Nitrilase mutant and application thereof in preparation of 1-cyanocyclohexyl acetic acid
A technology of nitrilase and mutants, which is applied in the field of preparation of antiepileptic drug gabapentin, can solve problems such as high risk, equipment corrosion, environmental pollution, etc., and achieve good application prospects and shorten the effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
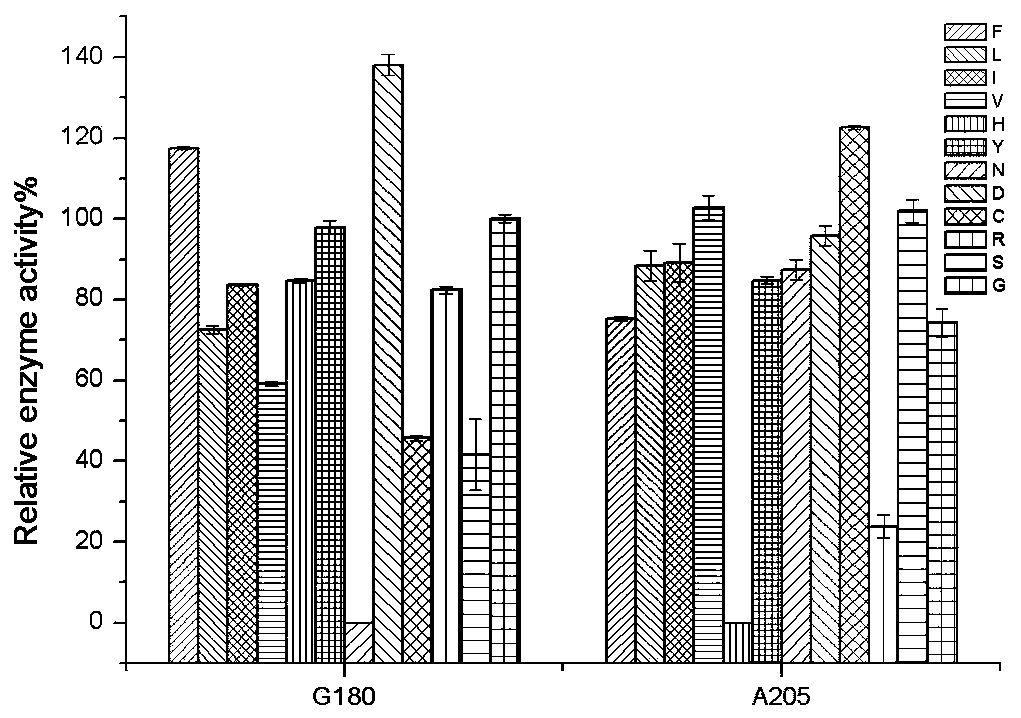
[0030] Example 1: Site-directed mutagenesis and screening
[0031] 1. Mutation site selection
[0032] The present invention utilizes site-directed mutation technique to carry out site-directed mutation at 168 sites on the nitrilase coding gene (GenBank Accession no.: AHW42593.1) derived from A. facilis CCTCC NO: M 029044, and mutates into E.coli BL21(DE3) / pET-28b(+)-AcN-F168V (see Zhang X H, et al. .ProcessBiochemistry, 2014.), based on this, mainly aimed at the amino acid site on the "A surface" as the mutation site, and after the site-directed mutation was successful by using the whole plasmid PCR, the expression vector where the target gene was located was transferred into E. coli Host, after induced expression, the positive mutants were screened out by enzyme activity detection method, and the enzyme activity was repeatedly detected to determine the mutant with improved enzyme activity, so as to obtain a self-assembly tendency and be able to efficiently catalyze the regi...
Embodiment 2
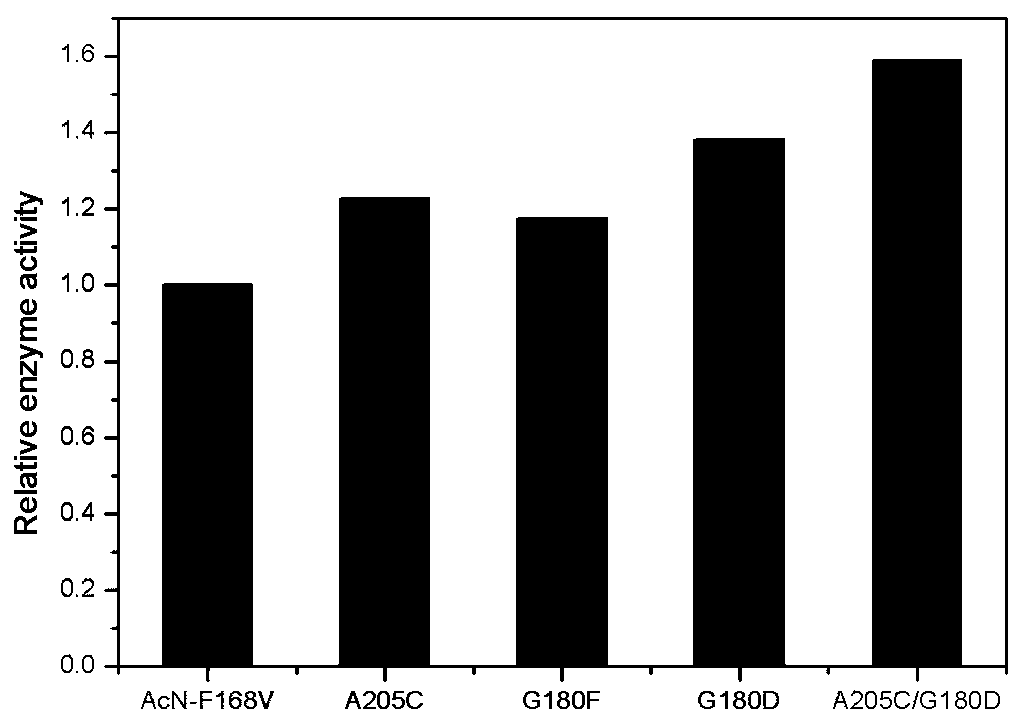
[0039] Example 2: Expression of nitrilase mutants
[0040] A plasmid pET-28b(+)-AcN-F168V containing nitrilase AcN-F168V (ie, SEQ ID No.1) from Acidovorax facilis CCTCC NO:M 029044 was constructed. The constructed pET-28b(+)-AcN-F168V expression plasmid was introduced into Escherichia coli E. Coli BL21(DE3) to realize overexpression. Site-directed mutagenesis was carried out by site-directed saturation mutagenesis, and recombined into the expression vector pET-28b(+), and then the recombinant plasmid was transferred into the expression host E. Coli BL21(DE3) to construct mutants. Obtain the mutant transformant E.coli BL21(DE3) / pET-28b(+)-AcN-G180F of embodiment 1, E.coli BL21(DE3) / pET-28b(+)-AcN-G180D, E.coli BL21(DE3) / pET-28b(+)-AcN-A205C and combined mutant E.coli BL21(DE3) / pET-28b(+)-AcN-G180D / A205C, and the original strain E.coli BL21(DE3) / pET-28b(+)-AcN-F168V (see Zhang X H, et al. Activity improvement of a regioselective nitrilase from Acidovorax facilis and its appli...
Embodiment 3
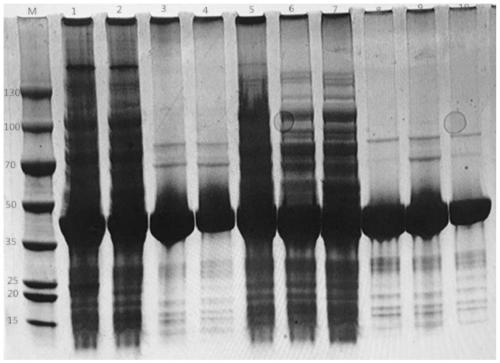
[0041] Embodiment 3: the purification of nitrilase and mutant protein thereof
[0042] (1) Add binding buffer (50mM NaH 2 PO 4 , 300mM NaCl, pH 8.0), resuspended bacteria and then ultrasonically disrupted (400W, 20min, 1s break and 1s pause). After the crushed product was centrifuged (8000rpm, 15min), the supernatant was taken as a crude enzyme solution for separation and purification.
[0043] (2) After prepacking 10mL Ni-NTA affinity chromatography column, use binding buffer (50mM NaH 2 PO 4 , 300mMNaCl, pH 8.0) for washing at a flow rate of 2mL / min.
[0044] (3) After cleaning 8-10 column volumes, pass the obtained crude enzyme solution through the Ni-NTA column at a flow rate of 1 mL / min, and the target protein is mounted on the chromatography column. After loading, a large number of unadsorbed foreign proteins will not be bound to the resin and will be removed directly.
[0045] (4) Use equilibration buffer (50mM NaH 2 PO 4 , 300mM NaCl, 50mM imidazole, pH 8.0) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com