Recombination preparation method of arginine deiminase
An arginine deiminase and amino acid technology, which is applied in the field of preparation of recombinant arginine deiminase invention, can solve the problems of high industrialization cost, renaturation rate and enzyme specific activity to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0062] Example 1. Artificial synthesis of arginine deiminase cDNA sequence
[0063] The cDNA sequence (SEQ ID NO:3) designed according to the amino acid sequence (SEQ ID NO:1) of arginine deiminase was commissioned to artificially synthesize the nucleotide sequence of the whole gene, embedded in the pMD 19-T vector, and named as
[0064] Named pMD 19-ADI.
example 2
[0065] Example 2. Construction of Arginine Deiminase Recombinant Plasmid and Engineering Strain
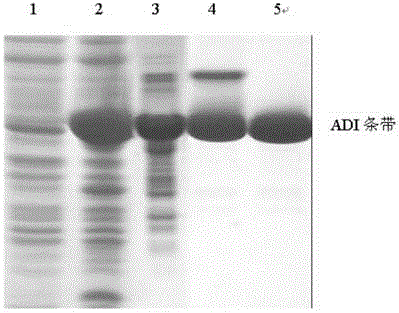
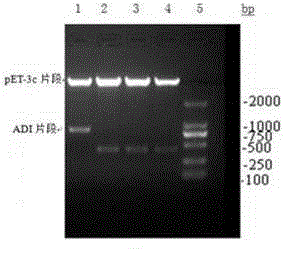
[0066] Using the pMD 19-ADI plasmid as a template, use NdeI endonuclease and BamHI endonuclease to double digest, recover the target fragment, and then use T4 DNA ligase and plasmid pET-3C (purchased from Invitrogen) to pass NdeI and BamHI The recovered fragments were digested and ligated, transformed into Escherichia coli cloning host strain Top10, and the recombinant plasmid pET-3c-ADI was screened by enzyme digestion and PCR verification (see attached figure 2 ). After DNA sequencing (TAKARA, Dalian) proved that the cDNA sequence of ADI in the recombinant plasmid was correct (see attached image 3 ), transformed into Escherichia coli expressing host strain BL21 Star (DE3) plysS (Novagen). Recombinant expression strains were obtained through expression screening. Construction diagram see attached figure 1 .
example 3
[0067] Example 3. Fermentation of recombinant arginine deiminase
[0068] Streak inoculate pET-3C-ADI / BL21 Star (DE3) plysS recombinant engineered bacteria with the best expression on LA agar plate and culture overnight at 37°C. Pick the bacterial lawn from the overnight cultured LA plate and inoculate it in a test tube containing LB liquid medium (tryptone 10g, yeast extract 5g, NaCl 10g, add water to 1000ml, 121°C, autoclave for 30min), 37°C Cultivate for 12 hours, then transfer to a 1000ml Erlenmeyer flask containing 200ml LB culture medium at a ratio of 1%, and cultivate overnight at 37°C to become the seed solution in the upper tank. Inoculate the seed solution in the upper tank into the YT-containing culture solution (tryptone 4g / L, yeast extract 3g / L, Na 2 HPO 4 12H 2 O 30g / L, KH 2 PO 4 3g / L, NaCl 1g / L, MgSO 4 ·7H 2 O 1g / L, glucose 8g / L, 121°C, autoclaved for 30min) in a 30L fermenter, cultured at 37°C, during the whole fermentation process, the dissolved oxygen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com