Construction of recombinant strain capable of producing arginine deiminase and directional modification method thereof
An arginine deiminase and a construction method technology, which can be applied in the directions of microorganism-based methods, botanical equipment and methods, biochemical equipment and methods, etc., and can solve problems such as limiting the application prospects of recombinant ADI enzymes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Construction of recombinant ADI
[0024] According to the gene sequence of the ADI, design primers:
[0025] F: 5'-GCT CATATG TCCGCTGAAAAACAGAAGTACG-3’ ( Nde I)
[0026] R: 5'-AT CTCGAG TTAGTAGTTGATCGGGTCGCGCA -3' ( xho I)
[0027] Introduce upstream and downstream separately Nde I and xho I enzyme cutting site (underlined), the primers used in the present invention were all synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0028] Using Pseudomonas mutans cells as a template, the ADI gene fragment was amplified by PCR, and the reaction system was 50 μL, as shown in Table 1:
[0029] Table 1 PCR amplification reaction system of ADI gene fragment
[0030] 10×Ex Taq Buffer
[0031] Reaction program: Denaturation at 94 °C for 10 min; reaction at 94 °C for 1 min, 56 °C for 45 s, and 72 °C for 2 min for 30 cycles, followed by extension at 72 °C for 10 min to obtain the PCR product ADI.
[0032]The PCR product A...
Embodiment 3
[0051] Example 3 Screening of ADI mutant library
[0052] A single colony in the ADI mutant library was transferred to an LB / Kan plate containing IPTG (final concentration 0.2 mmol / L), and cultured at 30°C until a single colony grew.
[0053] According to the reaction that ADI catalyzes arginine to produce citrulline and ammonia, a 96-well plate screening method for mutants with ADI activity was designed. Specific operation: Take a 96-well plate, first add 50 μl of 0.2mol / L phosphate buffer solution (pH 7.4) to each well, pick a single colony induced by IPTG and mix it in the well (the sample without bacteria is used as a control), Then add 50 μl of 1 mmol / L L-arginine hydrochloride, 0.2 mol / L phosphate buffer (pH 7.4), react at 37°C for 15 min, add 90 μl of mixed acid to terminate the reaction, and then add 30 μl of diacetylmonoxime-sulfur Amidurea solution, mix well, react at 37°C for 2 hours, measure OD 530 . The reaction solution in the well where the strain with ADI ac...
Embodiment 4
[0054] Example 4 ADI mutant strain M314
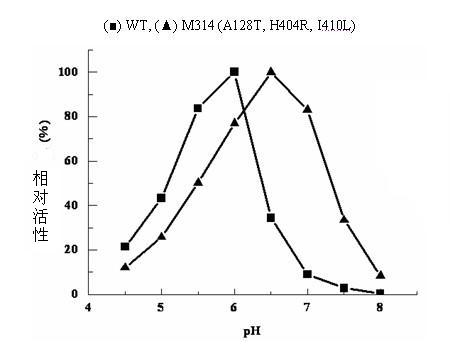
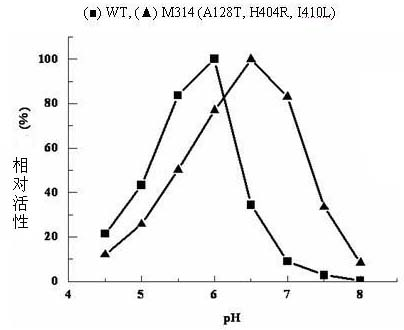
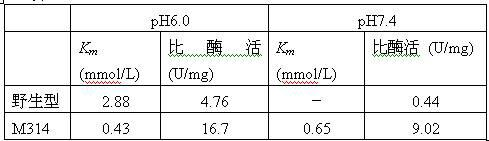
[0055] A single colony of the ADI mutant strain M314 was inserted into LB / Kan liquid medium and cultured overnight at 37°C and 200r / min. Bacteria were collected, plasmids were extracted, and purified. The plasmid was sequenced by Shanghai Sangon Bioengineering Technology Service Co., Ltd. See SEQ ID NO: 1 in the sequence table. It was determined that M314 carried three mutation sites, namely A128T, H404R and I410L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com