Lipase mutant with improved enzyme activity and regioselectivity and application of mutant
A lipase and mutant technology, applied in the field of genetic engineering, can solve the problems of poor position selectivity, high cost, and low lipase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Construction of lipase mutant
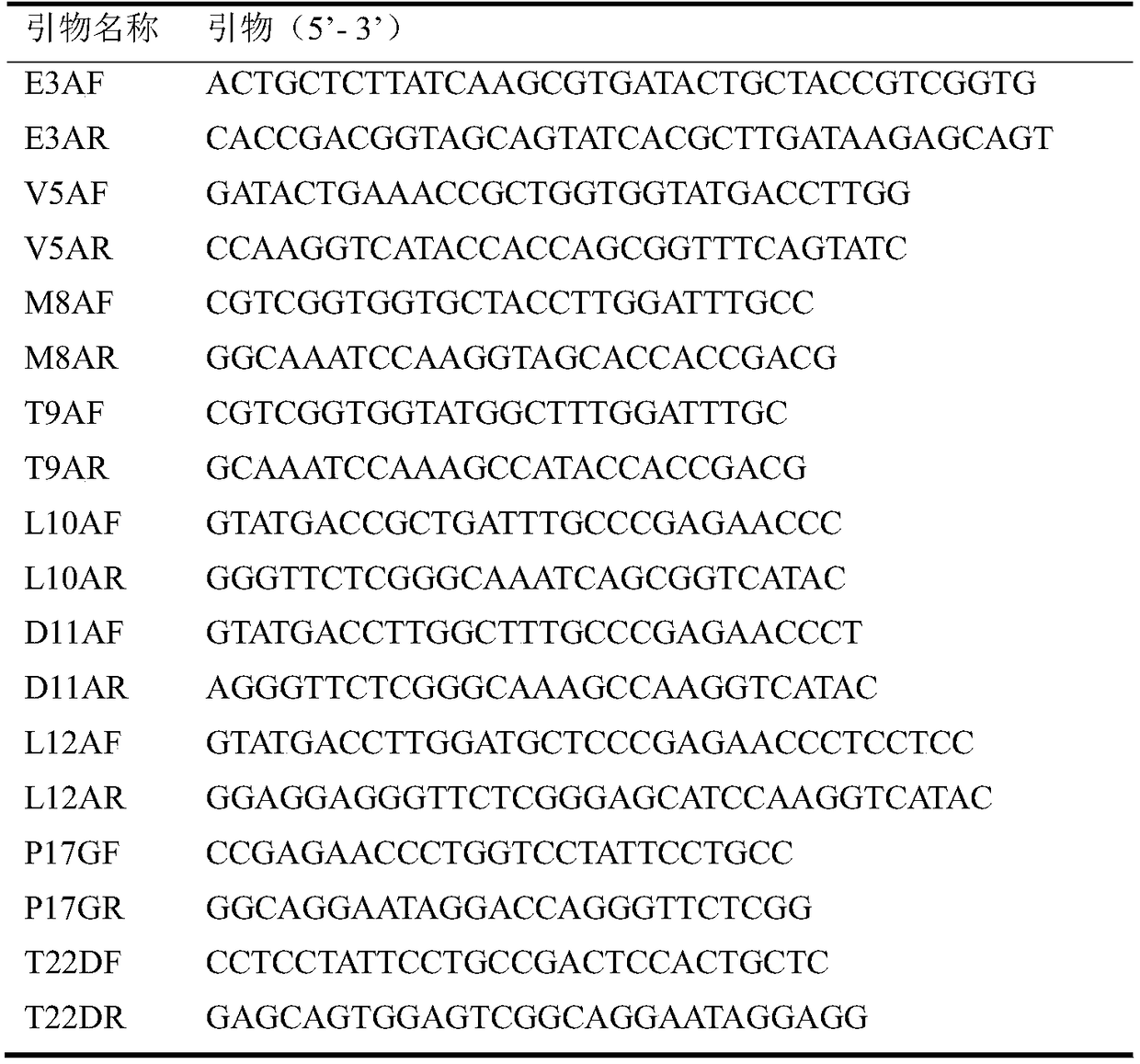
[0023] Through the analysis of the interaction between the lipase leader peptide and the main protein structure, the key sites are respectively determined: E3, V5, D11 that interact with the lid, M8, T9, T9 that interact with the active center or are relatively close. L12, P17, L10 interacts with the lid and the active center, and T22 interacts with the main protein but is far from the active center. Mutations were designed based on the interaction between lipase leader peptide amino acids and the host protein. Primers were designed as follows:
[0024] Table 1 Leader peptide mutation primers
[0025]
[0026] Using a point mutation kit ( Lightning Site Directed Mutagenesis Kit, Stratagene, Agilent technologies, La Jolla, CA, USA) used the MBP-proRCL plasmid (Sha Chong, Ph.D. dissertation, Jiangnan University, 2015) as a template for full-plasmid PCR, and heat shock transformed Escherichia coli JM109 competent, const...
Embodiment 2
[0027] Example 2: Expression and purification of lipase mutants
[0028] Expression and purification method of mutants E3A, V5A, M8A, T9A, L10A, D11A, L12A, P17G, T22D:
[0029] (1) Optimization of expression and purification conditions: Pick a single colony of Escherichia coli BL21trxB(DE3) carrying the recombinant plasmid of the mutant gene on LB solid medium, and inoculate 500 μL of LB liquid medium (containing 100 μg·mL -1 Ampicillin antibiotic and 50 μg·mL -1 Kanamycin antibiotic) 37°C, 200rpm for 4-6h. Take 10 μL and inoculate 10 mL of liquid LB medium, and culture overnight at 37°C and 200 rpm. Transfer all 10mL of liquid LB medium to 200mL of liquid LB medium and cultivate to OD at 37°C and 200rpm 600 When the temperature reaches 0.6-0.8, take 20mL and add 1mM IPTG to 17°C, 29°C, and 37°C, respectively, and induce overnight, 6h, and 4h respectively. 1 L of fermentation broth was collected, and the cells were collected by centrifugation at 6000 rpm for 30 min, and t...
Embodiment 3
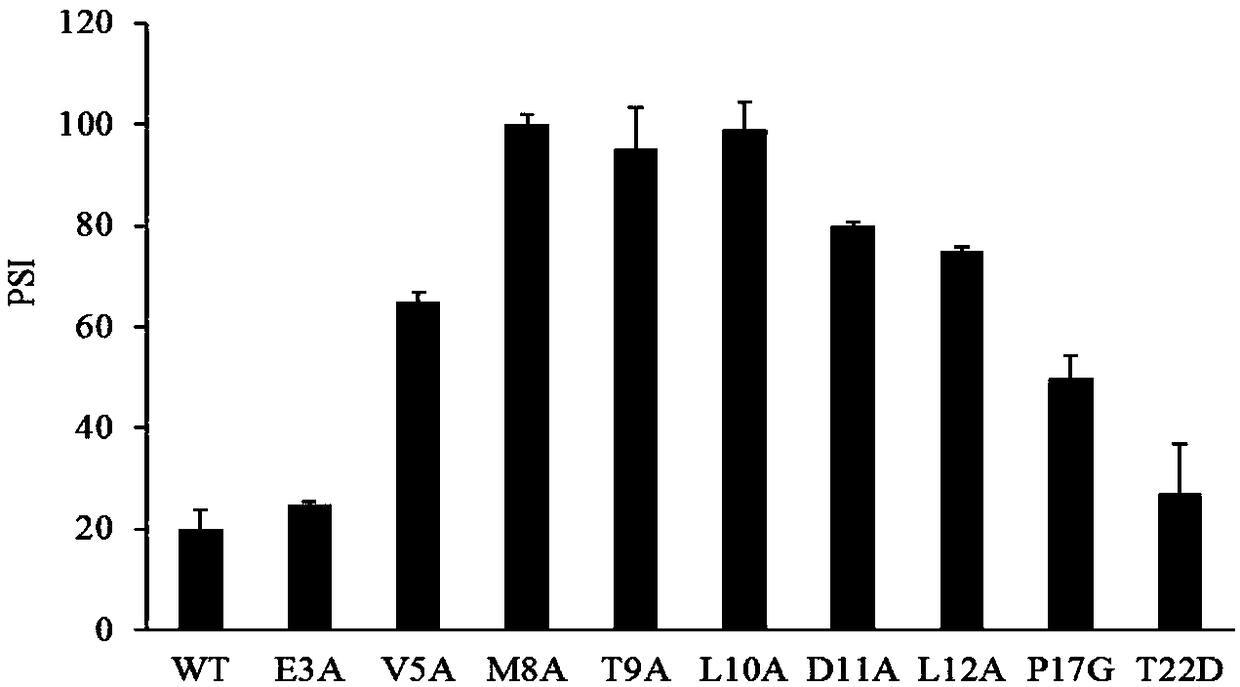
[0032] Example 3: Construction, expression and kinetic analysis of RCL leader peptide mutants
[0033] Pick a single colony of Escherichia coli BL21trxB(DE3) containing the recombinant plasmid on LB solid medium, and inoculate 500 μL LB liquid medium (containing 100 μg·mL -1 Ampicillin antibiotic and 50 μg·mL -1 Kanamycin antibiotic) 37°C, 200rpm for 4-6h. Take 50 μL and inoculate 50 mL of liquid LB medium, and culture overnight at 37°C and 200 rpm. Transfer all 50mL of liquid LB medium to a 2L shake flask containing 950mL of liquid LB medium and culture to OD at 37°C and 200rpm 600 After reaching 0.6-0.8, add 1mM IPTG to induce, and induce culture overnight at 17°C. SDS-PAGE was used to detect the whole cell protein and supernatant protein of each mutant.
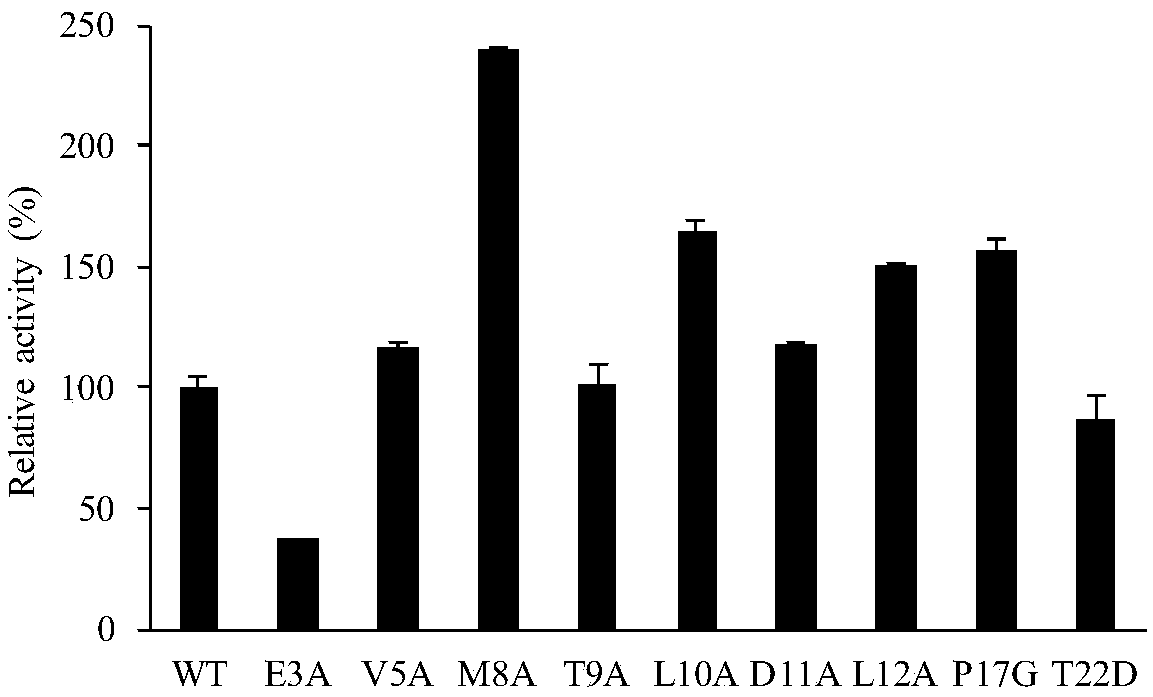
[0034] The specific activity of the wild-type enzyme is 156.15 U / mg, and the relative activity of the wild-type is set as 100%. like figure 1 As shown, the specific enzyme activities of the wild enzyme WT and T9A are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com