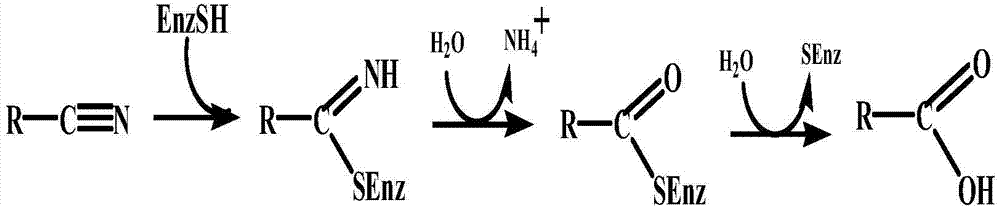
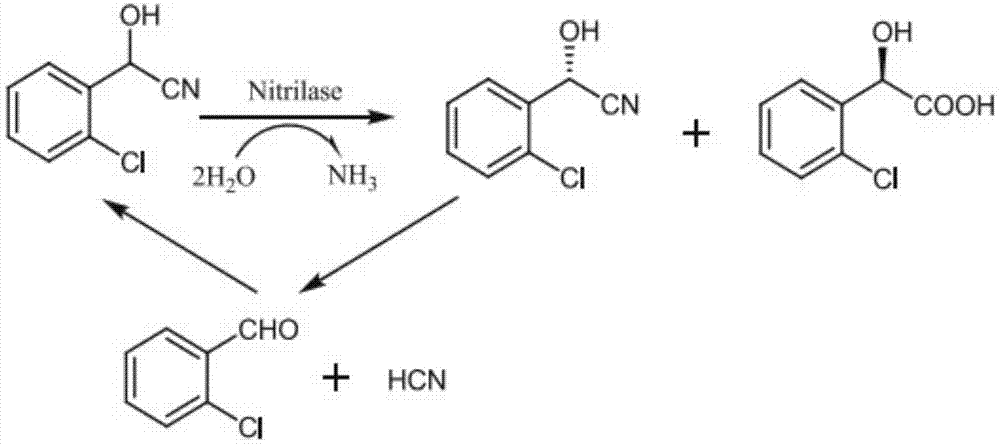
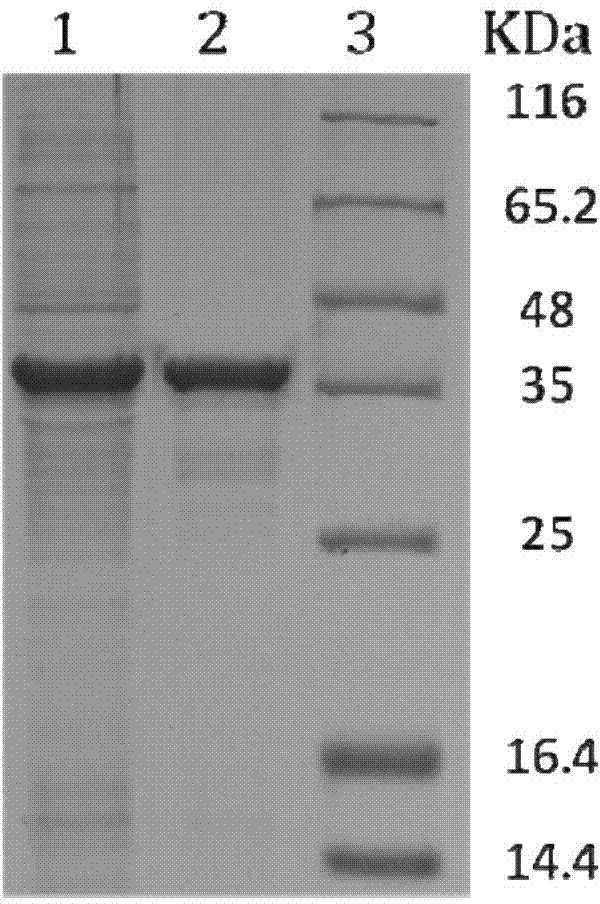
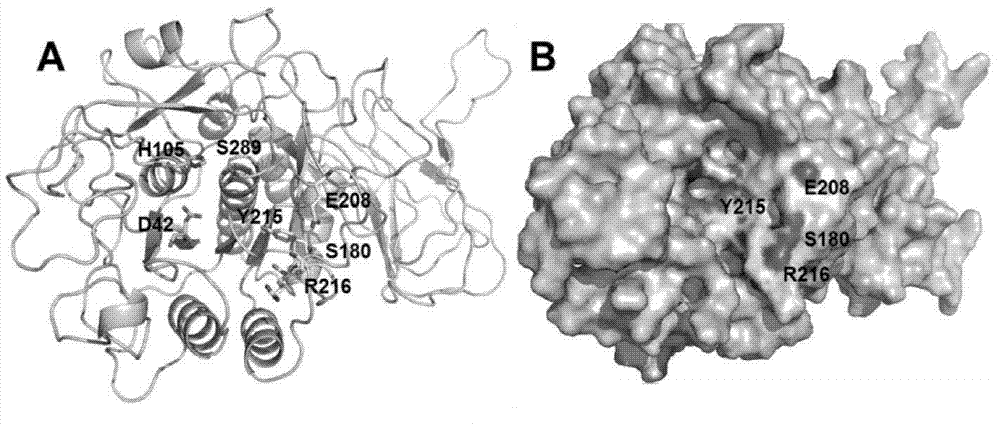
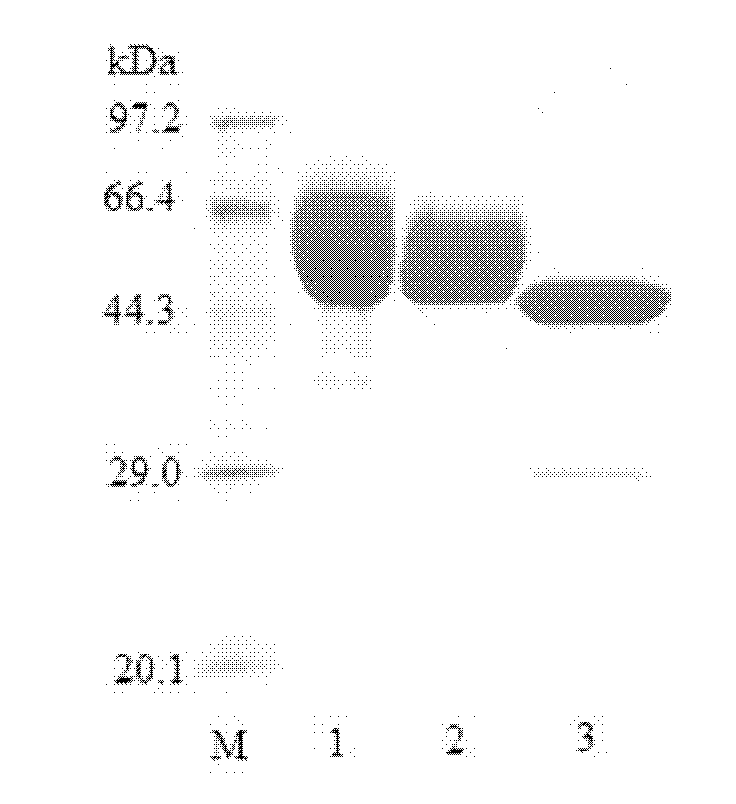
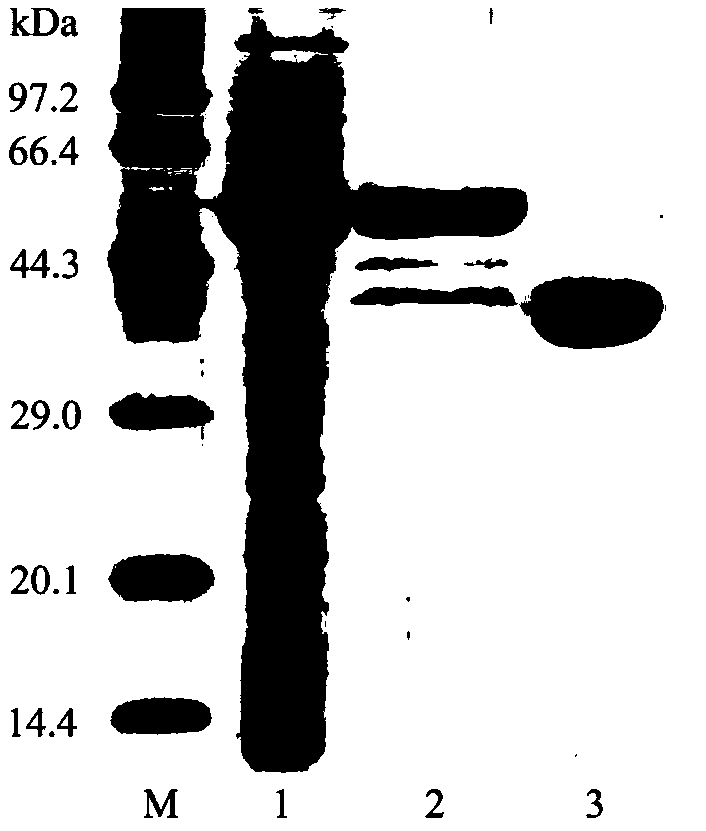
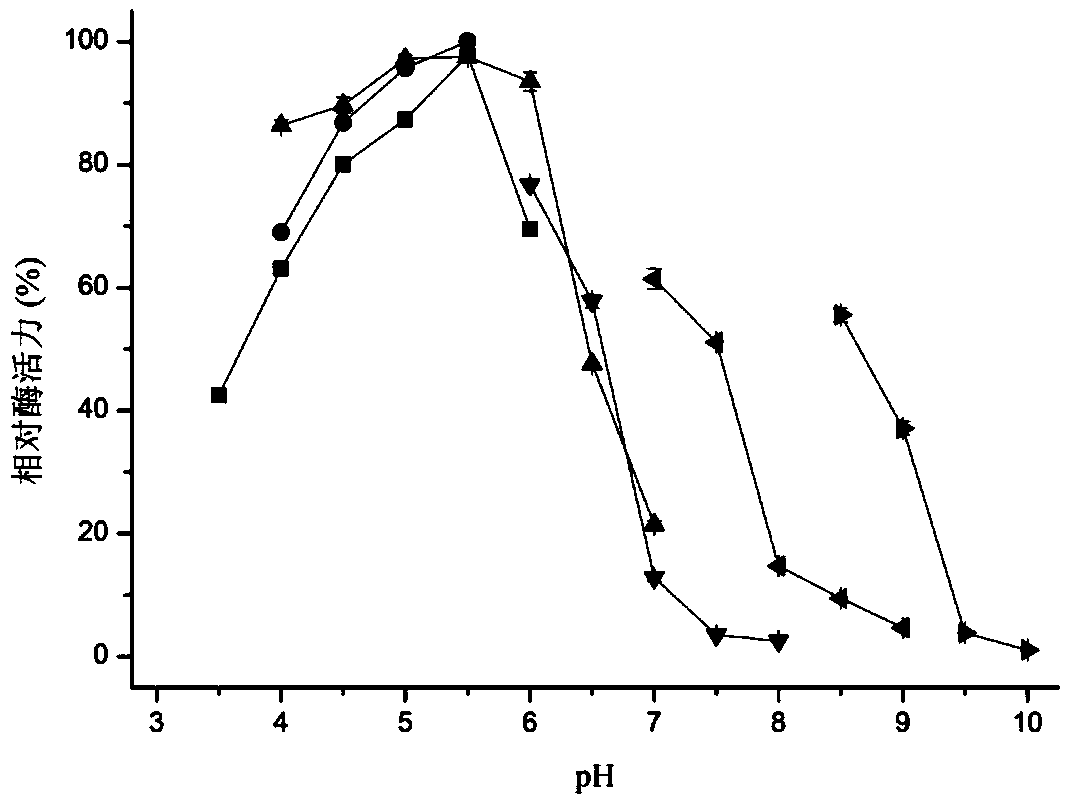
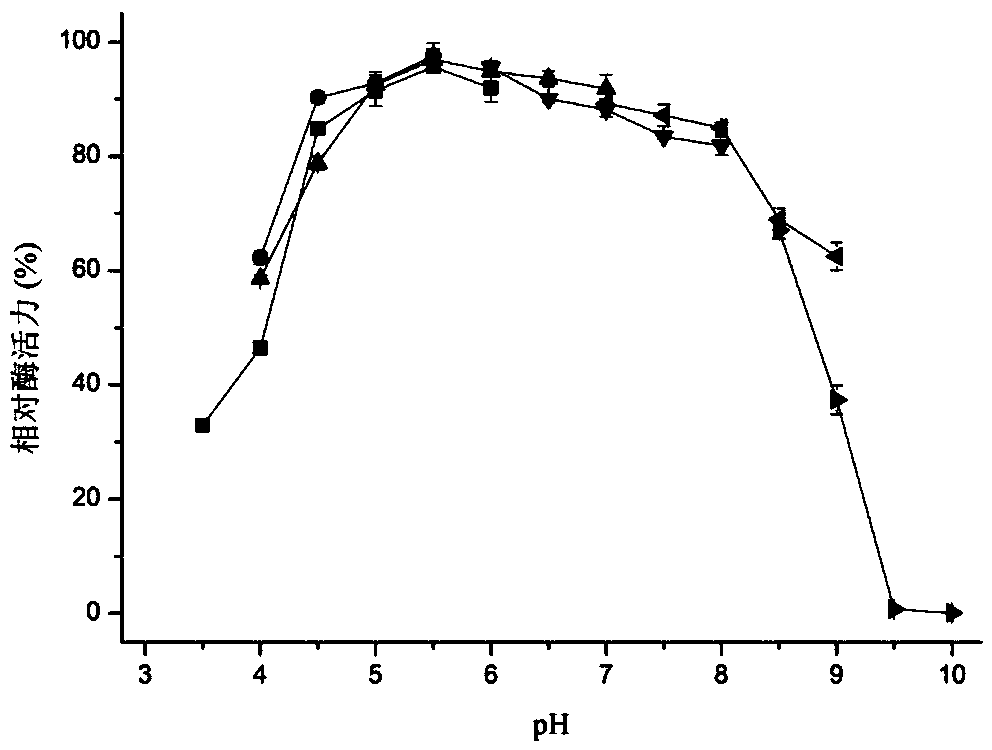
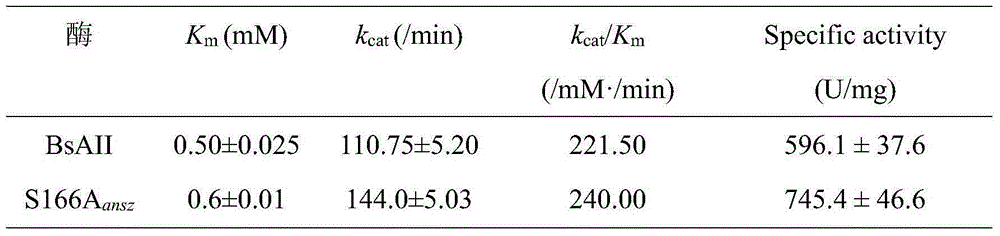
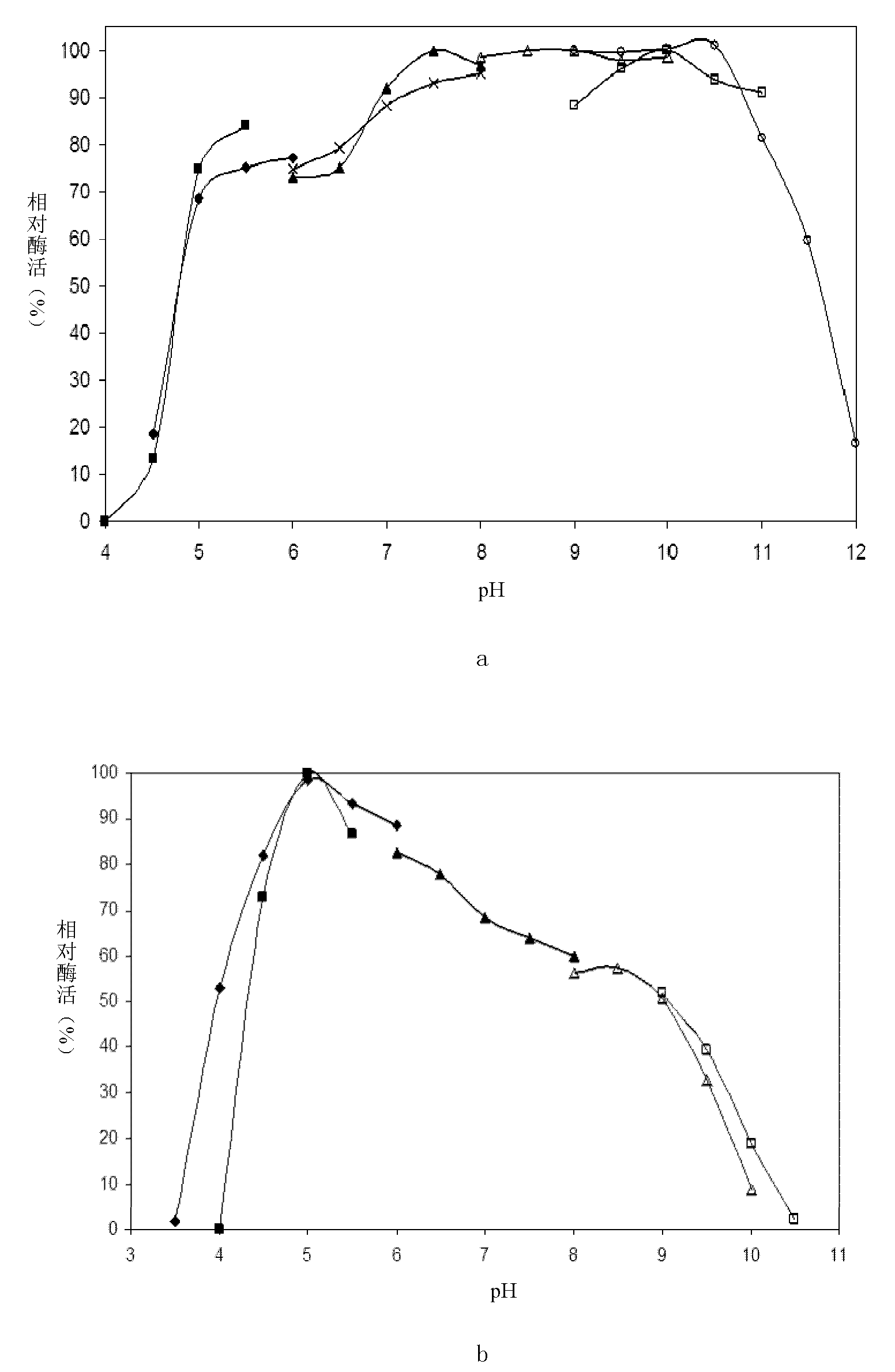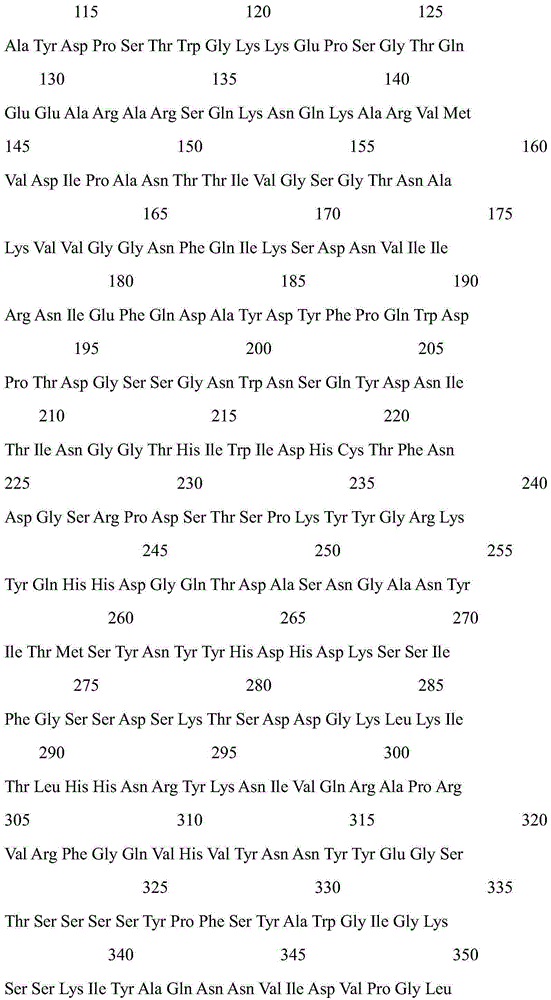Patents
Literature
92results about How to "Increased specific enzyme activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrilase mutant and application thereof
ActiveCN104774825AIncreased specific enzyme activityBacteriaHydrolasesDouble mutationSingle mutation
The invention discloses a nitrilase mutant and its application in R-o-chloromandelic acid synthesis. The mutant is obtained by single mutation or double mutation of the 132nd threonine or 189th phenylalanine on the amino acid sequence shown as SEQ ID NO.2. Compared with non-mutant nitrilase, the enzyme activity is increased by 3.70 times and reaches 1.08U / mg, and the enantioselectivity is up to 99%. Also, the result shows that in a two-phase system (with ratio of toluene to water being 2:8) the nitrilase mutant can catalyze 300mM o-chloromandelonitrile, and the yield is 90.8%. Through fed batch of the substrate, a maximum of 500mM o-chloromandelonitrile can be catalyzed, the yield is 90%, and the enantioselectivity is greater than 99%.
Owner:ZHEJIANG UNIV OF TECH
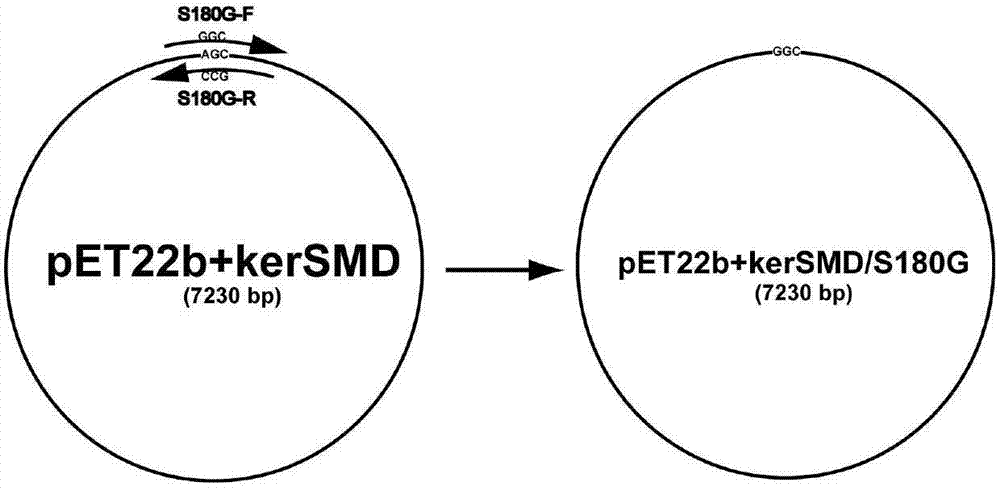
Higher-substrate-specificity keratinase mutant and preparation method thereof
ActiveCN104726436AIncrease enzyme activityStrong specificityHydrolasesAnimal feeding stuffWater solubleSite-directed mutagenesis
The invention discloses a higher-substrate-specificity keratinase mutant and a preparation method thereof, belonging to the field of enzyme engineering. Analysis is performed to obtain the optimal mutant site to perform site-directed mutagenesis, thereby obtaining the excellent mutant with higher keratinase enzyme activity and substrate specificity. The keratinase and mutant thereof can effectively hydrolyze feather, wool and other non-water-soluble keratin substrates, and can be used for leather textile industry and feed industry.
Owner:JIANGNAN UNIV
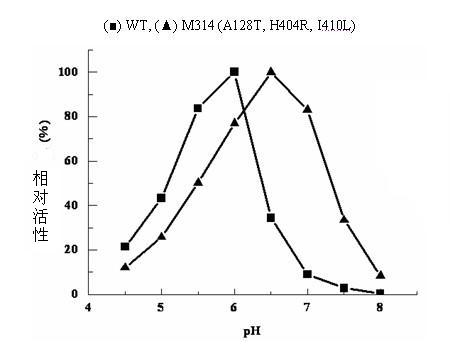
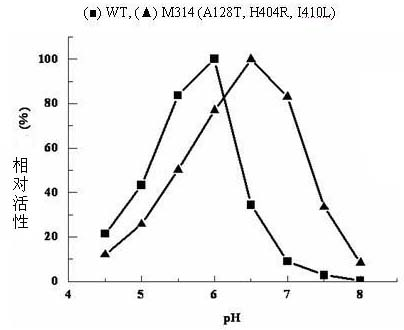
Construction of recombinant strain capable of producing arginine deiminase and directional modification method thereof
InactiveCN102061283AIncrease enzyme activityIncreased specific enzyme activityBacteriaHydrolasesBio engineeringRecombinase
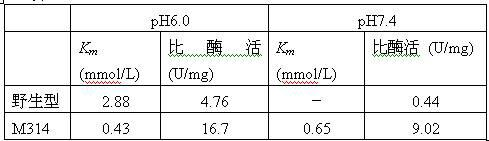
The invention relates to construction of a recombinant strain capable of producing arginine deiminase (ADI) and a directional modification method thereof, belonging to the technical field of medical biological engineering. The method comprises the following steps of: amplifying the ADI coding gene arcA of a pseudomonas plecoglossicida CGMCC (China General Microbiological Culture Collection Center) No. 2039 by adopting a PCR (polymerase chain reaction) method, and constructing an ADI recombination expression strain, researching the enzymology properties of the recombinant ADI enzyme, wherein Km is 2.88mmol / L (pH is 6.0), the optimal pH is 6.0, the enzyme activity is 20.85U / mg, and the enzyme activity is reduced by more than 90% when the pH is increased to the physiological pH (7.4). The directional modification is carried out on the recombinant ADI enzyme by adopting a prone PCR mutation technology, so as to improve the activity and substrate affinity of the enzyme under the physiological pH condition. An excellent mutant strain ADIM314 is obtained by screening through the directional modification. Compared with a wild enzyme, the activity of the ADIM314 enzyme under the physiological pH condition is improved by more than 20 times, the Km value is reduced to 0.65mmol / L (pH is 7.4), and the optimal pH is improved to 6.5 from 6.0.
Owner:JIANGNAN UNIV
Keratinase with improved thermal stability and specific activity as well as preparation method and application thereof
ActiveCN103602653AIncreased specific enzyme activityImprove thermal stabilityBacteriaHydrolasesWild typeKeratin
The invention discloses keratinase with improved thermal stability and specific activity as well as a preparation method and application thereof, and belongs to the field of genetic engineering. The keratinase having wide industrial application prospect is obtained by mutating a leading peptide cleavage site of keratin and transforming the N-terminal of the keratin. The t1 / 2 of the keratinase at the temperature of 60 DEG C is 20 minutes and is increased by nearly two times in comparison with that of wild type keratinase. Meanwhile, the specific activity of a keratinase mutant is increased by nearly 50% in comparison with that of the wild type keratinase, so that a good foundation is laid for the application of the keratinase.
Owner:JIANGNAN UNIV
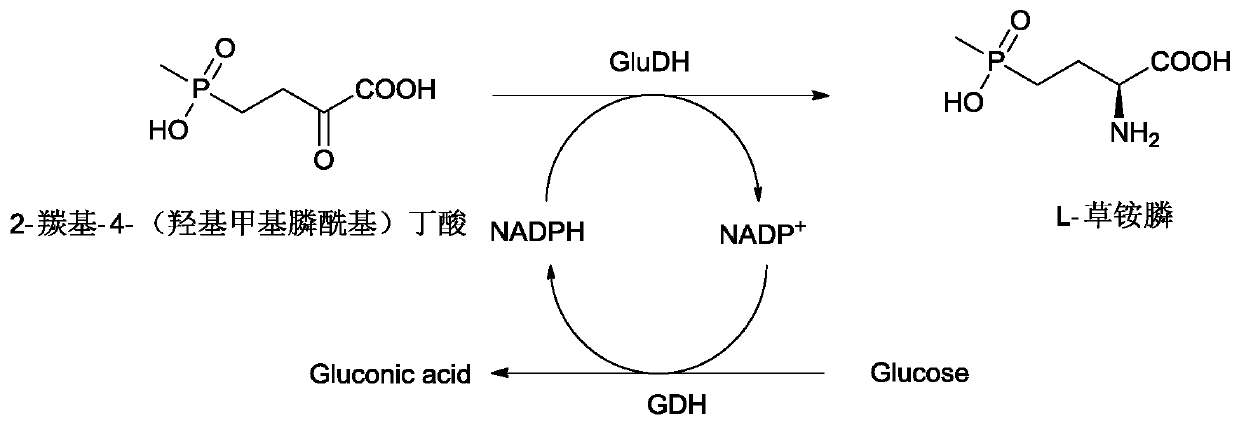
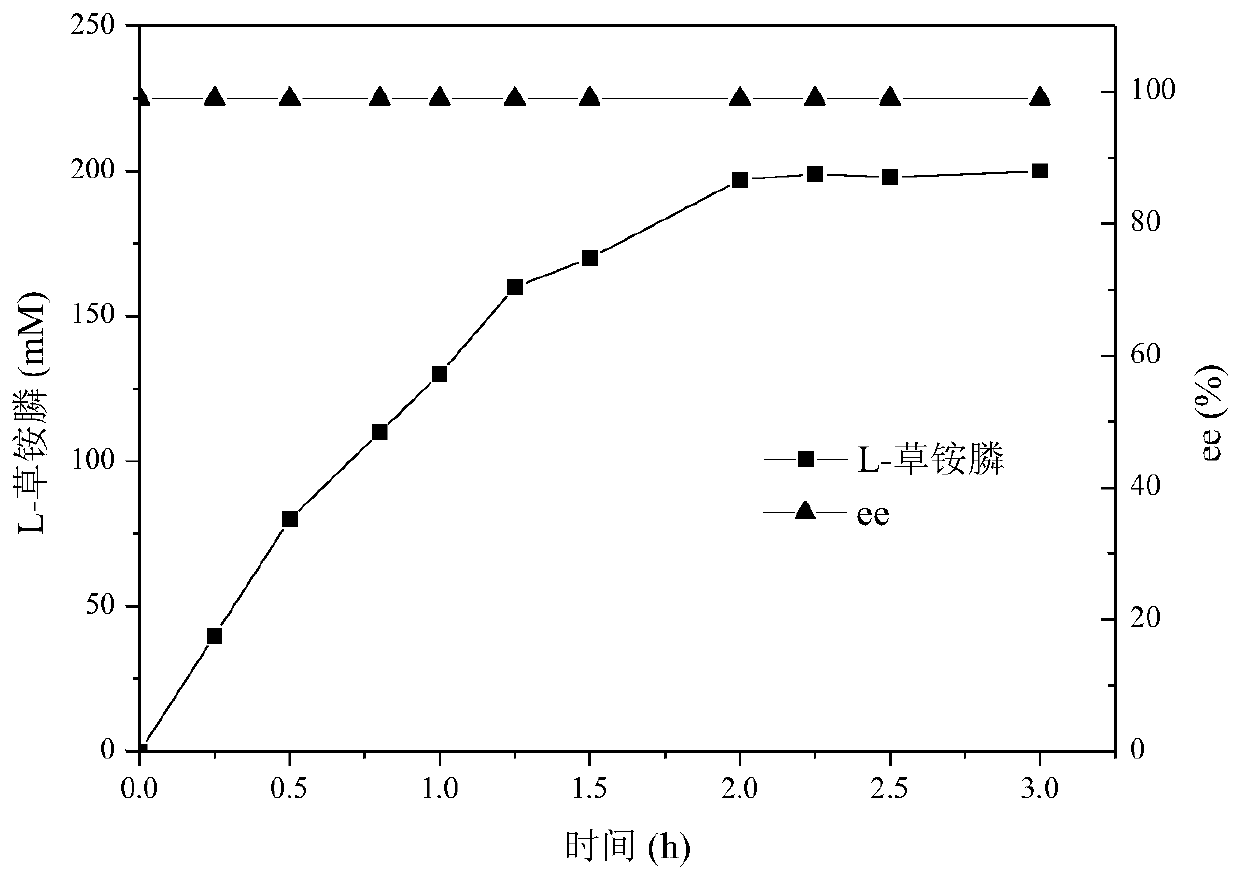
Glufosinate dehydrogenase mutant and application thereof
ActiveCN109750009AIncreased specific enzyme activityBacteriaMicroorganism based processesGlycineSpecific enzyme
The invention discloses a glufosinate dehydrogenase mutant and application thereof. The glufosinate dehydrogenase mutant is obtained by single mutation or multiple mutation of the 90th, 91st and 376thamino acid shown in SEQ ID No.2; the 90th lysine is mutated to serine; the 91th glycine is mutated to serine or valine; the 376th serine is mutated into arginine. The mutant utilizes a site-directedsaturation mutation technique for mutation of the glufosinate dehydrogenase gene shown in SEQ ID No.1, and finds that the 90th, 91st, and 376th positions are key sites affecting the enzyme activity, the obtained specific enzyme activity is much higher than the mutant of the parental glufosinate dehydrogenase, the specific enzyme activity of the mutant lvPDH-K90S-G91P-S376R is 8.4 times higher thanthat of the parental glufosinate dehydrogenase, and the mutant has industrial application prospects.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing phosphatidylserine with docosahexaenoic acid at sn-2 bit
ActiveCN104004797AGood application effectIncreased specific enzyme activityFungiBacteriaDocosahexaenoic acidPhospholipase A2
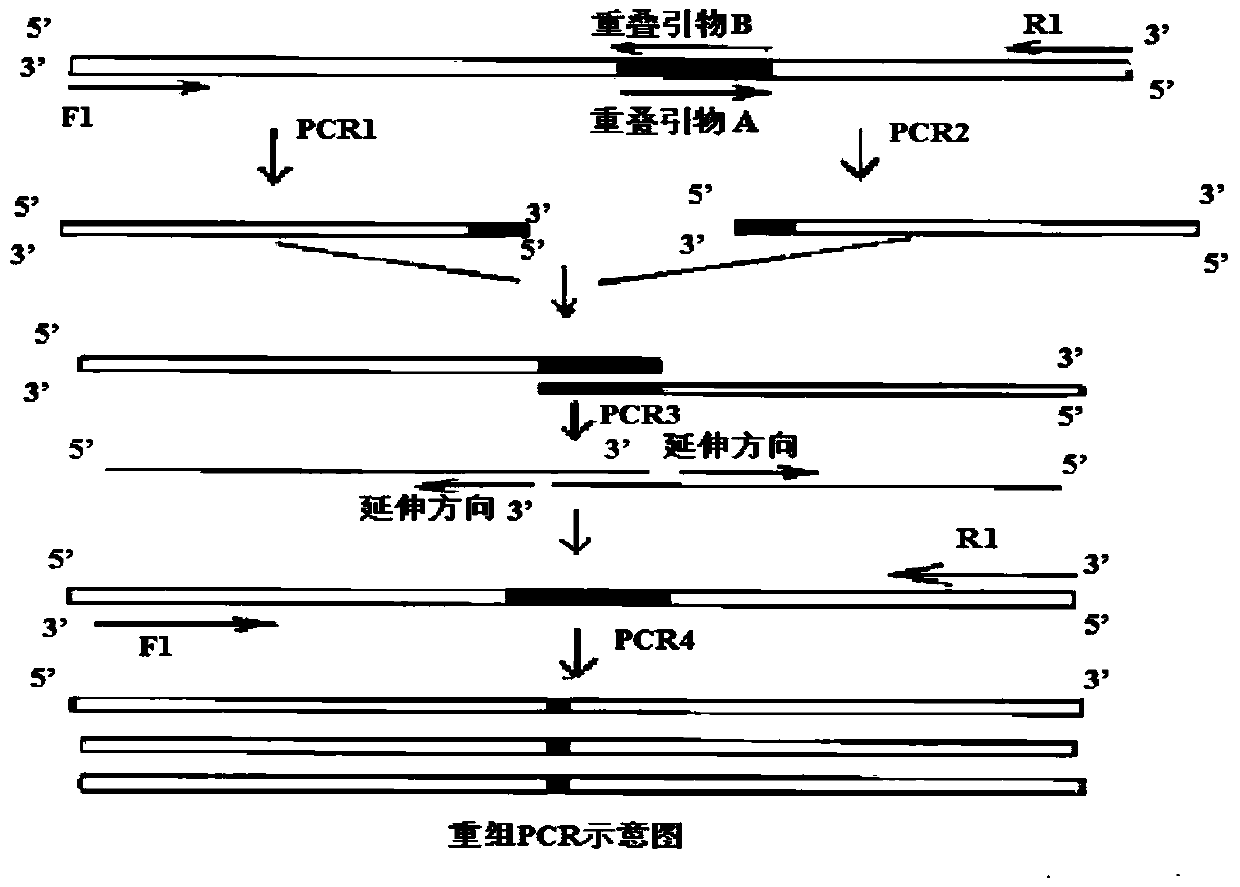
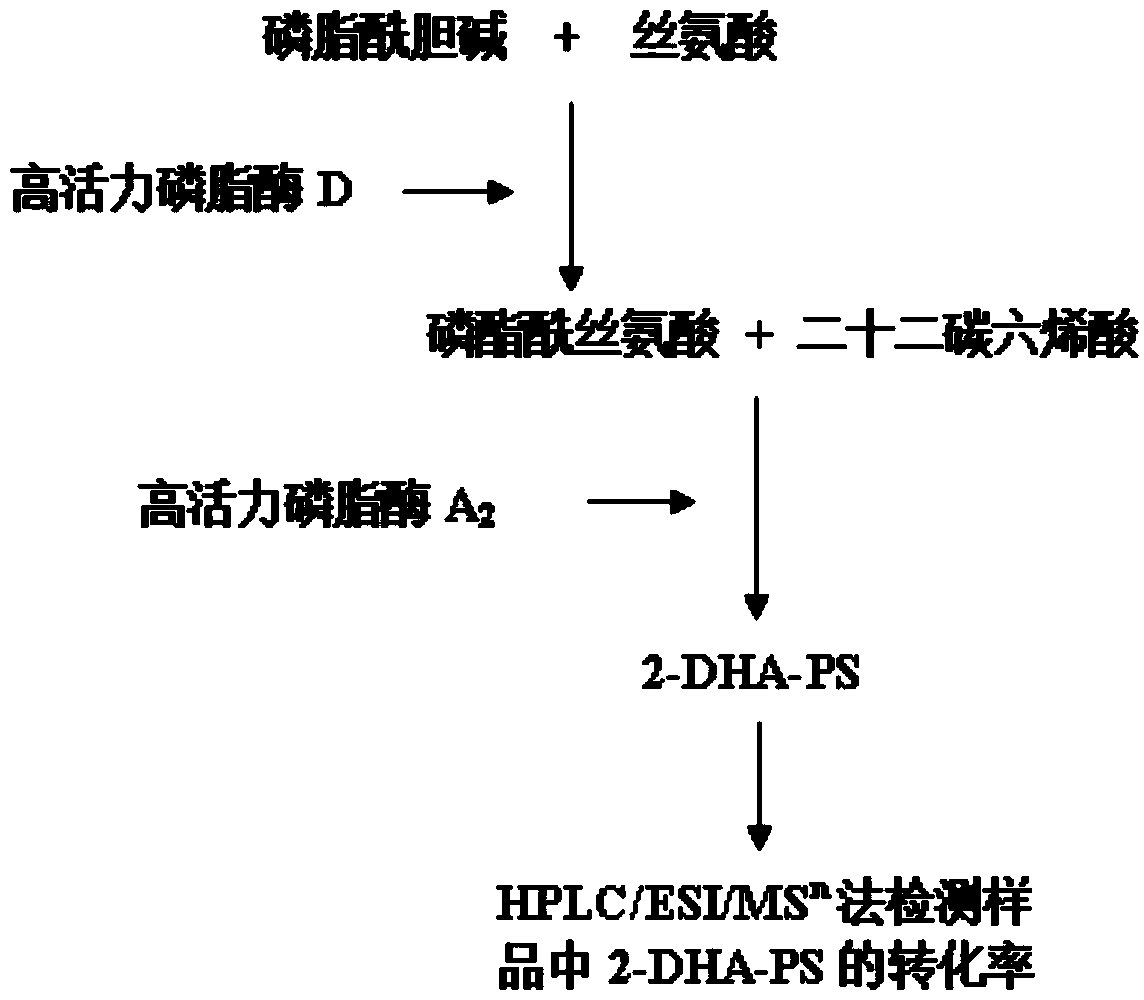
The invention relates to a method for preparing phosphatidylserine (2-DHA-PS) with docosahexaenoic acid at the sn-2 bit through high-activity phospholipase A2 and high-activity phospholipase D. Directed evolution is achieved through the overlapping PCR technology so that the high-activity phospholipase A2 and the high-activity phospholipase D can be obtained; the 2-DHA-PS is prepared through catalysis by means of the high-activity phospholipase A2 and the high-activity phospholipase D, phosphatidylserine is generated through phosphatidylcholine and serine under the catalysis of the high-activity phospholipase D first, and then the 2-DHA-PS is generated through the phosphatidylserine and the docosahexaenoic acid under the catalysis of the high-activity phospholipase A2. The relative content of the 2-DHA-PS in the product synthesized through the method is high, and the defects of an existing synthesizing method are effectively overcome.
Owner:TIANJIN UNIV OF SCI & TECH
Method for producing trans-4-hydroxy-L-proline
ActiveCN107674863AActiveIncreased specific enzyme activityBacteriaMicroorganism based processesBiotechnologyTrans-4-Hydroxy-L-proline
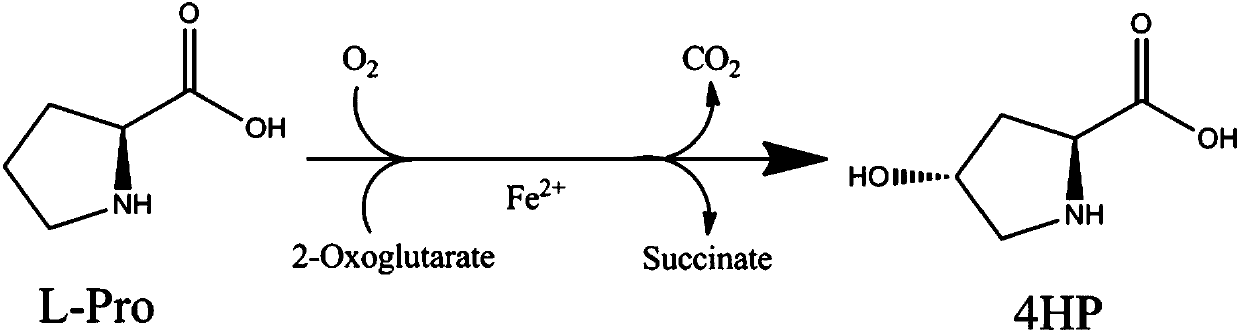
The invention provides a method for producing trans-4-hydroxy-L-proline. Specifically, the invention provides an application of polypeptide in producing the trans-4-hydroxy-L-proline or a downstream product taking the trans-4-hydroxy-L-proline as a precursor. The invention also provides the method for producing the trans-4-hydroxy-L-proline, wherein the method comprises a step of cultivating and expressing a strain of polypeptide, so that the trans-4-hydroxy-L-proline is obtained. The invention also provides a trans-4-hydroxy-L-proline producing strain and a construction method of the trans-4-hydroxy-L-proline producing strain. With the application of the method provided by the invention, the efficient production of the trans-4-hydroxy-L-proline with low cost is achieved.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
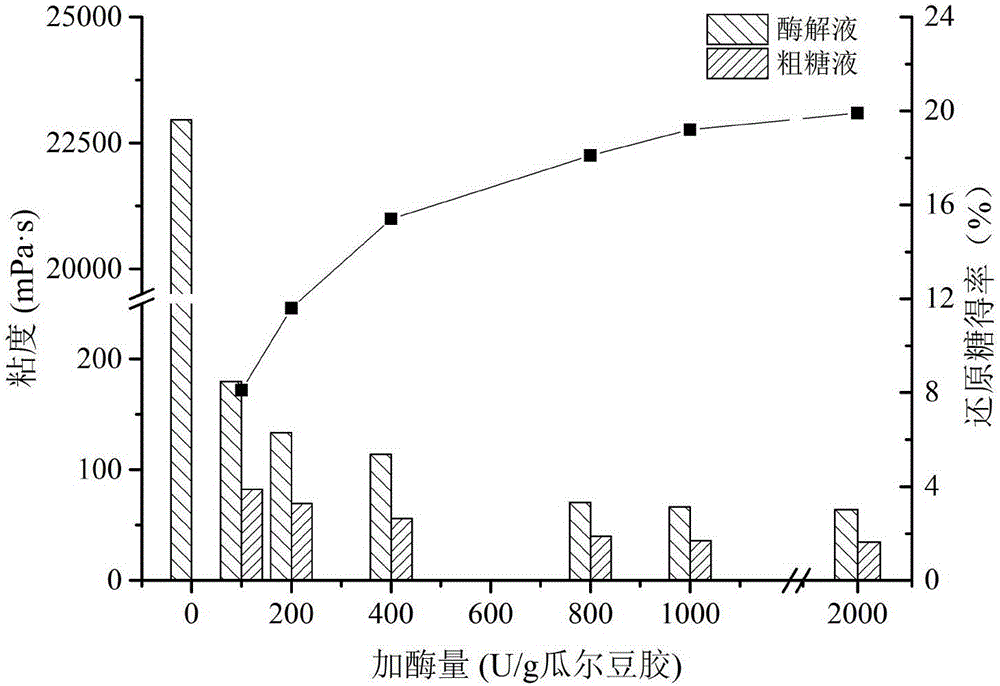
Soluble dietary fibers rich in galactomannan and preparation method of soluble dietary fibers
ActiveCN106387923AHigh activityImprove stabilityMicroorganism based processesFermentationIcing sugarSoluble dietary fiber
The invention discloses soluble dietary fibers rich in galactomannan and a preparation method of the soluble dietary fibers. The mass percentage of galactomannan is 20-30%. Beta-mannase provided by the invention is better in stability and higher in activity than enzyme, and has great application value in the trade of foods, feeds and the like. According to the preparation method provided by the invention, the hydrolysis rate and the galactomannan conversion rate are high, products are easy to separate, and the weight-average molecular weight of finished products is about 24800Da; and two kinds of products of soluble dietary fiber syrup and powdered sugar, containing the galactomannan, can be produced by the preparation method provided by the invention, besides, the yield of products is high, the yield of the soluble dietary fiber syrup is 74.2%, and the yield of the powdered sugar is 72.8%.
Owner:CHINA AGRI UNIV +1
S-adenosylmethionine synthetase mutant and preparation method thereof
ActiveCN110144336AIncreased specific enzyme activityBacteriaTransferasesS-Adenosyl-l-methionineS-Adenosylmethionine Synthetase
The invention discloses an S-adenosylmethionine synthetase mutant and a preparation method of the mutant. The mutant is prepared by single-point, double-point or three-point mutation of a site 65, a site 104 and a site 186 in an amino acid sequence shown as SEQ ID NO. (sequence identifier number) 1. Compared with wild S-adenosylmethionine synthetase, the mutant increases an accumulation amount ofSAM (S-adenosylmethionine) to be increased by 1.06mM in a 10mM substrate reaction system. At the same time, enzyme activity of the mutant is 3.3 times that of wild eMAT (escherichia coli S-adenosylmethionine synthetase), and is 6.02+ / -0.22U / mg. The possibility is further provided for enzyme method preparation of SAM, and a clue is provided for improving a product inhibition phenomenon of an enzymeby molecular modification.
Owner:ZHEJIANG UNIV
Xylanase mutant with improved specific enzymatic activity and coding gene and application thereof
ActiveCN106191083AIncreased specific enzyme activityReduce manufacturing costFermentationVector-based foreign material introductionXylanNucleotide
The invention relates to a xylanase mutant with improved specific enzymatic activity and a coding gene and application thereof. The xylanase mutant a protein shown in (a), (b) or (c): (a), a protein composed of amino acid sequences shown in SEQ ID NO. 1; a protein derived from (a) by subjecting the amino acid sequences shown in SEQ ID NO. 1 to substitution, deletion and / or addition of one or more amino acid residues and which has xylanase activity; (c), a protein coded by subjecting the amino acid sequences of code (a) and the amino acid sequences of code (b) to molecular hybridization and which has xylanase active amino acid sequences. The xylanase mutant according to the invention has specific enzymatic activity increased by 2.8 times, is applicable to degrading xylan substrates, has a wide range of acting temperature and pH, and has the advantages such as good resistance to acids and bases.
Owner:HUBEI UNIV
Beta-mannanase and preparation method thereof
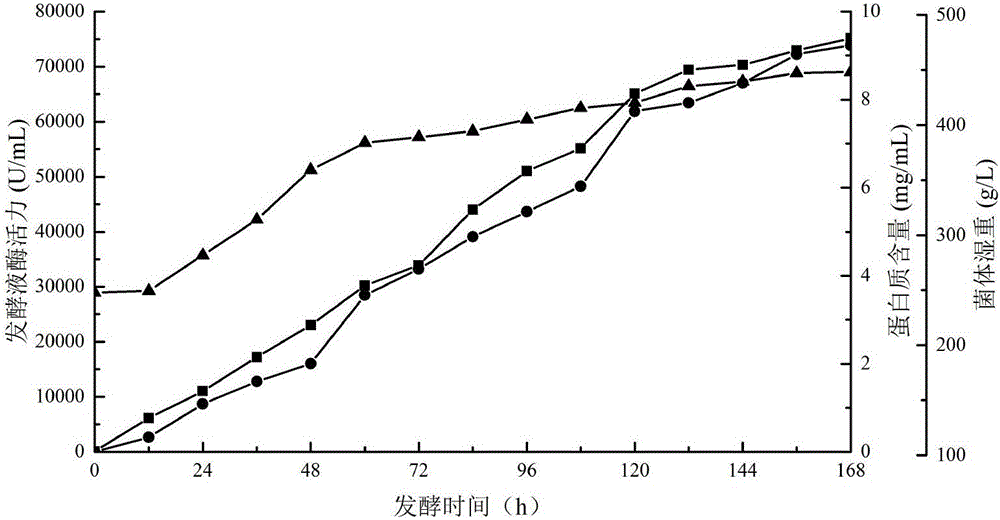
ActiveCN102732494AIncreased specific enzyme activityImprove thermal stabilityFungiMicroorganism based processesPichia pastorisPapermaking
The invention discloses beta-mannanase and a preparation method thereof. The beta-mannanase provided in the invention is a protein which is a protein 1, a protein 2 or a protein 3, wherein the protein 1 is composed of a 22-416 amino acid sequence shown in a sequence 1 in a sequence table; the protein 2 is composed of an amino acid sequence represented by the sequence 1 in the sequence table; and the protein 3 is obtained through substituting and / or deleting and / or adding one or more amino acid residues to an amino acid residue sequence of the protein 1 or the protein 2, has activities of the beta-mannanase, and is derived from the protein 1 or the protein 2. Engineering bacteria formed by introducing protein coding gen into Pichia pastoris are fermented in a 5L fermenting tank in a high density manner, and the enzymatic activity of a fermenting solution can reach 50029.6U / mL (the protein content is 6.1mg / mL), so efficient express is realized. The beta-mannanase of the invention has application potentials in the food industry, the medicine industry, the papermaking industry, the forage industry, the petroleum exploitation industry, the fine chemical engineering industry and the like.
Owner:CHINA AGRI UNIV
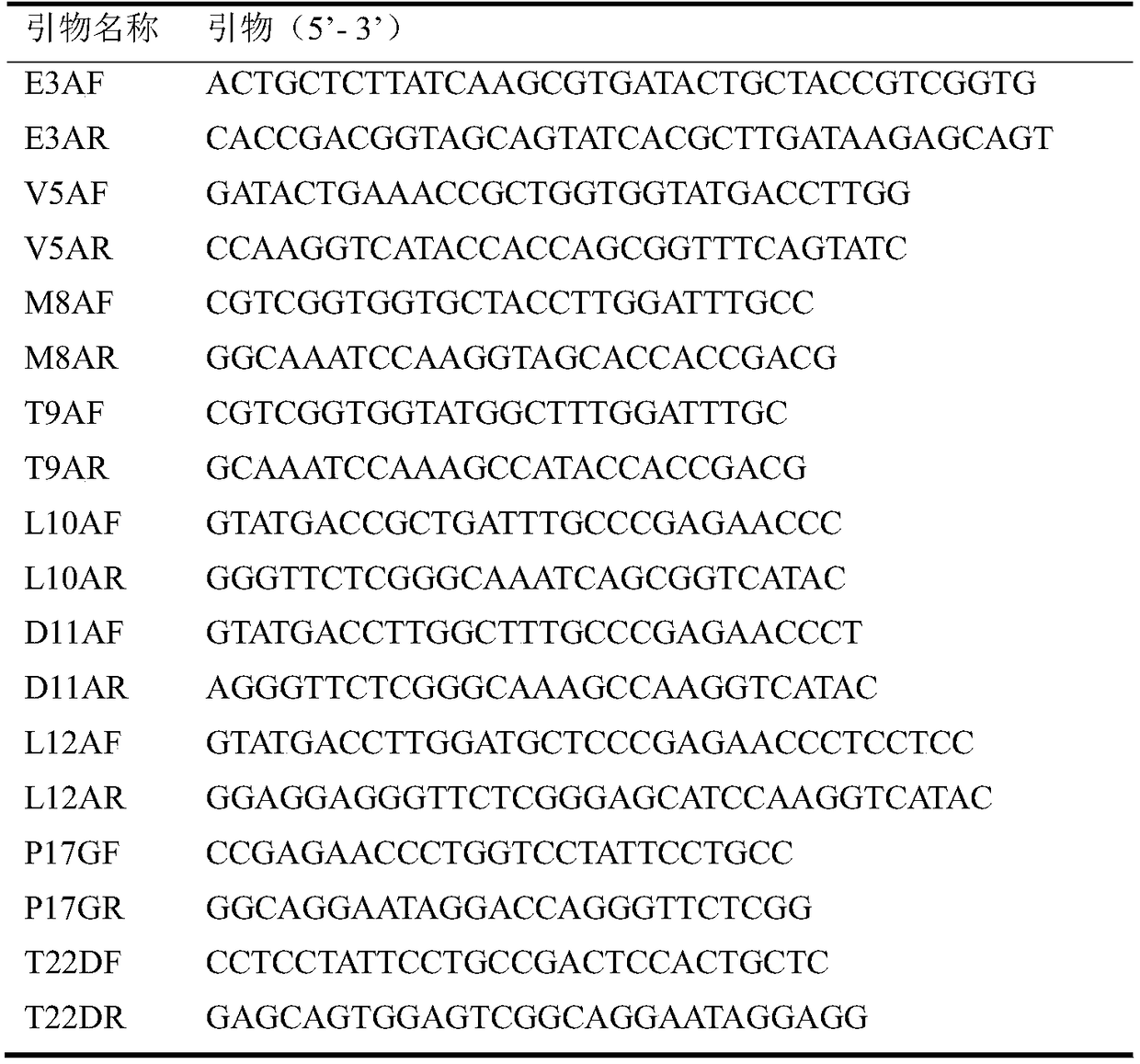
Lipase mutant with improved enzyme activity and regioselectivity and application of mutant
ActiveCN108642025AIncreased specific enzyme activityHydrolasesFermentationLipogenic enzymesRegioselectivity
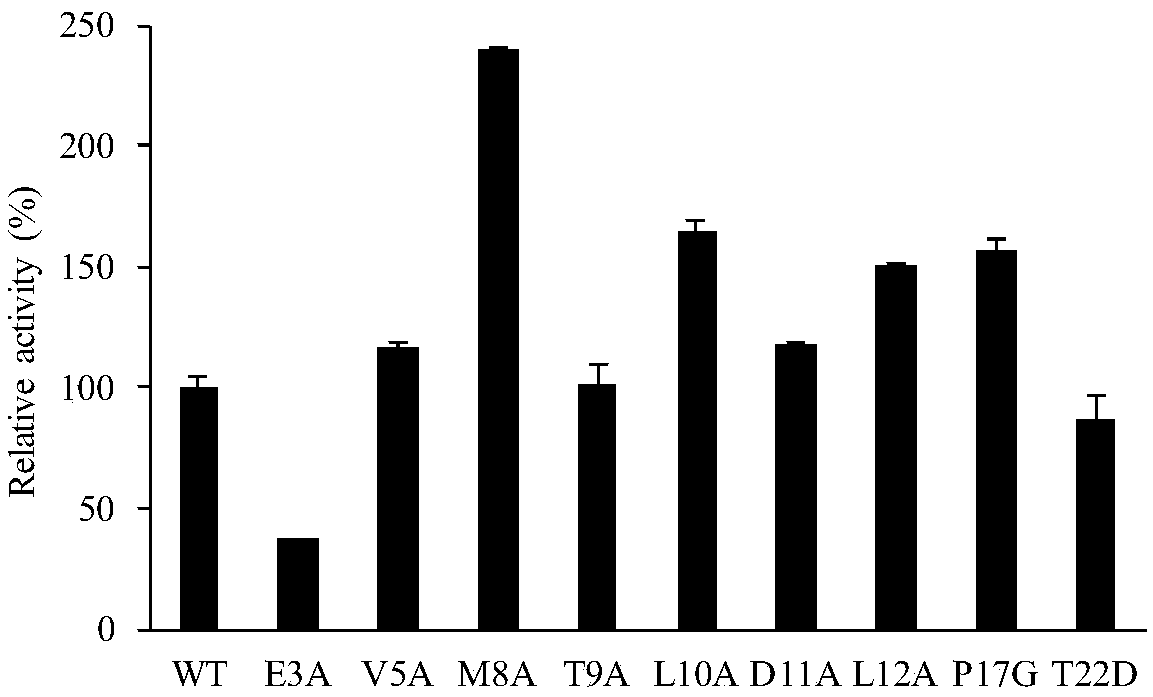
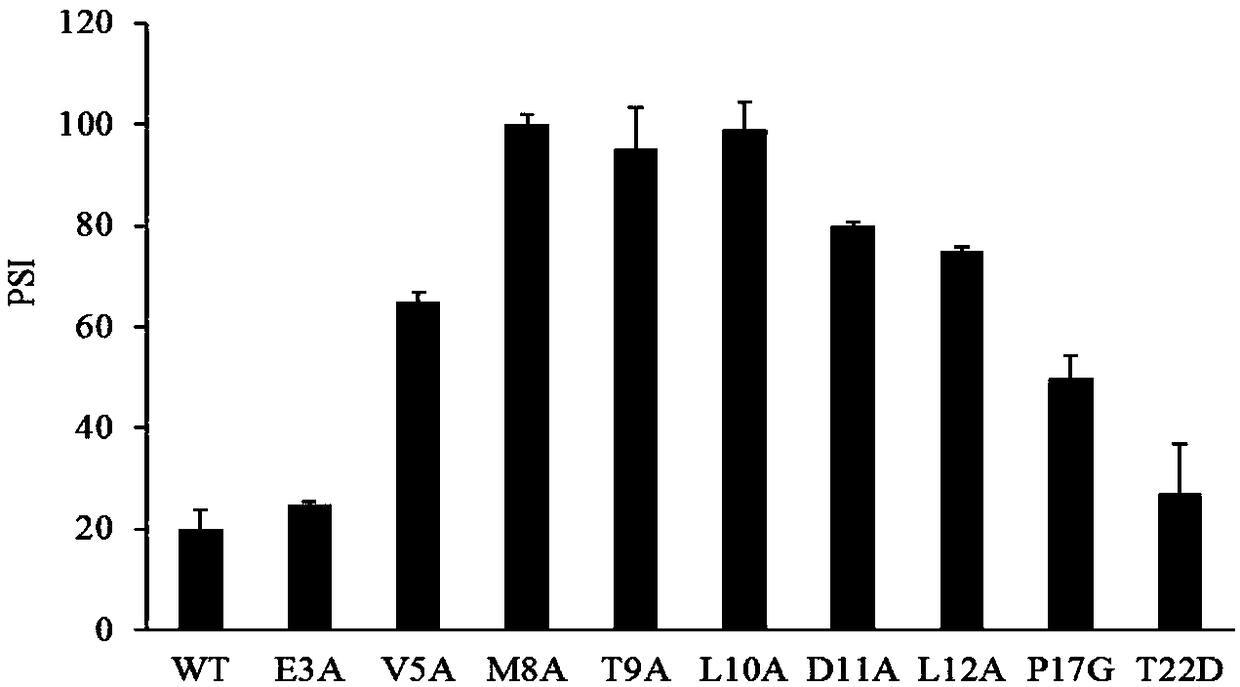
The invention discloses a lipase mutant with improved enzyme activity and regioselectivity and application of the mutant and belongs to the technical field of gene engineering. According to the lipasemutant, wild-type lipase is mutated, the enzyme activity of mutants M8A, L10A, D11A, L12A and P17G is increased by 1.1-2.4 times compared with the enzyme activity of wild-type lipase, and the 1,3-regioselectivity of the mutants M8A, L10A and T9A is increased by 4.75-5 times compared with the regioselectivity of wild-type lipase. The enzyme activity and regioselectivity are important parameters ofthe lipase mutant in structural lipid catalytic application. The lipase mutant is applied to a catalytic reaction of human milk fat substitutes and has an obvious effect.
Owner:无锡优普克生物科技有限公司
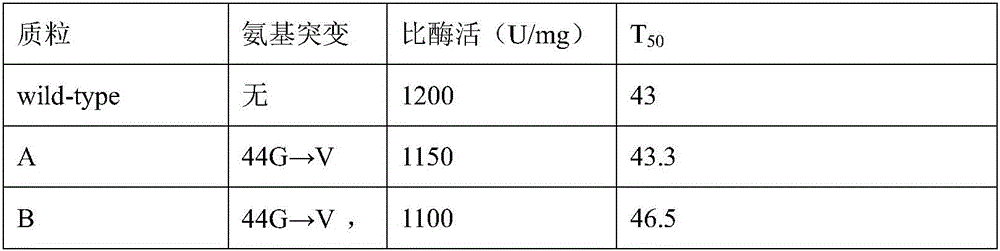
Glucose 6 phosphate dehydrogenase mutant
ActiveCN106190996AIncreased specific enzyme activityImprove thermal stabilityBiological material analysisOxidoreductasesMutantSorbitol-6-phosphate dehydrogenase
The invention relates to a glucose 6 phosphate dehydrogenase mutant. The mutant is obtained by mutating 44th-site glycine into valine and 319th-site glycine into valine in a glucose 6 phosphate dehydrogenase of which the amino acid sequence is disclosed as SEQ ID No.1; and compared with the glucose 6 phosphate dehydrogenase of which the amino acid sequence is disclosed as SEQ ID No.1 before mutation, the mutant has higher heat stability. The glucose 6 phosphate dehydrogenase mutant has the advantages of high heat stability and high inhibition rate; and the mutant enzyme can be used as a raw material for multiple small molecule detection kits, so that the obtained kit has higher stability and sensitivity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Alkaline pectinase mutant with improved specific enzyme activity
InactiveCN105316311AIncreased specific enzyme activityThermal stability is not affectedBacteriaMicroorganism based processesPectinaseFood industry
The invention discloses an alkaline pectinase mutant with improved specific enzyme activity, and belongs to the field of enzyme engineering. Compared with an existing mutant PGL-S1, the specific enzyme activity of the mutant PGL-S1-1 is improved by 5.7 times. Alkaline pectinase can be catalyzed under the alkaline condition, the alpha-1,4 glucosidic bond of polygalacturonic acid is split through the reverse eliminating effect, and the alkaline pectinase mutant can be widely applied to industries such as the food industry, the textile industry and the papermaking industry.
Owner:JIANGNAN UNIV
Thermophilic recombinant II type pullulanase and application thereof
ActiveCN109880783AGood pH toleranceIncreased specific enzyme activityBacteriaMicroorganism based processesChemistryMutant
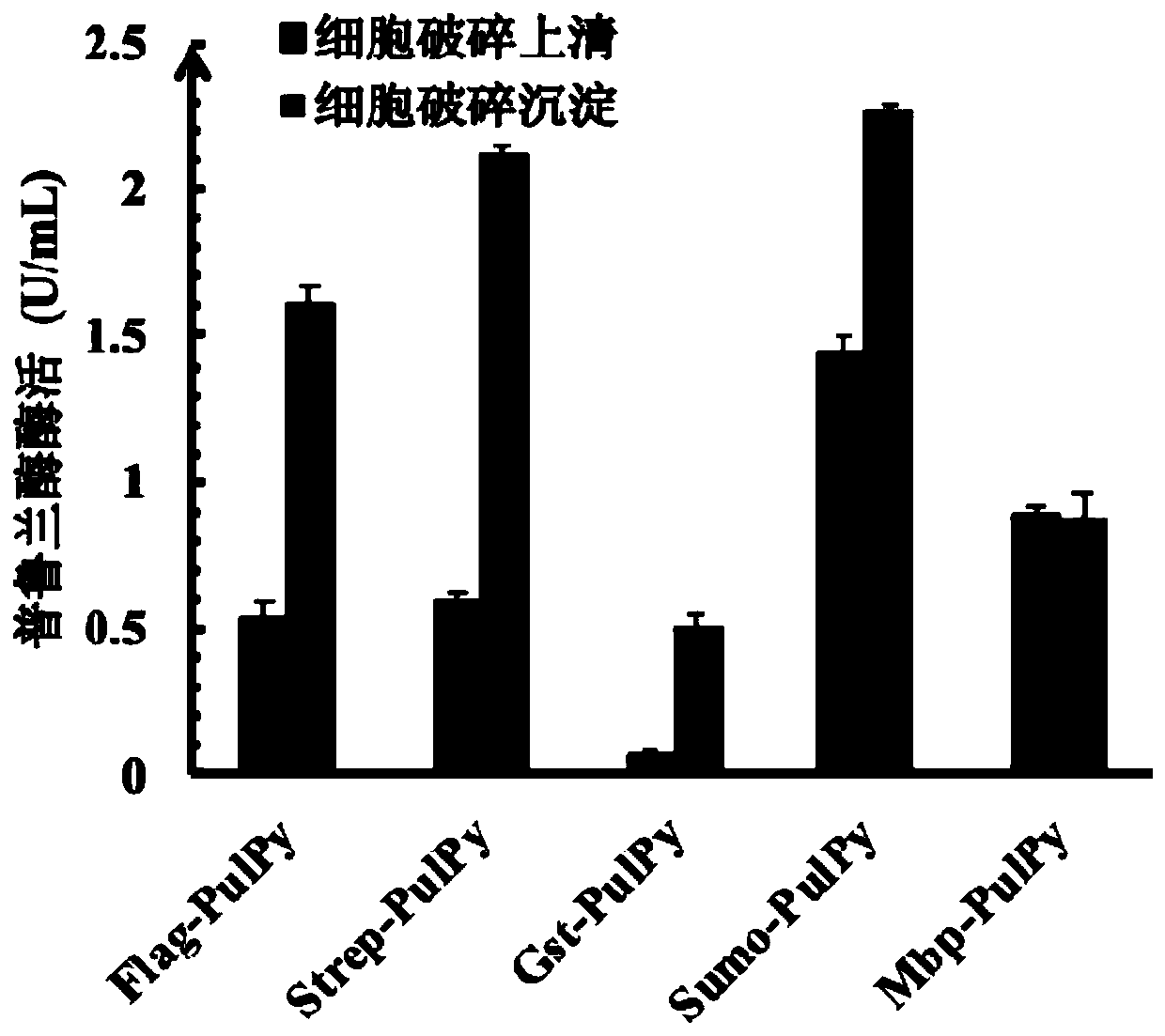
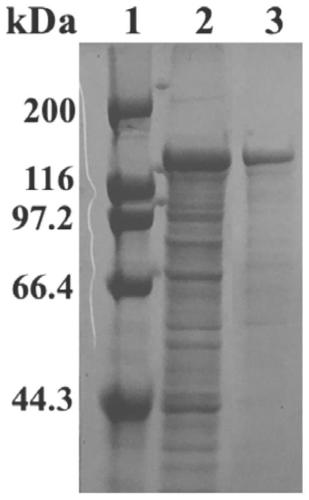
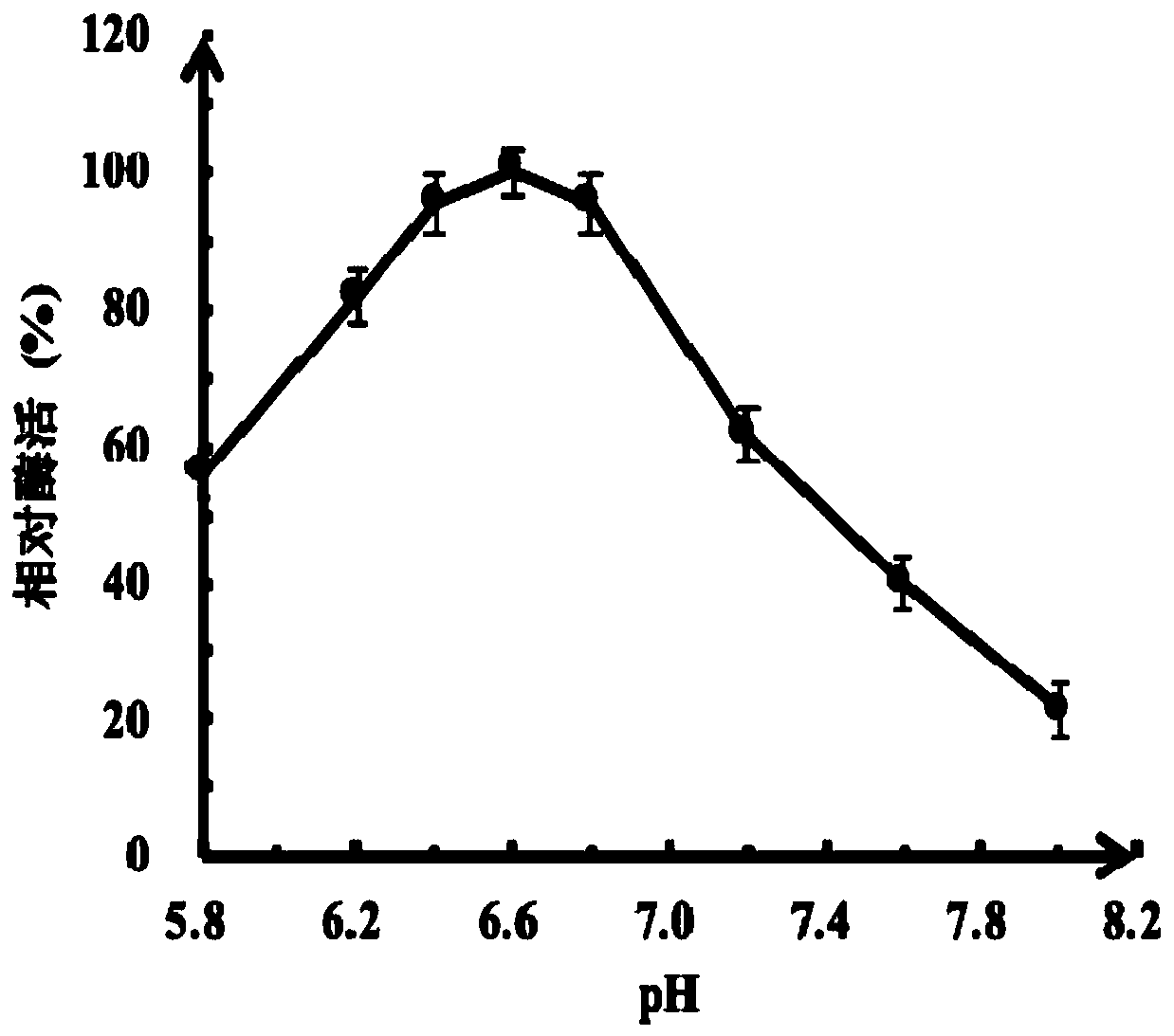
The invention discloses thermophilic recombinant II type pullulanase and application thereof, and belongs to the technical field of gene engineering. The thermophilic recombinant II type pullulanase is obtained through heterologous expression of II type pullulanase in escherichia coli, the most proper pH is 6.6, the thermophilic recombinant II type pullulanase has good pH tolerance under the condition that the pH value is 5.8-8.0, the most proper temperature is 95 DEG C, heat preservation is carried out for 10 h at 95 DEG C, the remaining enzyme activity is larger than 50%, and a relatively high specific activity can be shown under strong reduction conditions; if DTT is added into a culture environment, the specific activity of Sumo-PulPy can be increased by 237.2%. The invention also provides a combined truncated mutant delta28N+delta791C of the II type pullulanase Sumo-PulPy. The specific activity of the enzyme mutant is 32.18+ / -0.92 U / mg and is 5.99 times that of wild enzyme, and the mutant has important industrial application value and potential.
Owner:JIANGNAN UNIV
Beta-N-acetylglucosaminidase of Paenibacillus barungensis, encode gene thereof and application of beta-N-acetylglucosaminidase
ActiveCN109182303AGood enzymatic propertiesIncreased specific enzyme activityBacteriaMicroorganism based processesChitinaseThermal stability
The invention discloses a Beta-N-acetylglucosaminidase of Paenibacillus barungensis, an encode gene thereof and an application of beta-N-acetylglucosaminidase. The provided Beta-N-acetylglucosaminidase PbNag39 is excellent in the enzymatic property, and has a specific activity against chitosan is 28.3 U / mg, and the final products of hydrolysis of chitosan oligosaccharides are N-Acetyl glucosamine.The Beta-N-Acetylglucosaminidase and chitinase can hydrolyze chitin powder synergistically and obtain N-Acetyl glucosamine. The Beta-N-acetylglucosaminidase PbNag39 has the characteristics of high specific activity, good thermal stability and excellent hydrolysis characteristics. It can stabilize and play a catalytic role in a wide range of pH, and has important application value in chitin conversion.
Owner:CHINA AGRI UNIV
Genetic-engineering L-asparaginase amidohydrolase modified through site-specific mutagenesis
InactiveCN105062998AIncreased potential for industrial applicationsIncreased specific enzyme activityBacteriaHydrolasesGenetic engineeringL asparaginase
The invention discloses an activity-improved L-asparaginase amidohydrolase mutant and a construction method thereof, and belongs to the field of genetic engineering. The mutant is characterized in that the 166th serine is mutated into alanine on the basis of the amino acid shown in SEQ ID No.2. The mutant is expressed in bacillus subtilis, the activity is 657.1 U / ml after flask fermentation is carried out for 24 h, the mutation activity is improved by 23%, the substrate affinity is reduced by 20% compared with original amidohydrolase, the catalytic efficiency is improved by 8.4%, and meanwhile the specific activity is improved by 25%. The mutant and the construction method show that the 166th serine residue has large influences on the catalytic action of the amidohydrolase, a certain foundation is provided for researching the catalytic mechanism of the amidohydrolase, and the industrial application potential of the amidohydrolase is improved.
Owner:JIANGNAN UNIV +1
Alkaline pectinase mutant with improved specific enzyme activity and heat stability
ActiveCN105316310AIncreased specific enzyme activityExtended half-lifeBacteriaAntibody mimetics/scaffoldsFood industryPectinase
The invention discloses an alkaline pectinase mutant with improved specific enzyme activity and heat stability, and belongs to the field of enzyme engineering. Compared with an existing mutant PGL-S1, the specific enzyme activity of the mutant PGL-(GS)3-S1 is improved by 6 times and the half-life period at the temperature of 60 DEG C is prolonged by 1.3 times. Alkaline pectinase can be catalyzed under the alkaline condition, the alpha-1,4 glucosidic bond of polygalacturonic acid is split through the reverse eliminating effect, and the alkaline pectinase mutant can be widely applied to industries such as the food industry, the textile industry and the papermaking industry.
Owner:JIANGNAN UNIV
Transglutaminase mutants as well as genes, engineering bacteria and preparation method thereof
The invention belongs to the technical field of bioengineering and particularly relates to transglutaminase mutants as well as genes, engineering bacteria and a preparation method thereof. The mutantsare obtained from target genes through mutation with a sequential error-prone PCR method and screening, specific enzyme activity of the mutants expressed in a Bacillus subtillis expression system isincreased by 19% and 27% respectively compared with that of wild type transglutaminase, and the application prospect is broad.
Owner:TIANJIN UNIV OF SCI & TECH
Method for preparing straight-chain maltopentaose by using double-enzyme method
ActiveCN108300749AImprove thermal stabilityIncreased specific enzyme activityOrganic active ingredientsMetabolism disorderReaction temperaturePullulanase
The invention relates to a method for preparing straight-chain maltopentaose by using a double-enzyme method, and belongs to the technical field of the production of functional sugar. The number, in the GenBank, of the amino acid sequence of a used straight-chain maltooligosaccharide producing enzyme is AIV43245.1; the used pullulanase is purchased from the Japanese Amano Enzyme Inc.. The method comprises the following steps of preparing starch or maltodextrin solution with the pH (potential of Hydrogen) of 5.5 to 6.5 as a substrate, adding the straight-chain maltooligosaccharide producing enzyme according to an enzyme addition amount of 50U / g to 100U / g, adding the pullulanase according to an enzyme addition amount of 2U / g to 5U / g, and reacting for 24 to 72 hours at 60 to 70 DEG C to subsequently obtain straight-chain maltooligosaccharide syrup, wherein a conversion ratio (measured according to glucose to straight-chain maltoheptaose) reaches 90 percent or above; a main product is thestraight-chain maltopentaose; the percent of the main product can reach 40 percent or above. Two types of enzymes used by the method can be simultaneously added; the reaction temperature or the pH does not need to be regulated midway; the alpha-amylase is also not needed to liquefy the substrate; a calcium ion does not need to be added, and the method is simple in production process, is safe and further economical, and has higher application value.
Owner:JIANGNAN UNIV
Low temperature alkaline pectinase mutant with improved specific activity and thermal stability
ActiveCN108588061AHigh activityIncreased specific enzyme activityBacteriaFermentationMutantAmino acid
The invention discloses a low temperature alkaline pectinase mutant with the improved specific activity and thermal stability. The mutant has an amino acid sequence shown in SEQ ID NO.1, or an amino acid sequence with low temperature alkaline pectin lyase activity obtained by deletion, replacement, insertion or / and addition of conserved mutations of one to several amino acids on the basis of the amino acid sequence shown in SEQ ID NO.1. The mutant can greatly improve the specific activity and thermal stability, and is more suitable for industrial production in the fields such as textile degumming and washing and meets the needs of social production.
Owner:HUBEI UNIV
Specific enzyme activity improved directionally modified enzyme of heparinase I as well as molecular modification method and expression engineered bacterium
ActiveCN109321549AIncreased specific enzyme activityGreat application potentialBacteriaFermentationSpecific enzymeNucleotide
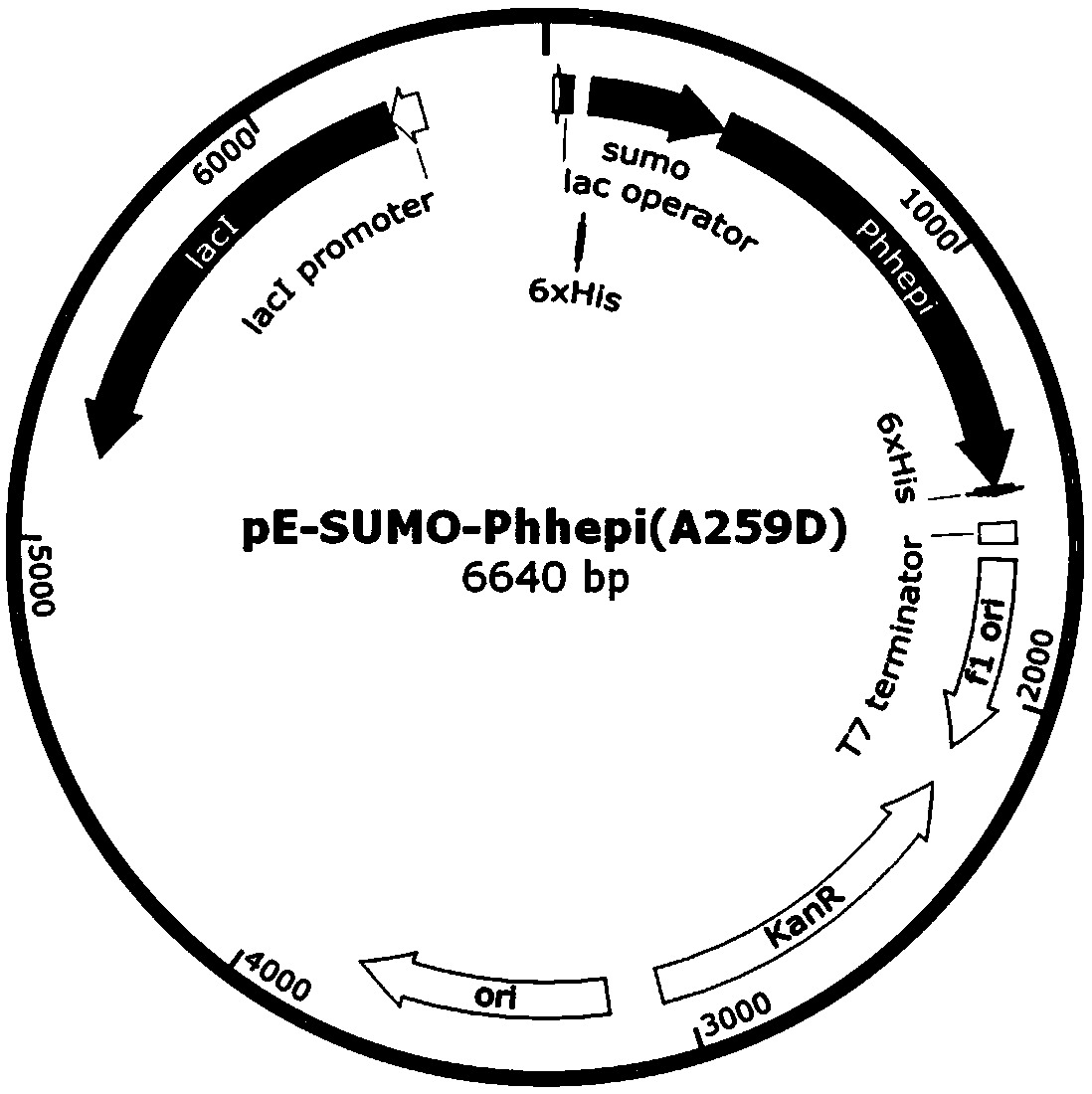
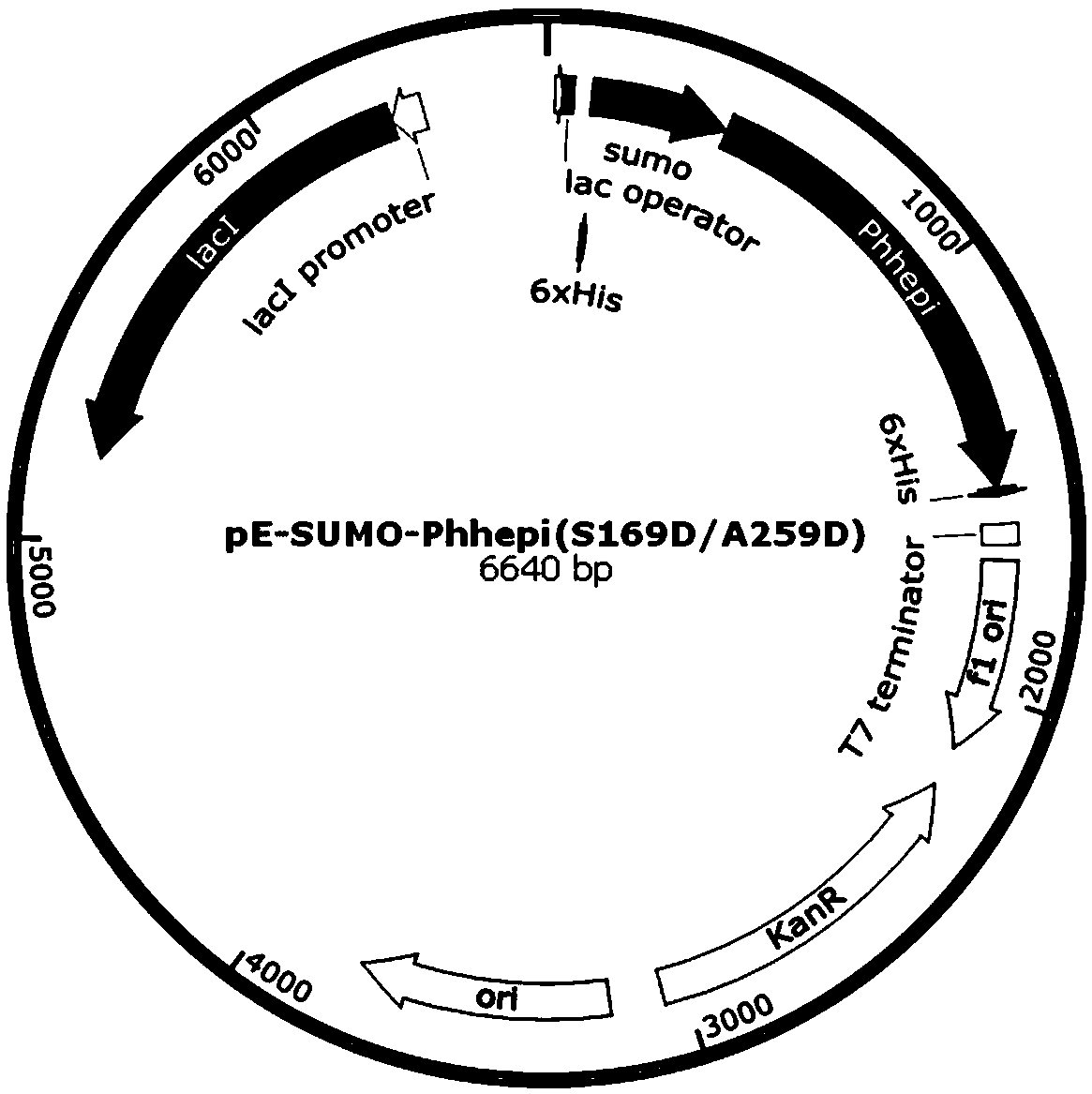
The invention relates to a specific enzyme activity improved directionally modified enzyme PhHepI<A259D> of heparinase I. The nucleotide sequence of the enzyme is SEQ ID NO: 1. The invention providesa design and construction method of two mutant enzymes PhHepI<A259D> and PhHepI<S169D / A259D>, and a method for the construction of a novel mutant-enzyme engineered bacterium and the high-efficiency expression of the mutant enzymes. Compared with a wild type, the mutant enzymes provided by the invention have higher specific enzyme activity and greater application potential, and the theoretical basis is also laid for the research of HepI.
Owner:TIANJIN UNIV OF SCI & TECH +1
Alkaline pectinase mutant with improved heat stability
ActiveCN106754848AIncreased specific enzyme activityBacteriaMicroorganism based processesPectinasePentagalacturonic acid
The invention discloses an alkaline pectinase mutant with improved heat stability and belongs to the field of enzyme engineering. The sequence of the alkaline pectinase mutant provided by the invention is as shown in SEQ ID NO. 1; with regard to an existing PGL, the enzyme activity of the mutant is improved by 3.4 times; and the half-life period at 60 DEG C is improved to 19min from previous 5.2min and is improved by 3.6 times. The alkaline pectinase disclosed by the invention can be used for catalyzing cracking of an alpha-1,4 glycosidic bond of polygalacturonic acid through a trans-elimination effect under an alkaline condition; and the alkaline pectinase mutant is widely applied to the industries including foods, textiles, papermaking and the like.
Owner:JIANGNAN UNIV
Laminarinase OUC-L1 and encoding gene and application thereof
ActiveCN111334488AEffective for hydrolysisGood coldnessHydrolasesFermentationNucleotideOligosaccharide
The invention discloses laminarinase OUC-L1. The amino acid sequence of the laminarinase OUC-L1 is shown as SEQ ID NO:1, the gene of the laminarinase OUC-L1 is encoded, and the nucleotide sequence ofthe laminarinase OUC-L1 is shown as SEQ ID NO. 2. The laminarinase OUC-L1 is explored from a human intestinal bacterium A. muciniphila genome, belongs to a glycoside hydrolase GH16 family, and can effectively hydrolyze laminarin, and the main product of the laminarinase OUC-L1 is three parts of oligosaccharides, has high specific enzyme activity and good biological catalysis efficiency, and has the highest reaction activity at the temperature of 35 DEG C. In addition, the laminarinase OUC-L1 also has good cold adaptability. The invention further discloses an enzyme preparation containing the laminarinase OUC-L1. The enzyme preparation still has considerable catalytic activity at low temperature and has good industrial application potential.
Owner:OCEAN UNIV OF CHINA
Fungus-derived laccase, recombinant pichia pastoris engineering bacteria thereof and application of fungus-derived laccase
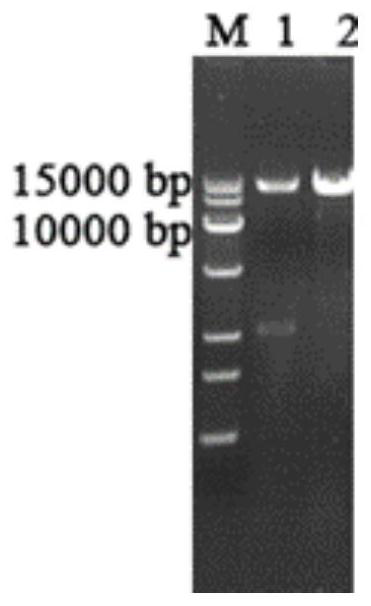
ActiveCN113215179AIncreased specific enzyme activityImprove heat resistanceFungiWater contaminantsPichia pastorisHeterologous
The invention discloses a fungus-derived laccase gene, and relates to the technical field of biology. The laccase gene is lac1, and the cDNA sequence of the lac1 is as shown in SEQ ID NO.2, or the lac1 has a base sequence for encoding an amino acid sequence as shown in SEQ ID NO.3. The invention also provides a recombinant pichia pastoris engineering strain carrying a laccase gene expression vector and an application of the recombinant pichia pastoris engineering strain. The fungus-derived laccase and the recombinant pichia pastoris engineering bacteria thereof have the beneficial effect that the laccase Lac1 newly identified in Trametes sp. Is expressed in a heterologous manner. The obtained recombinant laccase has good thermal stability and good tolerance to metal ions, organic solvents and inhibitors, can catalyze decoloration of indigo blue and other dyes, and has a good application prospect in industry.
Owner:ANHUI UNIVERSITY
Nitrilase mutant and application thereof in preparation of 1-cyanocyclohexyl acetic acid
ActiveCN111471668AIncreased specific enzyme activityShort reaction timeBacteriaHydrolasesEscherichia coliGabapentinoid
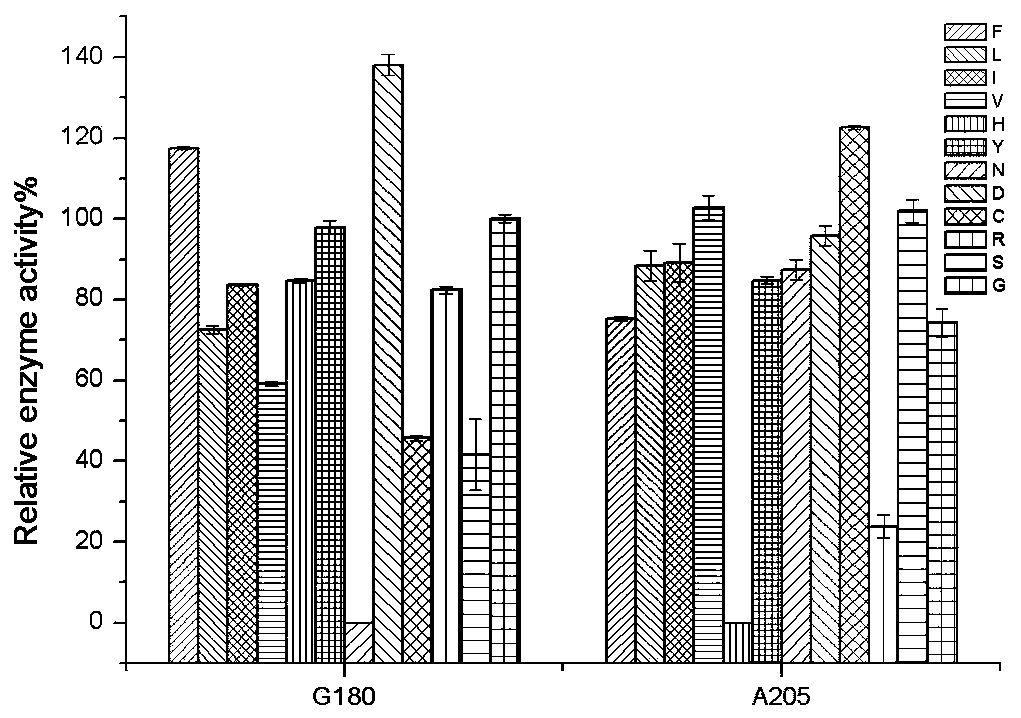
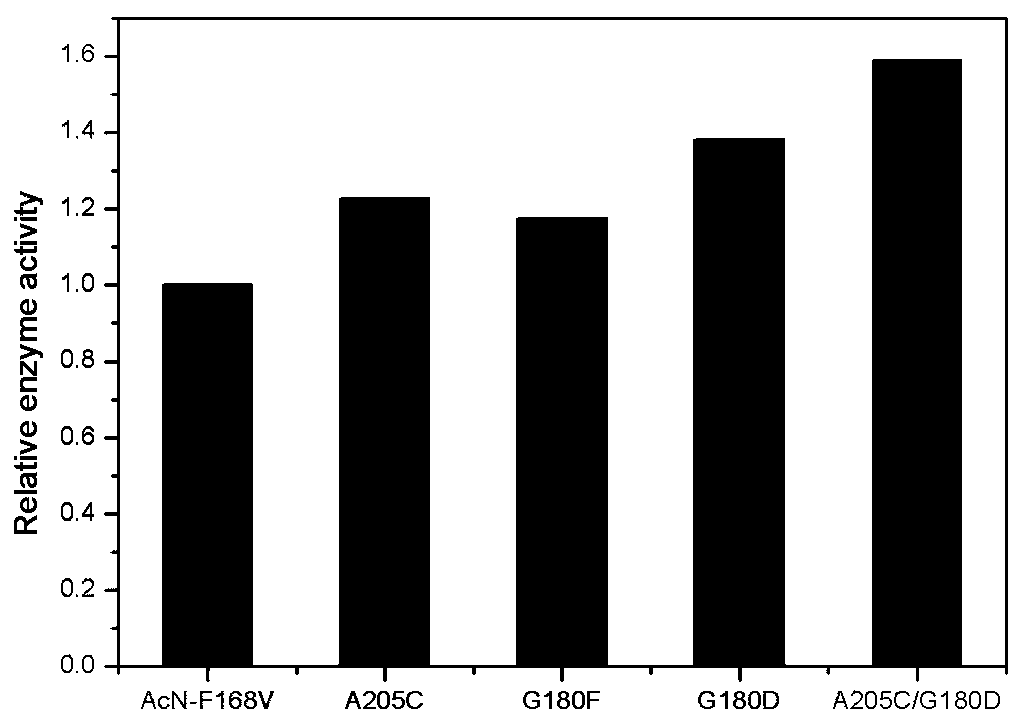
The invention discloses a nitrilase mutant and application thereof in preparation of 1-cyanocyclohexyl acetic acid. The nitrilase mutant is obtained by mutating one or more of 180th and 205th amino acids of an amino acid sequence shown as SEQ ID No.2. According to the invention, semi-rational design is adopted, proteins are subjected to molecular modification, the specific enzyme activity of nitrilase double mutant AcN-G180D / A205C is improved by 1.6 times to the maximum extent, the conversion rate is greater than 99%, and the reaction time is shortened to one fourth of the original reaction time by hydrolyzing 1-cyanocyclohexyl acetonitrile at a high temperature (50 DEG C) by using recombinant escherichia coli containing the nitrilase mutant. Therefore, the mutant obtained by the inventionhas a good application prospect in efficiently catalyzing the 1-cyanocyclohexyl acetonitrile to synthesize the gabapentin intermediate 1-cyanocyclohexyl acetic acid.
Owner:ZHEJIANG UNIV OF TECH
Method for improving specific activity and activation efficiency of transglutaminase
ActiveCN103540574AIncreased specific enzyme activityImprove cutting efficiencyAcyltransferasesMutagenic ProcessBiology
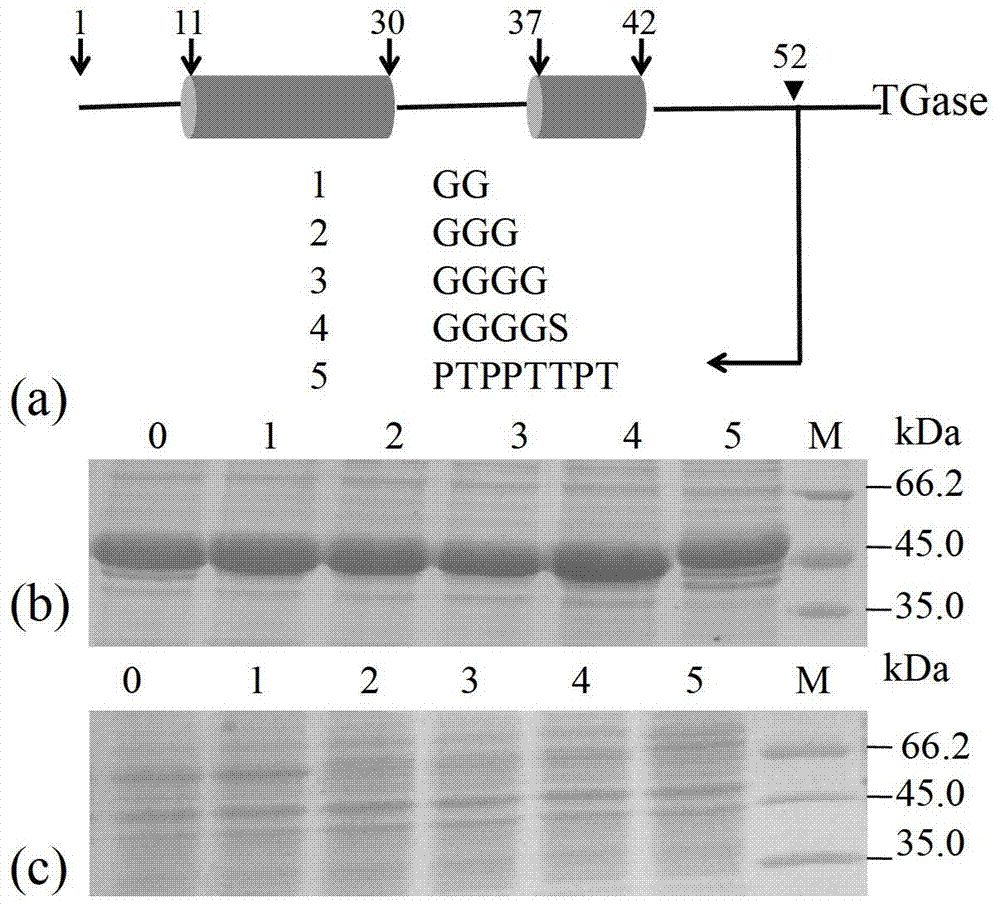
The invention discloses a method for improving specific activity and activation efficiency of transglutaminase. A connecting peptide frequently used in fusion protein is inserted into the C end of transglutaminase, the gene sequence of the connecting peptide is designed on a primer, and by the site-specific mutagenesis technology, the connecting peptide is inserted into the C end of leading peptide, and is preferably GS or PT. With the method disclosed by the invention, under the premise of keeping secretion efficiency of transglutaminase, the specific activity of transglutaminase is improved remarkably, which has important significance. Moreover, the invention also discovers that TGase leading peptide cutting efficiency can be improved remarkably due to application of the oligopeptide provided by the invention, so that the reaction time is decreased by 2 / 3.
Owner:TAIXING YIMING BIOLOGICAL PRODS
Production method for sucrose phosphorylase and application of production method
ActiveCN110656077AIncreased specific enzyme activityIncrease productionBacteriaMicroorganism based processesEscherichia coliSpecific enzyme
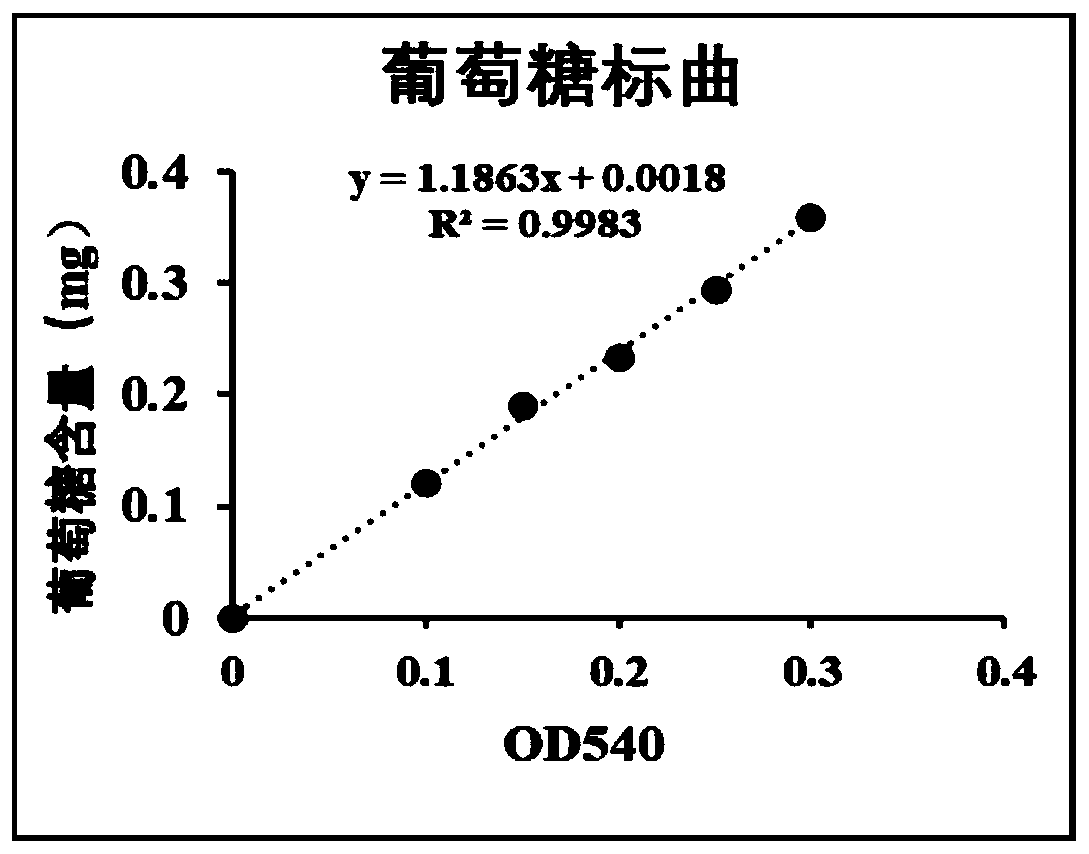
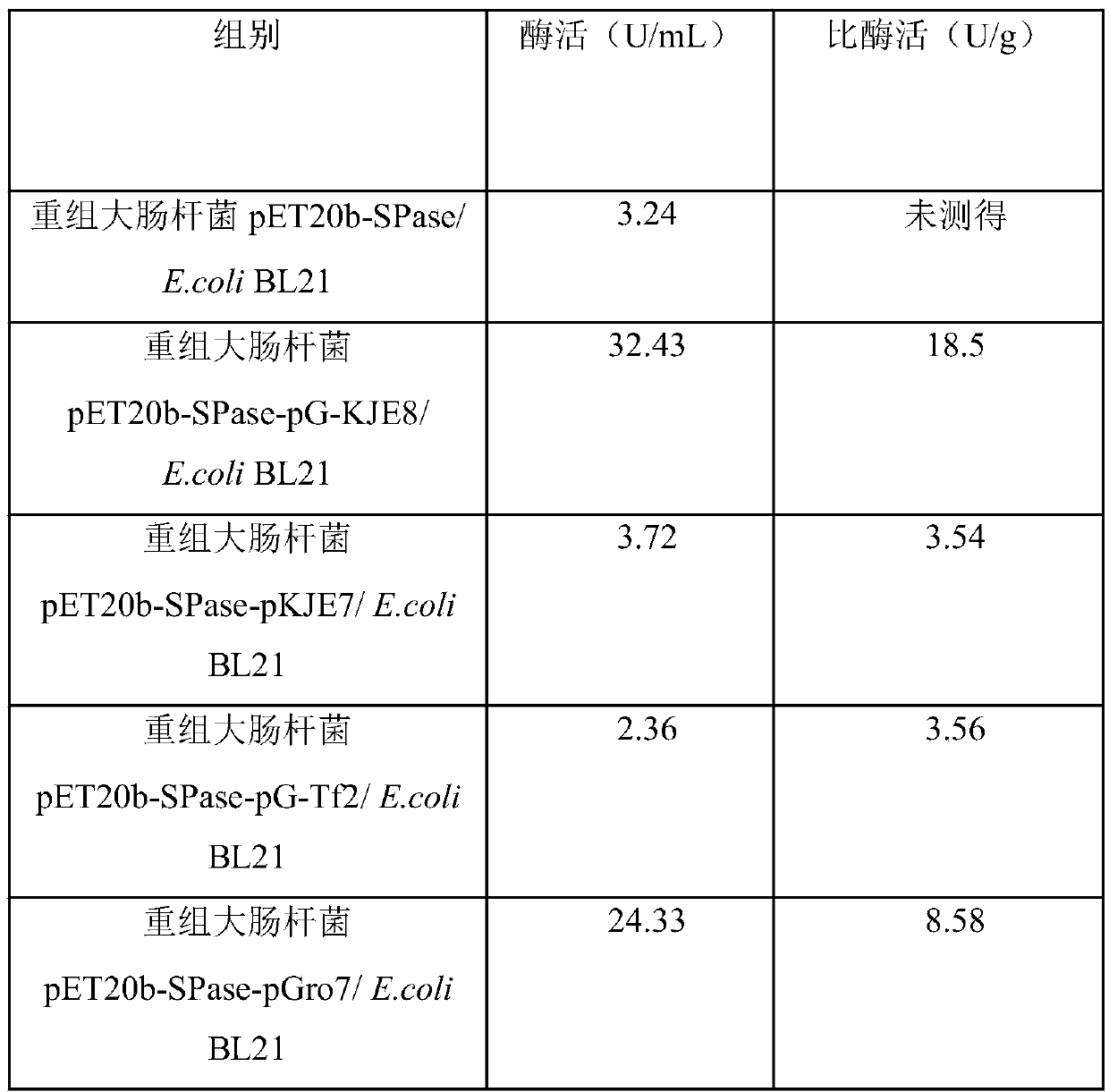
The invention discloses a production method for sucrose phosphorylase and an application of the production method, and belongs to the field of biotechnology. The invention provides the production method for sucrose phosphorylase. The method produces the sucrose phosphorylase with high yield and high specific enzyme activity. By the method, recombinant escherichia coli is expressed in a fermentation medium containing L-arabinose, tetracycline and IPTG for 12 hours by induction, allowing enzyme activity of the sucrose phosphorylase in a supernatant of cell disruption liquid up to 32.43U / mL, andspecific enzyme activity of the produced sucrose phosphorylase up to 18.5 U / mg.
Owner:JIANGNAN UNIV +1
Pullulanase enzyme production gene, carrier containing same and application of carrier
PendingCN105200070AIncreased specific enzyme activityImprove thermal stabilityEnzymesFermentationKlebsiella variicolaEnzyme Gene
The invention belongs to the technical field of gene engineering, and particularly relates to a pullulanase enzyme production gene capable of improving the specific activity and heat stability of pullulanase, a carrier containing the same and the application of the carrier in preparation of pullulanase. Compared with a klebsiella variicola HN7 wild pullulanase gene the preservation number of which is CGMCC NO.10357, base sequences corresponding to 31 amino acids are omitted at the N end after signal peptide is removed in the pullulanase enzyme production gene, and the specific sequence is shown in the sequence table. The invention further provides the carrier containing the gene by means of the gene engineering method. Compared with the original strain klebsiella variicola HN7, the pullulanase prepared by means of the carrier has the advantages that the specific activity, heat stability and affinity for pullulan substrates of the pullulanase are improved greatly, and the pullulanase has broad application prospects.
Owner:HENAN YANGSHAO BIOCHEM ENG
Glucan branching enzyme and coding gene and application thereof
ActiveCN103789281AStrong substrate specificityExcellent enzymatic propertiesBacteriaPre-baking dough treatmentProtein insertionPapermaking
The invention discloses a glucan branching enzyme and a coding gene and an application thereof. A protein disclosed by the invention is shown as a formula (1) or (2): (1) a protein shown as SEQ ID No.2; (2) a protein which is obtained by substituting and / or deleting and / or adding the amino acid sequence shown as SEQ ID No.2 by one or more amino acid residues and is same in function. The glucan branching enzyme RmGBE disclosed by the invention has excellent enzymatic characteristics, and has a greater application potential in industries such as foods, feed and papermaking.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com