Thermophilic recombinant II type pullulanase and application thereof
A technology of pullulanase and recombinant Escherichia coli, which is applied in the field of genetic engineering and can solve the problems of poor thermal stability and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1II
[0040] Cloning and recombinant expression of the type II pullulanase gene of embodiment 1
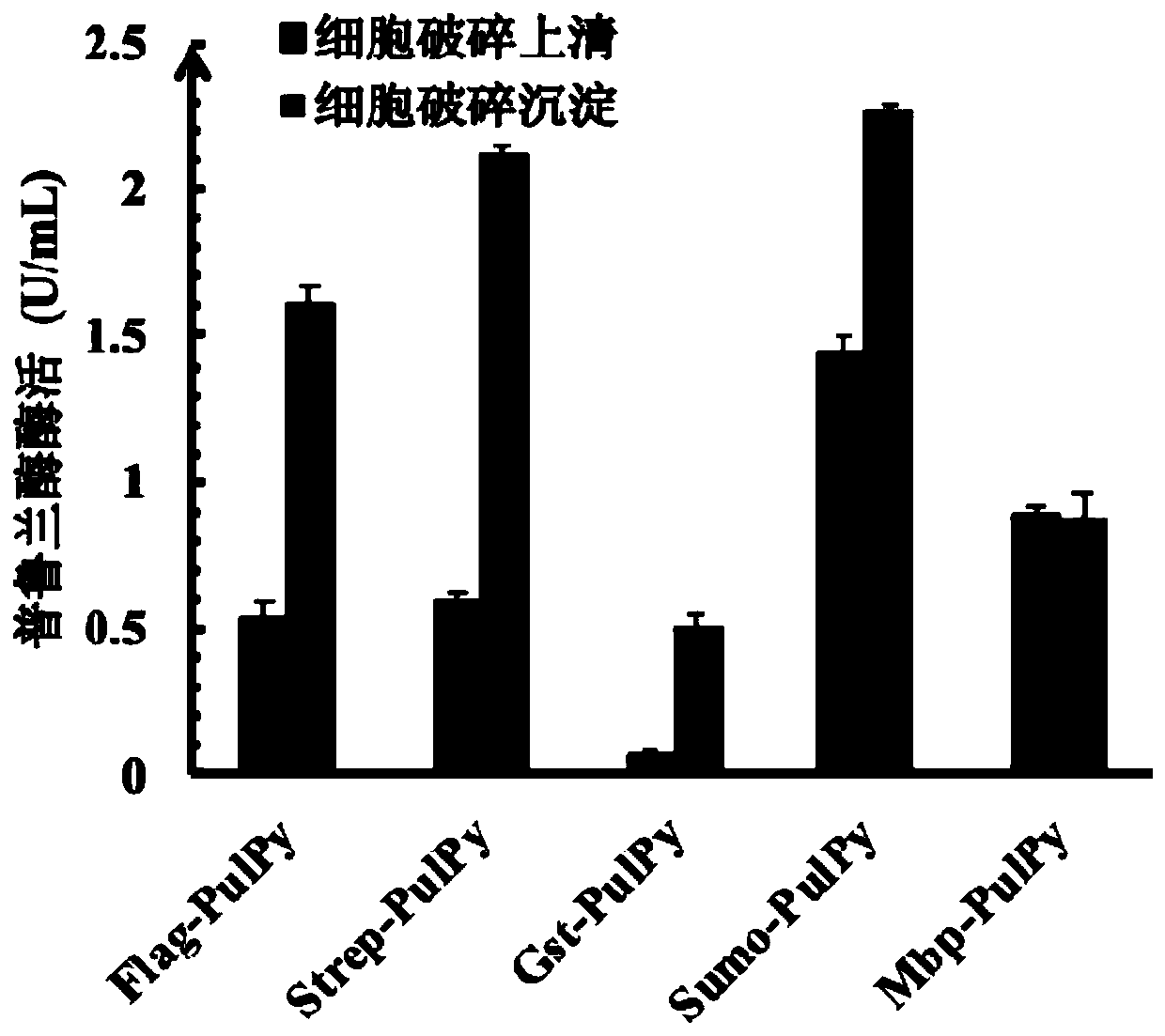
[0041] According to the suspected type II pullulanase gene (The Genbank accession number: WP_013906427.1) derived from the deep-sea hyperthermophilic and barophilic archaea Pyrococcus yayanosii CH1, the codon sequence of the gene sequence was carried out, and the optimized gene sequence (without The optimized gene sequence is almost not expressed in E. coli without a connexin tag.) SEQ ID NO.2 was synthesized by General Biosystems (Anhui) Co., Ltd., and connected to the expression vector pET-24a(+) Between BamH I and Xho I restriction sites, the recombinant plasmid pET-24a(+)-Pul Py . Next, we handed over the tag sequences Flag, Strep, Gst, Sumo, and Mbp to General Biosystems (Anhui) Co., Ltd. to synthesize them into pET-24a(+)-Pul Py Between the Nde I and BamH I restriction sites, the T7 tags of the above recombinant plasmids were replaced, and transformed into Escherichia coli BL21(DE...
Embodiment 2
[0044] Embodiment 2 recombinant type II pullulanase Sumo-Pul Py Determination of properties
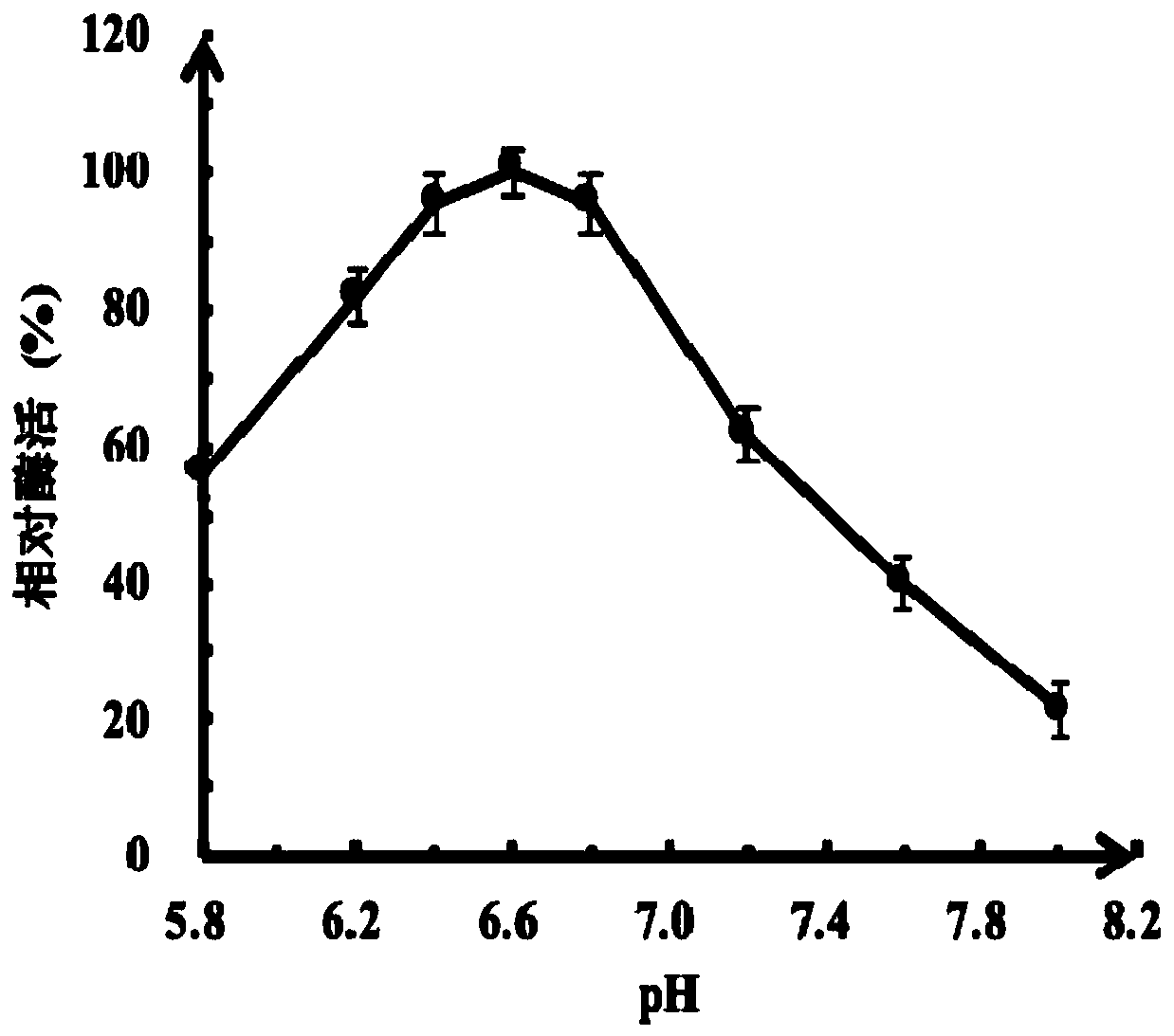
[0045] 1. Recombinant type II pullulanase Sumo-Pul Py Activity analysis of
[0046] Recombinant type II pullulanase Sumo-Pul Py 3,5-dinitrosalicylic acid (DNS) method was adopted for the determination of the activity of the method, and the specific method was as follows: 100 μL reaction system (10 μL final concentration of 100 mM potassium phosphate buffer with different pH, 50 μL final concentration of 3% pullulan and 40 μL Enzyme solution) reacted at a certain temperature for 15 minutes, added 100 μL DNS reaction solution, boiled water bath for 6 minutes, cooled to room temperature with running water, diluted with distilled water, mixed, and measured OD 476 . One enzyme activity unit (U) is defined as the amount of enzyme required to release reducing sugar equivalent to 1 μmol of glucose reducing power per minute under the assay conditions.
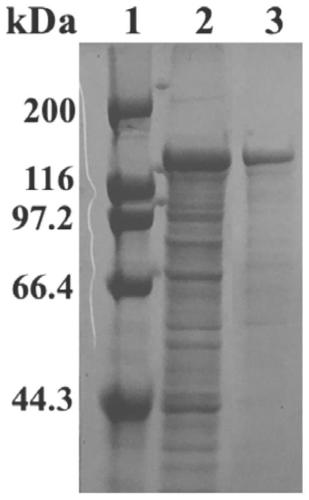
[0047] 2. Recombinant type II pullu...
Embodiment 3II
[0063] Example 3 Type II pullulanase Sumo-Pul Py Analysis of the enzymatic properties of the truncation mutant △28N+△791C
[0064] First, the Pul Py The first 28 and 33 amino acids at the N-terminal and the amino acids after the 734, 784, 790, 1021, 1025, 1068, and 1077 amino acids at the C-terminal were removed to obtain 9 Pul Py The truncated gene sequences were synthesized into the vector pET-24a(+)-His+Sumo-Pul by General Biosystems (Anhui) Co., Ltd. Py Between the BamH I and Xho I restriction sites, the 9 new recombinant plasmids obtained were transformed into Escherichia coli BL21(DE3). The results showed that the specific enzyme activity of △735C at 95°C was Sumo-Pul Py 0.33 times that of Sumo-Pul. Py 2.02 and 2.31 times of that of Sumo-Pul Py There is also a certain degree of improvement ( Figure 9 ).
[0065] Pul PyThe first 28 amino acids at the N-terminal and the amino acids after the 790th position at the C-terminal were removed to obtain a combined trunc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com