Selective androgen receptor modulators and methods of use thereof
An androgen receptor and selective technology, applied in diseases, drug combinations, metabolic diseases, etc., can solve problems such as vulnerability, weakened physical condition, and poor behavioral status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0289] The preparation of pharmaceutical compositions containing active ingredients is well known in the art, for example by mixing, granulating or tabletting techniques. The active therapeutic ingredient is often mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. For oral administration, the SARM drug or its physiologically tolerable derivatives such as salts, esters, N-oxides, etc. are mixed with additives customary for oral administration such as excipients, stabilizers or inert diluents , and converted into dosage forms suitable for administration such as tablets, coated tablets, hard or soft gelatin capsules, water, alcohol or oil solutions by conventional methods. For parenteral administration, the SARM drug or its physiologically tolerable derivatives, such as salts, esters, N-oxides, etc., can be converted together with substances customary and suitable here, such as solubilizers, etc. as a solution, suspension or em...
Embodiment 1
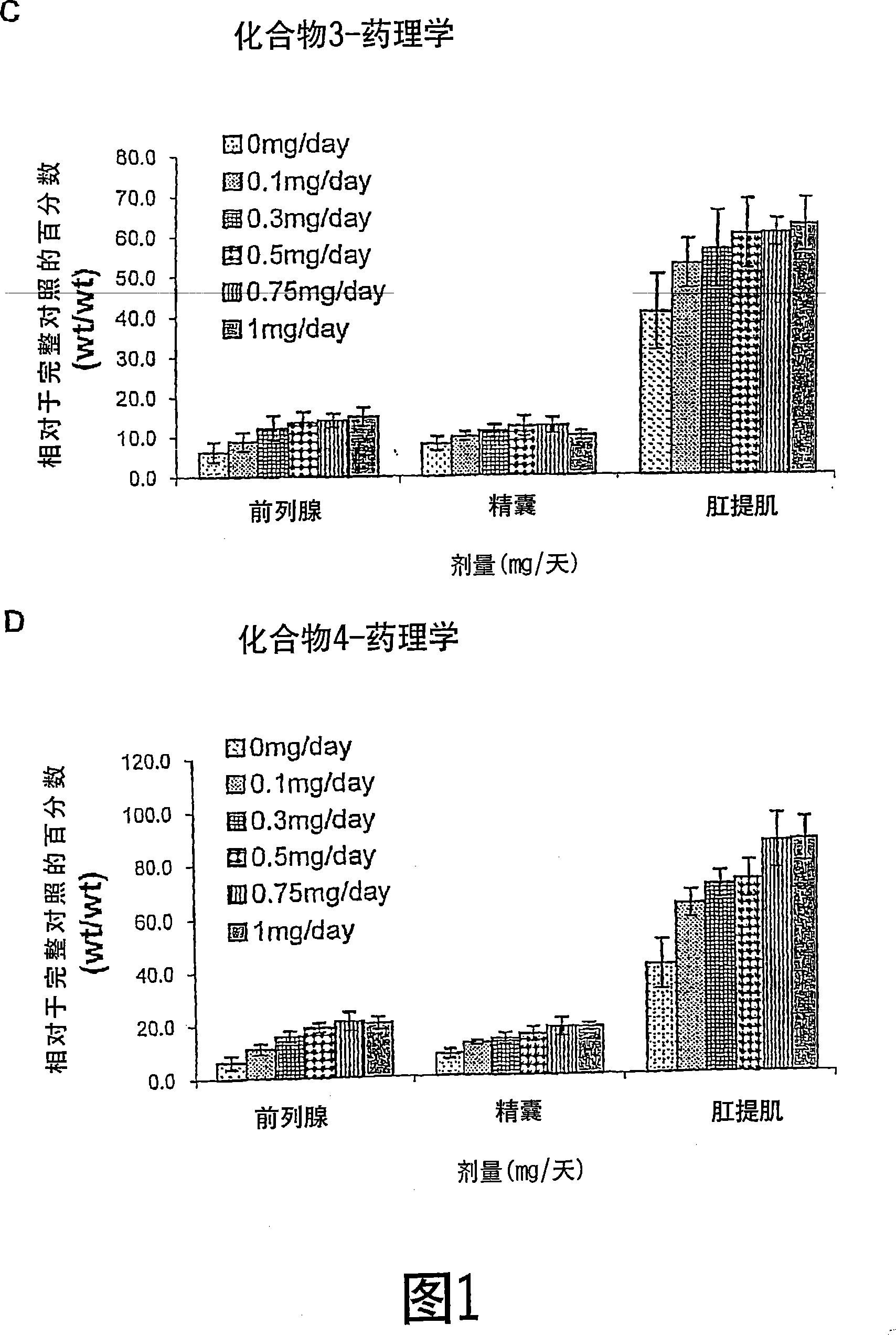
[0297] Androgenic and anabolic activities of compounds 1-4
[0298] The binding affinities of selected SARM compounds whose B rings were Fylated, Clyd, Bryd, or Iylated were determined and are shown in Table 1:
[0299] Table 1
[0300]
[0301]
[0302] experimental method
[0303] Animals. Immature male Sprague-Dawley rats, weighing 90-100 g, were purchased from Harlan Biosciences (Indianapolis, IN). Animals were maintained on a 12-hour light-dark cycle with ad libitum access to food and water. The animal experiment protocol was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee.
[0304] Study Design. Rats were randomly assigned to treatment groups. One day before starting drug treatment, animals were individually removed from cages, weighed, and anesthetized with ip ketamine / xylazine (87 / 13 mg / kg; approximately 1 mL / kg). When moderately anesthetized (ie, unresponsive to toe pinch), animals were earmarked for easy identification. ...
Embodiment 2
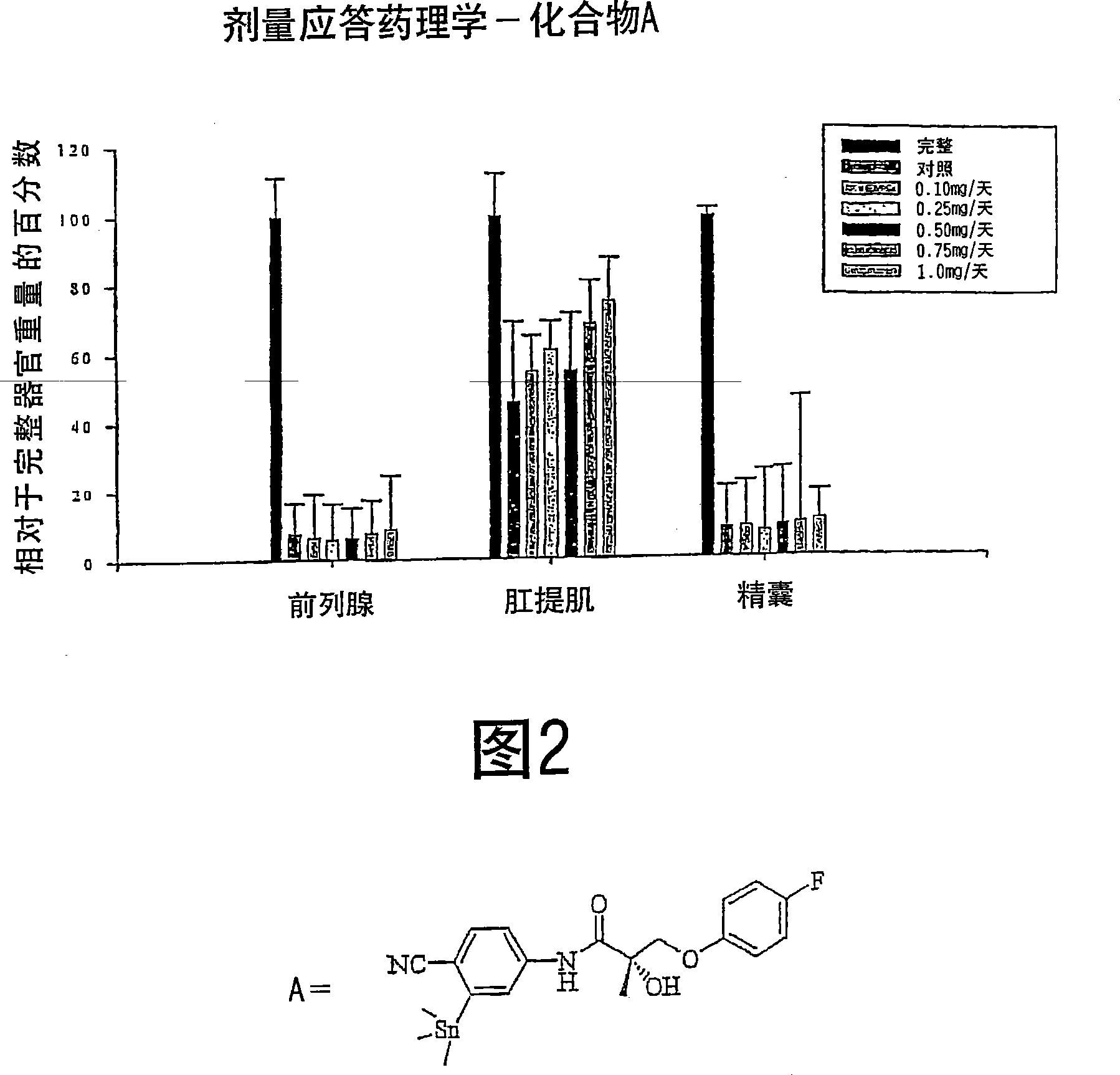
[0312] Androgenic and anabolic activity of compound 5
[0313] The binding affinities of selected compounds 5 are shown in Table 2.
[0314] Table 2
[0315]
[0316] After 14 days of administration, the androgenic and anabolic activity of Compound 5 in the castrated rat model were detected according to the method described in Example 1 above.
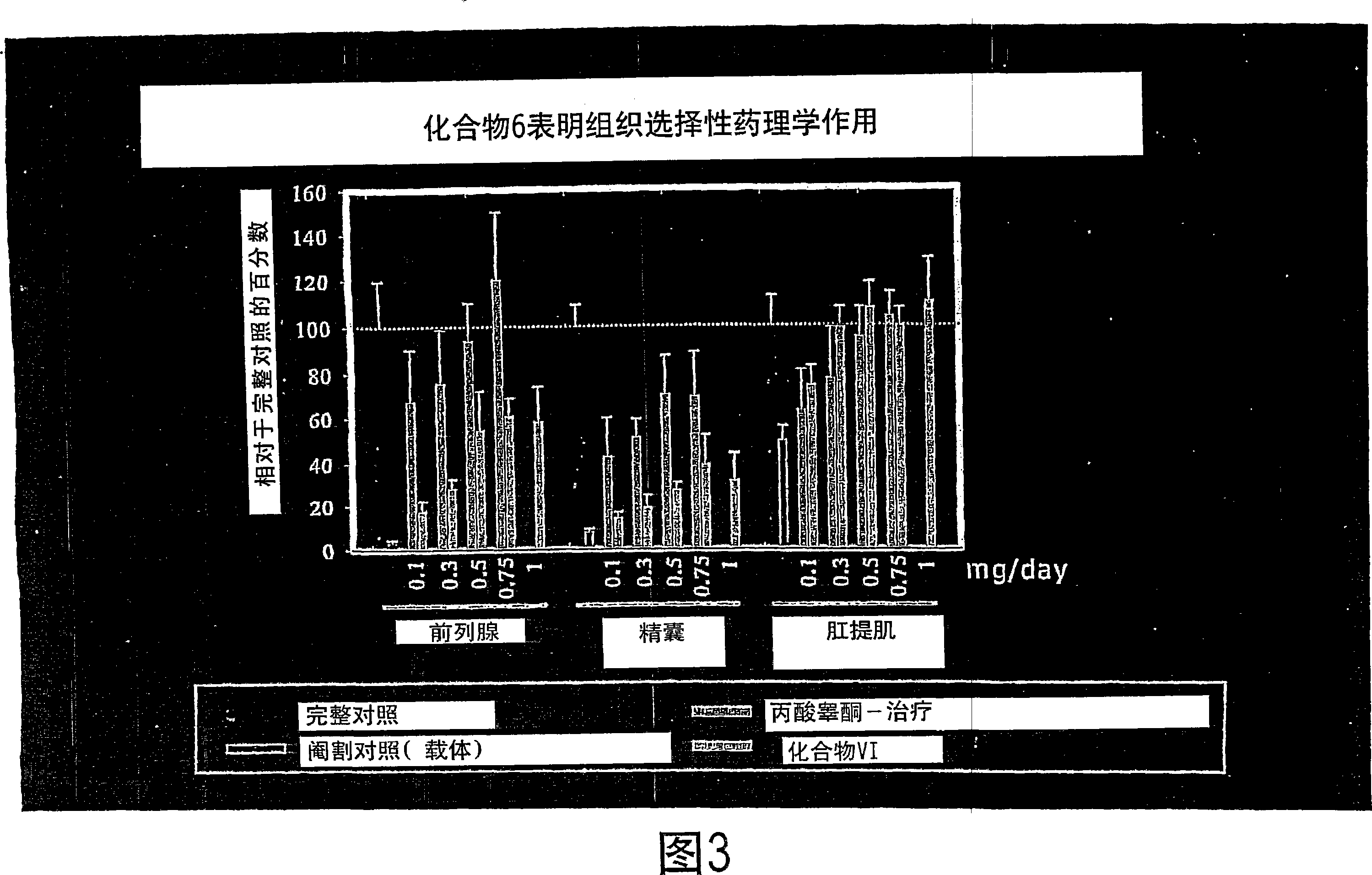
[0317] As shown in Table 3 and Figure 2, compound 5 exhibited tissue-selective pharmacological effects in castrated male rats, and in anabolic tissues (i.e. levator ani) compared to androgenic tissues (i.e. prostate and capsule) has higher efficacy. The compound showed little pharmacological activity in the prostate (8.7 ± 1.39% intact at a dose of 1.0 mg / day) and capsules (10.7 ± 0.91% intact at a dose of 1.0 mg / day), which It was shown to act as only a weak partial agonist in these tissues. Importantly, Compound 5 exhibited potent anabolic activity at a dose of 1.0 mg / day, restoring the levator ani muscle to 75.2 ± 9.51% of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com