Xylanase mutant with improved specific enzymatic activity and coding gene and application thereof
A xylanase mutation and coding gene technology, which is applied in the fields of application, genetic engineering, plant gene improvement, etc., can solve the problems of low specific enzyme activity, instability, high cost, etc., and achieve high enzyme activity, action temperature and pH range wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Error-prone PCR amplification gene xynHBN3
[0045] The information of xynHBN3 can refer to the patent literature with the patent publication number CN1924002A; the plasmid pHBM130 is used as a template (preserved by the Key Laboratory of Industrial Biotechnology in Hubei Province, which can be obtained by the public, and there is a xylanase gene on the plasmid. For details, see Zhang Guimin's doctoral thesis "Cloning, Expression and Enzymatic Properties of Xylanase Gene"), using primer 18N3F (sequence: 5'CGC GGATCC GCGGAAACG3') and 18N3R (sequence: 5'ACGC GTC GAC CTTTTATC3') for PCR amplification, the primers added a BamH I restriction site upstream of the gene, and a Sal I restriction site downstream, the PCR amplification conditions were 94°C, 5min; 94°C, 30s, 58°C, 30s, 72°C, 45s, 30 cycles; 72°C extension for 10min. After recovering the PCR product by 1% agarose gel electrophoresis, BamHI and SalⅠ double digestion. At the same time, the pET28a plasmi...
Embodiment 2
[0055] Embodiment 2 Construction of mutant xylanase recombinant expression vector
[0056] 1. Double digestion plasmid vector and gene fragment
[0057] Take 5 μg of the pET28a vector and 5 μg of the error-prone PCR product and digest them with Sal Ⅰ and BamH Ⅰ, respectively, and divide them into 5 tubes for enzyme digestion. The system for each tube is as follows:
[0058] Sal I
3μL
BamH Ⅰ
3μL
10×digestion buffer
10 μL
Error prone PCR product or pET28a vector
1μg
wxya 2 o
Up to 100μL
[0059] The reaction system was placed in a 1.5mL centrifuge tube and placed in a 37°C water bath for 3 hours. After detection by agarose electrophoresis, the carrier and error-prone PCR products were gel-recovered separately. The recovered products required A260 / A230>2.0 and A260 / A280≈1.8.
[0060] 2. Recombinant plasmid connection
[0061] Add the error-prone PCR-amplified target fragment (abbreviated as ep-PCR product) recovere...
Embodiment 3
[0070] The screening of embodiment 3 xylanase mutant libraries
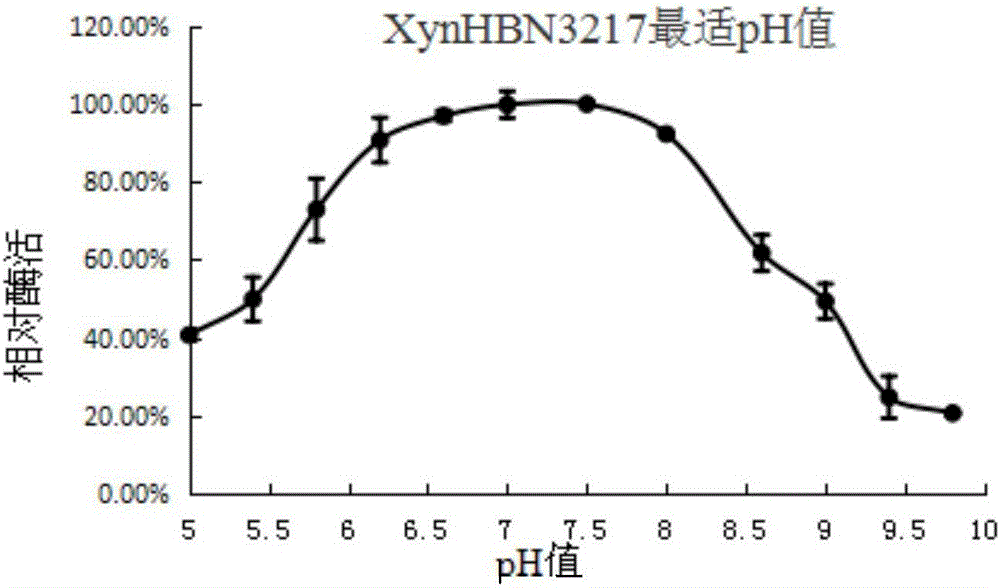
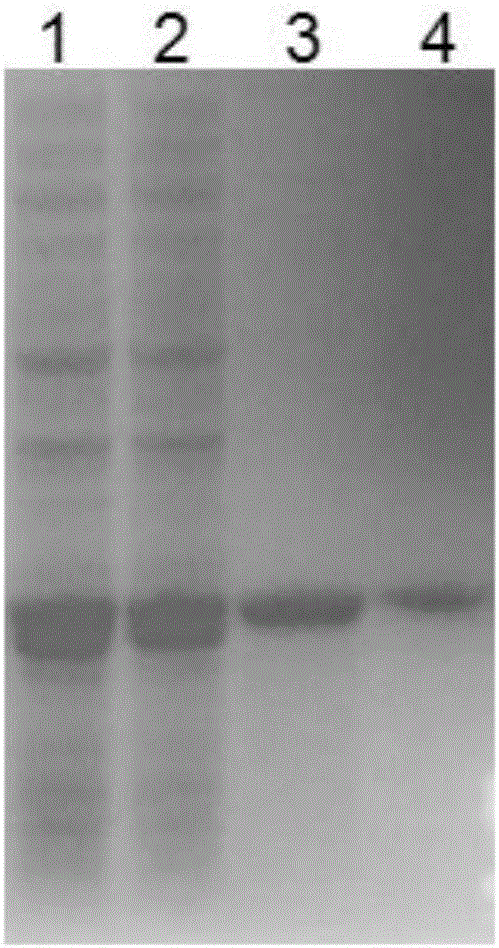
[0071] A total of about 20,000 transformants were obtained after the electric transformation of the recombinant plasmid in Example 2. Spot all the transformants onto a xylanase activity screening plate (0.5% cross-linked xylan, 1% peptone, 1 % sodium chloride, 0.5% yeast powder, 1.5% agar), and the culture temperature was 37°C, from which 46 mutants whose transparent circle was significantly larger than the original strain transparent circle were selected. First screened by the hydrolysis circle method, and then re-screened by expanding the culture of E. coli shake flasks to induce expression. figure 1 Finally, a strain with the highest enzyme activity was screened and its gene was named xynHBN3217.
[0072] For the preparation method of cross-linked xylan, please refer to the literature: Expression of xylanase XYNZG in Kluyveromyces lactis and its application in bread baking, Zhan Feixiang, Hubei University, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com