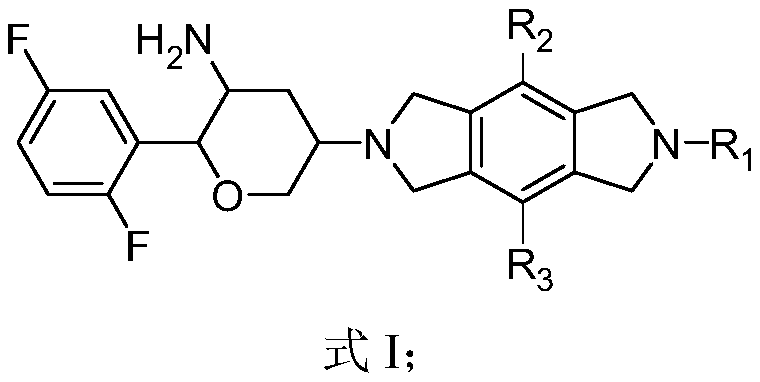
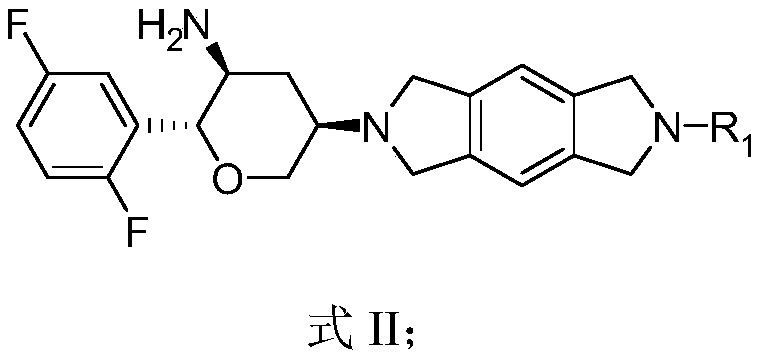
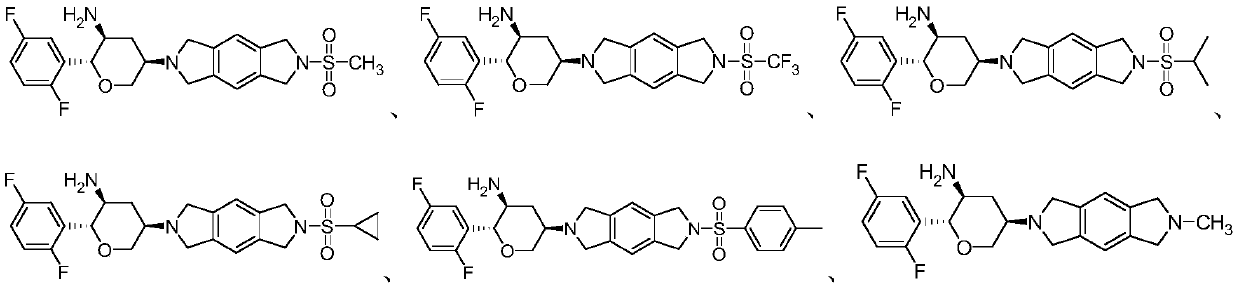
Benzodipyrrolidine compound or pharmaceutically acceptable salt thereof, and preparation method and application thereof
A technology of benzoditetrahydropyrrole and compound, which is applied in the field of medicine and can solve the problem that the drug has not been applied for listing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Synthesis of compound 1
[0073]
[0074] synthetic route:
[0075]
[0076] Synthesis of compound 1-1:
[0077] Tetrabromomethylbenzene (9.0g, 20mmol) was dissolved in 200mL tetrahydrofuran, trifluoroacetamide (9.0g, 80mmol) was added, sodium hydrogen (4.8g, 120mmol, 60wt% in oil) was slowly added at 0°C, and the temperature was gradually raised to room temperature Stir for 30 minutes, then heat up to reflux and stir for 3 hours. After TLC detects that there is no raw material, add 300mL ethyl acetate to the reaction solution for dilution, and the organic phase is washed once with 200mL water and saturated brine successively, then dried with anhydrous magnesium sulfate, pumped After filtration, the filtrate was evaporated to remove the solvent under reduced pressure, and the resulting concentrate was purified by column chromatography (200-300 mesh silica gel, eluent: petroleum ether / ethyl acetate, volume ratio: 4:1) to obtain a white solid (compound 1...
Embodiment 2
[0091] Example 2 Synthesis of compound 2
[0092]
[0093] Using intermediate 1-3 (compound 1-3) as a raw material, replace the raw material methanesulfonyl chloride in Example 1 with trifluoromethanesulfonyl chloride, refer to the synthetic method of the last two steps in Example 1, and synthesize compound 2, the latter two The step yield is 62%; MS: 504.1 [M+H+].
Embodiment 3
[0094] Example 3 Synthesis of compound 3
[0095]
[0096] Using intermediate 1-3 as the raw material, the raw material methanesulfonyl chloride in Example 1 was replaced with the raw material isopropylsulfonyl chloride, referring to the synthesis method of the last two steps in Example 1 to synthesize compound 3, and the yield of the last two steps was 65%. ; MS: 477.1 [M+H+].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com