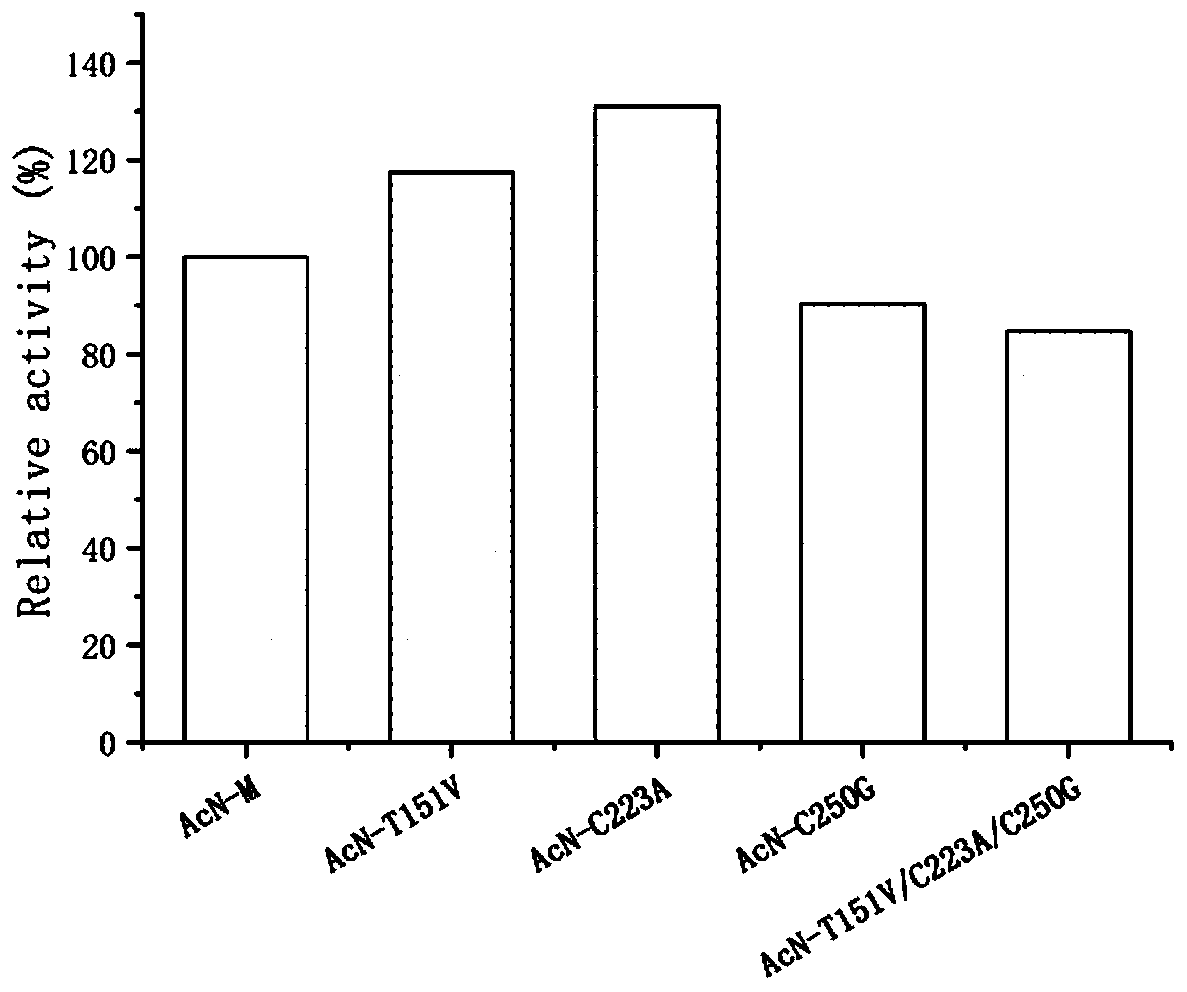
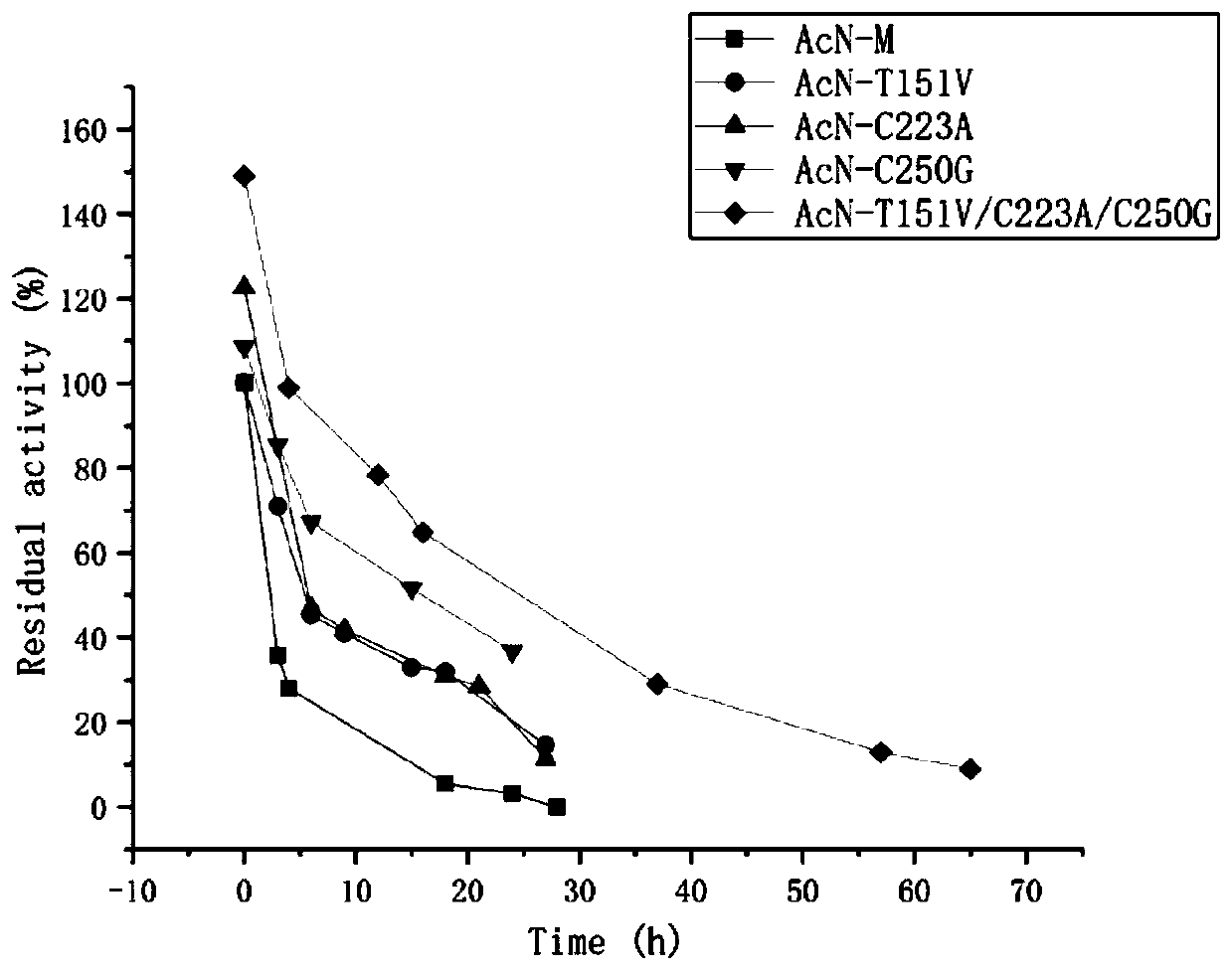
The invention discloses a nitrilase mutant and an application thereof in preparation of an anti-epileptic drug intermediate. The mutant is obtained by mutating one or more of 151th amino acid, 223th amino acid and 250th amino acid of an amino acid sequence shown in SEQ ID No.2. The thermal stability of the nitrilase mutant AcN-T151V / C223A / C250G is improved by 1.73 times, 1M 1-cyanocyclohexyl acetonitrile is hydrolyzed by use of recombinant escherichia coli containing the nitrilase mutant at the temperature of 35 DEG C to generate 1-cyanocyclohexylacetic acid, and the yield of a final product reaches 95%. When 1.2 M 1-cyanocyclohexyl acetonitrile is hydrolyzed at the temperature of 35 DEG C, the final yield reaches 97%. Gabapentin is synthesized by use of the nitrilase mutant, and the yieldof the final product reaches 80%.
Owner:ZHEJIANG UNIV OF TECH
Preparation method for trans 4-amino-cyclohexyl acetate derivative
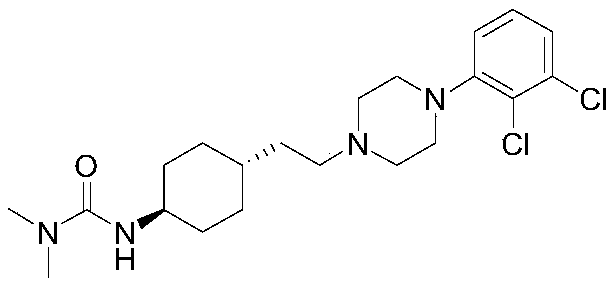
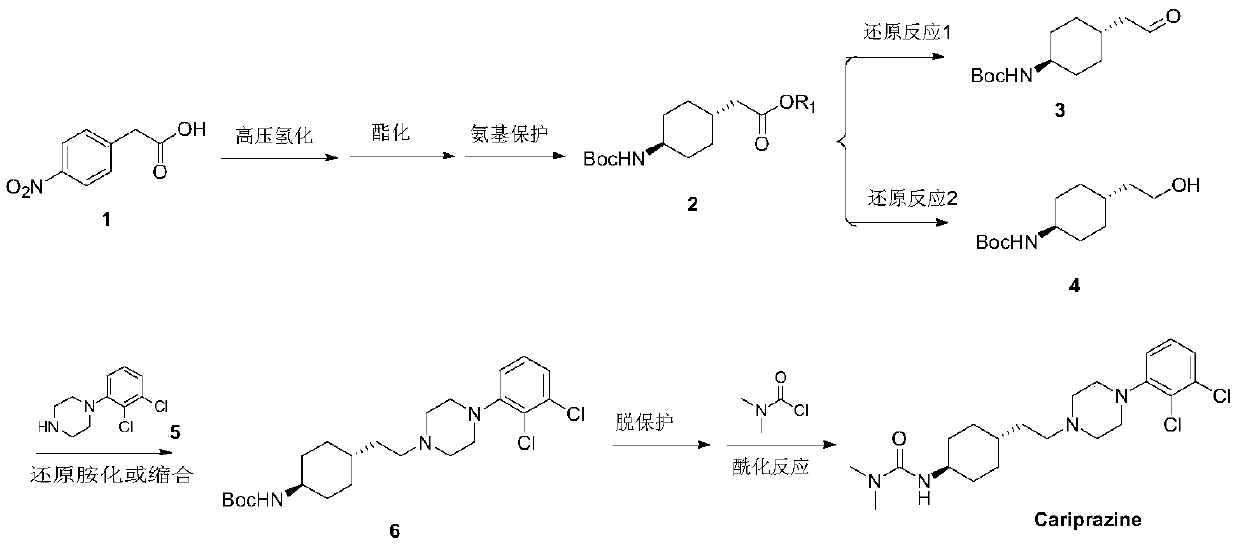
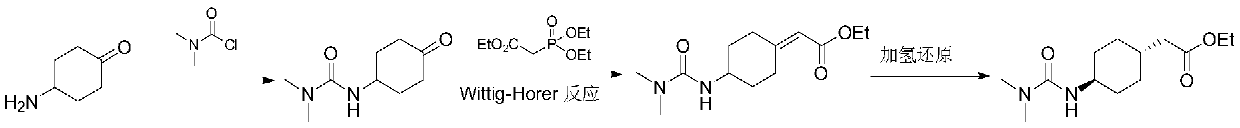
ActiveCN106565510AHigh purityGood effectOrganic compound preparationAmino-carboxyl compound preparationCariprazineCyclohexanone
The invention provides a preparation method for a trans 4-amino-cyclohexyl acetate derivative. The preparation method comprises the following steps that a formula (please see the description for the formula) is provided, wherein R is methyl or ethyl; a compound III is prepared from the raw materials of 4-amino cyclohexanone II and is subjected to hydrogenation reduction, a compound I crude product is obtained, acid is added, and salt is formed; and a compound I is prepared by adding alkali. According to the preparation method, 4-amino cyclohexanone not protected by amino is adopted as raw materials, witting reaction is conducted, and then through catalytic hydrogenation, the trans crude product of the preparation method is obtained. The crude product can be subjected to salifying crystallization so as to obtain the purer trans product, and the trans:syn ratio is 95-99.9:5-0.1. The product compound is high in purity and can be used for preparing cariprazine. Operation is simple, the raw materials are easy to obtain, industrial production is suitable, and large application value is achieved.
Owner:ZHEJIANG JINGXIN PHARMA +2
New method for synthesizing Gabapentin hydrochloride
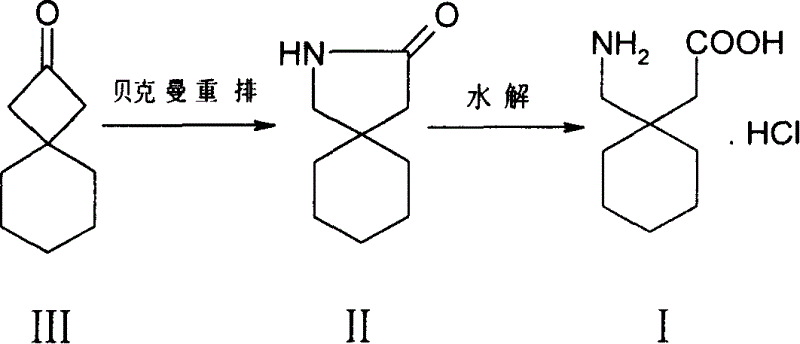
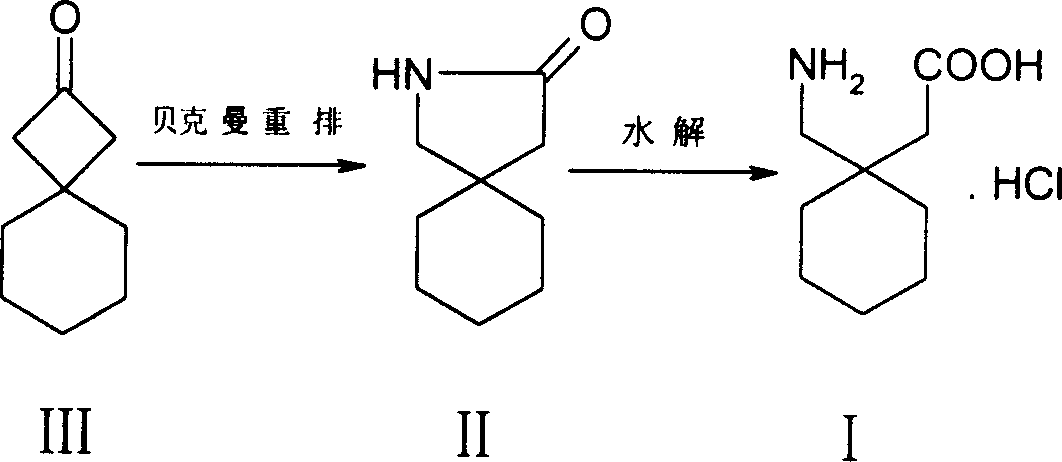
InactiveCN1727329ALow toxicityEasy to realize industrial productionOrganic compound preparationAmino-carboxyl compound preparationGabapentinCyclohexylacetic acid
A process for synthesizing gabapentin hydrochloride includes such steps as Backmann rearrangement reaction of spiro [3,5]-2-nonanone ó¾ to obtain 2-aza-spiro [4,5]-3-decanone ó�, hydrolyzing, and recrystallizing. Its advantages are simple process, low poison of raw material and low cost.
Owner:CHANGCHUN DISCOVERY SCI
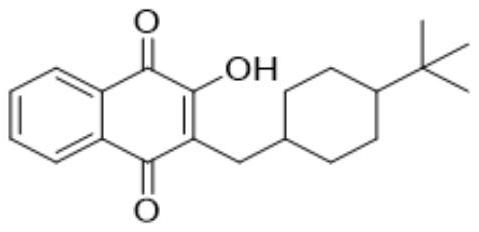
Preparation method for buparvaquone
InactiveCN105198718AShort reaction stepsLower reaction costOrganic compound preparationQuinone separation/purificationPhosphoric acidParvaquone
The invention discloses a preparation method for buparvaquone. The method comprises taking tert-butylcyclohexyl acetic acid, sodium omadine and 1,4-naphthoquinone as initial raw materials, firstly reacting tert-butylcyclohexyl acetic acid with sodium omadine to generate tert-butylcyclohexyl acetic acid 2-thioxo-pyridin-1-yl ester, then reacting with 1,4-naphthoquinone for addition, so as to generate 2-[(4-tert-butylcyclohexyl)methyl]-3-(2-pyridinylsulfanyl)-1,4-naphthalenedione, then mixing the obtained 2-[(4-tert-butylcyclohexyl)methyl]-3-(2-pyridinylsulfanyl)-1,4-naphthalenedione, methanol, tripotassium phosphate trihydrate and water, performing heating hydrolysis, processing the solution and acidifying, and filtering to obtain a buparvaquone crude product, and then recrystallizing by utilizing isopropanol, so as to obtain a buparvaquone competitive product. The method is simple in operation, relatively low in cost, high in product yield and relatively suitable for industrialized production.
Owner:山东川成医药有限公司
Process for the preparation of trans 4-amino-cyclohexyl acetic acid ethyl ester hcl
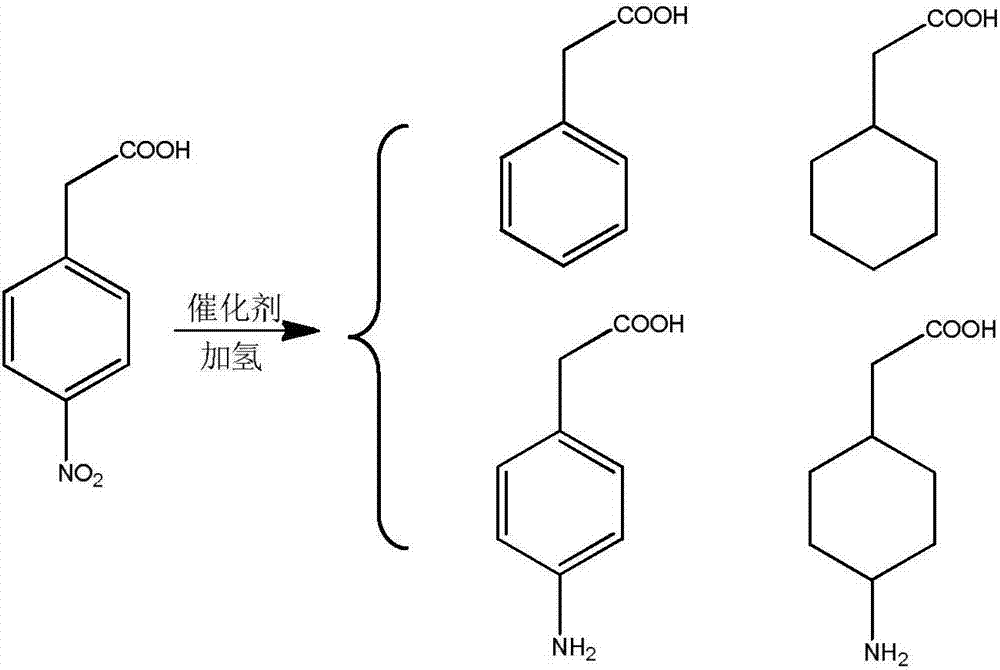
ActiveUS20110288329A1Organic compound preparationAmino-carboxyl compound preparationAcetic acidAcetonitrile
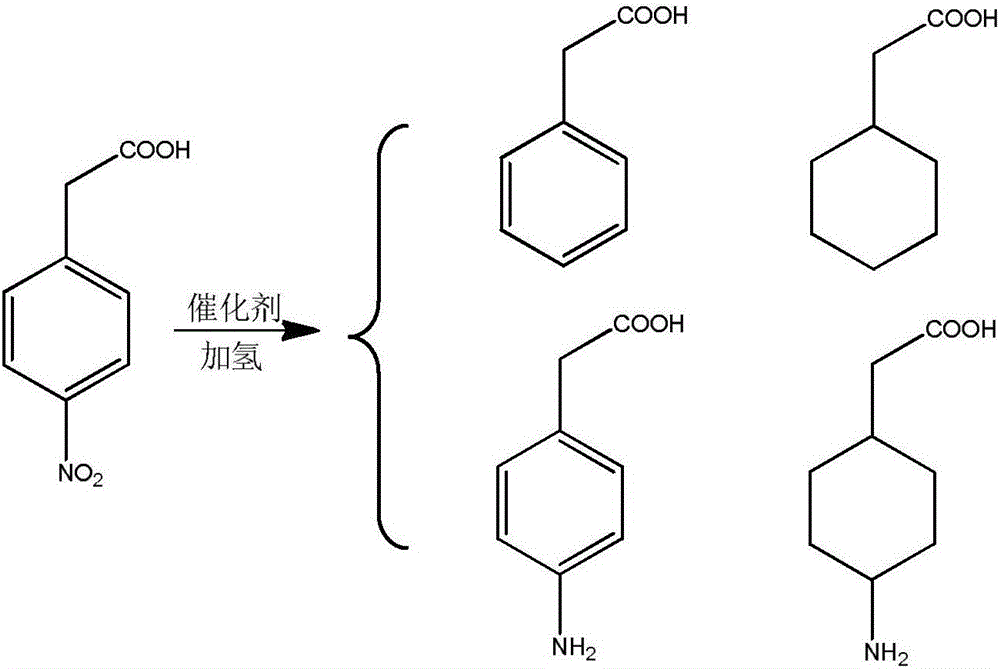
The invention relates to a process for the preparation of trans 4-amino-cyclohexil ethyl acetate HCl whereind) hydrogenating 4-nitrophenyl acetic acid in a protic solvent at a temperature between 40-50° C. in the presence of Pd / C under 0.1-0.6 bar overpressure, ande) further hydrogenating the 4-aminophenyl acetic acid obtained in situ in step a) at a temperature between 50-60° C. under 1-4 bar overpressures, thenf) heating to reflux the 4-aminocyclohexil acetic acid obtained in step b) for 1-3 hours in hydrochloric ethanol, and if desired after removing the solvent acetonitrile was added to the residue obtained and distilled off.
Owner:RICHTER GEDEON NYRT
Pantoea amidase, gene, vector, engineering bacterium and application thereof
The invention discloses a Pantoea amidase, a gene, a vector, an engineering bacterium and an application thereof in preparation of 1-cyancyclohexylacetic acid through biological catalysis of 1-cyancyclohexylacetamide. The amino acid sequence of the amidase is represented by SEQ ID NO.1. A synthesis technology of 1-cyancyclohexylacetic acid through biological catalysis by using the amidase is reported in the invention for the first time, and the technology has the substantial advantages of small catalyst dosage (2g / L), high substrate concentration (100g / L), short reaction time (20min), and high product conversion rate reaching 100%.
Owner:ZHEJIANG UNIV OF TECH
Nitrilase mutant and application thereof in preparation of 1-cyanocyclohexyl acetic acid
ActiveCN111471668AIncreased specific enzyme activityShort reaction timeBacteriaHydrolasesEscherichia coliGabapentinoid
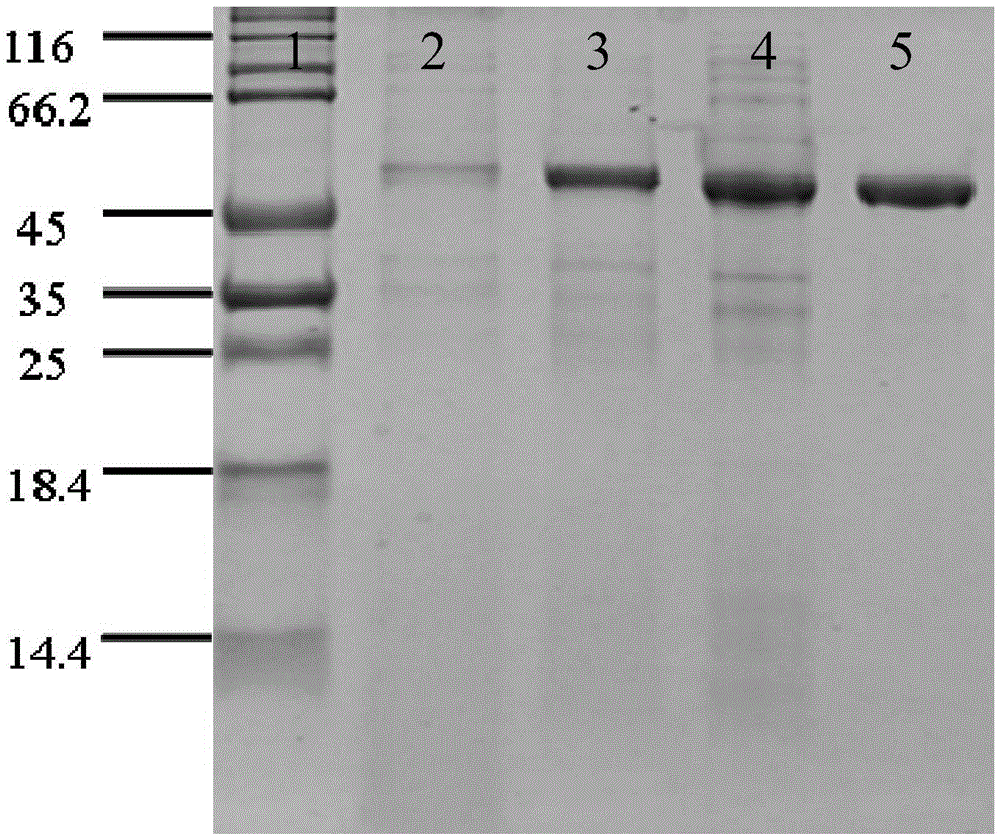
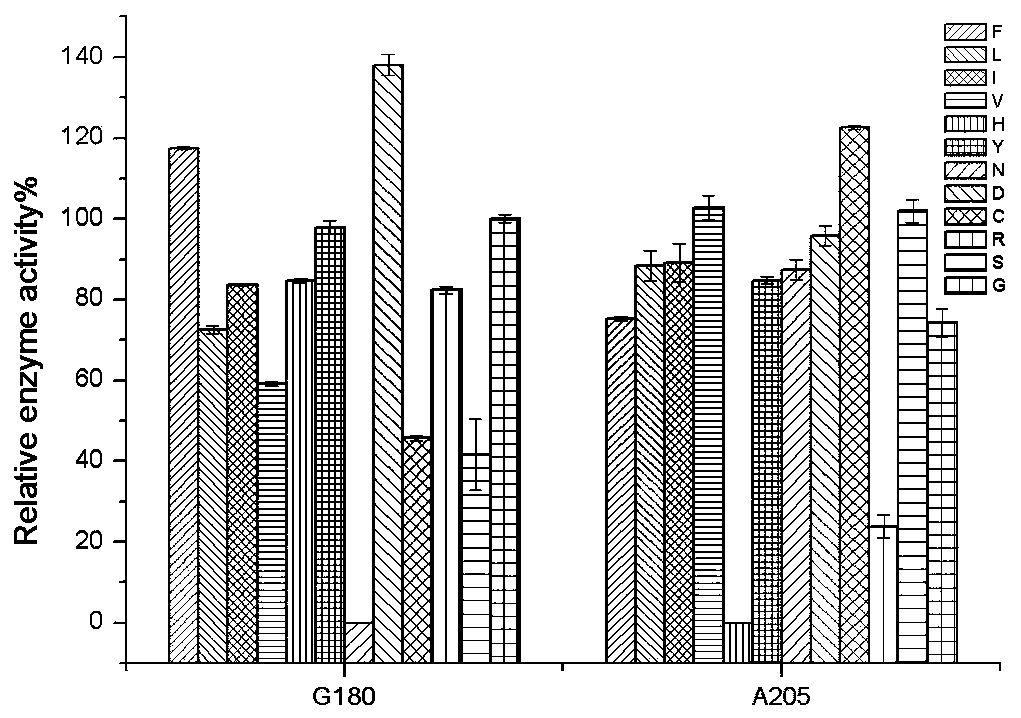
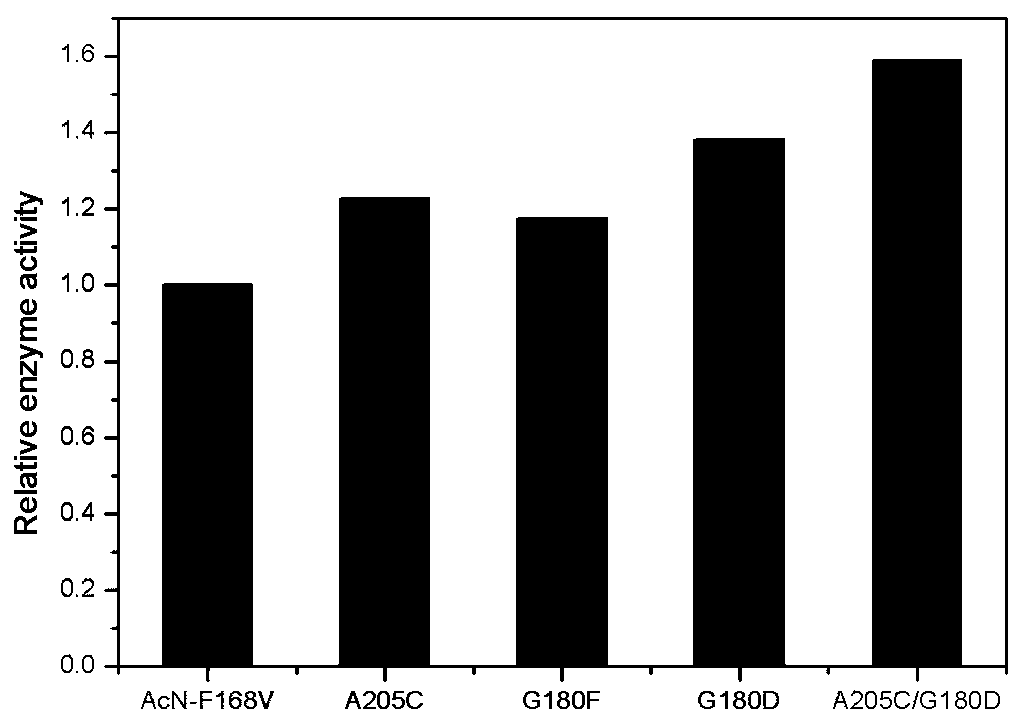
The invention discloses a nitrilase mutant and application thereof in preparation of 1-cyanocyclohexyl acetic acid. The nitrilase mutant is obtained by mutating one or more of 180th and 205th amino acids of an amino acid sequence shown as SEQ ID No.2. According to the invention, semi-rational design is adopted, proteins are subjected to molecular modification, the specific enzyme activity of nitrilase double mutant AcN-G180D / A205C is improved by 1.6 times to the maximum extent, the conversion rate is greater than 99%, and the reaction time is shortened to one fourth of the original reaction time by hydrolyzing 1-cyanocyclohexyl acetonitrile at a high temperature (50 DEG C) by using recombinant escherichia coli containing the nitrilase mutant. Therefore, the mutant obtained by the inventionhas a good application prospect in efficiently catalyzing the 1-cyanocyclohexyl acetonitrile to synthesize the gabapentin intermediate 1-cyanocyclohexyl acetic acid.
Owner:ZHEJIANG UNIV OF TECH
Process for the preparation of trans 4-amino-cyclohexyl acetic acid ethyl ester HCl
ActiveUS8802888B2Organic compound preparationAmino-carboxyl compound preparationAcetic acidEthyl ester
Owner:RICHTER GEDEON NYRT
Preparing method for 4-amino-cyclohexylacetic acid
ActiveCN106543017ALow priceHigh activityPhysical/chemical process catalystsOrganic compound preparationActive componentHydrogenation reaction
The invention discloses a 4-nitrophenylacetic-acid-catalyzing hydrogenation preparing method for trans-4-amino-cyclohexylacetic acid and hydrochloride. Under the condition of a multiple-active-component supported catalyst prepared with the method, a hydrogenation reaction has the high activity and selectivity, operating is easy, the cost of the catalyst is low, and other reaction steps are not required; the technology is 4-nitrophenylacetic-acid heterogeneous catalysis and hydrogenation, the reaction condition is mild, the method is efficient, simple, easy to carry out, economical and free of pollution, the technology is simple, and large-scale industrial production is facilitated.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for preparing DGAT-1 inhibitor intermediate
ActiveCN104496795ALow costCarbon chain lengthOrganic compound preparationCarboxylic acid esters preparationAcetic acidWolff rearrangement
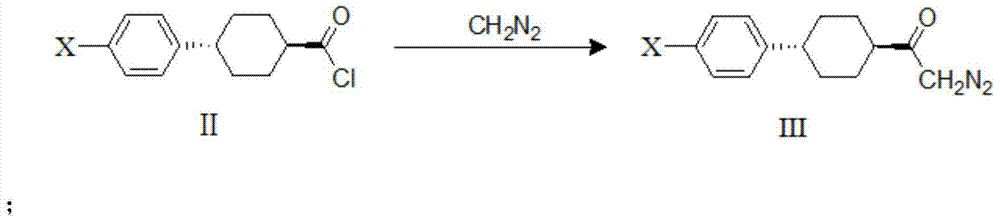
The invention discloses a method for preparing a DGAT-1 inhibitor intermediate. The method comprises the following steps: reacting trans-4-(4-halogeno phenyl)cyclohexanecarboxylic acid chloride with diazomethane, and generating trans-4-(4-halogeno phenyl)cyclohexyl diazo-ketone; and performing Wolff rearrangement on trans-4-(4-halogeno phenyl)cyclohexyl diazo-ketone, and generating trans-4-(4-halogeno phenyl)cyclohexyl acetic acid. According to the method disclosed by the invention, the reaction steps are reduced, and the raw material cost is reduced.
Owner:PORTON FINE CHEM
Polypeptide label, highly soluble recombinant nitrilase and application thereof in synthesis of pharmaceutical chemicals
ActiveCN112358530AImprove solubilityIncreased expression of solubleBacteriaHydrolasesGlycineAcetic acid
Owner:ZHEJIANG UNIV OF TECH
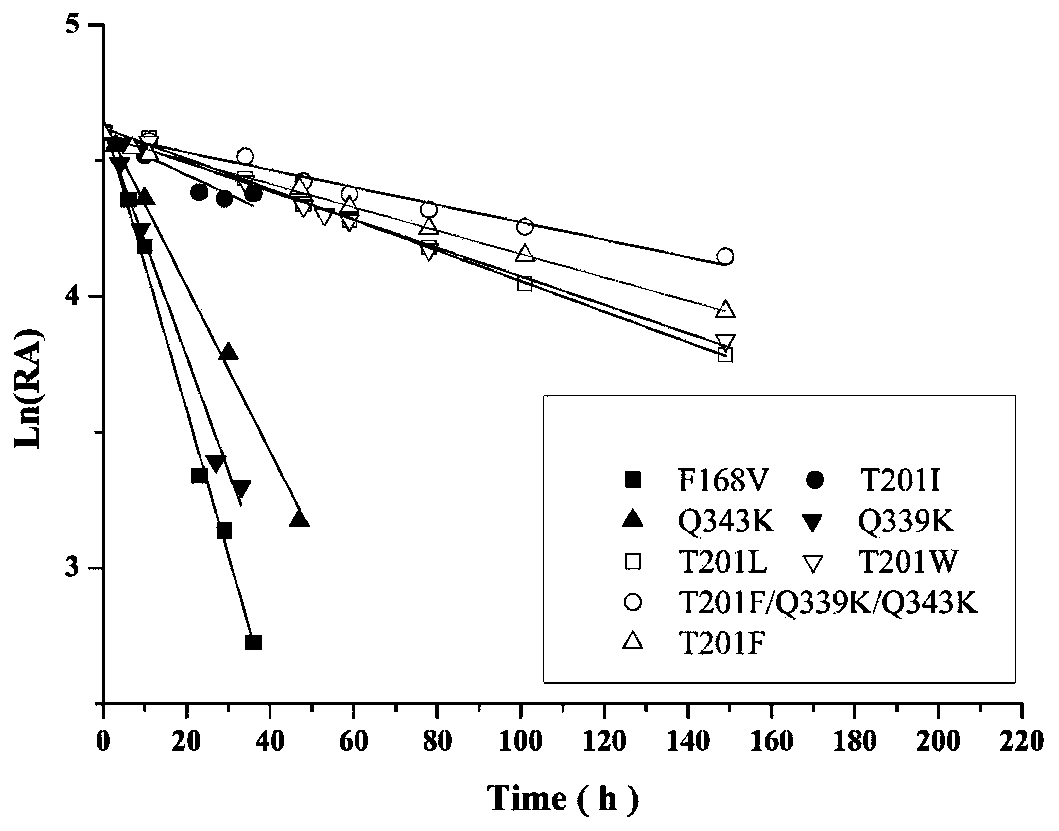
Nitrilase mutants and application thereof
ActiveUS20200115695A1Doubled conversion rateGood application prospectHydrolasesFermentationProtein moleculesGabapentinoid
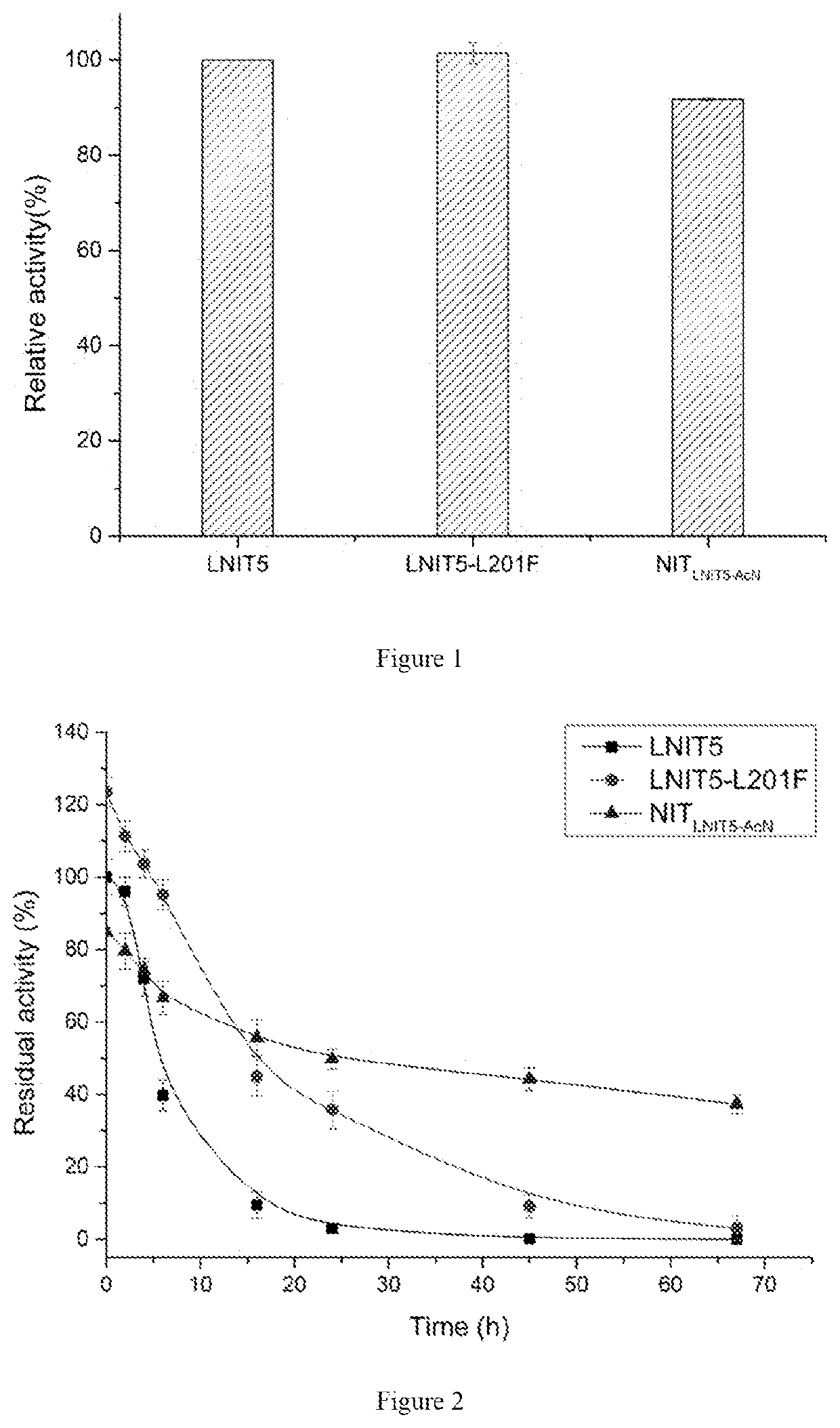
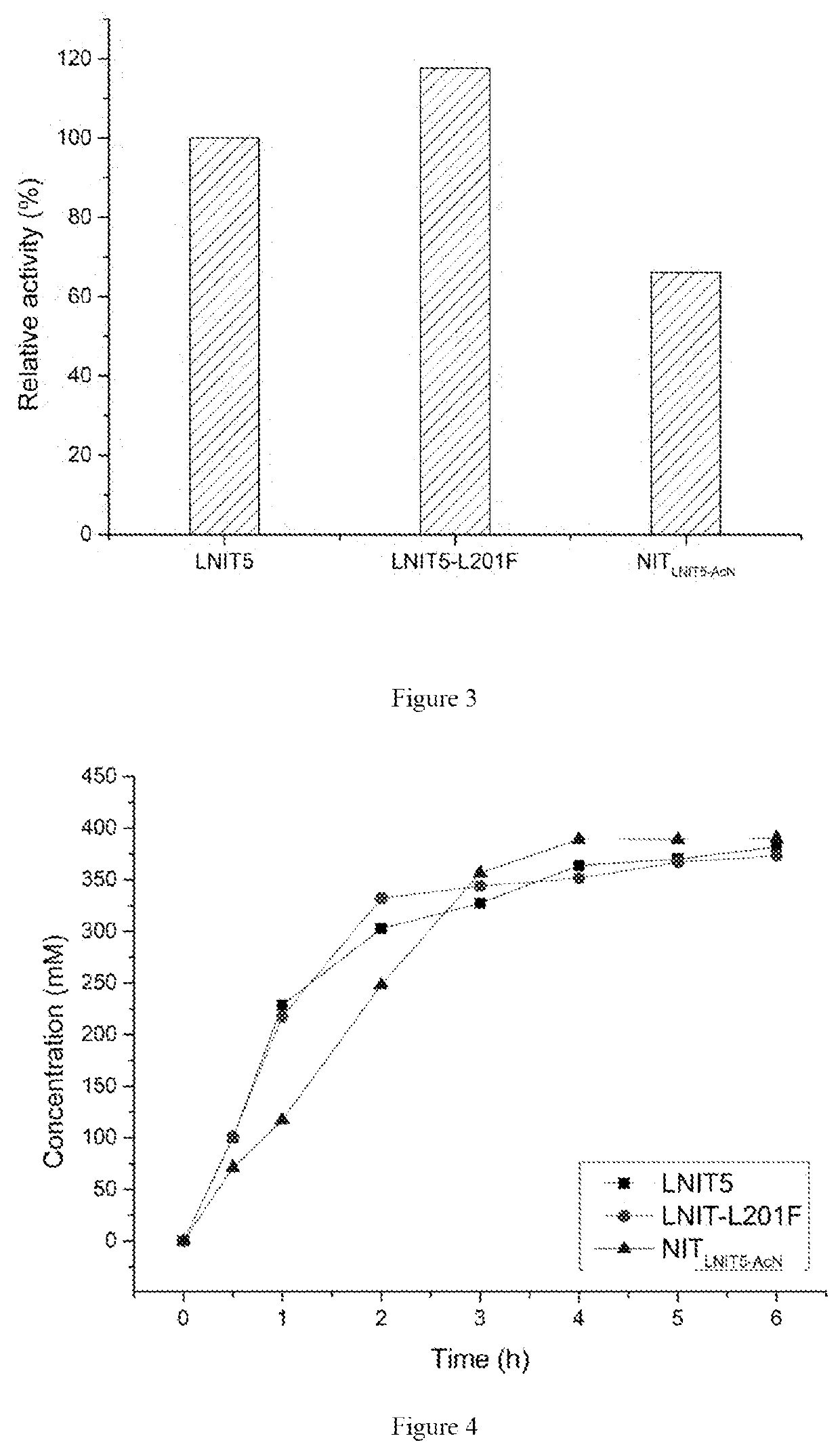
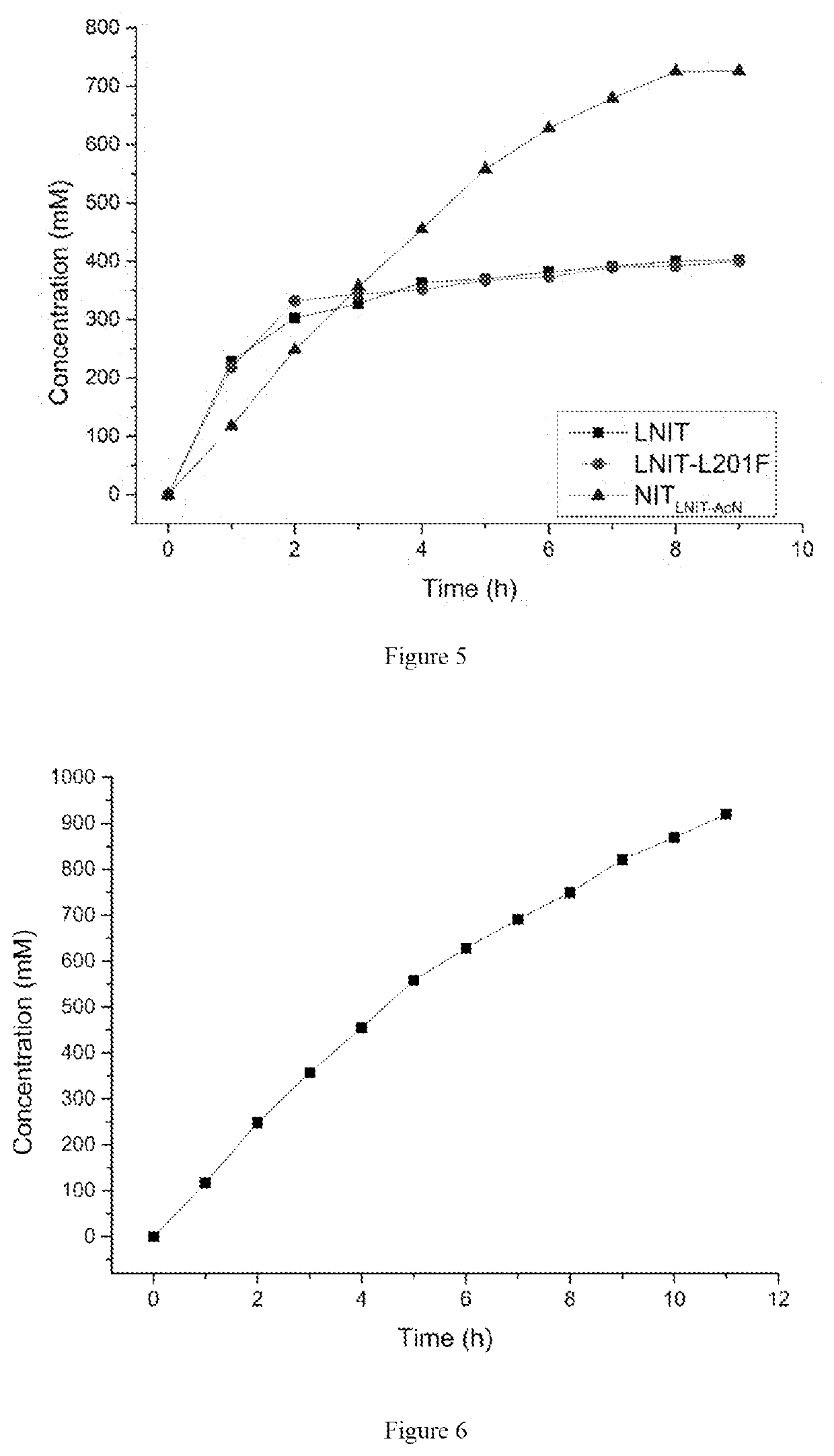
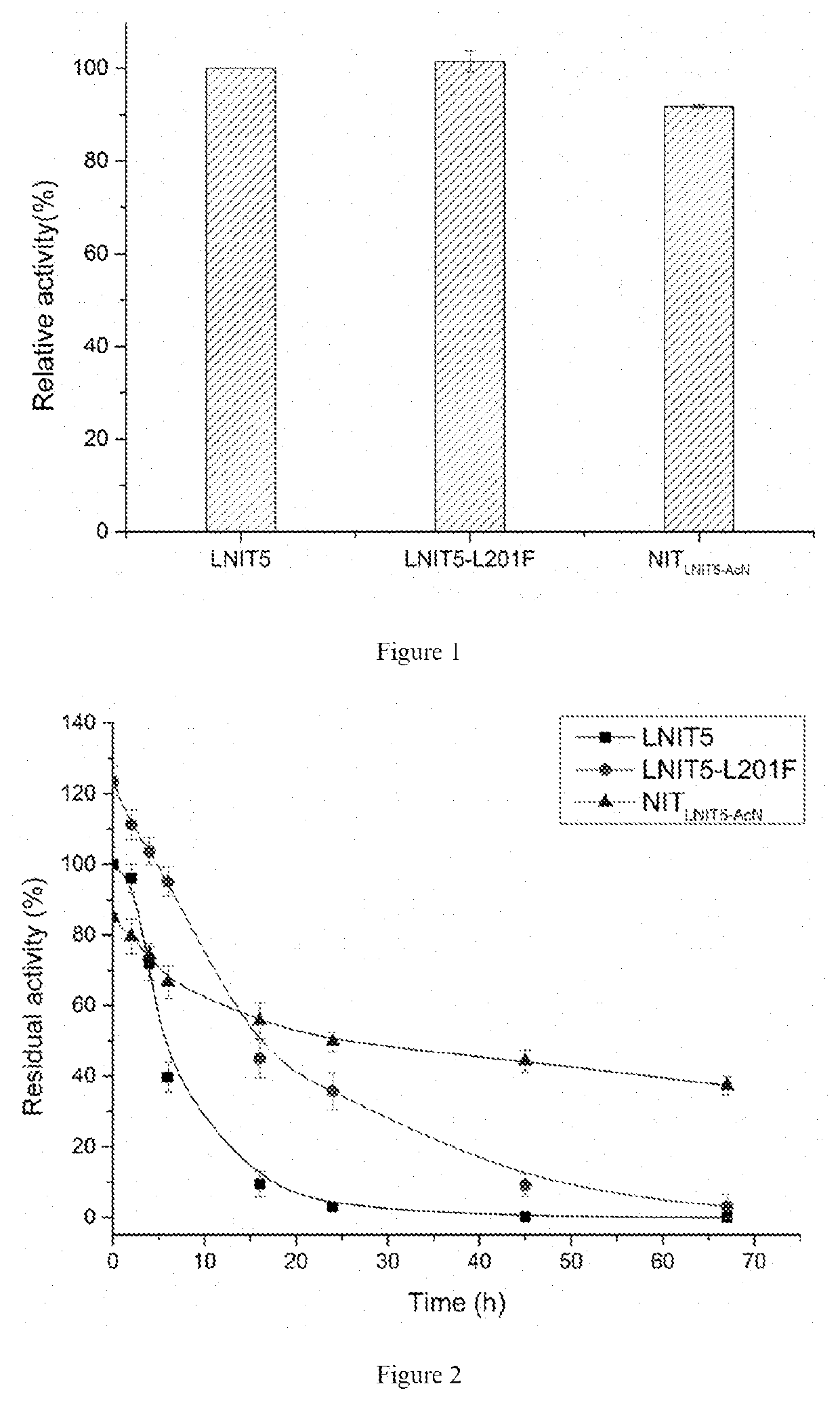
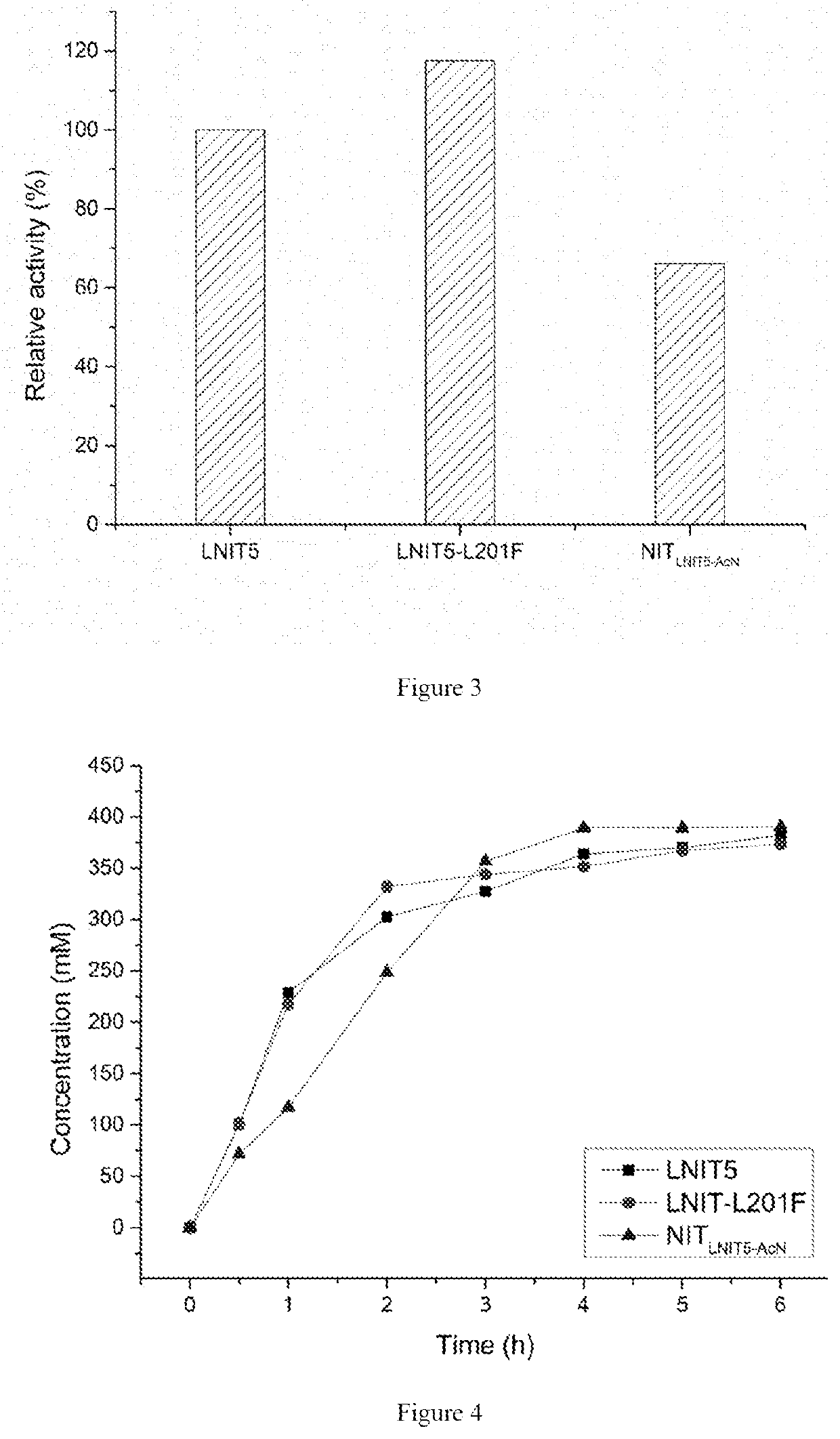
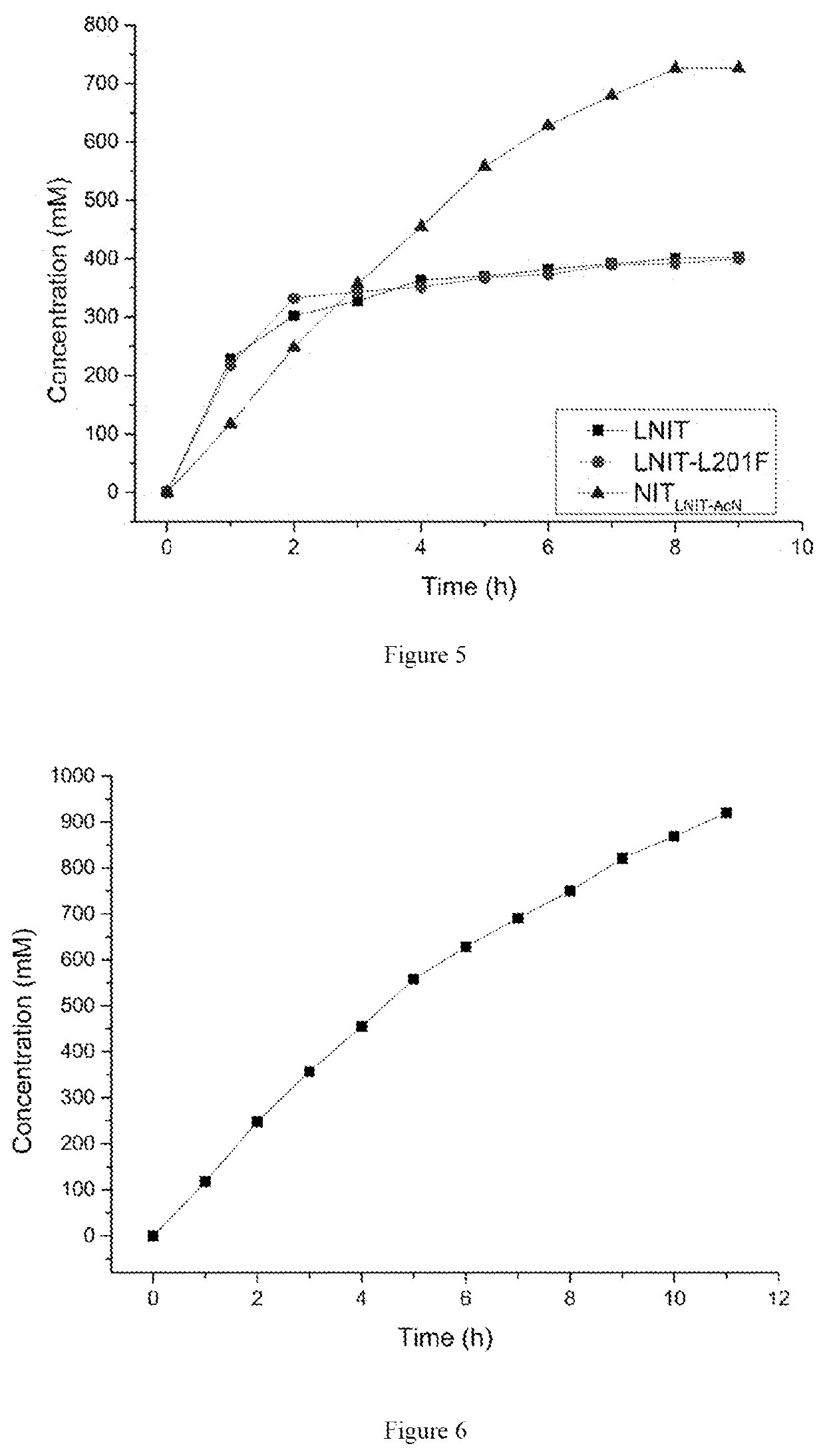
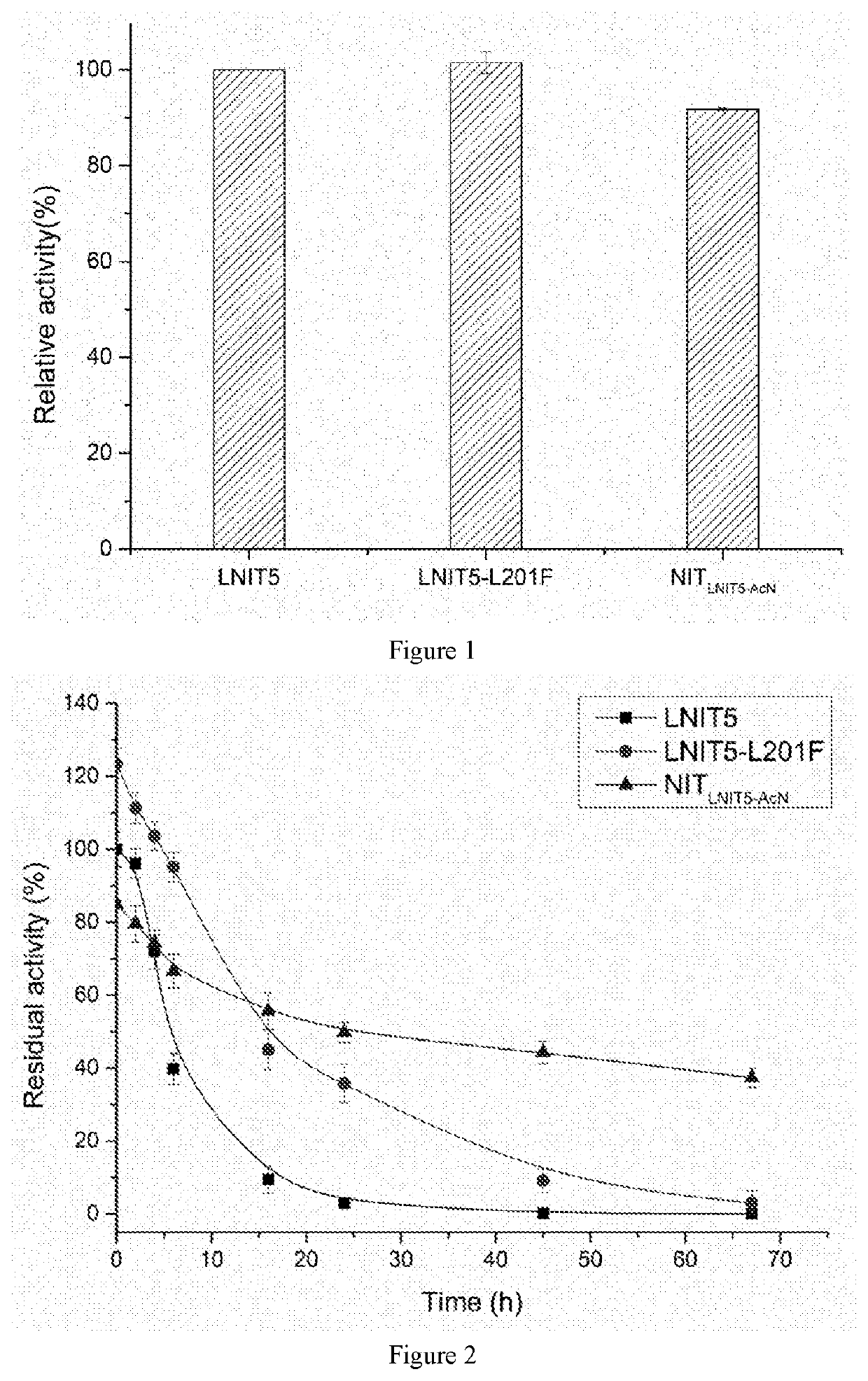
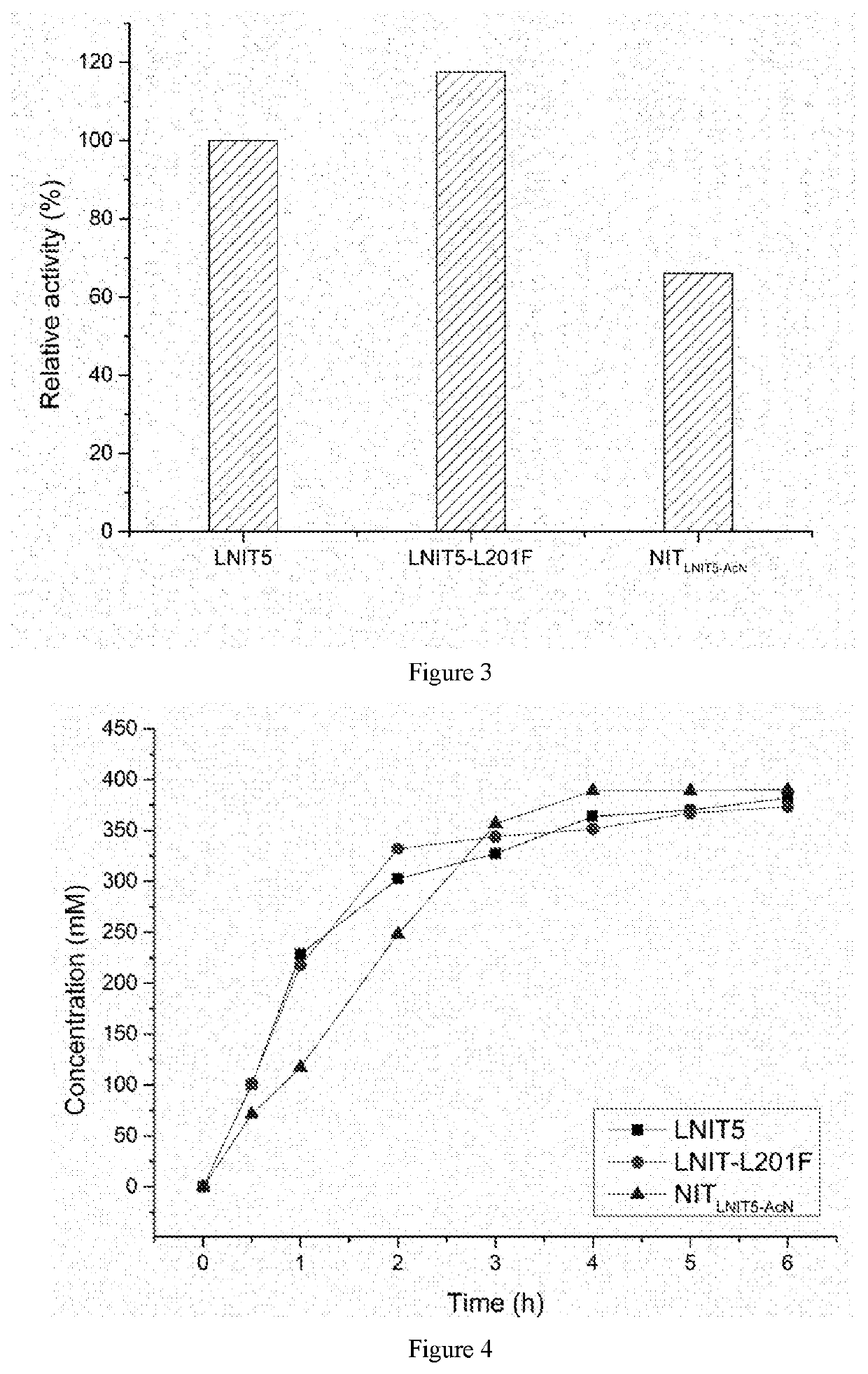
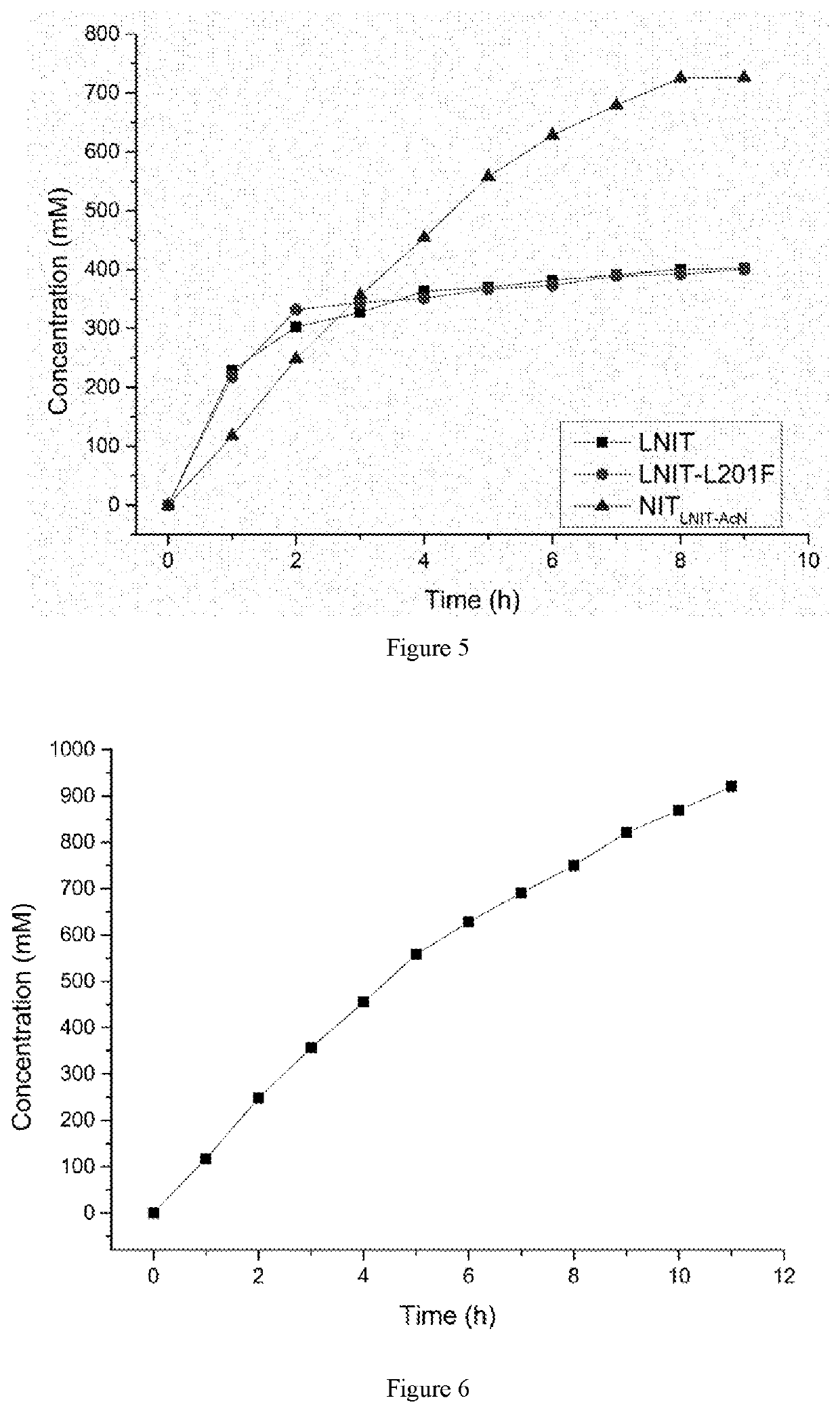
The present invention discloses a nitrilase mutant and application thereof. The mutant is obtained by mutating the amino acid at position 201 or replacing one or more amino acids at region 324-381 of the amino acid sequence shown in SEQ ID No. 2. In the present invention, by the protein molecular modification, thermostability of the purified nitrilase LNIT5 is increased by up to 4.5 folds; and by utilizing recombinant E. coli containing the nitrilase mutant to hydrolyze 1-cyanocyclohexylacetonitrile at a high temperature (45° C.), product tolerance is increased, activity of NIT5-L201F is increased by 20%, and the mutant NITLNIT5-AcN can completely hydrolyze 750 mM 1-cyanocyclohexylacetonitrile within 8 hours and achieve an doubled conversion rate. Therefore, the mutants obtained by the present invention have a good application prospect in efficiently catalyzing 1-cyanocyclohexylacetonitrile to synthesize gabapentin intermediate, 1-cyanocyclohexyl acetic acid. In the present invention, by protein molecular modification, thermal stability of pure nitrilase LNIT5 at 45° C. is increased up to 4.5 times, and while 1-cyanocyclohexylacetonitrile is hydrolyzed using recombinant Escherichia coli containing nitrilase mutant at high temperature(45° C.), the product yield is increased. Therefore, the mutants obtained in the present invention have a good application prospect in highly efficiently catalyzing 1-cyanocyclohexylacetonitrile to 1-cyanocyclohexyl acetic acid, the gabapentin intermediate.
Owner:ZHEJIANG UNIV OF TECH
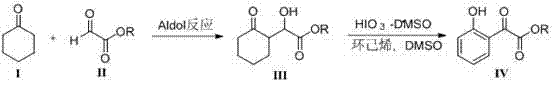
Synthetic method for 2-(2-hydroxyphenyl)-2-oxyacetate
ActiveCN104710305AMild reaction conditionsEasy to operateOrganic compound preparationCarboxylic acid esters preparationCyclohexanoneIodic acid
The invention discloses a synthetic method for 2-(2-hydroxyphenyl)-2-oxyacetate. The method comprises the following steps: taking cyclohexanone and glyoxylate as raw materials, producing an Aldol condensation reaction through catalysis at a room temperature, preparing an intermediate 2-hydroxy-2-(2'-oxocyclohexyl)acetate, producing oxidation and dehydro-aromatization reactions through iodic acid-dimethyl sulfoxide, and obtaining 2-(2-hydroxyphenyl)-2-oxyacetate. According to the synthetic method for 2-(2-hydroxyphenyl)-2-oxyacetate, iodic acid is used to produce the dehydro-aromatization reaction for the first time, oxidation and aromatization processes are performed in one step, reagents used in the method are all low in price and easily available, and the method is mild in reaction condition, easy to operate, simple in reaction post-treatment and relatively suitable for large-scale preparation of similar products of 2-(2-hydroxyphenyl)-2-oxyacetate and the like.
Owner:常熟市联创化学有限公司
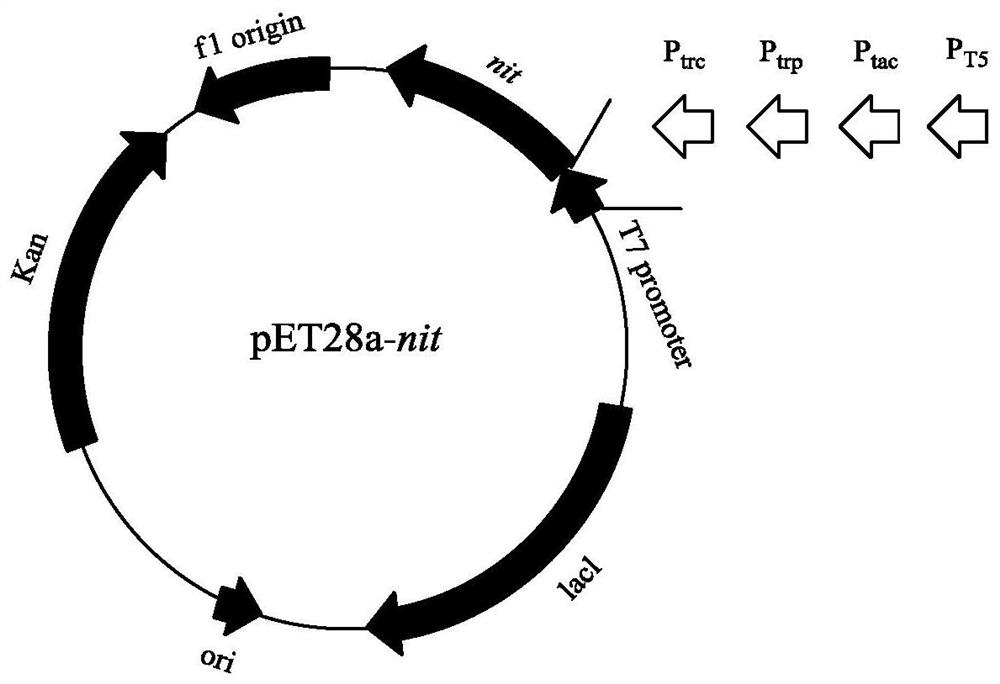
Optimized expression of nitrilase promoter and application thereof
PendingCN113025601AReduce fermentation costsReduce the amount of biocatalystHydrolasesFermentationHeterologousInclusion bodies
The invention discloses a method for screening and replacing a pET commercial plasmid promoter. According to the method, heterologous expression of nitrilase is adjusted from the transcriptional level, the correct folding proportion of nitrilase is increased, soluble expression is increased, and inhibition of inclusion body accumulation on thallus growth is relieved; the promoter mutant strain is subjected to shake-flask induced expression, and the thallus concentration, the specific enzyme activity and the fermentation liquor specific enzyme activity are 1.4 times, 1.6 times and 5.5 times higher than those of commercial plasmids respectively; meanwhile, in the reaction of catalytically preparing the gabapentin intermediate 1-cyanocyclohexyl acetic acid; and the catalytic efficiency of the promoter mutant strain is improved by more than 100% compared with that of the original strain.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
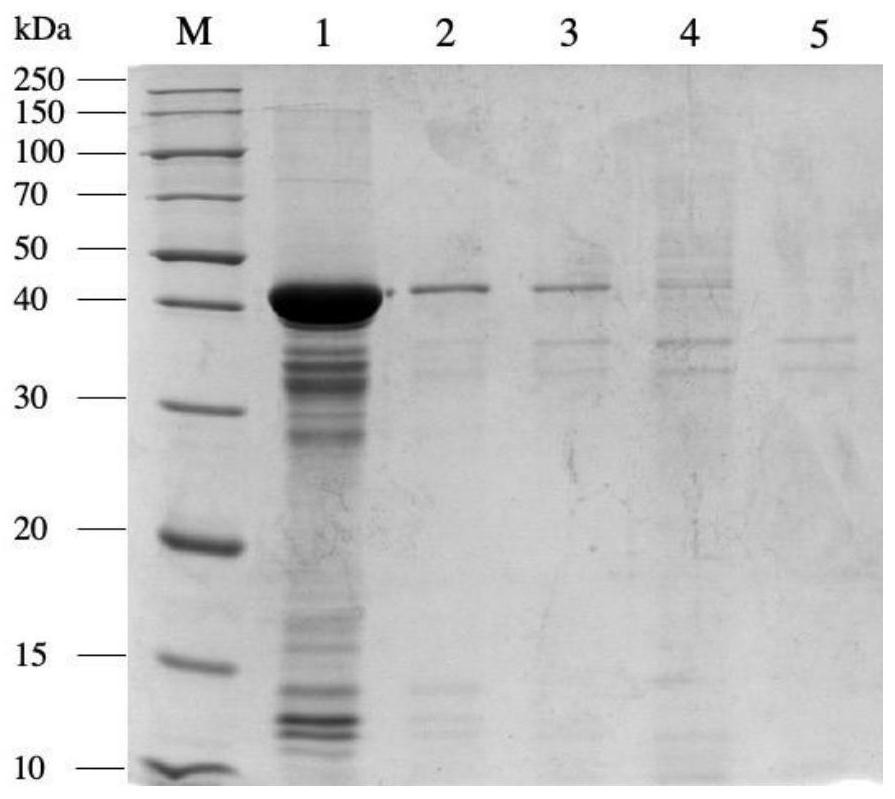
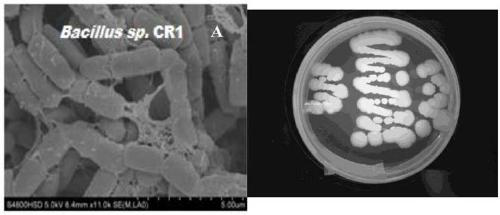
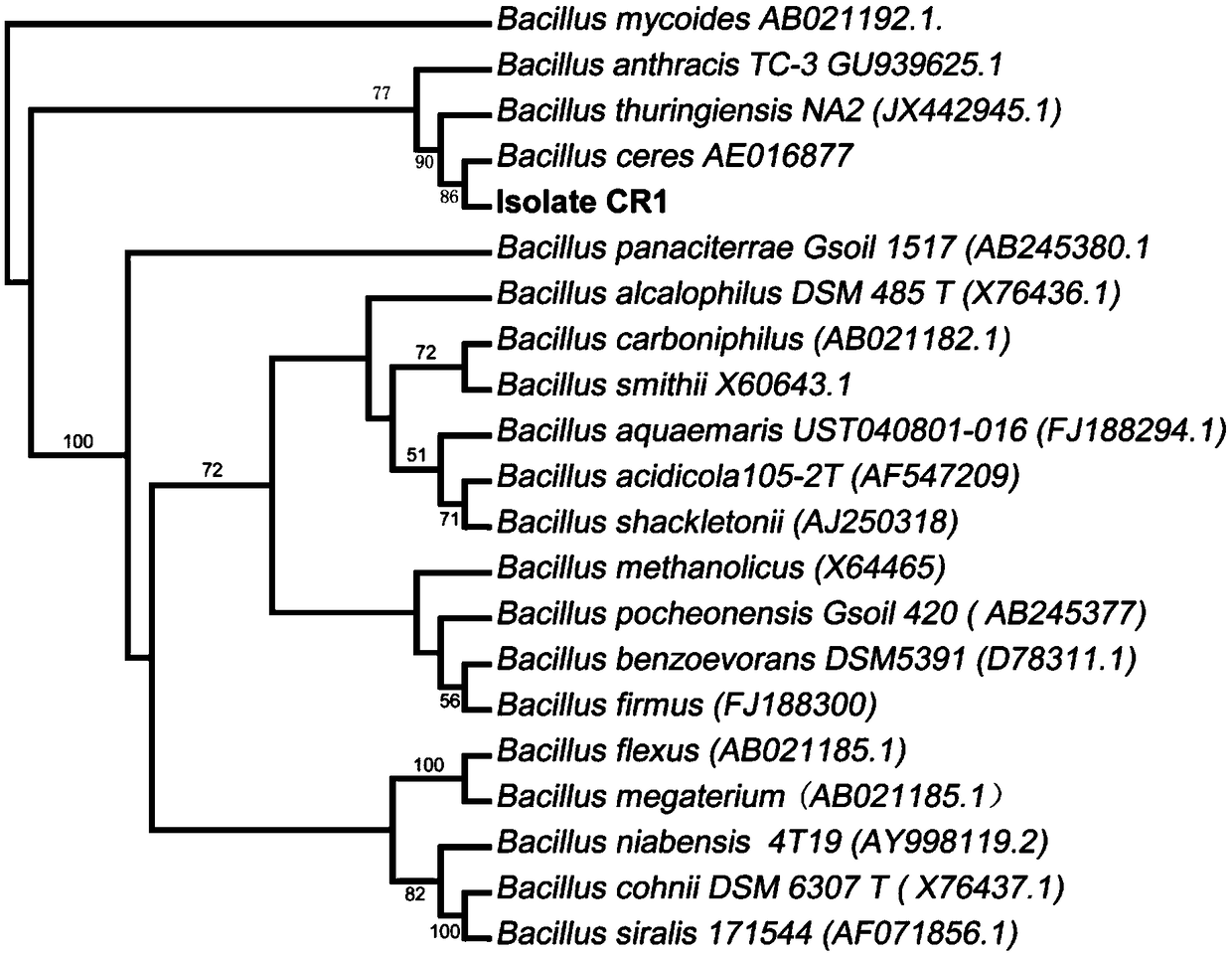
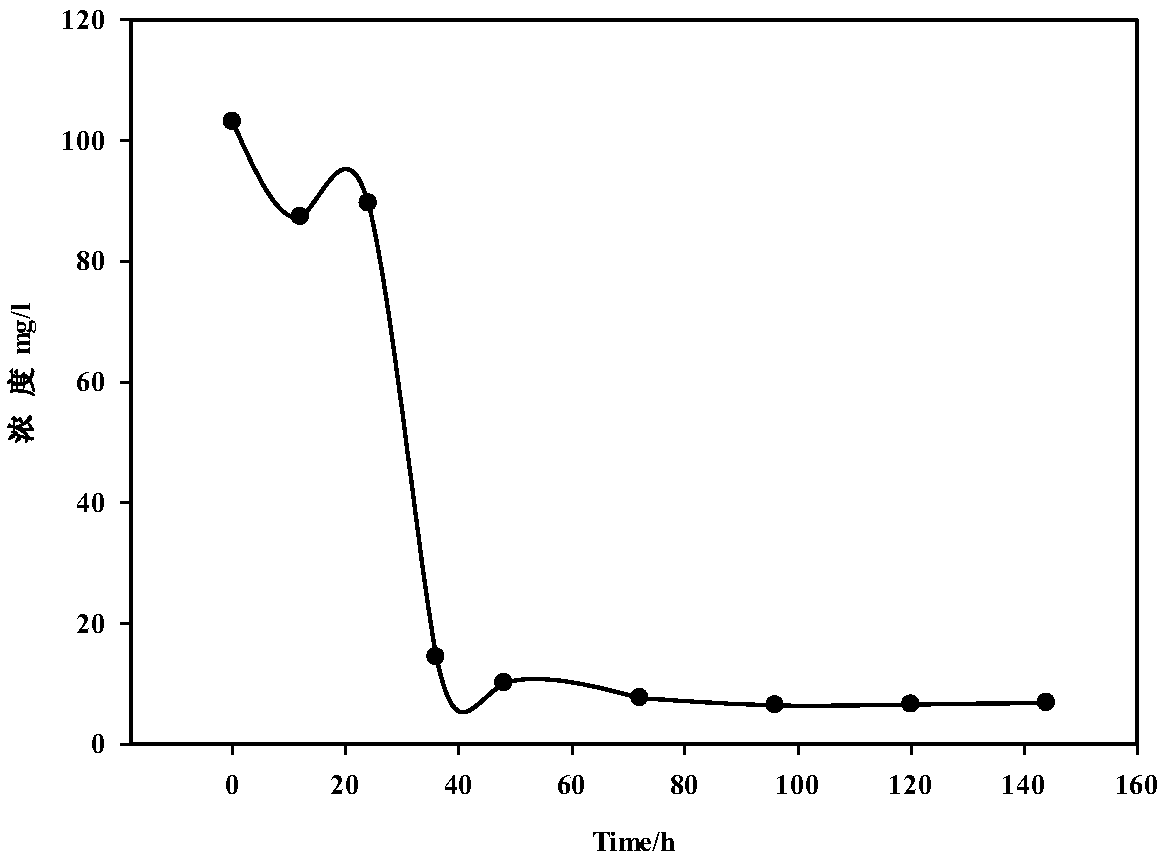
Bacillus capable of degrading naphthenic acids and application of bacillus capable of degrading naphthenic acids
The invention relates to a bacillus capable of degrading naphthenic acids and application of the bacillus capable of degrading the naphthenic acids and particularly relates, separates and identifies abacillus CR1 (in a preservation number of CGMCC NO.7368) capable of biologically degrading five types of naphthenic acids in different chemical structures. The bacillus grows by taking trans-4-methyl-cyclohexanecarboxylic acid, cis-4-methyl-cyclohexanecarboxylic acid, trans-4-tert-butylcyclohexanecarboxylic acid, trans-4-pentyl-cyclohexanecarboxylic acid and dicyclohexyl acetic acid as a unique carbon source, and trans-4-methyl-cyclohexanecarboxylic acid degrading rate and trans-4-pentyl-cyclohexanecarboxylic acid degrading rate are 93.2% and 42.2% respectively, and the bacillus can be applied to biodegradation of naphthenic acids.
Owner:NORTHEAST NORMAL UNIVERSITY
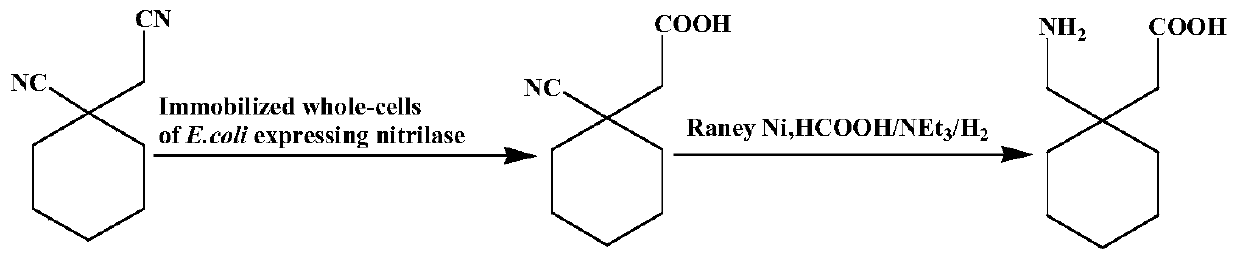
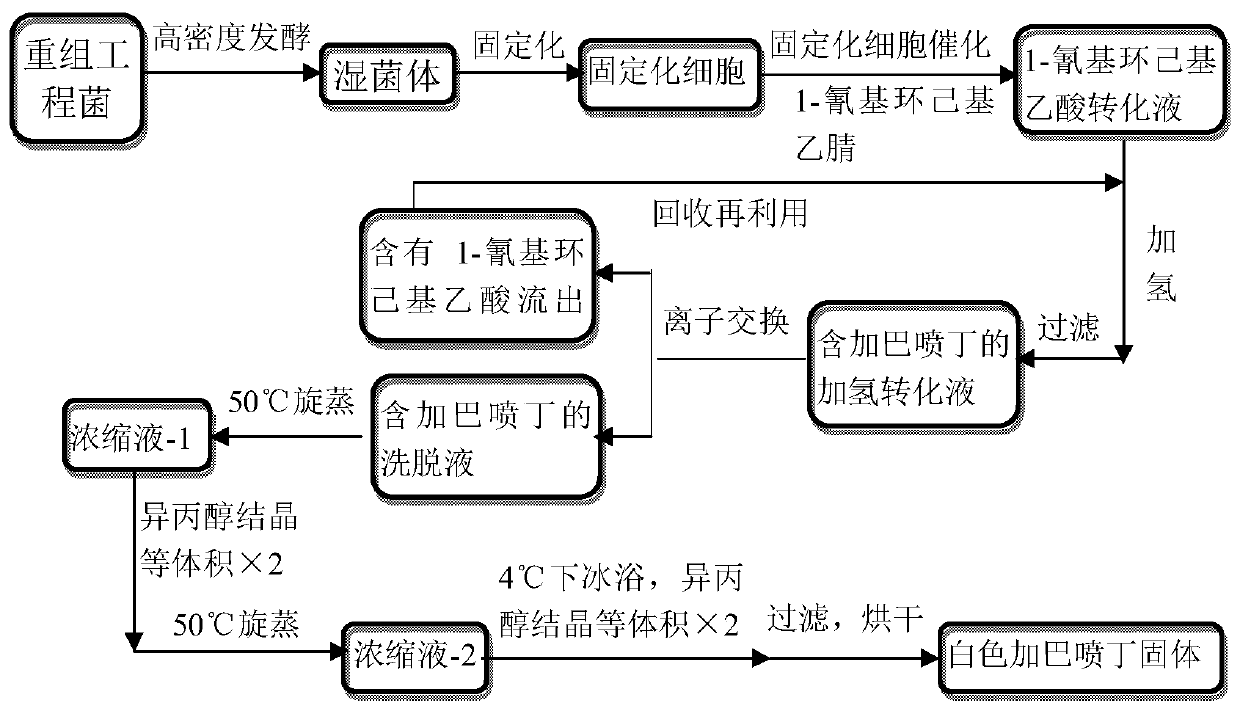
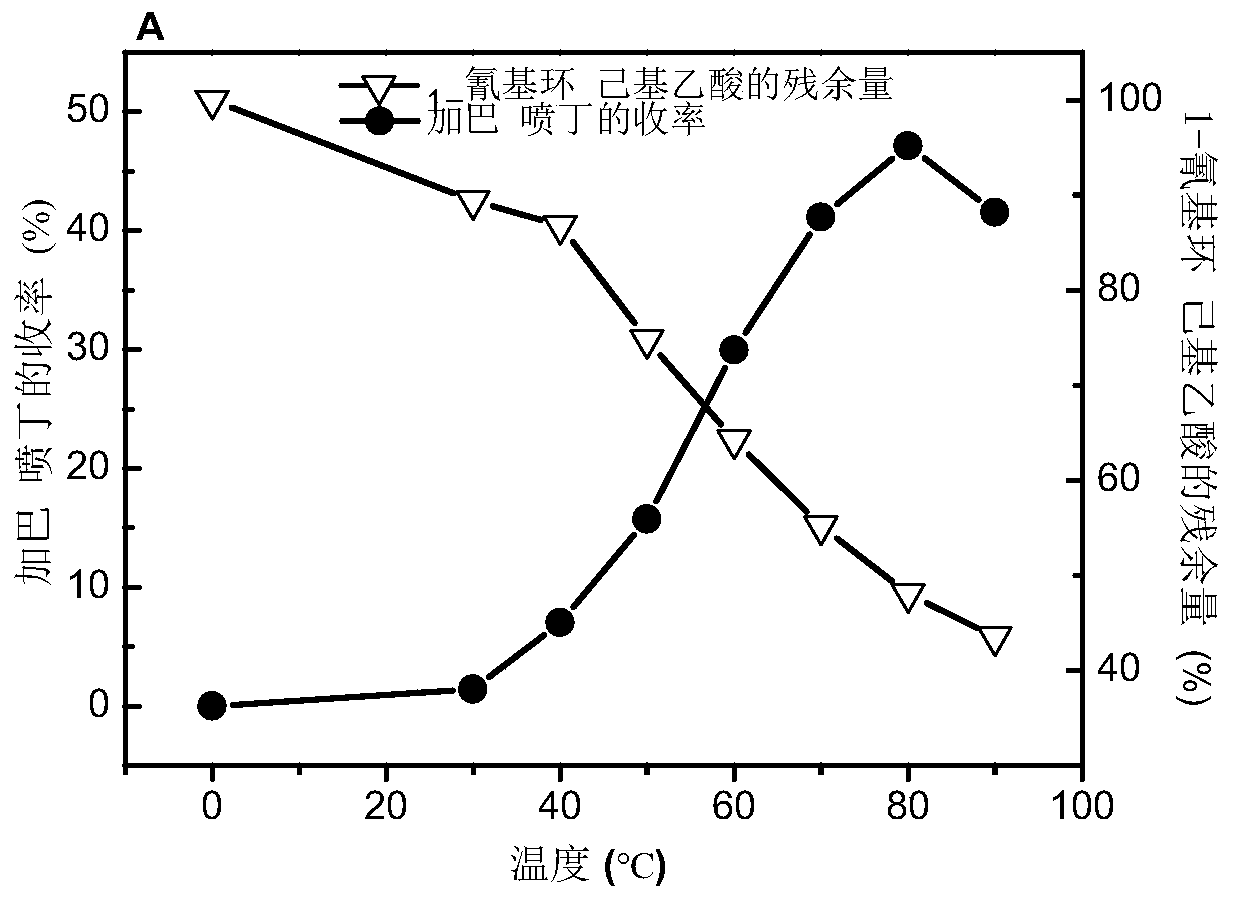
Utilize the method for directly synthesizing gabapentin with 1-cyano cyclohexyl acetic acid
ActiveCN107235850BHigh yieldAchieve conversionOrganic compound preparationAmino-carboxyl compound preparationBiotechnologyPtru catalyst
The invention discloses a method for directly synthesizing gabapentin by virtue of 1-cyanocyclohexyl acetic acid. The method comprises the following steps: dispersing immobilized cells of nitrilase genetically engineered bacteria into deionized water, adding 1-cyanocyclohexyl acetonitrile, stirring the completely react at 20-50 DEG C and 10rpm-350rpm, and carrying out suction filtration, so as to obtain filtrate a; adding the filtrate a into a hydrogenation reaction kettle, adding a catalyst and an aid, introducing nitrogen to completely react at 20-150 DEG C and 300rpm-1100rpm, and separating and purifying reaction liquid, so as to obtain gabapentin. By adding the aid and changing a heating strategy, the reaction is carried out for one batch under the optimal condition, and then the yield of gabapentin reaches 53.3%; 1-cyanocyclohexyl acetic acid is recycled and hydrogenated for five cycles, the yield of gabapentin reaches above 80% and is increased by 20% than that of a reported chemical process.
Owner:ZHEJIANG UNIV OF TECH
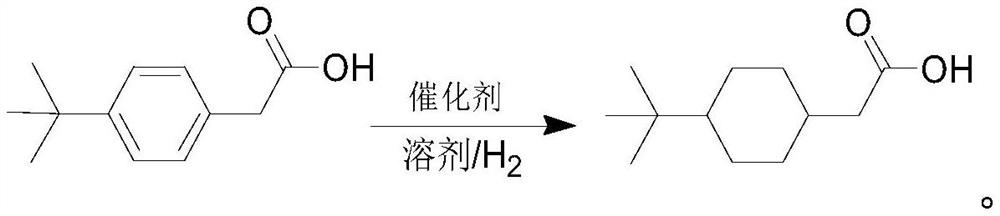
Synthesis method of 4-tert-butyl cyclohexyl acetic acid
InactiveCN114276231ARaw materials are cheap and easy to getThe synthesis process is simpleCarboxylic preparation by ozone oxidationChemical recyclingPhenyl acetic acidPtru catalyst
The invention discloses a synthesis method of 4-tert-butyl cyclohexyl acetic acid, which comprises the following steps: dissolving 4-tert-butyl phenylacetic acid in a solvent, adding a catalyst into the solvent, carrying out high-pressure catalytic hydrogenation reaction, after the reaction is finished, filtering out the catalyst from reaction liquid, desolventizing and concentrating, adding concentrated liquid into water to separate out solid, centrifuging, and drying to obtain the 4-tert-butyl cyclohexyl acetic acid. The refined 4-tert-butyl cyclohexyl acetic acid is obtained. According to the synthesis method provided by the invention, 4-tert-butylphenylacetic acid is adopted as a raw material, the raw material is cheap and easy to obtain, a target product can be obtained through a one-step catalytic hydrogenation reaction, the synthesis process is efficient and simple, the reaction steps are short, the production cost is relatively low, the product yield is high, the product purity is high, and ions harmful to subsequent coupling reaction are not contained; the method has obvious advantages on subsequent synthesis of buparvaquone. In the whole production process, reaction conditions are mild, safe and environment-friendly, equipment conditions are easy to meet, and industrial production is facilitated.
Owner:江苏云朴医药新材料科技有限公司
Nitrilase mutant and its application
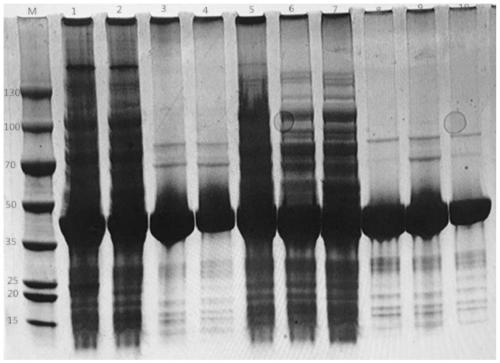
ActiveCN107177576BImprove thermal stabilityHigh activityBacteriaHydrolasesEscherichia coliProtein molecules
The invention discloses nitrilase mutant and application thereof. The mutant is obtained by carrying out mutation one or more of the sits including 201 site, 339 site and 343 site of an amino acid sequence as shown in SEQ ID No.2. By modification of protein molecules, the thermal stability of nitrilase AcN pure enzyme is improved by 14 times to a maximum extent, recombinant escherichia coli which contains the nitrilase mutant is used for hydrolyzing 1-cyanocyclohexane acetonitrile at the high temperature (45 DEG C), and the reaction time is shortened to be one third of the original reaction time. Therefore, the acquired mutant has good application prospect in efficient catalysis of the 1-cyanocyclohexane acetonitrile to synthesize 1-cyanocyclohexane acetic acid as a gabapentin intermediate.
Owner:ZHEJIANG UNIV OF TECH
A kind of method of synthesizing cariprazine
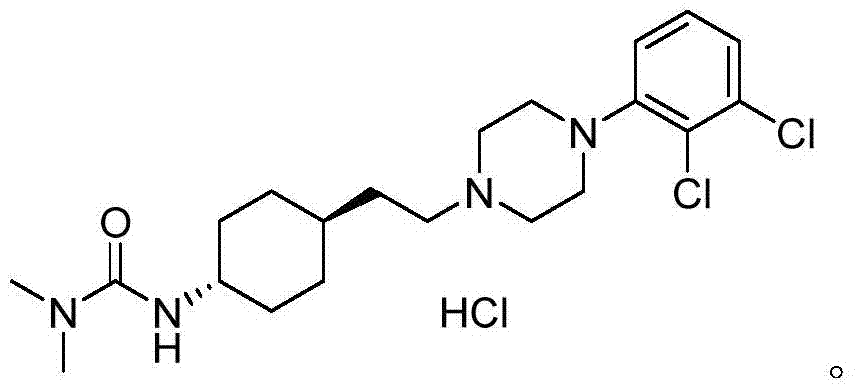
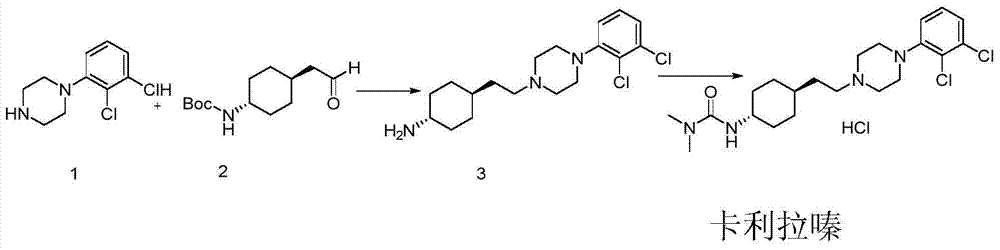
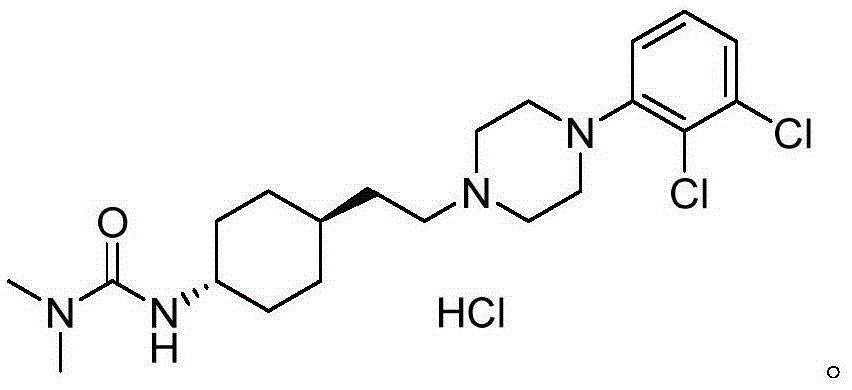
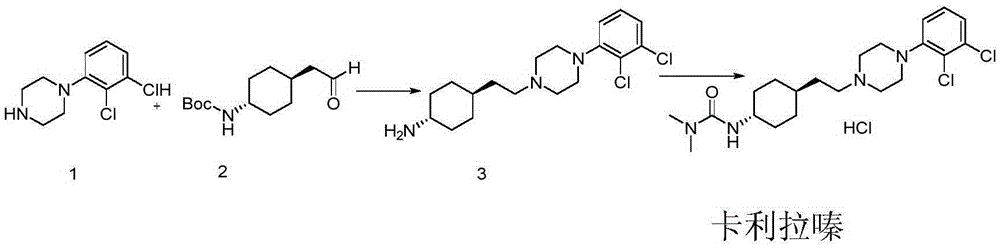
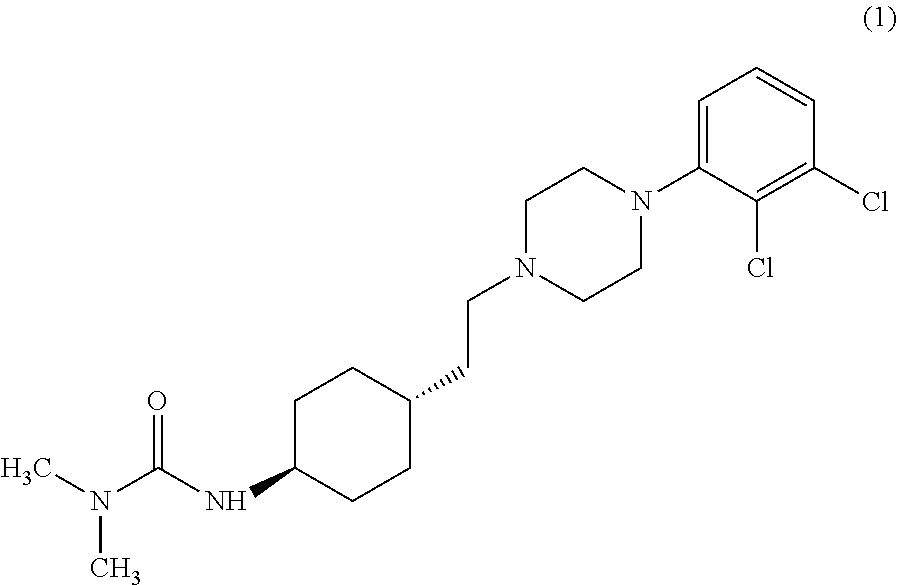
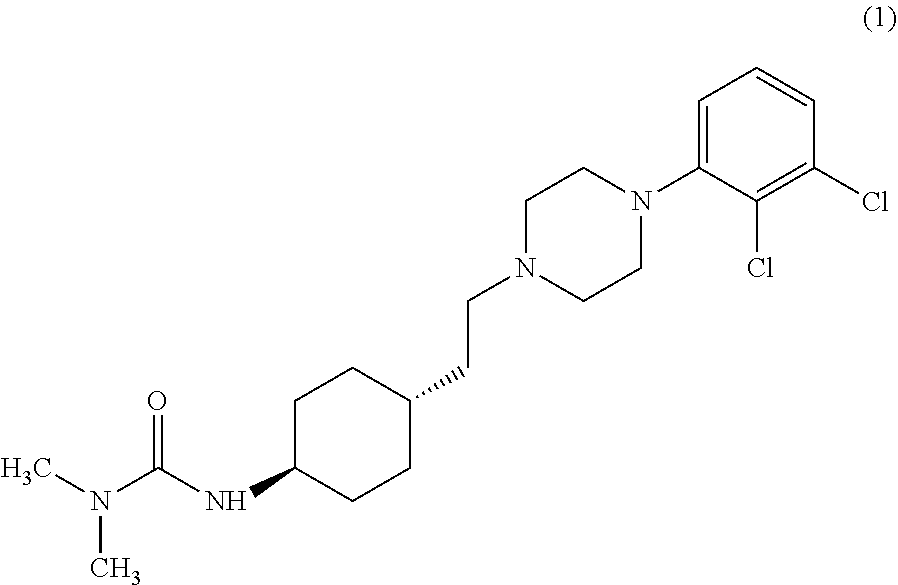
ActiveCN108586389BAvoid re-esterificationSteps to Avoid Reductive RehalogenationOrganic chemistryCariprazineAcetic acid
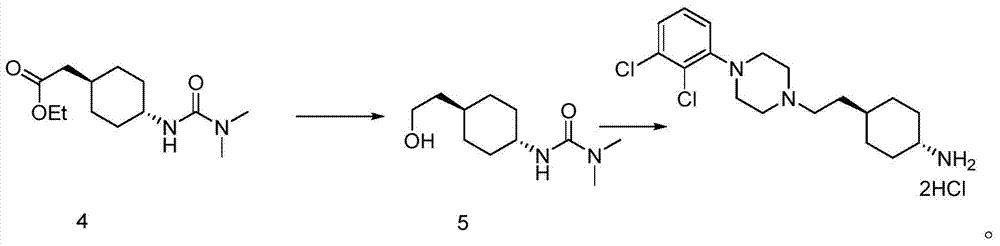
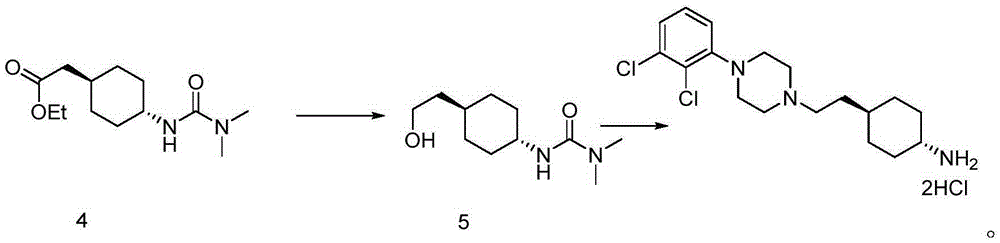
The invention belongs to the technical field of organic synthesis, and provides a novel method for synthesizing cariprazine. The novel method comprises the following steps: firstly, carrying out condensation reaction on trans-2-(4-(3,3-dimethyl ureido) cyclohexyl) acetic acid and 1-(2,3-dichlorophenyl) piperazine to obtain 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea; and secondly, reducing 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea by using borane to obtain the cariprazine. The method hasthe advantages that process steps are greatly shortened, the purity of a final product is ensured and the total yield is obviously increased.
Owner:成都福柯斯医药技术有限公司
A kind of preparation method of 4-amino-cyclohexylacetic acid
ActiveCN106543017BLow priceHigh activityPhysical/chemical process catalystsOrganic compound preparationActive componentHydrogenation reaction
The invention discloses a 4-nitrophenylacetic-acid-catalyzing hydrogenation preparing method for trans-4-amino-cyclohexylacetic acid and hydrochloride. Under the condition of a multiple-active-component supported catalyst prepared with the method, a hydrogenation reaction has the high activity and selectivity, operating is easy, the cost of the catalyst is low, and other reaction steps are not required; the technology is 4-nitrophenylacetic-acid heterogeneous catalysis and hydrogenation, the reaction condition is mild, the method is efficient, simple, easy to carry out, economical and free of pollution, the technology is simple, and large-scale industrial production is facilitated.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
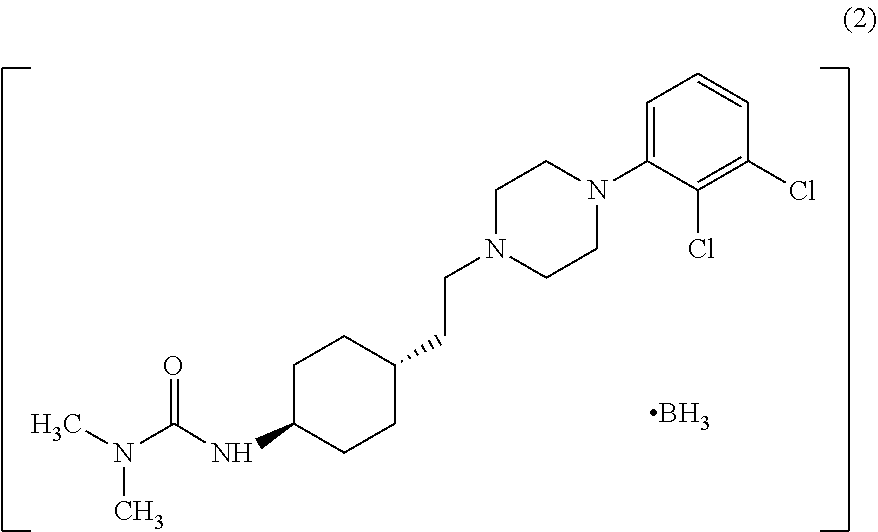
Industrial process for the preparation of cariprazine
In the process of the present invention, cariprazine is prepared by converting (trans-4-amino-cyclohexyl)-acetic acid ethyl ester hydrochloride to trans-4-aminocyclohexyl) acetic acid or its hydrochloride by hydrolysis, from the obtained product with addition of dimethylcarbamoyl derivative as a suitable reagent (trans-4-{[(dimethylamino)carbonyl]amino}cyclohexyl) acetic acid is formed, then the obtained compound is linked to 1-{2,3-dichlorophenyl)˜piperazine in the presence of carboxylic acid activating coupling reagent, and so 1,1-dimethyl-3-[trans-4-(2-oxo-2-(4-(2,3-dichlorophenyl)piperazin-1-yl-ethyl)cyclohexyl] urea is formed, which is converted to cariprazine borane adduct of formula (2) in the presence of reducing agent, and finally the product itself is eliminated directly or is obtained from its salt by a known method. The invention also relates to a group of intermediate compounds that are formed and / or used in the process according to the present invention.
Owner:RICHTER GEDEON NYRT
Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate
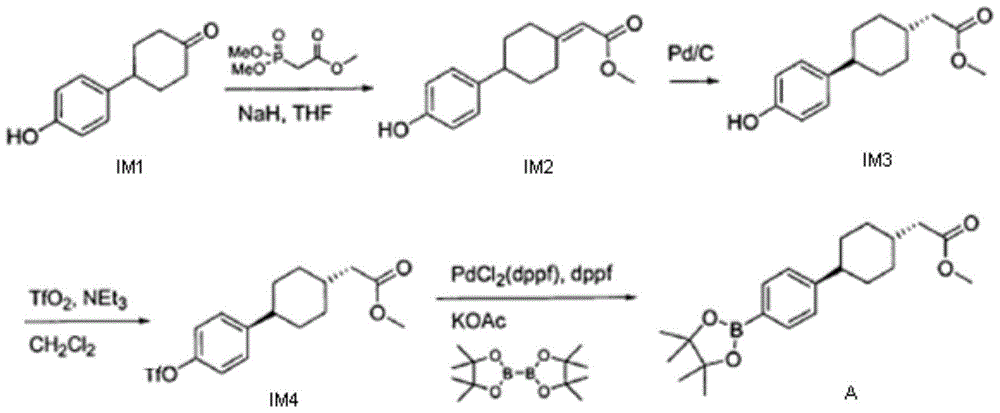
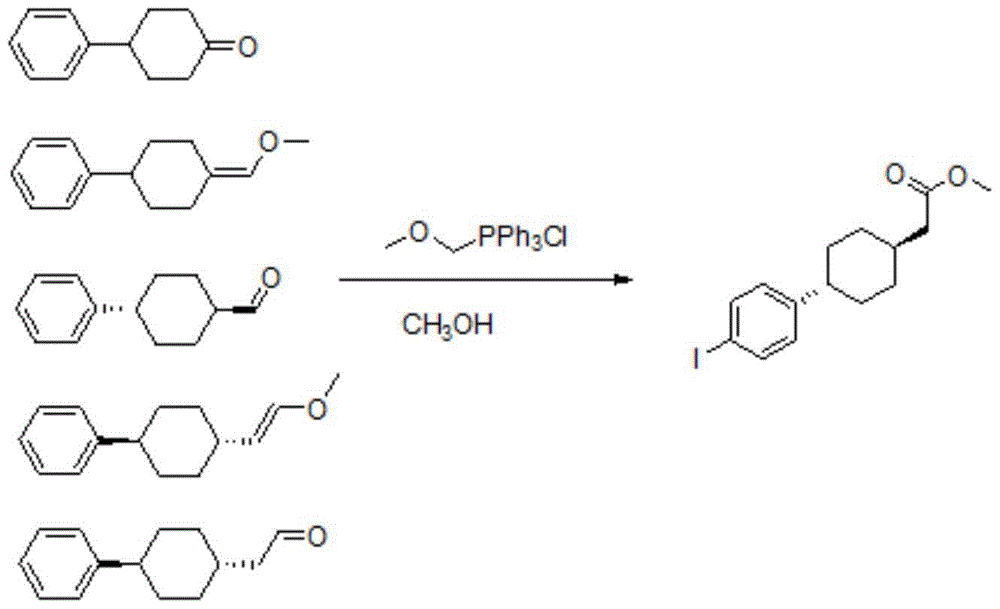
InactiveCN111689854AShort synthetic routeMild reaction conditionsOrganic compound preparationOrganic chemistry methodsFormateCombinatorial chemistry
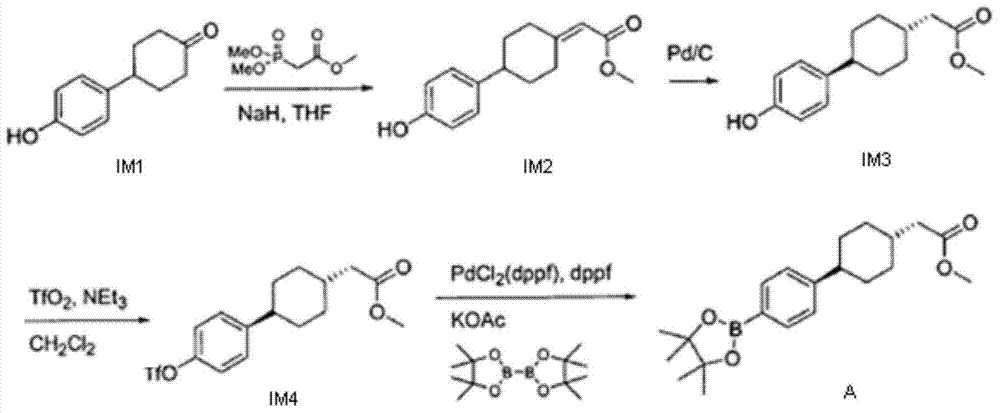
The invention discloses a method for preparing 3-carbonyl-bicyclo[2.2.2]octane-2-formate, 2-(4-alkoxycarbonyl cyclohexylidene)acetate is used as an initial raw material, catalytic hydrogenation and condensation are sequentially performed to obtain the 3-carbonyl-bicyclo[2.2.2]octane-2-formate, and X and Y are independently selected from alkyl or aryl. According to the method, the synthetic route is short, the reaction conditions are mild, and the total yield is greater than 80%; and the method has the advantages of accessible raw materials, lower production cost and low facility request, and is suitable for large-scale industrial production.
Owner:RAFFLES PHAMRMATECH CO LTD
A kind of preparation method of dgat-1 inhibitor intermediate
ActiveCN104496795BLow costCarbon chain lengthOrganic compound preparationCarboxylic acid esters preparationWolff rearrangementAcetic acid
Owner:PORTON FINE CHEM
Polypeptide tag, highly soluble recombinant nitrilase and application thereof in synthesis of pharmaceutical chemicals
PendingUS20220135960A1Increased expression of solubleImprove cell activityHydrolasesAntibody mimetics/scaffoldsPtru catalystArginine
The present invention provides a polypeptide tag and its application in the synthesis of pharmaceutical chemicals, the recombinant nitrilase was obtained by connecting a polypeptide tag to the N-terminus of the amino acid sequence of the nitrilase; wherein amino acids at both ends of the polypeptide tag are uncharged glycine G, and the rest are a random combination of any one or more of glycine G, histidine H, glutamic acid E, aspartic acid D, lysine K and arginine R; The activity of the recombinant nitrilase in the preparation of 1-cyanocyclohexyl acetic acid is up to 3034.7 U / g dcw, the polypeptide tag significantly improves the soluble expression of nitrilase, and the whole cell catalyst hydrolyzes 1M substrate with the same concentration 30 minutes faster than the mother enzyme. The method provided by the present invention can also be used for the biocatalytic reaction of other pharmaceutical intermediates as the substrate catalyzed by the nitrilase, improving the activity of the whole cell catalyst in reaction, and also improving the solubility of other types of nitrilases and the activity of the corresponding whole cell catalysts.
Owner:ZHEJIANG UNIV OF TECH
The preparation method of trans 4-amino-cyclohexyl acetate derivative
ActiveCN106565510BHigh purityGood effectOrganic compound preparationAmino-carboxyl compound preparationCyclohexanoneCariprazine
The invention provides a preparation method for a trans 4-amino-cyclohexyl acetate derivative. The preparation method comprises the following steps that a formula (please see the description for the formula) is provided, wherein R is methyl or ethyl; a compound III is prepared from the raw materials of 4-amino cyclohexanone II and is subjected to hydrogenation reduction, a compound I crude product is obtained, acid is added, and salt is formed; and a compound I is prepared by adding alkali. According to the preparation method, 4-amino cyclohexanone not protected by amino is adopted as raw materials, witting reaction is conducted, and then through catalytic hydrogenation, the trans crude product of the preparation method is obtained. The crude product can be subjected to salifying crystallization so as to obtain the purer trans product, and the trans:syn ratio is 95-99.9:5-0.1. The product compound is high in purity and can be used for preparing cariprazine. Operation is simple, the raw materials are easy to obtain, industrial production is suitable, and large application value is achieved.
Owner:ZHEJIANG JINGXIN PHARMA +2
Encoding genes of nitrilase mutants and application thereof
ActiveUS20210155919A1Improve thermal stabilityEfficiently catalyzing regioselective hydrolysisHydrolasesFermentationProtein moleculesNucleotide
Owner:ZHEJIANG UNIV OF TECH
Recombinant vector constructed from an encoding gene of a nitrilase mutant, a recombinant genetic engineered strain and application thereof
ActiveUS20210155920A1Improve thermal stabilityEffective compoundHydrolasesFermentationEscherichia coliProtein molecules
The present invention discloses a recombinant vector constructed from an encoding gene of a nitrilase mutant, a recombinant genetic engineered strain and application thereof. the nucleotide sequence of the gene is shown in SEQ ID No.5, and the amino acid sequence of the mutant is shown in SEQ ID No.6. In the present invention, by the protein molecular modification, thermostability of the purified nitrilase LNIT5 is increased by up to 4.5 folds; and by utilizing recombinant E. coli containing the nitrilase mutant to hydrolyze 1-cyanocyclohexylacetonitrile at a high temperature (45° C.), product tolerance is increased, activity of NIT5-L201F is increased by 20%, and the mutant NITLNIT5-AcN can completely hydrolyze 750 mM 1-cyanocyclohexylacetonitrile within 8 hours and achieve an doubled conversion rate. Therefore, the mutants obtained by the present invention have a good application prospect in efficiently catalyzing 1-cyanocyclohexylacetonitrile to synthesize gabapentin intermediate, 1-cyanocyclohexyl acetic acid.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



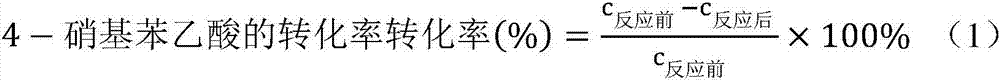

![Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate](https://images-eureka.patsnap.com/patent_img/7c60ae85-477c-495d-b54e-88f365bc2c85/560587DEST_PATH_IMAGE001.png)
![Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate](https://images-eureka.patsnap.com/patent_img/7c60ae85-477c-495d-b54e-88f365bc2c85/RE-GDA0002600399630000011.png)
![Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate Process for preparation of 3-carbonyl-bicyclo[2.2.2]octane-2-formate](https://images-eureka.patsnap.com/patent_img/7c60ae85-477c-495d-b54e-88f365bc2c85/RE-GDA0002600399630000021.png)