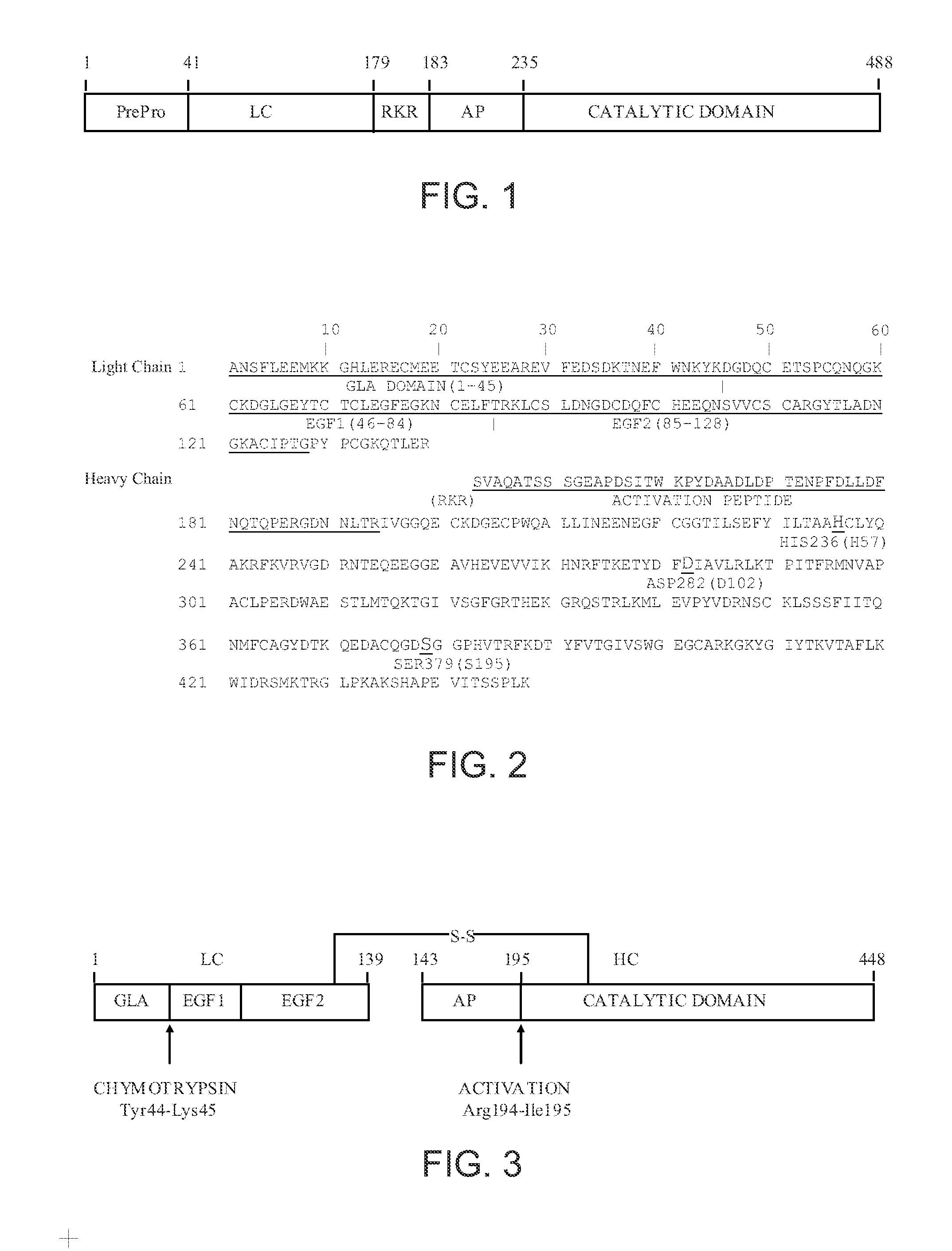
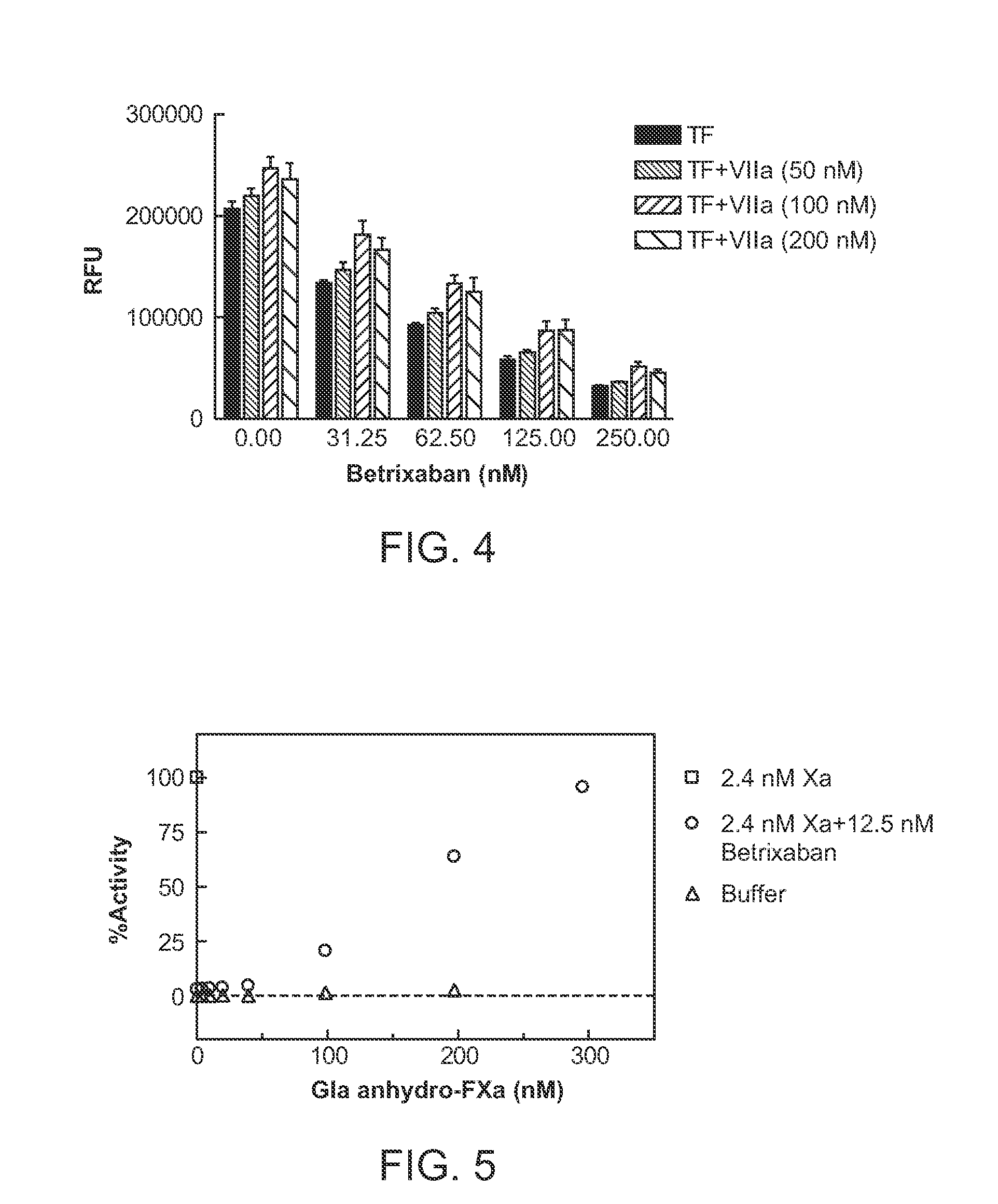
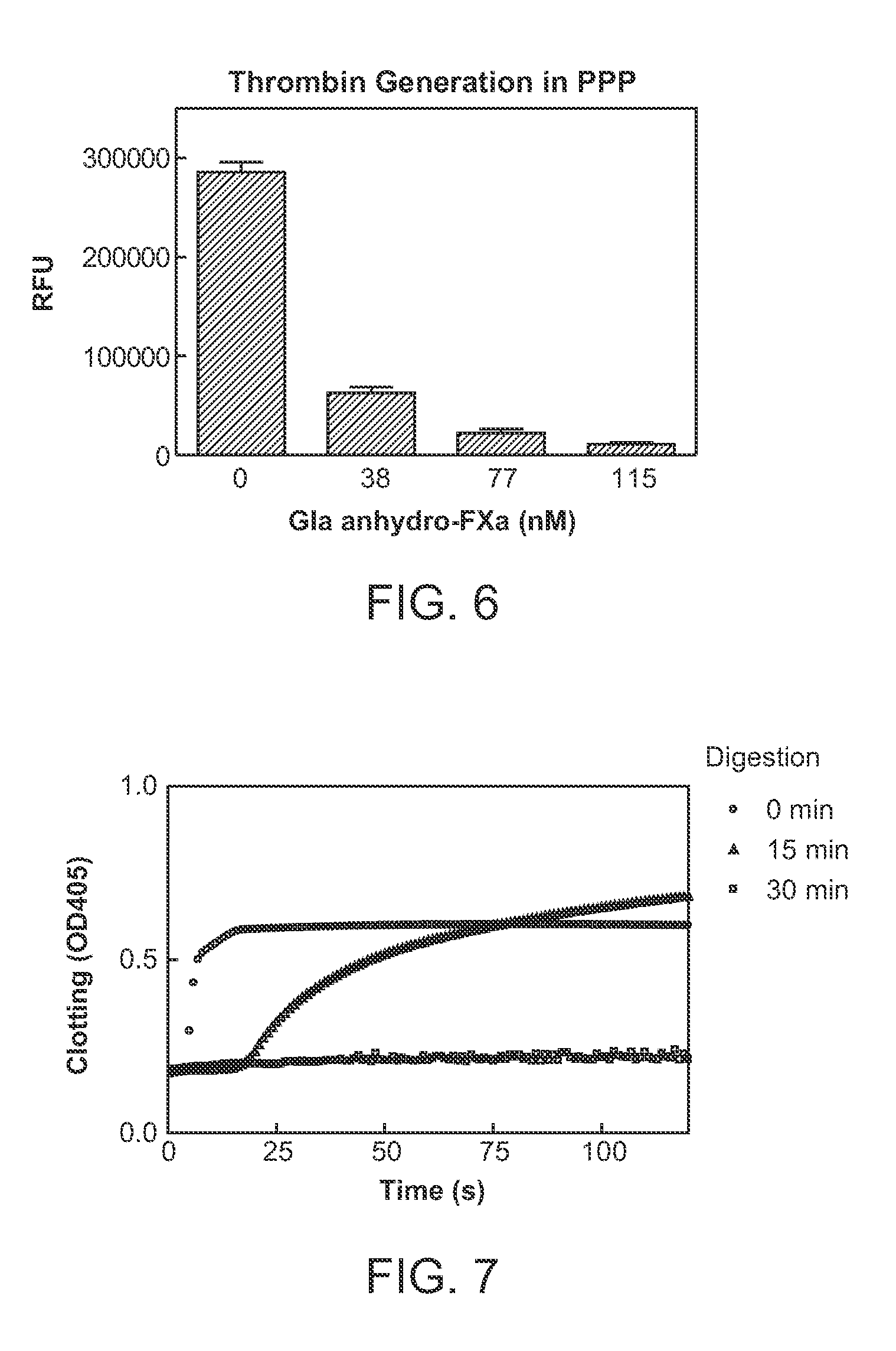
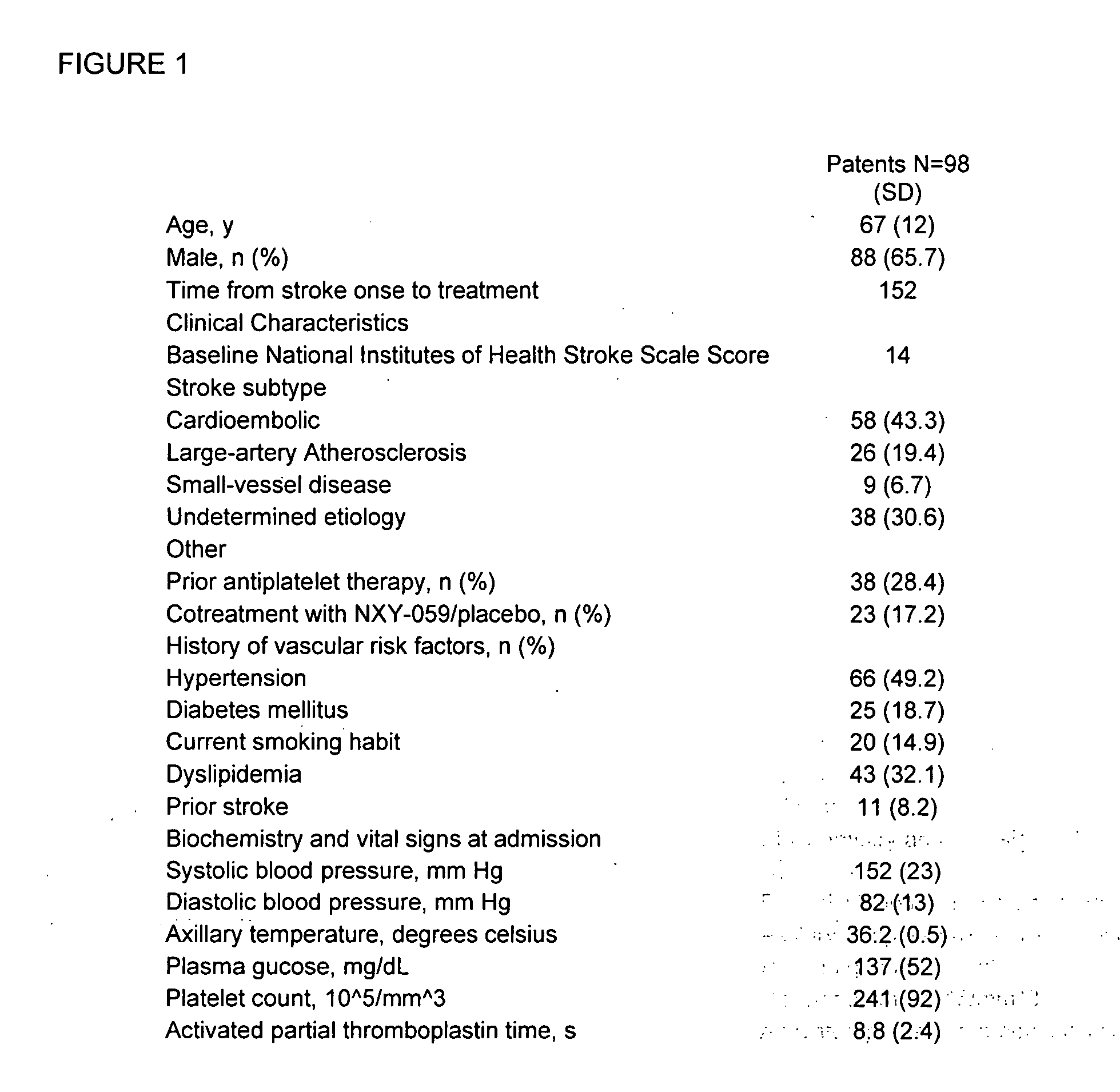
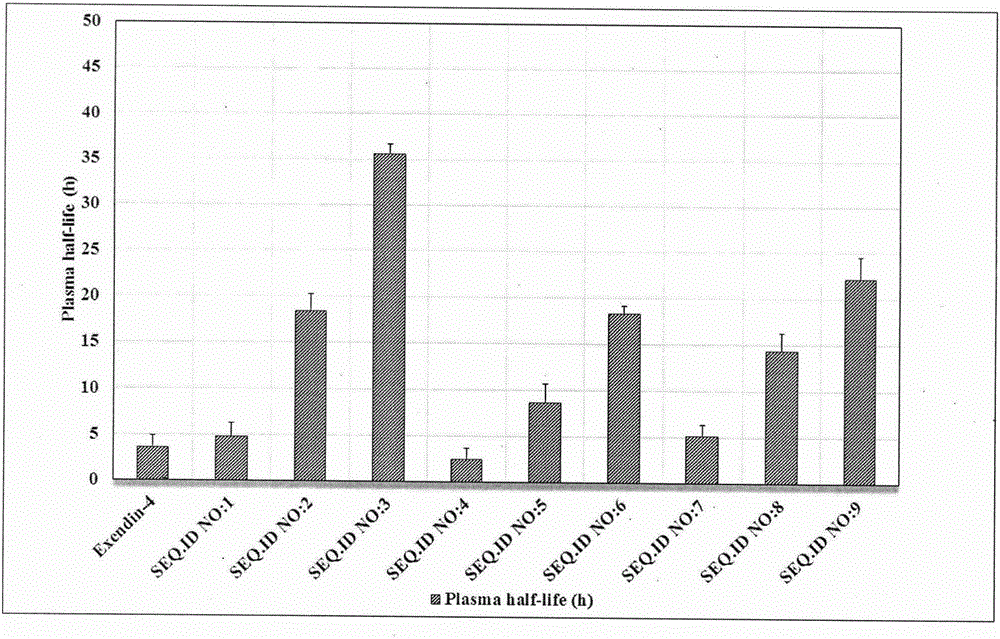
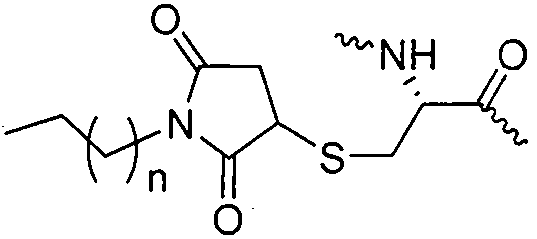
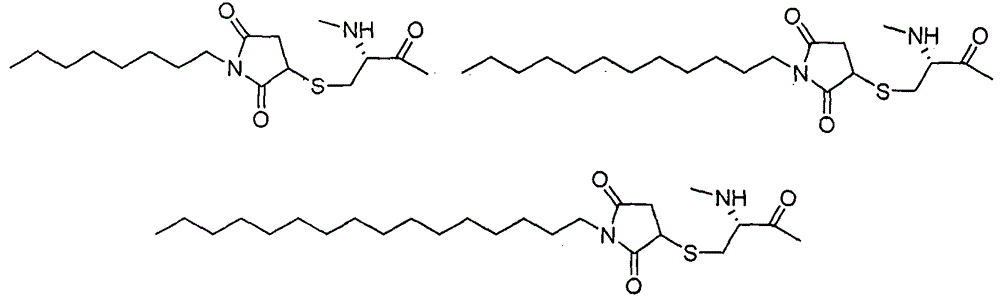
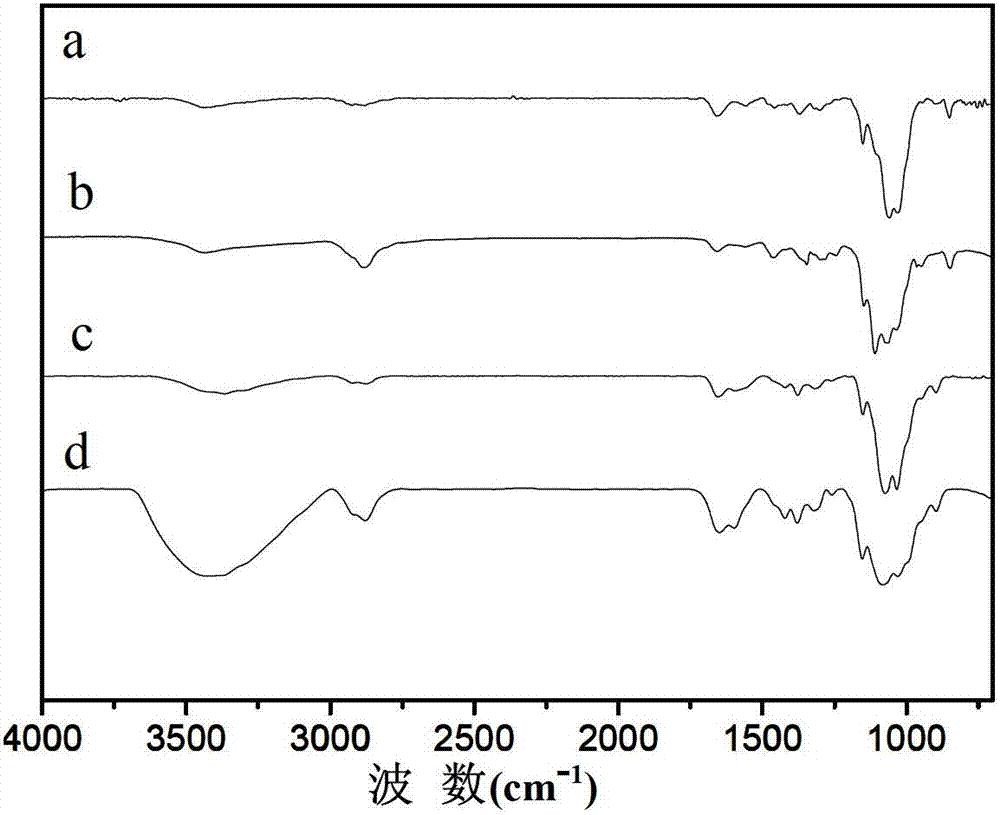
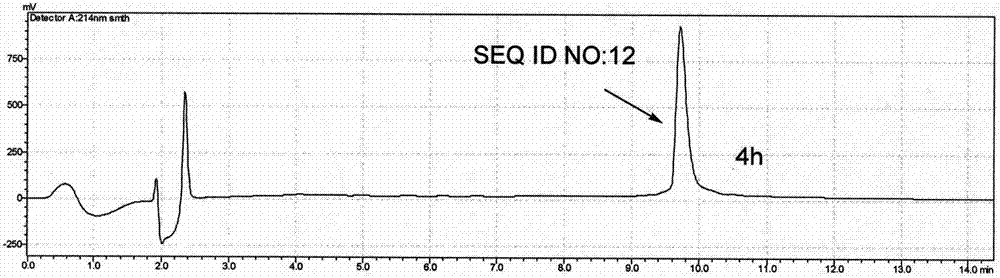
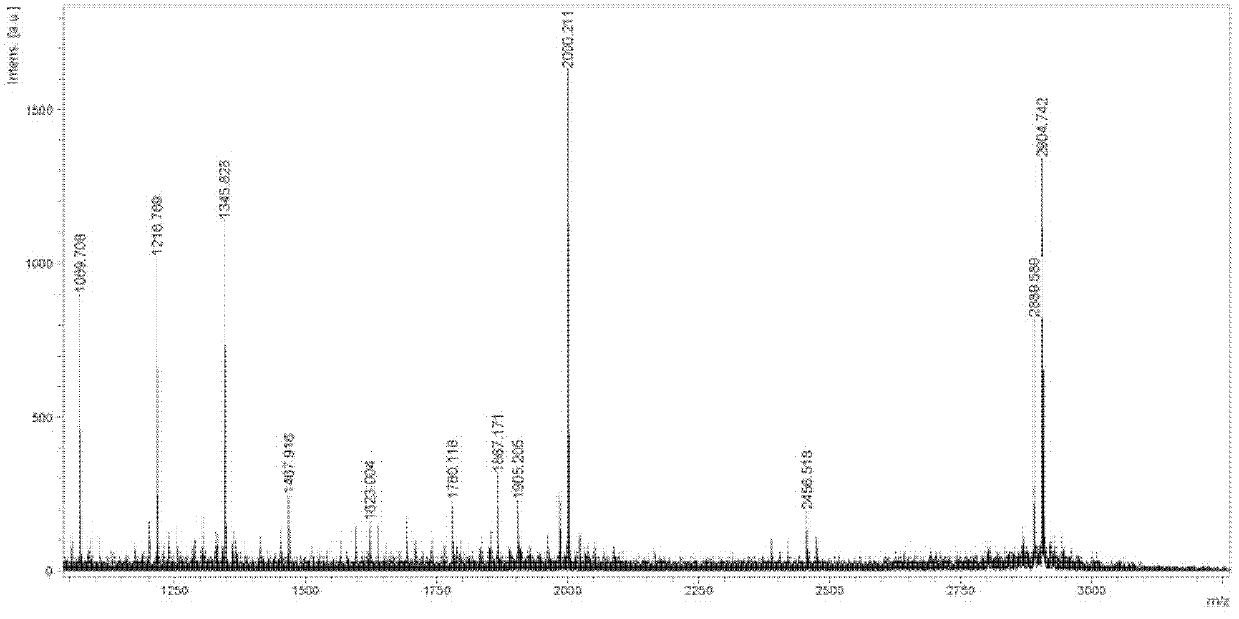
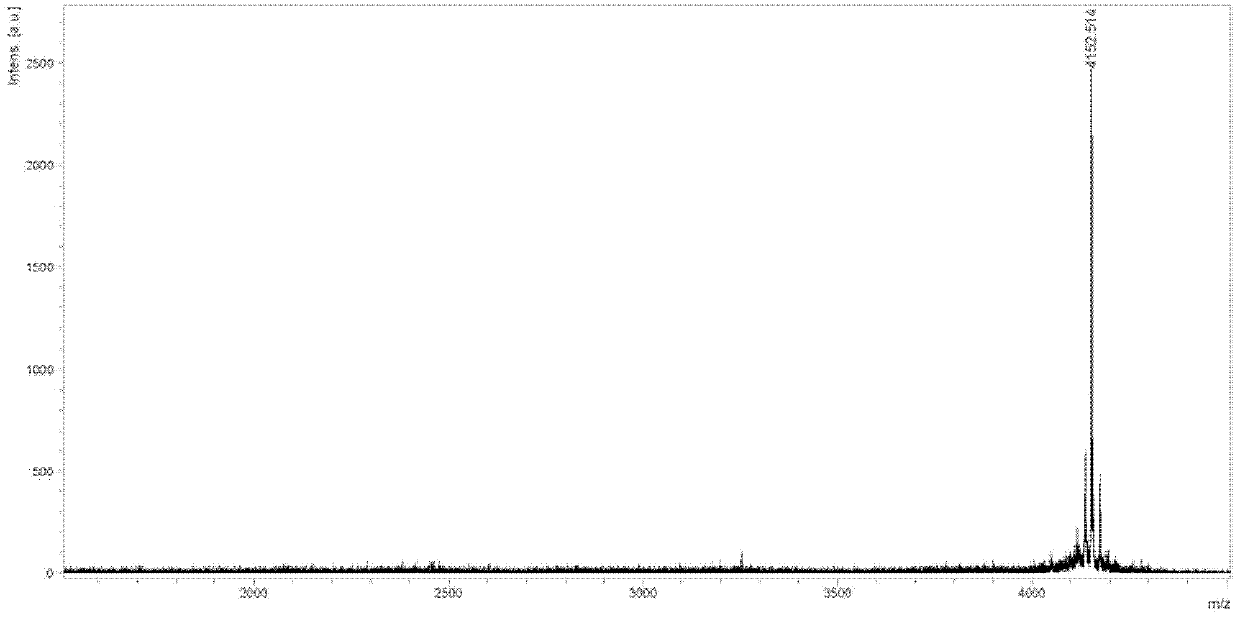
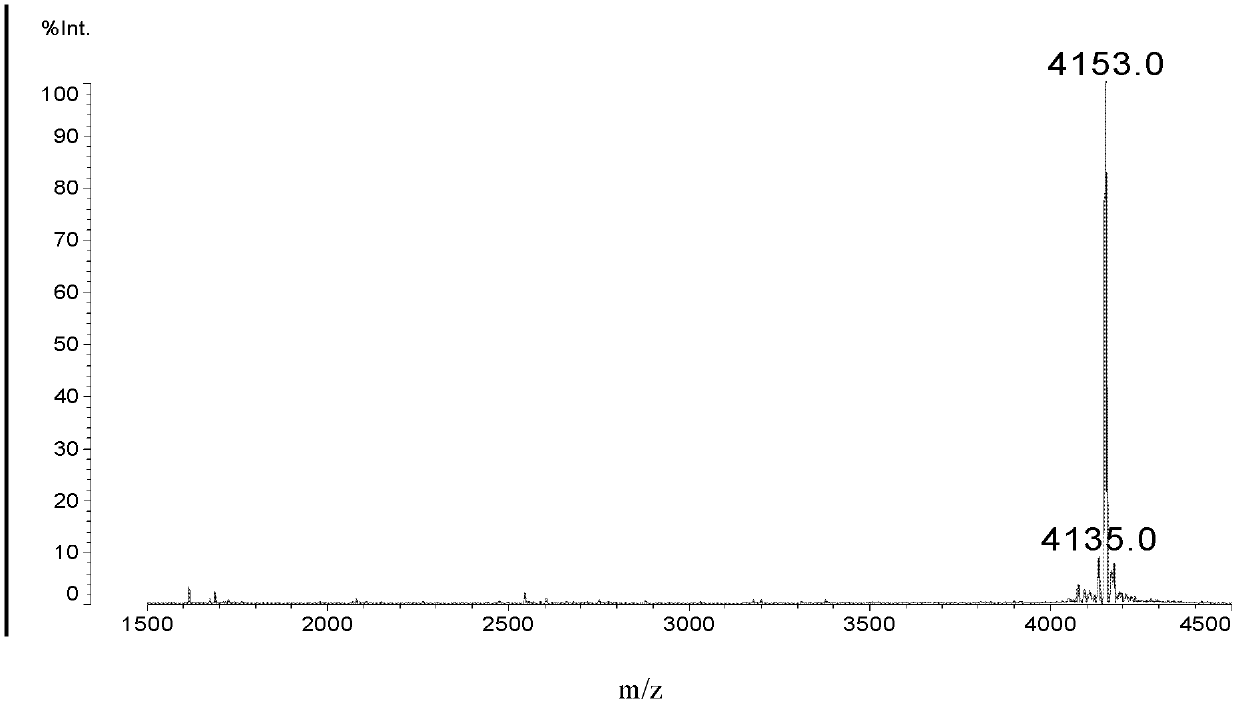
Patents
Literature
105results about How to "Prolonged plasma half-life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
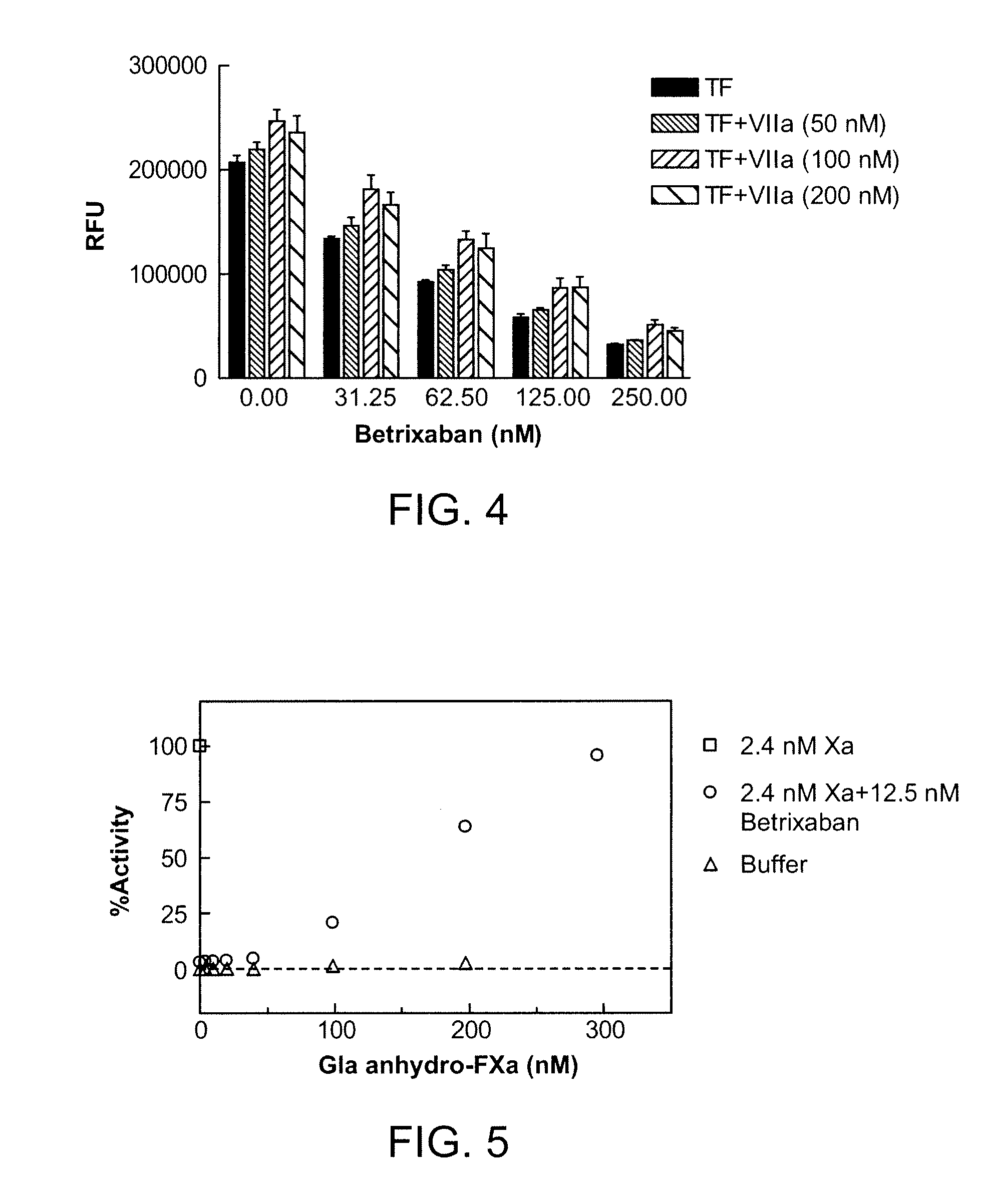
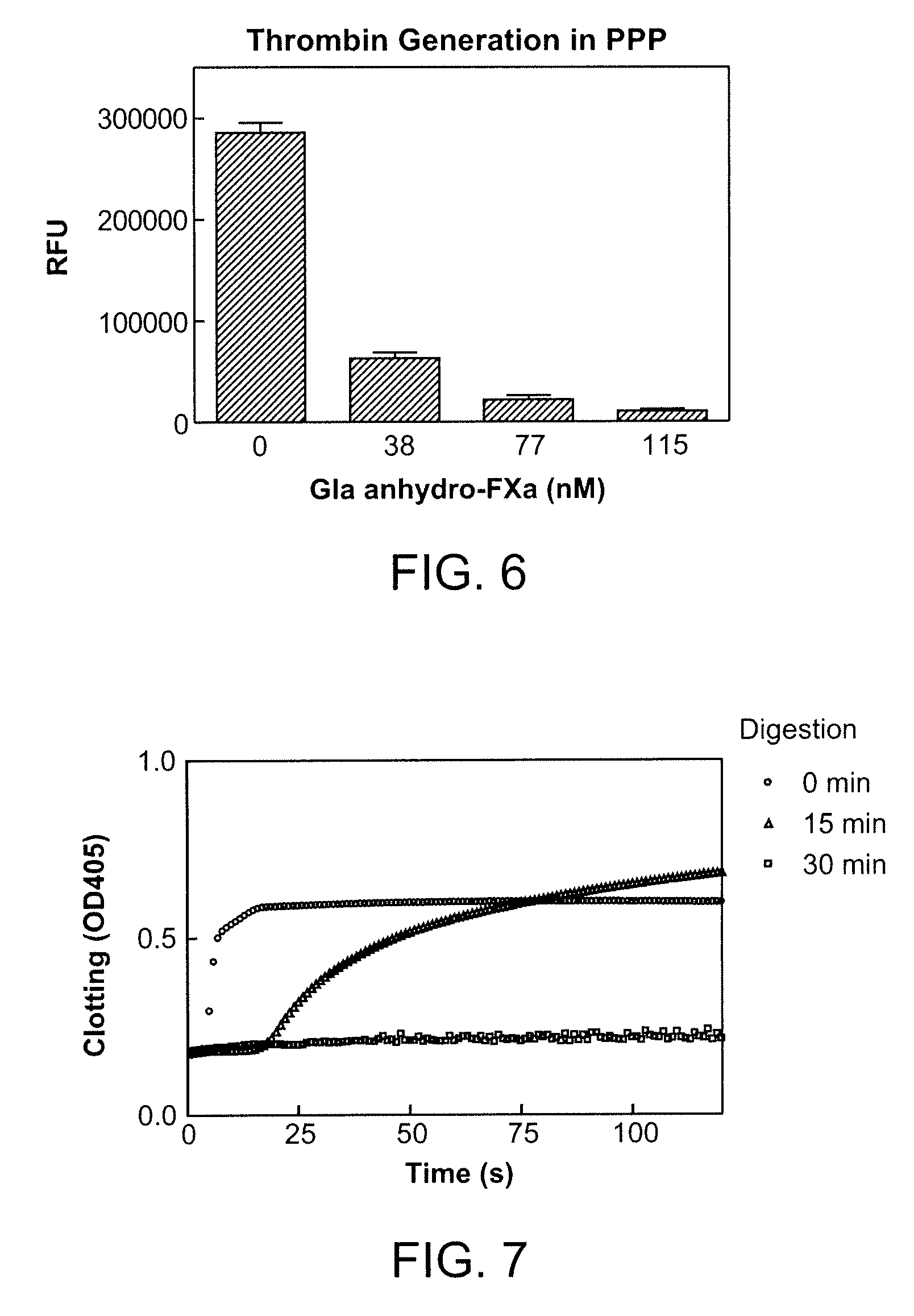
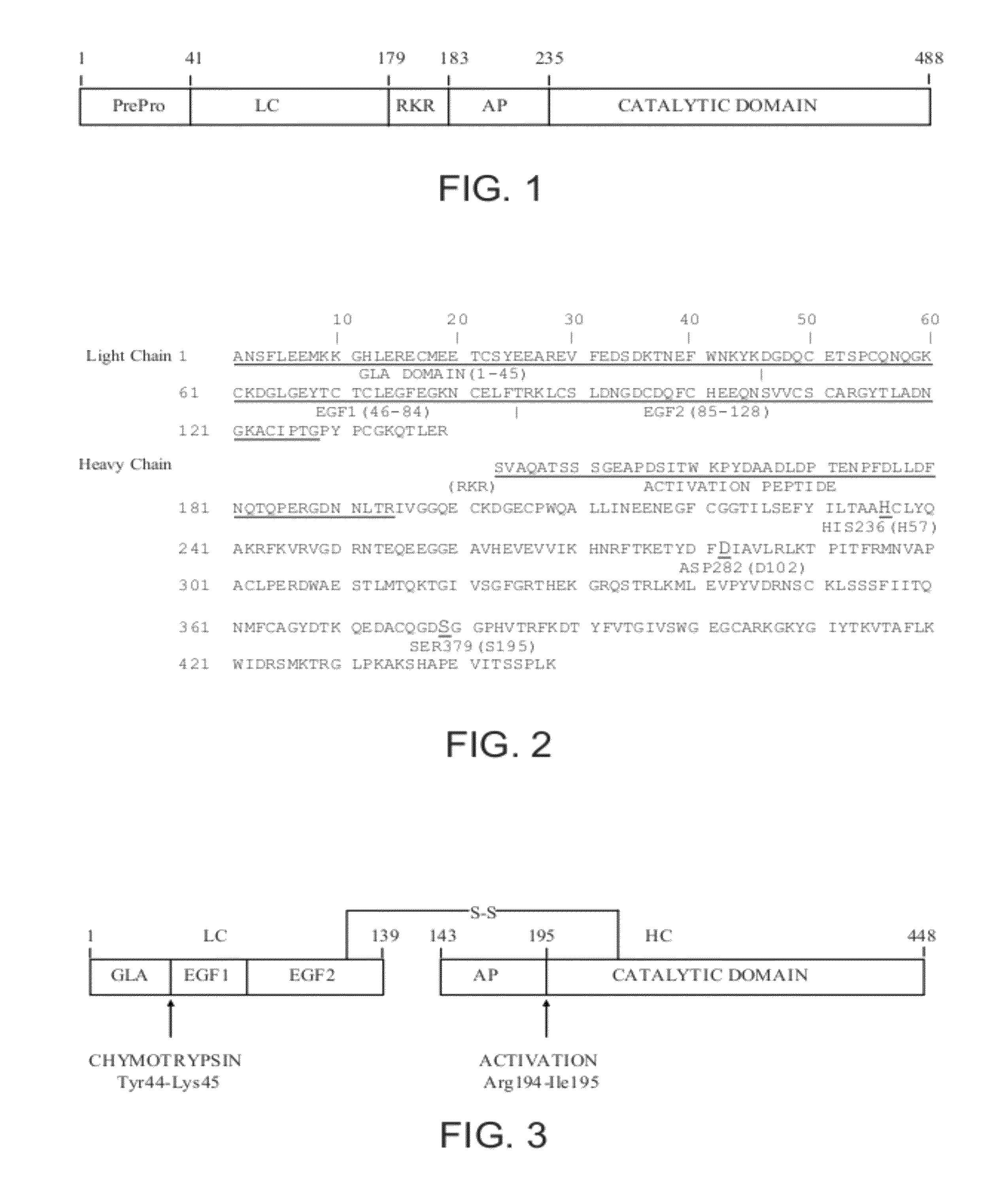
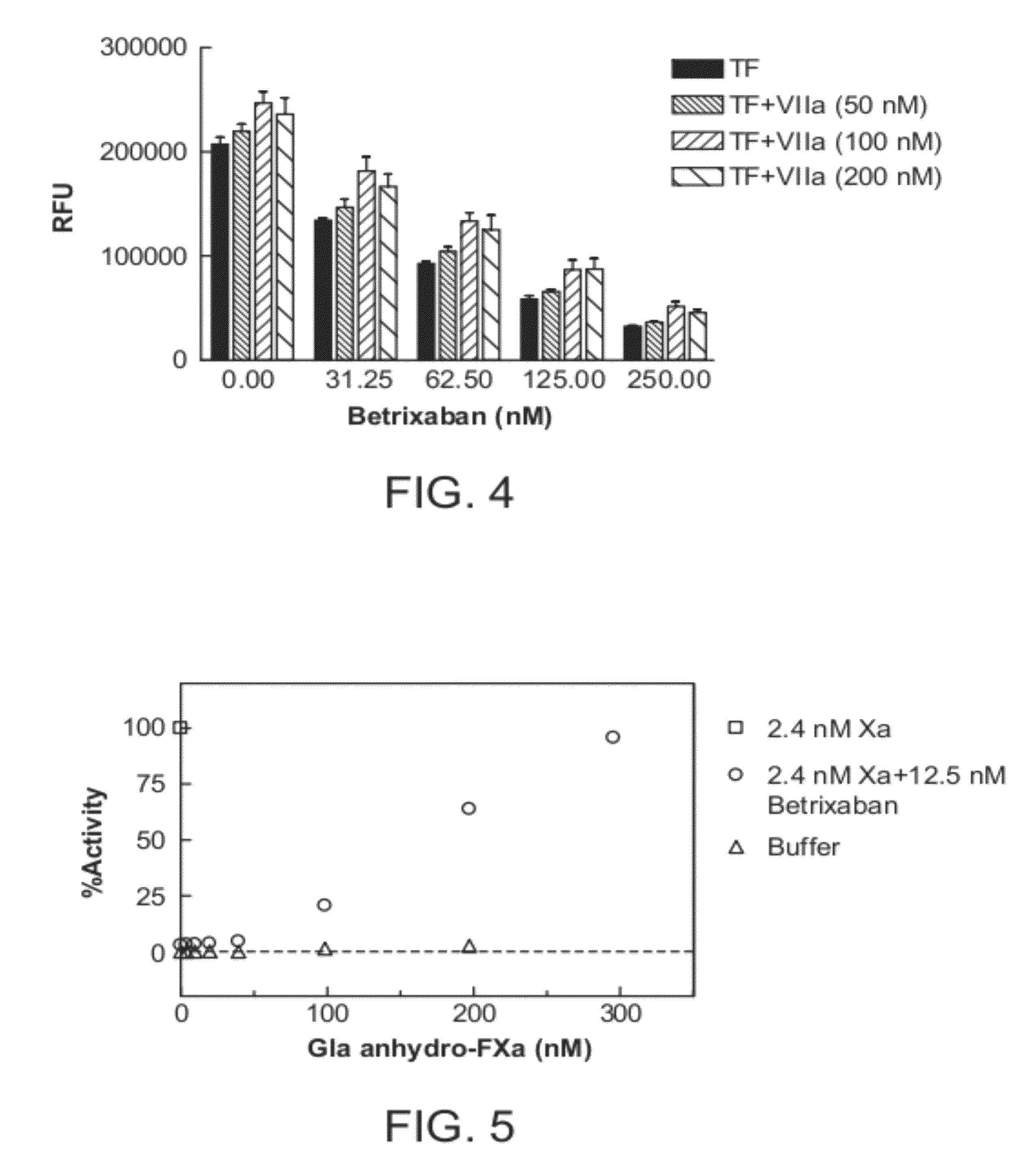
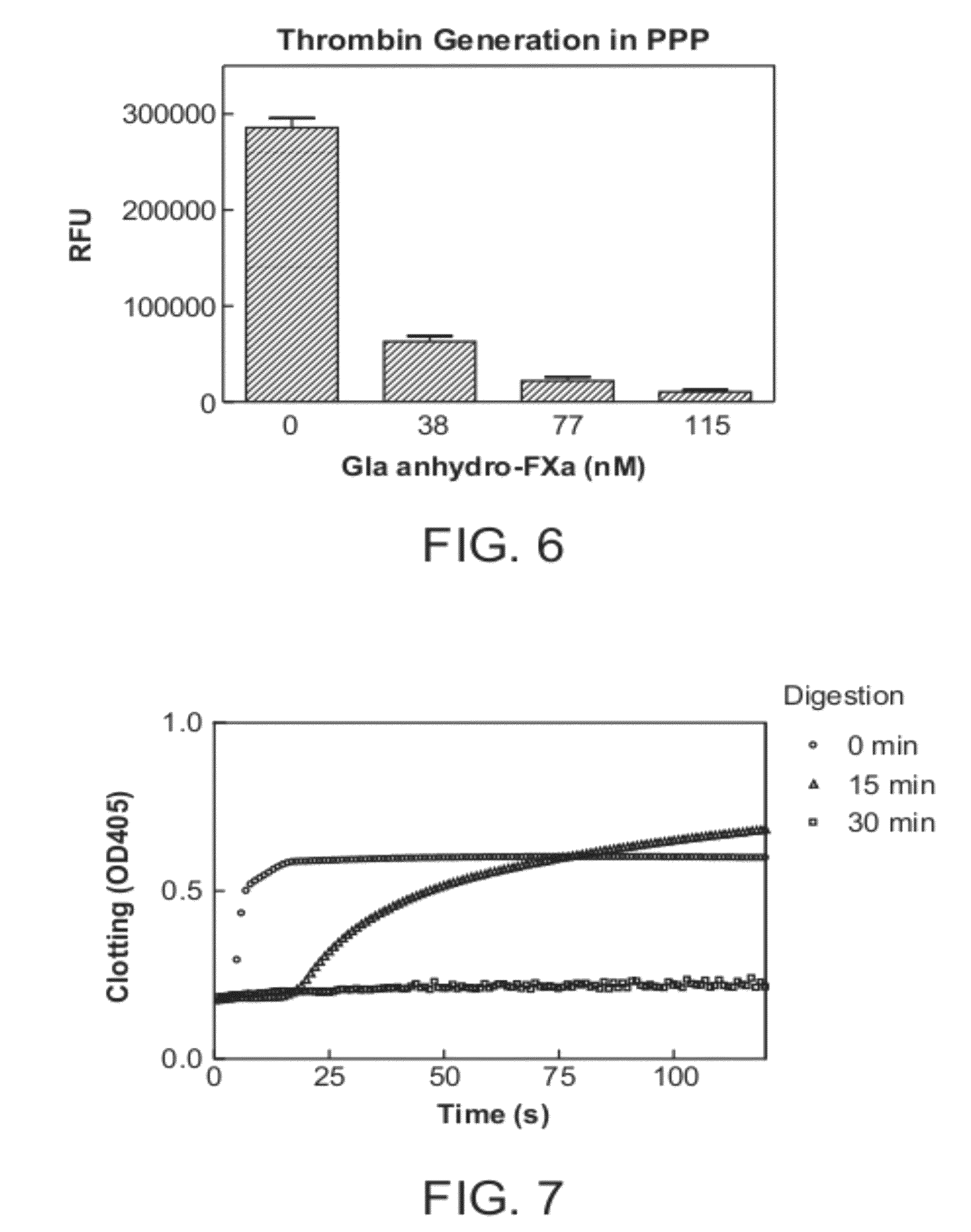
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
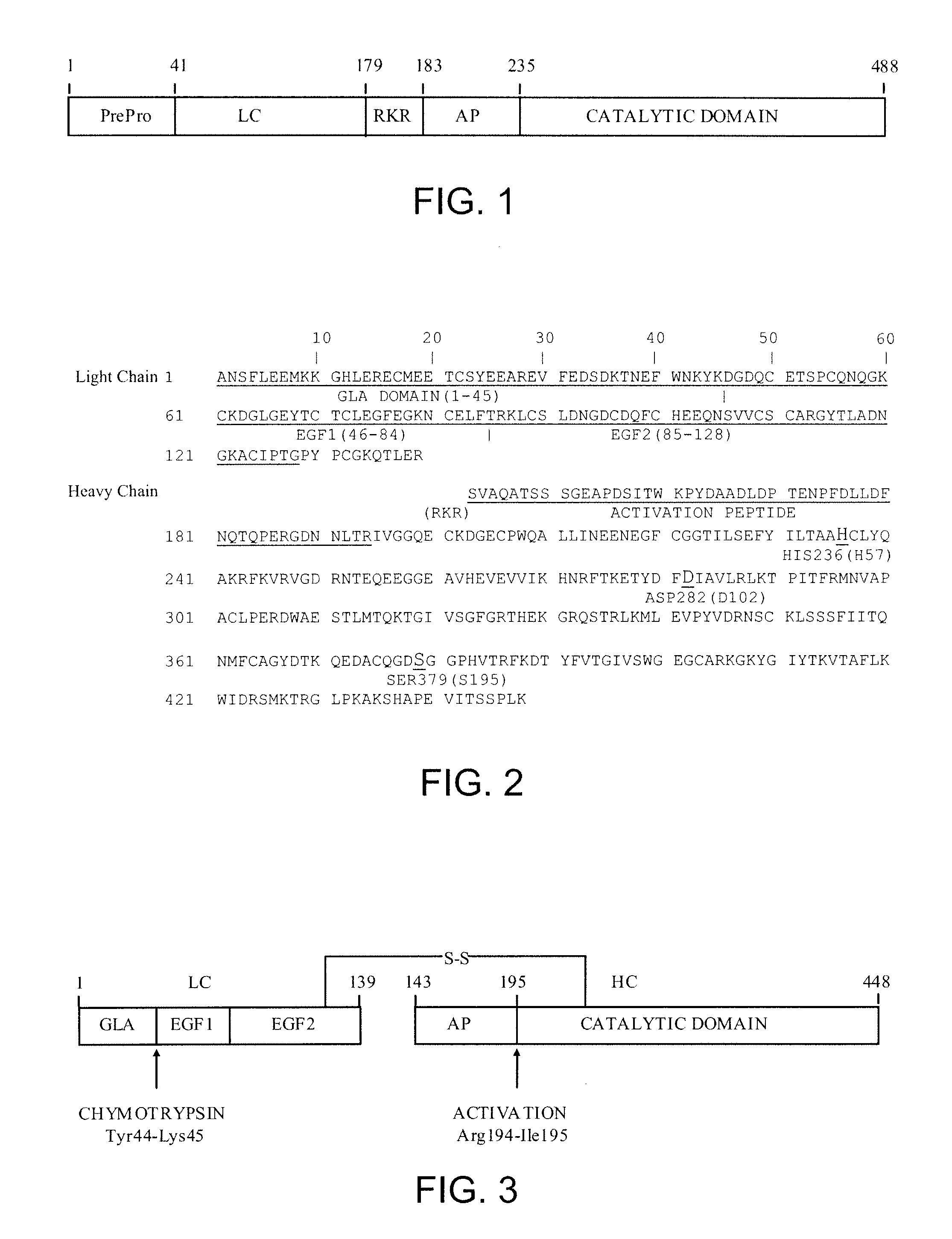
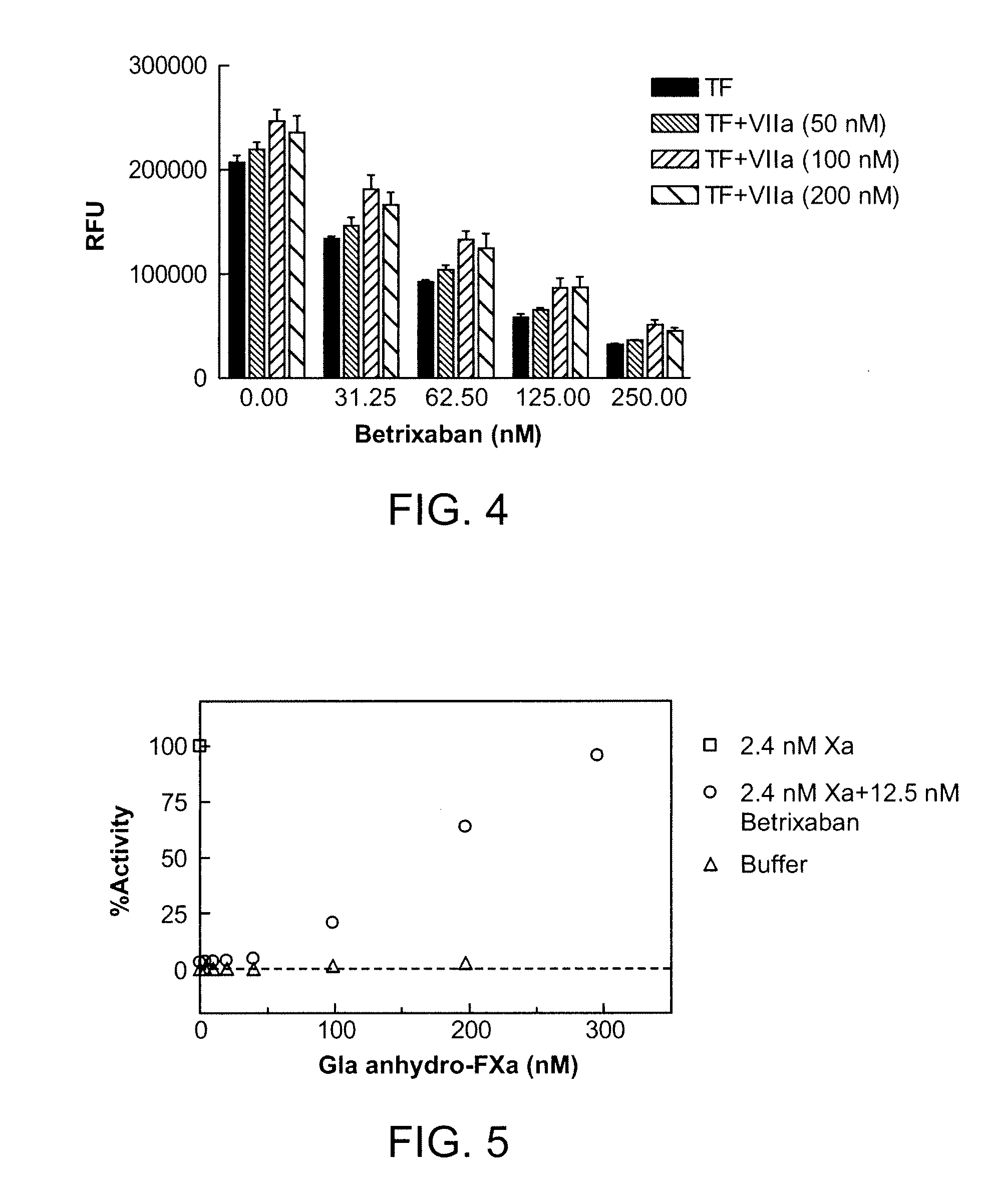
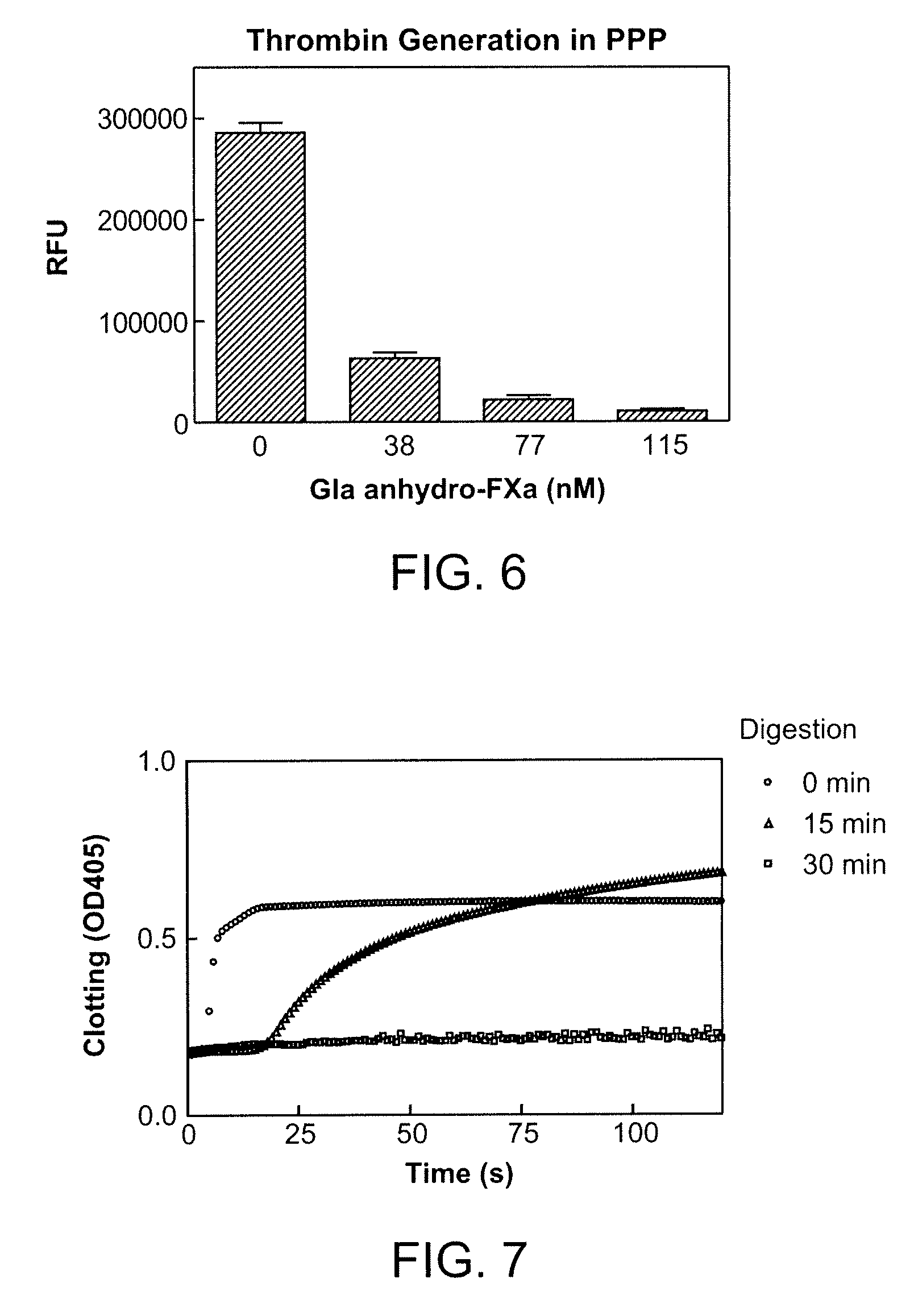
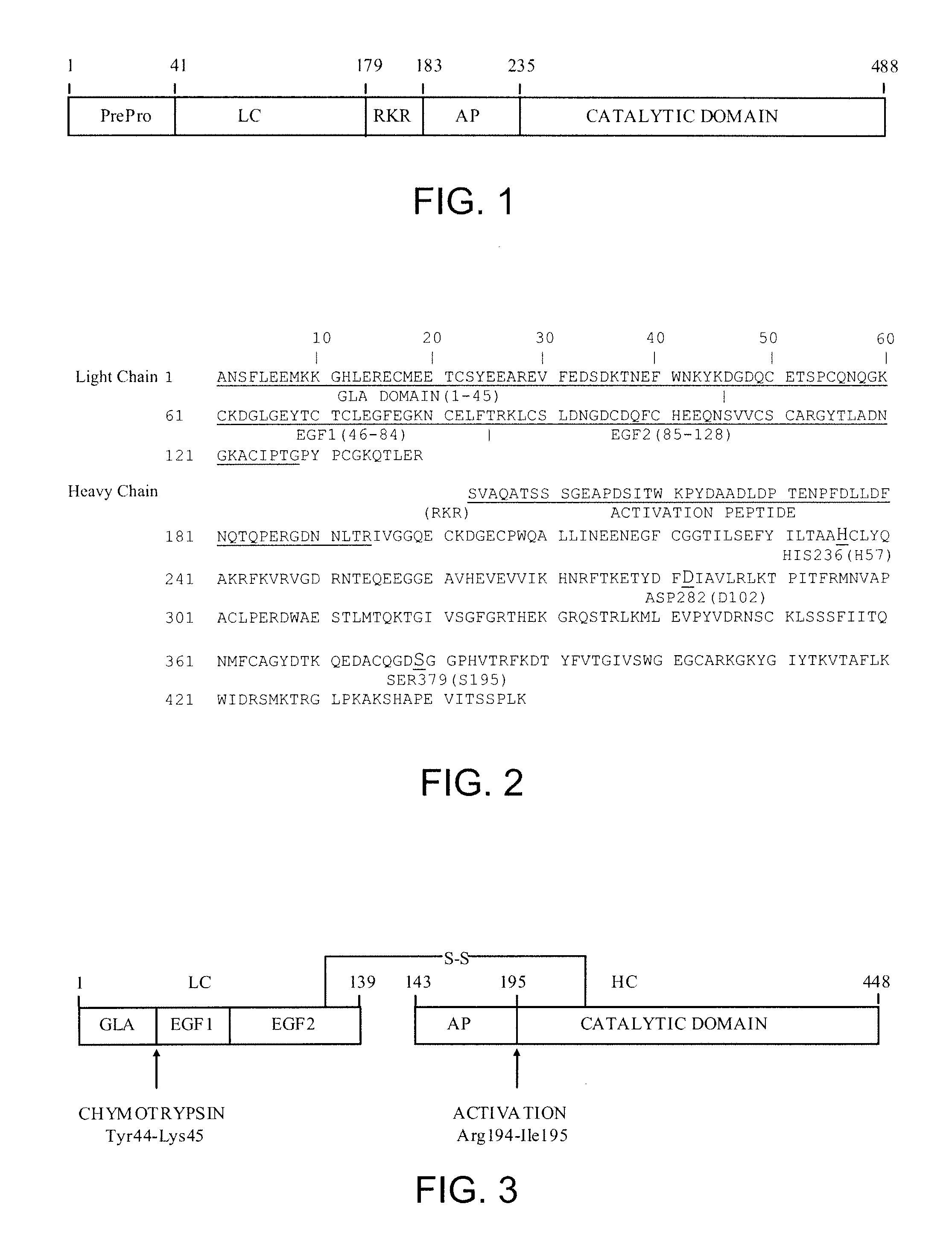
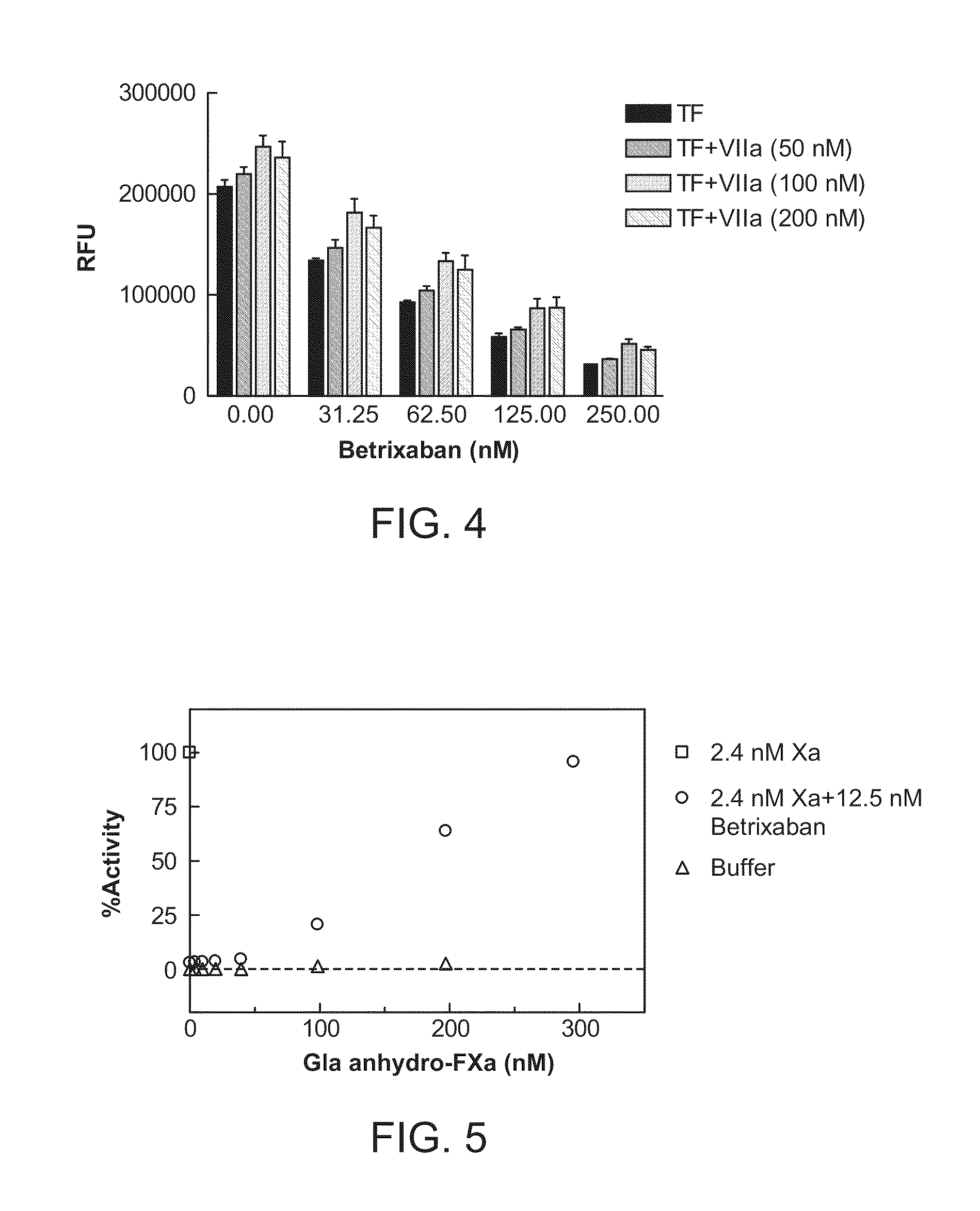
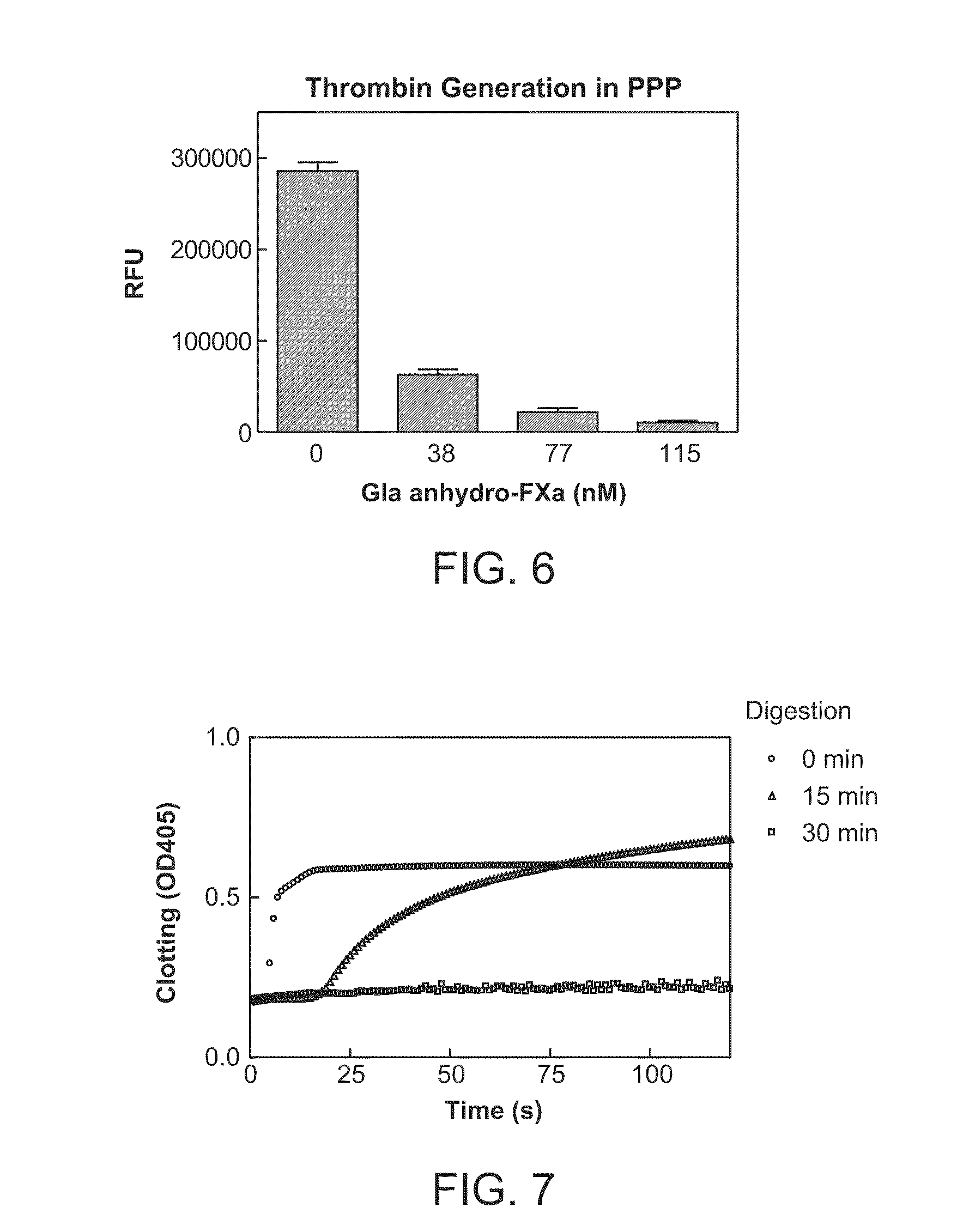
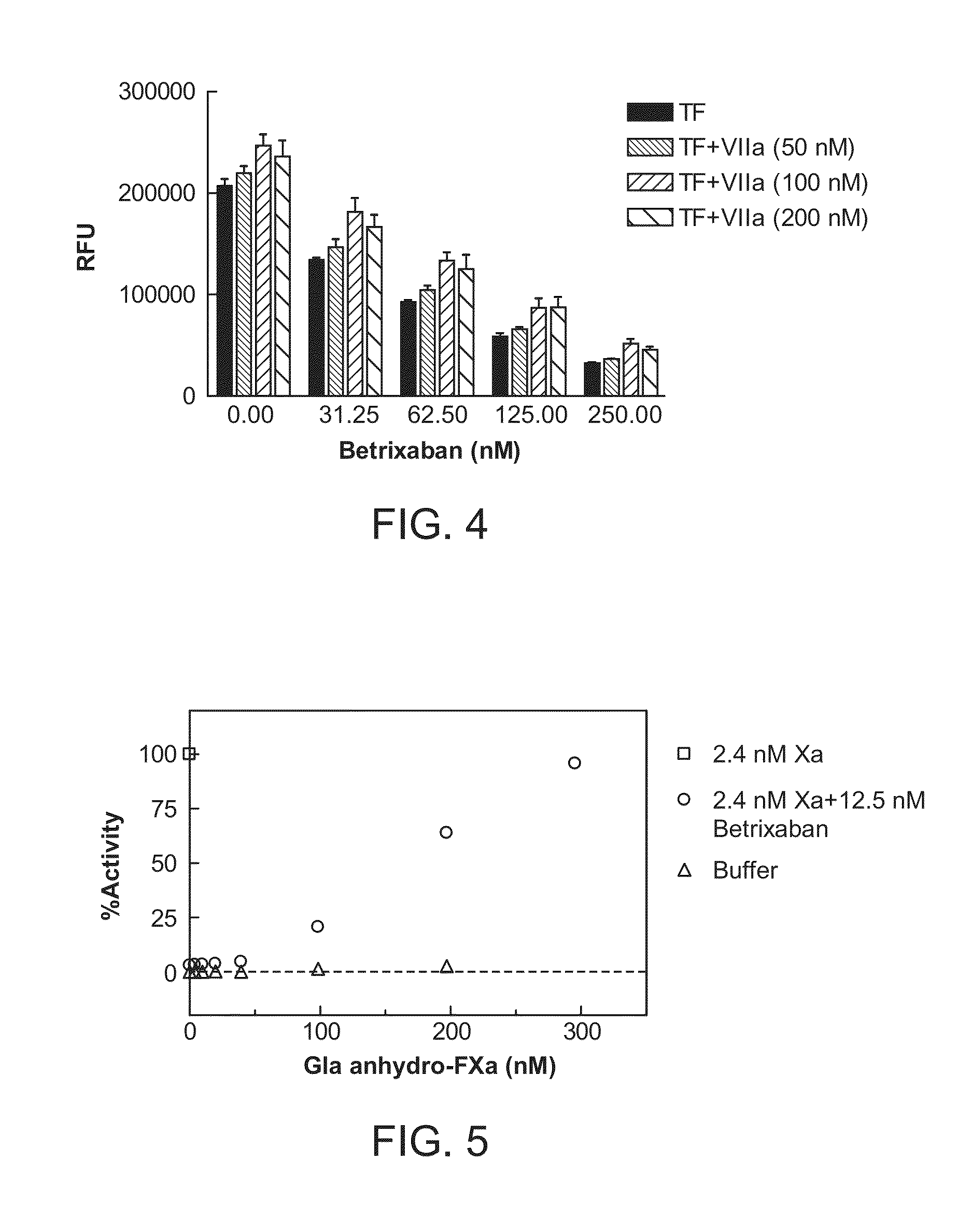
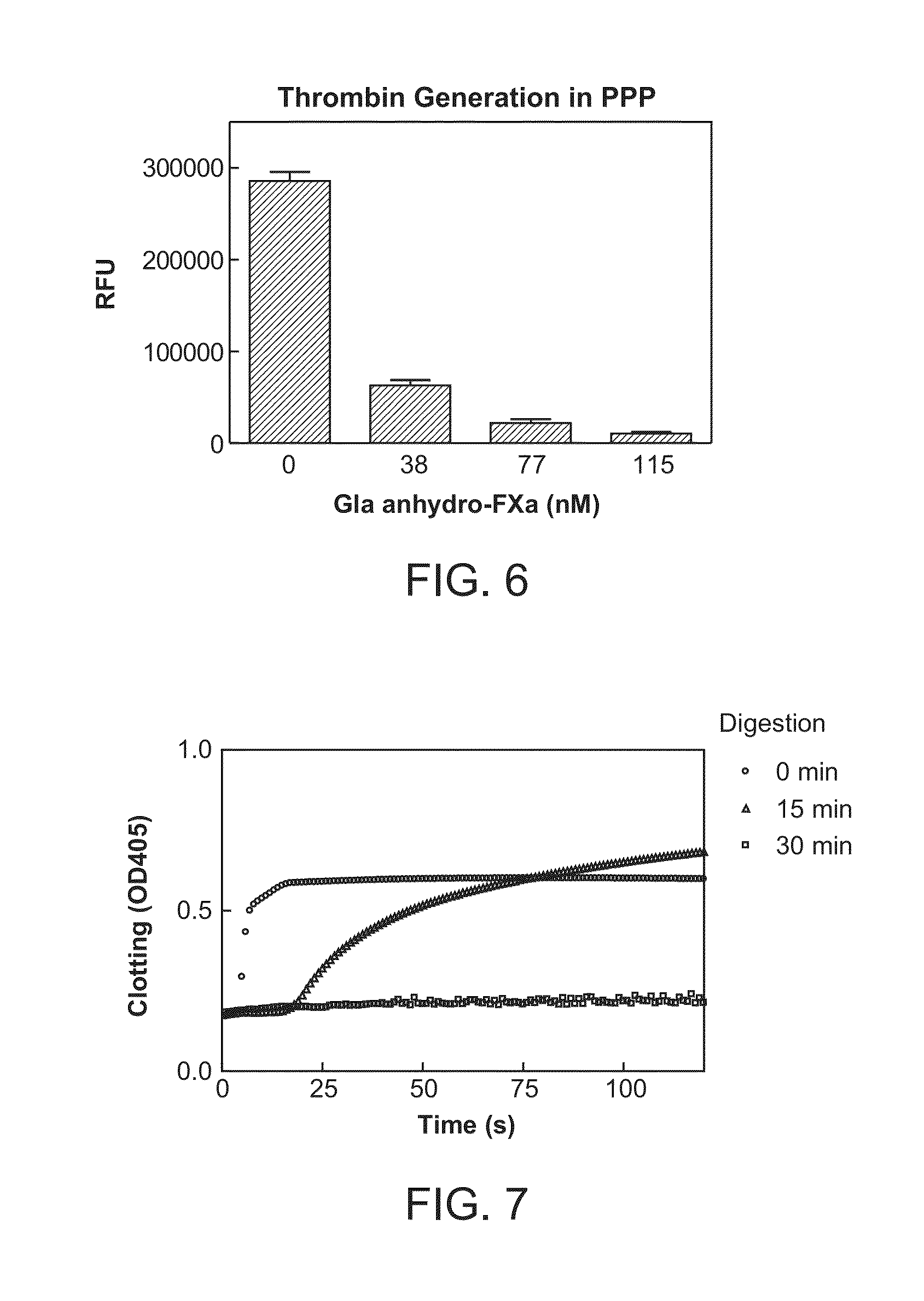
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8153590B2Reduces and removes anticoagulant effectReduced activityPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
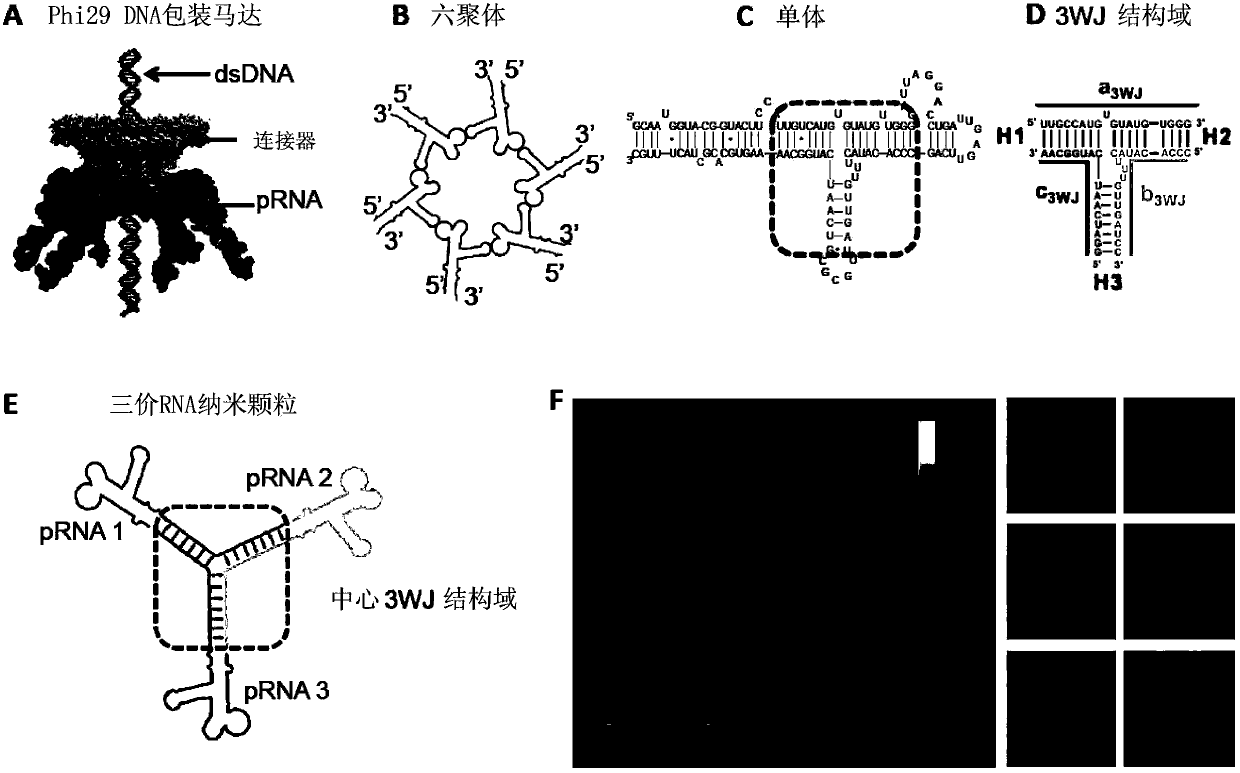
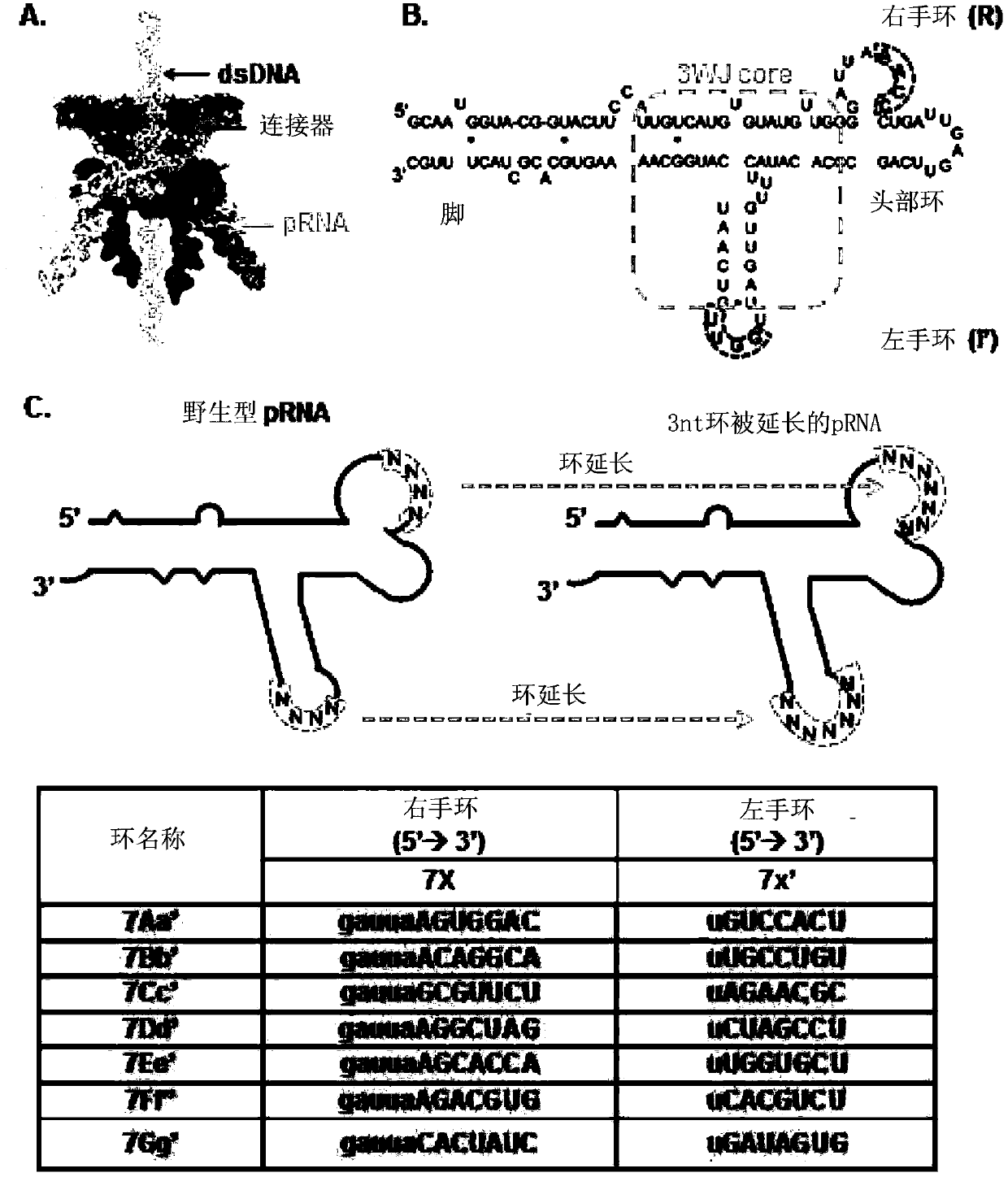
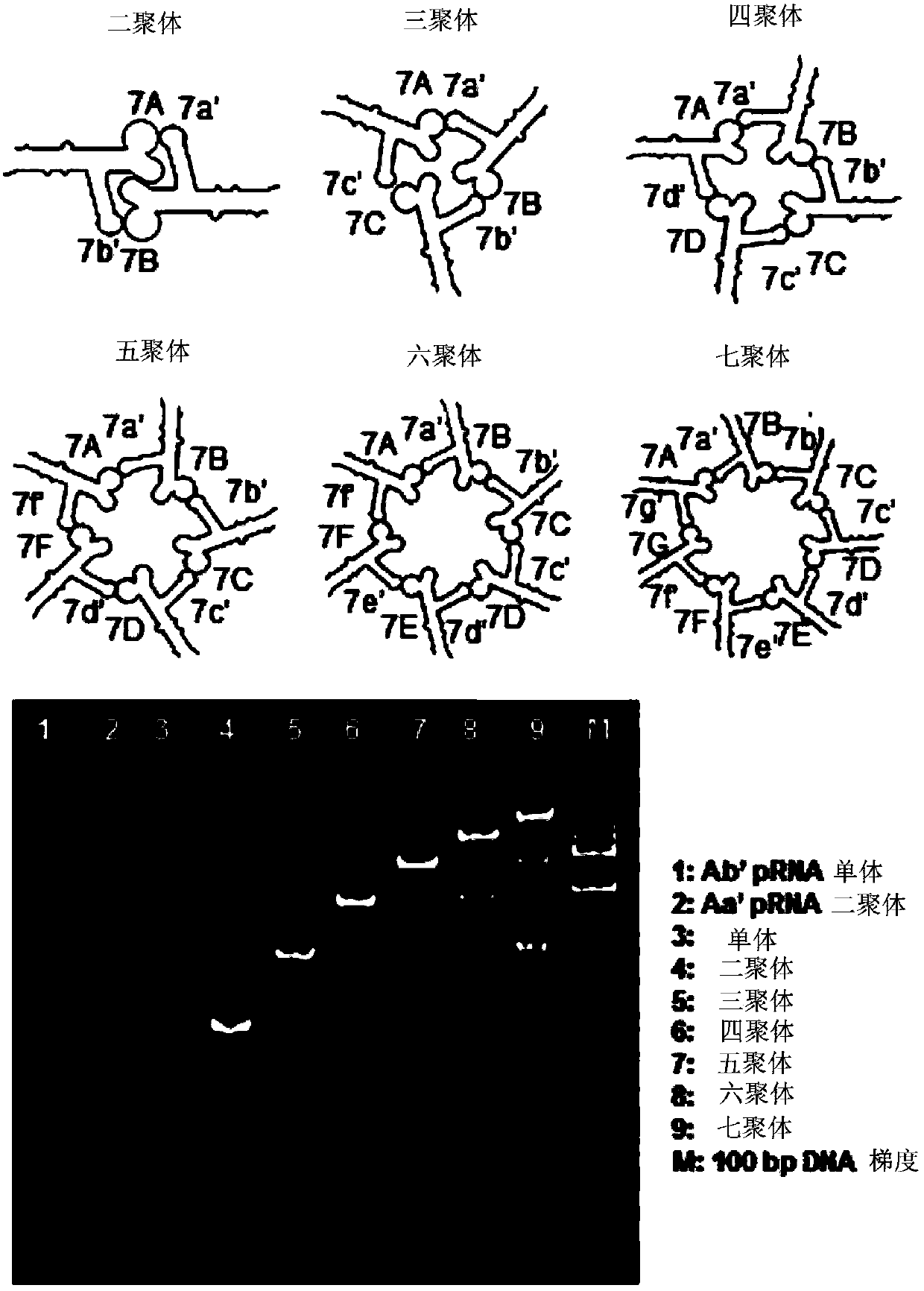
PRNA mutlivalent junction domain for use in stable multivalent RNA nanoparticles
ActiveCN103403189AStrong specificityImprove targetingMicrobiological testing/measurementDNA/RNA fragmentationDiagnostic agentNanoparticle
Trifurcate RNA junction domains derived from phi29 pRNA are described that assemble with high affinity and that are stable in vitro and in vivo. Further expansion of trifurcated RNA domains to multiple way junction scaffolds via creative designs enable an array of toolkit to construct nanoparticle architectures with diverse shapes and angles. The scaffolds can be used to form RNA nanoparticles having a wide variety of uses, including promotion of RNA crystallization, creation of RNA aptamer with high affinity to mimic antibody, delivery of therapeutic and / or diagnostic agents such as biologically active RNA-based moieties, including siRNA, ribozymes, aptamers, and others.
Owner:UNIVERSITY OF CINCINNATI
Novel PEGylation agent
InactiveUS20070184015A1Reduce compliancePromote degradationPeptide/protein ingredientsCalcitoninsHalf-lifeBlood plasma
To address the issue of degradation by enzymatic reactions to proteins and peptides, polyethylene glycol (PEGylation) of the proteins and peptides has been established. PEGylated proteins and peptides have increased plasma half-lives and reduced immunogenicity. To further improve and extend the plasma half-life of desired protein or peptide therapeutics, a novel branched molecule of PEG possessing three PEGs with a single point of attachment is designed in this invention disclosure.
Owner:HAHN SOONKAP
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8268783B2Reduces and removes anticoagulant effectReduced activityBiocidePeptide/protein ingredientsFactor XAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
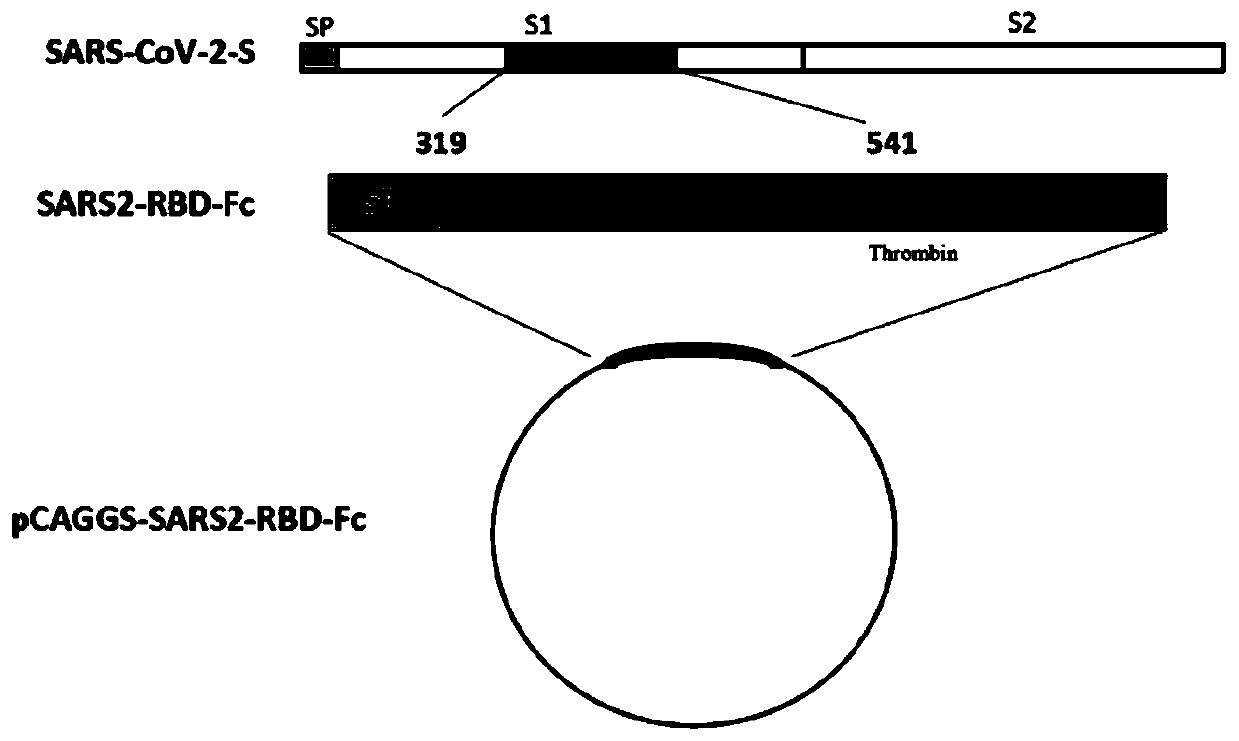
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20100255000A1Preventing and reducing bleedingReduces and removes anticoagulant effectPeptide/protein ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Cellular fibronectin as a diagnostic marker in cardiovascular disease and methods of use thereof
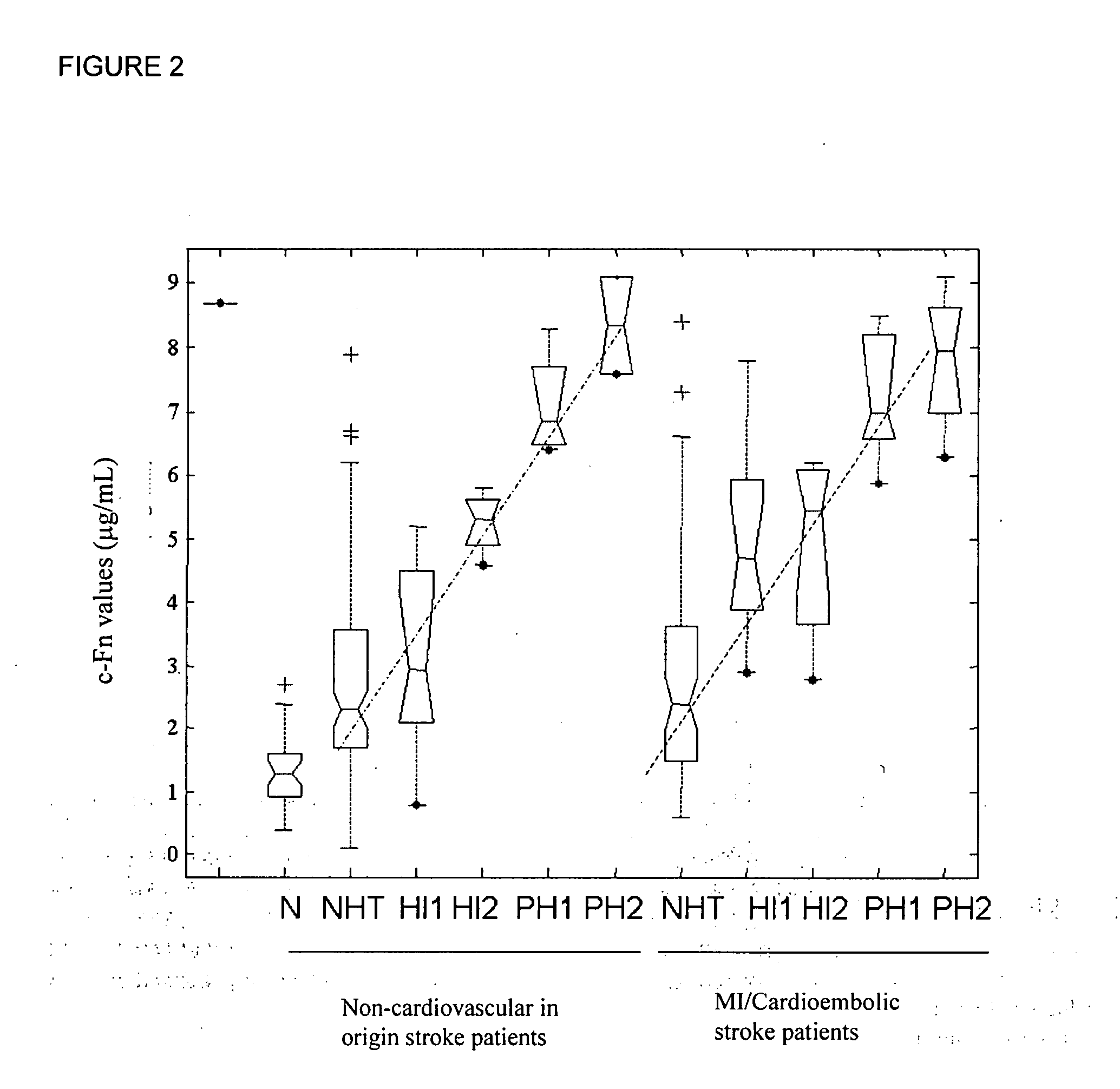
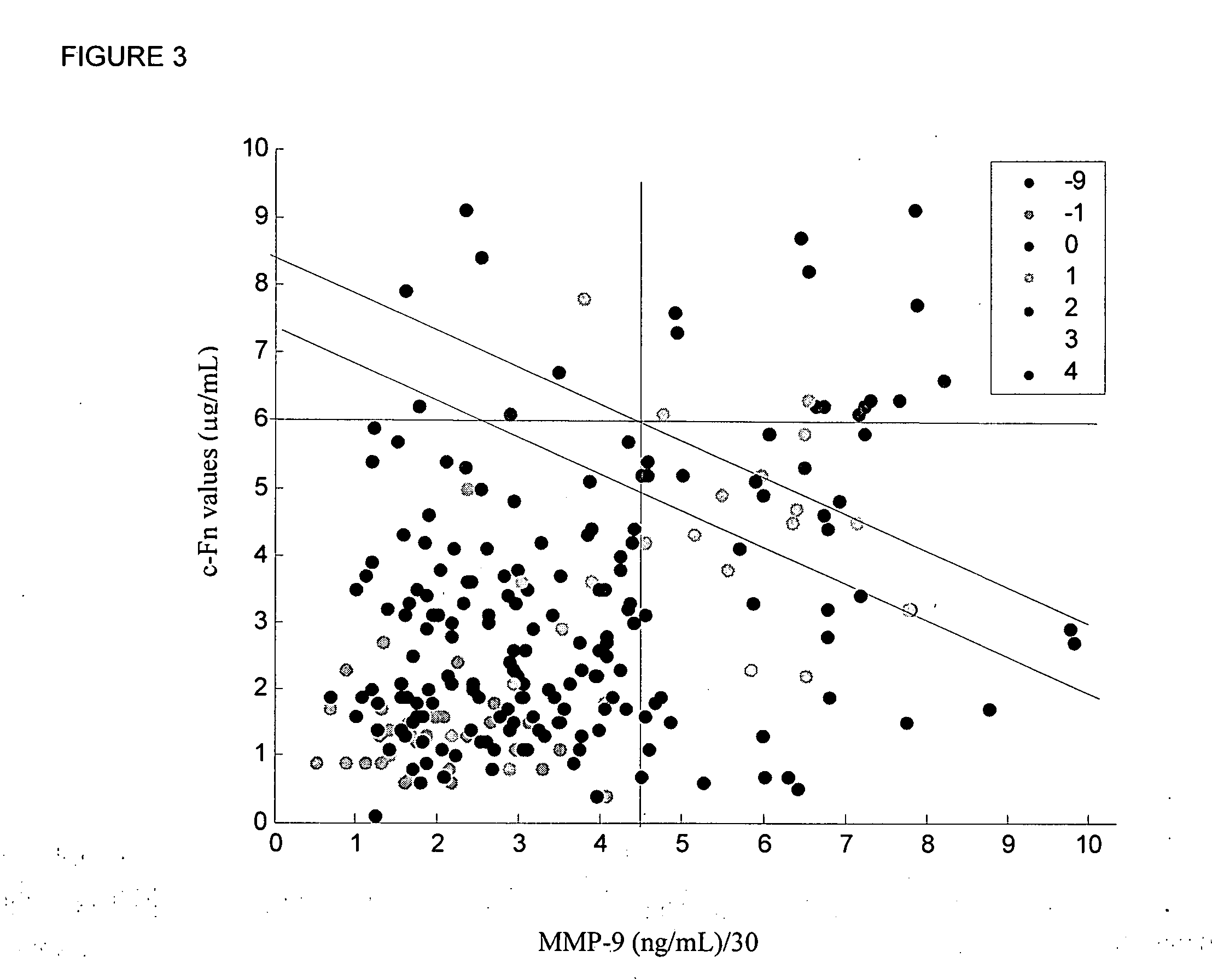
InactiveUS20080010024A1Reduce eliminateProlonged plasma half-lifeDisease diagnosisBiological testingCardiovascular InjuryCardiovascular Disorder
Thrombolytic therapy in the treatment of a cardiovascular event such as myocardial infarction (MI) carries with it a chance of suffering a hemorrhagic incident leading to severe disability and often death. Methods for the evaluation of proper therapy for a specific patient who has suffered a cardiovascular event employ a variety of bio-markers including cellular fibronectin (c-Fn) assembled as a panel for evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects the methods permit early detection of potential bleeding events, determination of the prognosis of a patient presenting cardiovascular damage, and identification of a patient at risk for hemorrhage when given thrombolytic therapy. The disclosed methods provide rapid, sensitive and specific assays to greatly reduce the risk of bleeding or the number of patients that can receive the most beneficial treatment for their cardiovascular event, and to reduce the human and economic costs associated with bleeding following such treatments.
Owner:PREDICTION SCI
Antidotes for factor xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS20100125052A1Reduce potential side effectsLow effective dosePeptide/protein ingredientsMammal material medical ingredientsMedicineAntidote
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS8455439B2Low effective doseReduce potential side effectsPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Viral core protein-cationic lipid-nucleic acid-delivery complexes
InactiveUS20090209037A1Improve liposome based gene transferImproved cationic liposome mediated gene transferNervous disorderPeptide/protein ingredientsHeterologousViral Core Proteins
A nucleic acid delivery complex is provided which comprises a condensed polypeptide / nucleic acid complex and a cationic lipid wherein the complex comprises (a) a nucleic acid sequence of interest (NOI); and (b) one or more viral nucleic acid packaging polypeptides, or derivatives thereof, said polypeptides or derivatives thereof being (i) capable of binding to the NOI; and (ii) capable of condensing the NOI; and wherein the NOI is heterologous to the polypeptide. Also provided is a method of introducing an NOI into a cell using the delivery vector.
Owner:TAGAWA TOSHIAKI +7
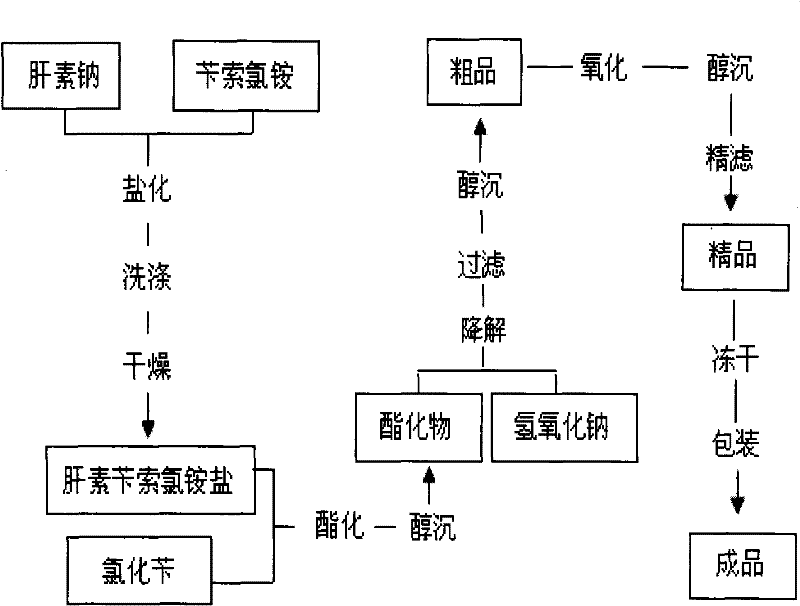
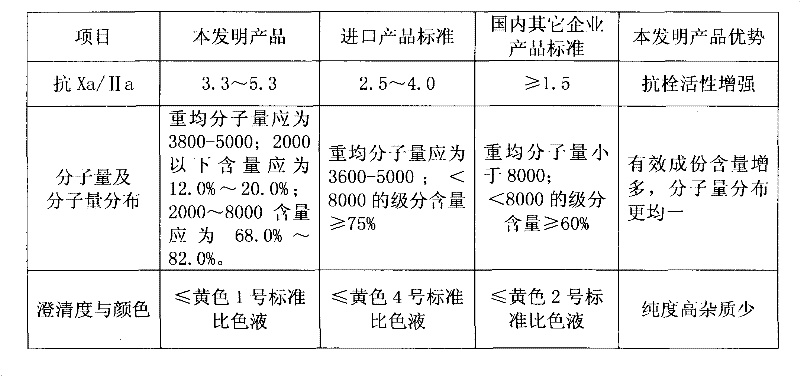
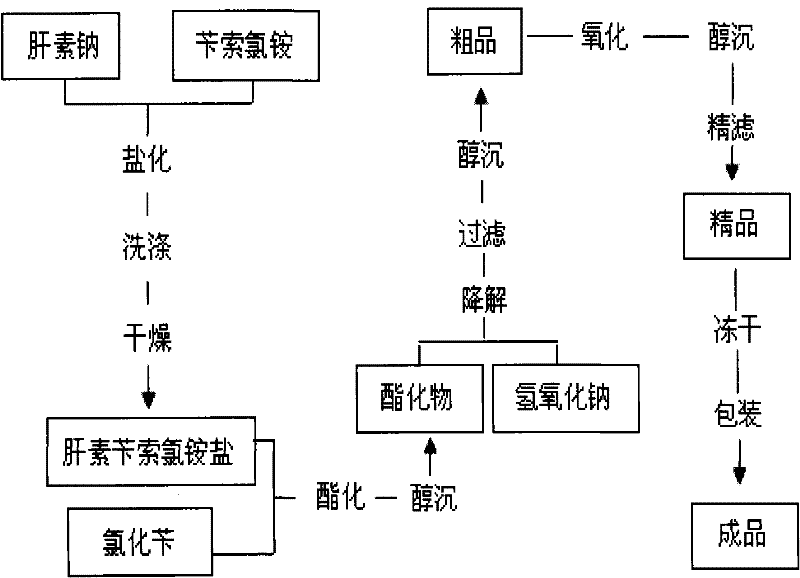
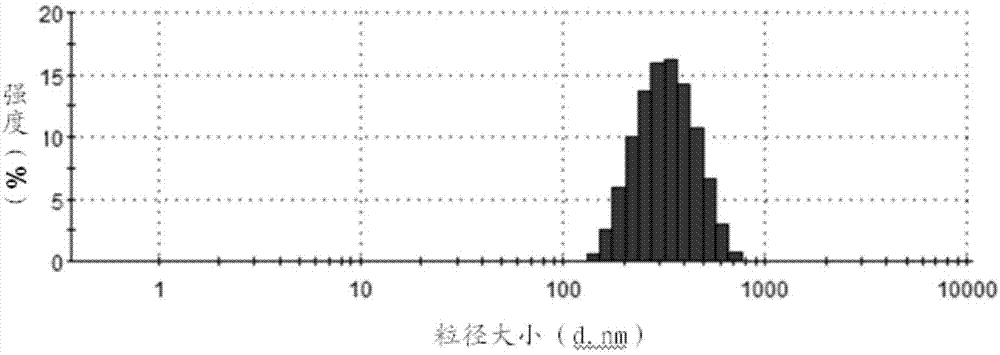
Method for preparing enoxaparin sodium
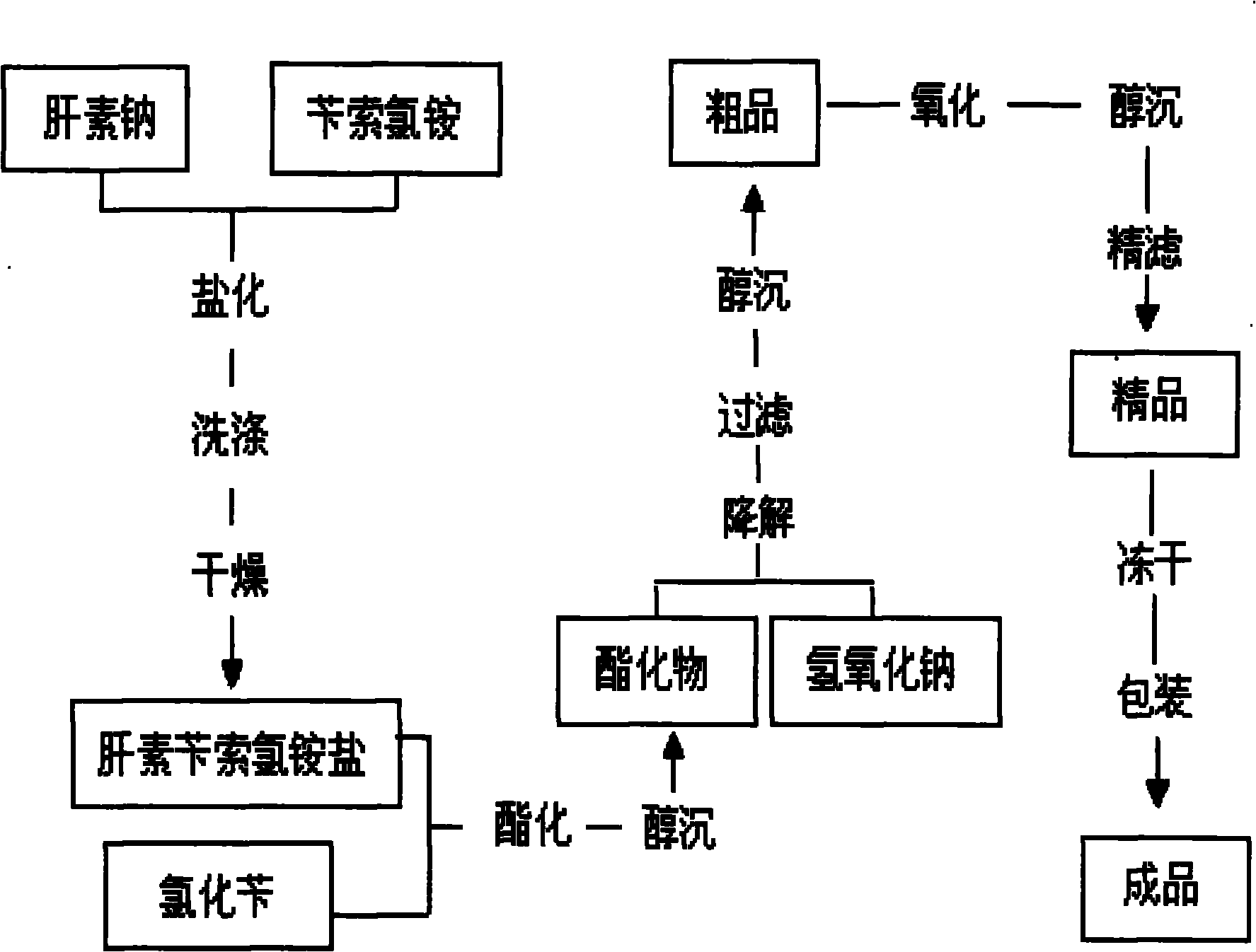
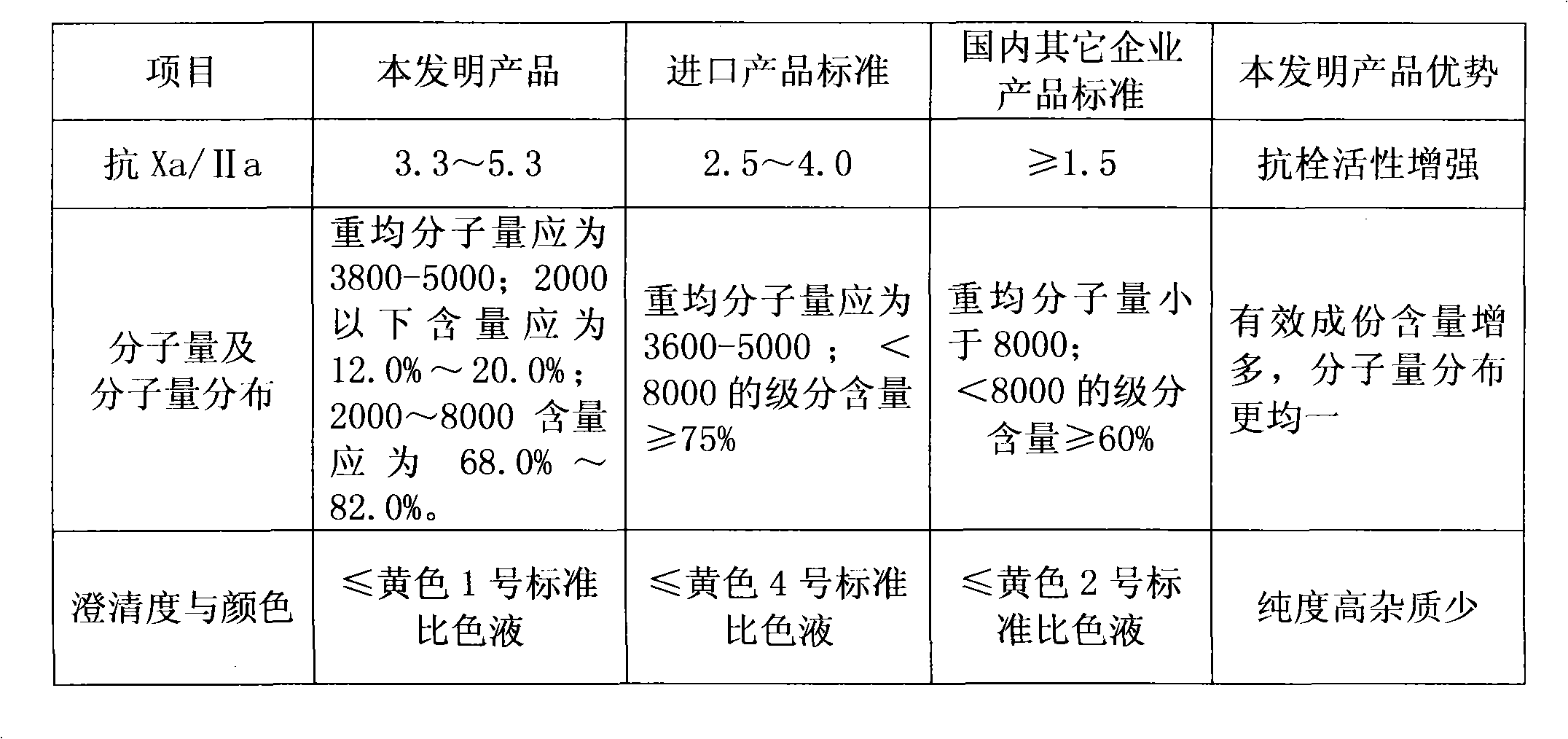
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminating the side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Modifications of peptide compositions to increase stability and delivery efficiency
ActiveUS20090042769A1Extended half-lifeGreat propensityPeptide/protein ingredientsPeptide sourcesPhysical stabilityDisulfide bond
The disclosed invention relates to methods of modifying peptide compositions to increase stability and delivery efficiency. Specifically, the disclosed invention relates to methods to increase the stability and delivery efficiency of protein kinase C (PKC) modulatory peptide compositions. A “therapeutic peptide composition” comprises a “carrier peptide” and a “cargo peptide.” A “carrier peptide” is a peptide or amino acid sequence within a peptide that facilitates the cellular uptake of the therapeutic peptide composition. The “cargo peptide” is a PKC modulatory peptide. Peptide modifications to either the carrier peptide, the cargo peptide, or both, which are described herein increase the stability and delivery efficiency of therapeutic peptide compositions by reducing disulfide bond exchange, physical stability, reducing proteolytic degradation, and increasing efficiency of cellular uptake.
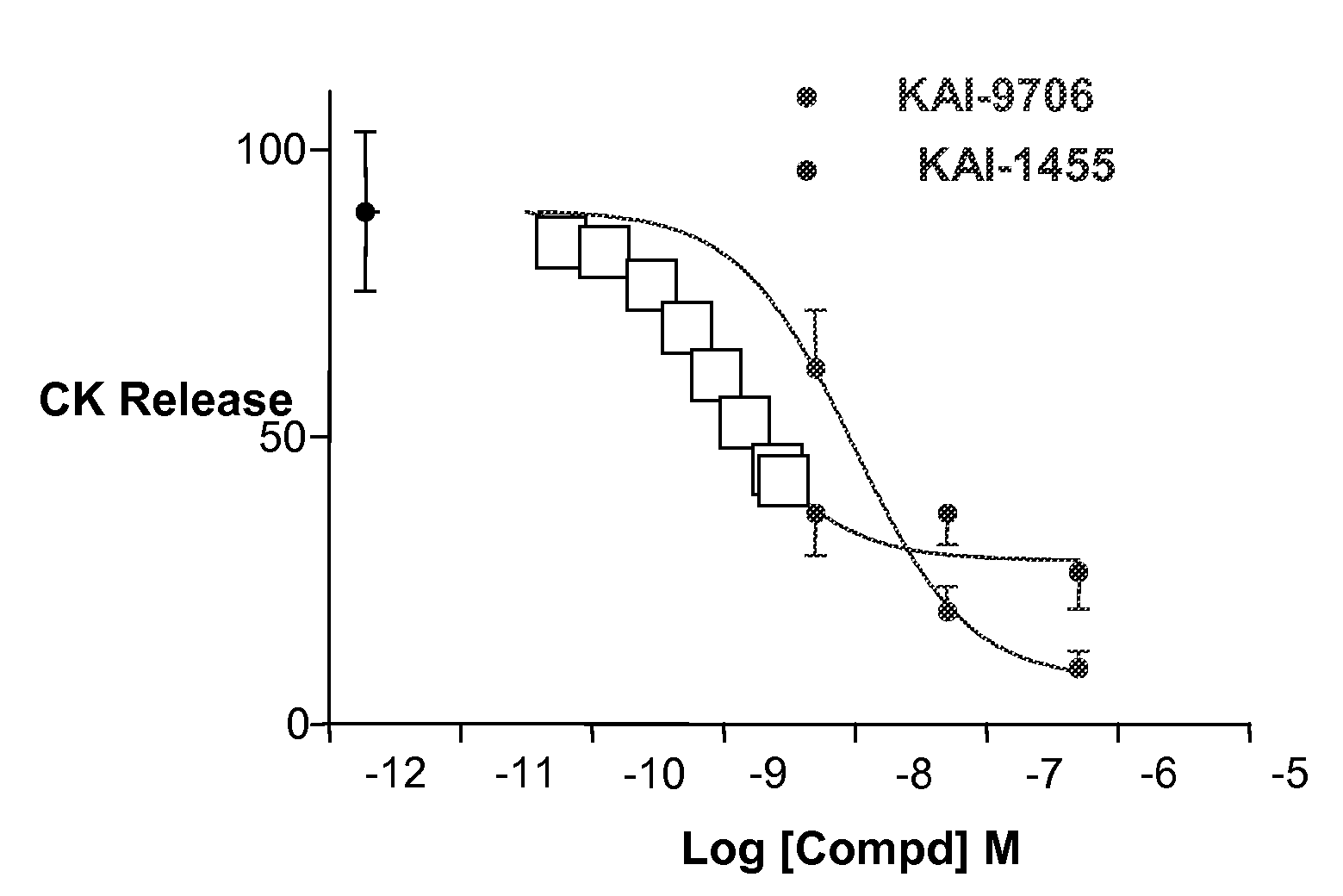
Owner:KAI PHARMA
Long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087178AChemically stableEasy to degradePeptide/protein ingredientsMetabolism disorderMicrowaveSynthesis methods
The invention relates to long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through adding a modified 37th amino acid to natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Organ and biological tissue preservation cold storage solution
InactiveUS20020115634A1Prolonged plasma half-lifePronounced growth-suppressing propertyBiocidePeptide/protein ingredientsMedicineOxygen
Cold storage solutions for the preservation of organs and biological tissues prior to implantation, including a cellular energy production stimulator under anaerobic conditions, an anti-inflammatory agent, and an oxygen free radical scavenger.
Owner:ORGAN RECOVERY SYST +1
Modified cytokines for use in cancer therapy
InactiveUS20030157056A1Improve biological activityImprove hydrophobicityBiocidePeptide/protein ingredientsAntigenCancer therapy
Owner:OSPEDALE SAN RAFFAELE SRL
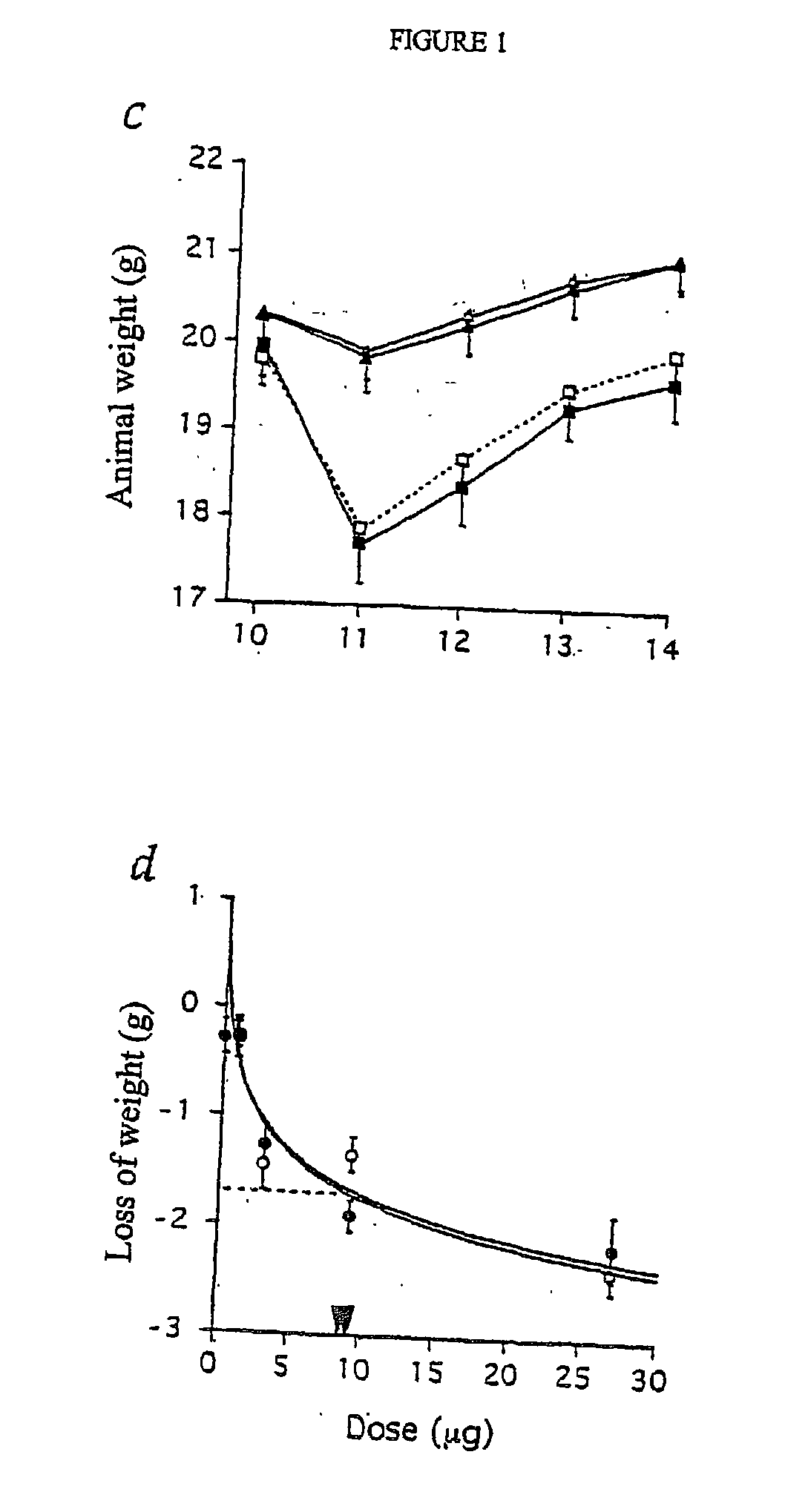
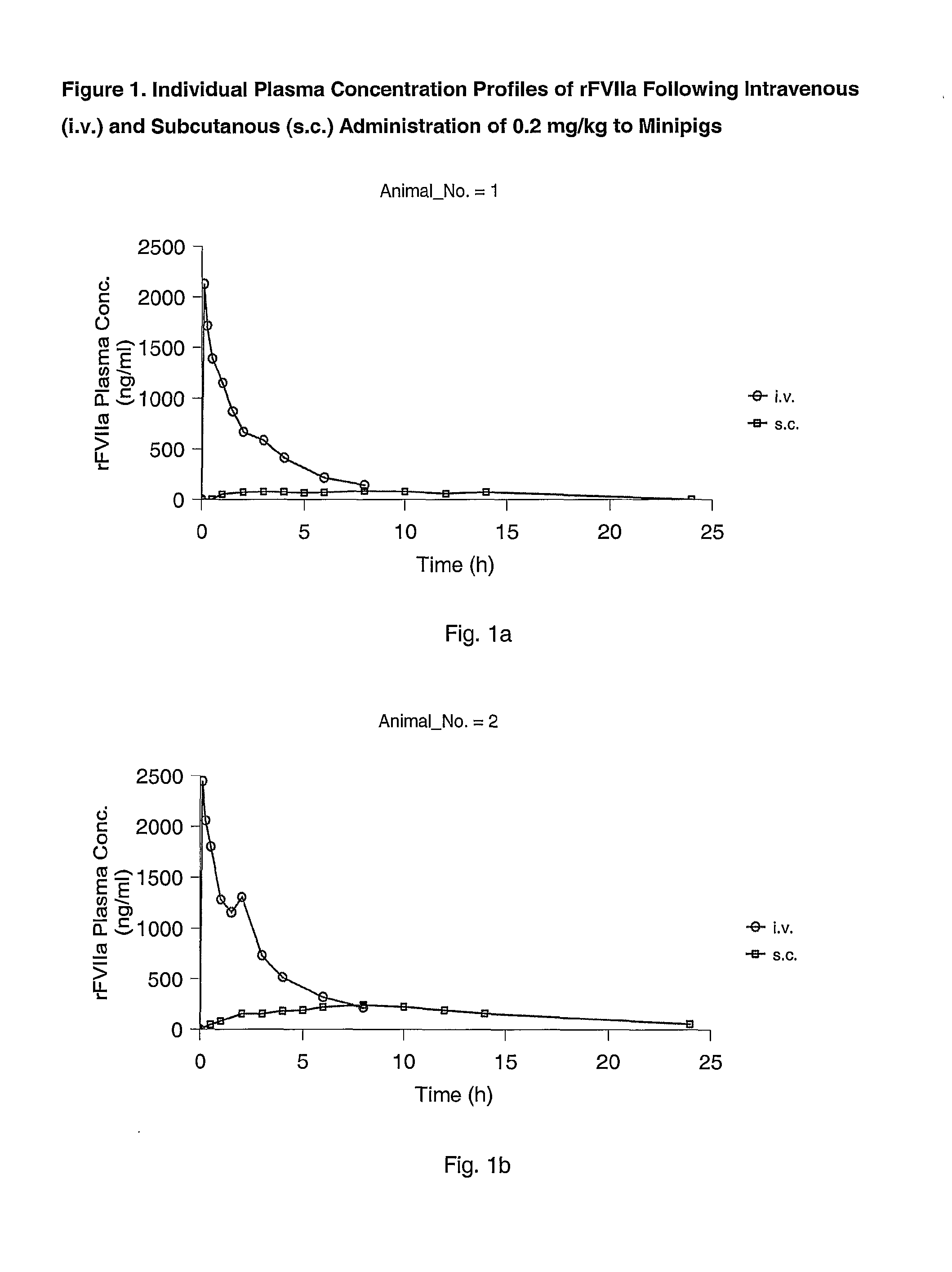
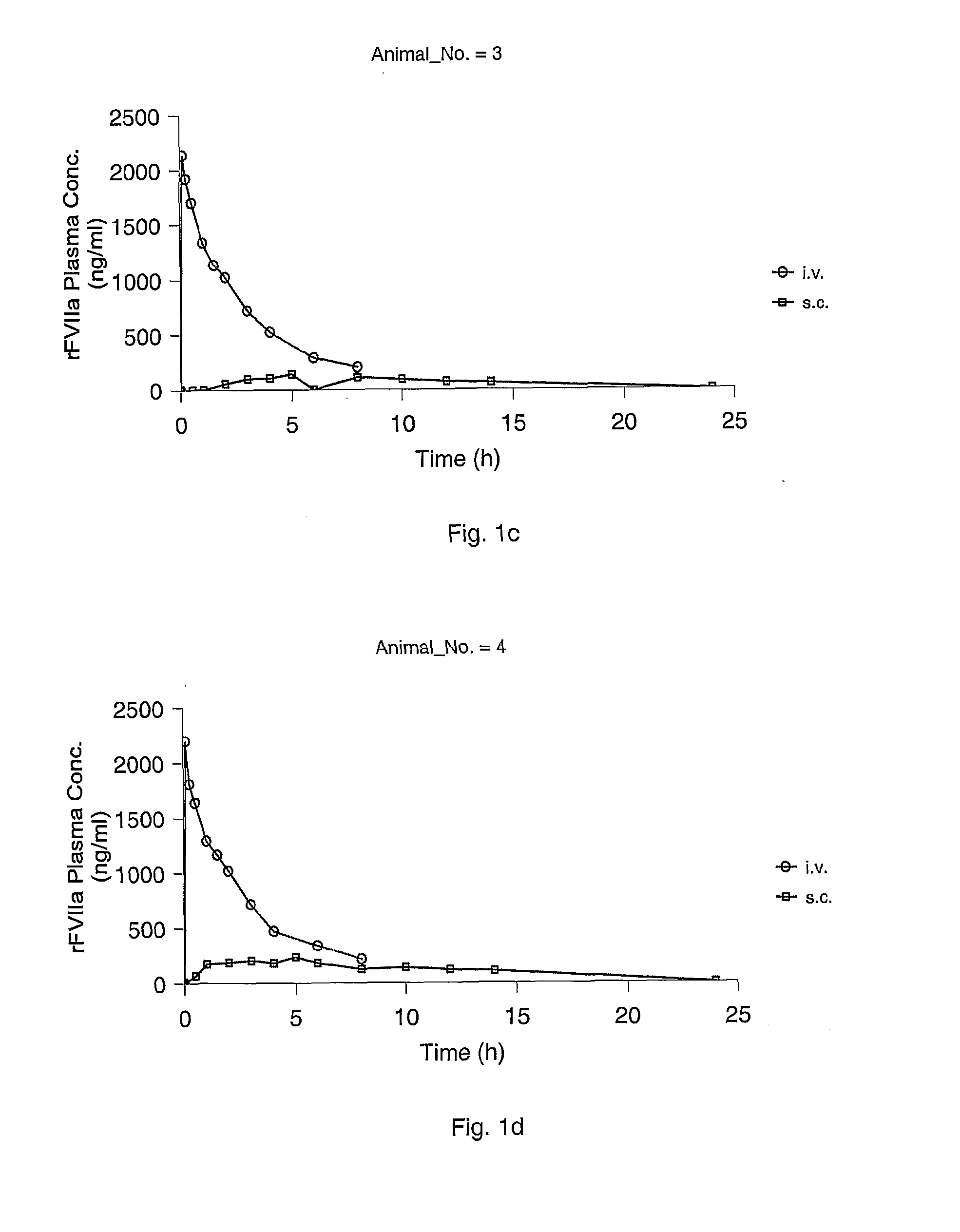
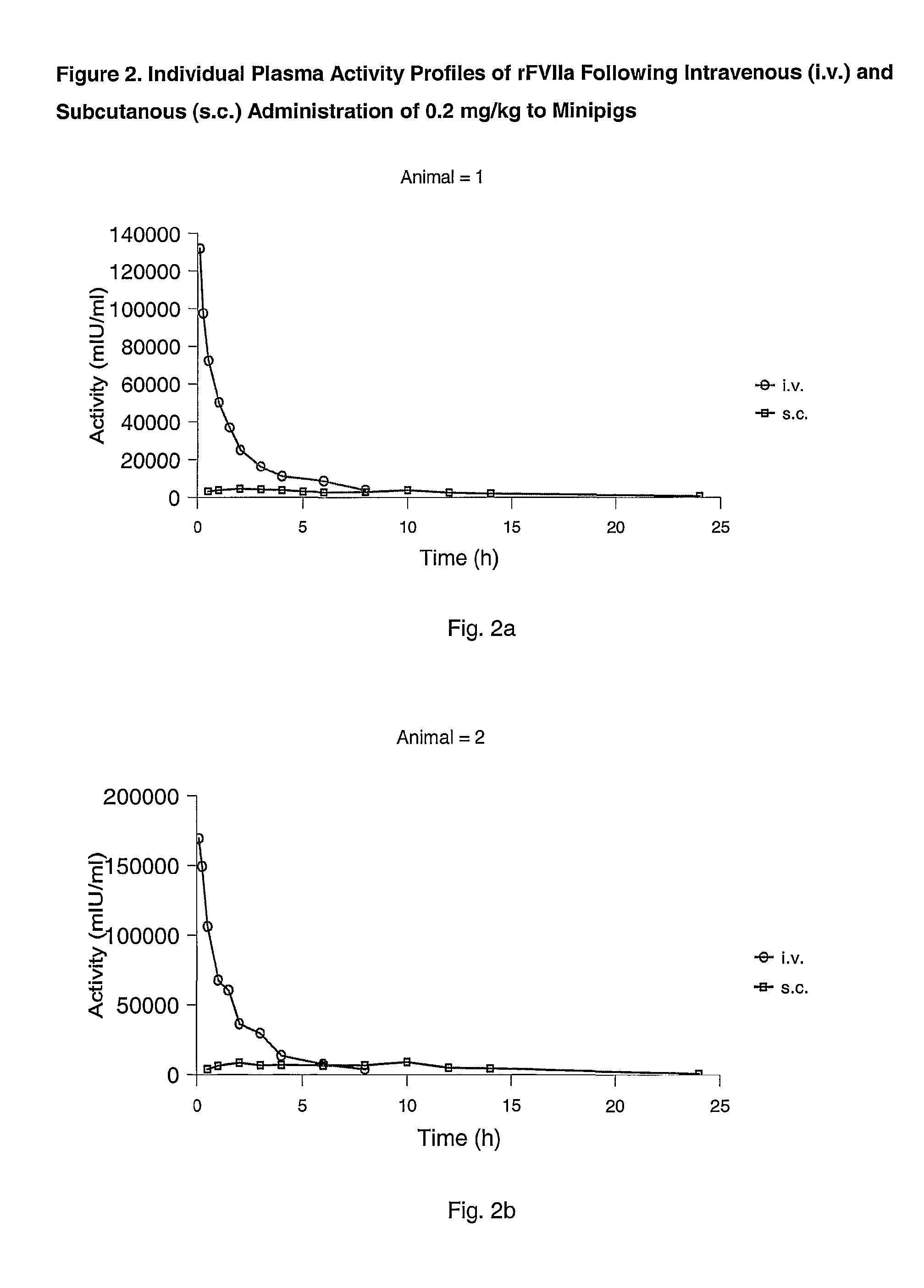
Subcutaneous administration of coagulation factor VII
InactiveUS7786070B2Acceptable absorptionImprove the level ofPeptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBiological half-life
The invention relates to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, said medicament being for subcutaneous, intramuscular or intradermal administration, and to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, wherein said medicament, when administered subcutaneously, intradermally or intramuscularly, shows a prolonged biological half-life.
Owner:NOVO NORDISK HEALTH CARE AG
Long-acting Exendin-4 analogue and application thereof
InactiveCN105936647AChemically stableProlonged plasma half-lifePeptide/protein ingredientsMetabolism disorderLong actingPharmacological action
The invention relates to a long-acting Exendin-4 analogue and a synthesis method thereof. Exendin-4 is transformed to obtain the Exendin-4 analogue having longer pharmacological action time, synthesis of a target polypeptide is rapidly achieved by a microwave-promoted solid phase synthesis method, and a crude product is purified and freeze-dried to obtain the Exendin-4 analogue.
Owner:CHINA PHARM UNIV
Novel anti-tumor nano-drug carrier and preparation method and application thereof
ActiveCN103083673AStrong volumeIncrease in sizePowder deliveryPharmaceutical non-active ingredientsPolyethylene glycolIn vivo
The invention discloses a novel anti-tumor nano-drug carrier and a preparation method and application thereof. The method comprises the following steps: carrying out an amidation reaction on chitosan and deoxycholic acid to form a copolymer chitosan-deoxycholic acid, forming an intermediate of the chitosan-deoxycholic acid and formaldehyde, carrying out a reaction on the intermediate and polyethylene glycol, carrying out an amidation reaction on the obtained chitosan-deoxycholic acid-polyethylene glycol so as to prepare the novel anti-tumor nano-drug carrier. The characteristics that a folate receptor on the surface of the tumor cells has high expression and the folate receptor hardly has excessive expression in normal tissues are utilized, the novel anti-tumor nano-drug carrier connected with folate and the tumor cells have high affinity, and the defect that the common nanoparticle function is single is overcome. The prepared novel anti-tumor nano-drug carrier can well avoid cytophagy of phagocyte, the carrier circulates in vivo for a long time and is not eliminated, the active targeting modification can be better combined with tumor cell specificity, and the harm of the drug to normal human cells is reduced.
Owner:JINAN UNIVERSITY
Glucagon-like peptide 1 (GLP-1) analogues with long-acting effect and application thereof
InactiveCN103087180AChemically stableProlonged plasma half-lifePeptide/protein ingredientsMetabolism disorderLong actingPharmacological action
The invention relates to glucagon-like peptide 1 (GLP-1) analogues with a long-acting effect and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through replacing / modifying 17th, 26th, 34th and 37th amino acids of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to obtain the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Glucagon-likepeptide1 derivatives and use thereof
ActiveCN102186881AChemically stableNot easy to degradeNervous disorderPeptide/protein ingredientsBlood plasmaBULK ACTIVE INGREDIENT
The invention relates to a type of Glucagon-likepeptide1 and the use thereof. The structure of the Glucagon-likepeptide1 derivatives is presented in the following formula. The invention also provides a medical salt, a solvolyte, a chelate or a non-covalent complex formed by the compounds shown by the structure, a prodrug based on the compound, or any mix of the above form. The chemical property of the compounds provided in the invention is stable, unlikely to be degraded by IV (DPP-IV) in body. When used for decreasing blood glucose concentration in body, the compound or the drug adopting the compound as an active ingredient have relatively long plasma half-life (above 30 hours) and an significant effect in decreasing blood glucose concentration. X, in the formula, is selected from glycin or glycinamide.
Owner:BETTA PHARM CO LTD
Method for preparing enoxaparin sodium
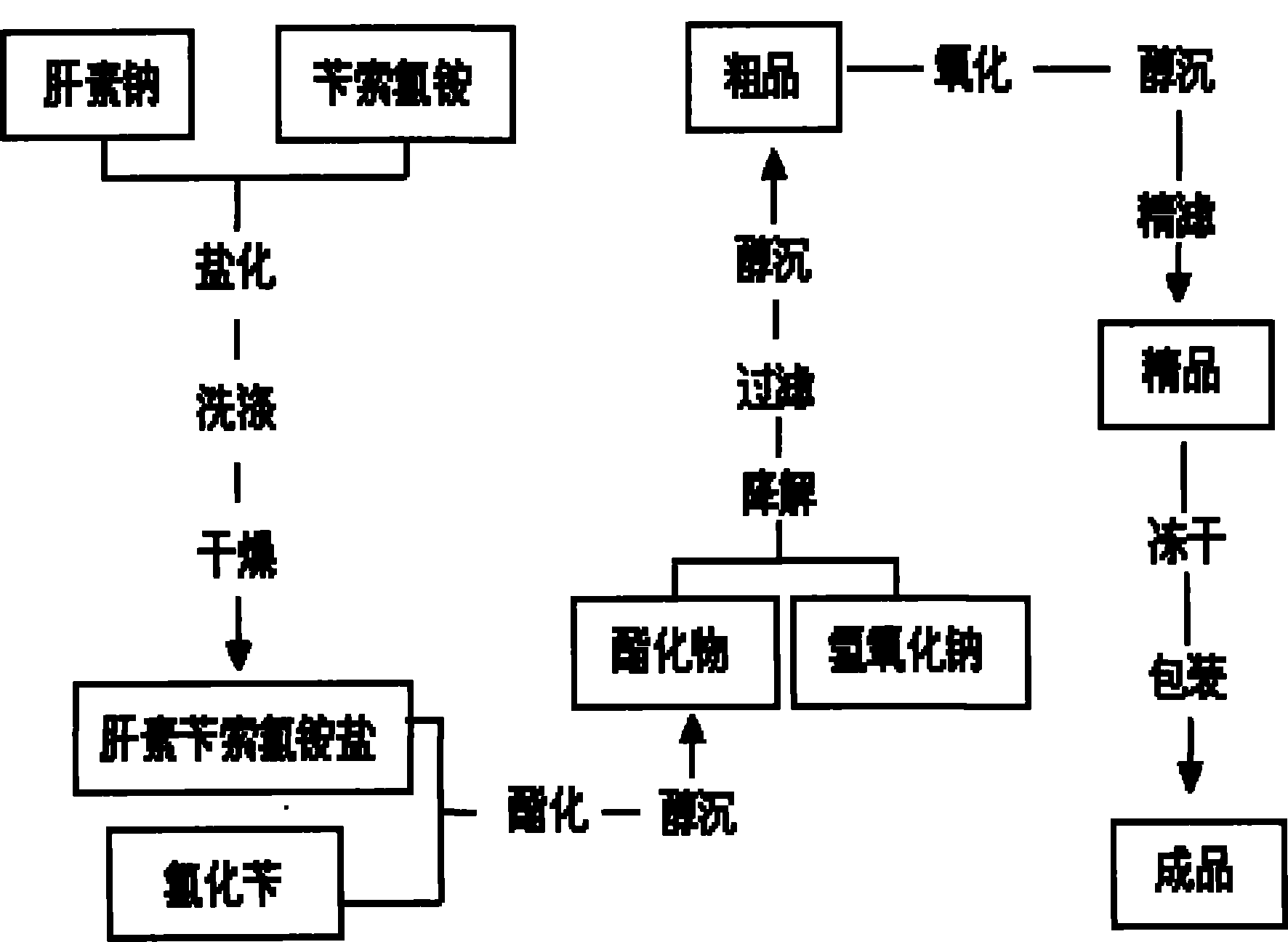
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminatingthe side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Folic acid-mediated (polyethylene glycol) PEG-graphene oxide doxorubicine-loaded nanoparticle and preparation method thereof
ActiveCN103784407AHigh drug loadingImprove targetingOrganic active ingredientsPowder deliverySide effectHalf-life
The invention relates to a folic acid-mediated (polyethylene glycol) PEG-graphene oxide doxorubicine-loaded nanoparticle and a preparation method thereof. The preparation method includes carrying out functional modification on graphene oxide with folic acid and PEG, and loading a model drug doxorubicine. According to the invention, the drug loading capacity of the nanoparticle is increased, drug targeting property is improved, and the half-life of plasma and mean residence time are remarkably prolonged, so as to improve the curative effect of doxorubicine. The invention can reduce the toxic and side effects of doxorubicine, and prolong the drug resistance time and improve drug targeting property, so as to enhance the antitumor effect of doxorubicine. The invention has important clinical significance for some medicaments with wide antitumor spectrum but severe adverse effect and high toxicity.
Owner:HARBIN MEDICAL UNIVERSITY
Synthesis and applications of an albumin bounding type 5-fluorouracil prodrug
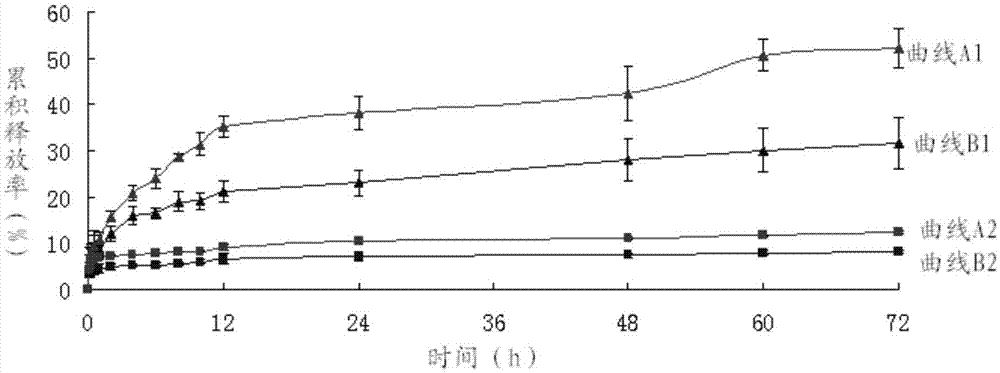
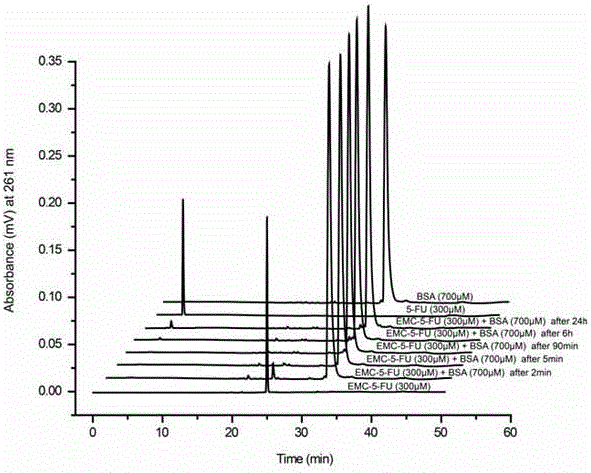
ActiveCN106632272AProlonged plasma half-lifeImprove anti-tumor activityOrganic active ingredientsOrganic chemistryHalf-lifeChemistry
The invention relates to an albumin bounding type 5-fluorouracil prodrug, and applications thereof in anti-tumor drug transfer. The prodrug is a compound formed by bridging 4-maleimidobutyric acid, 6-maleimidocaproic acid or 8-maleimido octanoic acid and N1-hydroxymethyl-5-fluorouracil through an ester bond, wherein the maleimido group is adopted as a bonding target of free sulfydryl of 34-site cysteine of albumin. The prodrug compound can be rapidly specifically bonded with albumin in blood to form an albumin prodrug composite, and therefore the drug metabolism speed is reduced, drug half life is significantly prolonged, and a long circulation function is achieved. In addition, under EPR effects and albumin acceptor mediation, tumor targeting is achieved and antitumor effects are improved. The 5-fluorouracil prodrug is used for intravenous injection and has a wide market application prospect.
Owner:SHENYANG PHARMA UNIVERSITY
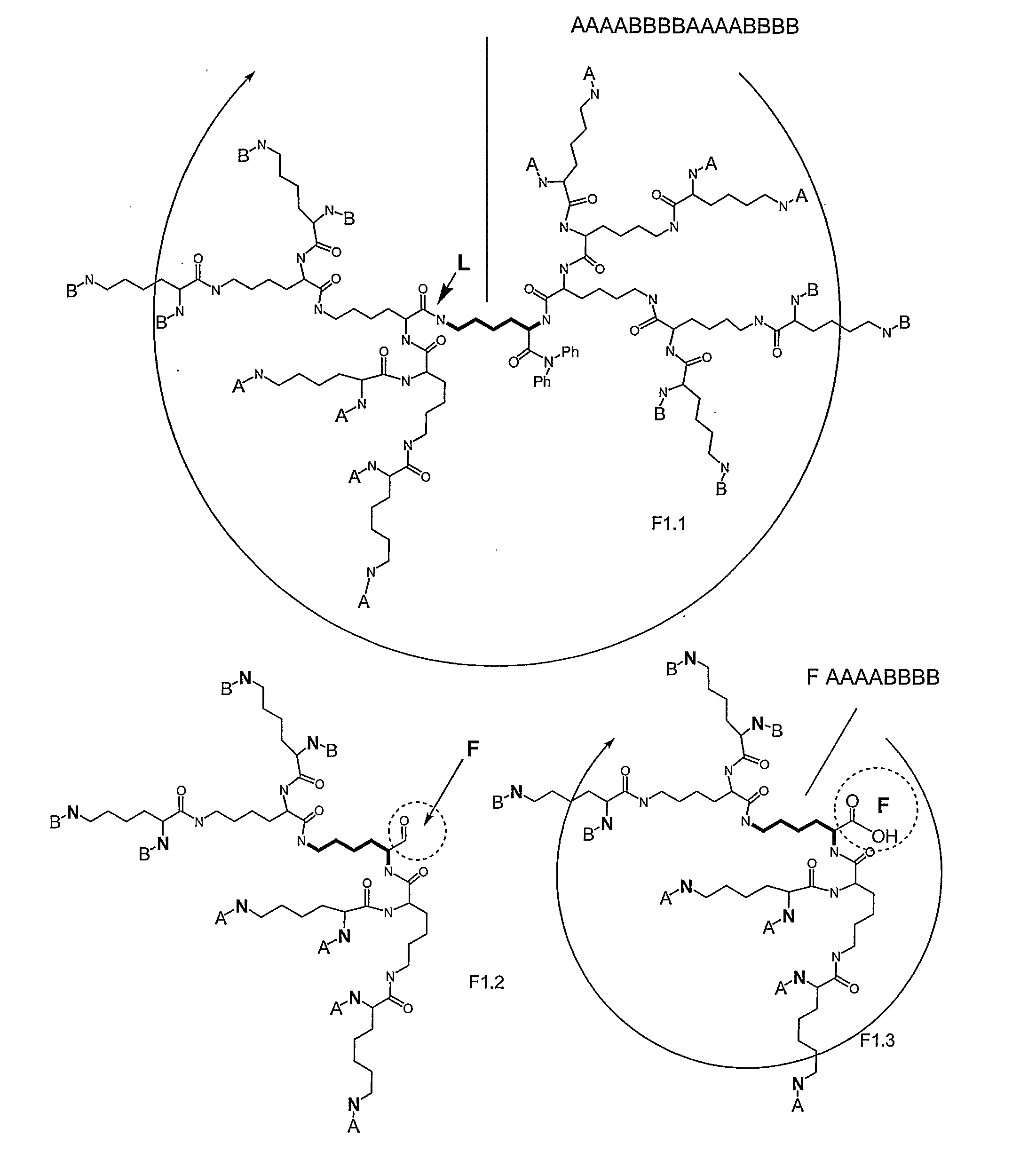
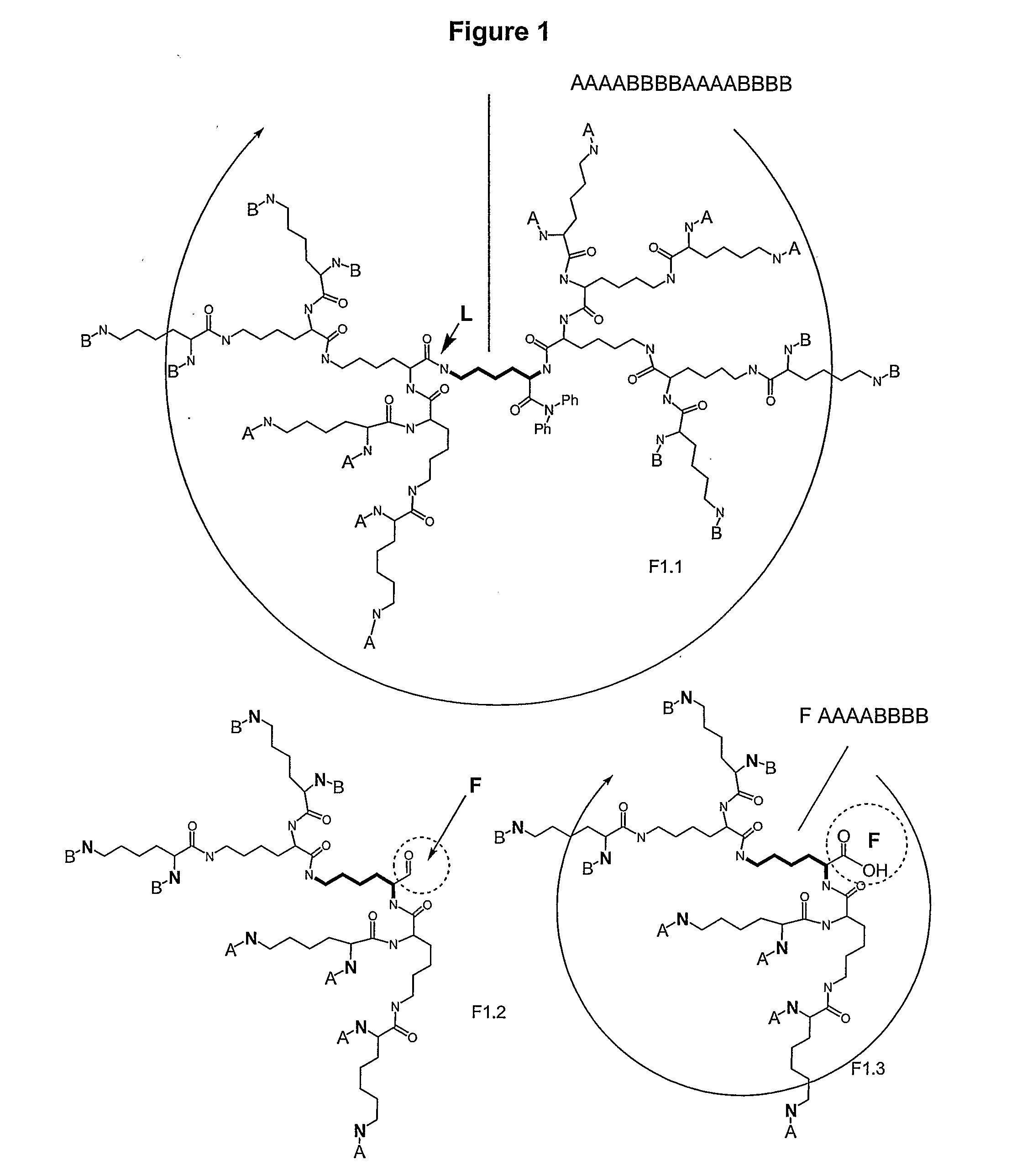
Macromolecular Compounds Having Controlled Stoichiometry
ActiveUS20090118467A1Overcome problemsDescribe wellOrganic active ingredientsPeptide/protein ingredientsPolymer scienceMoiety
The following invention is directed to macromolecules having controlled stoichiometry and topology, processes for their production, and applications for their use. The macromolecules have a controlled functional moiety stoichiometry and include at least one dendritic motif having a surface layer formed from at least one surface building unit and at least one subsurface layer formed from at least one building unit, the surface building unit and building units having a hydrocarbon backbone bearing a carbonyl group and at least one amine group; and at least two different functional moieties on the building unit and / or surface building unit; where functional moiety stoichiometry refers to the number and type of functional moieties.
Owner:STARPHARMA PTY LTD
Oxycontin controlled release formulations and methods of using same
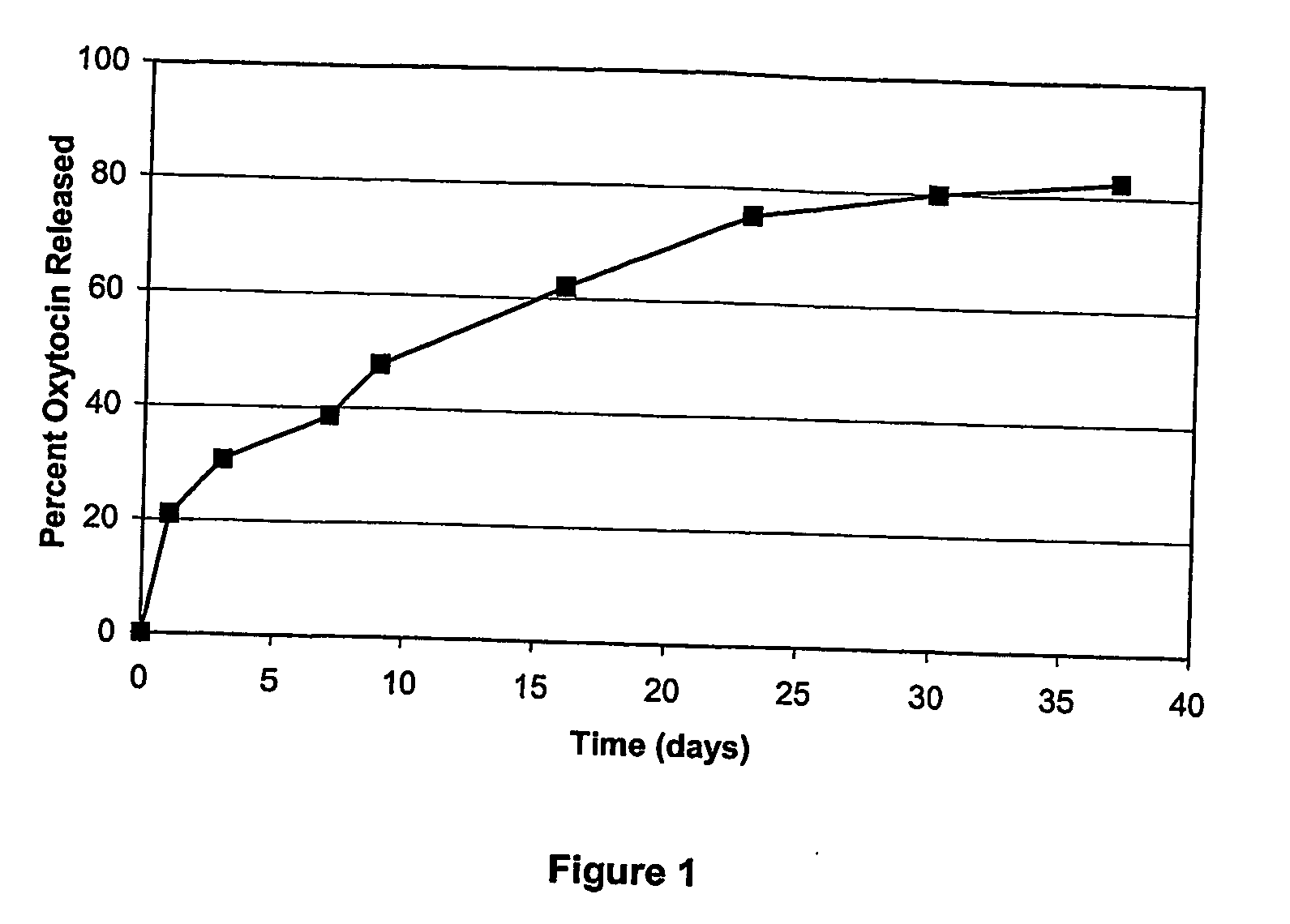
InactiveUS20100143485A1Prolonged plasma half-lifeImprove stabilityNervous disorderImpression capsDiseaseHalf-life
The compositions disclosed herein are of use for the treatment of a wide variety of diseases. In particular, the compositions provide oxytocin and oxytocin analogs in sustained release formulations. In particular embodiments, the disclosed compositions concern oxytocin and oxytocin analogs, each of which may be associated with a biodegradable polymer and / or attached to a hydrophilic polymer. The methods include treatment of a wide variety of diseases and conditions. In particular, the methods include treatment of sexual dysfunction and disorders associated with repetitive behaviors, such as autism. The usefulness of the present invention is that the oxytocin, oxytocin analogs and mixtures thereof can be administered in a pharmaceutical formulation that increases their half-life and also provides for sustained release.
Owner:PR PHARMA
Haptoglobin derivative for treatment of sepsis and acetaminophen-induced liver damage
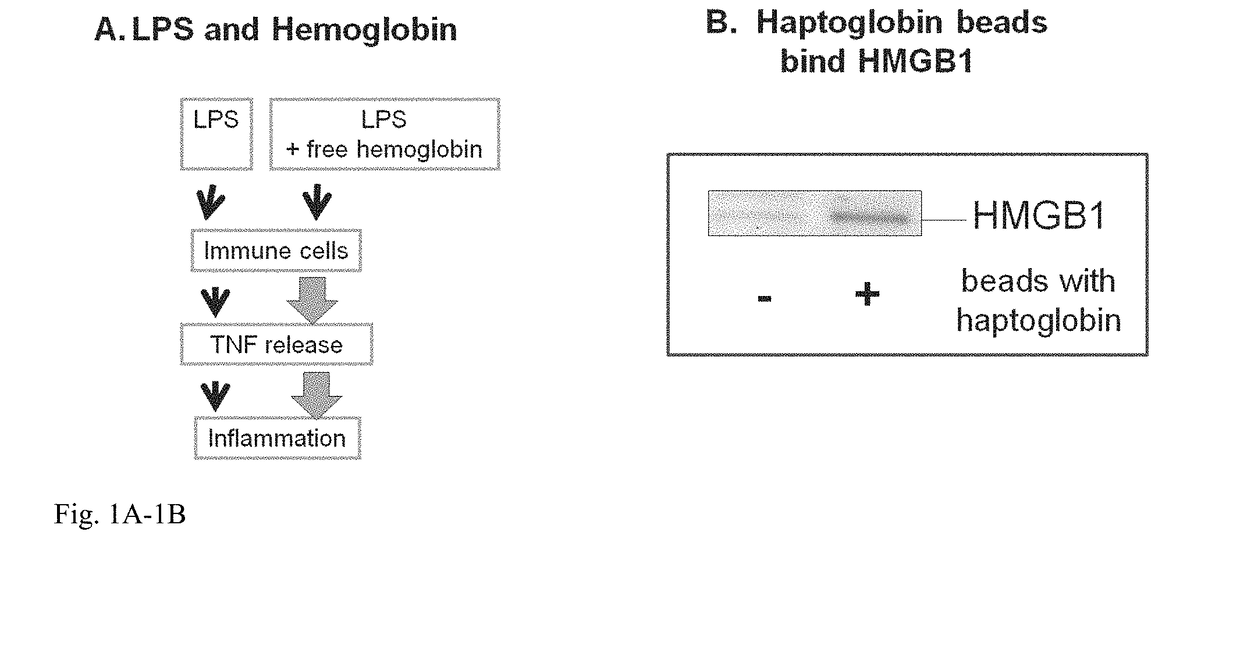
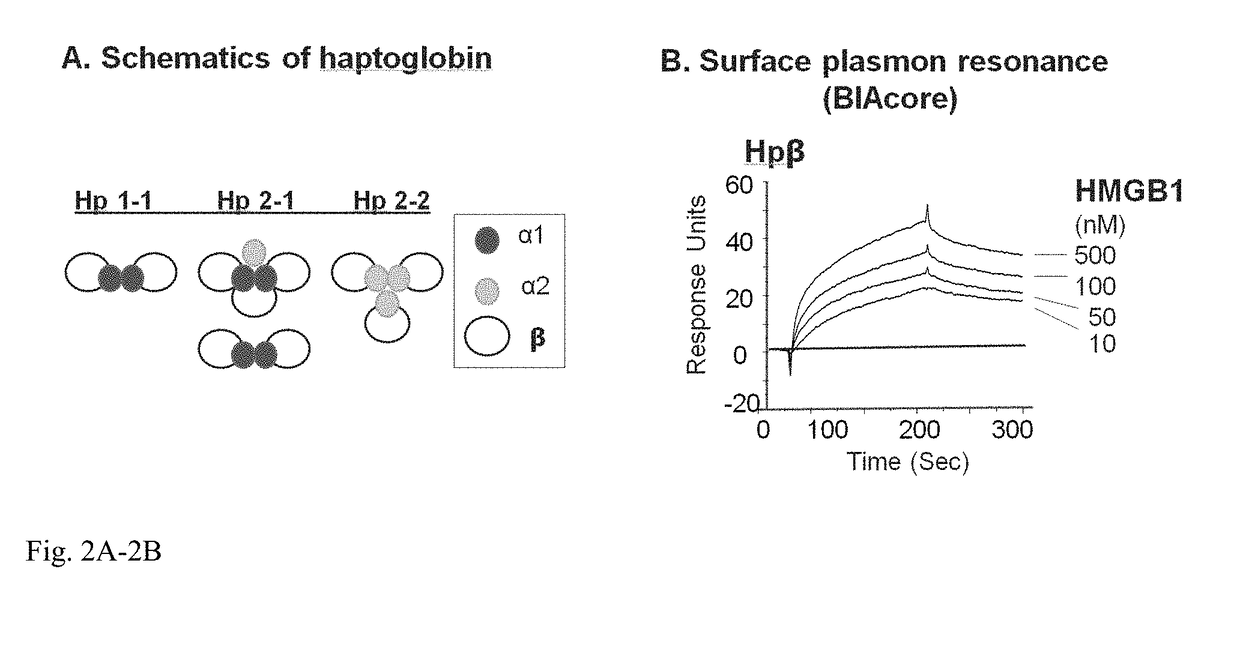
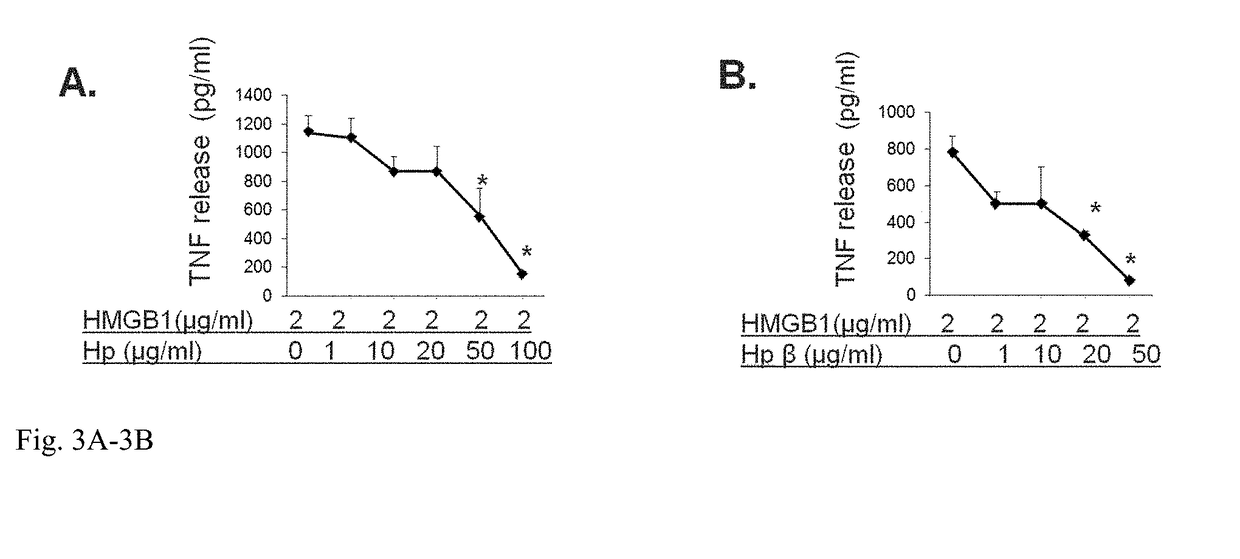
InactiveUS20180344808A1Reduce the possibilityProlonged plasma half-lifePeptide/protein ingredientsAntisepticsHaptoglobinGastroenterology
Methods for treating sepsis of acetaminophen-induced liver damage in a subject sing a haptoglobin derivative are provided. Compositions containing a haptoglobin derivative for treating sepsis of acetaminophen-induced liver damage are provided.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Novel long-acting glucagon-like peptide 1 (GLP-1) analogues and application thereof
InactiveCN103087175AChemically stableProlonged plasma half-lifePeptide/protein ingredientsMetabolism disorderLong actingPharmacological action
The invention relates to novel long-acting glucagon-like peptide 1 (GLP-1) analogues and a synthesis method thereof. GLP-1 analogues with longer pharmacological action time are obtained through replacing / modifying 17th, 26th, 34th and 37th amino acids of natural GLP-1, the synthesis of target polypeptides is quickly realized through a microwave-promoted solid-phase synthesis method, and crude products are purified and freeze-dried to the GLP-1 analogues.
Owner:CHINA PHARM UNIV
Isomorellic acid polylactic acid nano particle preparation and preparing method thereof
InactiveCN101229130ANanoparticles with low drug loadingPossesses liver passive targeting propertiesOrganic active ingredientsPowder deliverySide effectTherapeutic effect
The invention discloses a gamboges acid nanometer particle preparation and is prepared by the components of raw material gamboges acid 0.05 to 1.5 percent, polylactic acid 0.05 to 1.5 percent, surfactant 0.1 to 1.5 percent, organic solvent 33 to 50 percent and the allowance of water with the following steps: dissolving the polylactic acid into the organic solvent and dispersing the gamboges acid in the mixed solution of the polylactic acid and the organic solvent; the preparation is obtained by adding the mixed solution with the gamboges acid into water phase with the surfactant, stirring magnetically for 4 hours, vacuum distillation and filtering with a 0.22Mum micro-porous filtering film after the organic solvent and part of water are distillated. The drug with a clear system, the particle size between 10 to 100nm and good dispersion and stability can significantly solubilize drugs, has a targeting effect for reticuloendothelial cells and concentrate at lesion sites, thus improving the curing effect of the drug and reduces the side effect of the drug. Additionally, the drug has a slowly releasing effect, can maintain constant plasma concentration or pharmacologic effect for a long time and improve drug bioavailability.
Owner:NORTHWEST A & F UNIV
Nanocrystal of camptothecin drugs and preparation method thereof
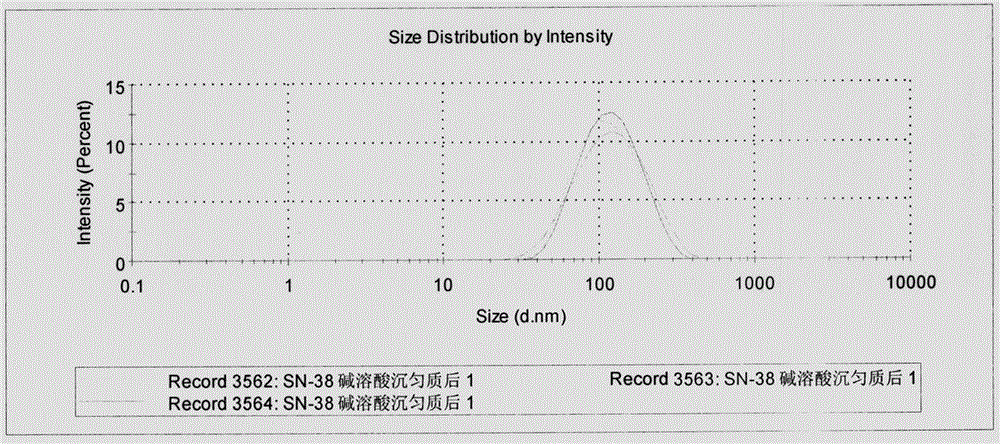
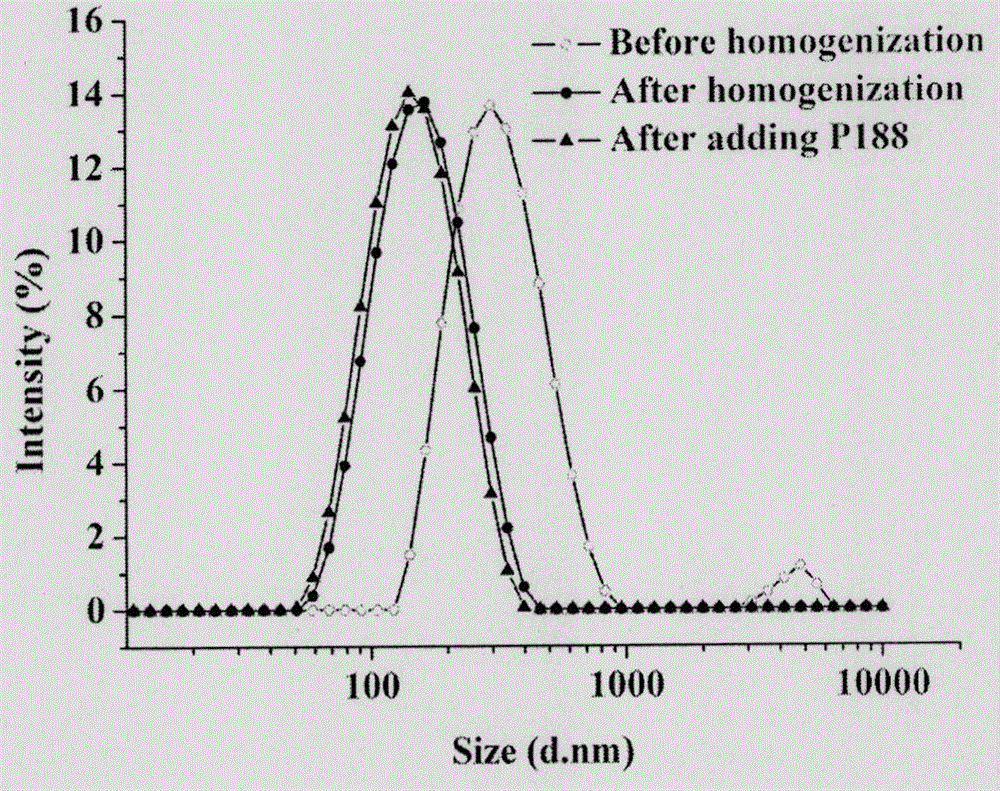
ActiveCN106466296AAdapt to clinical applicationImprove anti-tumor efficacyPowder deliveryOrganic active ingredientsFreeze-dryingDissolution
The invention provides a nanocrystal composition of a slightly-soluble antitumor drug, i.e., camptothecin and derivatives thereof, and a preparation method thereof. According to the invention, an optimized alkali-dissolution acid-precipitation high-pressure-homogenization process is employed; drug powder is subjected to alkali dissolution and then acid is added at a gradient speed to allow a nanocrystal to be precipitated; acid precipitation is carried out in two steps, and a produced salt is removed through a centrifugation step; and high-pressure homogenization is carried out to further decease a particle size. Preferably, the camptothecin nanocrystal has an average particle size of 100 to 150 m and is uniformly distributed; the camptothecin nanocrystal can be preserved for a long time in the form of freeze-drying powder only by adding a minute quantity of poloxamer 188 as a freeze-drying protection agent; and in use of the camptothecin nanocrystal, a glucose solution with a concentration of 5% is added and shaken out, so the camptothecin nanocrystal directly recovers to a state before freeze-drying. The method is simple, good in repeatability and suitable for large-scale production. The prepared nanocrystal has good slow release effect; and compared with commercially available injections, the nanocrystal has prolonged circulation time in blood plasma, improves distribution of the drug in human tissue and has beeter antineoplastic effect and good development prospects.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com