Selective androgen receptor modulators and methods of use thereof
An androgen receptor, selective technology, applied in the direction of plant growth regulators, botany equipment and methods, applications, etc., can solve the problem of non-steroidal androgen reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
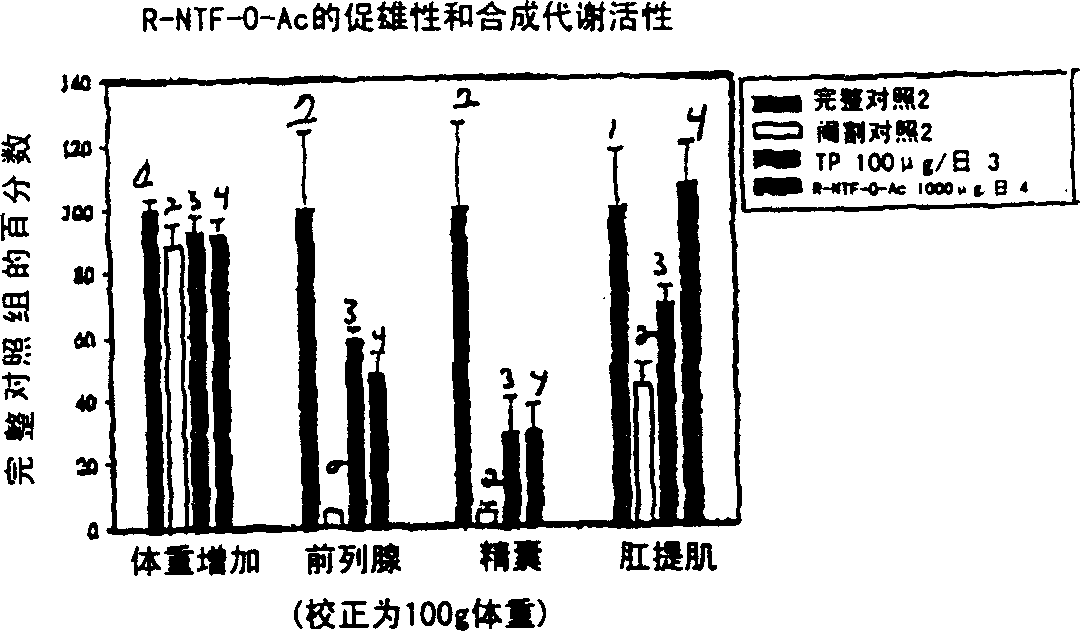
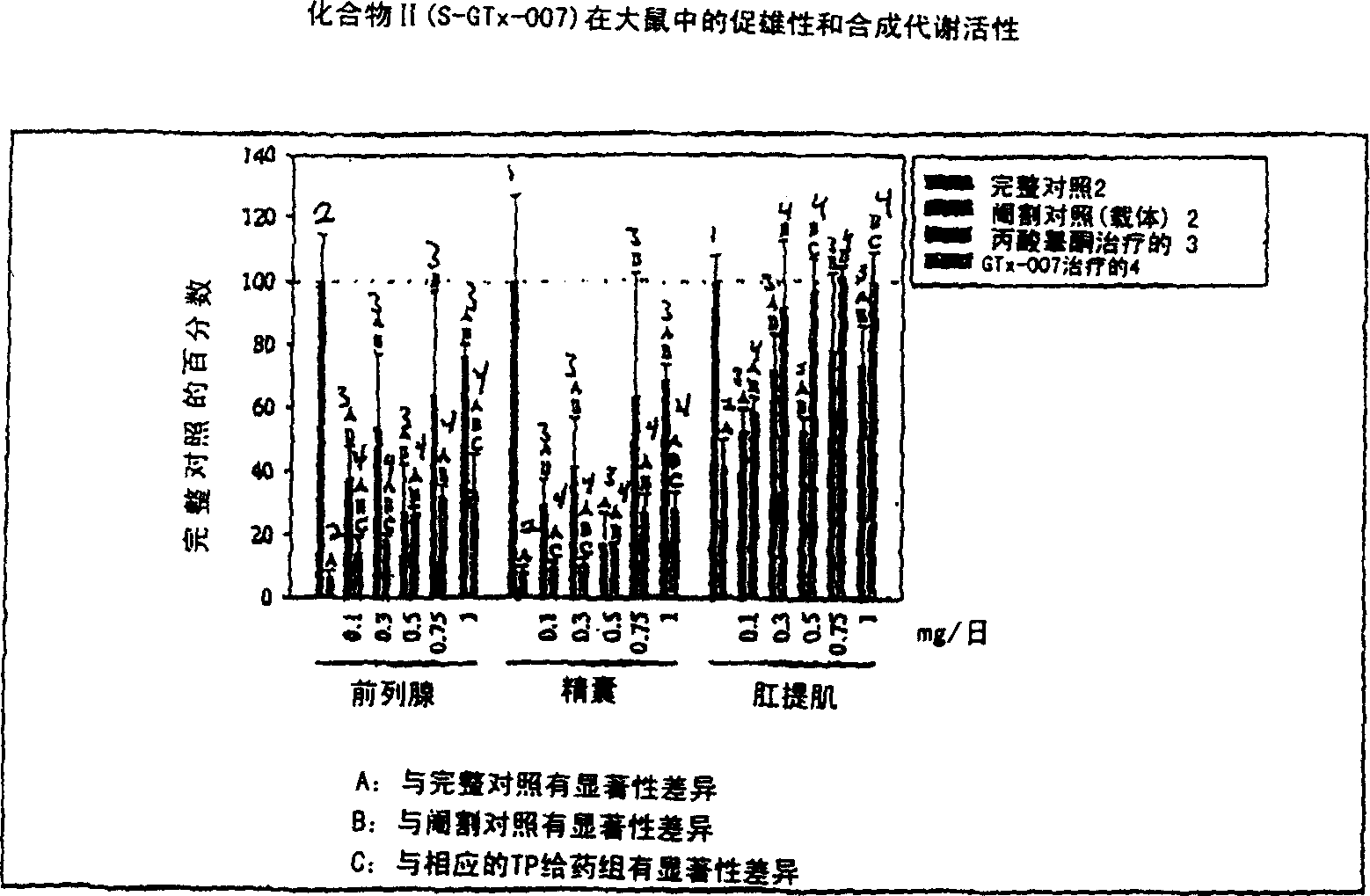
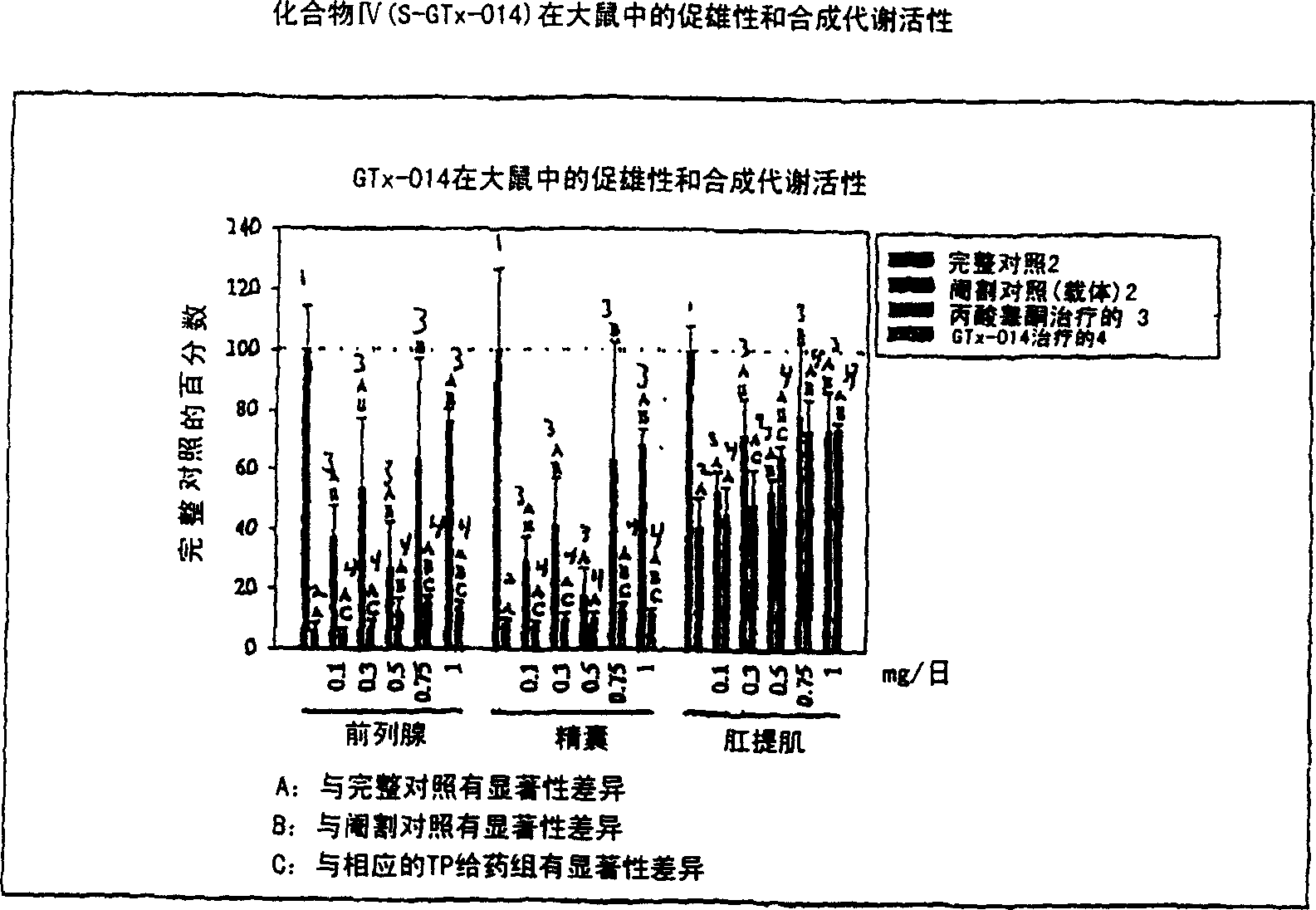
[0163] Non-steroidal ligands with androgenic and anabolic activity
[0164] The in vivo efficacy and acute toxicity of four new non-steroidal androgens (compounds IV, V, VI and VII) were examined in rats. In vitro experiments confirmed that these compounds bind the androgen receptor with high affinity. The structures and names of the four compounds are as follows:
[0165] GTx-014 R=F
[0166] GTx-015 R=COCH 3
[0167] GTx-016 R=COC 2 h 5
[0168] GTx-017 R=NHCOCH 3 experiment method
[0169] material.according to Figure 9 The shown route synthesizes compound GTx-014 (compound IV), GTx-015 (compound V), GTx-016 (compound VI) and GTx-007 (compound VII, wherein R is NHCOCH 3) and the R-isomer of GTx-014. Testosterone propionate (TP), polyethylene glycol 300 (PEG300, reagent grade) and neutral buffered formalin (10% w / v) were purchased from Sigma Chemical Company (St Louis, MO). Alzet osmotic pum...
Embodiment 4
[0186] HPLC analysis of GTx-007
[0187]A reverse-phase high-pressure liquid chromatography (HPLC) assay was developed to quantify GTx-007 concentrations in canine plasma. Canine blood samples were obtained by venipuncture and centrifuged at 1000g for 15 minutes. Samples were stored frozen at -20°C until analysis. Each sample was thawed quickly, and an aliquot (0.5 ml) was taken to elute the peak together with the internal standard (2 μl 200 μg / ml CM-II-87 aqueous solution). Aliquots of 1 ml of acetonitrile were added to the samples to precipitate plasma proteins. After vortexing the samples, centrifuge at 1000g for 15 minutes. Gently pour the supernatant into a glass extraction tube and add 7.5 ml of ethyl acetate. The extraction mixture was left at room temperature for 20 minutes, during which time it was vortexed several times. Then, the samples were centrifuged at 1000 g for 10 min, and the organic phase was removed and loaded into conical bottom glas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com