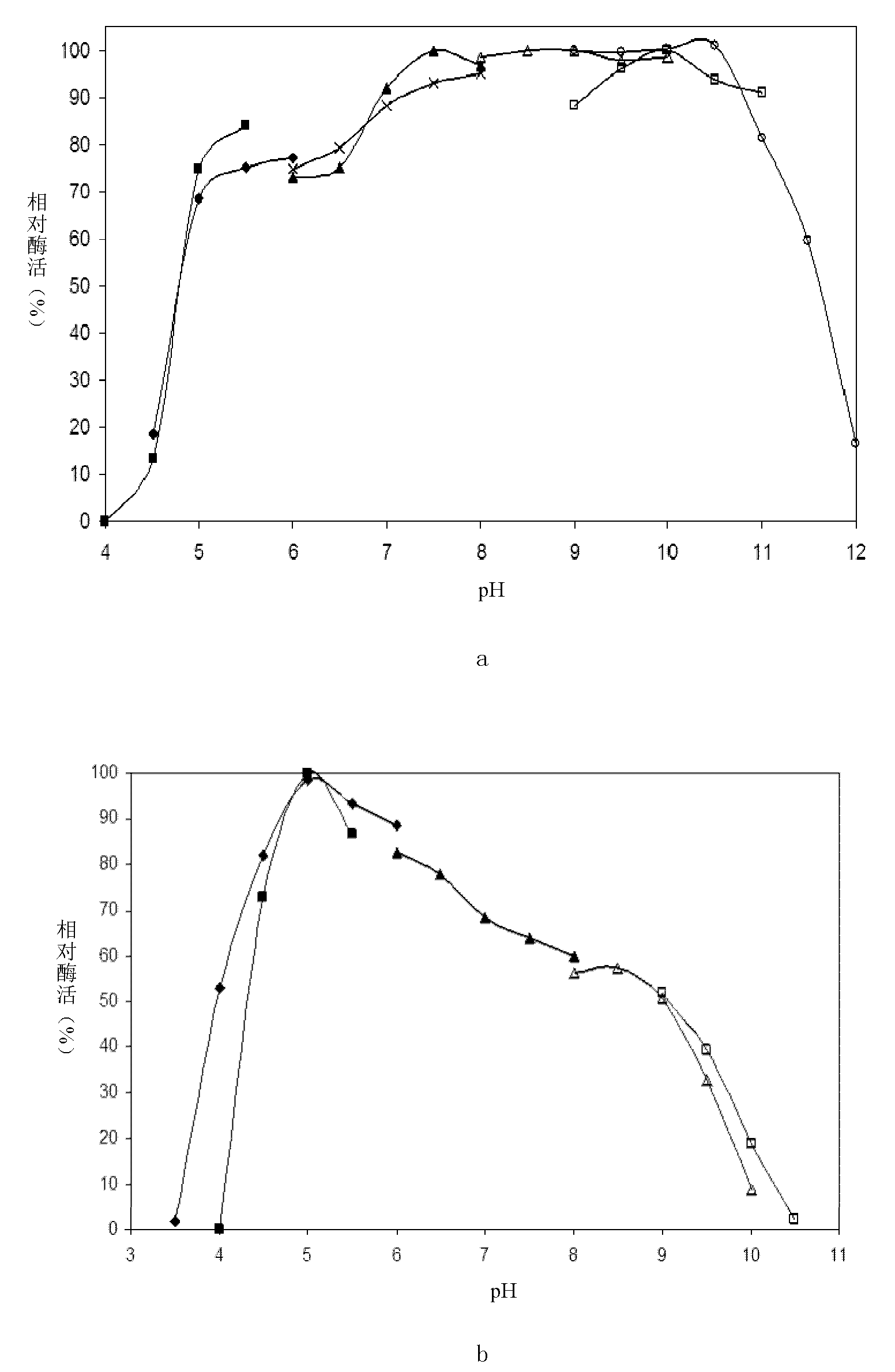Beta-mannanase and preparation method thereof
A technology of mannanase and glycerol, which is applied in the fields of botany equipment and methods, biochemical equipment and methods, and microbial-based methods, can solve the problems of unseen and unseen production of mannanase, and achieve high-efficiency expression , good thermal stability and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Cloning of the β-mannanase gene of Chaetomium CQ31
[0049] The obtaining of the full-length gene sequence of the β-mannanase of Chaetomium CQ31 comprises the following steps:
[0050] 1. Cloning of β-mannanase gene fragment
[0051] According to the amino acid sequence of the fungal β-mannanase announced in GenBank, the conserved sequence was analyzed by comparison, and Codehop software ( http: / / bioinformatics.weizmann.ac.i1 / blocks / codehop.html ) online design of degenerate primers, the sequences of degenerate primers and corresponding conserved amino acids are as follows:
[0052] DP1 (upstream primer): CCTGCGCGTCTGGGGNTTYAA(LRVWGF)
[0053] DP2 (downstream primer): CGTTGGCCAGCTCCCCANGCRAA(FAWELANE)
[0054] Where Y: A / G, N: A / T / G / C, R: C / T
[0055] In the PCR reaction, the total DNA of Chaetomyces CQ31 was used as a template, DP1 and DP2 were used as primers, and Ex taq DNA polymerase (Takara Company) was used to amplify. The program was: 94°C pre-de...
Embodiment 2
[0064] Example 2: Construction and high-efficiency expression of Chaetomium CQ31 β-mannanase expression engineering bacteria
[0065] 1. Construction of engineering bacteria expressing β-mannanase
[0066] Design expression primers according to the sequence of the yeast expression vector and β-mannanase, and the upstream and downstream primers are respectively added with EcoR I and Not I restriction sites. The upstream and downstream primers are as follows:
[0067] Upstream primers: GAATTC CCAAGCCGAGCTGTGCAGG (EcoR I)
[0068] Downstream primers: GCGGCCGC TTAGTCACTTCTCGCCTCGCTA (Not I).
[0069] Use the above primers to amplify the eDNA obtained from the reverse transcription of the total RNA of Chaetomyces CQ31 in Example 1. After the PCR product is detected by agarose gel electrophoresis, it is recovered and connected to the pMD-18T vector, and transformed into E.coli From JM109 (purchased from Bomed Biological Company), a single colony was selected for sequencing. Th...
Embodiment 3
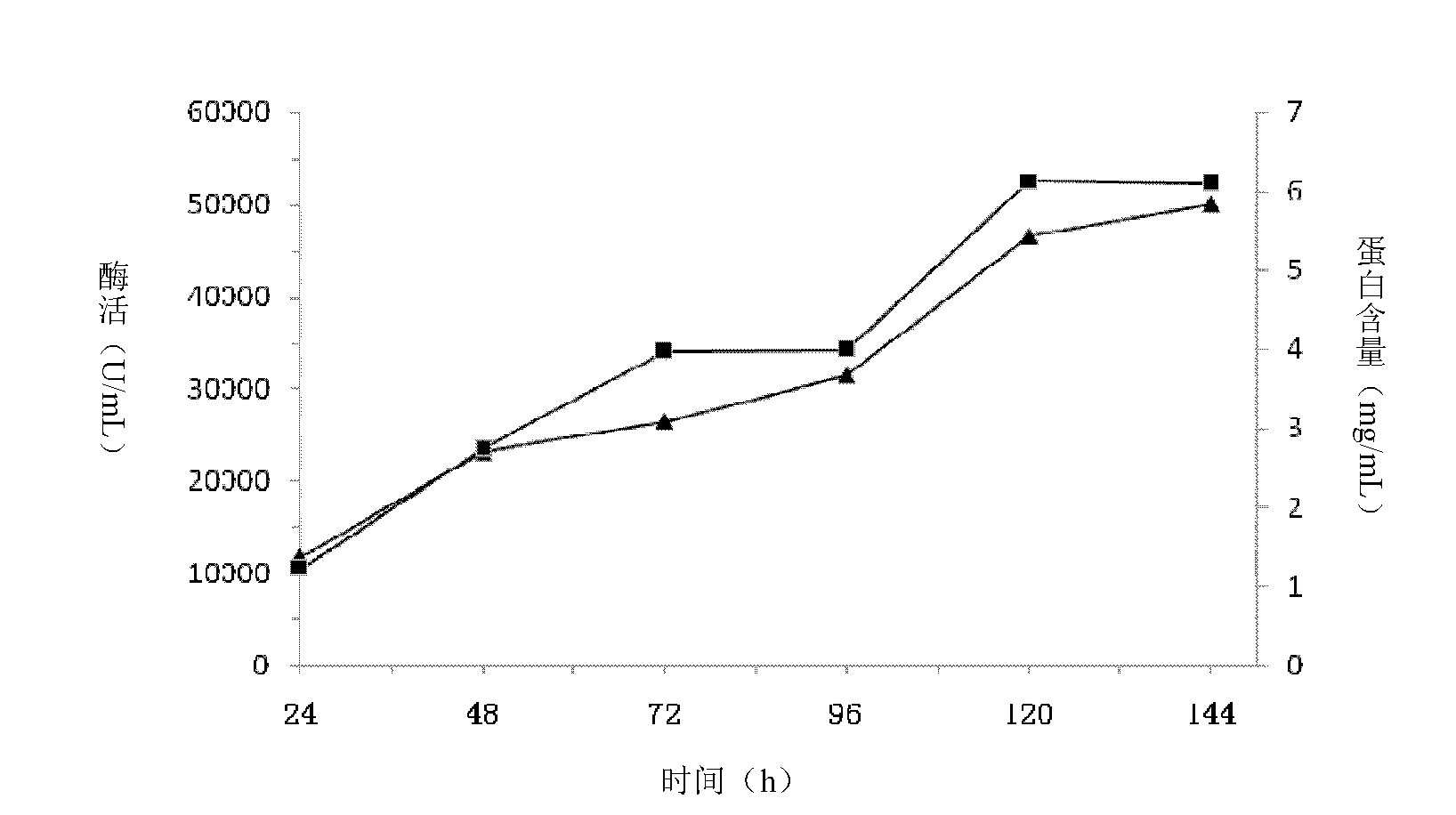
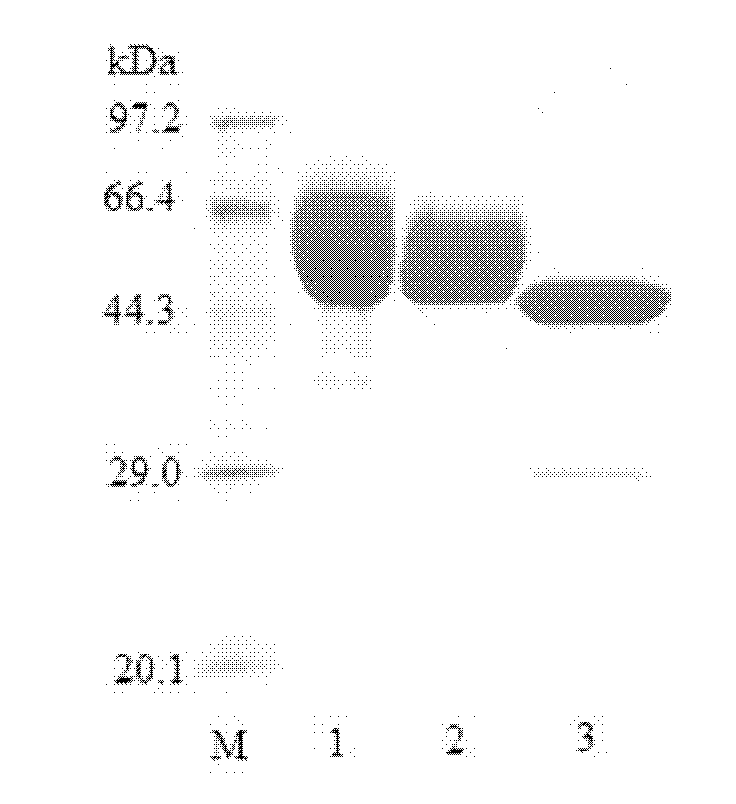
[0078] Embodiment 3: Purification and enzymatic properties of Chaetomium β-mannanase
[0079] 1. Definition and determination method of enzyme activity
[0080] Enzyme activity was determined by DNS method. 100 μL of appropriately diluted enzyme solution was added to 900 μL of 0.5% locust bean gum (LBG) prepared in 50 mM pH 5.0 citrate buffer, and reacted at 55° C. for 10 min. After the reaction, the reaction was terminated with DNS reagent and reacted with reducing sugar. With mannose as the marking line, define the amount of enzyme needed to generate 1 μmol mannose per minute as 1U.
[0081] 2. Purification of β-mannanase
[0082] 10mL of the fermentation broth supernatant of the fermenter, after dialysis equilibration, was loaded on a Q-Sepharose column equilibrated with 20mM pH 6.5 phosphate buffer. After washing unbound protein with 5 column volumes of buffer, 0-500 mM NaCl was linearly eluted and fractionated. After the obtained pure enzymes were combined, they were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com