Methods and Devices for Capturing Circulating Tumor Cells
a tumor cell and cell technology, applied in the field of circulating tumor cells, can solve the problems of increasing the mortality of cancer patients, increasing the risk of recurrence, and increasing the risk of metastasis, so as to improve the effect of active targeting, improve the effect of interacting surface area, and improve the flexibility of conformational deformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Multivalent Effect
[0103]The recent development of nanotechnology has demonstrated many breakthroughs in a range of biomedical applications—particularly for cancer treatment. For a new design of effective targeted drug delivery / imaging vectors based on nanotechnology, multivalent effects are desirable as they dramatically enhance active targeting efficacy. The similar enhancement can be also achieved in specific capturing when the delivery vectors are immobilized on the surfaces.
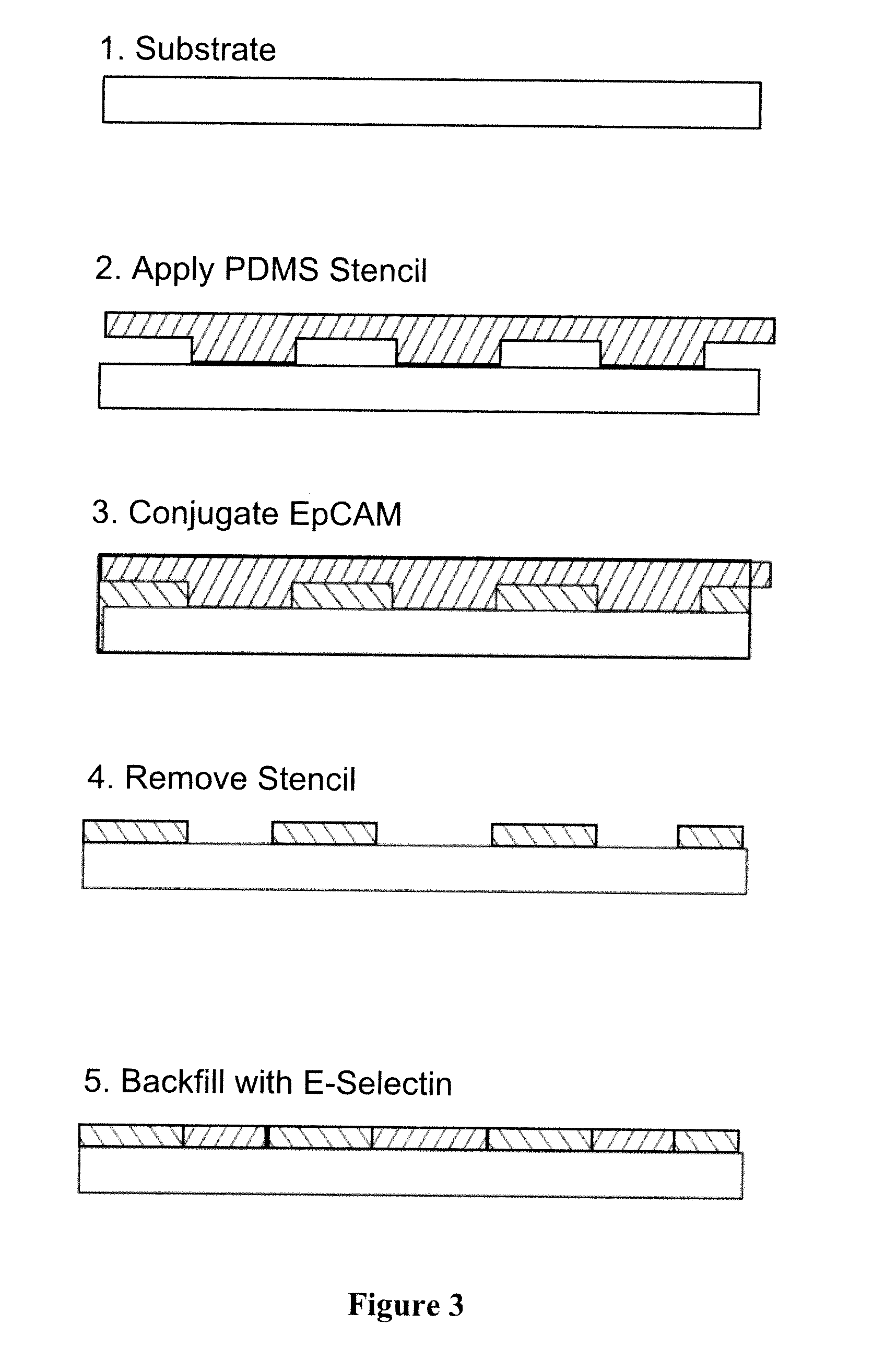
[0104]Preparation of PAMAM dendrimer-based nanodevice: the PAMAM dendrimer-based folate receptor (FAR) targeting nanodevices were synthesized as summarized in FIG. 3. Briefly, G5 PAMAM dendrimers were partially acetylated (70 of the 110 total primary amines), resulting in G5-Ac70. The remaining 40 primary amine groups were used for reaction to further functionalize the dendrimers. Note that a G5 PAMAM dendrimer molecule has approximately 110 primary amine termini according to the previous titration measuremen...
example 2
Controlled Immobilization of P-Selectin
[0110]Covalent immobilization of biologically active species has a number of advantages such as controlling the density, conformation, and enhanced stability of the species. Although covalent immobilization procedures for peptides and enzymes have been extensively studied for decades, covalent immobilization of large molecular weight biomolecules such as selectins present significant challenges due to the increase of binding to non-specific sites and due to the requirement for mild processing conditions to prevent protein inactivation. Given that preparation of devices proposed in this work requires a high level of control over the selectin presentation on surfaces, it is desirable to control density and conformation of selectin, and to introduce controlled co-immobilization capacity for secondary molecules that facilitates selective separation of target CTCs. We have developed covalent immobilization chemistries along with a set of appropriate...
example 3
Rolling Assays of a Tumor Cell Line as a CTC Model
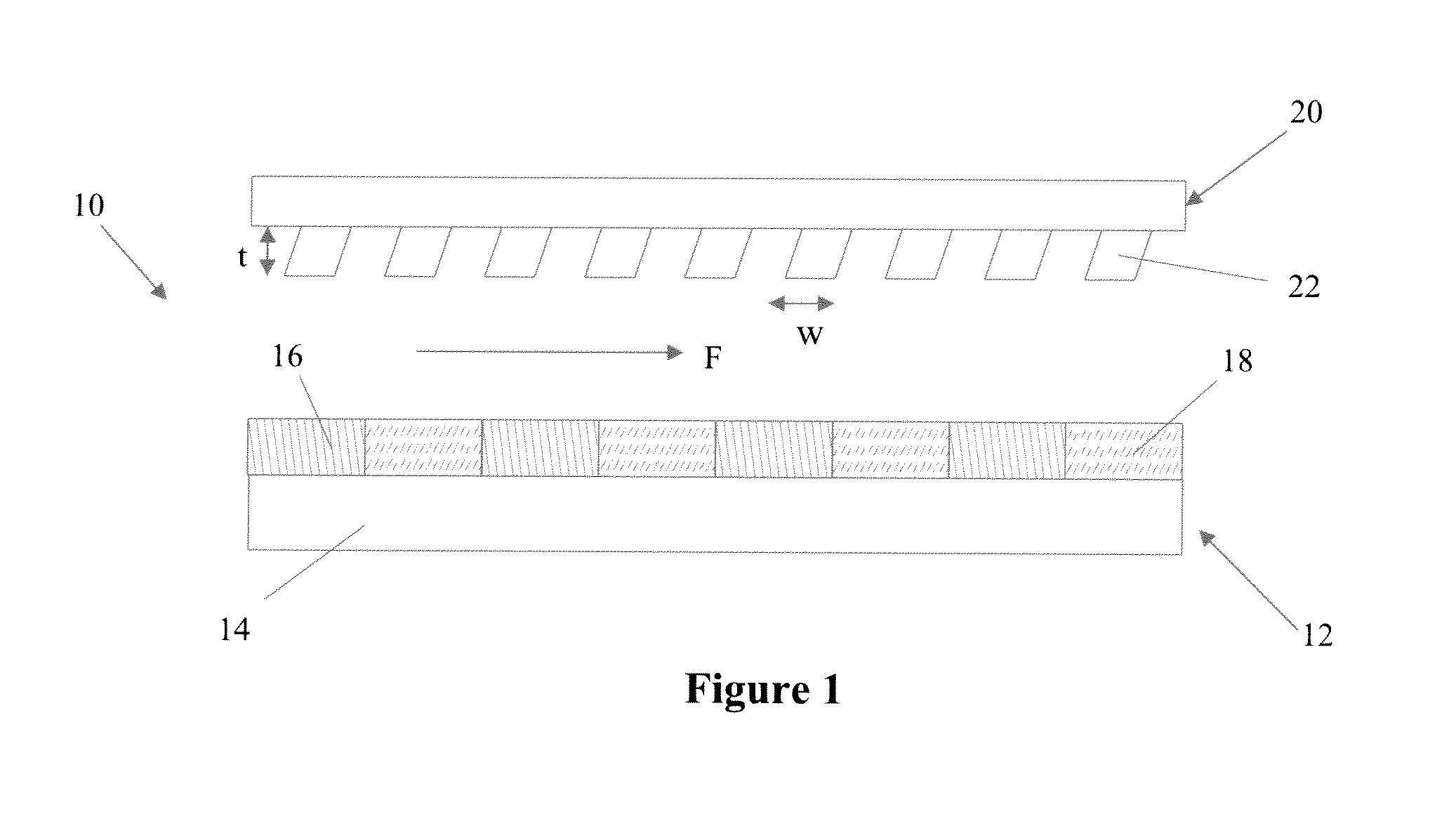
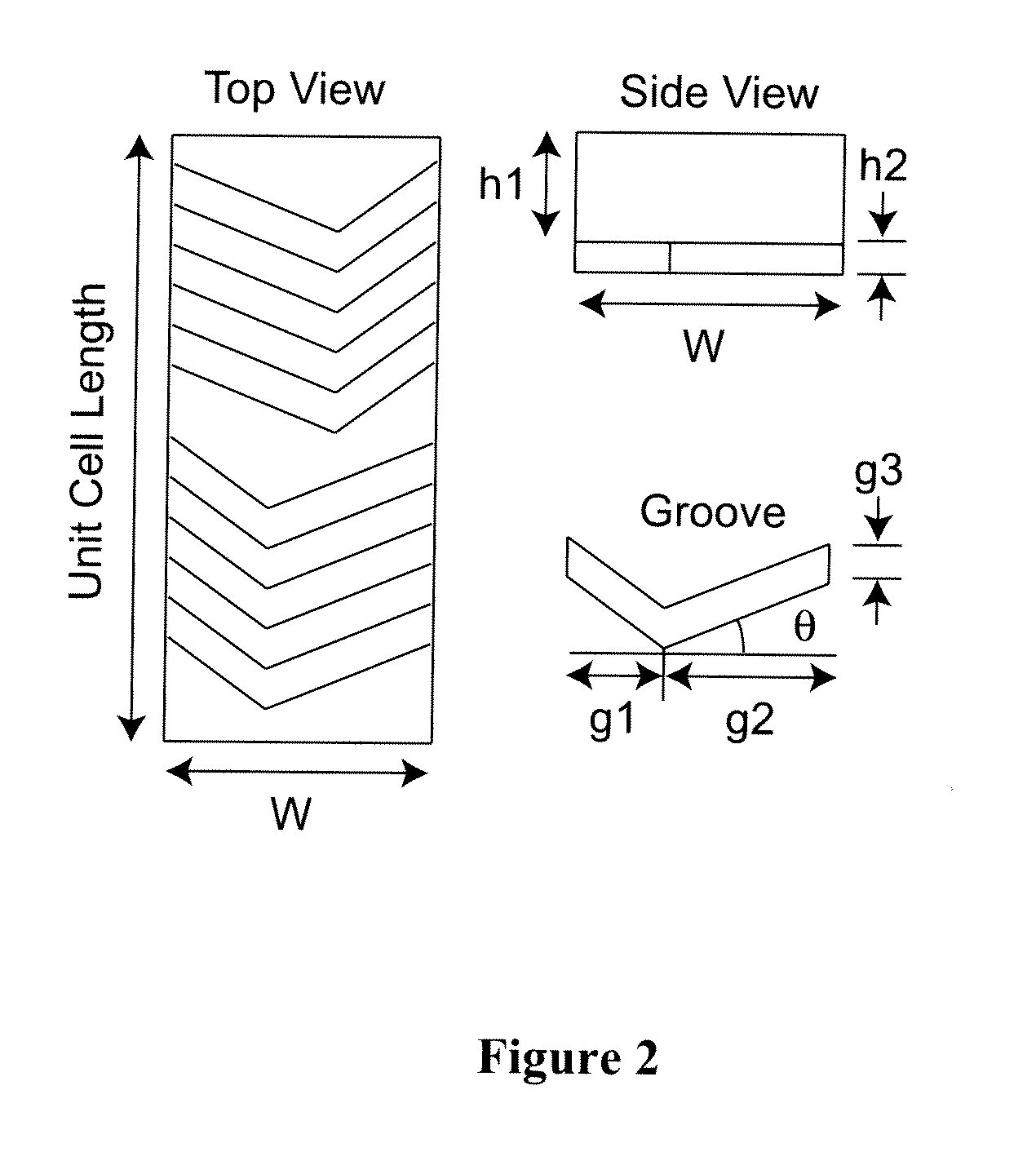
[0113]Patterning of E-selectin on a glass substrate: Recombinant human E-selectin chimera (R&D systems, Minneapolis, Minn.) was patterned on an epoxy functionalized glass surface (SuperEpoxy®, ArrayIt Inc, Sunnyvale, Calif.) using a silicone gasket to block a part of the glass substrate during E-selectin immobilization, resulting in the clear interface between E-selectin coated and uncoated regions as shown in FIG. 9 (the yellow dotted lines). An 1.5 cm×6 cm silicone gasket was placed on a SuperEpoxy® glass slide, along with a small piece (0.3 cm×1 cm) of silicone in the center of the slide. The larger gasket was filled with PBS to rinse the surface, followed by incubation with 5 μg / mL E-selectin at RT overnight. The slide was then rinsed with PBS, the small piece was removed, and the entire surface was blocked with 1% BSA solution.
[0114]Cell rolling of tumor cells on E-selectin: The rolling response of tumor cells on E-selectin coat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear stress | aaaaa | aaaaa |
| shear stress | aaaaa | aaaaa |
| shear stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com