Polypeptide label, highly soluble recombinant nitrilase and application thereof in synthesis of pharmaceutical chemicals
A technology of polypeptide labeling and nitrilase, which is applied in the fields of genetic engineering and protein engineering, can solve the problems of insufficient enzyme activity for large-scale industrial applications and poor thermal stability of enzymes, and achieve the effects of improving activity, excellent stability, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Construction of recombinant plasmids containing polypeptide tags
[0053] 1. The design principle is: the solubility of the protein is closely related to the hydrophobicity of the residues, and is also affected by the net charge of the protein or the ratio of helical residues. Polar amino acids have an important effect on the solubility of proteins. Palindromic element sequences usually consist of multiple repeating units containing one or two polar amino acids, have positive or negative charges, have been reported to facilitate protein folding, and are usually smaller than 15 residues.
[0054] Based on the above principles, we first designed a pentapeptide tag, in which both ends and the middle (i.e. 1, 3, 5 amino acids) are uncharged glycine (G), and the rest (i.e. 2, 4 amino acids) are glycine ( G), any one or two random combinations of histidine (H), glutamic acid (E), aspartic acid (D), lysine (K), arginine (R), specifically Is one of: GDGDG, GDGEG, G...
Embodiment 2
[0065] Embodiment 2: Construction of recombinant escherichia coli
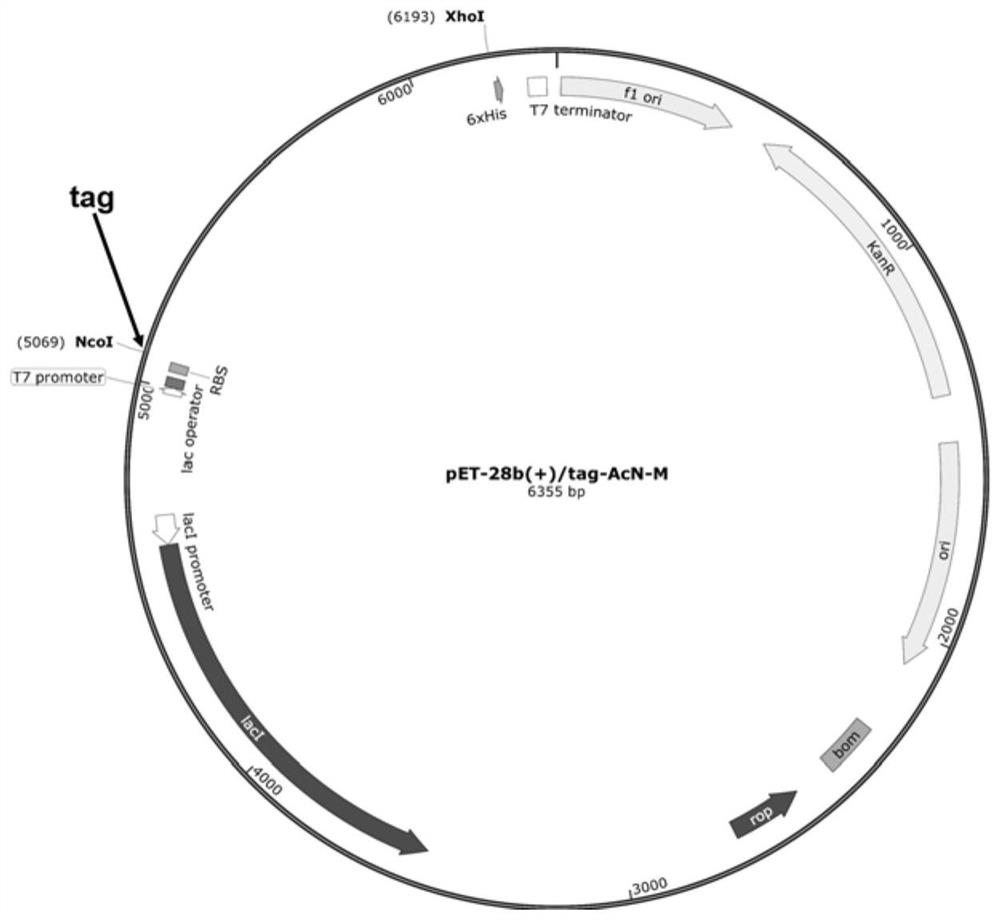
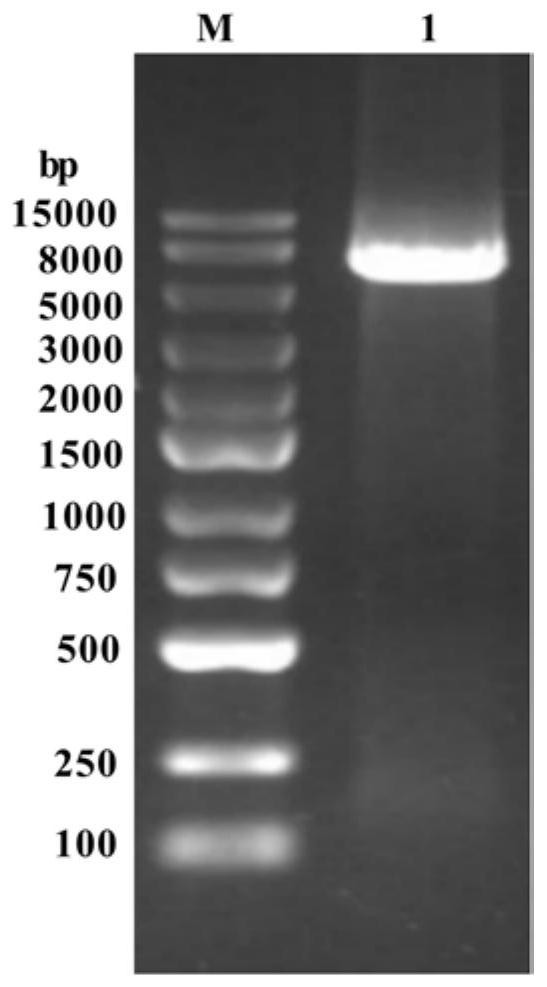
[0066] Axygen clean-up kit (purchased from Corning Life Science (Wujiang) Co., Ltd.) was used to purify (clean-up) the PCR product containing recombinant plasmid pET-28b(+) / tag-AcN-M in Example 1, The specific operation is: add three times the volume of PCR-A buffer to the 5 μL PCR product of Example 1 and mix well, transfer to the preparation tube, centrifuge at 12000 rpm for 1 min, discard the filtrate, add 700 μL W2 buffer to the preparation tube, and centrifuge at 12000 rpm 1min, discard the filtrate, wash twice with W2 buffer; add 5μL to pre-thawed competent cells E.coli BL21(DE3), ice-bath for 30min, then heat shock at 42°C for 90s, ice-bath again for 3-5min, add 700μL LB Liquid medium, incubate at 37°C for 1h. Inoculate 500 μL of the culture solution onto the LB solid medium with 0.5 μg / mL kana-resistance, spread evenly, culture at 37°C for 12-14 hours, and verify by sequencing to obtain recombinant Es...
Embodiment 3
[0067] Embodiment 3: Recombinant escherichia coli expresses nitrilase
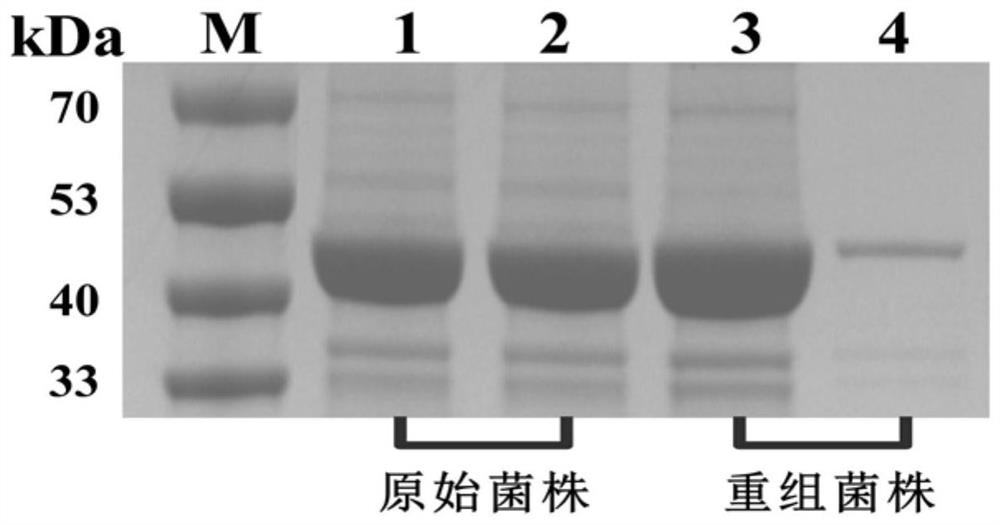
[0068] 1. Resting cells: Take out the recombinant Escherichia coli E. coli BL21(DE3) / pET-28b(+) / tag-AcN-M strain constructed in Example 2 and inoculate it to a concentration of 0.5 Streak the μg / mL kana-resistant LB plate and culture at 37°C for 12-14 hours to obtain a single colony. Pick a single colony into 10mL LB test tube medium containing 0.5μg / mL kanamycin, culture at 37°C for 8h, then transfer 2mL to 100mL fermentation medium containing 0.5μg / mL kanamycin, 37°C After culturing for 2 hours, 100 μL of IPTG (final concentration 0.1 mM) was added to induce enzyme production at 28° C. for 12-14 hours. After centrifugation at 12,000 rpm for 10 min, the obtained bacteria were collected, washed twice with 0.9% normal saline, and then suspended to obtain a resting cell suspension, and the relative enzyme activity was determined.
[0069] 2. Pure enzyme: Take 1g of resting cells prepared by the method in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com