Arginine deiminase mutant and preparation and application thereof
A technique for arginine deiminase and mutants, which is applied in the field of arginine deiminase mutants and their preparation and application, and can solve the problems of decreased activity of modified proteins, heterogeneous mixture of products, and uncontrollable modification site and other issues, to achieve high activity retention, high product uniformity, and high in vitro activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
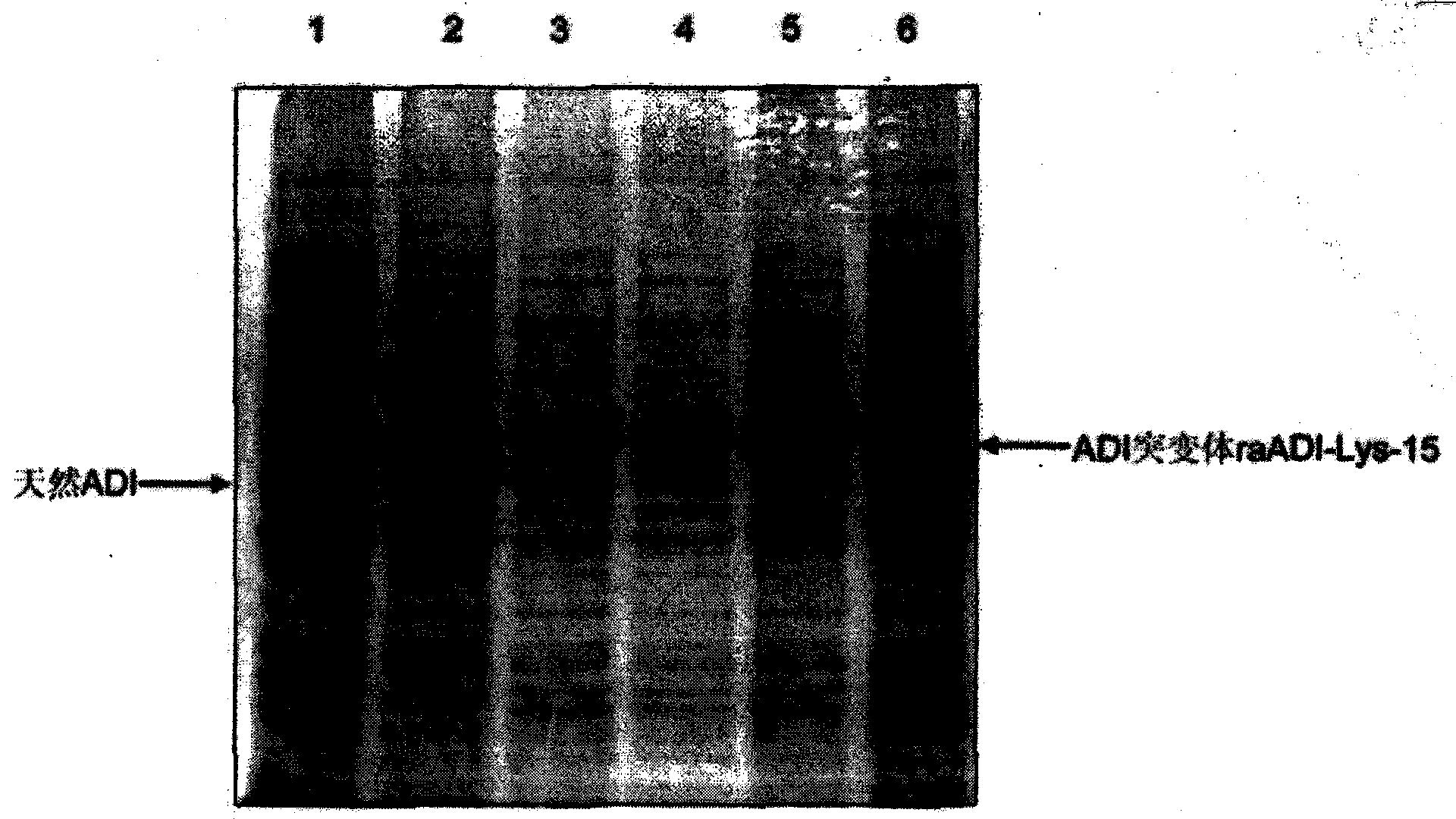
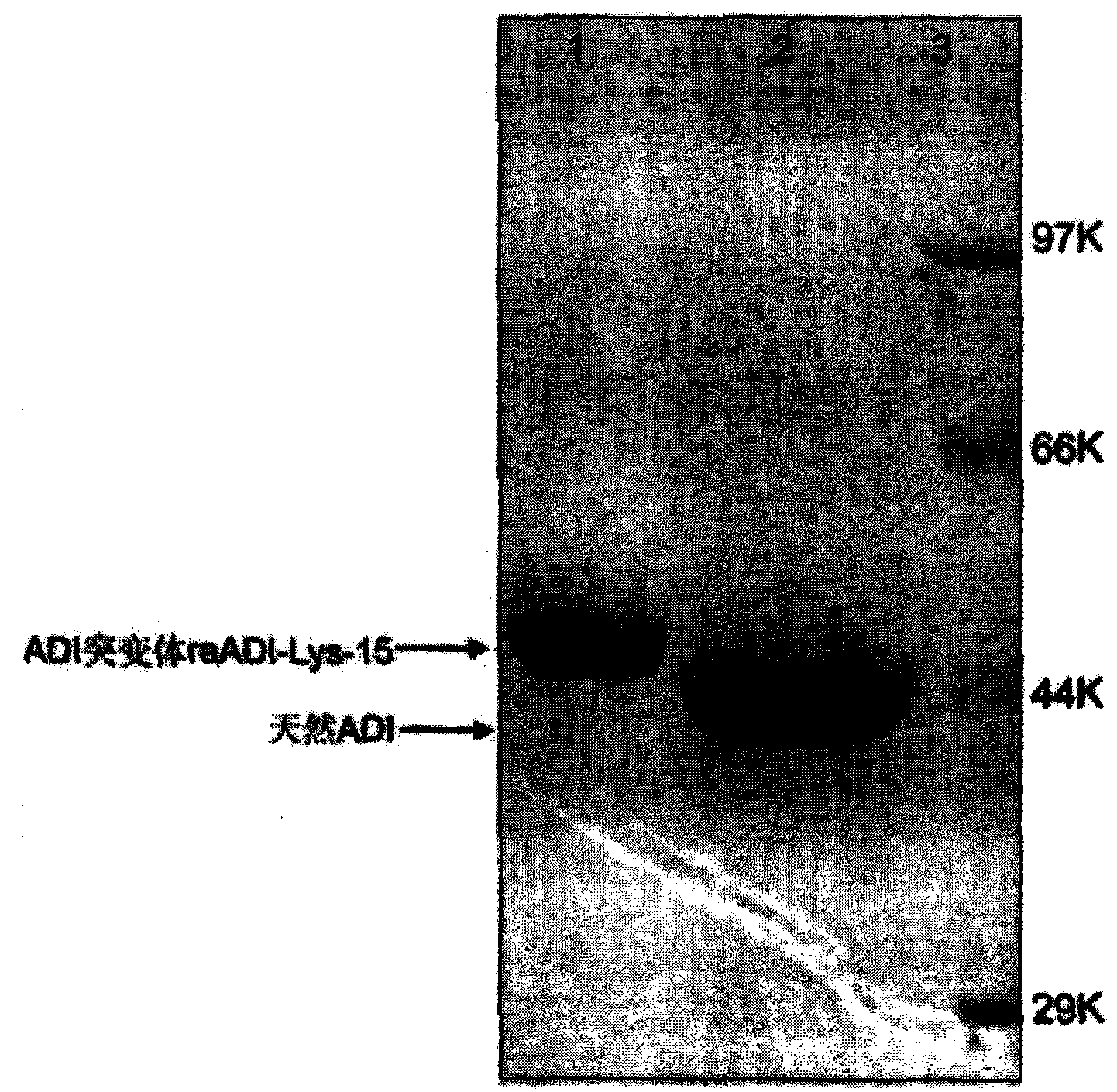
[0042] Example 1: Expression, purification and identification of recombinant arginine deiminase mutants
[0043] 1.1 Expression and renaturation of recombinant arginine deiminase mutants
[0044] In order to obtain a recombinant arginine deiminase with a partial mutation of lysine with high biological activity, the researchers of the present invention screened out a mutant of arginine deiminase with a random point mutation of lysine. A mutant of arginine deiminase with partial mutation of lysine, compared with the original sequence of SEQ ID NO: 1, the mutant of arginine deiminase with partial point mutation only retains a part that does not significantly affect the activity Lys residues.
[0045] Sequencing shows that the amino acid sequence of the above mutant is shown in SEQ ID NO: 2, in which 15 lysines are replaced by other amino acids, named raADI-Lys -15 , the specific mutation sites are: K9N, K59Q, K66R, K93E, K111R, K119Q, K121Q, K122E, K126E, K178I, K196I, K209G, K...
Embodiment 2
[0055] According to the mutation site in the mutant obtained in Example 1, the protein after the single point mutation of 15 mutation points was prepared with reference to the method of Example 1, and the activity residue after the single mutation of each mutation point was checked. The results are shown in the following table:
[0056] Table 1 Activity residues of arginine deiminase mutants after single point mutation
[0057] amino acid
[0058] The results showed that the arginine deiminase mutant with the above single point mutation can also retain more than 85% of the residual activity. In view of the results of Examples 1 and 2, those skilled in the art know that no matter whether these mutations are single mutations or multiple mutations, they have basically no effect on the arginine degradation activity of arginine deiminase, so even if only Partial mutations in these 15 lysines, the obtained mutants can still maintain the enzyme activity of degrading arginin...
Embodiment 3
[0060] According to the results of Example 2, another arginine deiminase mutant was artificially synthesized. The amino acid and DNA sequences are shown in SEQ ID NO: 3 and SEQ ID NO: 6 respectively. Compared with SEQ ID NO: 2, this mutation Only K9N, K59Q, K66R, K93E, K111R, K119Q, K121Q, K122E, K126E, K178I, K196I, K209G mutations occurred in the body sequence, a total of 12, named raADI-Lys -12 . Its expression, renaturation, purification and activity determination are the same as in Example 1, and the specific activity is still about 30 U / mg. It is proved that the 15 lysine mutations in Example 1 can be combined at random without significantly affecting their activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com