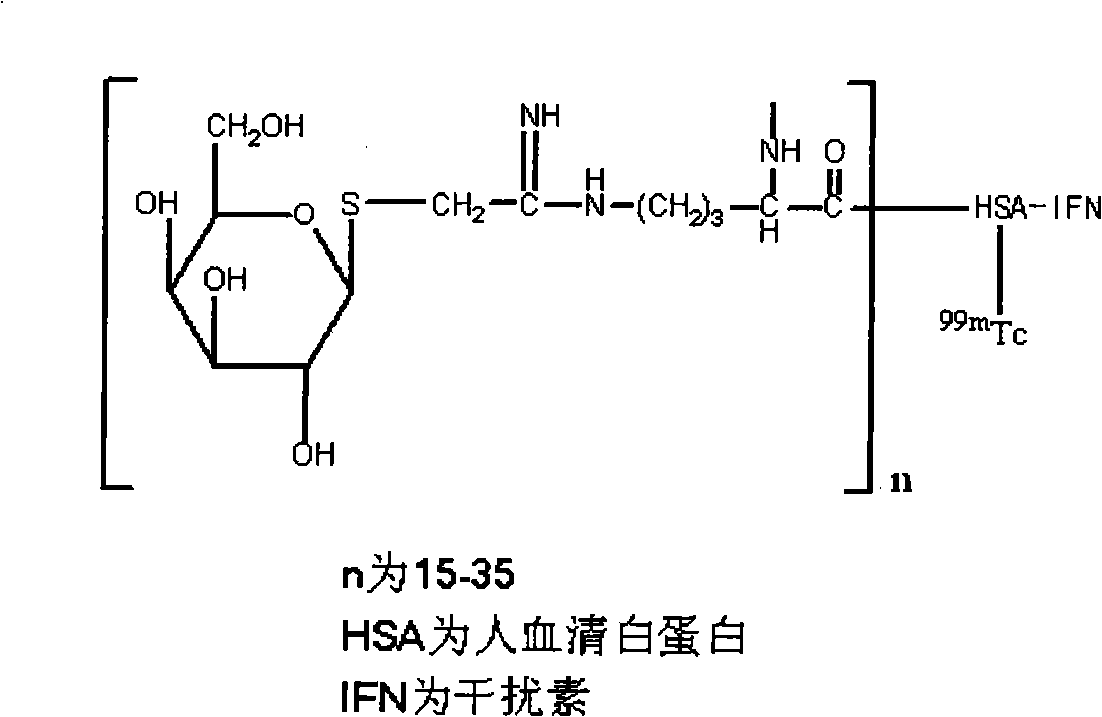
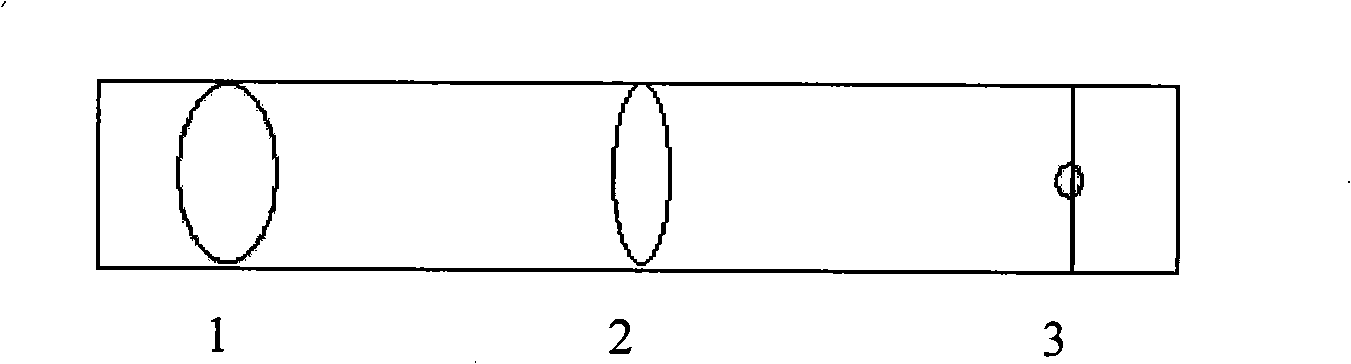Preparation of <99m>Tc galactosyl human serum albumin fusion interferon of liver receptor developer and uses thereof
A technology of serum albumin and 99mtc-ghsa-ifn, which is applied in the direction of interferon, peptide preparation, animal/human protein, etc., can solve the serious harm of hepatitis C, achieve convenient clinical application, reduce side effects, pain relieving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: 99m Preparation of Tc-GHSA-IFN
[0044] by 99m The preparation of Tc-GHSA-IFN α2b is illustrated as an example 99m Preparation of Tc-GHSA-IFN.
[0045] (1) 0.3mg GHSA-IFNα2b (sugar density is 24) lyophilized product, dissolved with 0.2mol / L buffer solution containing 10mmol / L vitamin C;
[0046] (2) Add 50-100 μg stannous reducing agent freshly prepared with HCl, and mix well;
[0047] (3) Sterilize by filtration with a medical sterile disposable filter containing a 0.22 μm filter membrane;
[0048] (4) Quickly add an appropriate amount (0.3mL) of Na 99m TCO 4 Eluent, reacted for 30 minutes;
[0049] The buffer described in step (1) can be acetic acid-sodium acetate buffer, citric acid-sodium citrate buffer, disodium hydrogen phosphate-citric acid buffer, phthalic acid-hydrochloric acid buffer and glycine-hydrochloric acid buffer etc., the buffer solution pH is 3.0-4.5; the stannous reducing agent described in step (2) comprises the one in stannous fl...
Embodiment 2
[0050] Example 2: Preparation of GHSA-IFNα2b freeze-dried kit
[0051] The kit can be conveniently used in clinical technetium[ 99m Tc] labeled, prepared 99m Tc-GHSA-IFN. The preparation of the freeze-dried kit should be carried out in the radiopharmaceutical factory workshop meeting the GMP standard, and its specific steps are as follows:
[0052] (1) 30mg GHSA-IFN, dissolved in 90mL 0.2mol / L buffer containing 10mmol / L vitamin C;
[0053] (2) Add 8mL, 5-10mg / mL excipient (mannitol) solution and mix well;
[0054] (3) Add 2mL, 5mg / mL stannous reducing agent (freshly prepared with HCl), mix well;
[0055] (4) Sterilize by filtration with a medical sterile disposable filter containing a 0.22 μm filter membrane;
[0056] (5) Aliquot 1 mL of each vial, freeze-dry to make a freeze-dried kit, and store at -20°C. Just before use, just add 0.3mL [99mTc] Sodium Pertechnetate Injection to each bottle of lyophilized kit, and let it stand at room temperature for 30 minutes.
[0057...
Embodiment 3
[0058] Example 3: 99m Tc-GHSA-IFNα2b analysis
[0059] Determination by thin layer chromatography on polyamide-66 thin film chromatography paper 99 Tc m -The radiochemical purity of GHSA-IFNα2b, the development system is 20mmol / L PBS pH7.2: 10% sodium dodecyl sulfate (SDS): acetone (V:V:V)=3:1:1, under this condition Under , the Rf of reduced technetium, hydrolyzed technetium and colloidal technetium are all 0.0, free Na 99m TCO 4 The Rf is 0.4~0.5, 99m The Rf of Tc-GHSA-IFNα-2b is 0.8-1.0. The result shows that its radiochemical purity is 91.8%. After being placed at room temperature for 30 minutes, the radiochemical purity is still greater than 90%, which meets the requirements of clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com