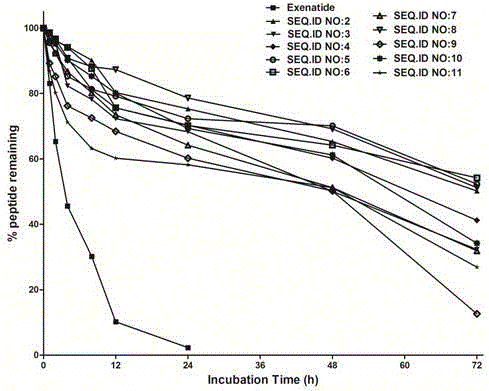
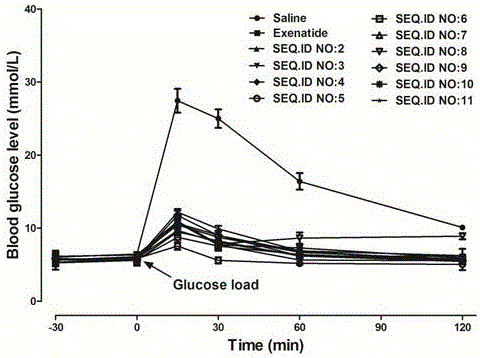
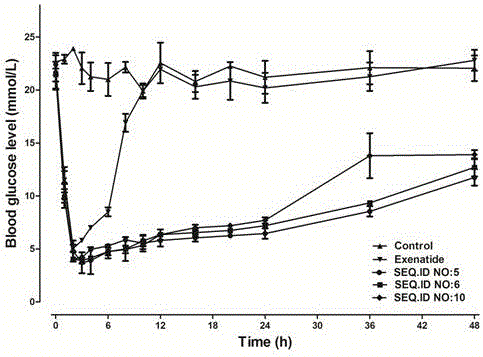
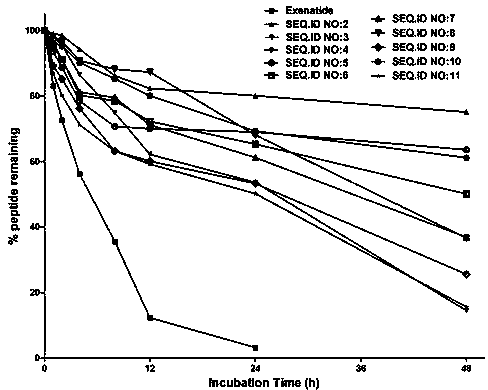
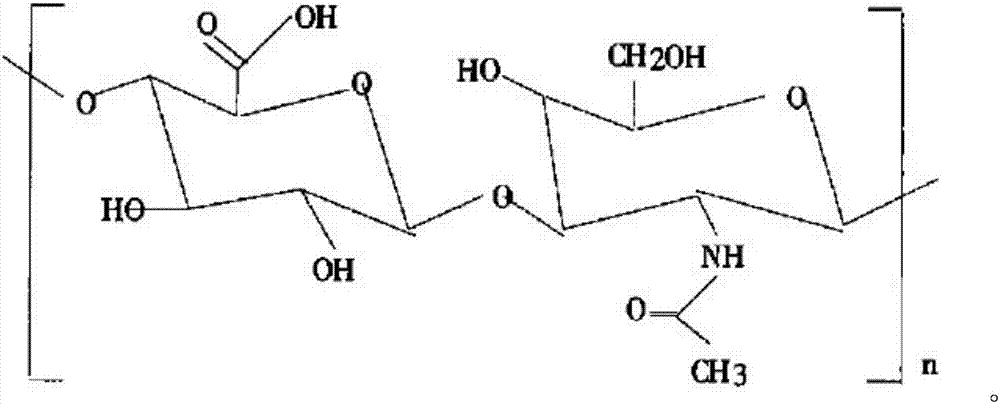
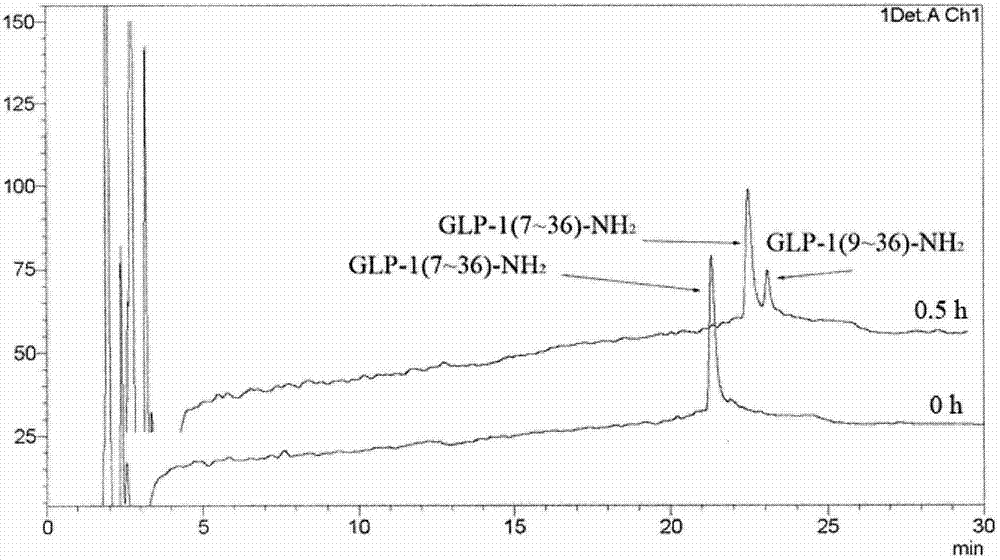
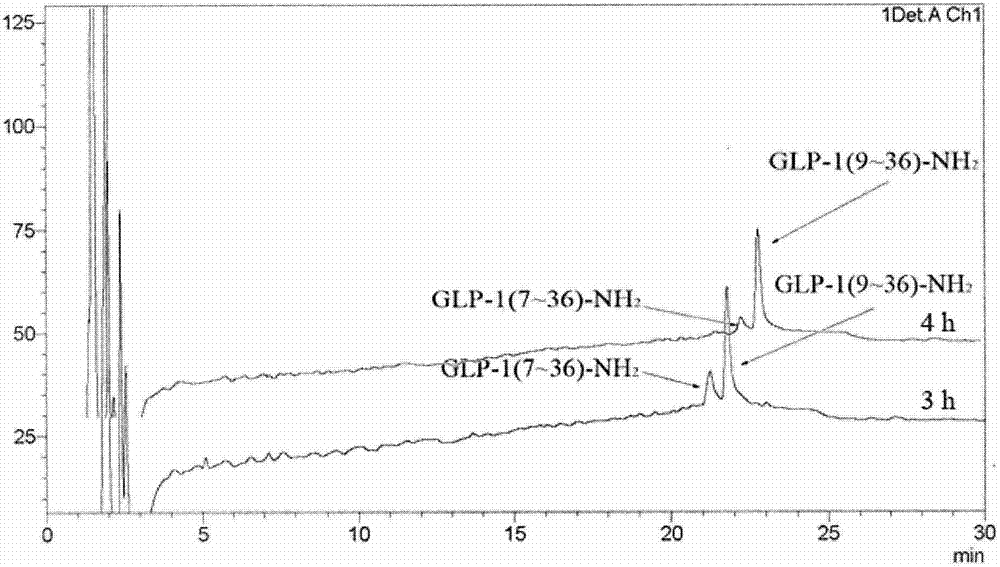
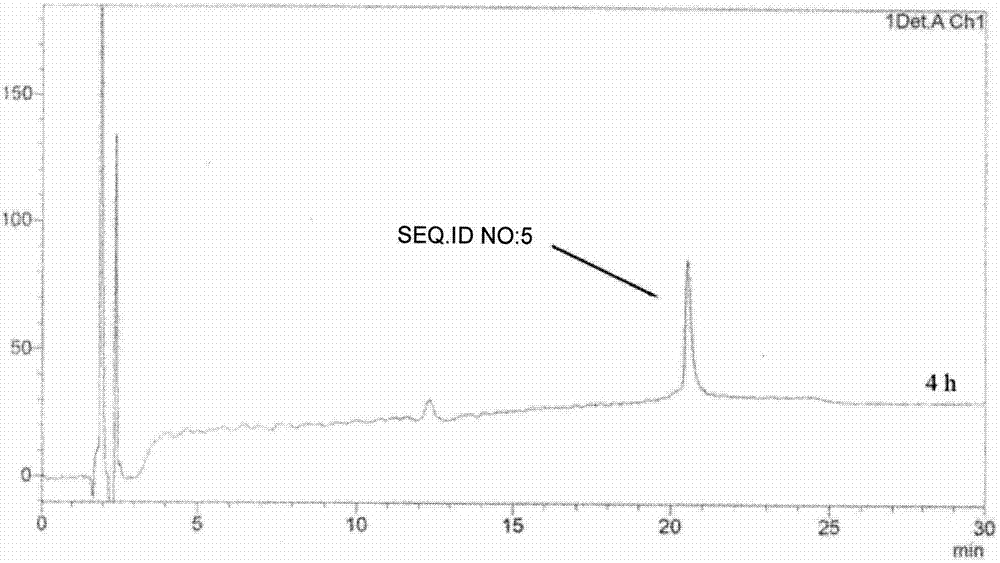
Patents
Literature
64results about How to "Prolong biological half-life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
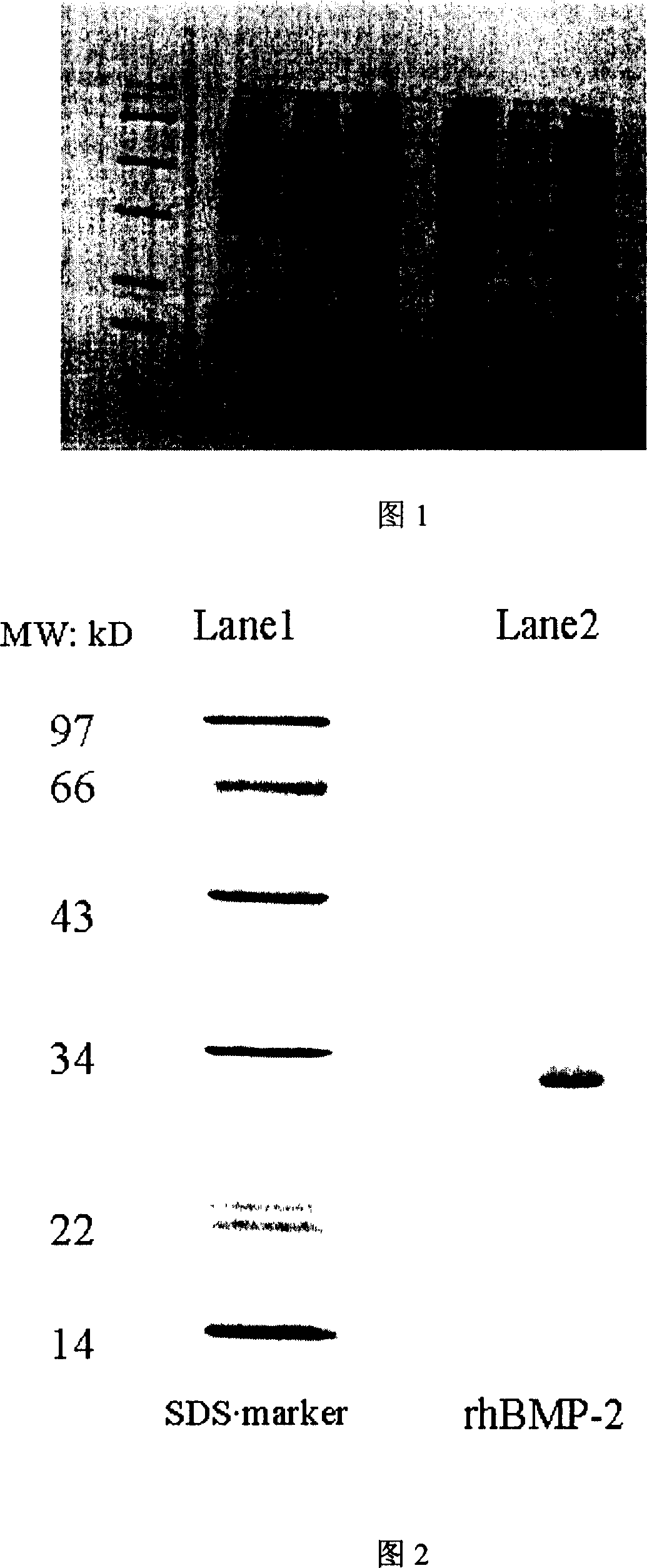
Long chain recombinant human bone morphogenesis protein-2 and its preparation method and uses
ActiveCN1951964AIncreased affinity binding sitesEasy to useBacteriaBone-inducing factorEscherichia coliNucleotide
The invention discloses a long-chain recombination human bone pattern generating protein-2 and preparing method and application, which is characterized by the following: utilizing gene project technique to grow the protein in the expressing system of escherichia coli and bacillus subtilis; adopting human bone sarcoma cell mRNA to do reverse transcription to obtain the cDNA as form; augmenting nucleotide sequence of entire 114 amino acids naturally from carboxyl end; adding a segment of nucleotide in front of the first codon of primer 5' end; increasing a segment of polypeptide at N end corresponding to amino acid sequence; obtaining long-chain rhBMP-2 gene with molecular weight at 30KD and purity over 95%.
Owner:SHANGHAI REBONE BIOMATERIALS
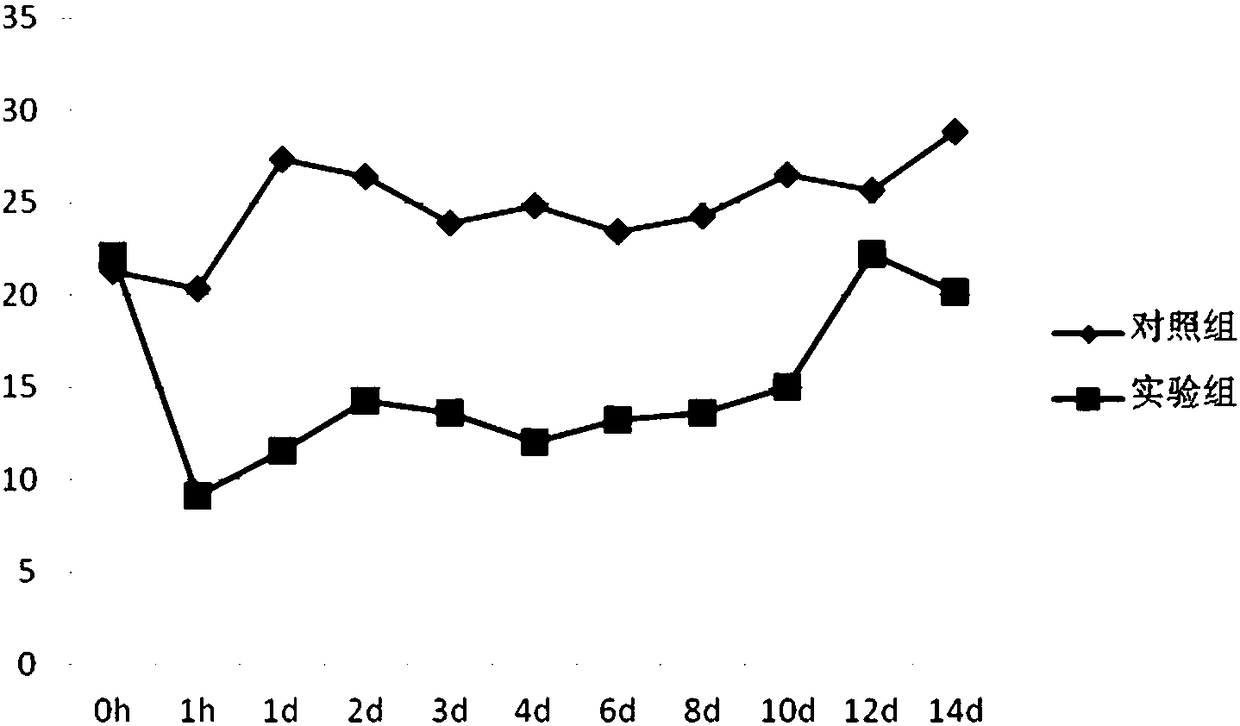
Long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087178AChemically stableEasy to degradePeptide/protein ingredientsMetabolism disorderMicrowaveSynthesis methods
The invention relates to long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through adding a modified 37th amino acid to natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Polyethylene glycol modified L-Asparaginasum and modification method thereof
InactiveCN101586099AStrong ligation reactionHigh reaction productHydrolasesFiltrationPolyethylene glycol
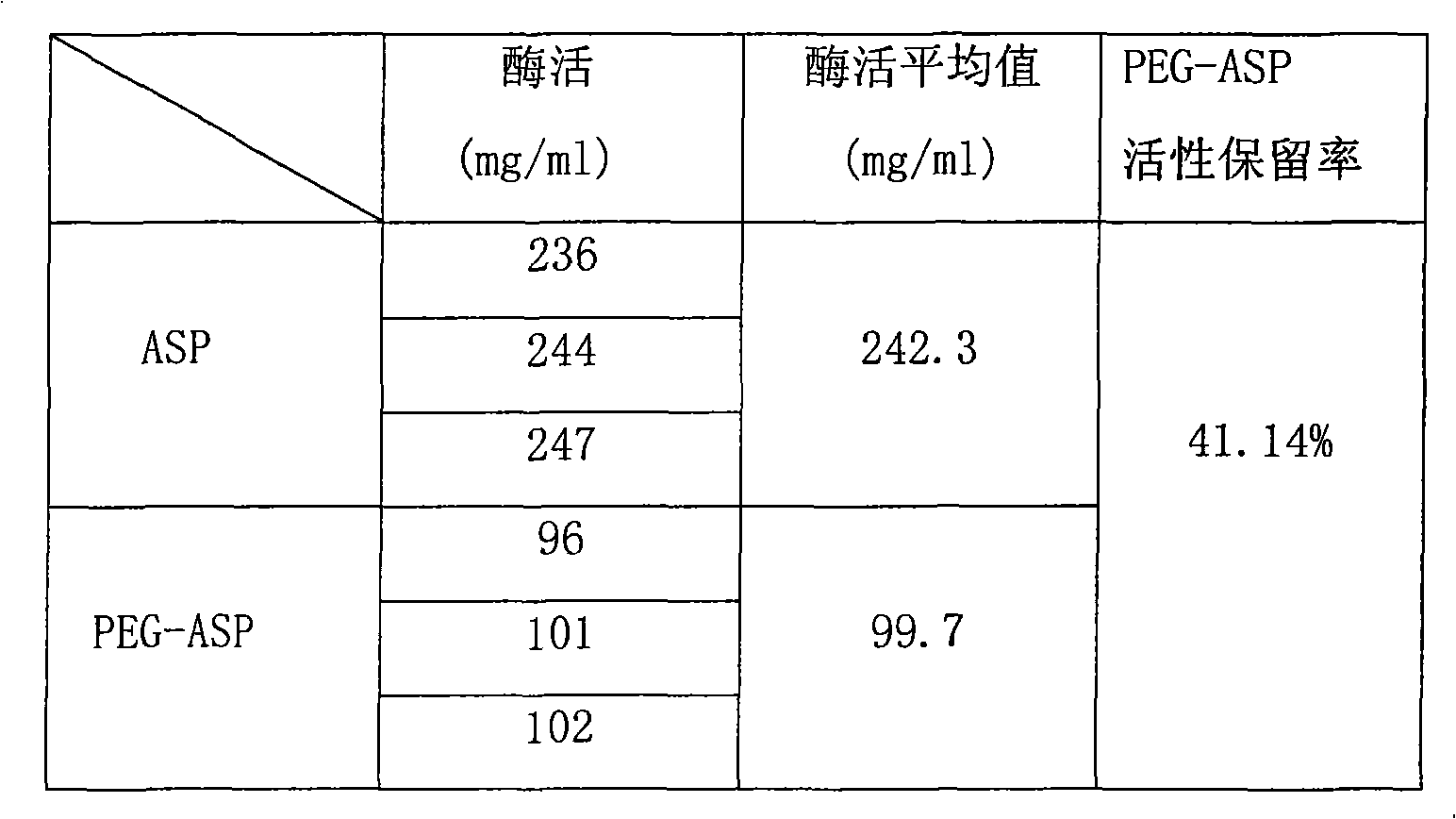
The invention provides polyethylene glycol modified L-Asparaginasum shown as formula (1) and a modification method thereof. The method uses the modification reaction property of polyethylene glycol molecules to modify the L-Asparaginasum under the condition of proper pH value of a reaction system. The polyethylene glycol modified L-Asparaginasum is characterized in that two polyethylene glycol molecules are covalently bound in a reaction product; and the substance has lower glomerular filtration rate and lower immunogenicity compared with a single polyethylene glycol molecule modifier so as to provide better antitumor effect.
Owner:BEIJING SL PHARMA
Method for preparing hydrophobic medicament nuclear shell granule-type solid dispersion by static electricity spraying method
InactiveCN103099785AAvoid degradationInhibitory activityPowder deliveryPharmaceutical product form changeOrganic solventPropeller
The invention discloses a method for preparing a hydrophobic medicament nuclear shell structure micro / nano granule-type solid dispersion by a high-voltage static electricity spraying method, relating to the pharmaceutics and biomedical field. The method comprises the following steps of: dissolving hydrophobic medicaments and fat soluble high polymer materials together into an organic solvent, and emulsifying and dispersing the mixture into an aqueous solution containing water soluble high polymer material; then placing oil-in-water emulsion into a trace propeller, and carrying out electrostatic spraying under the effect of high-voltage electrostatic field, thereby obtaining the hydrophobic medicament nuclear shell structure micro / nano granule-type solid dispersion finally. According to the preparation method, the operation is simple, the cost is low, environmental protection is realized and industrial production is facilitated. The particle size of the prepared hydrophobic medicament nuclear shell structure micro / nano granule-type solid dispersion is controllable, and the solid dispersion has tissue and organ targeting; and the granules of the nuclear shell structure is beneficial to prolonging the medicament action time.
Owner:杨晔
Glucagon-like peptide 1 (GLP-1) analogues with long-acting effect and application thereof
InactiveCN103087180AChemically stableProlonged plasma half-lifePeptide/protein ingredientsMetabolism disorderLong actingPharmacological action
The invention relates to glucagon-like peptide 1 (GLP-1) analogues with a long-acting effect and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through replacing / modifying 17th, 26th, 34th and 37th amino acids of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
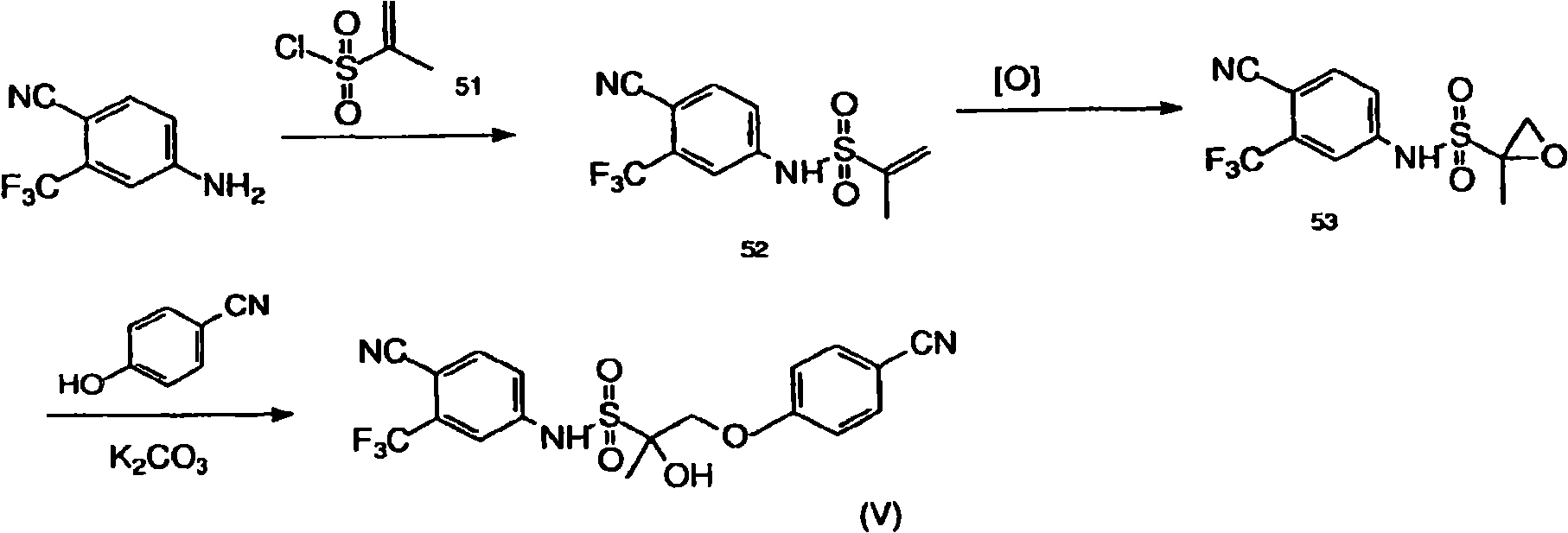
Selective androgen receptor modulators, analogs and derivatives thereof and uses thereof
InactiveCN101516835AImprove drug deliveryReduce manufacturing costOrganic chemistrySelective androgen receptor modulatorSelective receptor modulator
Owner:THE OHIO STATE UNIV RES FOUND +1
Total bufadienolide solid lipid nanometer particle freeze-dried injection and preparation method thereof
ActiveCN104706622AImprove targetingSmall toxicityAmphibian material medical ingredientsPowder deliverySide effectFreeze-drying
The invention discloses total bufadienolide solid lipid nanometer particle freeze-dried injection and a preparation method thereof and belongs to field of the solid lipid nanometer particle freeze-dried injection and the preparation method thereof. The invention firstly discloses a total bufadienolide solid lipid nanometer particle which comprises the following components: total bufadienolide, solid lipid, a lipid-soluble emulsifying agent, a water-soluble emulsifying agent and water for injection. The total bufadienolide solid lipid nanometer particle is added with a freeze-drying protective agent and then freeze-dried to obtain the total bufadienolide solid lipid nanometer particle freeze-dried injection. After the total bufadienolide solid lipid nanometer particle freeze-dried injection disclosed by the invention is redissolved, the measured particle size of the total bufadienolide solid lipid nanometer particle is 190nm to 250nm, the polydispersity index is 0.18 to 0.3, the encapsulation efficiency is 30% to 80%, the tumor cell targeting property of the total bufadienolide can be effectively improved, and toxic and side effects are alleviated.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Selective androgen receptor modulators and methods of use thereof
InactiveCN1471508AHas oral bioavailabilityProlong biological half-lifeOrganic active ingredientsBiocideHormone Receptor ModulatorsSpermatogenesis
This invention provides a novel class of androgen receptor targeting agents (ARTA). The agents define a new subclass of compounds which are tissue-selective androgen receptor modulators (SARM), which are useful for oral testosterone replacement therapy, male contraception, maintaining sexual desire in women, treating prostate cancer and imaging prostate cancer. These agents have an unexpected in-vivo activity for an androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. These agents may be active alone or in combination with progestins or estrogens. The invention further provides a novel class of nonsteroidal agonist compounds and methods of binding an androgen receptor, modulating spermatogenesis, treating and imaging prostate cancer, and providing hormonal therapy for androgen-dependent conditions.
Owner:UNIV OF TENNESSEE RES FOUND
Novel long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087175AChemically stableProlonged plasma half-lifePeptide/protein ingredientsMetabolism disorderLong actingPharmacological action
The invention relates to novel long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through replacing / modifying 17th, 26th, 34th and 37th amino acids of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to the GLP-1 analogues.
Owner:CHINA PHARM UNIV
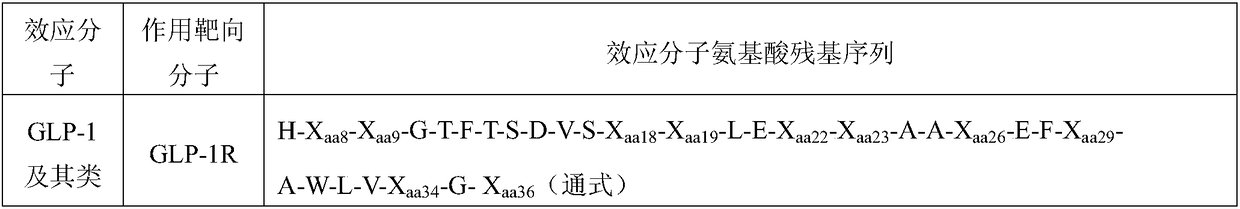
IgG-like long-acting immunological fusion protein and applications thereof
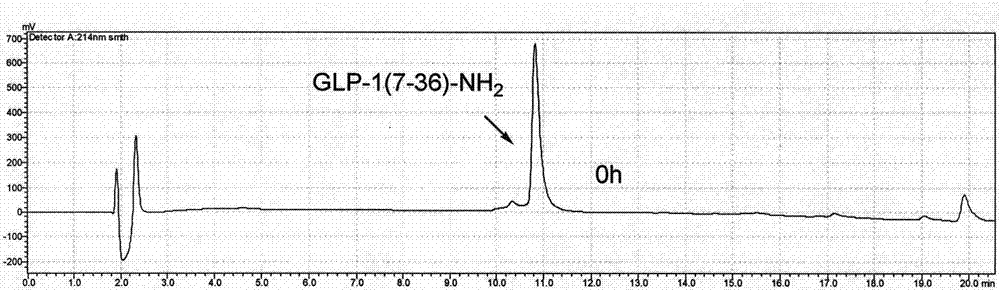
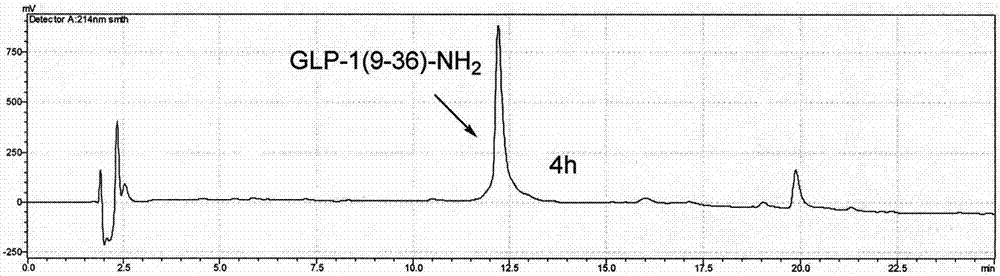
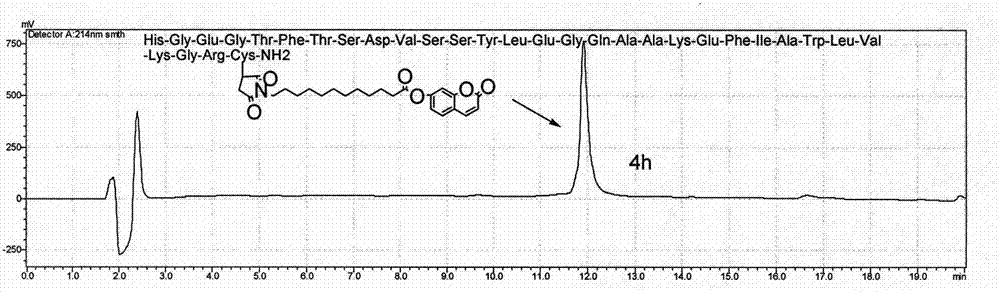
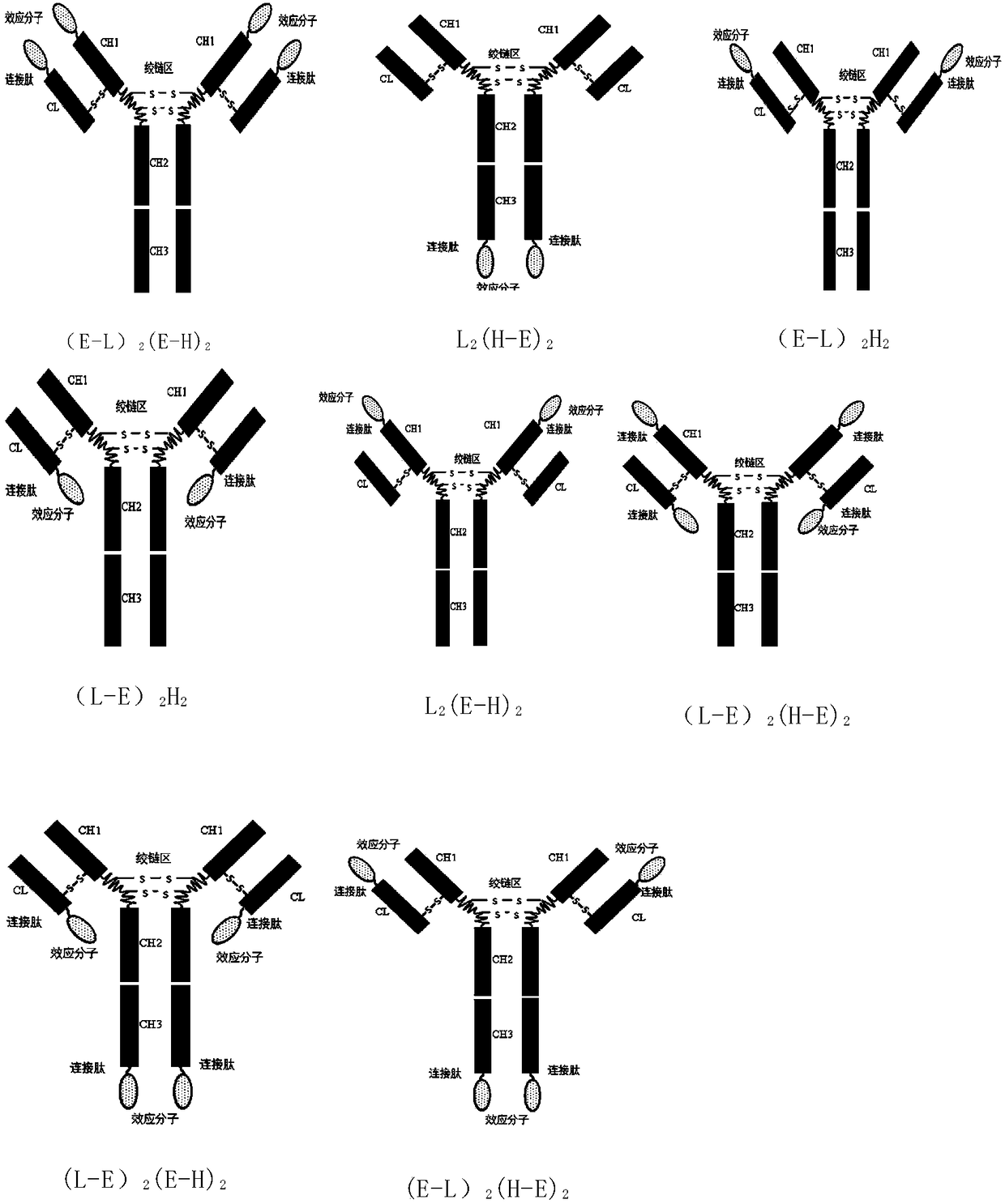
ActiveCN108623691AProlong biological half-lifeHigh affinityPeptide/protein ingredientsMetabolism disorderDiseaseAutoimmune disease
The invention discloses an IgG-like long-acting immunological fusion protein and applications thereof. The IgG-like long-acting immunological fusion protein comprises an effector molecule and an IgG antibody constant region, wherein the effector molecule is linked to the IgG antibody constant region through a linker peptide, the effector molecule is a protein capable of exerting physiological functions in vivo, and the IgG antibody constant region is a structure obtained by removing two heavy chain variable regions and two light chain variable regions from an IgG antibody. According to the present invention, the IgG-like immunological fusion protein can effectively prolong the biological half-life of the protein drug (effector molecule) under the premise of the ensuring of the high affinity to the targeting molecule and the good in vivo activity, is far better than the similar Fc immunological fusion protein, and can be used for the treatment of diabetes, tumors, autoimmune diseases, endocrine and various diseases.
Owner:BEIJING BIYANG BIOTECH
Polyglycols modified disinfection/neutralization endotoxin polypeptide, preparation and uses thereof
InactiveCN101402676AProlong biological half-lifeRetain the ability to bindPeptide/protein ingredientsPeptide preparation methodsMonomethoxypolyethylene glycolHalf-life
The invention discloses a sterilization / endotoxin neutralization polypeptide modified by polyethylene glycol, which consists of a formula as follows: X-Y-BNEP-OH or H-BNEP(i-Y-X)-OH, wherein, X is polyethylene glycol or single-methoxy polyethylene glycol, Y is a linking group, i is 1 or 3 or 13 or 14, representing the position number of lysine with a modified on side-chain amino group in an amino acid sequence; and BNEP is the sterilization / endotoxin neutralization polypeptide which has an amino acid sequence shown by SEQ ID No.1; the modification polypeptide, while maintaining the endotoxin neutralization activity of BNEP, prolongs the half-life in vivo of BNEP, and shows potential for being developed into new endotoxin neutralization medicaments, thus being capable of providing new curative medicaments for treating patients of severe diseases such as pyaemia, septic shock and the like. The invention also discloses a preparation method of the modification polypeptide, which has high product yield and high purity, thus being capable of realizing fixed-point modification of polyethylene glycol of synthetic polypeptide.
Owner:SOUTHWEST UNIV
Aqueous solution of recombinant human serum albumin-interferon alpha fusion protein and preparation method thereof
InactiveCN101954067AReduced clearance rateProlong biological half-lifeOrganic active ingredientsPeptide/protein ingredientsArginineInterferon alpha
The invention relates to stable aqueous solution of a recombinant human serum albumin-interferon alpha fusion protein and belongs to the technical field of biological preparations. The aqueous solution of the recombinant human serum albumin-interferon alpha fusion protein is prepared by dissolving the recombinant human serum albumin-interferon alpha fusion protein and pharmaceutically acceptable stable auxiliary material in pharmaceutically acceptable buffer solution, and is characterized in that the pharmaceutically acceptable stable auxiliary material is arginine at a concentration of 5 to 30 g / L. The aqueous solution of therecombinant human serum albumin-interferon alpha fusion protein can prevent the aggregation, degradation, oxidation, denaturation and the like of the recombinant fusion protein effectively so as to retain bioactivity and is suitable to be used in clinic.
Owner:QILU PHARMA
Method for producing iodine radioisotopes fraction, in particular of I-131, iodine radioisotopes fraction, in particular of I-131
PendingCN109416952AImprove selectivityImprove environmental safetySpecific isotope recoveryEnriched uraniumAluminium
A method for producing an iodine radioisotopes fraction, comprising the steps of dissolving enriched uranium targets forming a slurry, filtering the slurry, absorbing salts of iodine radioisotopes onan aluminium resin doped with silver and recovering the iodine radioisotopes fraction. The recovery of the iodine radioisotopes fraction, in particular of I-131, comprises washing the aluminium resindoped in silver using a solution of NaOH and eluting of iodine radioisotopes by a solution of thiourea, and collecting an eluate containing the iodine radioisotopes in a theourea solution.
Owner:国家放射性元素研究所
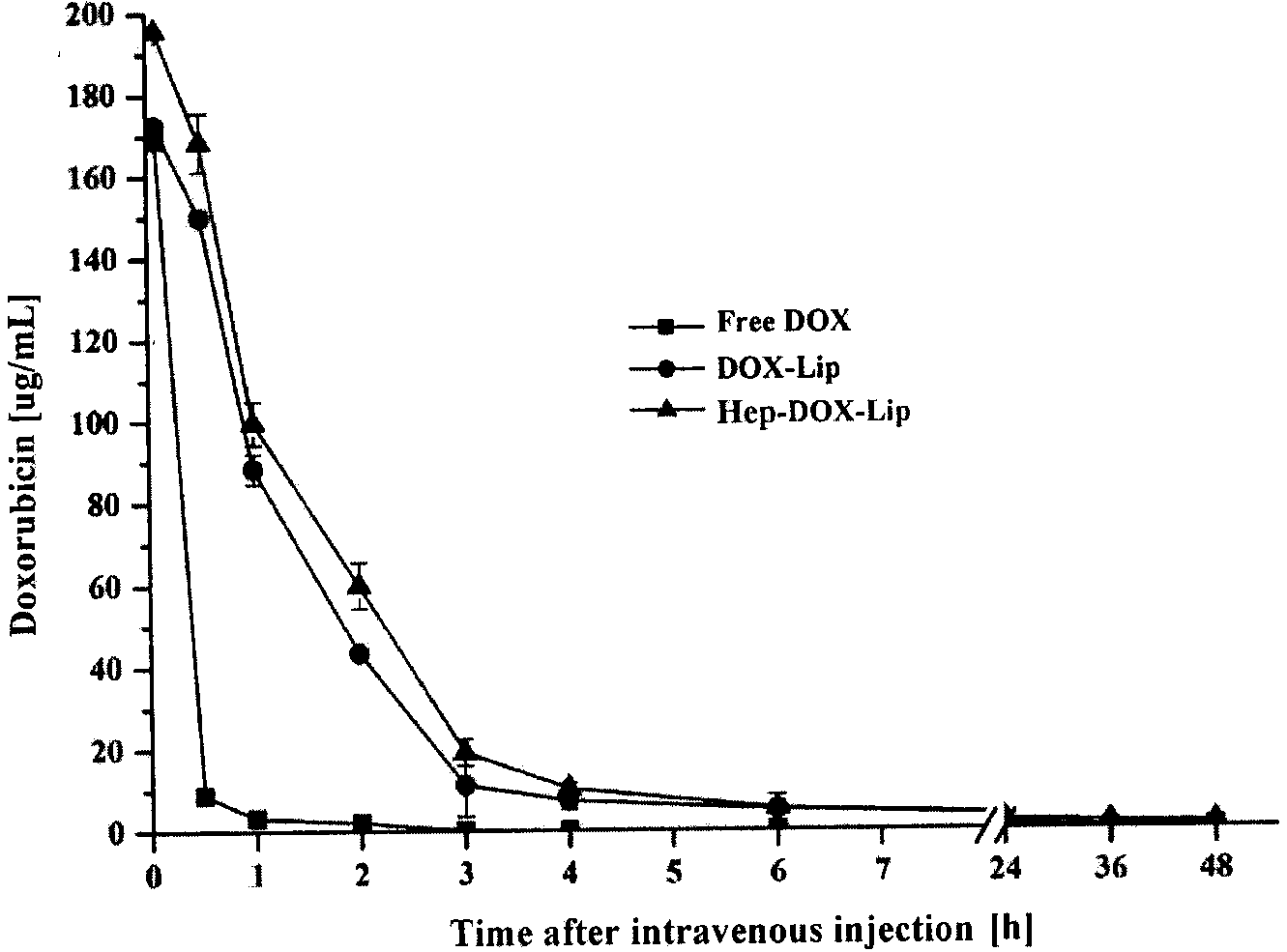
Heparin-modified adriamycin liposome preparation and preparation method thereof
InactiveCN103720658AImprove stabilityProlong half-life in vivoOrganic active ingredientsPowder deliveryDrugHalf-life
The invention relates to the field of medicinal preparations, and in particular relates to a heparin-modified adriamycin liposome (Hep-DOX-Lip) preparation. The preparation is characterized by consisting of 1 part of adriamycin, 1-4 parts of heparin, 5-30 parts of soybean lecithin, 0.5-4 parts of cholesterol and 0.5 part of a cationic material. The invention further discloses a preparation method of the heparin-modified adriamycin liposome preparation. The heparin-modified adriamycin liposome preparation has an effect similar to pegylation, and can remarkably enhance the stability of an adriamycin liposome, prolong the in-vivo half-life period of a medicament, and enhance the bioavailability of the medicament. Meanwhile, the heparin-modified adriamycin liposome preparation can remarkably lower the toxic and side effects of chemotherapeutic drugs and enhance the compliance of patients.
Owner:CHINA PHARM UNIV
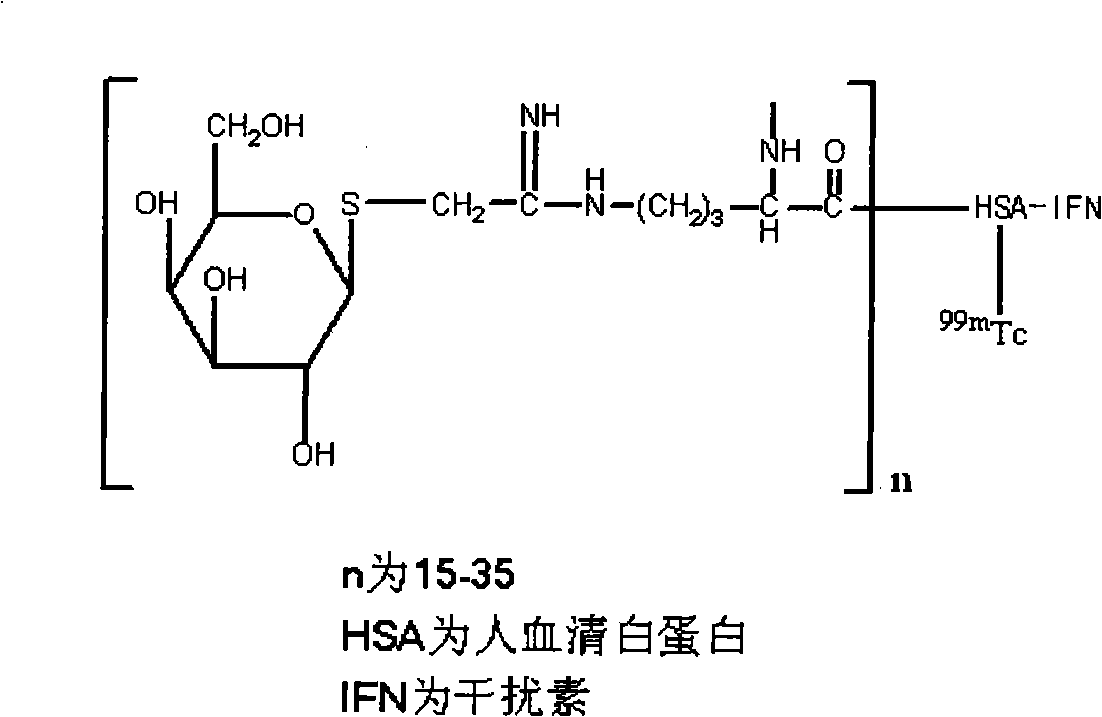
Preparation of <99m>Tc galactosyl human serum albumin fusion interferon of liver receptor developer and uses thereof
InactiveCN101333259AClear imagingMeet scientific researchIn-vivo radioactive preparationsPeptide/protein ingredientsDiseaseImaging agent
Disclosed are a preparation method for hepatic receptor imaging agent 99mTc-galactosyl-human serum albumin fusion interferon and the application of the albumin fusion interferon, belonging to the nuclear medicine technical field. The invention adopts a stannous reduction method for the radioactive technetium tagging of the hepatic receptor imaging agent 99mTc-galactosyl-human serum albumin fusion interferon GHSA-IFN; upon polyamide-66 thin layer chromatography analysis, the radiochemical purity of the prepared 99mTc-GHSA-IFN is more than 90% and can be stabilized for more than 6 hours at room temperature, in line with the requirements of scientific research and clinical use. The distribution in mice and the imaging results of rats show that the 99mTc-GHSA-IFN can be incepted by liver in a specific way, and the liver imaging is very clear within 10-30 minutes which is the best time for liver imaging. Therefore, the 99mTc-GHSA-IFN is a good new liver receptor function SPECT imaging agent and can be used to evaluate the liver receptor functions, diagnose liver diseases and evaluate the liver disease treatment drug efficacy; at the same time, the 99mTc-GHSA-IFN is also used to prepare liver targeted therapy drugs and the treatment drugs for viral hepatitis.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
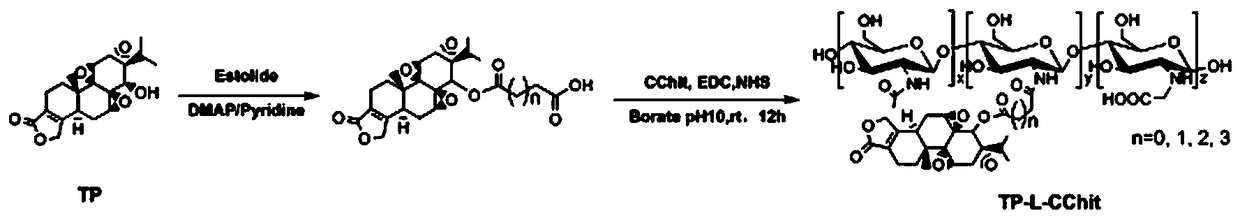
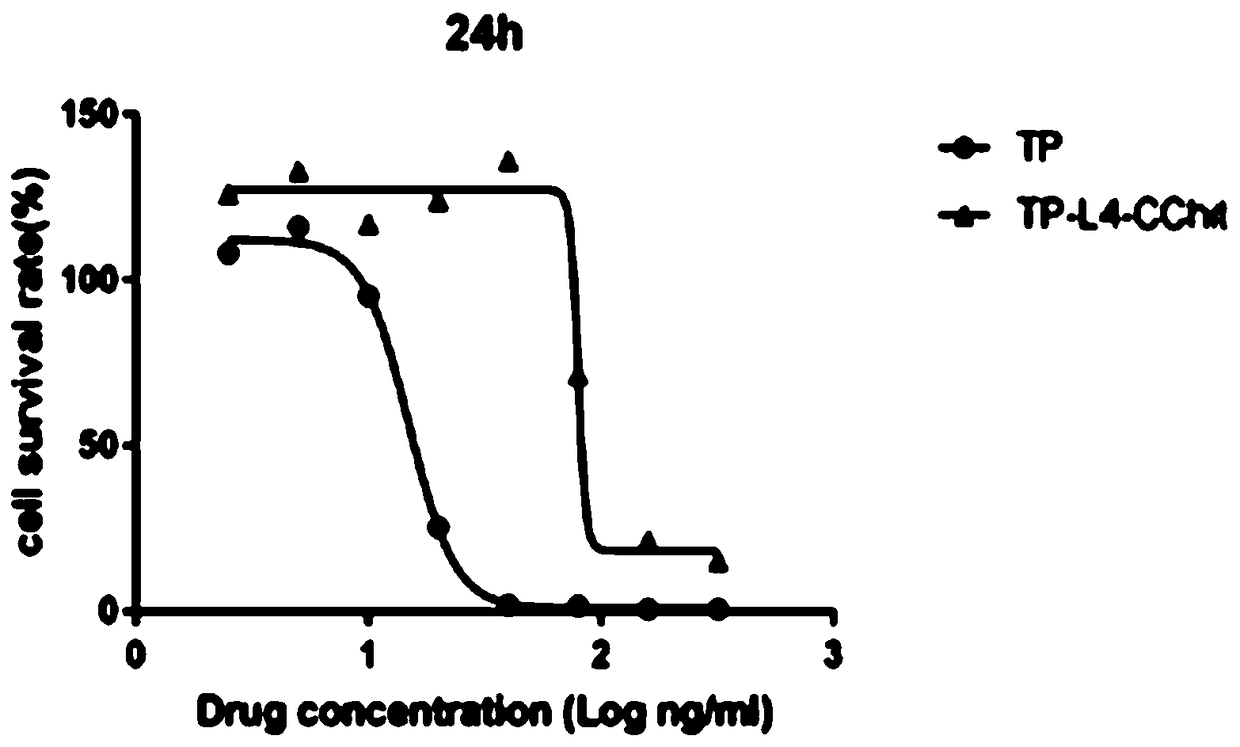
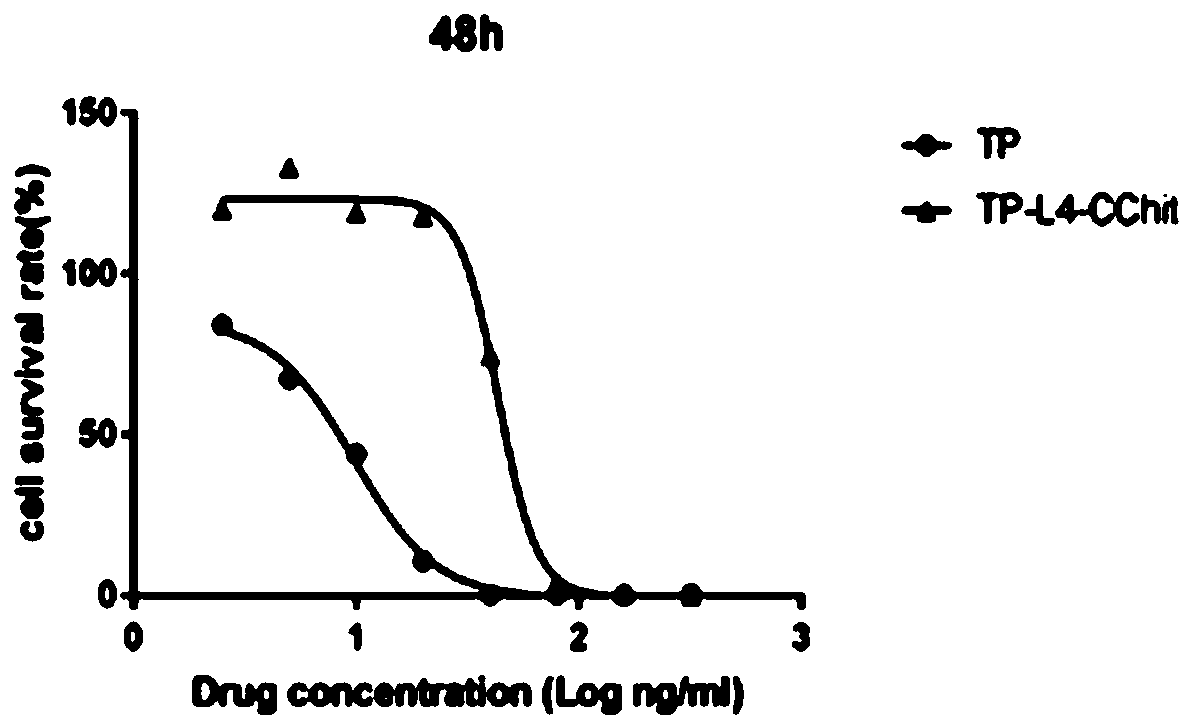
Preparation method and application of triptolide-carboxylation chitosan coupling drug
ActiveCN109464675ASimple methodEasy to produceOrganic active ingredientsNervous disorderSolubilityDisease
The invention discloses a preparation method and application of a triptolide-carboxylation chitosan coupling drug. The preparation method comprises the following steps: (1) synthesis of intermediate carboxylation triptolide (TP-L); and (2) synthesis of the triptolide-carboxylation chitosan coupling drug (TP-L-CChit). The triptolide-carboxylation chitosan coupling drug (TP-L-CChit) prepared by themethod, as a pro-drug, has application for preparing drugs for treating rheumatoid arthritis, tumors and Alzheimer's disease; the drug is simple in method, easy for production preparation, high in yield and rich and easily obtained in raw materials, the product is high in water solubility, long in biological half life period, high in availability, free of toxic or side effect and good in curativeeffect and is innovation for preparing drugs for treating rheumatoid arthritis, tumors and Alzheimer's disease, and the economic and social benefits are obvious.
Owner:HENAN UNIV OF CHINESE MEDICINE
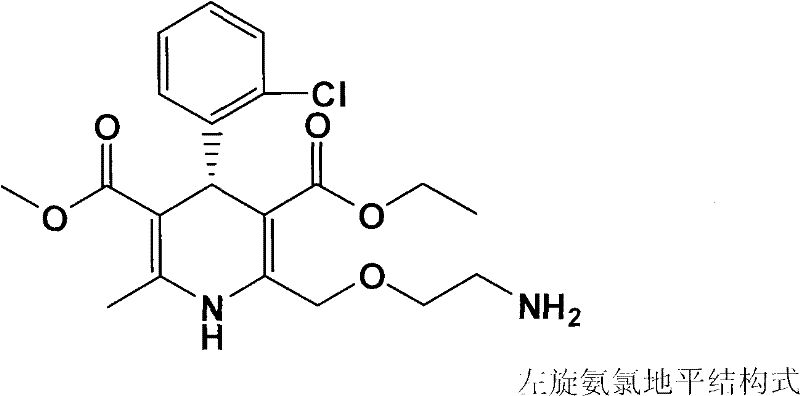
Compound medicinal composition for reducing blood pressure, and compound tablet for reducing blood pressure
ActiveCN102327263AProlong biological half-lifeReduce the frequency of takingOrganic active ingredientsPill deliveryHypotensive actionCompounded preparations
The invention discloses a compound medicinal composition for reducing blood pressure and a compound tablet for reducing blood pressure. The compound medicinal composition for reducing blood pressure comprises levamlodipine or pharmaceutically acceptable salt thereof and indapamide, wherein the weight ratio of indapamide to levamlodipine is 1:(1.5-12). The novel compound preparation for reducing blood pressure improves the effect of reducing blood pressure by using synergism among medicines, has a stable effect of reducing blood pressure and excellent cost performance, reduces a side effect, and can be widely popularized and applied to crowds. The compound tablet for reducing blood pressure has high stability and can effectively prolong a validity period.
Owner:KANGYA OF NINGXIA PHARMA +1
Process for preparing nanometer particle of adriacin-PACA-PBCA
InactiveCN1732962ALow toxicityReduce dosagePowder deliveryOrganic active ingredientsPolymer scienceFiltration
The invention provides a process for preparing nanometer particle of adriacin-PACA-PBCA, which comprises, reacting HCl solution with doxorubicin hydrochloride powder, stirring to dissolve, charging Dextran70 and charging alpha-butylcyanoacrylate, stirring and adjusting pH to be 7, passing through 0.45um filtration film and drying.
Owner:张阳德
Total bufogenin nano cubic liquid crystal and preparation method thereof
InactiveCN105106118AImprove targetingSmall toxicityAmphibian material medical ingredientsPowder deliveryCrystallographySolubility
The invention discloses a total bufogenin nano cubic liquid crystal and a preparation method thereof. The total bufogenin nano cubic liquid crystal is composed of a lipid material, an emulsifying agent and purified water. The total bufogenin nano cubic liquid crystal provided by the invention is characterized in that amphipathic lipid with certain concentration spontaneously forms double layers of the lipid in water, and the double layers are assembled into a cubic liquid crystal form with thermodynamic stability. The total bufogenin nano cubic liquid crystal disclosed by the invention increases the medicine solubility, realizes the oral administration of lipid-soluble medicine total bufogenin, adjusts and controls the medicine release, improves the medicine bioavailability, effectively improves the tumor cell targeting property of total bufogenin, and reduces toxic or side effects.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
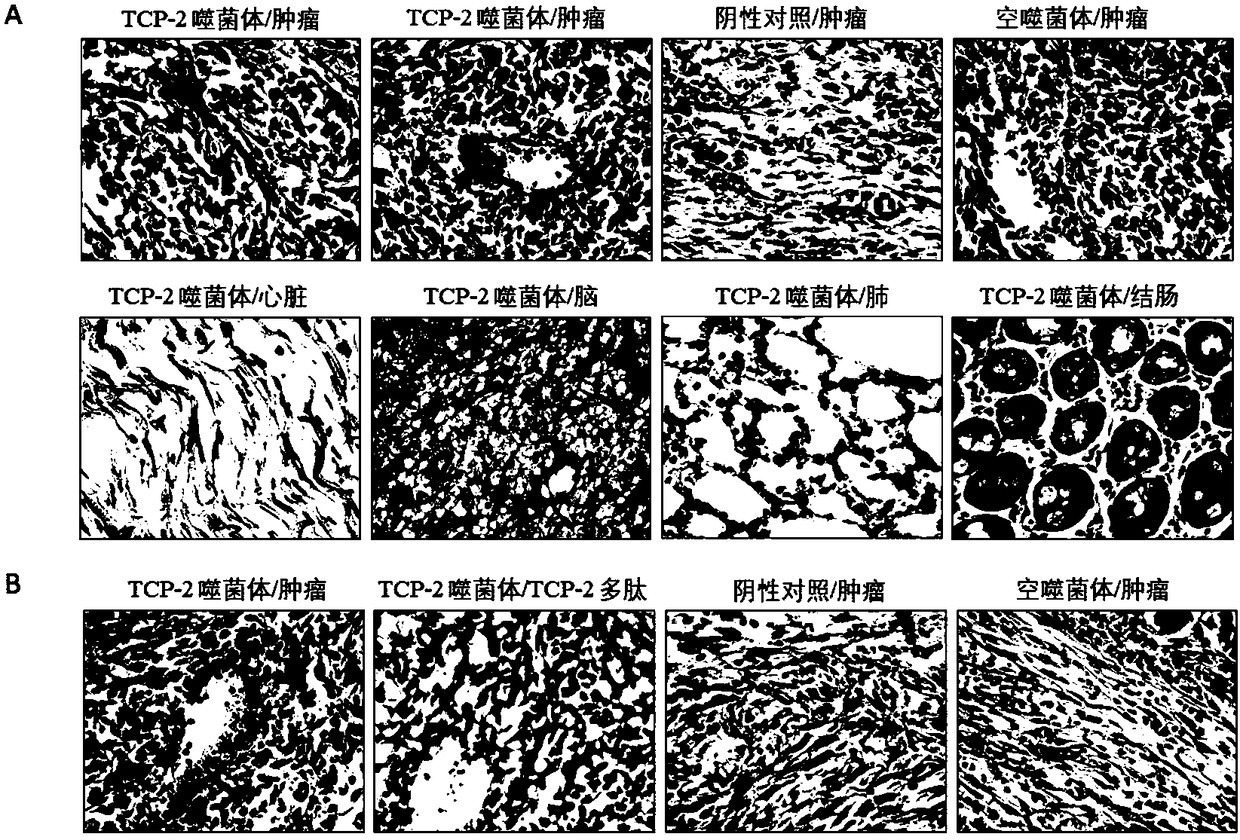
Tumor blood vessel and M1 type macrophage targeting peptide and application thereof
ActiveCN108178783AProlong biological half-lifeImprove pharmacological activityAntipyreticAnalgesicsMolecular targetingMacrophage targeting
The invention belongs to the technical field of molecular biology and in particular relates to a tumor blood vessel and M1 type macrophage targeting peptide and application thereof. Aiming at the problem that a small molecular targeting peptide for preparing a medicine for diagnosing or treating tumors is lacked, the invention provides the tumor blood vessel and M1 type macrophage targeting peptide TCP-2 and a carrier thereof. The small molecular targeting peptide has an amino acid sequence shown as SEQ ID NO: 1, can be used for specifically targeting tumor blood vessels and M1 type macrophages and can be used for preparing the targeting medicine for diagnosing or treating the tumors; especially, the mall molecular targeting peptide is used for treating digestive tract tumors and providesa new choice for targeting therapy of the tumors.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Method for preparing polyethylene glycol-modified alpha-interferon 1b
InactiveCN1654478AProlong biological half-lifeImprove bioavailabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsPolyethylene glycolImmunogenicity
The present invention relates to a sort of making method for the Alpha-interferon modified by the covalent bond of the activating polyethyleneglycol. By changing the acid-base scale of the reaction system it can achieve the purpose that modifies the histidine or lysine in the surface of the Alpha-interferon protein. During the course of reaction there is no need to separate the raw material of Alpha-interferon 1 b from the intermediate product, the novel compound which is modified by the polyethyleneglycol of Alpha-interferon 1 b can retain the biological activity of the former Alpha-interferon 1 b, but in pharmacology, immunogen character, medicine substituting character, medicine effect and the like aspects it gains an advantage over the former protein.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Novel peptide conjugate of African clawed frog GLP-1 (glucagon like peptide-1), as well as application thereof
ActiveCN106432471AProlong biological half-lifeExcellent hypoglycemic activityPeptide/protein ingredientsMetabolism disorderHalf-lifePharmacological action
The invention relates to a novel peptide conjugate of African clawed frog GLP-1 (glucagon like peptide-1), as well as a synthesis method and application thereof. A spiral promoting sequence is introduced to a terminal C of the African clawed frog GLP-1, and meanwhile, PEG (polyethylene glycol)-based modification is performed to obtain an analogue, with reserved hypoglycemic activity and longer pharmacological action time, of the African clawed frog GLP-1. The analogue of the African clawed frog GLP-1 has the advantages of high synthesis yield and low cost, the half-life of the analogue is remarkably prolonged, and the bioactivity is remarkably improved.
Owner:XUZHOU NORMAL UNIVERSITY
Long-acting African clawed frog glucagon-like peptide-1 (GLP-1) analogue and application thereof
PendingCN106699870AChemically stableLow immunogenicityPeptide/protein ingredientsMetabolism disorderHalf-lifeSynthesis methods
The invention relates to a long-acting African clawed frog glucagon-like peptide-1 (GLP-1) analogue as well as a synthesis method and application thereof. An African clawed frog glucagon-like peptide-1 is subjected to PEG (Polyethylene Glycol) modification to obtain the African clawed frog GLP-1 analogue which can keep blood glucose lowering activity and has longer pharmacological action time. The African clawed frog GLP-1 analogue provided by the invention is high in synthesis yield and low in cost; and the half-life period of the analogue is remarkably prolonged and the biological activity is remarkably improved.
Owner:XUZHOU NORMAL UNIVERSITY
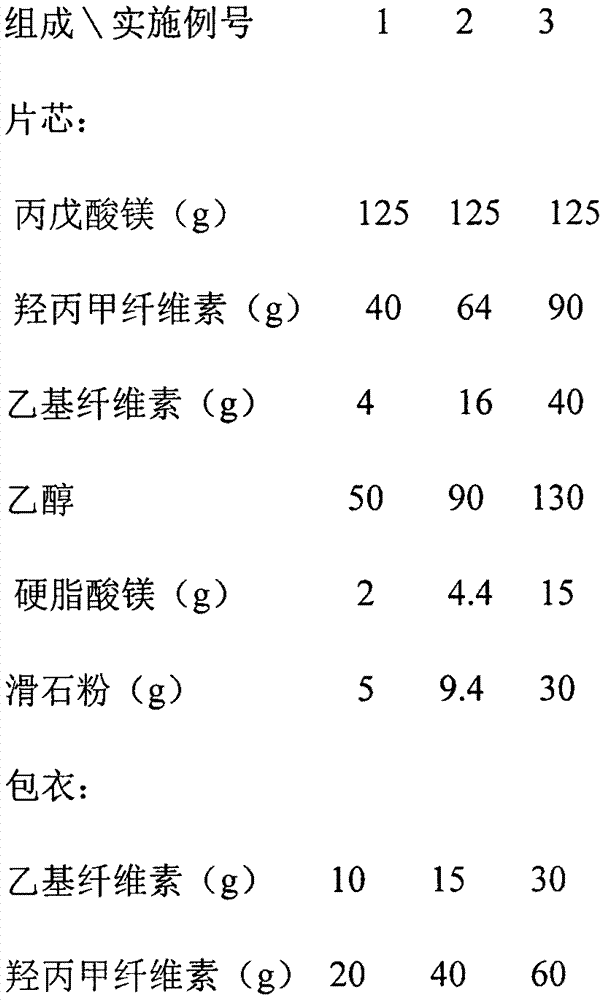
Magnesium valproate sustained release tablet and preparation process thereof
InactiveCN107875133AImprove complianceReduce the number of dosesNervous disorderPharmaceutical non-active ingredientsSustained Release TabletAlcohol
The invention relates to a magnesium valproate sustained release tablet and a preparation process thereof. The magnesium valproate sustained release tablet is prepared from the following two parts: A,a tablet core prepared from the following substances: 29 to 54 percent of magnesium valproate, 17 to 21 percent of hydroxypropyl methylcellulose, 2 to 9 percent of ethylcellulose, 22 to 30 percent ofethyl alcohol, 0.8 to 4 percent of magnesium stearate, and 3 to 7 percent of talcum powder; B, a sustained release tablet coating formula prepared from the following substances: 0.7 to 2 percent of ethylcellulose, 0.7 to 1.3 percent of glycerinum, 1.3 to 4 percent of hydroxypropyl methylcellulose, and 0.3 to 1.3 percent of talcum powder. The preparation process comprises: A, a preparation processof the tablet core, comprising the steps of weighing raw materials according to tablet core formula amounts uniformly mixing, adding an appropriate amount of the ethyl alcohol, preparing a soft material, sieving and pelletizing, then baking and drying at the temperature of 55 DEG C to 65 DEG C, adding the magnesium stearate and the talcum powder, uniformly mixing, measuring the content, and pressing to form an oval tablet; B, a preparation process of the coating comprising the steps of weighing ingredients according to sustained release tablet coating formula amounts, dissolving through an appropriate amount of 50 to 100 percent ethyl alcohol, and then spraying a coating on the tablet core.
Owner:湖南省湘中制药有限公司
Protein or polypeptide composition and preparation method and use thereof
ActiveCN107362352AImprove biological activityProlong biological half-lifeCosmetic preparationsPeptide/protein ingredientsHyaluronic acidProtein C
The invention discloses a composition and a preparation method and a use thereof. The composition contains protein or polypeptide and oligomerization hyaluronic acid (oligomerization HA) or salt of the oligomerization hyaluronic acid; and when being in a liquid form, the protein or polypeptide is 0.1-100 [mu]g / ml, and the oligomerization hyaluronic acid or the salt of the oligomerization hyaluronic acid is 1-20%.
Owner:上海建华精细生物制品有限公司
Preparation method and application of engineering bionic exosome for delivering specific protein
PendingCN112458119AGood biocompatibilityStrong targeting abilityGenetically modified cellsNGF/TNF-superfamilyMacromolecular drugProtein protein
The invention discloses a preparation method and application of an engineering bionic exosome for delivering specific protein. The preparation method comprises the following steps: S1, packaging viruses by using engineering cells to prepare specific lentiviral particles; S2, infecting target cells by using the obtained lentivirus, and adding a resistant drug for screening, so as to prepare a stable cell line for expressing a specific gene with high purity; and S3, culturing the obtained stable cell line, extracting cell membranes of the stable cell line, preparing the stable cell line into membrane nano-vesicles, and finally performing centrifugal purification to obtain the engineering bionic exosome. The invention provides a synthetic method for preparing a large number of specific protein drug delivery systems, the method can effectively enhance the stability of protein biomacromolecule drugs in vivo, prolong the drug effect duration and realize effective delivery of the drugs, a newthought is provided for the administration route of the protein biomacromolecule drugs, the engineering bionic exosome can be used for immunotherapy for treating tumors, is good in safety and strongin action effect, and has very high application value and clinical transformation prospect.
Owner:NANCHANG UNIV
Long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087177AProlong biological half-lifeProlong hypoglycemic action timePeptide/protein ingredientsMetabolism disorderMicrowaveSynthesis methods
The invention relates to long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through modifying the 17th residue of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Recombinant human serum albumin-interferon alpha fusion protein water solution and preparation method thereof
ActiveCN103432569AReduced clearance rateProlong biological half-lifePeptide/protein ingredientsDigestive systemAdjuvantArginine
The invention relates to a stable recombinant human serum albumin-interferon alpha fusion protein water solution, belonging to the technical field of biological agents. The stable recombinant human serum albumin-interferon alpha fusion protein water solution is prepared by dissolving a recombinant human serum albumin-interferon alpha fusion protein and a pharmaceutically acceptable stability adjuvant in a pharmaceutically acceptable buffer solution. The invention is characterized in that the pharmaceutically acceptable stability adjuvant is arginine, and the concentration is 5-30 g / L. The recombinant human serum albumin-interferon alpha fusion protein water solution provided by the invention can sufficiently prevent the recombinant fusion protein from aggregation, degradation, oxidation, denaturization or the like, thereby keeping the biological activity of the recombinant fusion protein; and the recombinant human serum albumin-interferon alpha fusion protein water solution is suitable for clinical application.
Owner:QILU PHARMA CO LTD
Method for preparing hydrophobic drug-carrying core-shell structure micro/nanoparticles through electrostatic spraying
InactiveCN103948568AAvoid degradationInhibitory activityOrganic active ingredientsPharmaceutical product form changeSolubilityPolymer science
The invention discloses a method for preparing hydrophobic drug-carrying core-shell structure micro / nanoparticles through electrostatic spraying, and relates to the field of pharmaceutics. The method comprises the following steps: jointly dissolving a hydrophobic drug and a lipid solubility high polymer material in an organic solvent, emulsifying and dissolving in an aqueous solution containing a water-soluble polymer material; and putting the obtained oil-in-water emulsion in a trace propeller, carrying out electrostatic spraying under the effect of a high-voltage electrostatic field, and thus finally obtaining the hydrophobic drug-carrying core-shell structure micro / nanoparticles. The preparation method is simple to operate, low in cost, environment-friendly and suitable for industrial production. The prepared hydrophobic drug-carrying core-shell structure micro / nanoparticles are controllable in particle sizes, have targeting property on tissues and organs and are beneficial to prolonging of action time of the drug.
Owner:杨晔
Application of covalent organic nanosheet material
ActiveCN112107594AQuick captureImprove stimulation rateOrganic active ingredientsNanomedicinePharmaceutical drugRadiation damage
The invention relates to application of a covalent organic nanosheet material to preparation of a medicine for preventing and / or treating radiation damage caused by radionuclide. The covalent organicnanosheet material comprises a plurality of amidoxime groups. The invention discloses the novel application of the covalent organic nanosheet material. The radionuclide is chelated and coordinated byutilizing the synergistic effect of multiple pieces of amidoxime, the covalent organic nanosheet material has very high selectivity on radionuclide, and the bottlenecks of fast metabolism, strong toxicity and poor excretion promoting effect of a conventional small molecular ligand are broken through.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com