Genetic-engineering L-asparaginase amidohydrolase modified through site-specific mutagenesis
An asparaginase and mutant technology, applied in the field of genetic engineering, can solve the problems of low L-asparaginase enzyme activity and low protein expression, and achieve the effect of improving industrial application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Contains the construction of the recombinant vector of L-asparaginase mutant
[0019] (1) Obtaining of the S166A mutant: using the nucleotide sequence shown in SEQIDN0.4 as a template, Fprimer (sequence shown in SEQIDN0.5), and Rprimer (sequence shown in SEQIDN0.6) as primers, PCR is obtained The recombinant gene shown in SEQIDN0.3.
[0020] (2) Digest the recombinant gene and pMA5 with BamHI and MluI, respectively, and ligate with T4 DNA ligase overnight at 16°C after purification. The ligation product was chemically transformed into JM109 competent cells. The transformation solution was applied to an LB plate containing kanamycin (50 mg / L), the plasmid was extracted, and the recombinant plasmid constructed was verified by double enzyme digestion, which was named pMA5-S166A. The sequencing work was completed by Shanghai Sangong.
Embodiment 2
[0021] Example 2 Production of L-asparaginase Bacillus subtilis Engineering Bacteria Construction
[0022] The recombinant plasmid pMA5-S166A obtained in Example 1 was chemically transformed into B. subtilis168 competent cells, and the specific method was as follows:
[0023] (1) The solution required for the transformation experiment is as follows (g / L):
[0024] Sp-A: (NH 4 ) 2 SO 4 4,K 2 HPO 4 28. Sodium citrate 12Sp-B: MgSO 4 ·7H 2 O0.4
[0025] 100×CAYE: Casaaminoacid20, yeast powder 100SpI medium: Sp-A49%, Sp-B49%, 50% glucose 2%, 100×CAYE2% SpII medium: SpI medium 98%, 50mmol / LCaCl 2 1%, 250mmol / LMgCl 2 1%. 115°C damp heat sterilization.
[0026] (2) Inoculate a single colony of B.Subtilis168 into 2mL SpI medium (50mL centrifuge tube), and culture overnight at 37°C and 200r / min;
[0027] (3) Take 100 μL of the culture solution into 5 mL of SpI medium, and culture at 37°C and 200 r / min until the logarithmic phase (OD600 value is about 1), about 4 to 5 hours; ...
Embodiment 3
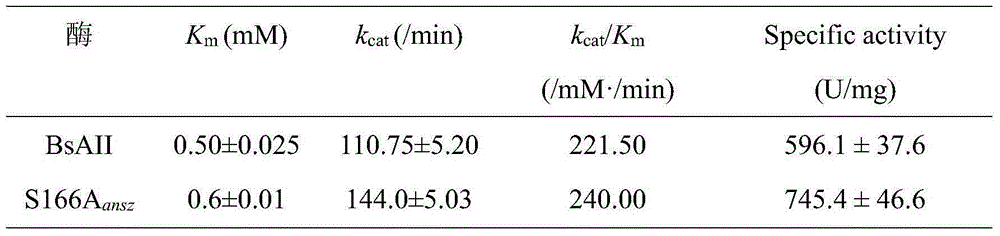
[0029] Example 3: High expression and enzyme activity determination of recombinant bacteria pMA5-S166A / B.subtilis168L-asparaginase.
[0030] (1) The recombinant strain pMA5-S166A / B.subtilis168 constructed in Example 2 and the original strain pMA5-ansz / B.subtilis168 were respectively inoculated in 10 mL of LB medium containing kanamycin, cultured with shaking at 37°C overnight, and Transfer to the fermentation medium of Bacillus subtilis at 4% daily inoculum, culture at 37°C for 24 hours, take the fermentation broth at 4°C, and centrifuge at 10,000r / min for 10min, the supernatant is extracellular crude enzyme liquid, and the supernatant of broken cells The solution is the intracellular crude enzyme solution, which is used for the determination of enzyme activity.
[0031] (2) Bacillus subtilis fermentation medium: soybean peptone 10g / L, K 2 HPO 4 2.3g / L,KH 2 PO 4 1.7g / L, corn steep liquor 15g / L, urea 3g / L, glucose 40g / L, MgSO 4 0.75g / L, NaCl5g / L. Adjust pH6.8-7.0.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com