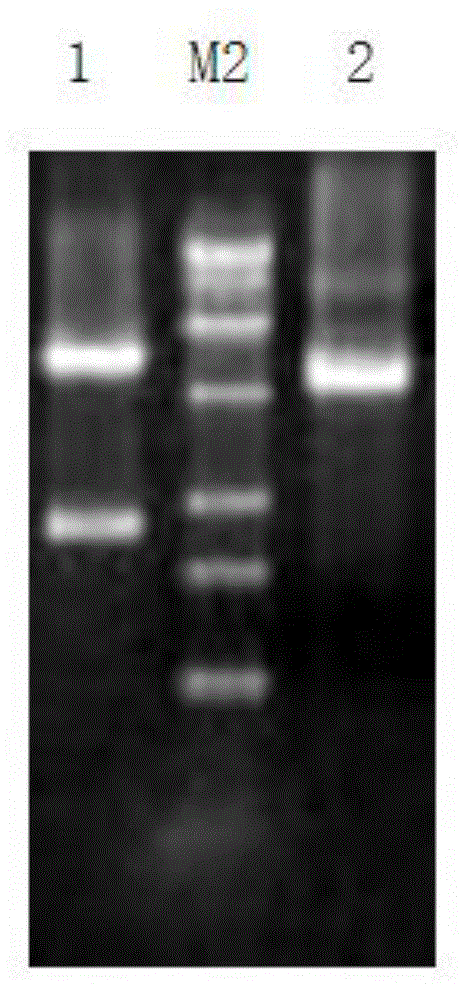
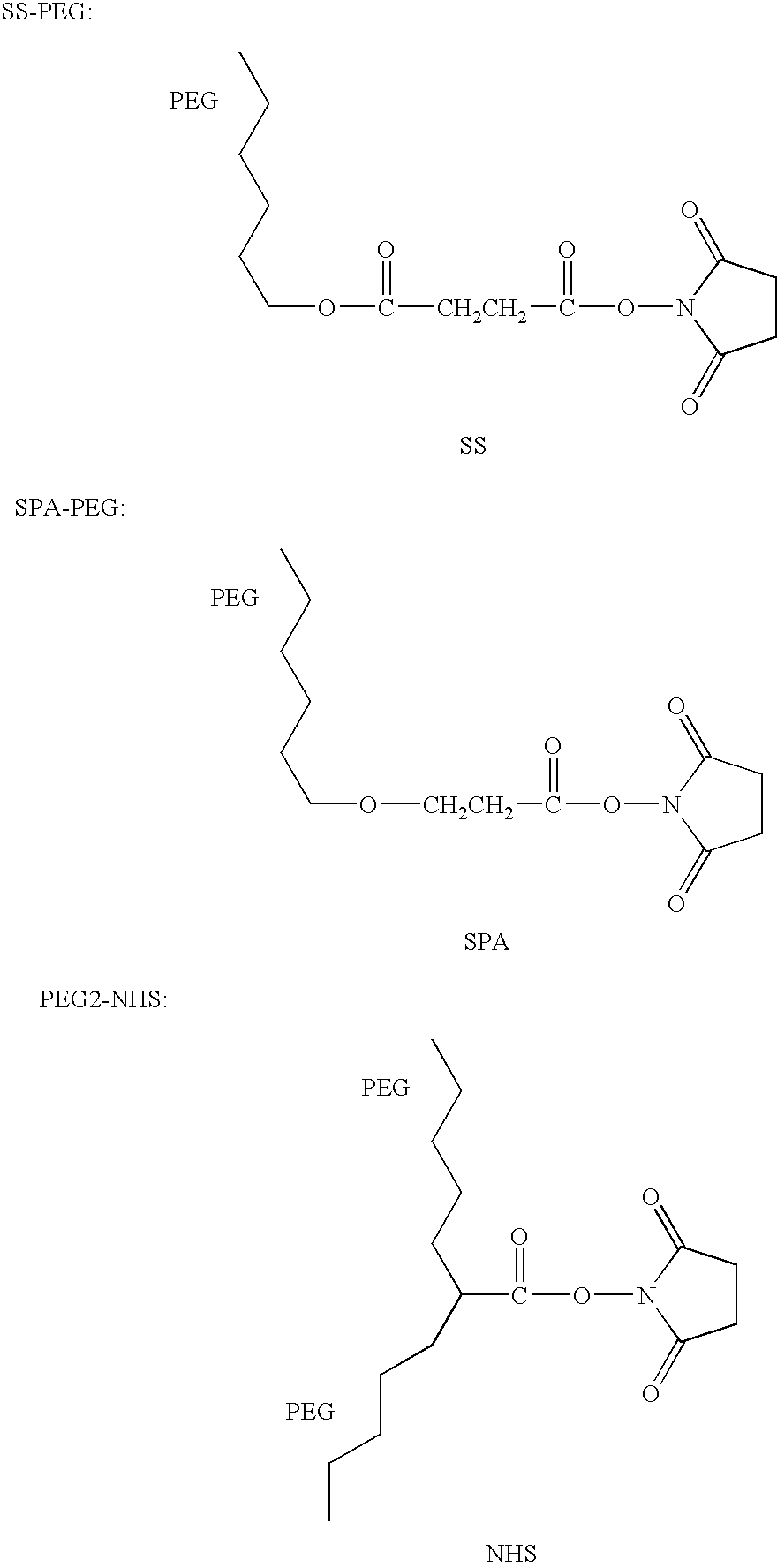
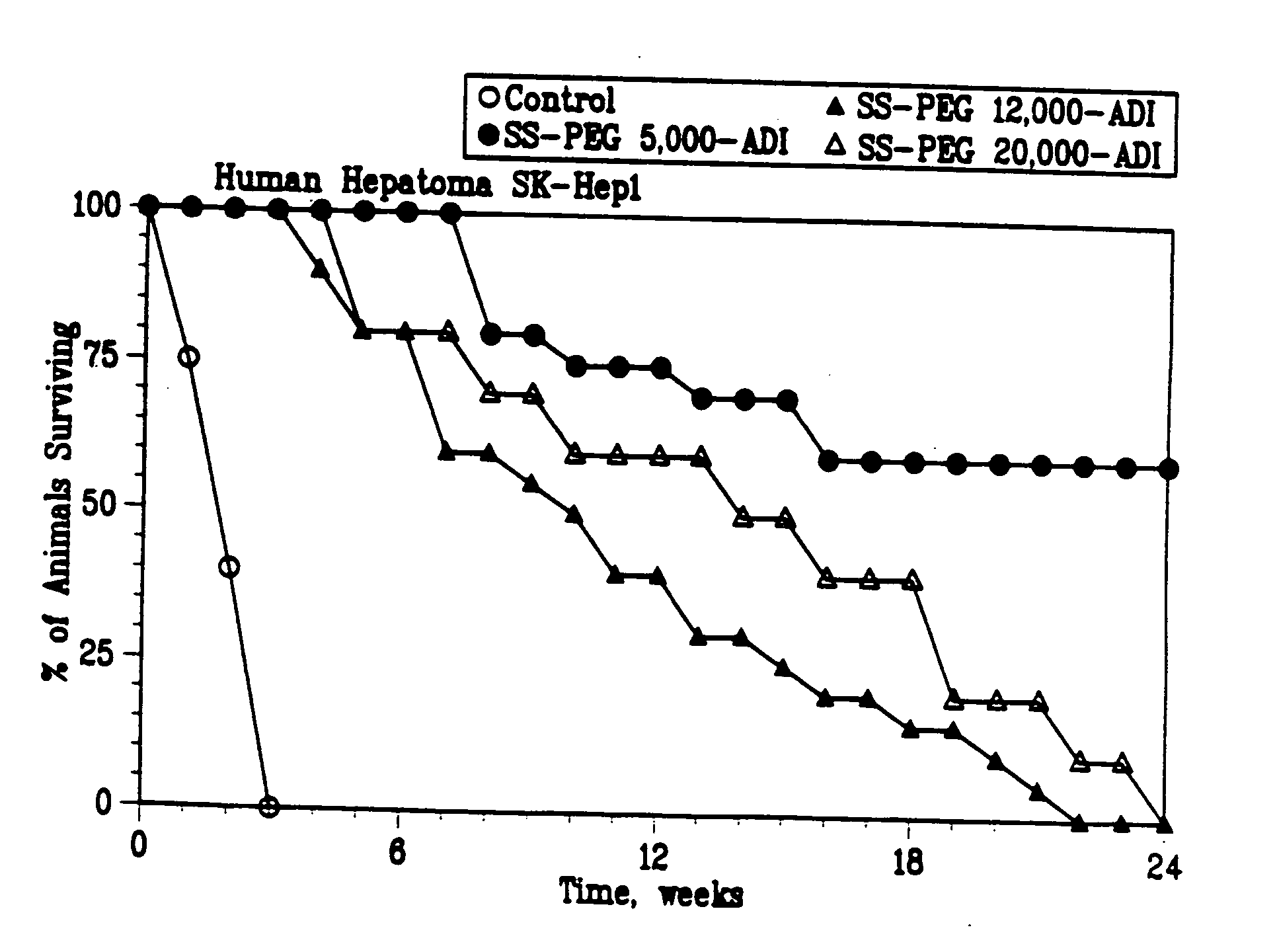
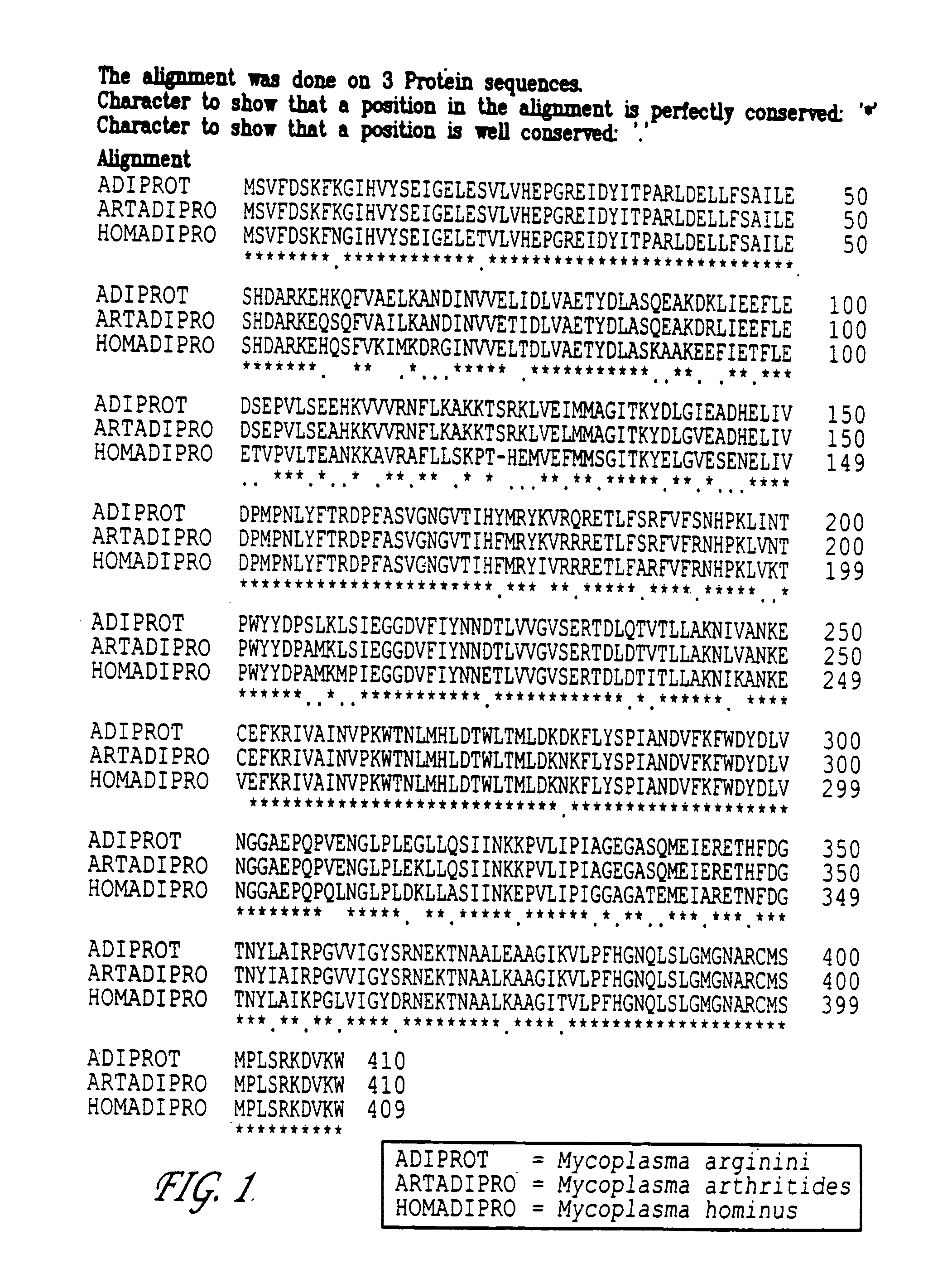
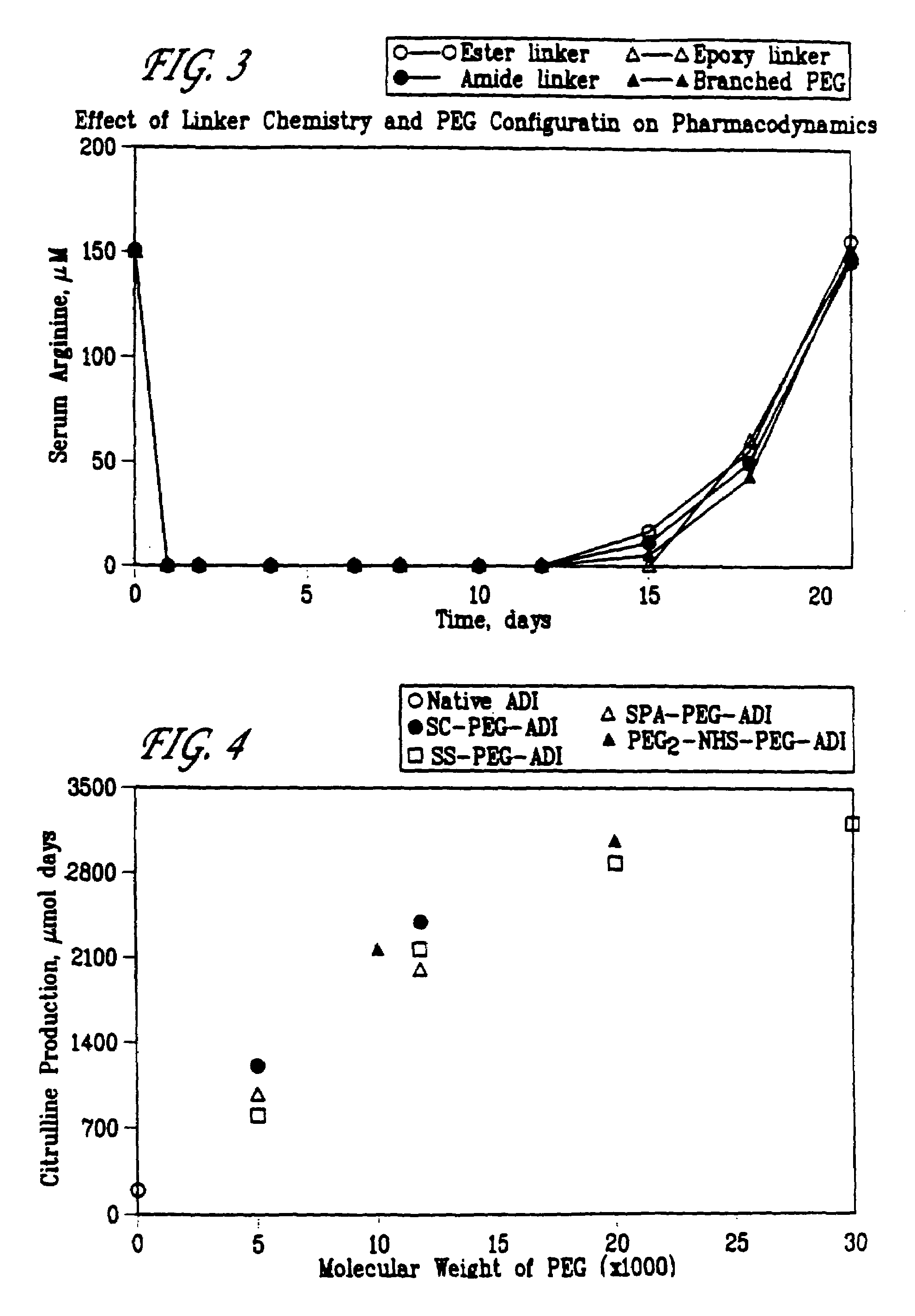
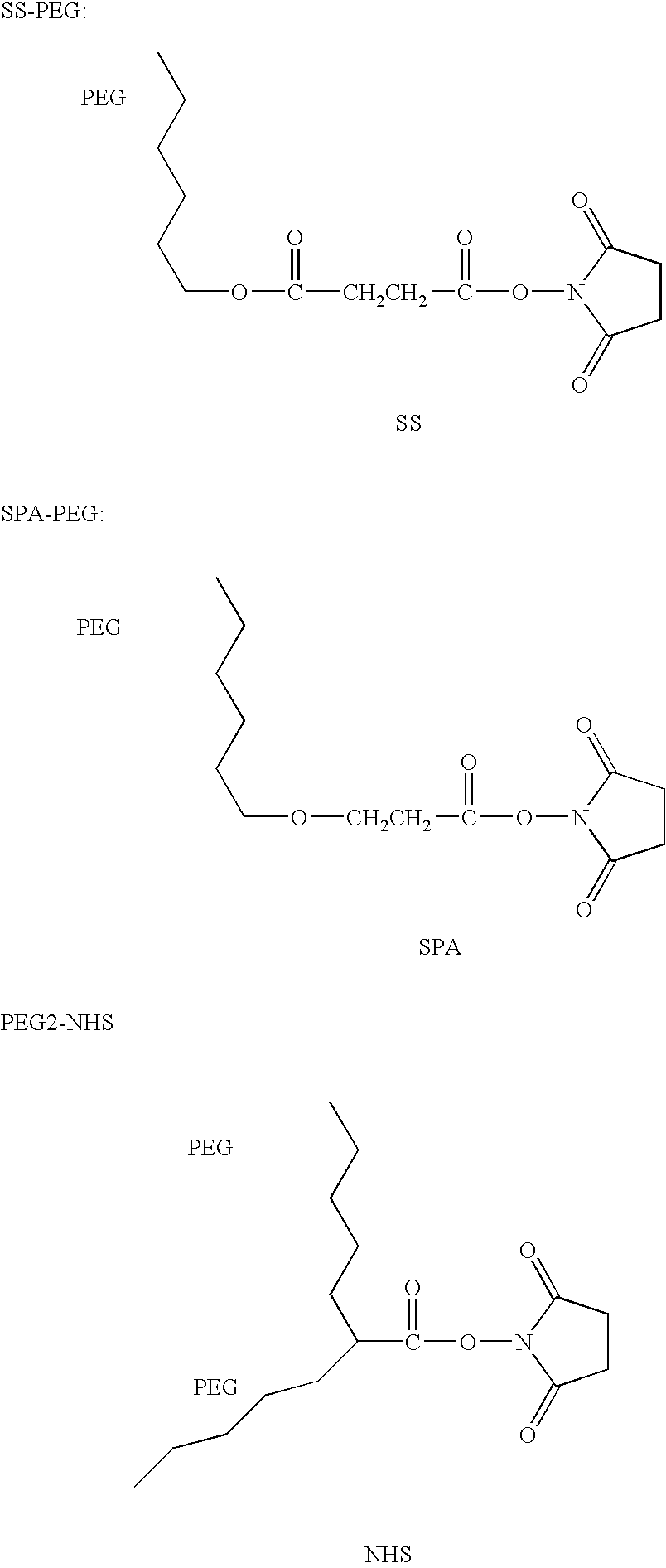
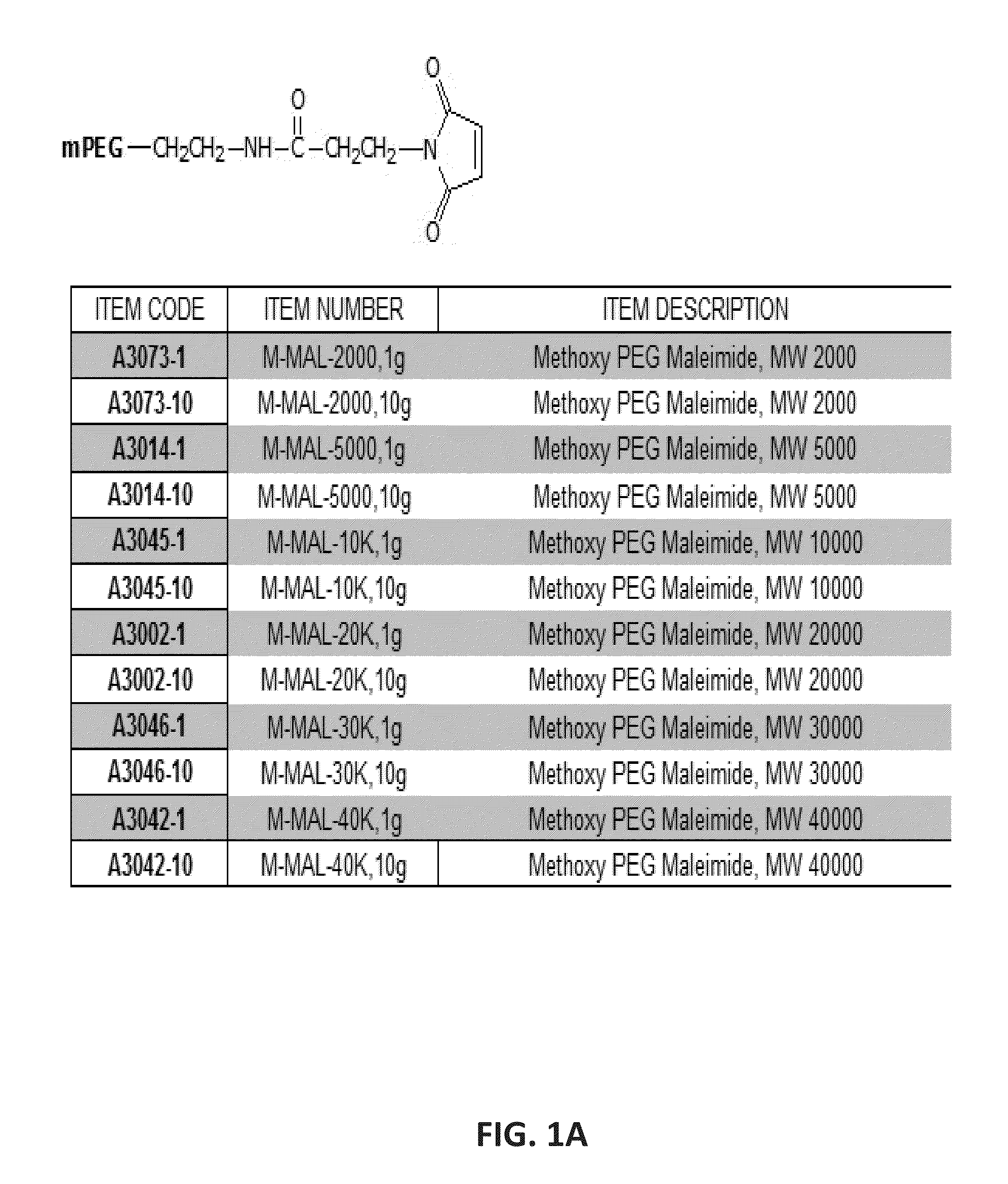
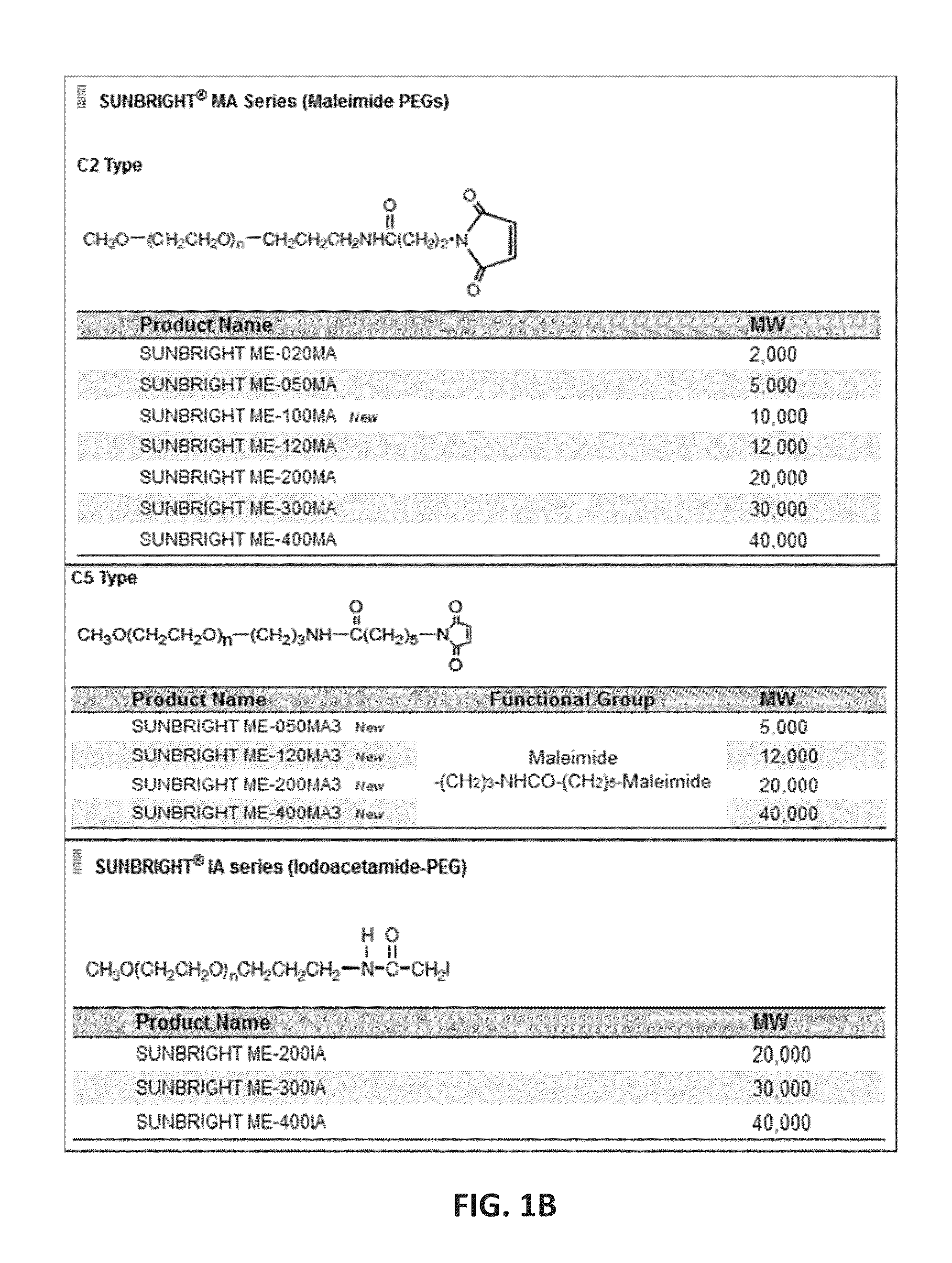
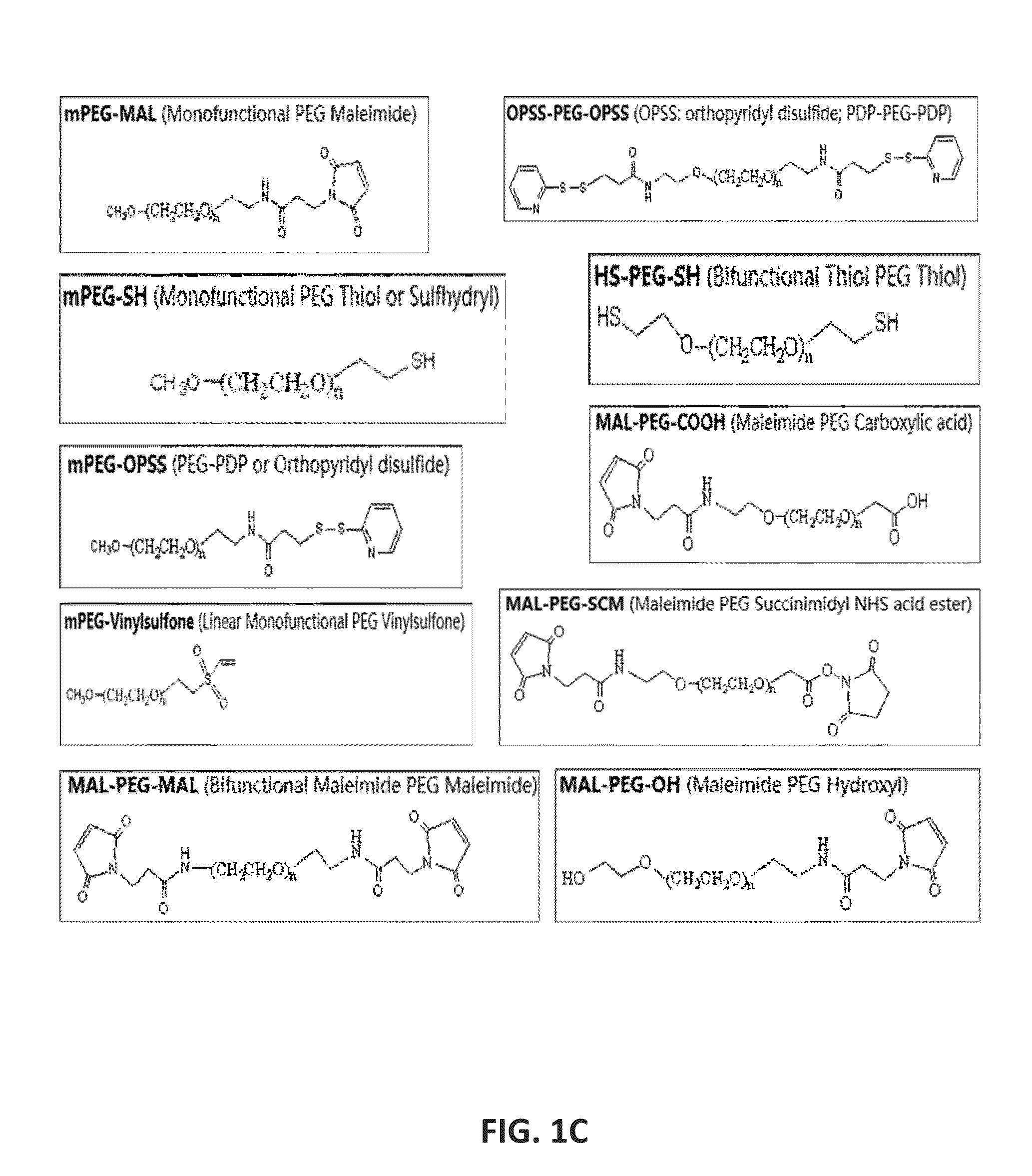
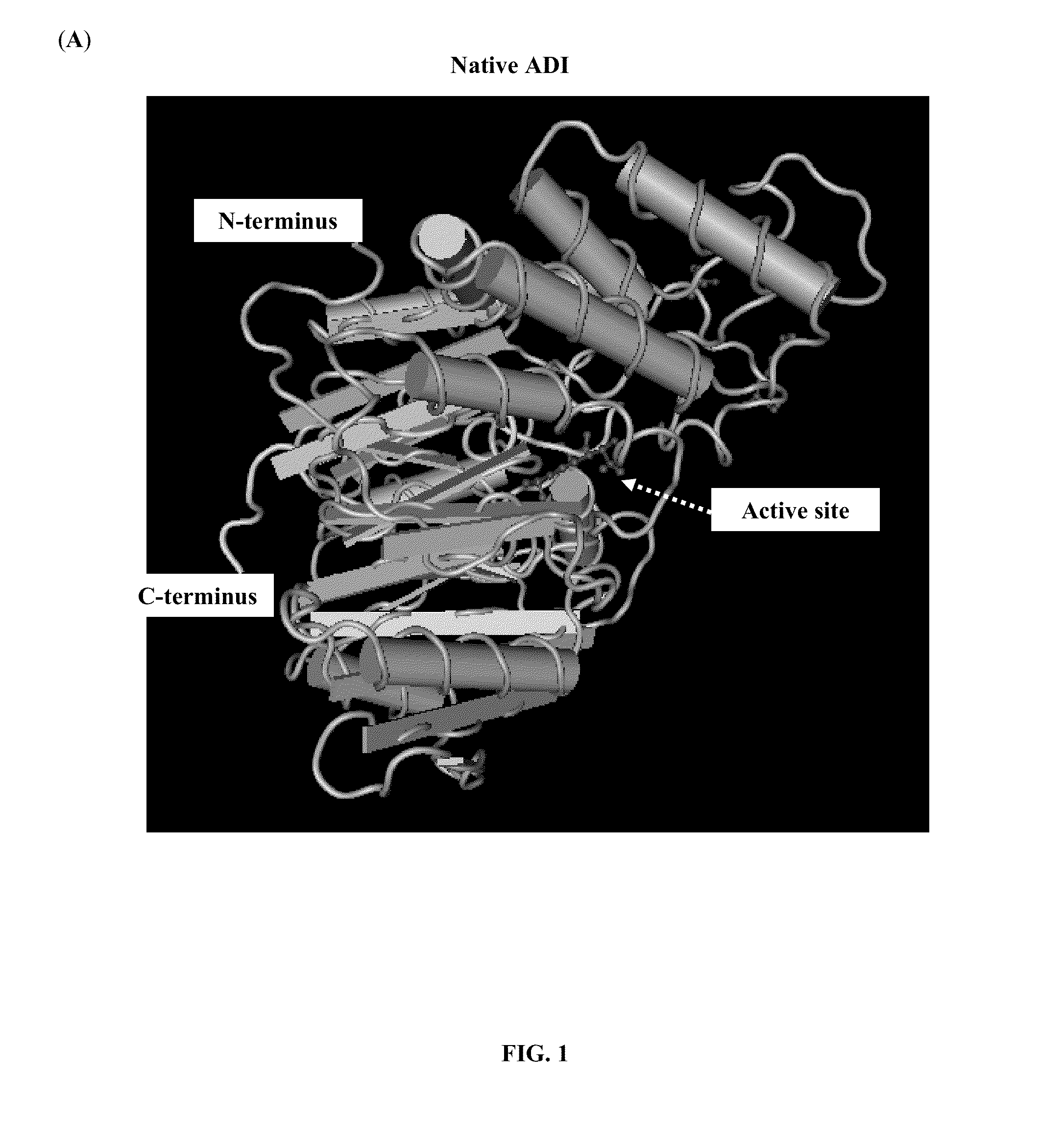
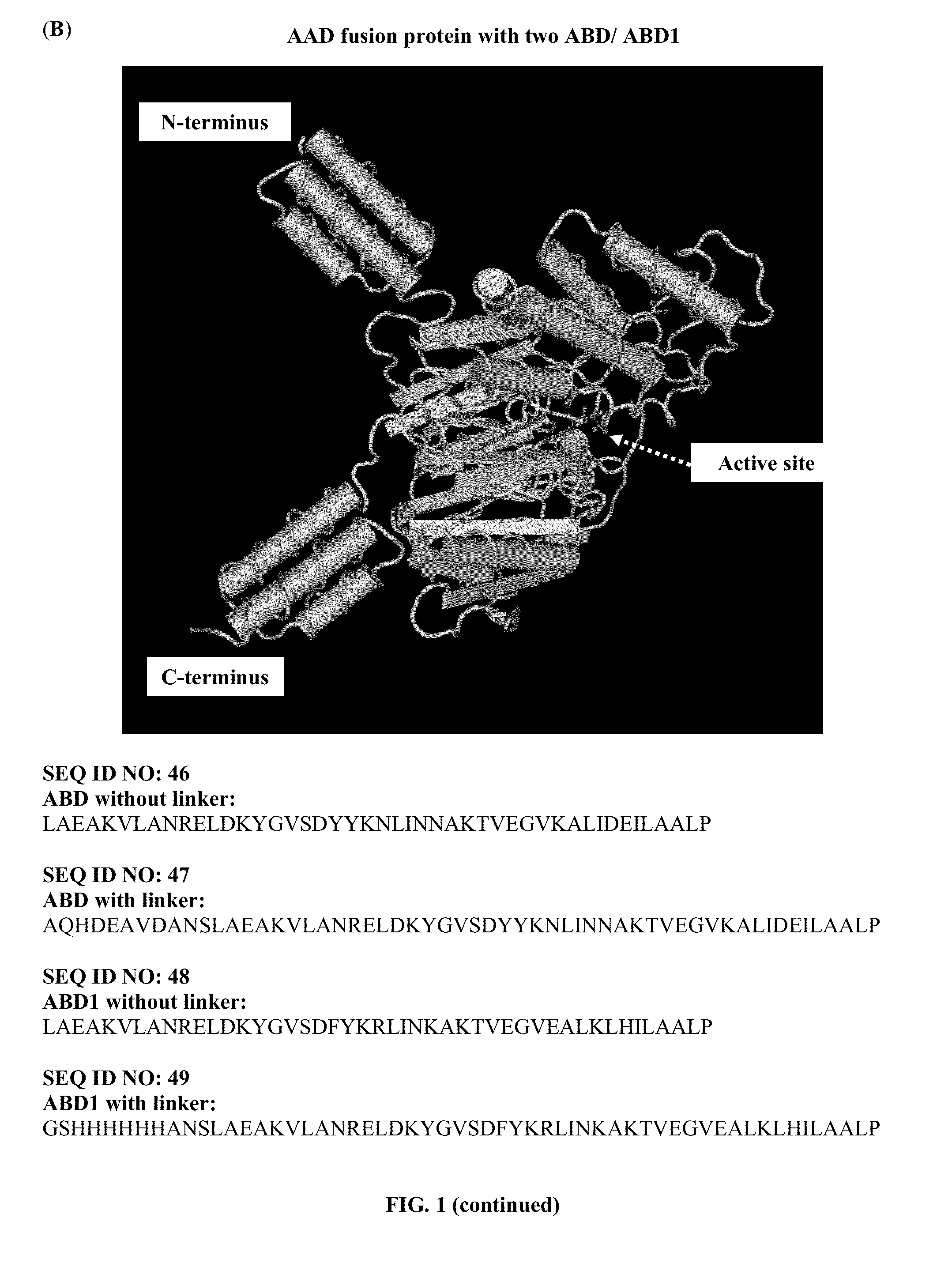
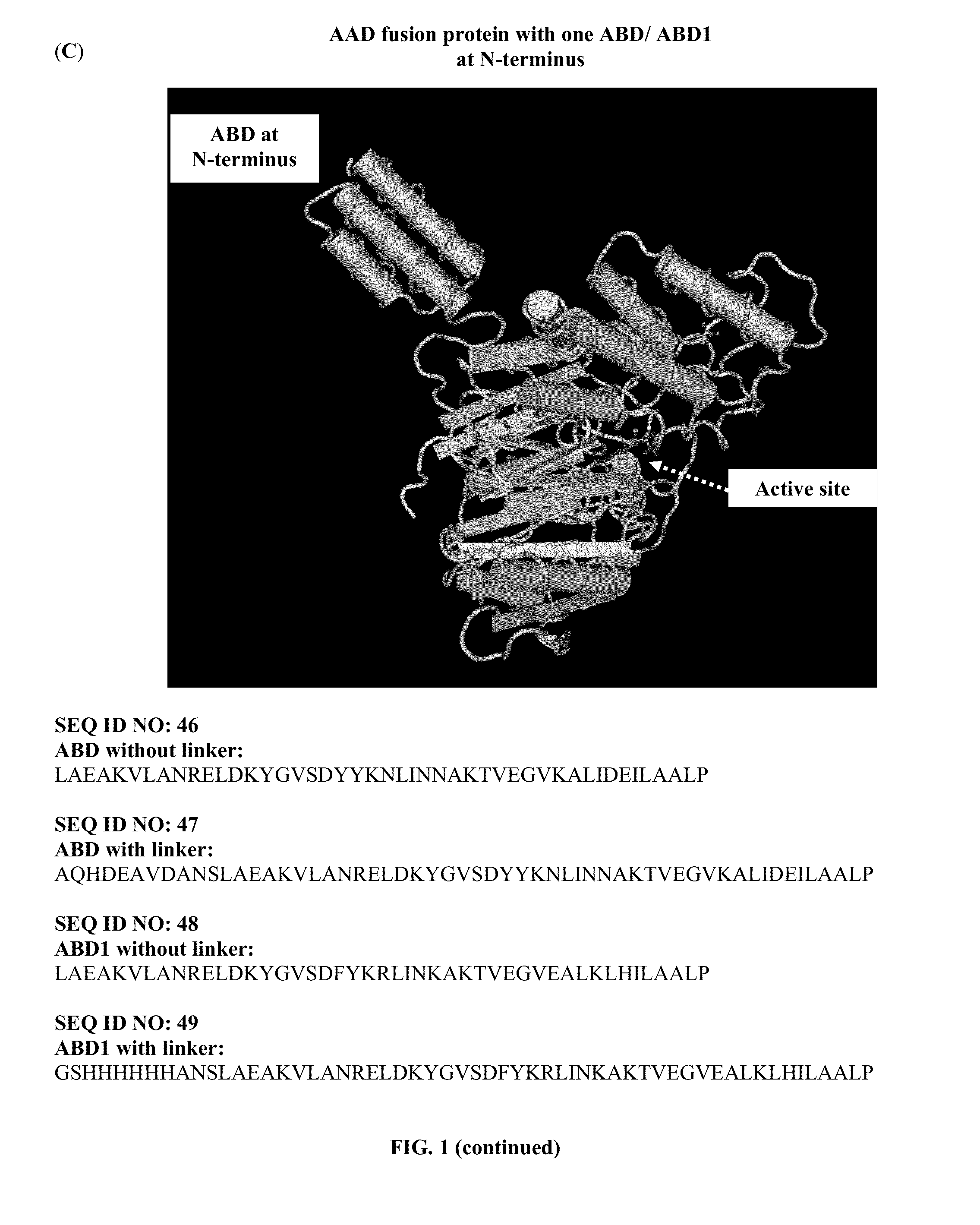
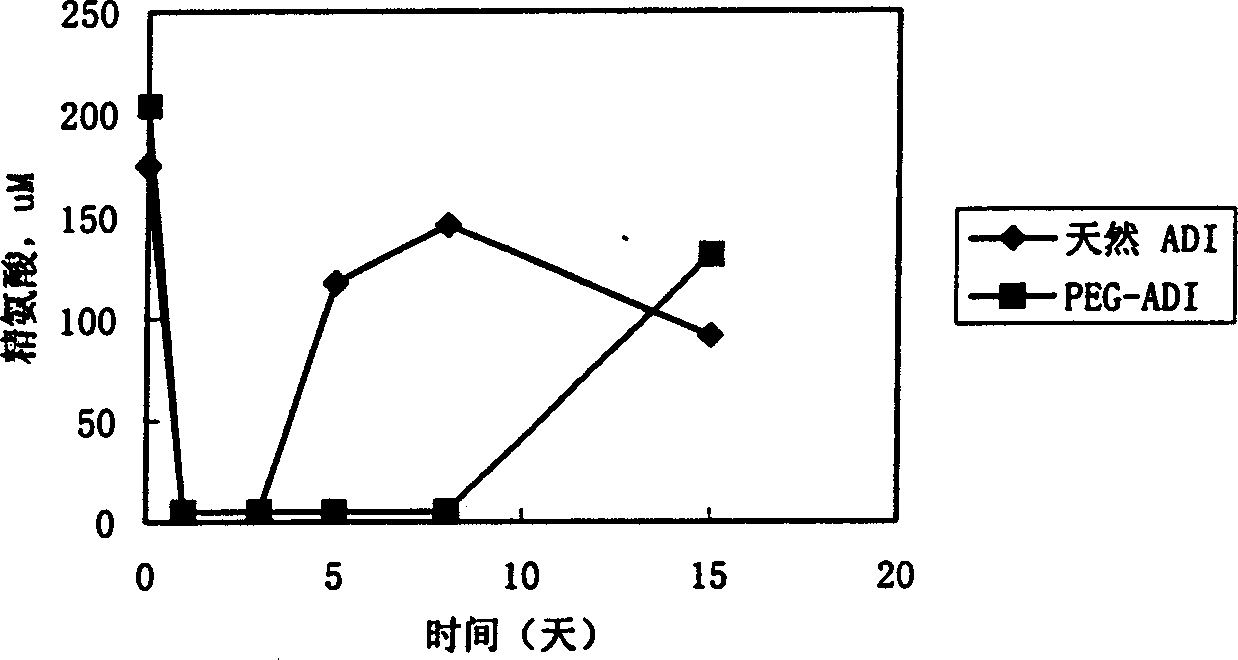
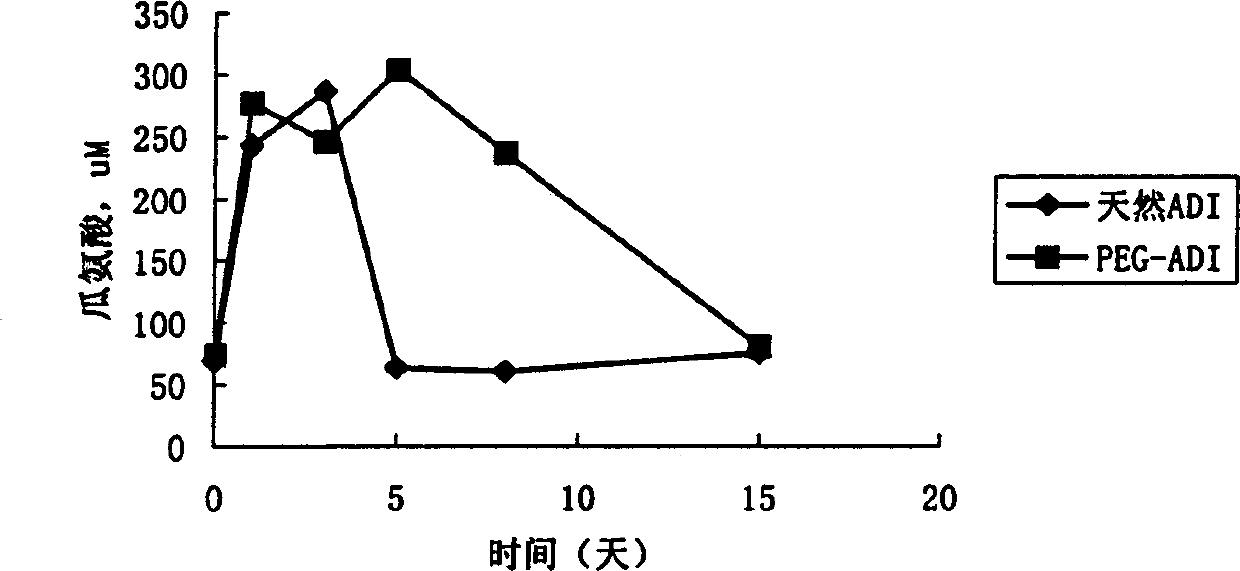
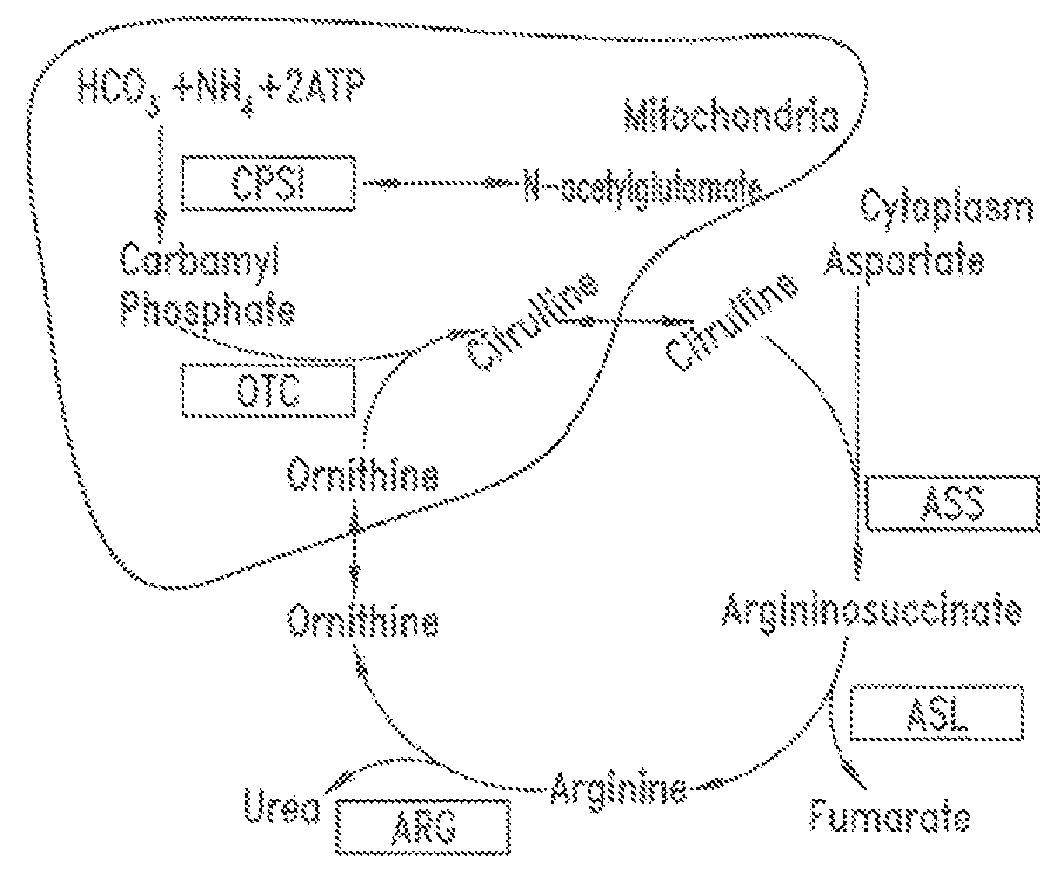
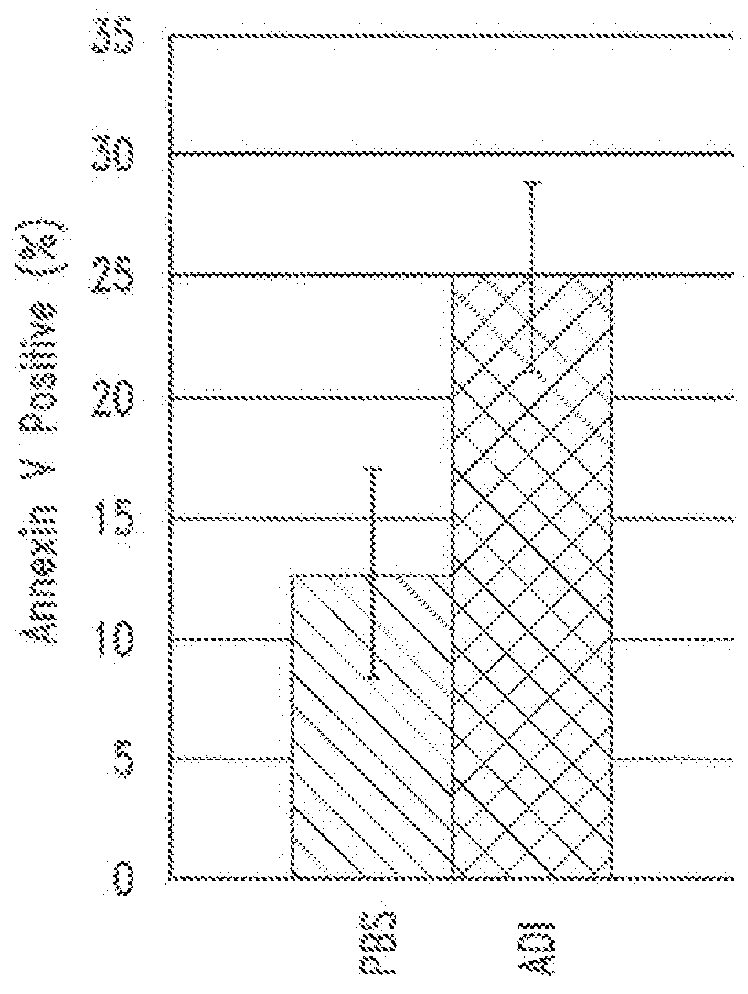
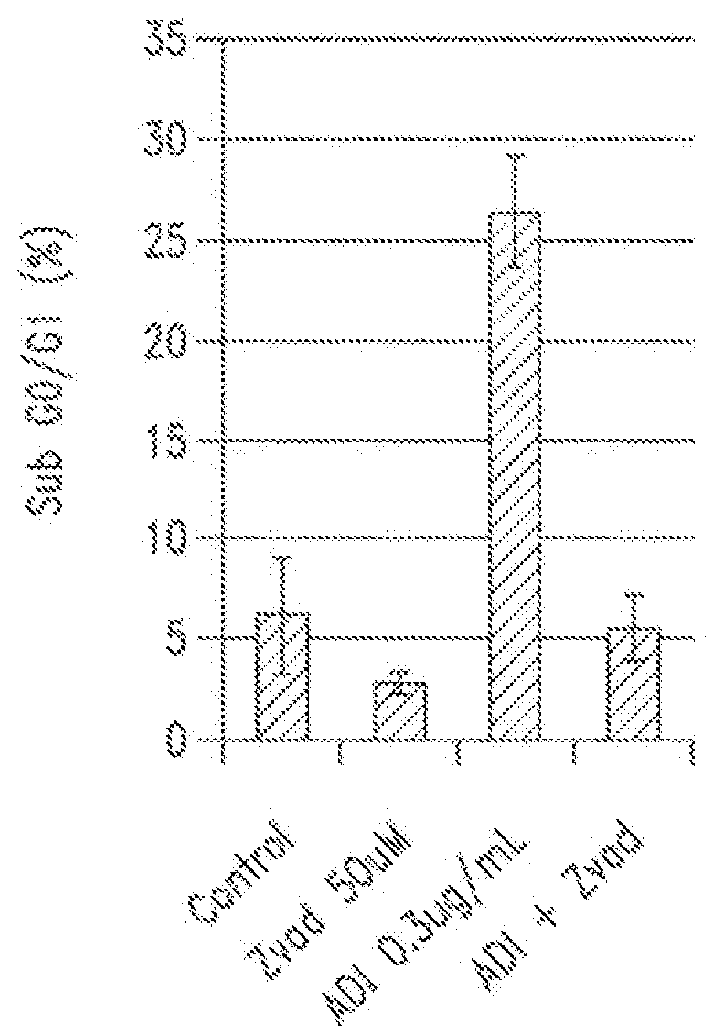
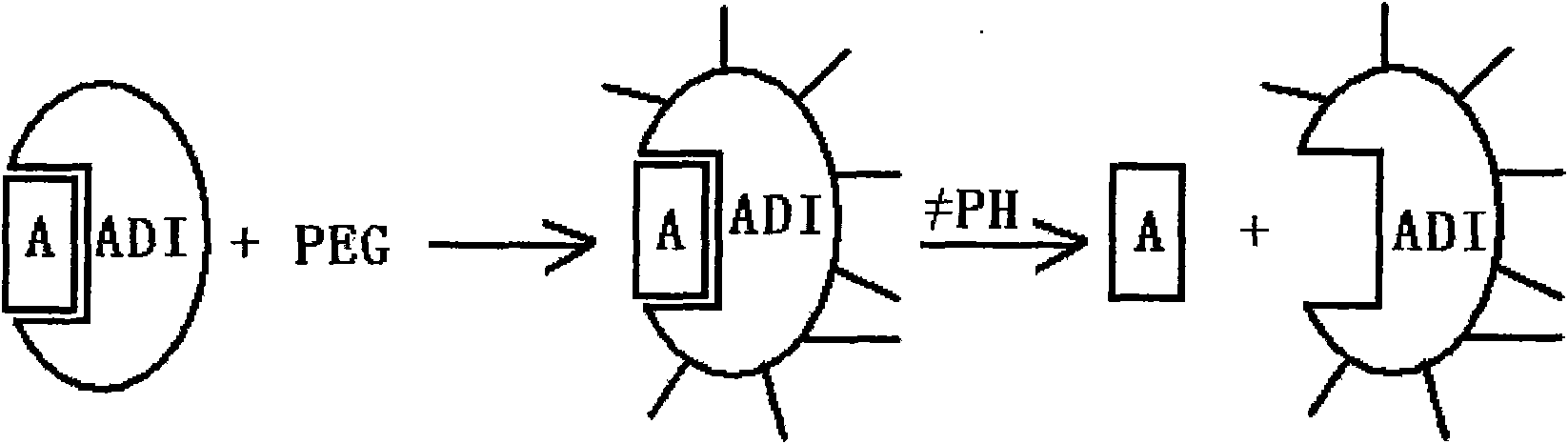
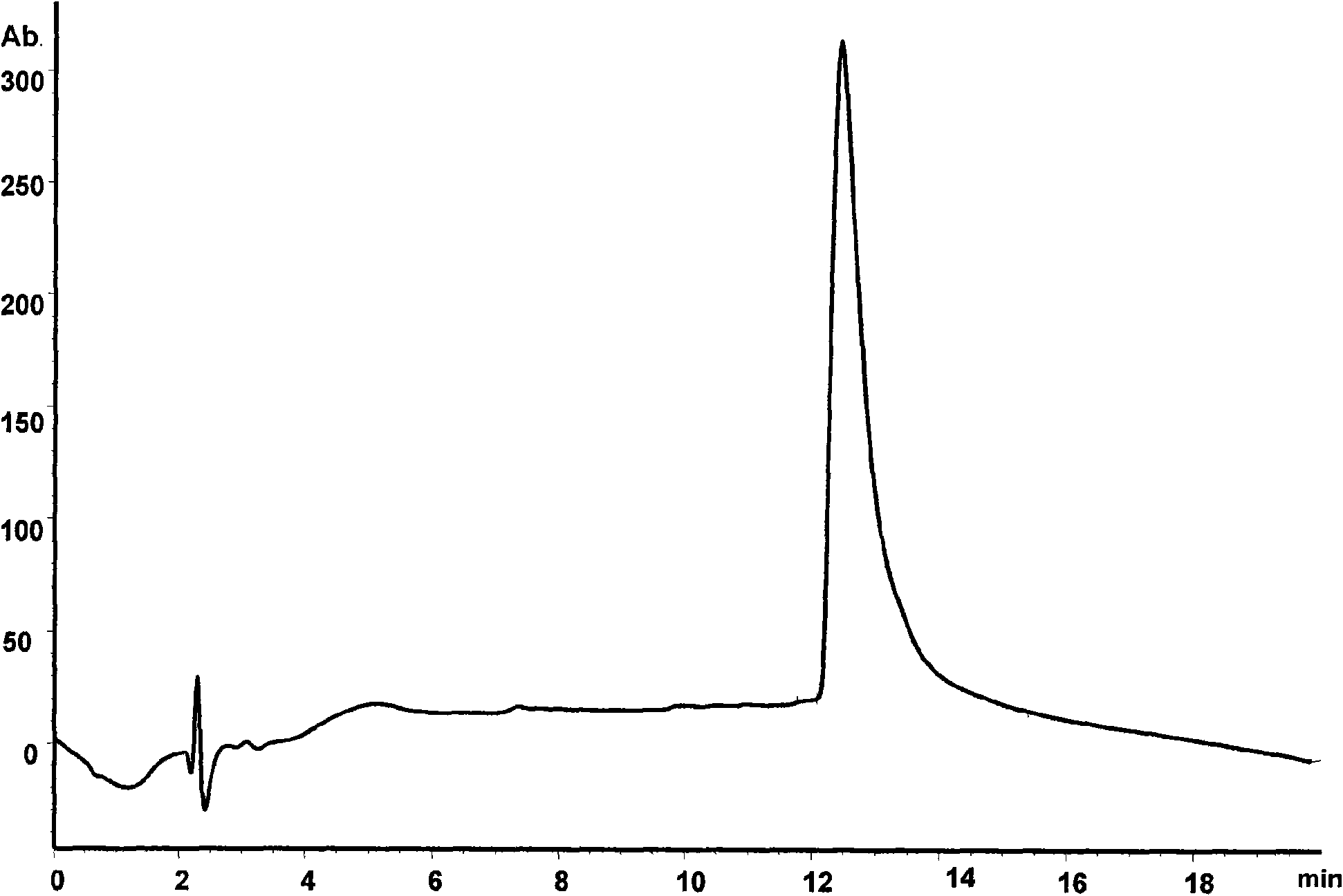
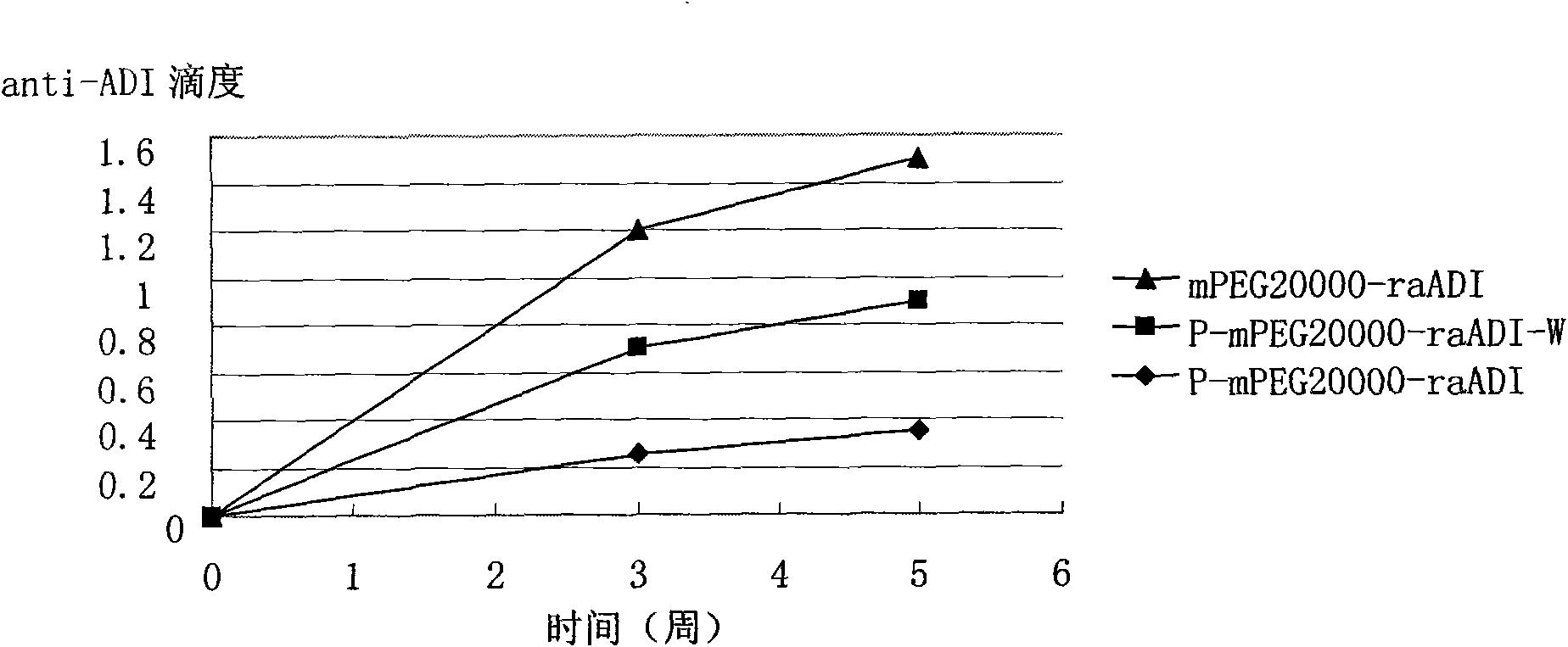
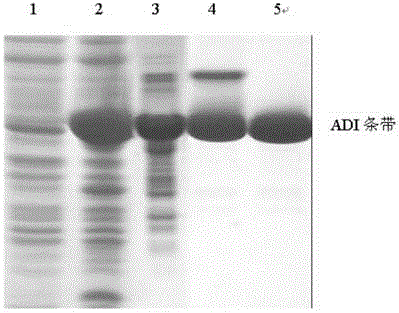
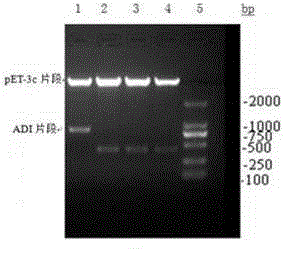
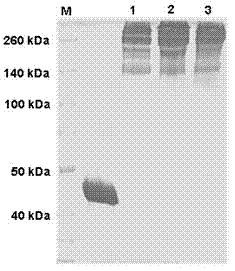
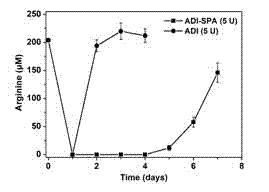
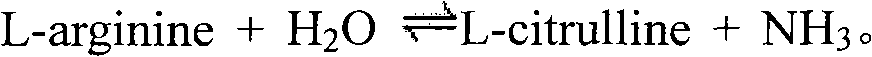Patents
Literature
79 results about "Arginine deiminase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
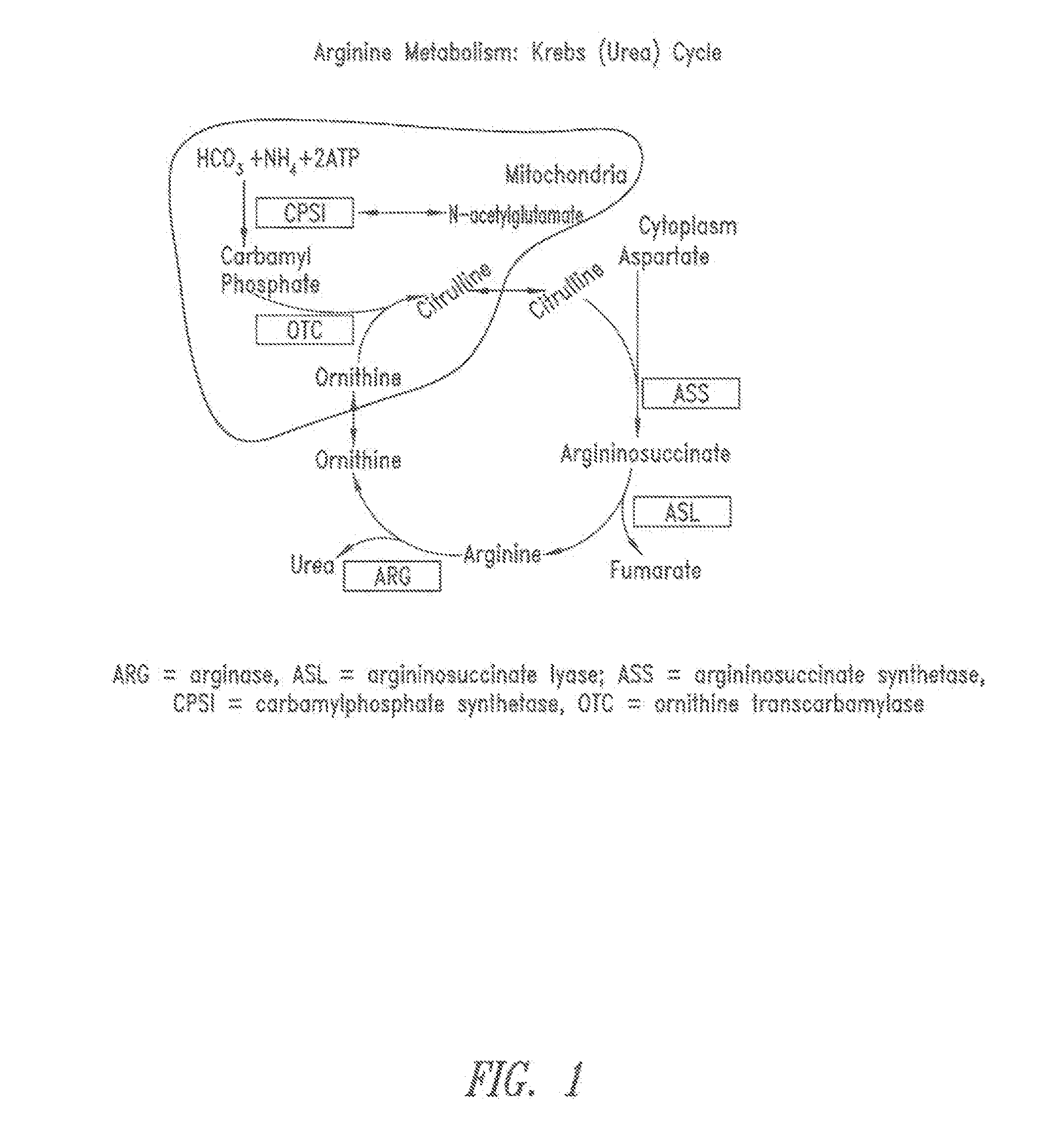
In enzymology, an arginine deiminase (EC 3.5.3.6) is an enzyme that catalyzes the chemical reaction L-arginine + H₂O ⇌ L-citrulline + NH₃ Thus, the two substrates of this enzyme are L-arginine and H₂O, whereas its two products are L-citrulline and NH₃. This enzyme belongs to the family of hydrolases, those acting on carbon-nitrogen bonds other than peptide bonds, specifically in linear amidines. The systematic name of this enzyme class is L-arginine iminohydrolase.
Arginine deiminase mutant and preparation and application thereof
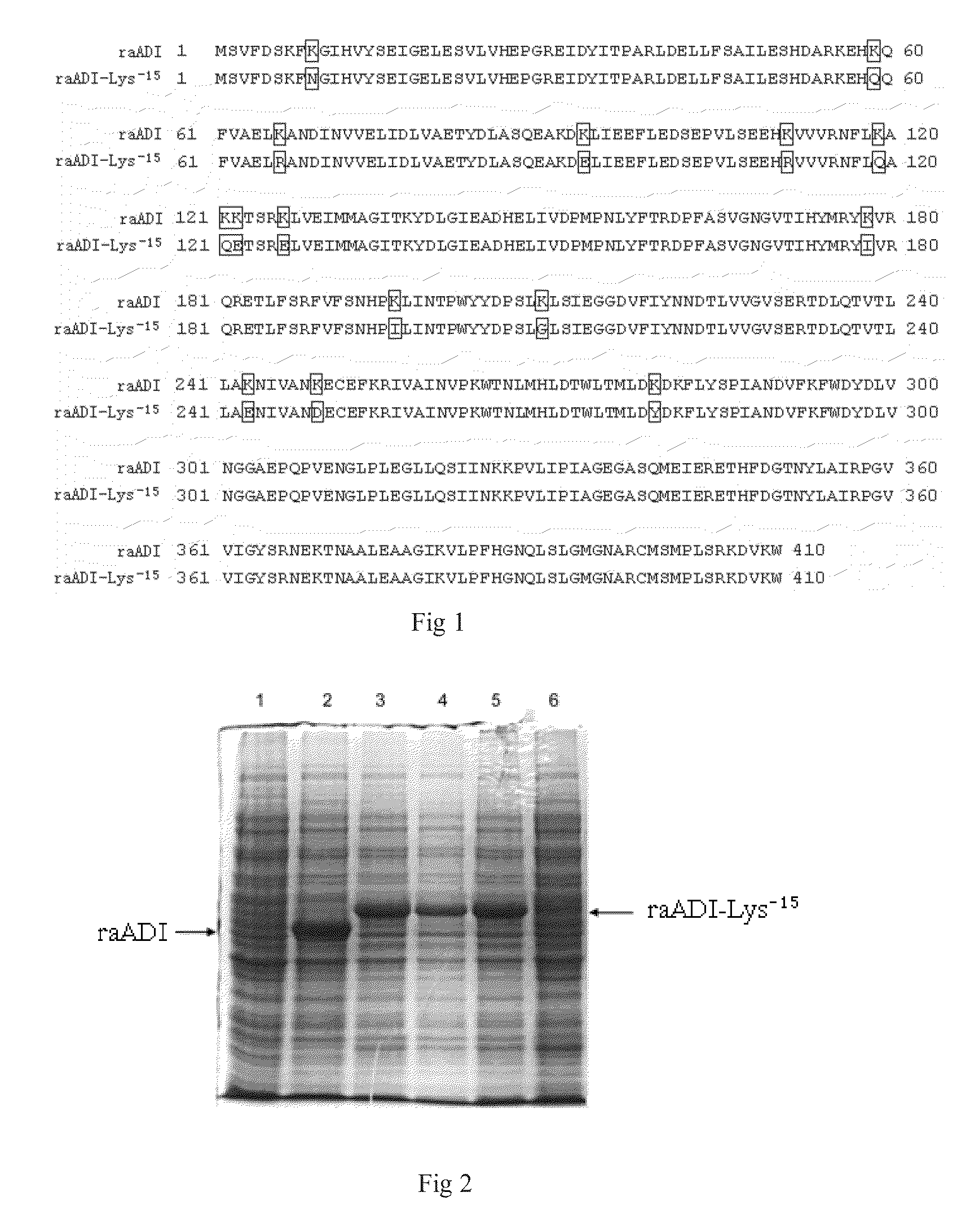
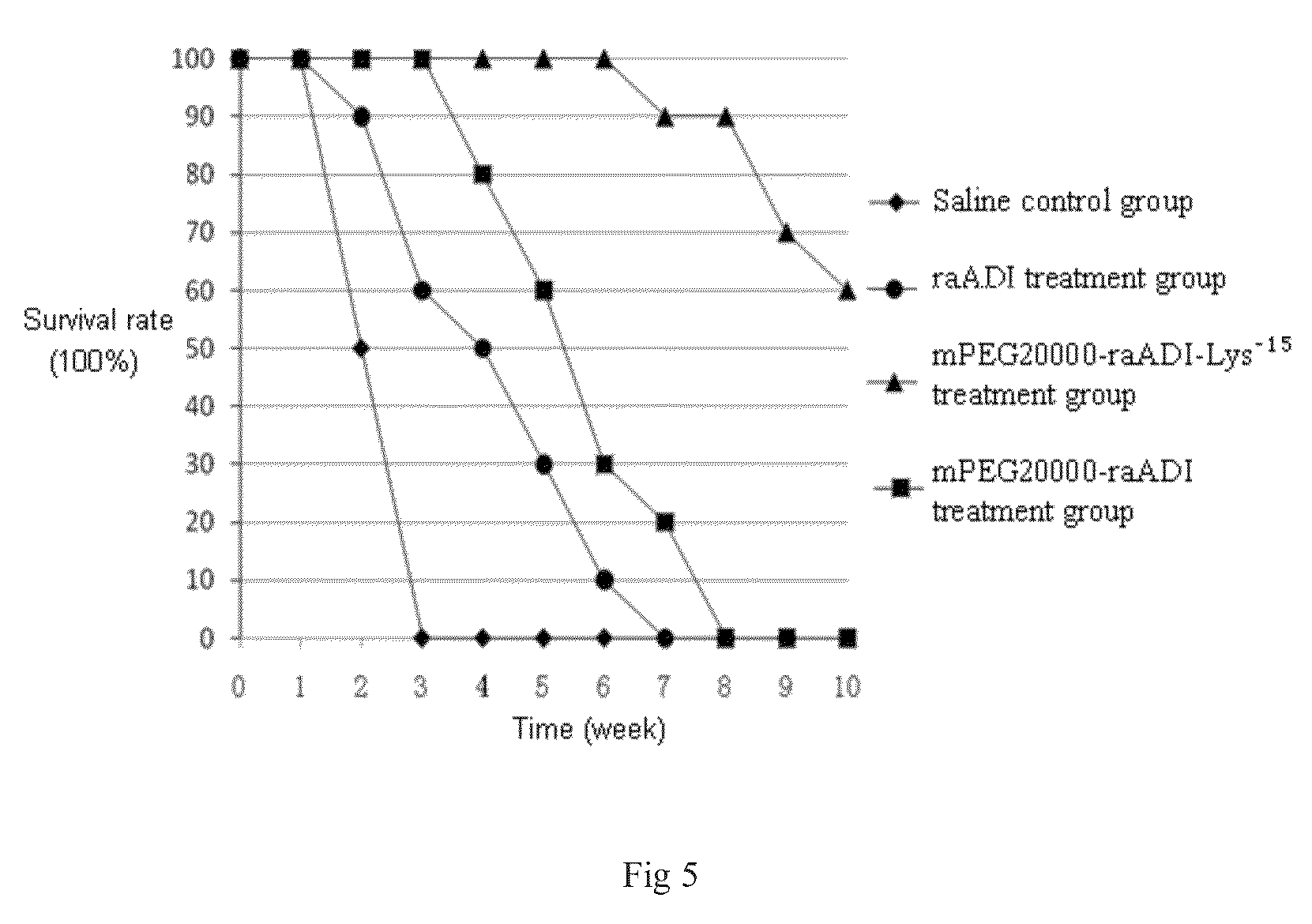
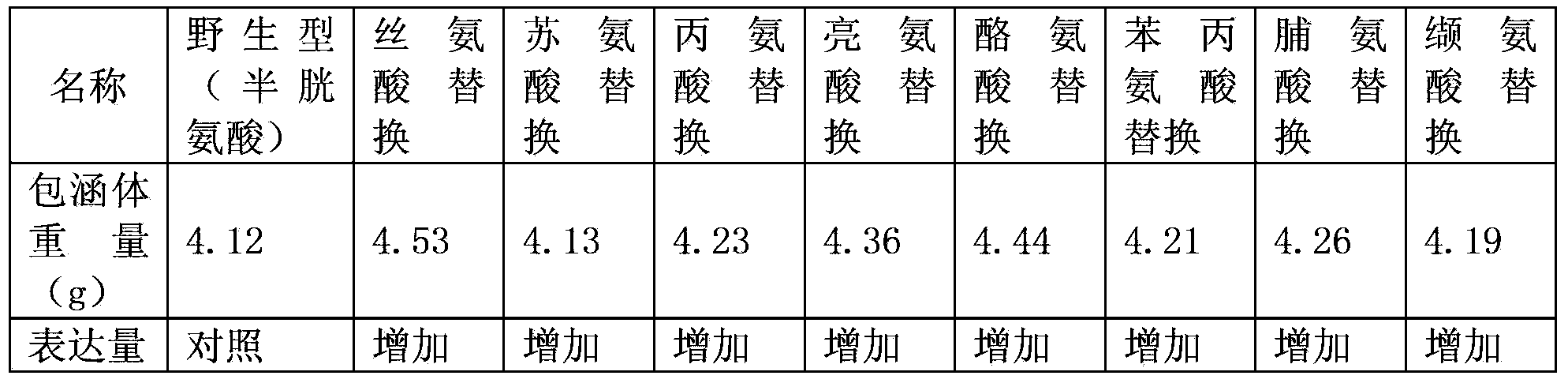
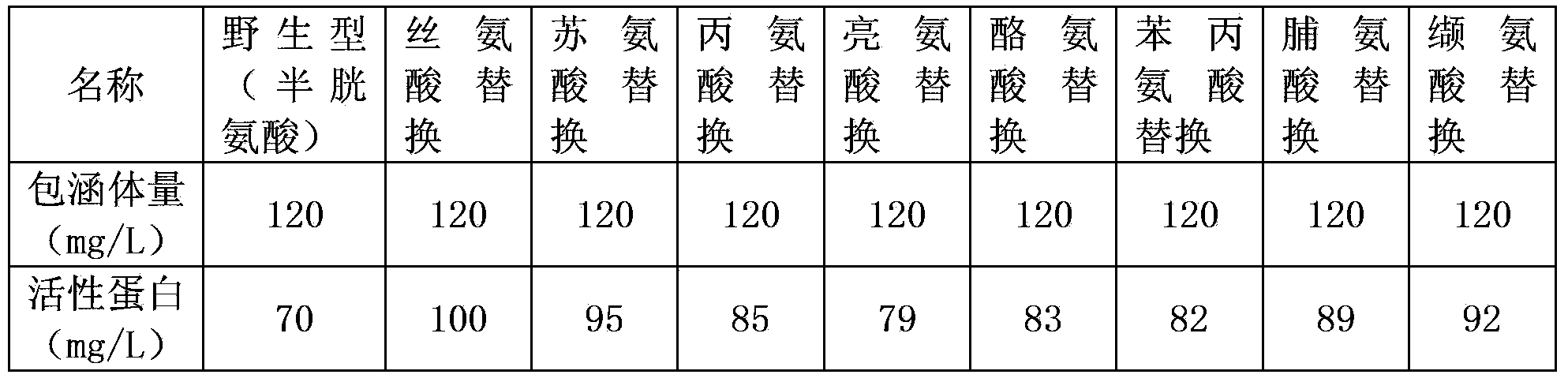
The present invention relates to an arginine deiminase mutant with partial lysine-deficient and preparation and application thereof. The arginine deiminase mutant of the present invention has enzymatic activity of degrading arginine into citruline; compared with the arginine deiminase with the amino acid sequence of SEQ ID NO: 1, the amino acid sequence comprises one or more of K9N, T, K59Q, K66R, A, K93E, A, Q, K111R, A, K119Q, L, M, K121Q, I, K122E, L, K126E, S, R, K178I, E, D, K196I, R, K209G, T, D, K243E, V, R, K249D, Q, K263N, Q, K279Y, T, K293R, H, E, K325V, I, K380T, R, E, and K406E, D, S substitutions. Compared with PEG modified natural derived arginine deiminase, the PEG modified arginine deiminase mutant of the present invention retain better bioactivity; and because the quantity of lysine in arginine deiminase is reduced, the PEG modified products are more uniform and can be applied to clinical treatment of hepatoma, melanoma and the like.
Owner:JIANGSU T MAB BIOPHARMA
Methods for inhibiting viral replication in vivo
InactiveUS7204980B2Bacterial antigen ingredientsPeptide/protein ingredientsPolyethylene glycolNitric oxide
The present invention is directed to methods of modulating viral replication in vivo comprising administering to an individual a therapeutically or prophylactically effective amount of a composition comprising arginine deiminase modified with polyethylene glycol, to methods of concurrently modulating viral replication and treating cancer, and to methods of modulating nitric oxide levels in a patient, among others.
Owner:POLARIS GROUP
Arginine deiminase mutant and preparation and application thereof
ActiveCN101812438AImprove in vitro activityHigh activity retentionFungiNervous disorderMelanomaMutant
The invention relates to an arginine deiminase mutant with partial mutated lysine and preparation and application thereof. The arginine deiminase mutant of the invention has enzymatic activity of degrading arginine into citruline; compared with the arginine deiminase with the amino acid sequence of SEQ IDNO:1, the amino acid sequence comprises mutants of K9N, K59Q, K66R, K93E, K111R, K119Q, K121Q, K122E, K126E, K178I, K196I and one or more of K209G, K243E, K249D and K279Y. Compared with natural arginine deiminase, after the arginine deiminase mutant of the invention is modified by PEG, the retention rate of active residues is improved; and as the quantity of lysine is reduced, products are more uniform and can be applied to clinical treatment of hepatoma, melanoma and the like.
Owner:JIANGSU T MAB BIOPHARMA
Pharmaceutical composition comprising arginine deiminase for inhibiting angiogenesis
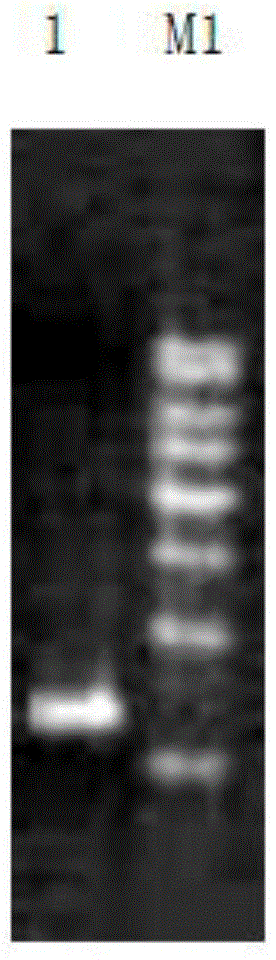
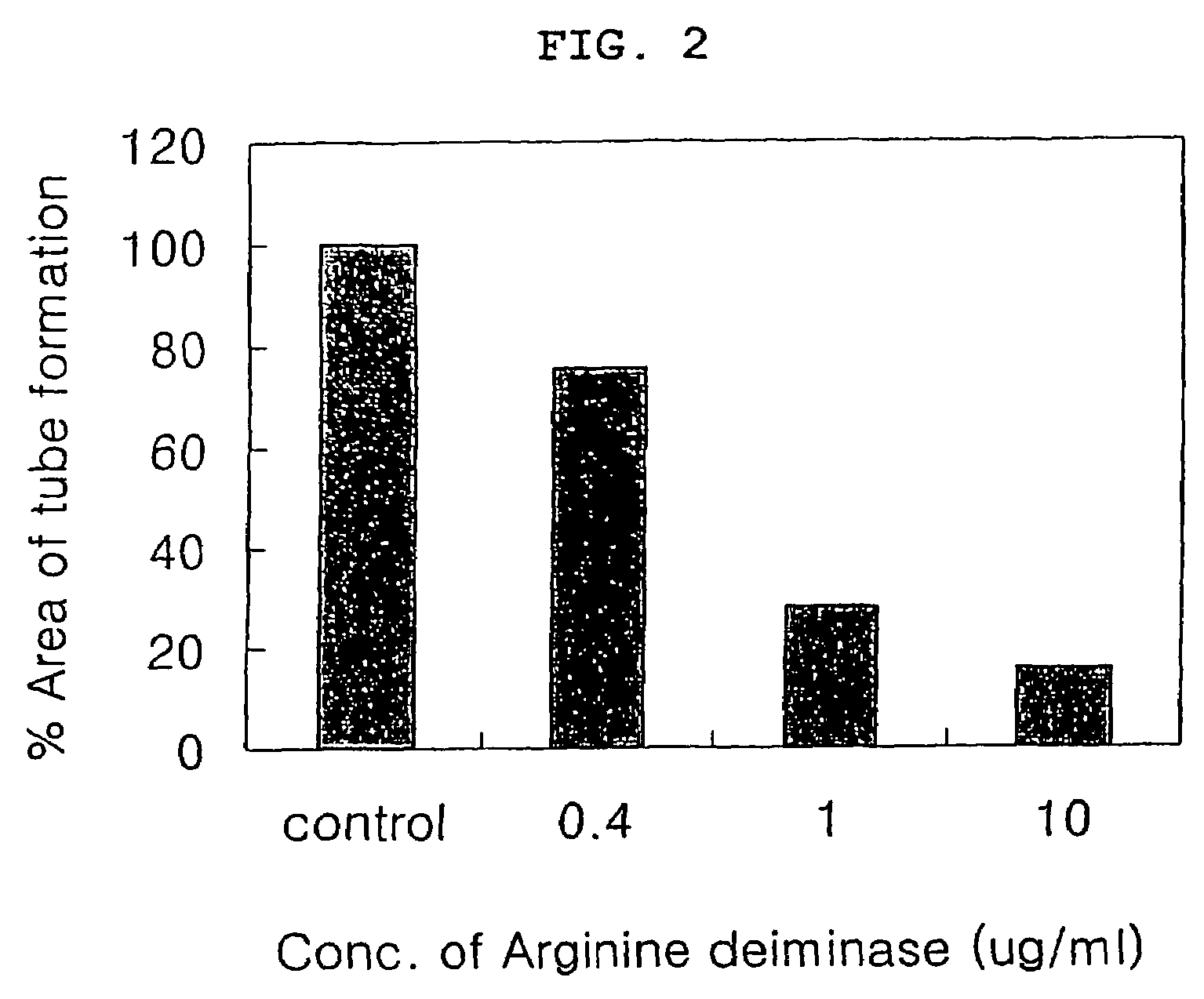
InactiveUS20060002915A1Tube formation was suppressedInhibited tube formationSugar derivativesHydrolasesAngiogenesis growth factorBULK ACTIVE INGREDIENT
The present invention relates to a pharmaceutical composition for inhibiting angiogenesis which comprises arginine deiminase as an active ingredient, where the arginine deiminase, obtained from Mycoplasma arginini or prepared by a genetic recombination technique, may be conjugated to an activated polymer to lower its immunogenecity and increase its life time. The pharmaceutical composition of the present invention exhibits an excellent inhibitory activity against angiogenesis.
Owner:ANGIOLAB
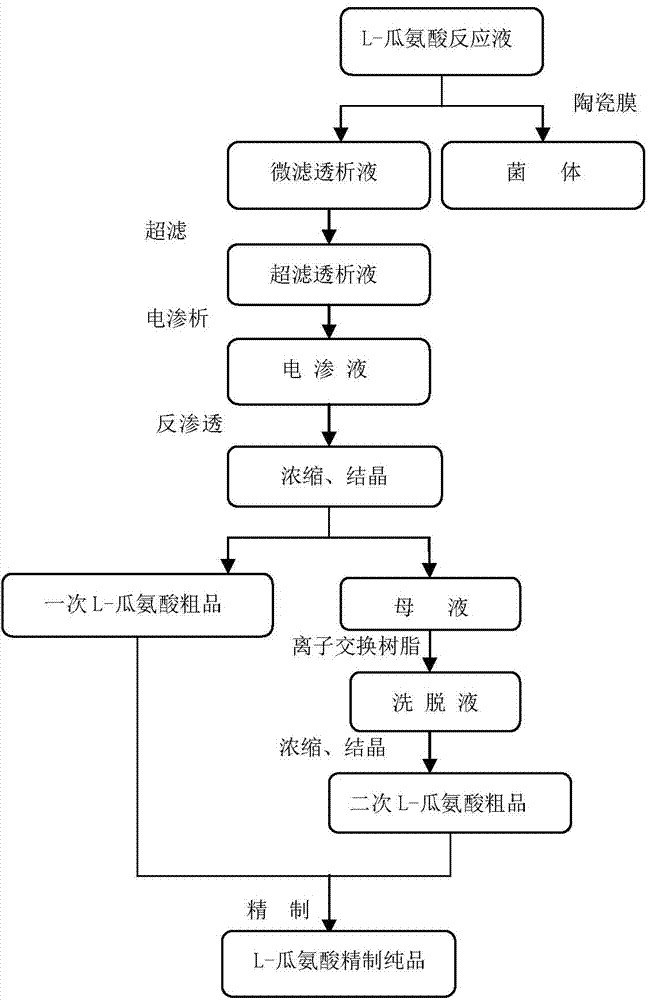
High-yield arginine deiminase bacterial strain and method for producing L-citrulline by same
ActiveCN102703339AHigh acid productionStable traitsBacteriaMicroorganism based processesContinuous fermentationMicrobiology
The invention discloses a high-yield arginine deiminase bacterial strain and a method for producing L-citrulline by the same. The high-yield arginine deiminase bacterial strain of the invention is streptococcus faecalis BM-2 CGMCC No.4990. After small-scale fermentation of the bacterial strain in a 30-L fermentation tank, the L-citrulline yield is up to 98 g / L, which is increased by 50% when compared with the L-citrulline yield of the original strain; after continuous fermentation for 5 batches, the L-citrulline yield of the bacterial strain is stable, and the L-citrulline yield level is obviously higher than that of reported bacterial strains; and the bacterial strain of the invention has important industrial production application value.
Owner:山东恩贝生物工程有限公司
Mutated arginine deiminase as well as preparation method and application thereof
The invention discloses a mutated arginine deiminase as well as a preparation method and an application thereof. Amino acids in one or more sites of K137A, F198W, V230A, R257L and A260D of arginine deiminase mutate, and the mutated arginine deiminase is improved in activity significantly, can be used for catalyzing L-arginine to produce L-citrulline and can achieve the conversion rate of more than 95%. The mutated arginine deiminase is prepared by adopting an error-prone PCR (polymerase chain reaction) system.
Owner:石药集团中诺药业(石家庄)有限公司
Arginne deiminase with reduced cross-reactivity toward adi - peg 20 antibodies for cancer treatment
ActiveUS20140348814A1Low cross-reactivityInhibit progressSugar derivativesBacteriaAnti-CEA AntibodyCancer treatment
The present invention relates generally to isolated to arginine deiminase (ADI) proteins that have reduced cross-reactivity with anti-ADI-PEG 20 antibodies as compared to ADI-PEG 20, but which can have functional characteristics comparable to or better than ADI-PEG 20, compositions comprising the ADI proteins, and related methods of treating arginine-dependent diseases or related diseases such as cancer.
Owner:POLARIS GROUP
Modified arginine deiminase
Owner:POLARIS GROUP
Peptidylarginine deiminase and uses thereof in the production of citrullinated proteins and peptides
The present invention relates to a protein, peptide or protein hydrolysate wherein the molar ratio of citrulline and arginine residues, being part of protein or peptide, is at least 0.15, preferably at least 0.30, more preferably at least 0.5, still more preferably at least 1.0, even still more preferably 2.0 and most preferably at least 4.
Owner:DSM IP ASSETS BV
Construction of recombinant strain capable of producing arginine deiminase and directional modification method thereof
InactiveCN102061283AIncrease enzyme activityIncreased specific enzyme activityBacteriaHydrolasesBio engineeringRecombinase
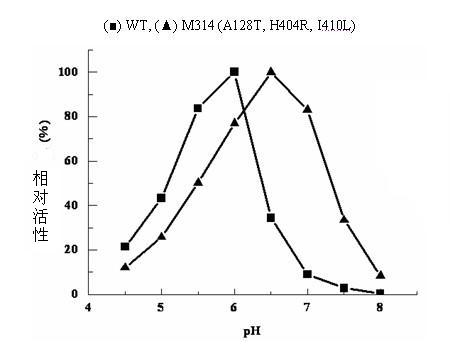
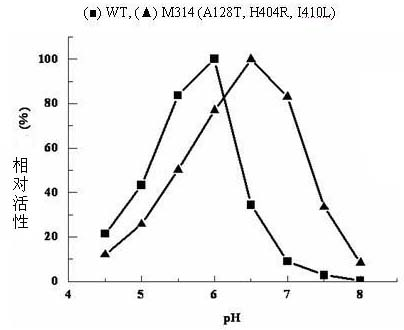
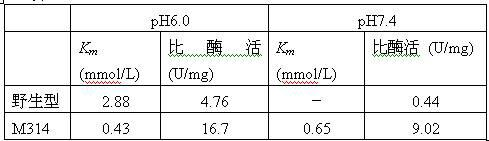
The invention relates to construction of a recombinant strain capable of producing arginine deiminase (ADI) and a directional modification method thereof, belonging to the technical field of medical biological engineering. The method comprises the following steps of: amplifying the ADI coding gene arcA of a pseudomonas plecoglossicida CGMCC (China General Microbiological Culture Collection Center) No. 2039 by adopting a PCR (polymerase chain reaction) method, and constructing an ADI recombination expression strain, researching the enzymology properties of the recombinant ADI enzyme, wherein Km is 2.88mmol / L (pH is 6.0), the optimal pH is 6.0, the enzyme activity is 20.85U / mg, and the enzyme activity is reduced by more than 90% when the pH is increased to the physiological pH (7.4). The directional modification is carried out on the recombinant ADI enzyme by adopting a prone PCR mutation technology, so as to improve the activity and substrate affinity of the enzyme under the physiological pH condition. An excellent mutant strain ADIM314 is obtained by screening through the directional modification. Compared with a wild enzyme, the activity of the ADIM314 enzyme under the physiological pH condition is improved by more than 20 times, the Km value is reduced to 0.65mmol / L (pH is 7.4), and the optimal pH is improved to 6.5 from 6.0.
Owner:JIANGNAN UNIV
Method of treatment with modified arginine deiminase
The present invention is directed to arginine deiminase modified with polyethylene glycol, to methods of treating cancer, and to methods of treating and / or inhibiting metastasis.
Owner:POLARIS GROUP
Methods For Inhibiting Viral Replication In Vivo
The present invention is directed to methods of modulating viral replication in vivo comprising administering to an individual a therapeutically or prophylactically effective amount of a composition comprising arginine deiminase modified with polyethylene glycol, to methods of concurrently modulating viral replication and treating cancer, and to methods of modulating nitric oxide levels in a patient, among others.
Owner:POLARIS GROUP
Strain for producing arginine deiminase and application thereof
InactiveCN101121925AIncrease enzyme activityBacteriaPeptide/protein ingredientsBacteroides speciesArginine deiminase
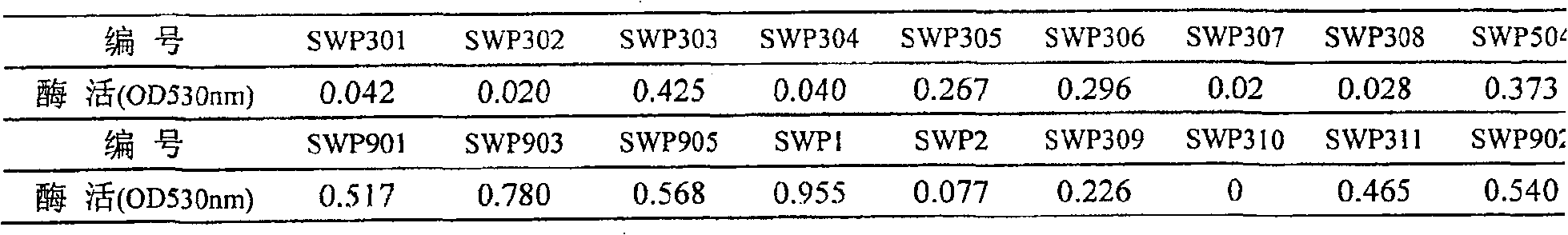
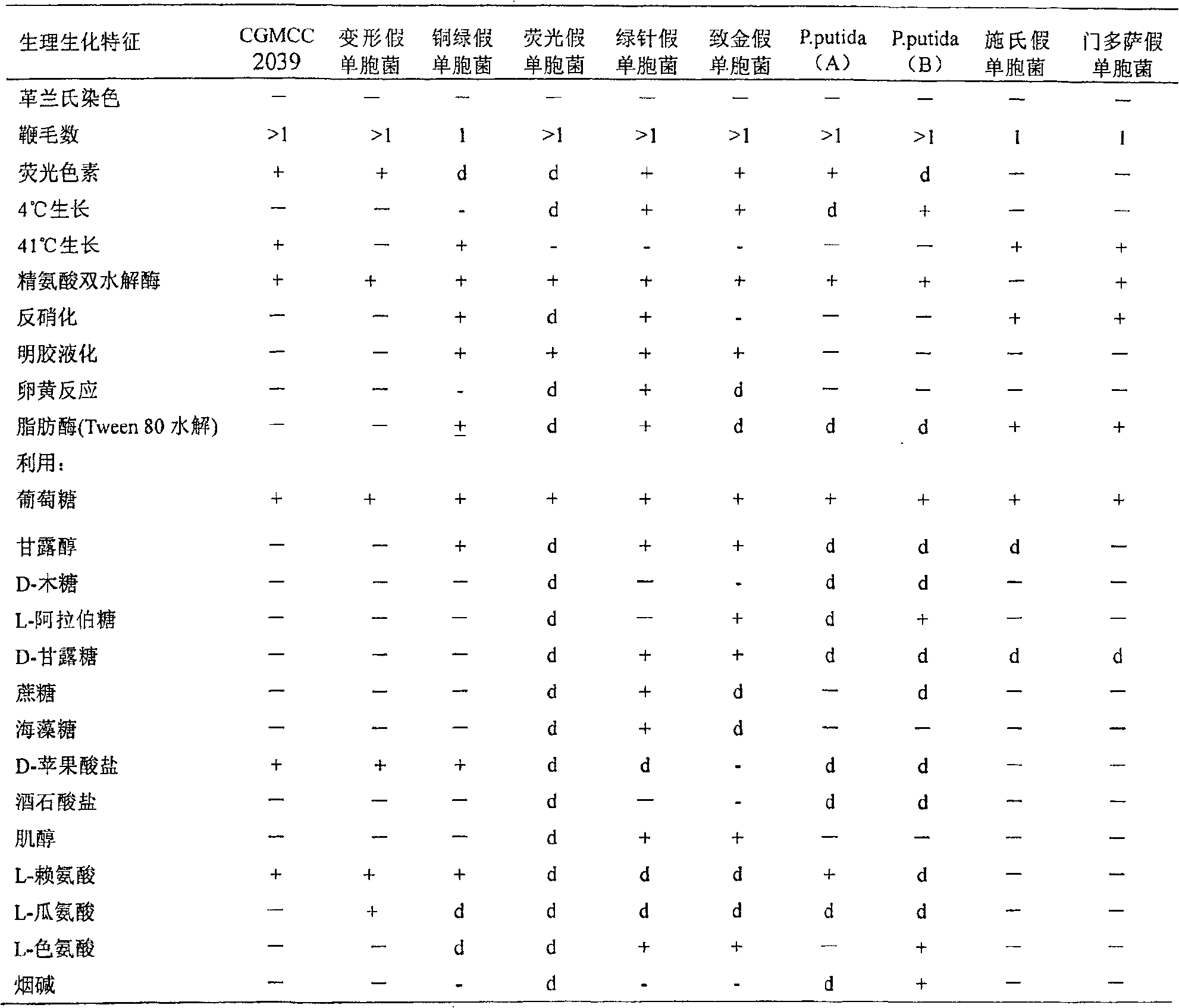
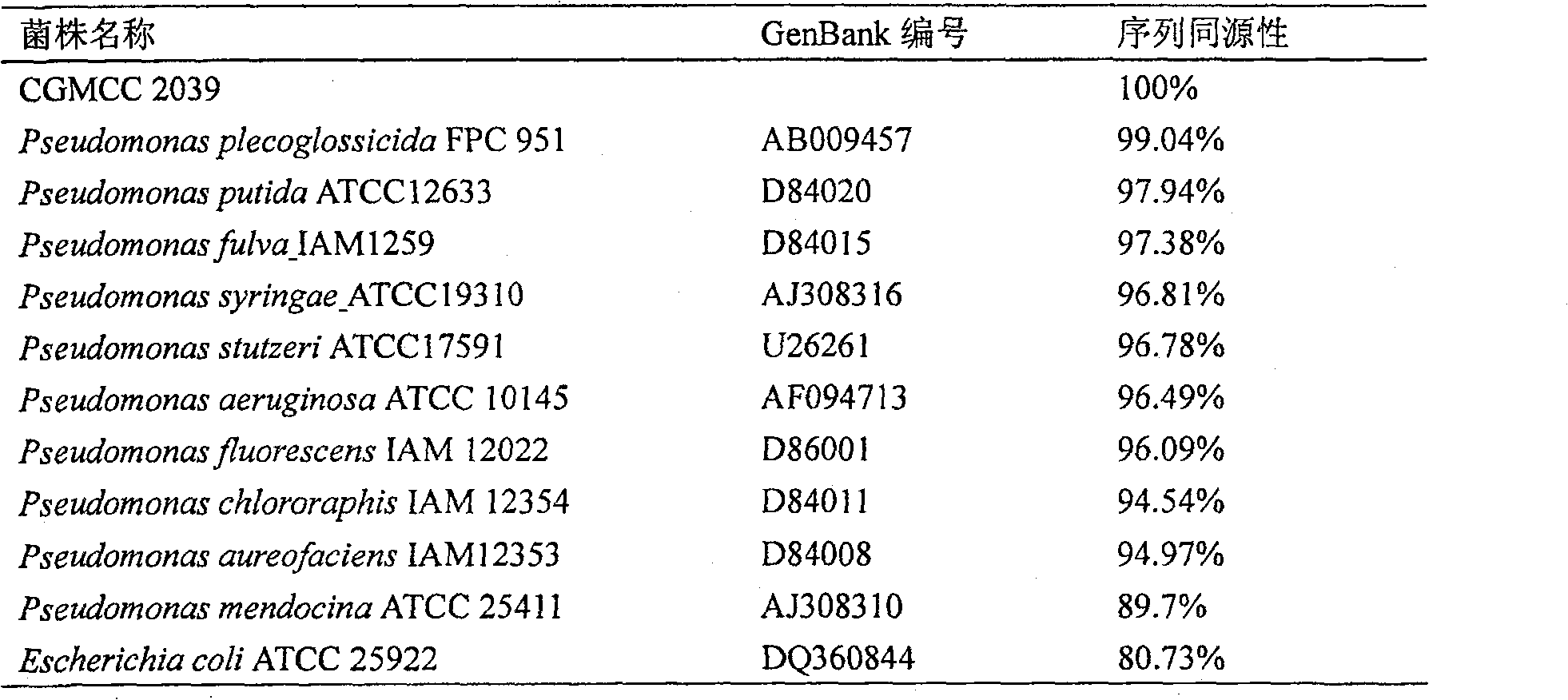
The invention discloses a method to produce ADI strain by screening and the application of the method, pertaining to the field of bioengineering technology. The invention particularly relates to a rapid method to screen ADI strain and produce Pseudomonas plecoglossicida (CGMCC No.2039) of ADI, as well as a method to produce ADI by using the microorganism. Under an aerobic condition and an environment maintained at pH6.5-8.0, enzyme activity can achieve 1.6U / mL fermentation broth after 20-24h fermentation culture. ADI produced with such microorganism has very strong inhabitation agaisnt liver cancer cell strain (HepG2). ADI genes can be obtained from the chromosome DNA of such microorganism, and the gene order can be achieved.
Owner:JIANGNAN UNIV
Arginine deiminase with reduced cross-reactivity toward adi - peg 20 antibodies for cancer treatment
ActiveUS20160074487A1Low cross-reactivitySymptoms improvedBacteriaSugar derivativesProtein-arginine deiminaseDisease
The present invention relates generally to isolated to arginine deiminase (ADI) proteins that have reduced cross-reactivity with anti-ADI-PEG 20 antibodies as compared to ADI-PEG 20, but which can have functional characteristics comparable to or better than ADI-PEG 20, compositions comprising the ADI proteins, and related methods of treating arginine-dependent diseases or related diseases such as cancer.
Owner:POLARIS GROUP
Pharmaceutical composition comprising albumin-binding arginine deiminase for cancer targeting treatment
ActiveUS20140255377A1Efficiently depletedHigh activityHydrolasesPeptide/protein ingredientsDiseaseHalf-life
The present invention provides a pharmaceutical composition containing albumin-binding arginine deiminase fusion protein (AAD) for treating cancer or other arginine-dependent diseases. The AAD fusion protein can be purified from both soluble and insoluble fractions of crude proteins, it binds to human serum albumin (HSA) and has its high activity with longer half life for efficient depletion of arginine in cancer cells. The specific activities of wild-type ADI and AAD in the present invention are 8.4 and 9.2 U / mg (at physiological pH 7.4), respectively. The AAD used in the present invention can be used in the treatment of various cancers (e.g. pancreatic cancer, leukemia, head and neck cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal cancer, esophageal cancer, prostate cancer, stomach cancer & brain cancer) and curing arginine-dependent diseases. The composition can be used alone or in combination with at least one chemotherapeutic agent to give a synergistic effect on cancer treatment and / or inhibiting metastasis.
Owner:VISION GLOBAL HLDG
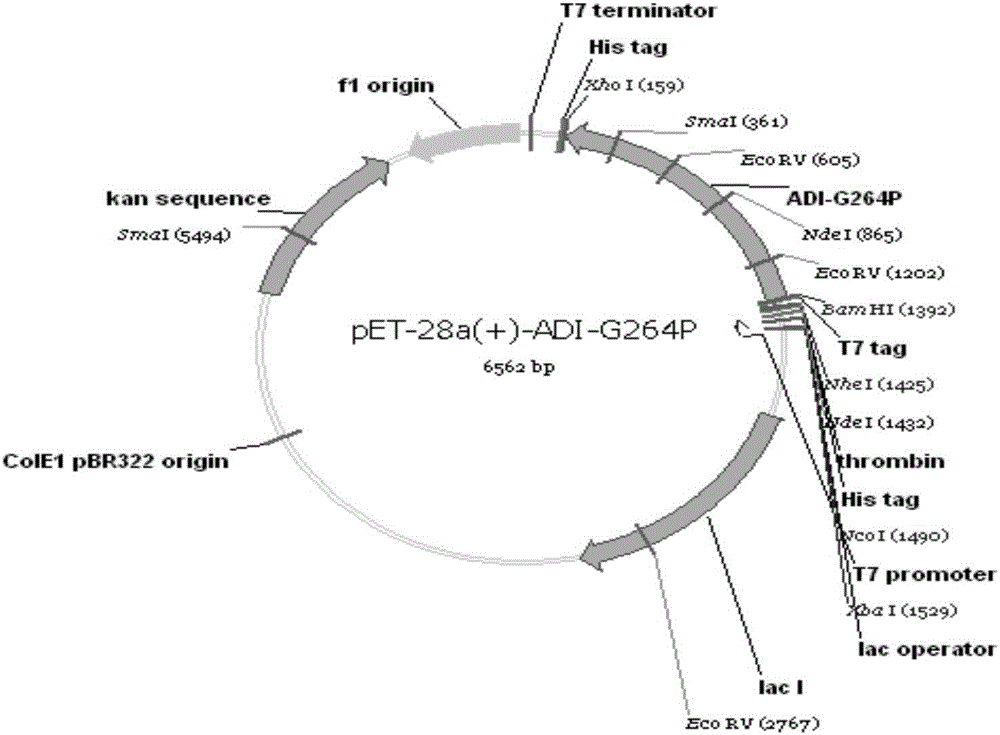
Gene engineering arginine deiminase reformed through site directed mutagenesis
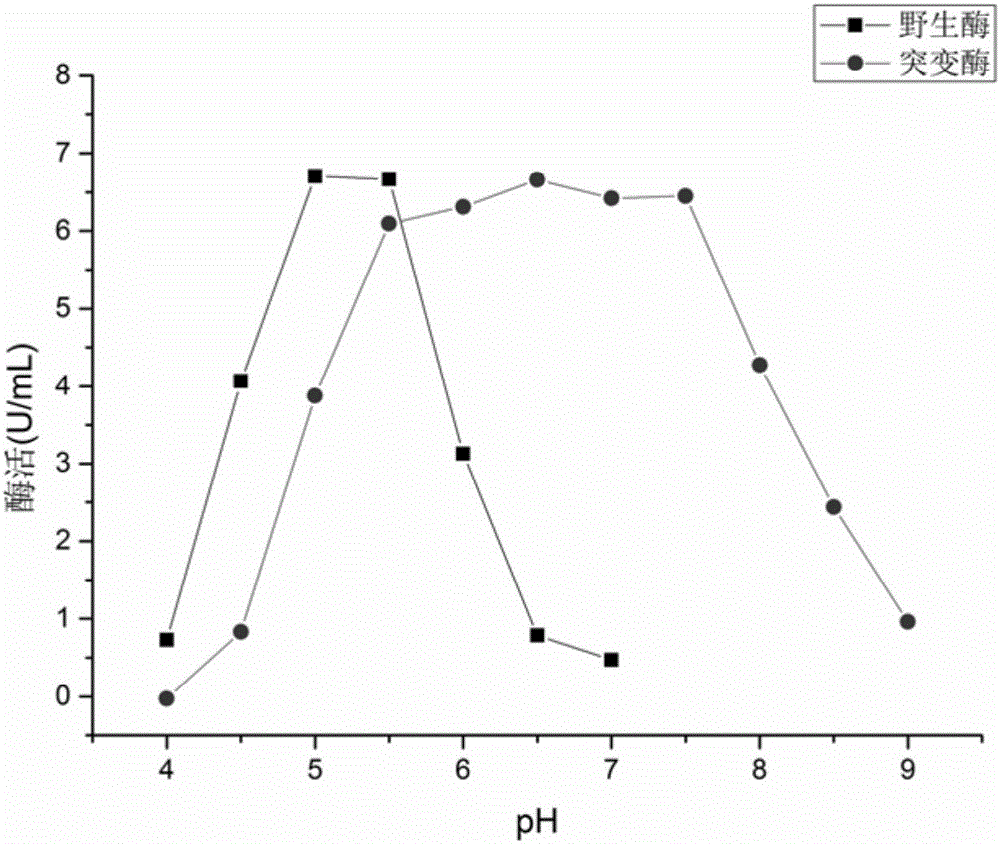
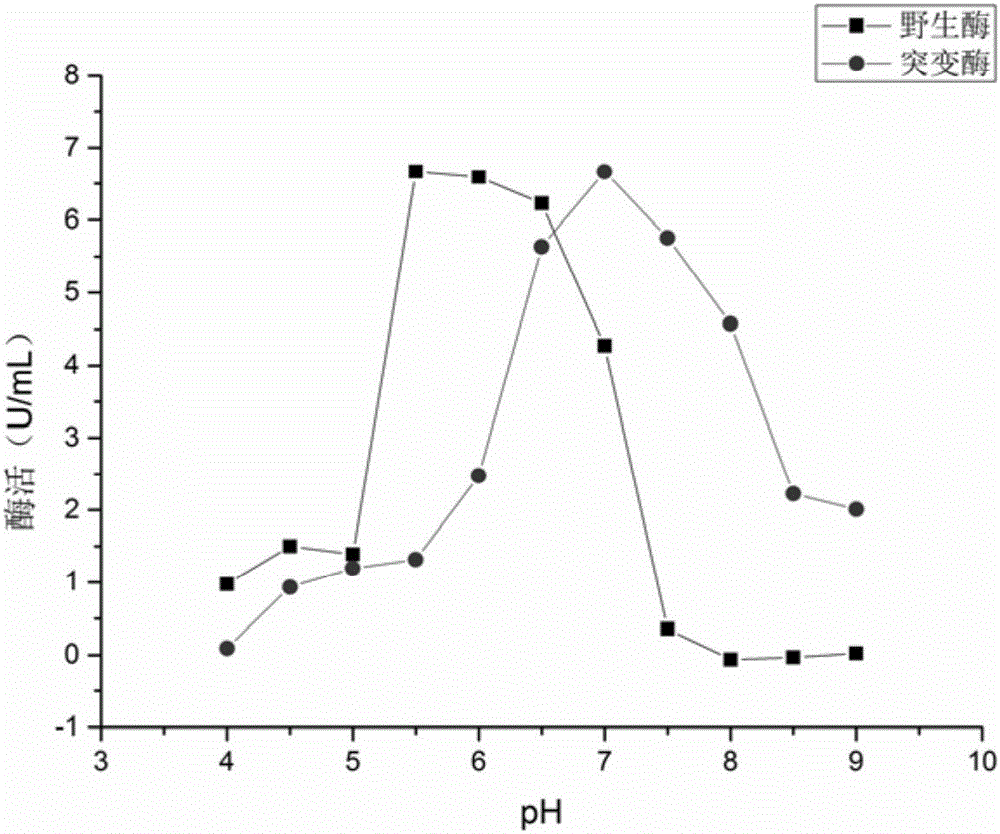
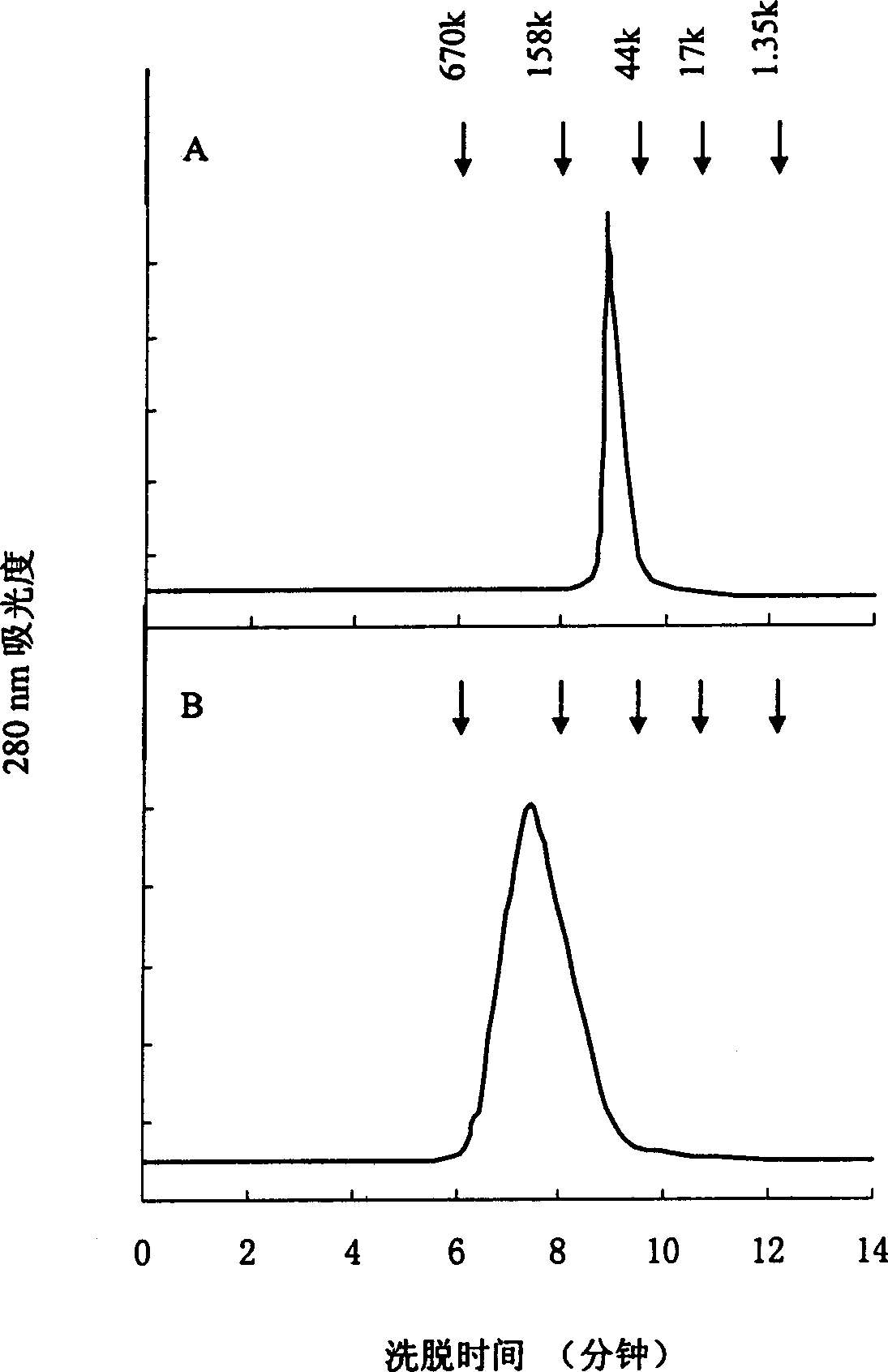
The invention relates to a strain of gene engineering arginine deiminase reformed through site directed mutagenesis, and belongs to the technical field of gene engineering, wherein the amino acid sequence is SEQ ID No.1, and the 264th site glycine in the amino acid sequence of the arginine deiminase reformed through site directed mutagenesis mutates into the proline relative to the amino acid sequence of the natural arginine deiminase. According to the present invention, compared to the wild enzyme, the mutant arginine deiminease has the following characteristics that the effective pH value action range is expanded to a certain degree, particularly the good enzyme activity is provided at the physiological pH value of 7.4, and the mutant enzyme has the high stability under the pH value of 5.5-7.5 along with the broadening of the effective pH value action range, such that the problems of low enzyme activity and short half-life in vivo under the physiological conditions of the clinical applications of the arginine deiminase in tumor treatment are solved, and the good conditions are created for the applications of the arginine deiminase and the coding gene thereof in the clinical treatment.
Owner:JIANGNAN UNIV
Modified arginine deiminase
InactiveCN1536079AChemically stableExcellent characteristicsPeptide/protein ingredientsHydrolasesPolyethylene glycolImmunogenicity
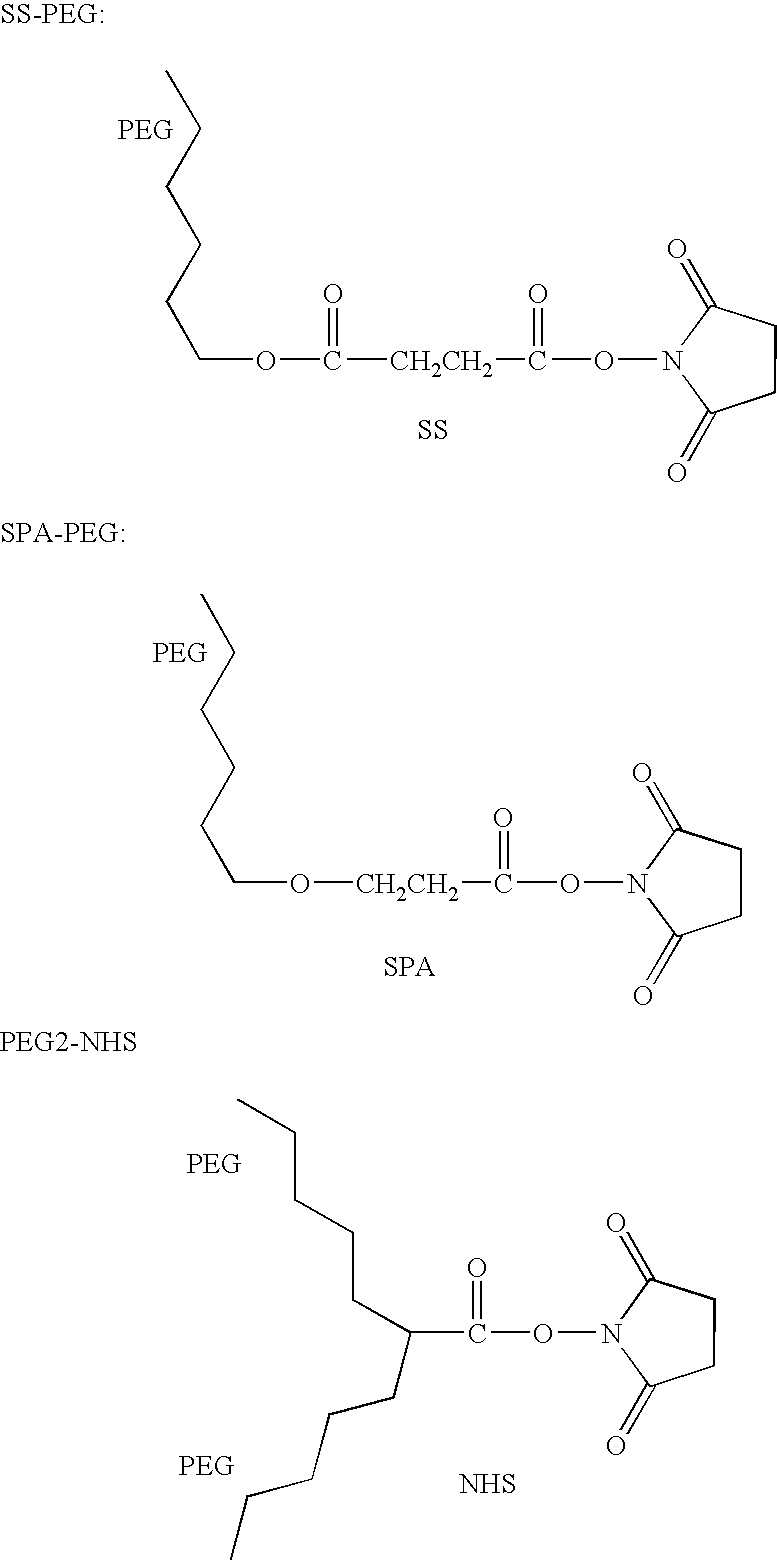
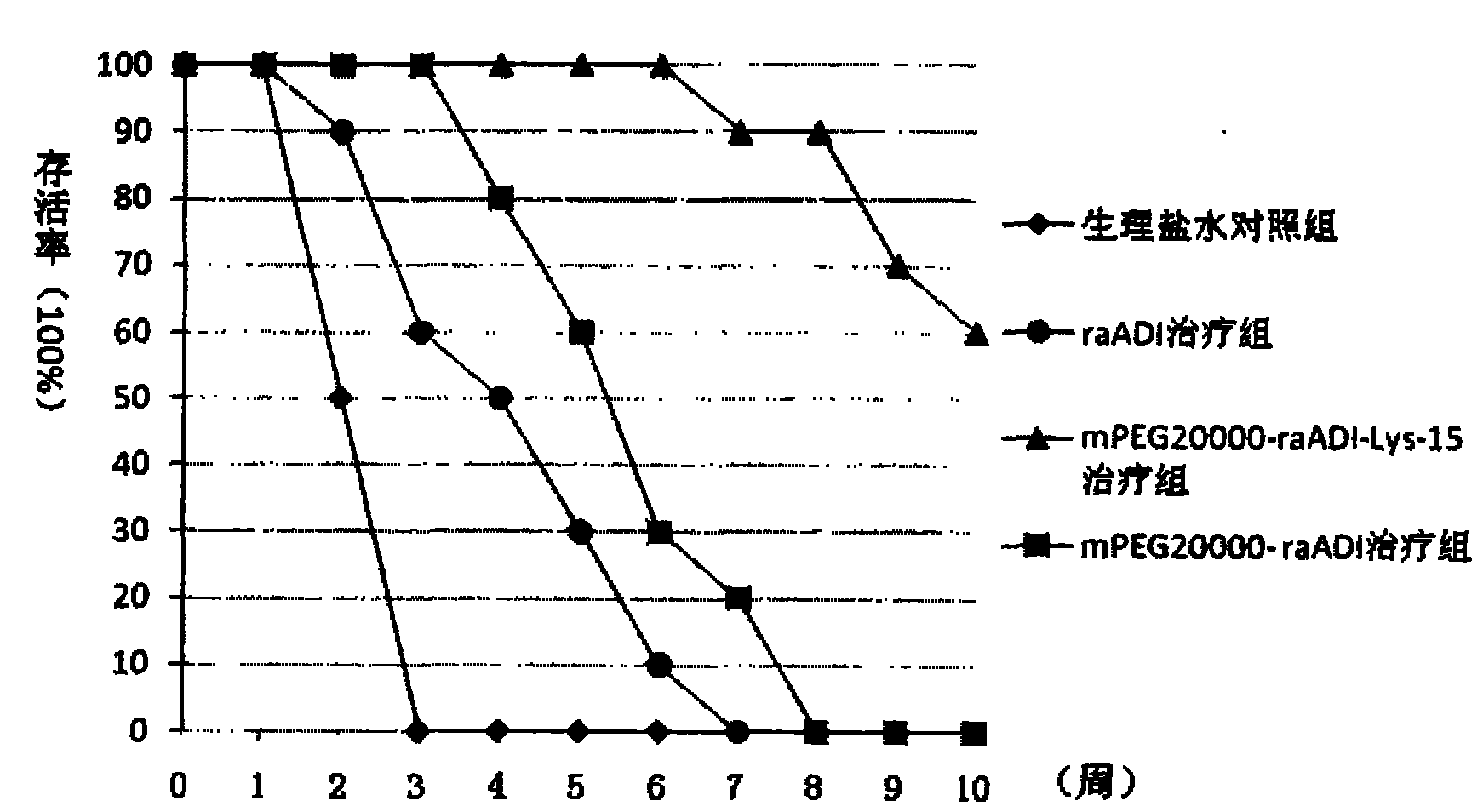
The present invention relates to a modified arginine deiminase having no connecting gene, it is a compound with formula (1) structure, said formula (1) is ADI-(PEG)n (I): in which PEG represents polyethylene glycol whose average molecular weight is 1000-20000 Da, ADI represents arginine deiminase, and 'represents covalent bond between PEG and ADI, and n is integral number of 2-30. Said invention also provides preparation method of modified arginine deiminase and its correspondent medicine composition. Said invented compound has obvious effect for resisting tumor, more stable chemical property and lower immunogenicity.
Owner:上海复旦张江生物医药股份有限公司
Methods of treatment with arginine deiminase
ActiveUS9333268B2Increase progression free survival timeInhibit angiogenesisOrganic active ingredientsHeavy metal active ingredientsPegylated arginine deiminaseOncology
Owner:POLARIS GROUP
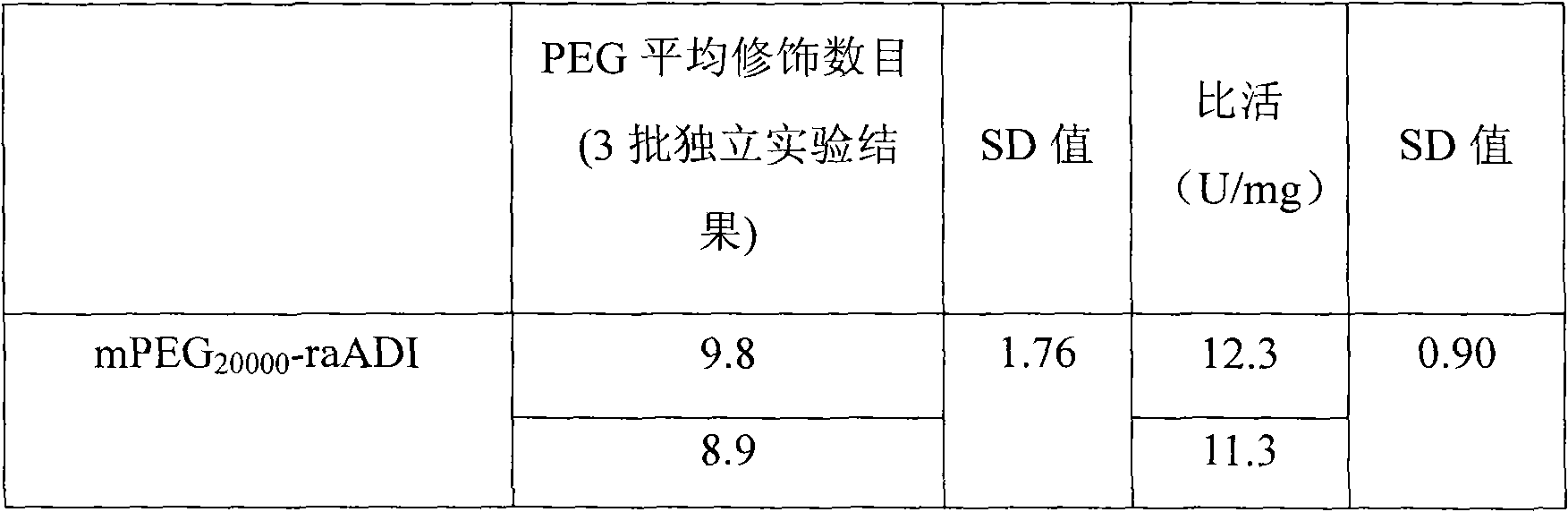
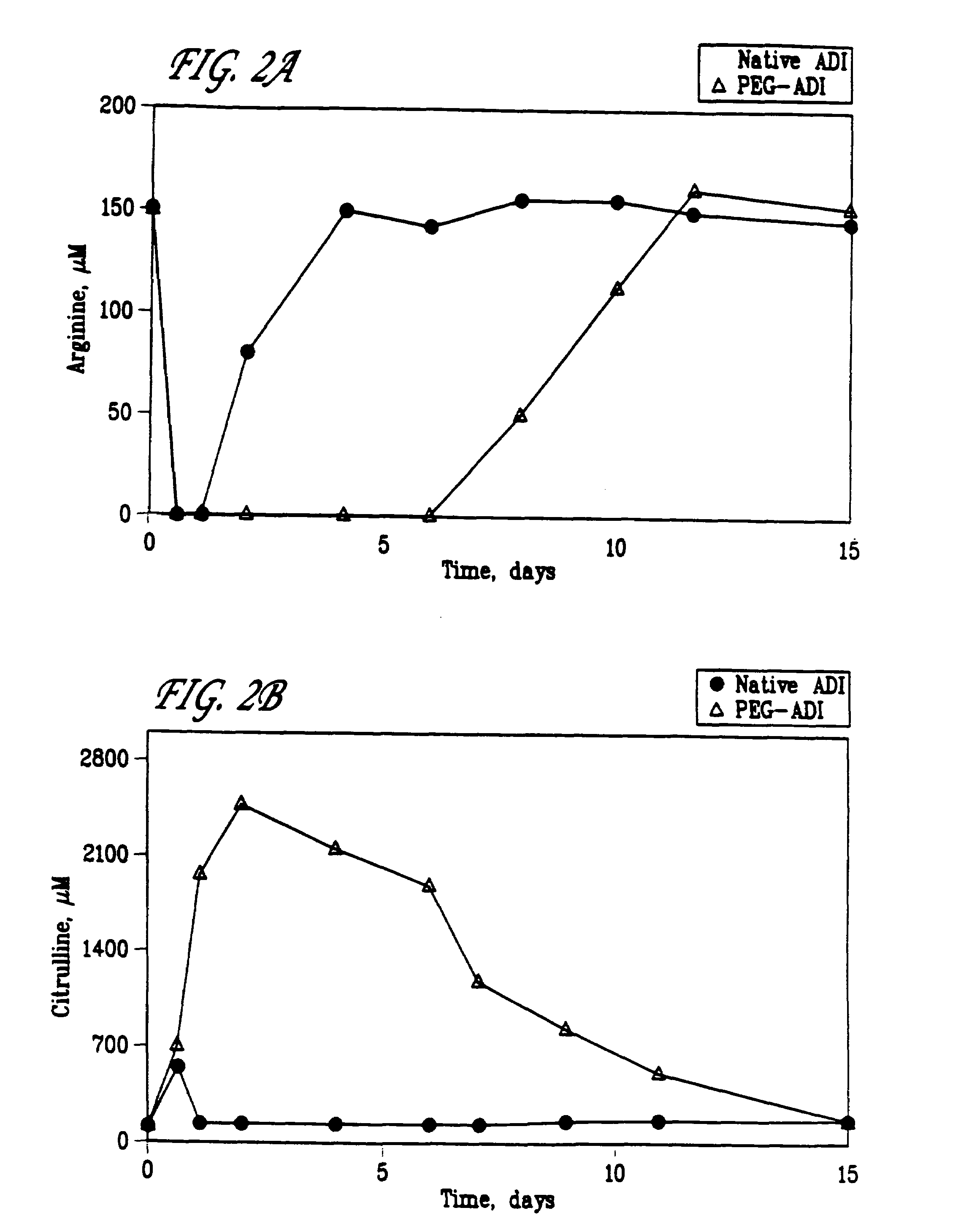
Methoxypolyethylene glycol-modified arginine deiminase, preparation thereof and use thereof
ActiveCN101591649ANon-immunogenicImmunological uniformityPeptide/protein ingredientsPharmaceutical non-active ingredientsIn vivoHigh activity
The invention discloses a methoxypolyethylene glycol-modified arginine deiminase, preparation thereof and use thereof. As a gratuitous substrate is added in a modification reaction to protect active loci, the modified PEG-ADI has higher biological activity compared with the prior EG-ADI modified at the same modification rate. In addition, as specific affinity chromatography is used to remove immunogenic PEG-ADI, the finally prepared PEG-ADI has high activity, high immunologic uniformity and no in-vivo immunogenicity, thereby being more safe and effective in clinic.
Owner:JIANGSU T MAB BIOPHARMA
Recombination preparation method of arginine deiminase
The invention discloses a recombination preparation method of arginine deiminase (hereinafter referred to as ADI), and particularly discloses the preparation method for escherichia coli to conduct recombination expression on the ADI. The method mainly comprises a recombination expression method and an inclusion body renaturation method using urea as a denaturing agent. The N tail end of the ADI prepared through recombination expression does not contain methionine.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
PEG (polyethylene glycol) modified recombinant arginine deiminase (ADI) as well as preparation method and application thereof
InactiveCN103923898AUniform finishGood stability in vitroHydrolasesAntineoplastic agentsCancer cellPolyethylene glycol
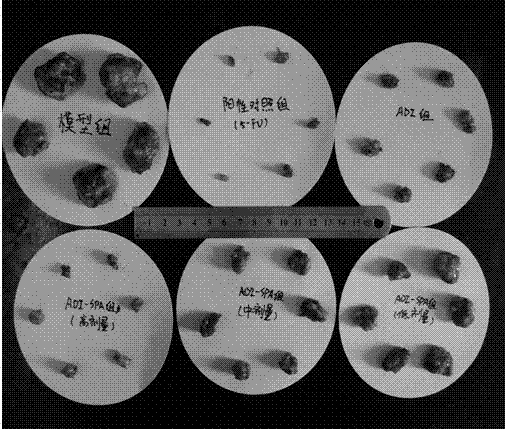
The invention discloses a PEG (polyethylene glycol) modified recombinant arginine deiminase (ADI) as well as a preparation method and application thereof, belonging to the technical field of biomedical engineering. The stability of ADI-PEG obtained by modifying ADI with PEG in vitro and in mouse plasma is remarkably improved. A pharmacokinetics / pharmacodynamics study shows that compared with free ADI before modification, the half-life period of ADI-PEG in a mouse is prolonged by more than 11 times; the concentration of arginine in the plasma can be reduced and maintained for more than 5 days in the limiting level through single injection of 5U ADI-PEG and can be maintained for 1 day only through injection of unmodified ADI; an H22 liver cancer model mouse is treated for 15 days, and the cancer cell inhibition rate of ADI-PEG (15U) is 95.02% close to 98.34% (the inhibition rate) of a positive control group treated with 5-fluorouracil. PEG modified recombinant arginine deiminase is relatively long in in-vivo half-life period and relatively low in immunogenicity, and serves as an excellent tumor treating or inhibiting drug.
Owner:JIANGNAN UNIV
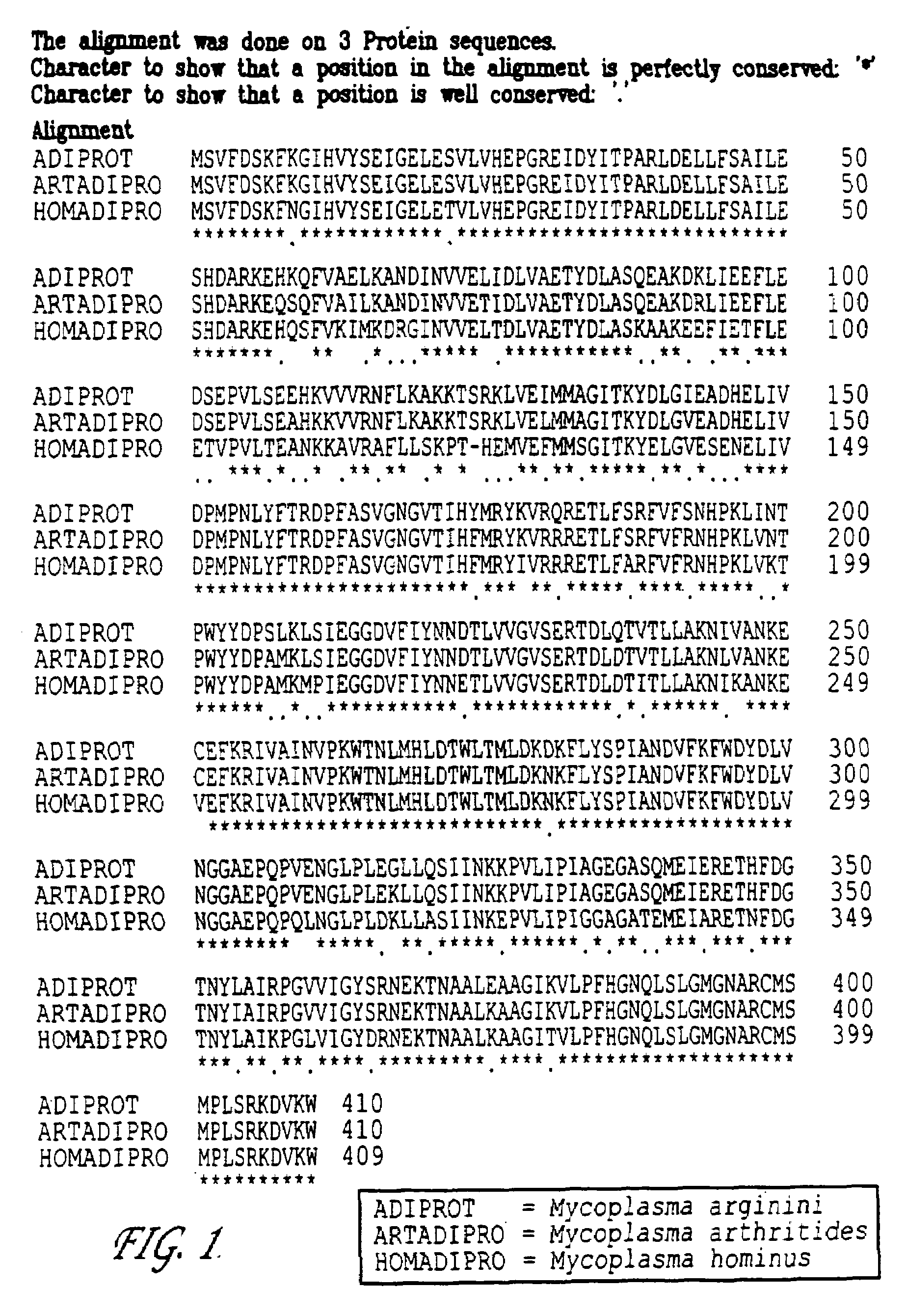
Arginine deiminase mutant from arthritis-type mycoplasma and application thereof
InactiveCN104130996ARetain enzyme activityHigh expressionBacteriaHydrolasesEscherichia coliBiotechnology
The invention discloses an arginine deiminase mutant from an arthritis-type mycoplasma, a gene, a polyethylene glycol modified substance and an application thereof. The arginine deiminase mutant is a dipolymer which is formed by two protein subunits, wherein amino sequences of the protein subunits are represented as SEQ ID No.2 in a sequence table. The arginine deiminase mutant is increased in expression quantity in escherichia coli, is increased in renaturation efficiency, is not changed in enzyme activity and is suitable for industrial production. The polyethylene glycol modified substance of the arginine deiminase mutant can be used for preparing anti-tumor drugs, such as anti-hepatoma drugs, anti-leukemia drugs and anti-melanoma drugs.
Owner:SHANGHAI INST OF PHARMA IND +1
Recombinant Escherichia coli for expressing arginine deiminase gene and application of recombinant Escherichia coli
InactiveCN104726478AHomoarginine deiminase activityReduce dosageBacteriaMicroorganism based processesEscherichia coliInduced mutation
The invention discloses recombinant Escherichia coli for producing arginine deiminase at high yield. The recombinant Escherichia coli is collected with the serial number of CCTCC (China Center for Type Culture Collection) NO:M2015048 in CCTCC; an arginine deiminase gene is from Bacillus cereus which is bred after being subjected to induced mutation of N<+> ion beams; and then, a mutant strain for producing arginine deiminase at high yield is obtained through screening, wherein the collection number of the mutant strain is CCTCC NO:M2015047. The invention also discloses application of the recombinant Escherichia coli to production of L-citrulline and a method for producing L-citrulline. By using the recombinant Escherichia coli disclosed by the invention, more than 99% of arginine can be converted into L-citrulline, and the purity of a product can be up to more than 99%.
Owner:WUHAN GRAND HOYO
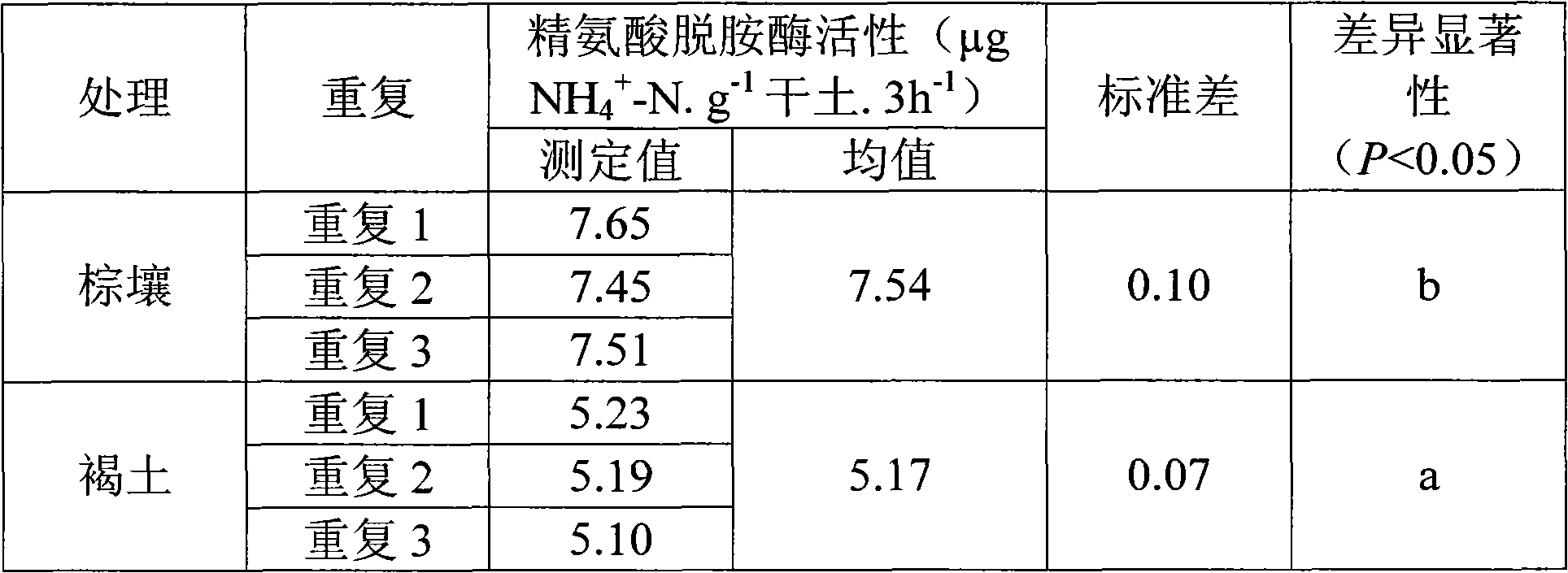
Method for determining activity of soil arginine desaminase
InactiveCN102108377AReduce testing costsEasy to operateMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPotassiumChloride
The invention relates to a method for determining the activity of soil arginine desaminase, which comprises the following steps: 1) weighting n fresh soil or precultured soil samples, placing in n conical flasks having plugs (wherein n is more than or equal to 4), respectively adding arginine substrate solution into each conical flask, uniformly mixing the arginine substrate solution with the soil, blocking each flask with a rubber plug, and then culturing for 3 hours at constant temperature; 2) after the culturing process is finished, adding a certain volume of potassium chloride solution into the flasks, oscillating, and filtering; 3) determining the quantity of generated ammonium ions by using the indophenol blue colorimetry; and 4) calculating the activity of arginine desaminase. The method for determining the activity of soil arginine desaminase has the following advantages: 1) compared with the traditional biochemical determining method, the method provided by the invention simplifies the operation steps; 2) the dependence on high equipment demand is reduced, and a common spectroscopic instrument can meet the demand; 3) the accuracy is high, and the operation can be easily performed; and 4) the result is stable and reliable, and the repeatability is good.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
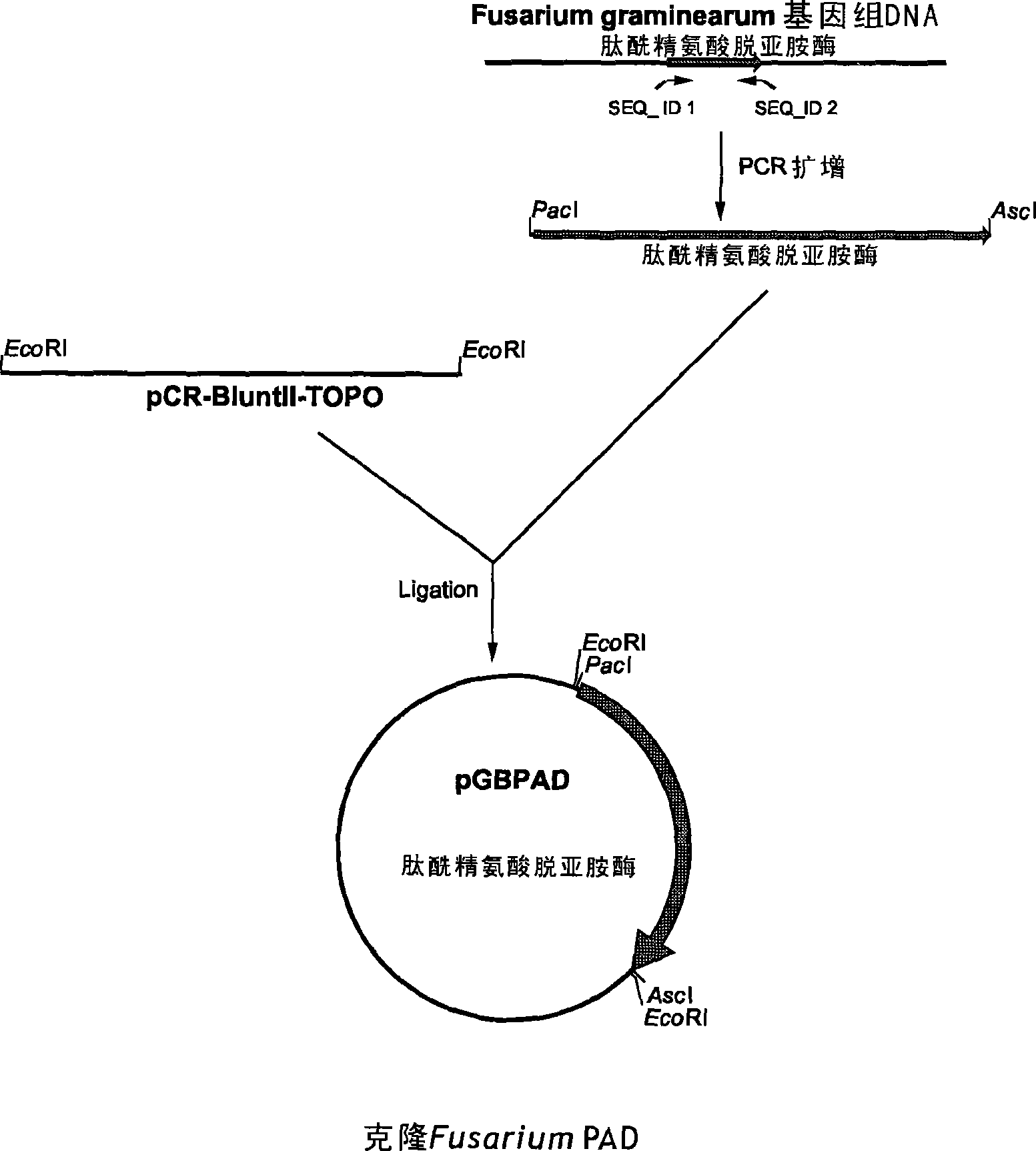
Peptidylarginine deiminase and uses thereof in the production of citrullinated proteins and peptides
InactiveCN101479380AAllergenicity reductionReduce emulsificationHydrolasesMicroorganism based processesGibberella zeaeProtein hydrolysates
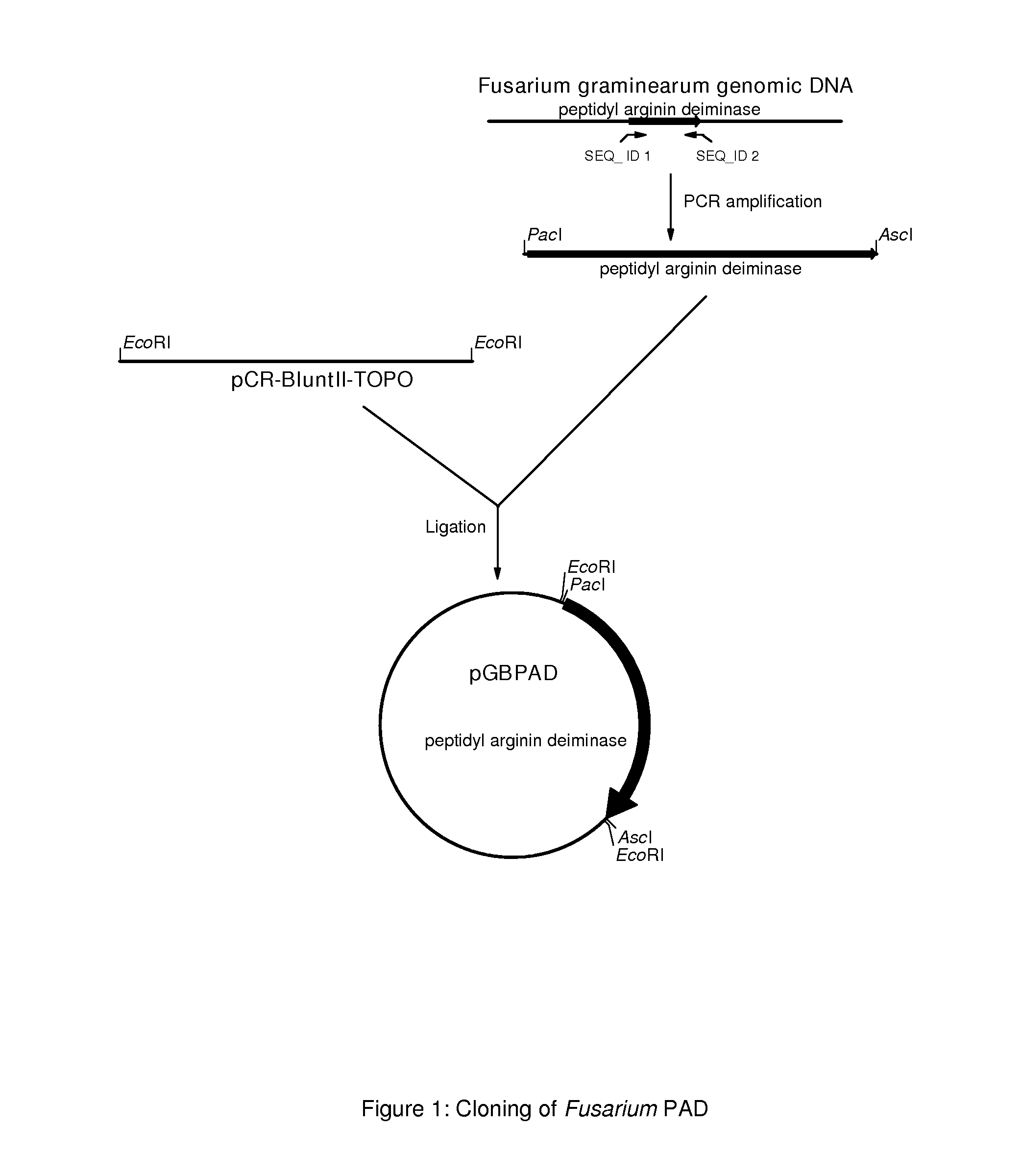
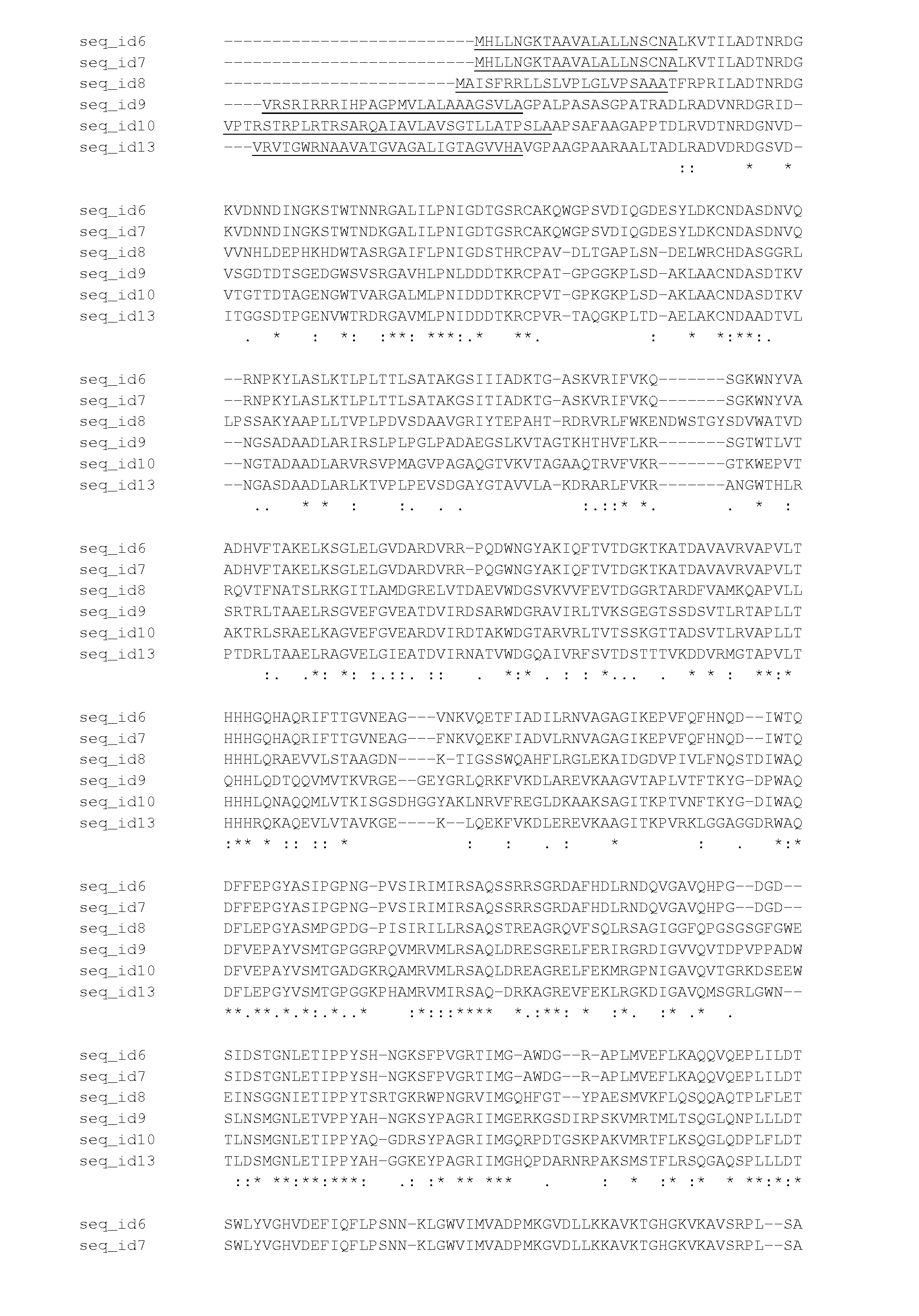
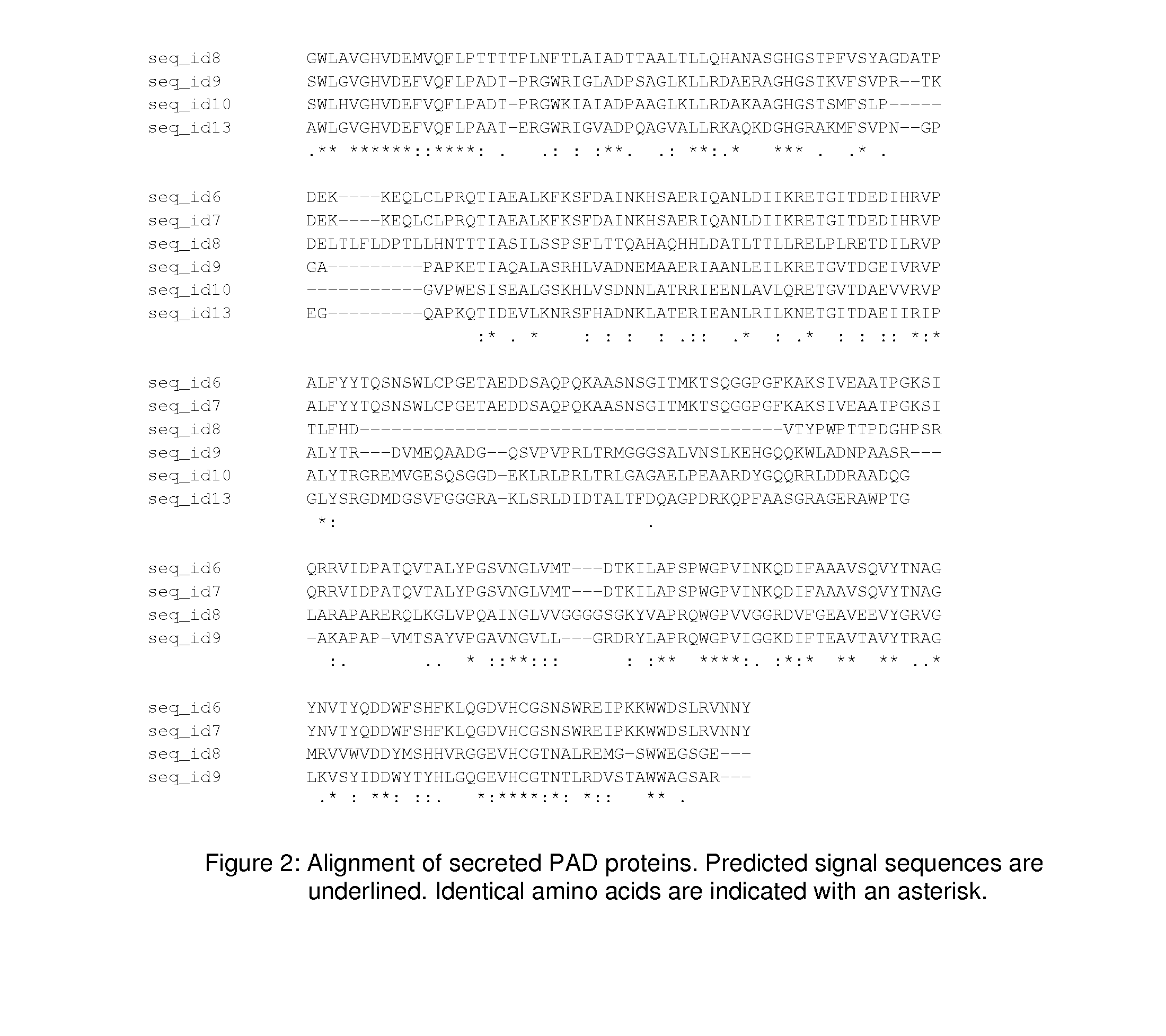
The present application concern the isolation of a peptidylarginine deiminase enzyme (EC 3.5.3.15) from Gibberella zeae (also called Fusarium graminearum). The application also discloses citrullinated proteins and peptides produced enzymatically using said arginine deiminase and uses of such citrullinated proteins and peptides in foodstuff. The present invention relates to a modified protein, peptide or protein hydrolysate wherein at least 15%, preferably at least 30%, more preferably at least 45%, still more preferably at least 60%, and most preferably at least 80% of the arginine residues which are originally present in the protein, peptide or protein hydrolysate is transformed into a citrulline residue.
Owner:DSM IP ASSETS BV
Pharmaceutical composition comprising arginine deiminase for inhibiting angiogenesis
InactiveUS7413735B2Bacterial antigen ingredientsSugar derivativesAngiogenesis growth factorBULK ACTIVE INGREDIENT
The present invention relates to a pharmaceutical composition for inhibiting angiogenesis which comprises arginine deiminase as an active ingredient, where the arginine deiminase, obtained from Mycoplasma arginini or prepared by a genetic recombination technique, may be conjugated to an activated polymer to lower its immunogenecity and increase its life time. The pharmaceutical composition of the present invention exhibits an excellent inhibitory activity against angiogenesis.
Owner:ANGIOLAB
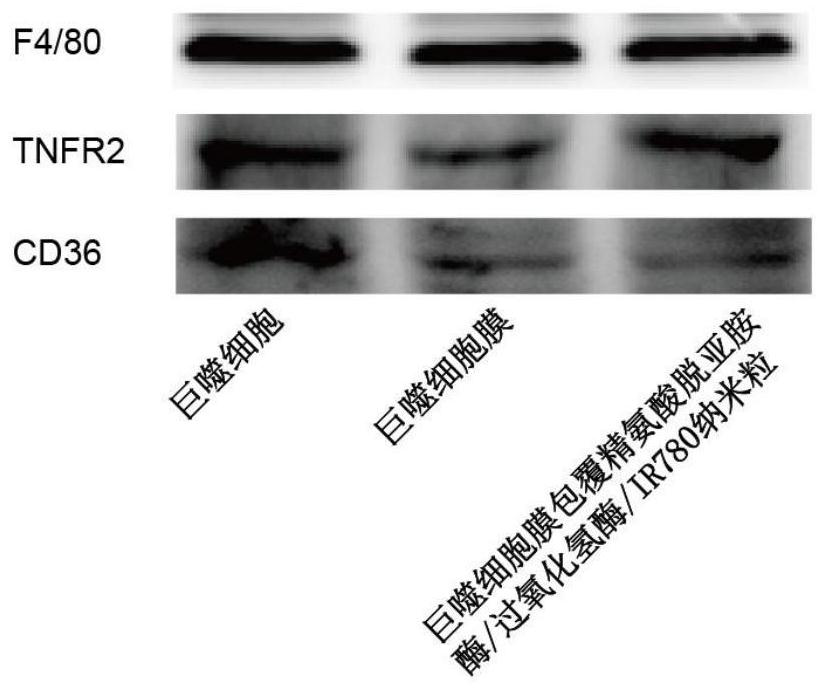
Macrophage membrane coated arginine deiminase/catalase/IR780 nanoparticle, preparation method and application
ActiveCN113750232AImprove stabilityLow immunogenicityPeptide/protein ingredientsHydrolasesCell membraneCatalase
The invention belongs to the field of pharmaceutical preparations. The invention relates to a preparation method and application of a macrophage membrane coated arginine deiminase / catalase / IR780 nanoparticle. The prepared macrophage membrane coated arginine deiminase / catalase / IR780 nanoparticle is good in biocompatibility, the preparation method is simple, the particle size distribution of the nanoparticle is uniform, the stability of the arginine deiminase can be improved, the protease hydrolysis resistance ability of the arginine deiminase is improved, an anti-tumor effect is enhanced in cooperation with photothermal therapy or photodynamic therapy, and a potential application value is achieved in the fields of treatment of cancers (especially arginine-dependent diseases) and the like.
Owner:CHONGQING MEDICAL UNIVERSITY
Methods of treatment with arginine deiminase
ActiveUS20150231272A1Increase progression free survival timeInhibit angiogenesisHeavy metal active ingredientsOrganic active ingredientsPegylated arginine deiminaseOncology
Owner:POLARIS GROUP
Engineered chimeric pegylated adi and methods of use
ActiveUS20170000862A1Stable expressionHigh yield productionHydrolasesPeptide/protein ingredientsVirologyPEGylation
Owner:POLARIS GROUP
Strain for producing arginine deiminase and application thereof
InactiveCN100584940CIncrease enzyme activityBacteriaPeptide/protein ingredientsBio engineeringChromosomal dna
The invention discloses a method to produce ADI strain by screening and the application of the method, pertaining to the field of bioengineering technology. The invention particularly relates to a rapid method to screen ADI strain and produce Pseudomonas plecoglossicida (CGMCC No.2039) of ADI, as well as a method to produce ADI by using the microorganism. Under an aerobic condition and an environment maintained at pH6.5-8.0, enzyme activity can achieve 1.6U / mL fermentation broth after 20-24h fermentation culture. ADI produced with such microorganism has very strong inhabitation agaisnt liver cancer cell strain (HepG2). ADI genes can be obtained from the chromosome DNA of such microorganism, and the gene order can be achieved.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com