Selective androgen receptor modulators, analogs and derivatives thereof and uses thereof
A compound, unsaturated technology, applied in organic chemistry and other directions, can solve problems such as non-ideal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0914] The preparation of pharmaceutical compositions containing the active ingredients is well understood in the art, for example by mixing, granulating or tabletting. The active therapeutic ingredient is often mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. For oral administration, the compounds according to the invention or their physiologically tolerated derivatives such as salts, esters, N-oxides etc. are mixed with additives customary for this purpose such as carriers, stabilizers or inert diluents and reacted in a conventional manner. Conversion into a form suitable for administration, such as tablets, coated tablets, hard or soft capsules, aqueous, alcoholic or oily solutions. For parenteral administration, the compounds of the present invention or their physiologically tolerated derivatives such as salts, esters, N-oxides, etc. are converted into solutions, suspensions or emulsions (if necessary, with conventional...
Embodiment 15
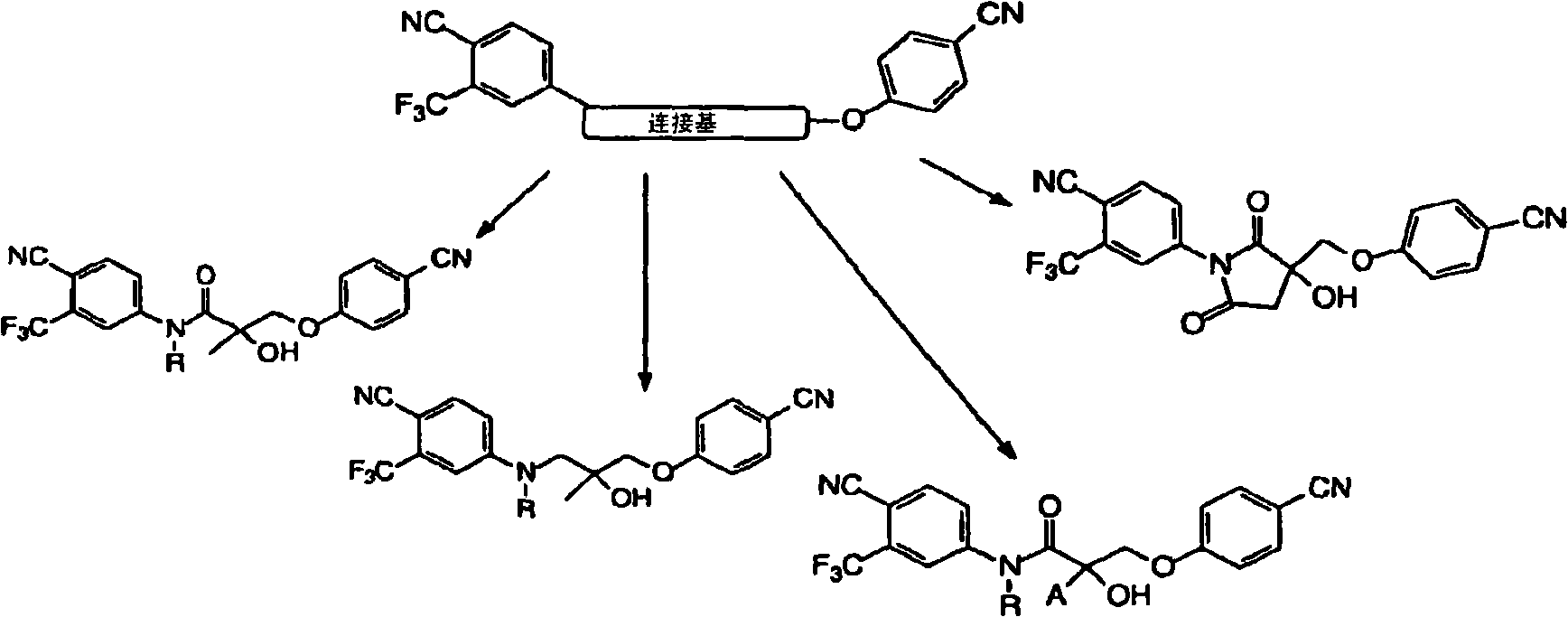
[1097] Example 15 shows that compounds of formula (I) are anabolic, but minimally androgenic, and that such compounds may therefore be useful in the treatment of patient populations for whom androgens were contraindicated in the past. Compounds of formula (I) have been shown to promote muscle growth irrespective of the presence or absence of testosterone while exerting an antiproliferative effect on the prostate, thus, in one embodiment, the methods of the invention are used to restore loss in individuals suffering from sarcopenia or cachexia of muscle mass.
[1098] In one embodiment, the compounds described herein alter leptin levels in a subject. In another embodiment, the compounds described herein lower the levels of leptin. In another embodiment, the compounds described herein increase leptin levels in a subject. Leptin is known to have an effect on appetite, weight loss in obese mice and thus has been associated with obesity.
[1099] In one embodiment, the compounds...
Embodiment 1
[1176] I
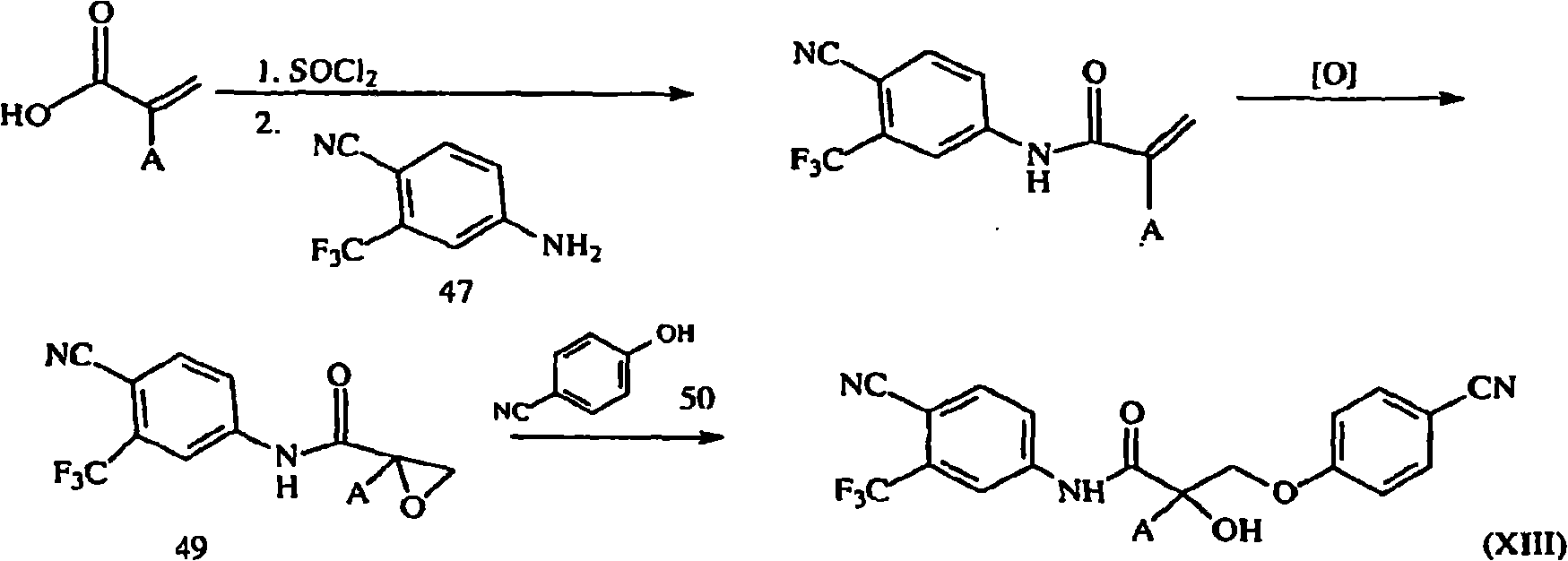
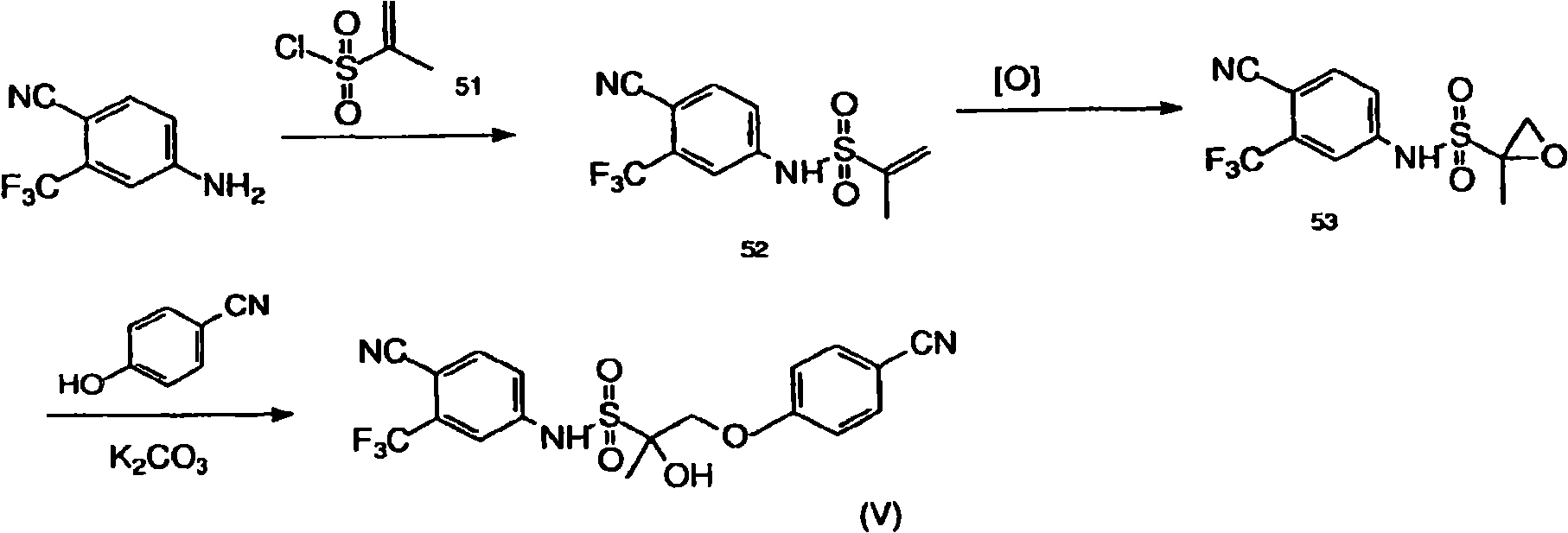
[1177] Synthesis of Compound XIII
[1178] 2-Substituted acrylic acid (compound 46) was reacted with 4-cyano-3-trifluoromethylaniline followed by epoxidation. Opening of the epoxide with p-CN-phenol in the presence of potassium carbonate yields compound XIII as Figure 1B shown in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com