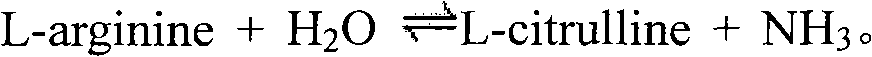Arginine deiminase mutant and preparation and application thereof
A technology for arginine deiminase and mutants, which is applied in the fields of arginine deiminase mutants and their preparation and application, and can solve the problems of uncontrollable modified sites, decreased activity of modified proteins, and unsatisfactory products. Uniform mixture and other problems, to achieve the effect of high activity retention rate, high product uniformity and high in vitro activity
Active Publication Date: 2010-08-25
JIANGSU T MAB BIOPHARMA
View PDF2 Cites 22 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Conventional PEG modification not only leads to a decrease in the activity of the modified protein, but also results in an inhomogeneous mixture of products due to the inability to control the modified site
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
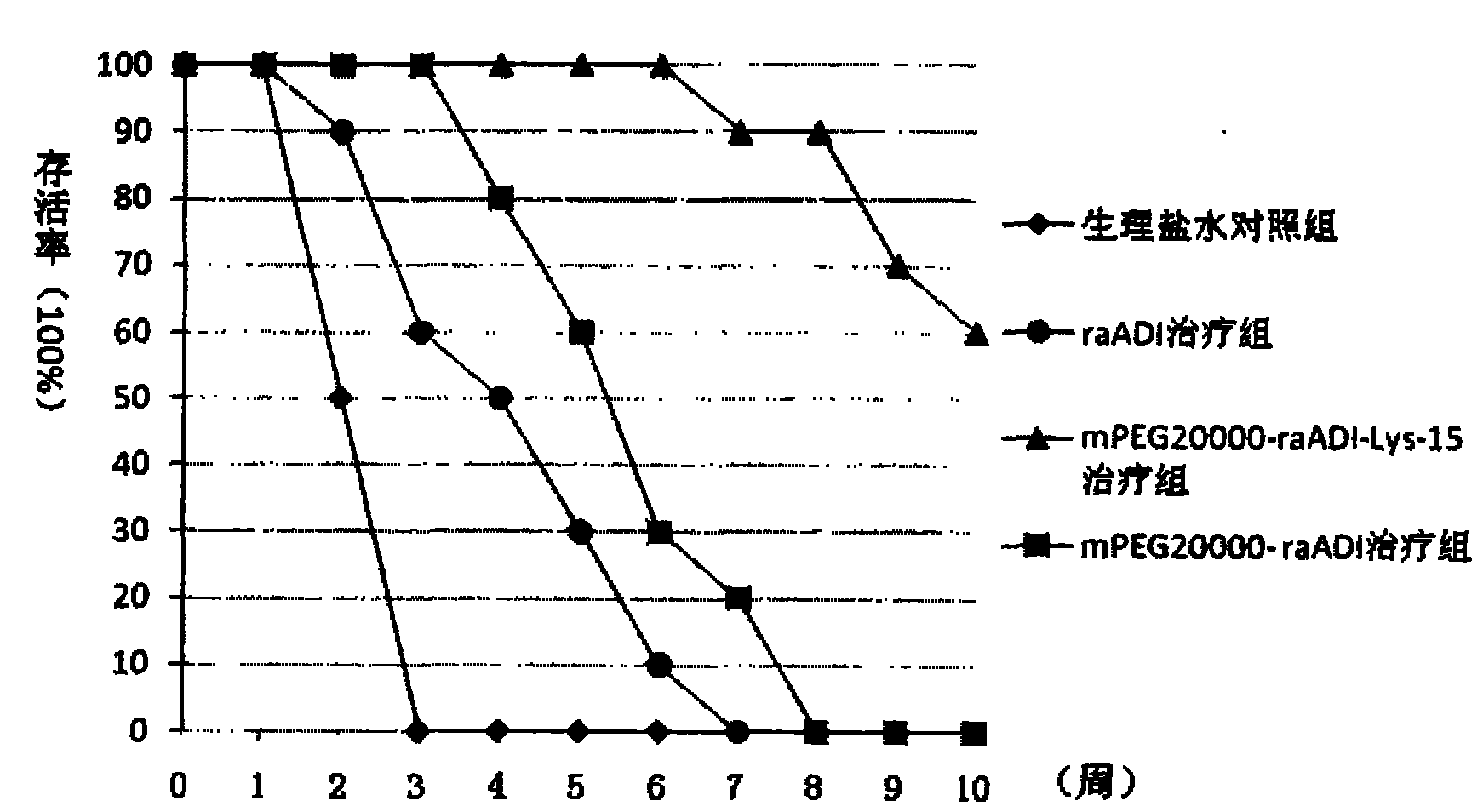
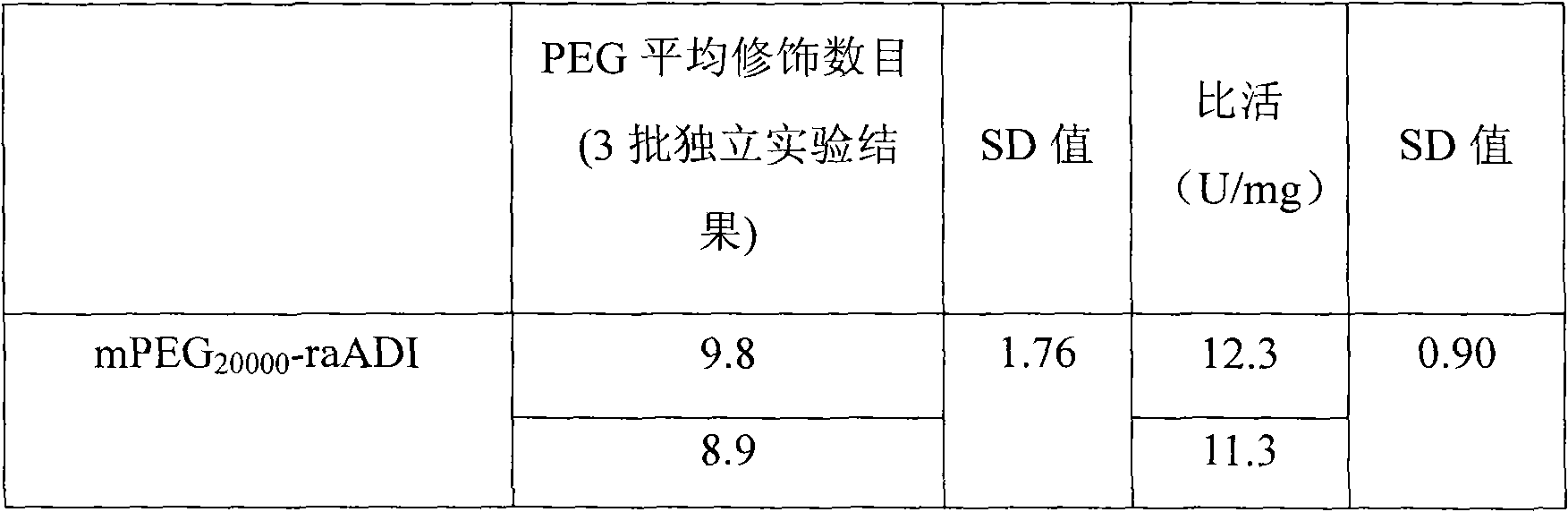
The invention relates to an arginine deiminase mutant with partial mutated lysine and preparation and application thereof. The arginine deiminase mutant of the invention has enzymatic activity of degrading arginine into citruline; compared with the arginine deiminase with the amino acid sequence of SEQ IDNO:1, the amino acid sequence comprises mutants of K9N, K59Q, K66R, K93E, K111R, K119Q, K121Q, K122E, K126E, K178I, K196I and one or more of K209G, K243E, K249D and K279Y. Compared with natural arginine deiminase, after the arginine deiminase mutant of the invention is modified by PEG, the retention rate of active residues is improved; and as the quantity of lysine is reduced, products are more uniform and can be applied to clinical treatment of hepatoma, melanoma and the like.
Description
technical field The invention relates to the field of biotechnology, in particular to an arginine deiminase mutant and its preparation and application. Background technique Arginine is not required for normal cell growth because they can be synthesized from guanidine through a two-step reaction catalyzed by argininosuccinate synthetase (ASS) and argininosuccinate lyase (AL). Arginine: However, hepatocellular carcinomas (HCC), melanomas (melanomas) and some other sarcomas do not express argininosuccinate synthase, so they are auxotrophic for arginine and can only be used in the presence of arginine In the presence of enzymes that degrade arginine, the removal of arginine makes these tumor cells enter a "starvation" state, and their growth is inhibited. Therefore, arginine-degrading enzymes can be used as potential clinical drugs for diseases such as liver cancer and melanoma. Arginine deiminase (arginine deiminase, ADI) can catalyze the conversion of arginine to guanidine, ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C12N9/78C12N15/55C12N15/63C12N1/15C12N1/19C12N1/21C12N5/10A61K38/50A61P31/12A61P35/00A61P25/28
Inventor 黄岩山裘霁宛凌建群温晓芳尚玉栓傅晓瑜范敏王玉姣王叶飞
Owner JIANGSU T MAB BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com