Fungus-derived laccase, recombinant pichia pastoris engineering bacteria thereof and application of fungus-derived laccase
A technology of Pichia pastoris and engineering strains, applied in the fields of application, fungi, genetic engineering, etc., can solve the problems of pollution, high price, etc., and achieve the effect of improving decolorization rate, high specific enzyme activity, and high oxidation capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Cloning of target gene
[0047] Strain Trametes sp.AH28-2 was activated by PDA plate, inoculated in 100mL / 250mL cellobiose-asparagine liquid medium at 28℃, 120rpm for 4 days, after homogenization treatment, inoculated in 100mL / 250mL cellobiose-asparagine Paragine liquid medium. After 3 days of culture, vanillic acid was added for induction, and samples were taken every 12 hours. The isolated supernatant and bacterial cells were stored in refrigerators at 4°C and -80°C, respectively. After being treated with a liquid nitrogen freezer, the RNA was extracted according to the instructions of the RNAiso Plus kit of TaKaRa Company, and the cDNA was prepared according to TaKaRa Company PrimeScript TM RT reagent kit with gDNA Eraser (Perfect Real Time) kit instructions require to carry out, to obtain the laccase gene template.
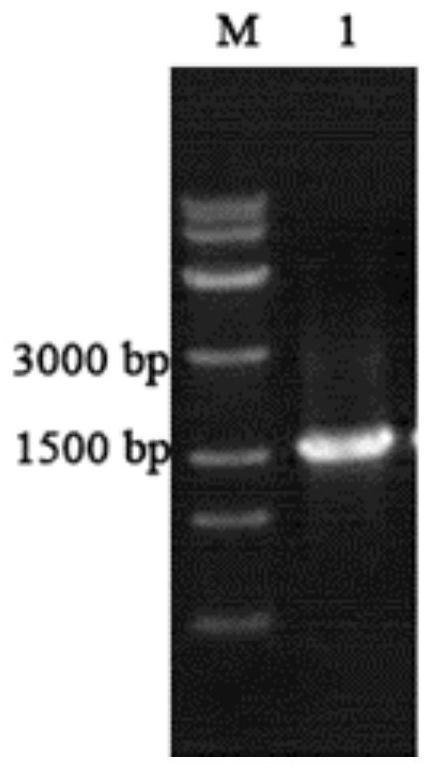
[0048] Using the first strand of the prepared laccase cDNA as a template, the laccase lac1 was amplified by PCR, and the amplification PCR diagram is...
Embodiment 2
[0056] Construction of lac1-pPIC9K expression vector
[0057] The lac1 and the vector pPIC9K were double digested with restriction endonucleases SnaB I and Not I. The enzyme digestion system is shown in Table 2. The reaction was carried out in a water bath at 37°C for 30 minutes, and the enzyme digestion product was recovered using a purification kit.
[0058] Table 2 Double Enzyme Digestion System
[0059]
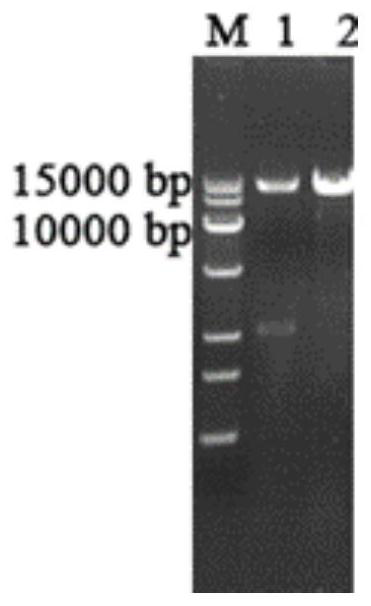
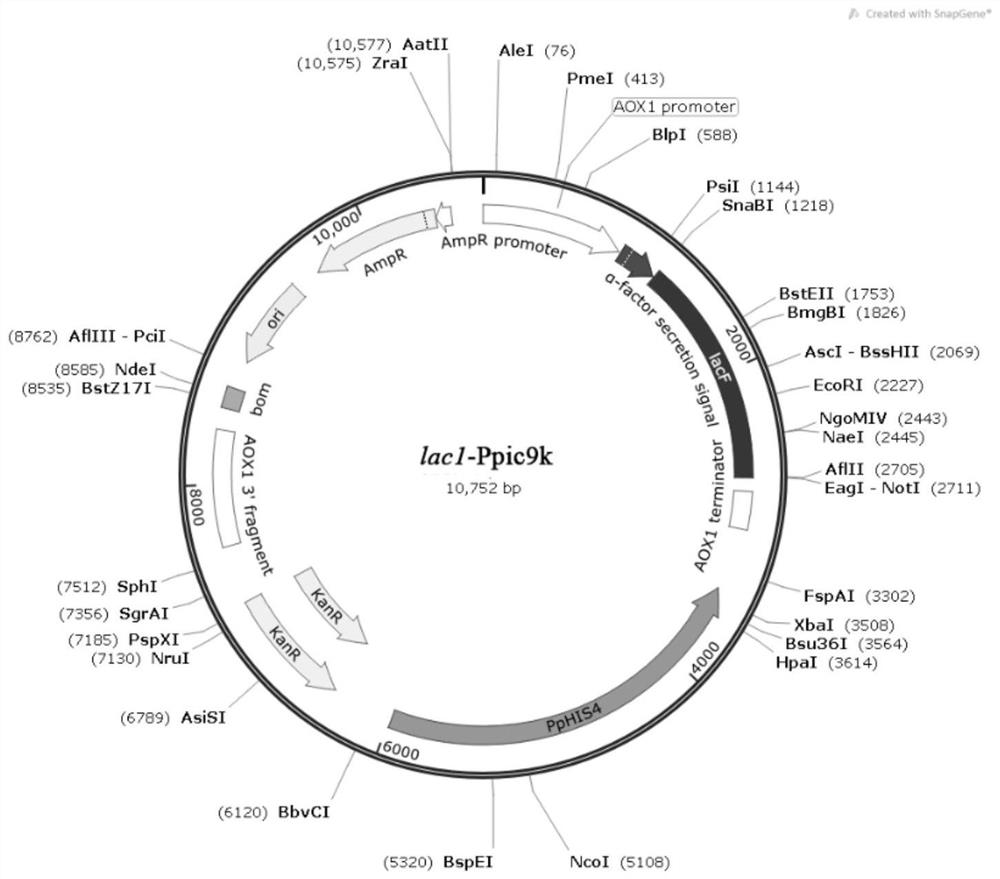
[0060] The pPIC9K and lac1 double digestion products were added in proportion, and ligated overnight at 16°C. The connection system is shown in Table 3. Transform into Trans T1 Escherichia coli competent cells, use the 5' end restriction site SnaB I, the 3' end restriction site Not I, insert the pPIC9K plasmid vector promoter AOX1 downstream, construct the lac1-pPIC9K expression vector, the expression vector See atlas image 3 , double enzyme digestion verification such as figure 2 , the expression vector was constructed successfully.
[0061] Table 3 connection...
Embodiment 3
[0064] Construction of recombinant Pichia pastoris
[0065] 1. Linearize the recombinant expression plasmid lac1-pPIC9K with restriction endonuclease Stu I. The linearization system is shown in Table 4, and react in a water bath at 37°C for 30 minutes. For detection of linearized plasmids by nucleic acid electrophoresis, see Figure 4 . The linearized plasmid was transformed into Pichia pastoris GS115 by electroporation and spread on MD plate to obtain transformants.
[0066] Table 4 Stu I digestion system
[0067]
[0068] 2. To extract the yeast genome, refer to the Instruction Manual of the Quick Extraction Kit of Yeast Genome DNA of Shanghai Sangon Bioengineering Co., Ltd., No. B518227. For the genome PCR map, see Figure 5 . The clones on the MD plate (50 clones in total) were picked and transferred to the BMGY plate, and cultured at 28°C for 2 days. Then copy the clone on BMGY to the BMM plate containing 1mM guaiacol, and culture it at 28°C until a color circle a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com