Large-scale synthesis of selective androgen receptor modulators
An androgen receptor and selective technology, which is applied in the field of large-scale synthesis of selective androgen receptor modulators, can solve the problems of low yield and unsuitability for large-scale preparation, and achieve high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
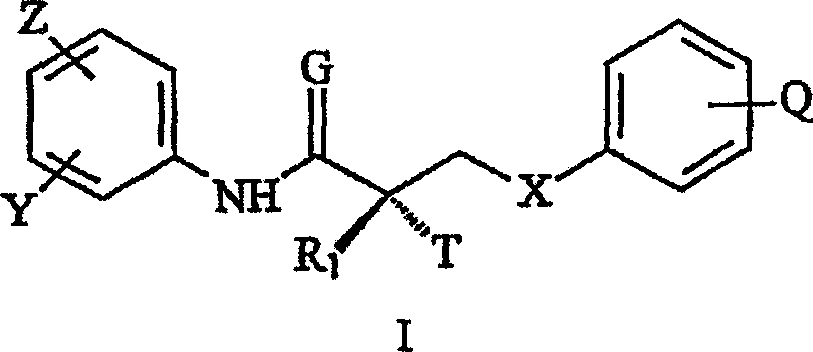
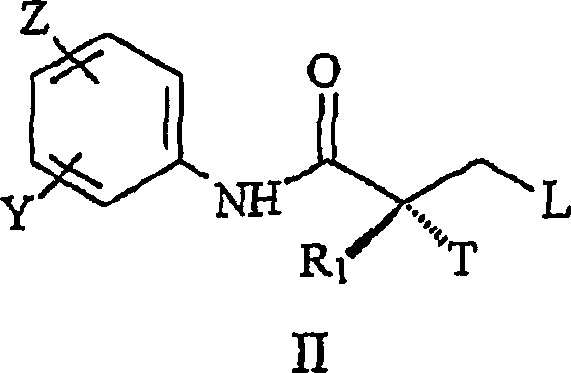
[0091] Accordingly, in one embodiment, the present invention provides a synthetic method for the preparation of the SARM compounds described herein, which involves a purification step comprising crystallization of the SARM product from a mixture of non-toxic organic solvents and water. In one embodiment, the non-toxic organic solvent is ethanol. In a particular embodiment, the crystallization step comprises mixing an alcoholic solution containing the SARM compound and water to crystallize the SARM compound. In another embodiment, the method further comprises the step of collecting the SARM compound by filtration.
[0092] The method of the present invention is suitable for large-scale preparations, since all steps give high-purity compounds, thus avoiding complicated purification processes which ultimately reduce yields. Accordingly, the present invention provides a method for the synthesis of non-steroidal agonist compounds, which is applicable to industrial large-scale synt...
Embodiment 1
[0270] Embodiment 1: the synthesis of compound (1)
[0271] Compound (1) was synthesized according to the description below and the scheme of Scheme 1.
[0272]
[0273] Route 1
[0274] (2R)-1-methacryloylpyrrolidine-2-carboxylic acid (R-129). Dissolve D-proline (R-128, 14.93 g, 0.13 mol) in 71 mL of 2N NaOH and place in an ice bath Cool in medium; the basic solution obtained was diluted with acetone (71 mL). A solution of methacryloyl chloride 127 (13.56 g, 0.13 mol) in acetone (71 mL) and 2N NaOH solution (71 mL) were added simultaneously to an aqueous D-proline solution in an ice bath over 40 minutes. During the addition of methacryloyl chloride, the pH of the mixture was maintained at 10-11°C. After stirring (3 hours, room temperature), the mixture was evaporated under vacuum at a temperature of 35-45°C to remove acetone. The resulting solution was washed with ether and acidified to pH 2 with concentrated HCI. The acidic mixture was saturated with NaCl and extract...
Embodiment 2
[0282] Large-Scale Synthesis of Compound (1)
[0283]Compound (1) (3-[4-(acetylamino)phenoxy]-2-hydroxy-2-methyl-N-[3-trifluoromethyl-4-nitro-phenyl]-propionamide) is a member of the oxolutamide family of androgen receptor agonists and is a non-steroidal selective androgen receptor modulator (SARM). In vitro, it binds to the androgen receptor with high affinity (Ki=7.5±0.5nM). In vivo, it is a partial agonist of the androgen receptor and causes strong anabolic and weak androgenic effects. Compound (1) has no other known endocrine activity.
[0284]
[0285] Compound (2) was synthesized according to the following synthetic steps:
[0286] Step 1 - Synthesis of (2R)-1-methacryloylpyrrolidine-2-carboxylic acid (R-129)
[0287]
[0288] route 2
[0289] A 72 L flask with mechanical stirrer and inlet for inert atmosphere was mounted in a cooling bath. Place the flask under argon and add 5000 g (43.4 mol) D-proline (ICN Lot #7150E, > 99%), 11.9 L 4N NaOH and 12 L acetone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com