Method for improving specific activity and activation efficiency of transglutaminase
A technology of transglutaminase and glutamine is applied in the field of improving the specific enzyme activity and activation efficiency of transglutaminase, which can solve the problem of low heterologous expression and secretion, inability to secrete TGase, and affecting the secretion and catalytic activity of TGase. and other problems to achieve the effect of improving cutting efficiency, shortening reaction time, and improving specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Simulation of MTG crystal structure derived from Streptomyces hygroscopicus
[0028] Using the reported S. mobaraensis pro-TGase (PDB code: 3IU0) as a template (the amino acid similarity between the two is 73.1%), the online simulation software SWISS-MODEL was used to simulate the crystal structure of S. hygroscopicus TGase.
Embodiment 2
[0029] Embodiment 2: the acquisition of mutant
[0030] The gene sequence of the short peptide was designed on the primer, and inserted into the C-terminus of the leading peptide by site-directed mutagenesis. Using the S. hygroscopicus pro-TGase expression plasmid pBB1-1011 as a template (in the previous study, our laboratory screened out a new strain of transglutaminase production (Streptomyces hygroscopicus CCTCC M203062), through gene cloning method, Obtained the MTG gene sequence and its upstream and downstream sequences, including MTG's own promoter and terminator (Genbank: EU477523), the specific documents are Liu S, Zhang D, Wang M, Cui W, Chen K, Liu Y, Du G, Chen J, Zhou Z (2011) The pro-region of Streptomyces hygroscopicus transglutaminase affects its secretion by Escherichia coli. FEMS Microbiol Lett324 (2): 98-105), the whole plasmid PCR. The primers are listed in Table 1 and were synthesized by Shanghai Sangon Bioengineering Company. The primer Pro-52-R is the d...
Embodiment 3
[0034] Embodiment 3: Detection of mutant enzymatic properties
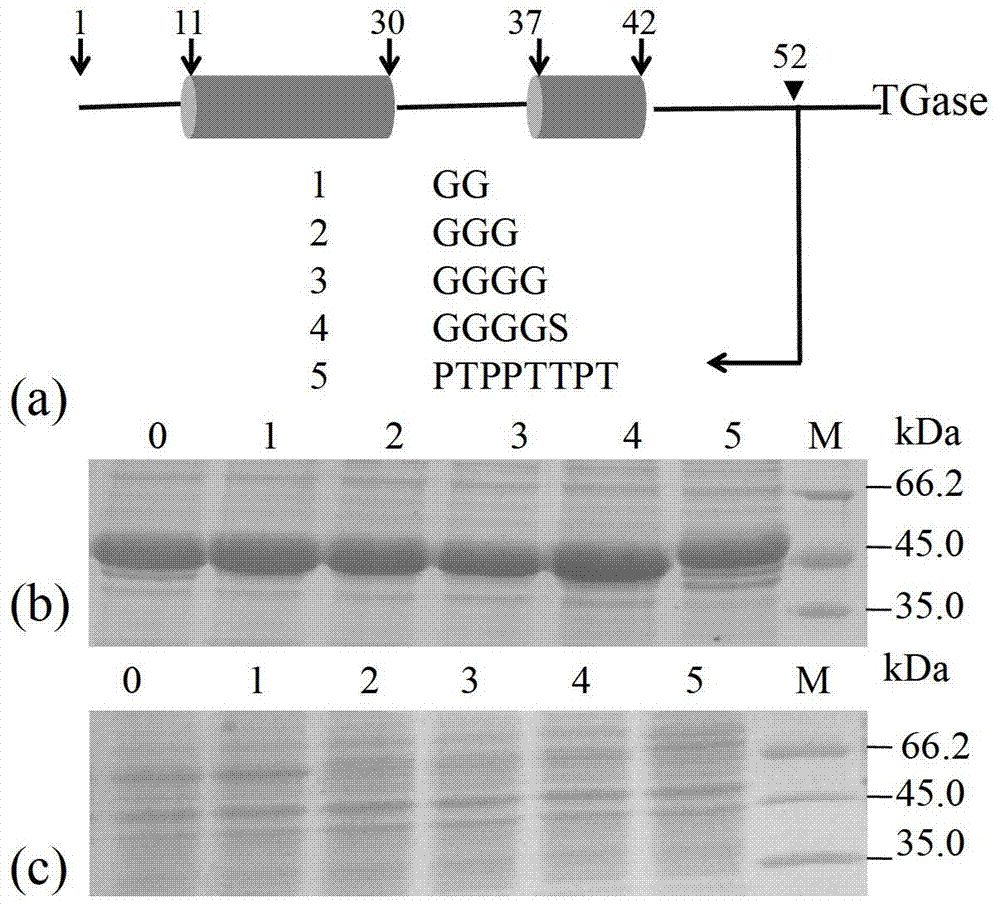
[0035] In order to allow the leader peptide to be cleaved normally after mutation, the short peptide insertion site is selected before the leader peptide cleavage site (L53-F54), that is, before L53 ( figure 1 a). GG, GGG, GGGG, GGGGS and PTPPTTPT were selected as insertion short peptides, and the corresponding mutants were pro-52GG, pro-52GGG, pro-52GGGG, pro-52GS and pro-52-PT, respectively. The above-mentioned mutants were fermented, and intracellular and extracellular pro-TGase were detected by SDS-PAGE, and all mutant enzymes could be secreted extracellularly ( figure 1 b), and no intracellular accumulation ( figure 1 c), showing that the insertion of the above short peptide at the C-terminus of the leader peptide has no obvious effect on its secretion.
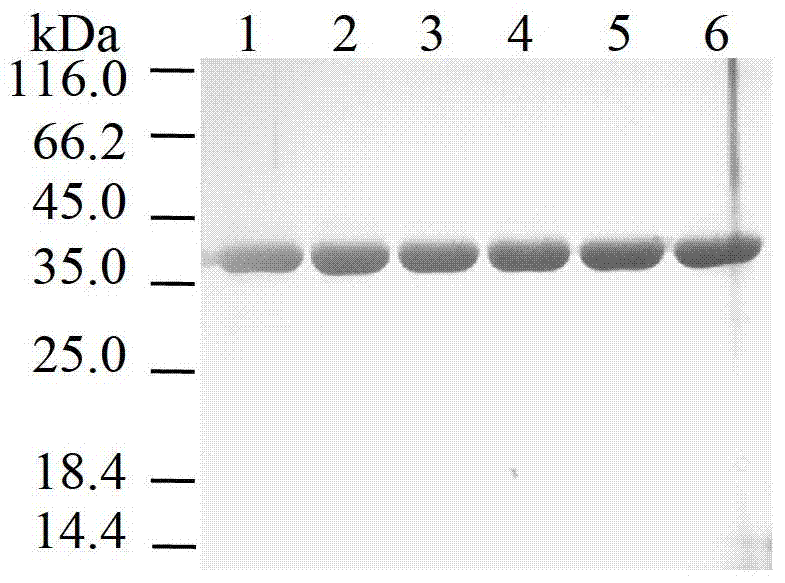
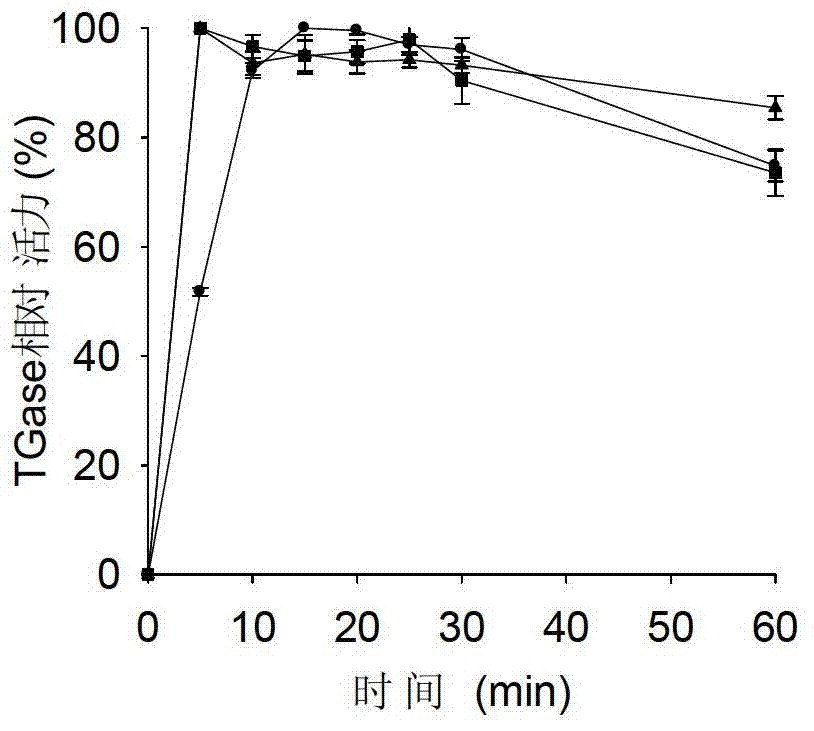
[0036] Purification of the above mutant enzymes ( figure 2 ), to detect enzymatic properties. Compared with the wild enzyme, inserting short peptides ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com