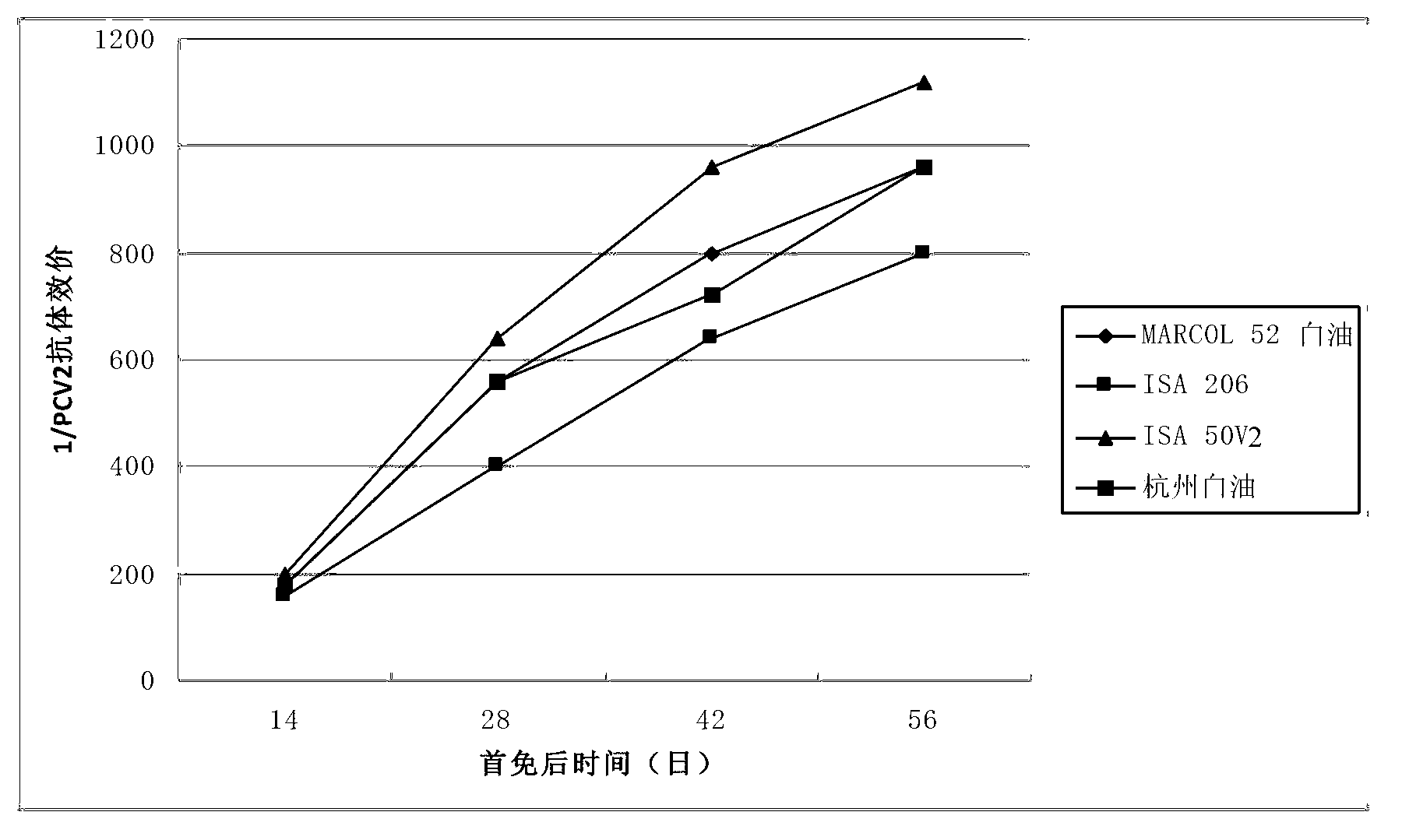
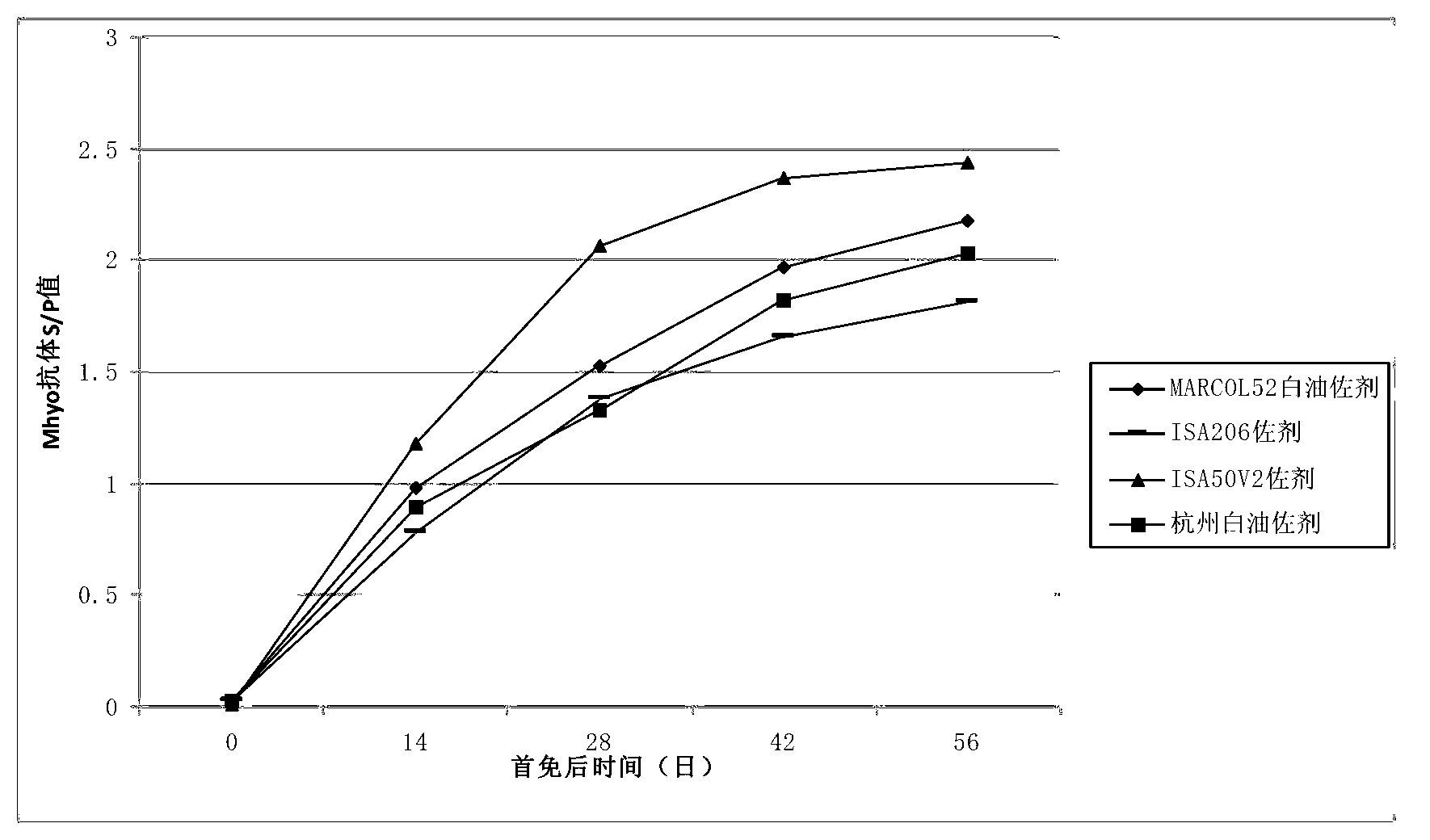
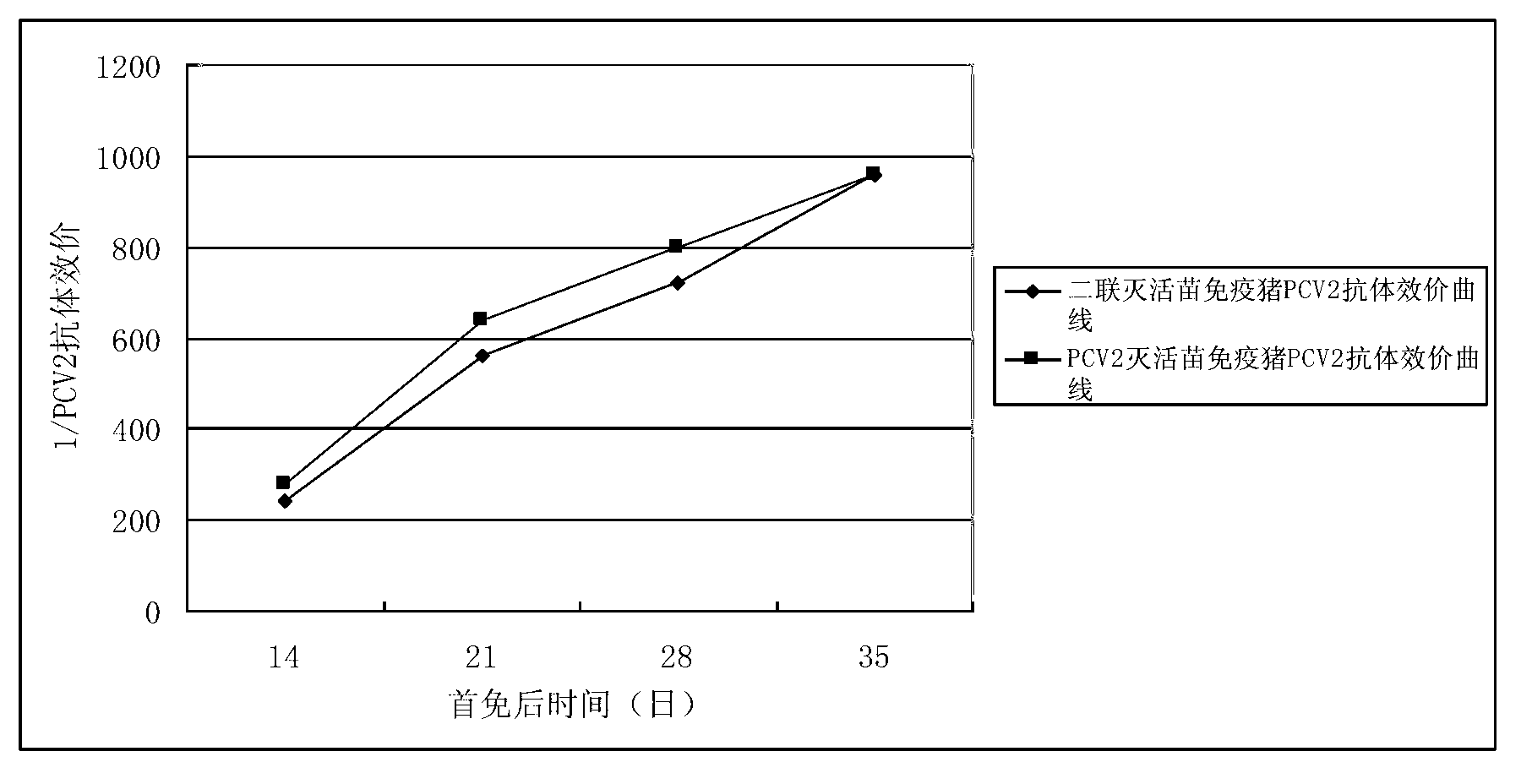
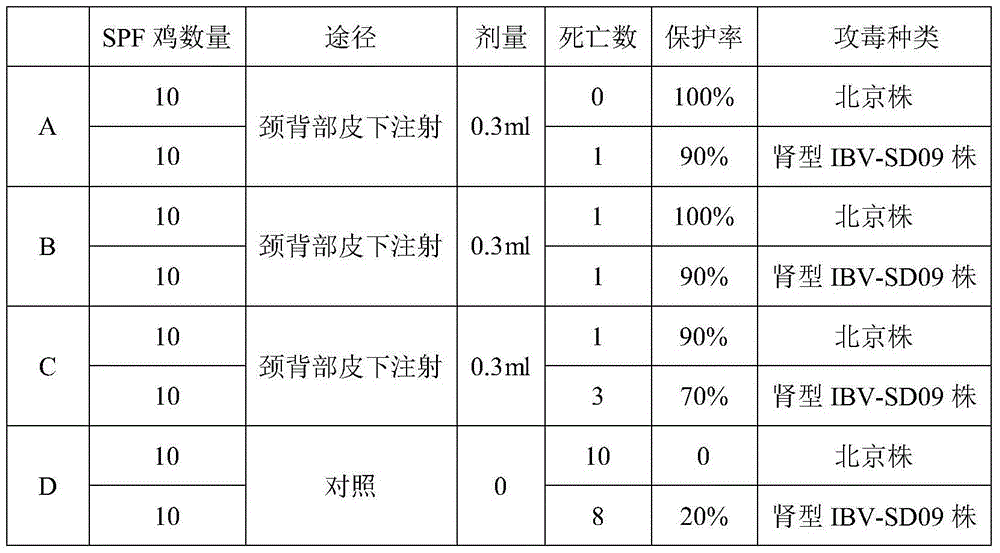
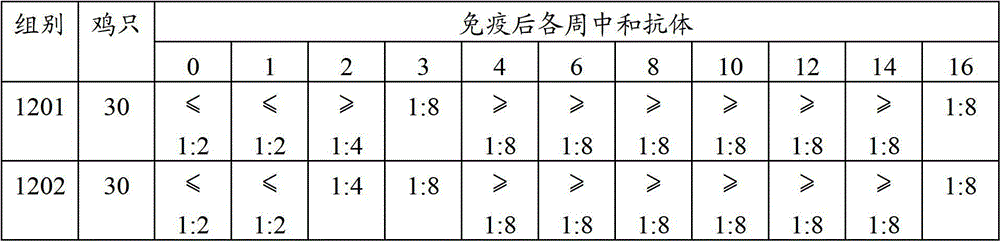
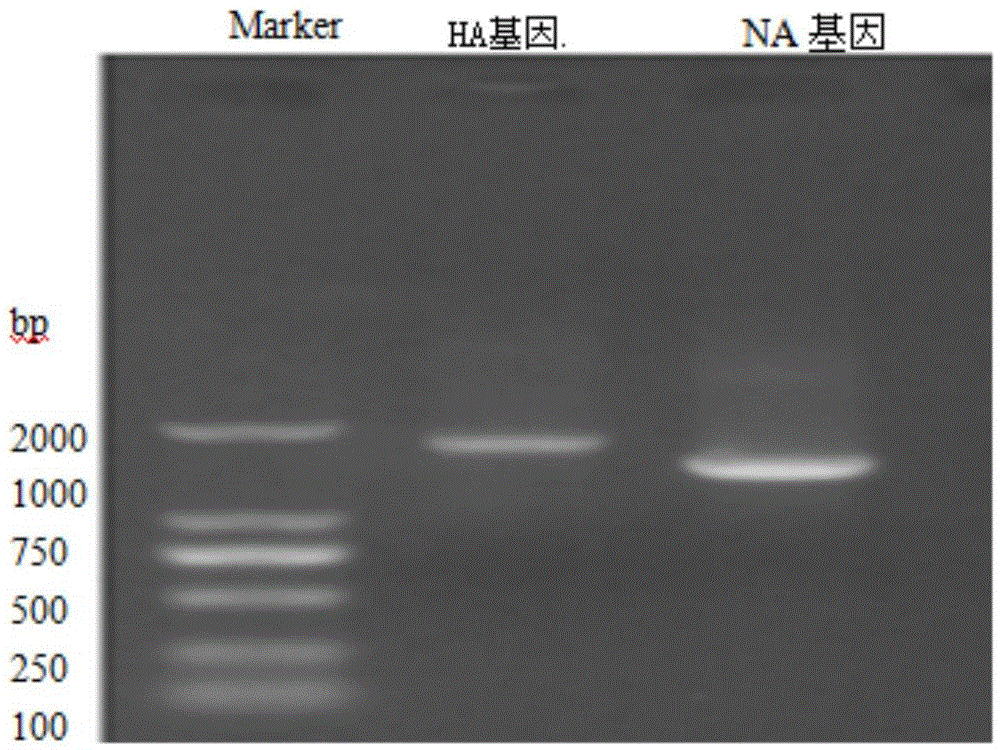
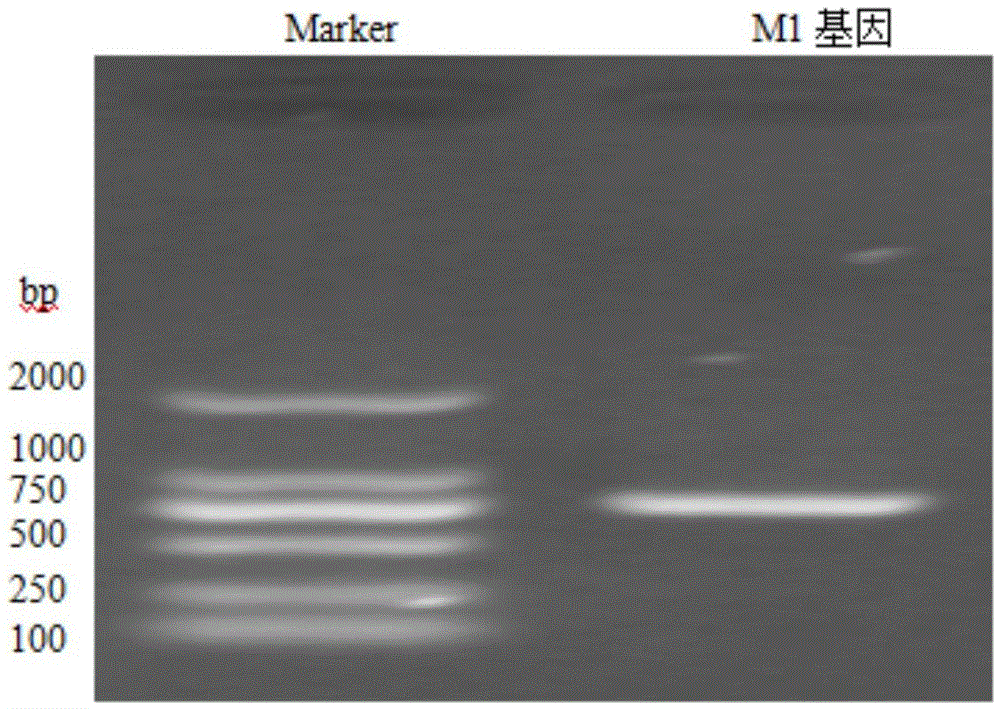
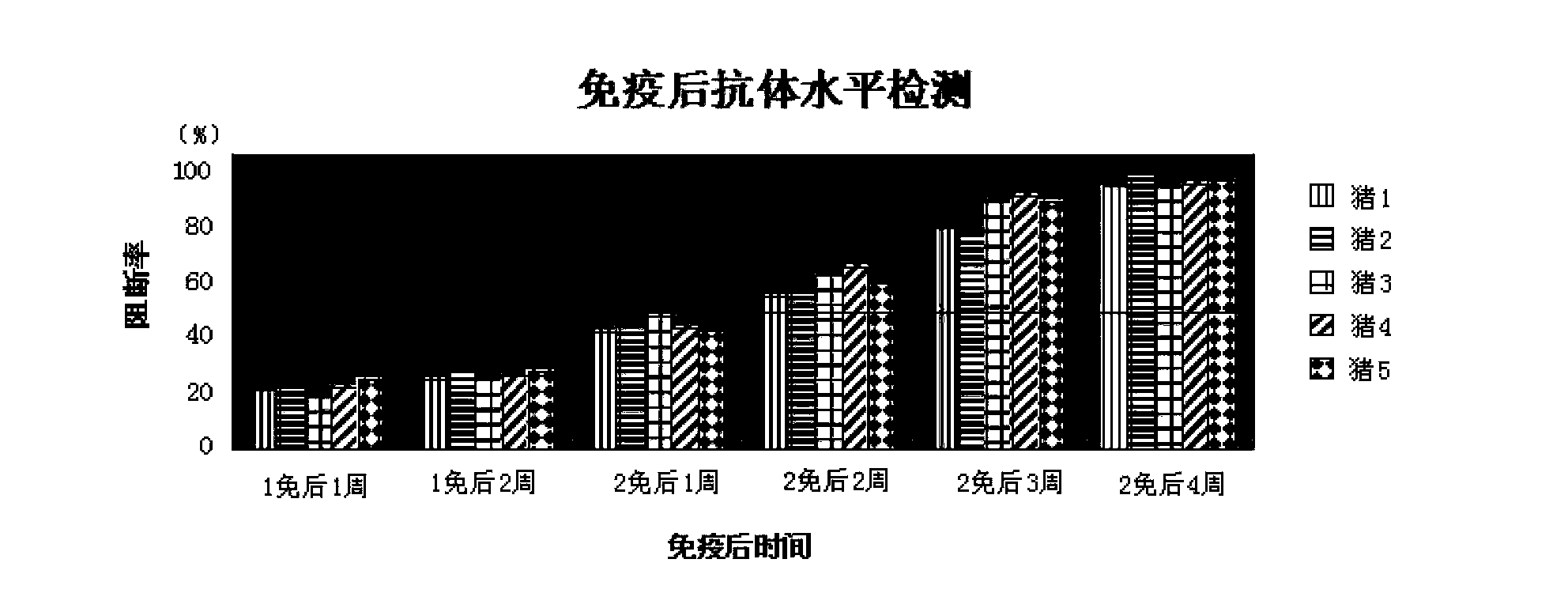
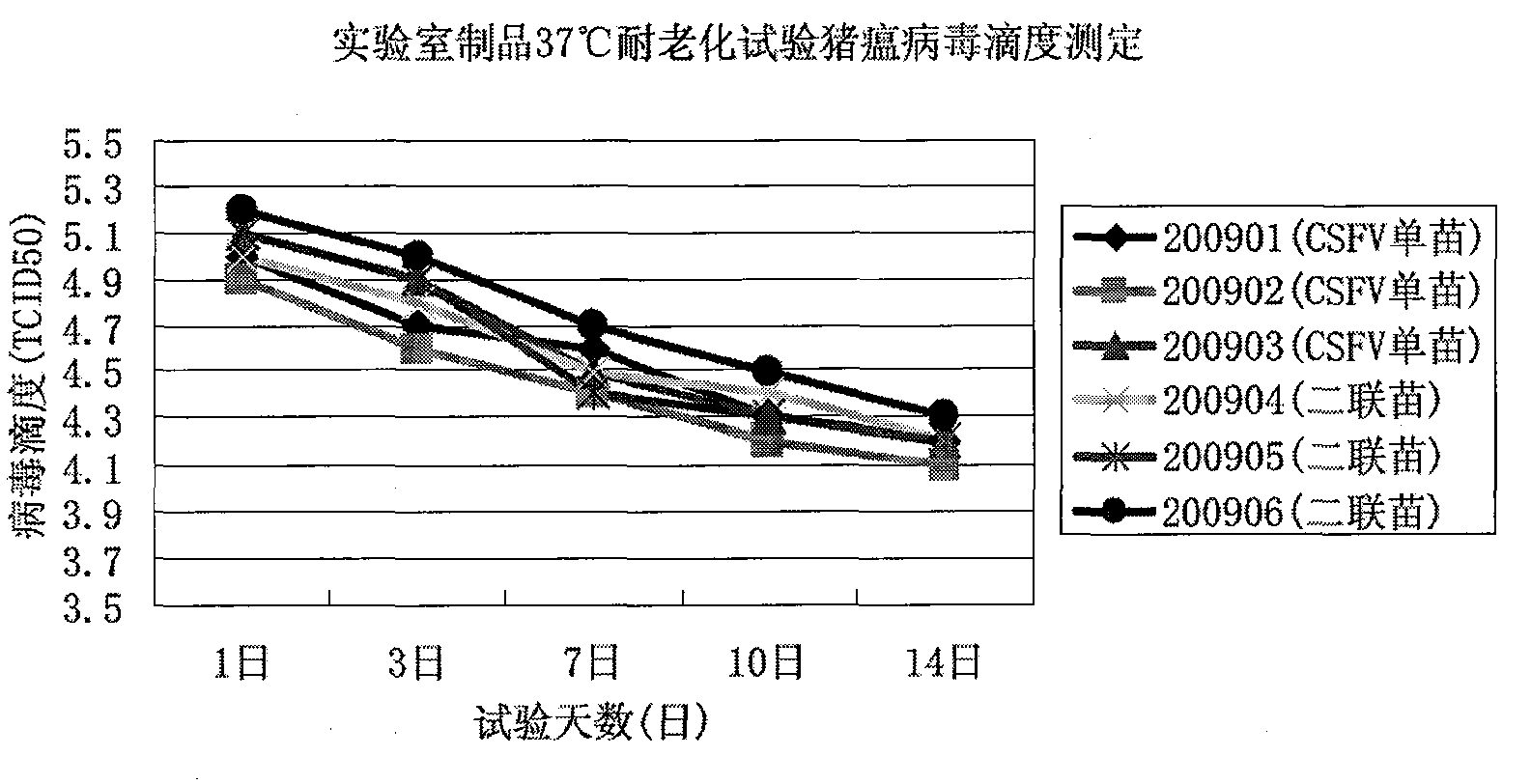
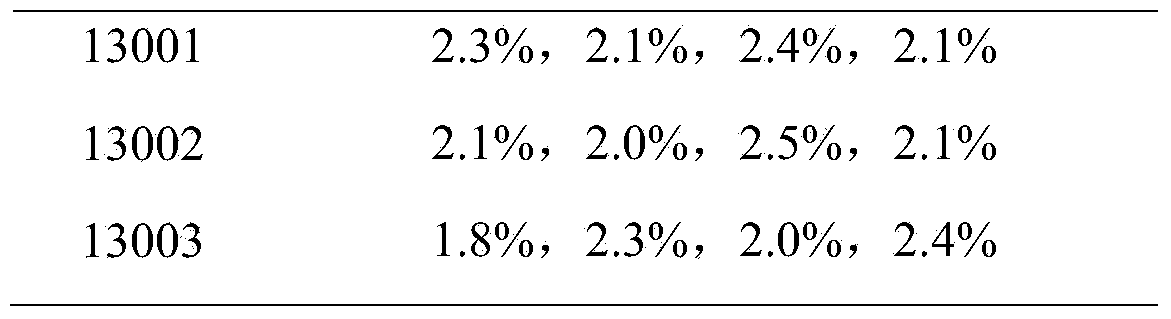Patents
Literature
56results about How to "Reduce the number of immunizations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
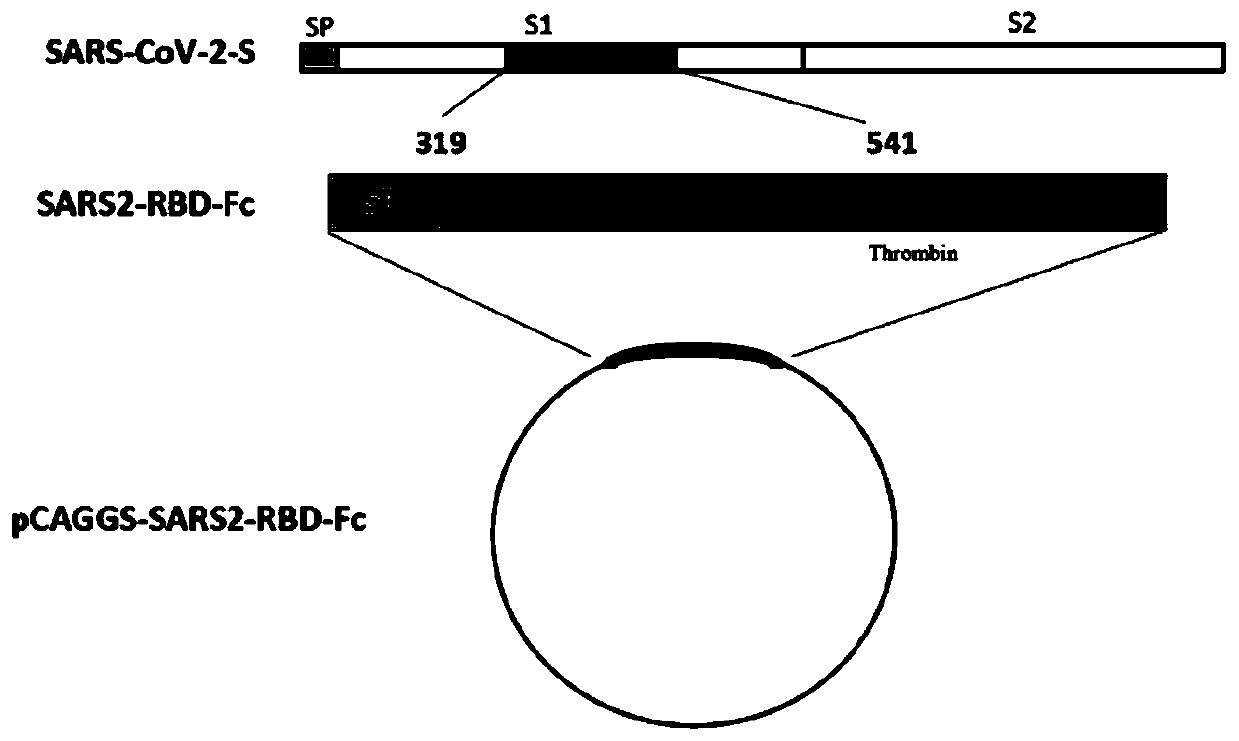
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
Method for preparing bivalent vaccine of newcastle disease virus La Sota strain and infectious bronchitis virus N-S multi-epitope protein
InactiveCN104548088AGood immune effectReduce adverse reactionsViral antigen ingredientsAntiviralsAntibody levelInfectivity
The invention provides a preparation method of a bivalent vaccine by taking newcastle disease virus La Sota strain and infectious bronchitis virus N-S multi-epitope protein as antigen. To immunize 21-day-old chickens, the bivalent vaccine prepared by the method disclosed by the invention can be used for simultaneously preventing newcastle disease and nephrotropic infectious bronchitis virus infection, and the antibody level of the bivalent vaccine is higher than that of respectively immunizing by single vaccine, so that immunological stress is effectively relieved and immunity time is reduced.
Owner:TIANJIN RINGPU BIO TECH
Triple vaccine for feline calicivirus infection, feline infectious rhinotracheitis and feline panleukopenia as well as preparation method and application thereof
ActiveCN111632137ASolve the requirements of cultivating large-scale productionGood immune effectPowder deliverySsRNA viruses positive-senseFeline calicivirus infectionFeline panleukopenia
The invention belongs to the technical field of veterinary biological products, and particularly relates to a triple vaccine for feline calicivirus infection, feline infectious rhinotracheitis and feline panleukopenia as well as a preparation method and application thereof. The triple vaccine comprises antigen components and a freeze-drying protective agent, and the antigen components comprise a feline calicivirus BJ strain inactivated antigen, a feline infectious rhinotracheitis virus SH strain inactivated antigen and a feline panleukopenia virus SY strain inactivated antigen. The feline calicivirus antigen, the feline infectious rhinotracheitis virus antigen and the feline panleukopenia virus antigen are prepared by adopting a full-suspension technology, the triple inactivated freeze-dried vaccine for feline calicivirus, feline infectious rhinotracheitis and feline panleukopenia is prepared through the processes of inactivation, concentration, freeze-drying and the like, three diseases are avoided by one injection, the animal immunization frequency is reduced, the animal stress frequency is reduced, and the economic cost of feeders is greatly reduced.
Owner:郑州爱科生物科技有限公司
Combined Newcastle disease and infectious bronchitis inactivated vaccine and method for preparing same
InactiveCN102716478AOvercome the adverse effects of interferenceSimple production processViral antigen ingredientsAntiviralsInfectious bronchitisAdjuvant
The invention discloses a method for producing a combined oil emulsion vaccine, and specifically relates a method for producing a combined Newcastle disease and infectious bronchitis inactivated vaccine, comprising the following steps of: carrying out homeomorphous inoculation, regulating the concentrations and proportions of Newcastle disease and infectious bronchitis viruses to achieve rational collocation, and regulating the components of an adjuvant in the vaccine so as to avoid aluminium stearate. The vaccine is capable of causing simultaneous production of Newcastle disease and infectious bronchitis antibodies, reducing disturbance, free of aluminium stearate, and capable of keeping high antibody production capability in a long time; and besides, the problems of stress reaction of animals and vaccine residue caused by vaccine injection can be solved.
Owner:PU LIKE BIO ENG
H9N2 type flu virus-like particle vaccine for prevention and preparation method thereof
InactiveCN105457023AImprove securityStrong immunogenicitySsRNA viruses negative-senseViral antigen ingredientsAdjuvantCell strain
The invention provides an H9N2 type flu virus-like particle vaccine for prevention and a preparation method thereof. The vaccine composition comprises H9N2 type flu virus-like particles, the virus-like particles are of the virus-like shell particle structure of the H9N2 type flu virus, and the shell particle structure capsule membrane surfaces contain one or more structural protein of the H9N2 type flu virus; the preparation method includes the steps that firstly, HA, NA and M1 gene DNA are synthesized, recombinant plasmid pFastBac-DuaL-HA-NA-M1 is constructed, the constructed recombinant plasmid is led into E.coLi competent state cell strain DH10Bac cells, the plasmid rBacmid-HA-NA-M1 is obtained through blue-white selection, transfection of SF9 insect cells is conducted, the virus-like particles are obtained, an adjuvant is dissolved in the virus-like particles, and the vaccine is obtained after incubation and centrifugal cleaning are obtained. By means of the H9N2 type flu virus-like particle vaccine, an organism can be stimulated to generate a strong immune response, and immunogenicity is high.
Owner:CHONGQING UNIV OF TECH +1
Swine fever and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
InactiveCN103505724ASolve the problem of low early potencyImprove securityAntiviralsAntibody medical ingredientsDiseaseRabies
The invention provides a swine fever and porcine pseudorabies bivalent vaccine. The swine fever and porcine pseudorabies bivalent vaccine contains at least one swine fever virus antigen and at least one porcine pseudorabies virus antigen, wherein the two antigens coordinate well, are excellent in immune effect and can promote each other. The swine fever and porcine pseudorabies bivalent vaccine is simple in preparation method, is convenient and efficient in immunization and has the advantages that immunization cost is reduced, an immunization procedure is simplified and economy and reliability are realized compared with a vaccine which can be used for preventing and treating more than two diseases only when immunization is carried out in steps and at least two injections are taken and an immune method of the vaccine in the prior art. The immune effect of the swine fever and porcine pseudorabies bivalent vaccine is better than that of a single vaccine and better in safety and avoids adverse effects caused by multiple immunizations. Besides, the invention also provides a simple testing method for determining swine fever effect in the bivalent vaccine by adopting an indirect immunofluorescence method, so that quality of bivalent live vaccines in each batch is guaranteed, and economic benefit is obviously increased.
Owner:PU LIKE BIO ENG
Vaccine of swine fever-porcine contagious pleuropneumoniae bivalent subunit and preparation method and applications thereof
ActiveCN110038124AAvoid infectionNo cross interferenceAntibacterial agentsSsRNA viruses positive-senseSerum igeClassical swine fever virus E2
The invention relates to the technical field of veterinary drugs, in particular to a vaccine of swine fever-porcine contagious pleuropneumoniae bivalent subunit and a preparation method and applications thereof. The vaccine comprises classical swine fever virus E2 protein, serum type-7 actinobacillus pleuropneumoniae toxin protein ApxII, serum type-1 actinobacillus pleuropneumoniae outer membraneprotein Oml and vaccine adjuvant. The vaccine is obtained by mixing and emulsifying the proteins and the vaccine adjuvant in equal volume. The vaccine can simultaneously prevent swine fever and porcine contagious pleuropneumoniae caused by serum type-1 and type-7 porcine actinobacillus pleuropneumoniae, and has good effect and low cost.
Owner:天康生物制药有限公司
Chicken subunit four-combination vaccine and preparation and application thereof
ActiveCN103007271AReduce the number of immunizationsReduce stressViral antigen ingredientsAntiviralsNewcastle disease vaccineAvian virus
The invention aims to prepare a chicken subunit four-combination vaccine which is capable of simultaneously preventing newcastle disease, infectious bronchitis, avian influenza, infectious bursal disease and particularly super-strong virus infection. The vaccine comprises infectious bursal disease VP2 protein with the sequence of SEQ ID NO:1, newcastle disease virus, infectious bronchitis virus and avian influenza virus. The four-combination vaccine can be applied to vaccine immunity of chickens of 21 days, can be used for simultaneously preventing the newcastle disease, the infectious bronchitis, the avian influenza and the infectious bursal disease, has immune effects similar to those of a single newcastle disease vaccine, the inactivated vaccine of the infectious bronchitis, the inactivated vaccine of the avian influenza and single infectious bursal disease vaccine, and fulfills the aims of reducing immunization frequency and lowering stress.
Owner:QINGDAO VLAND BIOTECH INC +1
Rabbit staphylocosis and bordetellosis duplex tissue inactivated vaccine and preparation method
InactiveCN108721614AImmune effectReduce the number of immunizationsAntibacterial agentsBacterial antigen ingredientsAntigenPositive control
The invention relates to a rabbit staphylocosis and bordetellosis duplex tissue inactivated vaccine and a preparation method thereof. The rabbit staphylocosis and bordetellosis duplex tissue inactivated vaccine consists of rabbit staphylocosis tissue inactivated positive control antigen and rabbit bordetellosis tissue inactivated positive antigen according to the volume ratio of 3: 1. The rabbit staphylocosis tissue inactivated positive control antigen is prepared from pathological tissues of a rabbit affected with the rabbit staphylocosis by homogenizing, filtering, centrifugation sedimeantation and inactivating; and the rabbit bordetellosis tissue inactivated positive antigen is prepared from pathological tissues of a rabbit affected with rabbit bordetellosis by homogenizing, filtering,centrifugation sedimeantation and inactivating. The duplex tissue inactivated vaccine is highly targeted and high in safety, and can effectively prevent rabbit staphylocosis and rabbit bordetellosis.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Porcine circovirus type 2, porcine reproductive and respiratory syndrome bivalent vaccine and preparation method thereof
ActiveCN104056265AEasy to useStrong stress responseViral antigen ingredientsAntiviralsAdjuvantVaccine antigen
The invention relates to a porcine circovirus type 2, porcine reproductive and respiratory syndrome bivalent vaccine and a preparation method thereof. The bivalent vaccine is a lyophilized vaccine, and comprises a porcine circovirus type 2 SH strain inactivated virus and a respiratory syndrome virus R98 strain live virus, while an adjuvant is added into the vaccine diluent, so that the immunizing effect of the vaccine can be further improved. Immune interference does not exist among the bivalent lyophilized vaccine antigens, and the quality inspection of the vaccine finished product can achieve the quality standard of a single vaccine, and the immune protection effect of the vaccine finished product is better than that of a single vaccine; the bivalent vaccine can be used for simplifying the immune procedure, reducing immune cost and reducing the stress reaction of an immunized pig, and thus the bivalent vaccine has great market application values.
Owner:JIANGSU NANNONG HI TECH
Chicken subunit triple vaccine, as well as preparation and application thereof
InactiveCN102973935AReduce the number of immunizationsReduce stressViral antigen ingredientsAntiviralsInfectious bursal diseaseImmune effects
The invention aims at providing a vaccine capable of simultaneously preventing infection of newcastle disease, infectious bronchitis and infectious bursal disease, in particular to very virulent strains. The vaccine comprises a infectious bursal disease VP2 protein with sequence of SEQ ID NO:1, newcastle disease and infectious bronchitis virus. The triple vaccine is used for immunizing a 21-day chick, which can simultaneously prevent newcastle disease, infectious bronchitis and infectious bursal disease, and has similar immune effect as newcastle disease single vaccine, infectious bronchitis inactivated vaccine and infectious bursal disease single vaccine, so that the aims of reducing the number of immunization times and reducing stress are achieved.
Owner:QINGDAO VLAND BIOTECH INC +1
Combined vaccine for anthrax and black death
InactiveCN1966074AImprove complianceReduce the number of immunizationsAntibacterial agentsFungiEpitopeMicrobiology
The invention relates to a coupled vaccine for protecting human or animal from bacillus anthracis and Yersinia, wherein it comprises bacillus anthracis PA antigen, Yersinia V antigen, and Yersinia F1, or their protective epitope. The antigen is recombined protein separated and / or purified. The DNA of integral or partial PA antigen, integral or partial F1 antigen, and integral or partial V antigen can be directly used as nucleic acid vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Construction method and application of avian influenza universal vaccine with spore probiotics as mucosal delivery vector
InactiveCN105950646AAdjuvant effectRealized the adjuvant effect and realized nasal mucosal immunizationSsRNA viruses negative-senseBacteriaCtl epitopeAntigen
The invention provides host bacteria, and also provides an avian influenza universal vaccine; the avian influenza universal vaccine takes spore probiotics as a mucosal delivery vector. A CTL epitope peptide of H7N9 or other type A avian influenza virus subtypes, a cytokine IL-2 and a brevibacterium mucosal immune vaccine vector are organically combined for the first time and have complementary advantages. For the H7N9, H5N1, H5N6, H7N9, H7N2, H7N3, H7N7, H9N2, H10N7 and other type A avian influenza virus subtypes, a CTL epitope antigen is designed and screened, the spore vector is used for delivery of the antigen epitope; a spore and a spore-displayed recombinant IL-2 are used as adjuvants, and the immune type and immunoreaction level are regulated.
Owner:SUN YAT SEN UNIV
Chimeric virus-like particle vaccine and preparation method therefor and application of chimeric virus-like particle vaccine
ActiveCN111647087AIncrease productionHigh puritySsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseEpitope
The invention relates to a chimeric virus-like particle vaccine and a preparation method therefor and application of the chimeric virus-like particle vaccine, in particular to a porcine Seneca valleyvirus and porcine circovirus-2 chimeric virus-like particle vaccine. According to the chimeric virus-like particle vaccine, sequences of VP0, VP3 and VP1 are connected in tandem for expression, wherein part of the VP3 sequences are replaced with porcine circovirus-2 ORF2 gene C-terminal epitope sequences to form a recombination sequence; the recombination sequence transfects sf9 cells after beingconstructed on recombinant bacmid for further expression to obtain porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particles. It is the first time for the invention to developthe porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine by utilizing the chimeric virus-like particles, and the porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine can exert a relatively good immune protection effect on the porcine Seneca valley virus and the porcine circovirus-2; and the vaccine of the invention is economical and practical, can effectively reduce the epidemic prevention cost of diseases, and provides a new method of preventing two diseases at the same time for breeding enterprises in China.
Owner:CHINA ANIMAL HUSBANDRY IND
Vaccine composition for porcine circovirus and swine influenza and its preparation method and use
ActiveCN105709220AImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The invention relates to a vaccine composition for preventing and / or treating porcine circovirus diseases and swine influenza and its preparation method and use. The vaccine composition comprises i, a porcine circovirus subunit antigen or a nucleic acid which is used for coding the porcine circovirus subunit antigen and can be expressed in a pig, and ii, a swine influenza virus subunit antigen or a nucleic acid which is used for coding the swine influenza virus subunit antigen and can be expressed in a pig. The invention also relates to a nucleic acid molecule, a recombinant plasmid and a host cell for treating and / or preventing two types of pig diseases.
Owner:PU LIKE BIO ENG
Adenosine triphosphate (ATP) and aluminium hydroxide composite adjuvant and vaccine containing same
ActiveCN102526725AReduce the number of immunizationsImprove immune efficiencyAntiviralsAntibody medical ingredientsAdjuvantAluminium hydroxide
The invention provides an adenosine triphosphate (ATP) and aluminium hydroxide composite adjuvant and a vaccine containing the same. The composite adjuvant consists of the following components: ATP and aluminium hydroxide, wherein the mass ratio of the ATP to the aluminium hydroxide is equal to (5-10):(1.8-2.7). The vaccine containing the composite adjuvant is prepared by adding 0.68 to 1.27mg ofcomposite adjuvant into per 100 microlitres of vaccine and mixing. The composite adjuvant is high in safety and repeatability and low in cost, and is convenient to prepare; humoral immunity response of antigenic specificity can be well induced, and the induction efficiency and the specific antibody production level are higher than those of an aluminium adjuvant which is independently used in effect; and when the vaccine is used by matching with a rabies vaccine, the humoral immunity response level of the rabies vaccine can be effectively improved, the immunity time of the vaccine can be reduced, and the immune efficiency of the vaccine can be improved.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Optimized PCV2d ORF2 gene and preparation method of virus-like particles
PendingCN111647609AEasy to operateShort cycleVirus peptidesImmunoglobulins against virusesOrf2 geneVirus-like particle
The invention belongs to the molecular biology field, and discloses an optimized PCV2d ORF2 gene and a preparation method of virus-like particles of the optimized PCV2d ORF2 gene. After a coding genesequence for coding porcine circovirus 2d subtype Cap proteins is optimized, an expression engineering bacteria expression system is constructed and is used for expressing the Cap proteins. Expressionbacteria is capable of expressing a large number of dissoluble and active porcine circovirus 2d subtype Cap proteins under a proper expression condition, the expressed proteins are purified by virtueof a monoclonal antibody column, and then the high-purity and high-concentration Cap proteins are obtained and are capable of forming the virus-like particles. According to the preparation method, efficient soluble expression of the porcine circovirus 2d subtype full-length Cap proteins in a prokaryotic expression system is realized, the operation is simple, the cost is low, the prepared monoclonal antibody column can be repeatedly used, and the Cap proteins obtained through purification are high in concentration and purity, are capable of spontaneously forming the virus-like particles, havegood immunogenicity and are suitable for industrial application.
Owner:SHANGHAI ACAD OF AGRI SCI
Applications of fullerene derivatives, vaccine adjuvant, and vaccine preparation thereof
ActiveCN103495164AImprove securityNo obvious stimulating effectAntibody medical ingredientsNanoparticleMedicine
The invention provides fullerene derivatives, the fullerene derivatives are fullerol represented by a formula of C60OxHy (x is less than 50 and more than or equal to y; y is more than 10 and less than or equal to x) or fullerene carboxyl derivatives represented by a formula of C60(C(COOH)2)n (n is an integer from 1 to 4), and the diameter range of nano particles of the fullerene derivatives in a solvent is from 1 to 400 nm. The invention also provides applications of a vaccine adjuvant of the fullerene derivatives in the vaccine field; the vaccine adjuvant has a good safety, can prominently improve the vaccine immunogenicity through different immunity ways, and has a very large application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA +1
Preparation method for monoclonal antibody of riemerella anatipestifer
PendingCN110172096AGood immune effectImprove immunityImmunoglobulins against bacteriaSpleen cellImmune effects
The invention provides a preparation method for a monoclonal antibody of riemerella anatipestifer. The method comprises the following specific steps: (1) expanding the culture of the riemerella anatipestifer, inactivating formaldehyde, and then preparing suspension by using PBS to complete antigen preparation; (2) mixing the newly prepared antigen with a freund's complete adjuvant uniformly and stirring for 4 hours until a mixture is emulsified, then performing subcutaneous injection on mice, mixing the newly prepared antigen with the freund's incomplete adjuvant uniformly and stirring for 4 huntil a mixture is emulsified after two weeks of immunization, performing subcutaneous injection on the mice again to obtain immunized mice after one week of immunization, and detecting antibody titer is 1 to 6400; (3) performing cell fusion on the spleen cells of the immunized mice and SP2 / 0 cells; 4) performing positive screening and cloning on fusion cells in step (3). According to the method,the antibody titer is increased by improving the immune effect, so that the immunization times are reduced, thereby achieving the effects of saving time and reducing materials.
Owner:FOSHAN UNIVERSITY
Chicken subunit four-combination vaccine and preparation and application thereof
ActiveCN103007271BReduce the number of immunizationsReduce stressViral antigen ingredientsAntiviralsAvian virusNewcastle disease vaccine
The invention aims to prepare a chicken subunit four-combination vaccine which is capable of simultaneously preventing newcastle disease, infectious bronchitis, avian influenza, infectious bursal disease and particularly super-strong virus infection. The vaccine comprises infectious bursal disease VP2 protein with the sequence of SEQ ID NO:1, newcastle disease virus, infectious bronchitis virus and avian influenza virus. The four-combination vaccine can be applied to vaccine immunity of chickens of 21 days, can be used for simultaneously preventing the newcastle disease, the infectious bronchitis, the avian influenza and the infectious bursal disease, has immune effects similar to those of a single newcastle disease vaccine, the inactivated vaccine of the infectious bronchitis, the inactivated vaccine of the avian influenza and single infectious bursal disease vaccine, and fulfills the aims of reducing immunization frequency and lowering stress.
Owner:QINGDAO VLAND BIOTECH INC +1
Porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine
PendingCN112957460AReduce the chance of side effectsHigh antigen contentAntibacterial agentsBacterial antigen ingredientsUltrafiltrationVirus Protein
The invention discloses a porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine which comprises an antigen and a vaccine adjuvant, the antigen is composed of a porcine circovirus type 2 antigen, a porcine pseudorabies virus antigen and a mycoplasma antigen, the porcine circovirus type 2 antigen is a purified, concentrated and inactivated porcine circovirus type 2 protein antigen solution, and the content of Cap protein is more than or equal to 160 [mu]g / ml; the porcine pseudorabies virus antigen is a purified, concentrated and inactivated porcine pseudorabies virus protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; the mycoplasma antigen is an inactivated mycoplasma protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; and the vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. Foreign protein is removed through clarification filtration and ultrafiltration concentration, and the side reaction probability of the vaccine is greatly reduced; and three-proofing can be achieved through one needle, so that the number of immunization times and stress are reduced. The method is economical and practical, the immunization procedure is simplified, and the epidemic prevention cost is reduced.
Owner:JIANGXI ZHENGBANG TECHNOLOGY CO LTD +1
Deoxy nucleotide containing CpG single chain as adjuvant of hepatitis B virus vaccine
InactiveCN1781930AImproving immunogenicityImprove immune activitySugar derivativesDigestive systemImmune effectsAdjuvant
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Recombinant human papillomavirus vaccine composition and use thereof
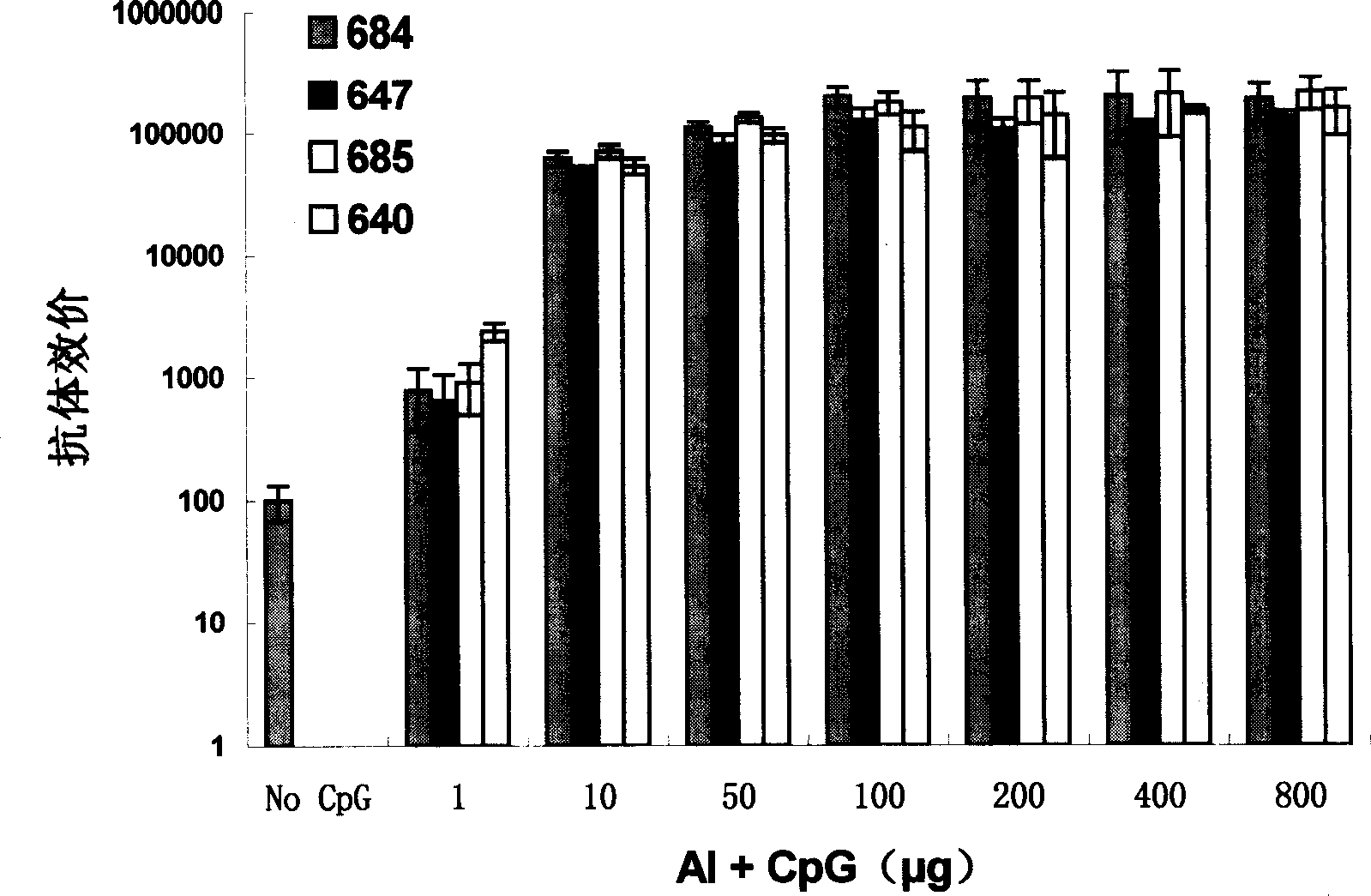
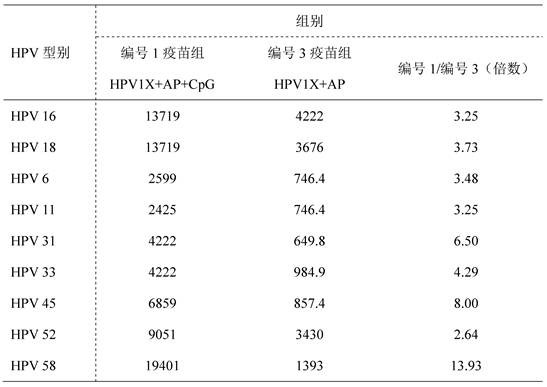
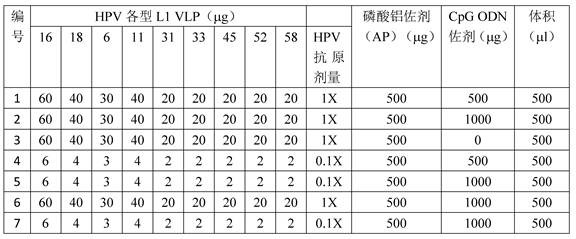
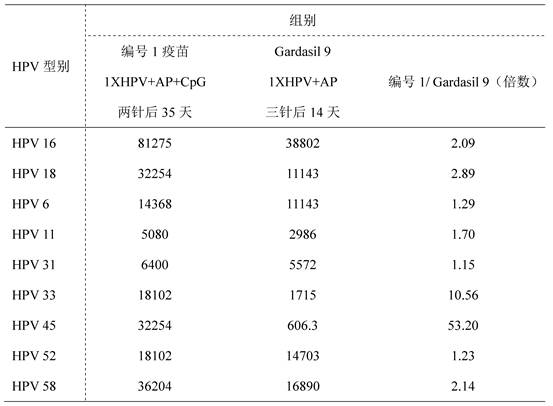
ActiveCN112439059BSmall dose of immunizationImprove immune activityViral antigen ingredientsVirus peptidesImmune effectsAdjuvant
The invention discloses a recombinant human papillomavirus vaccine composition and its application. Compared with the combination of other antigens and adjuvants, the novel vaccine composition provided by the present invention has more beneficial immune effects, reduces the number of immunizations, reduces the dose of antigen immunization, reduces immunization costs, and is beneficial to improve the immunization coverage. Therefore, the vaccine composition of the present invention becomes a new generation of human papillomavirus vaccine.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Combined live vaccine against porcine reproductive and respiratory syndrome and swine fever, and application thereof
ActiveCN102727883BEffective protectionImprove protectionViral antigen ingredientsAntiviralsDiseaseAttenuated vaccine
Owner:华威特(江苏)生物制药有限公司
Adjuvant for vaccine, vaccine composition containing adjuvant and application of vaccine composition
ActiveCN106421775ALow antigen contentStimulate immune responseAntibacterial agentsBacterial antigen ingredientsSide effectAdjuvant
The invention provides an adjuvant for a vaccine. The adjuvant is prepared from alumina gel, chitosan and lecithin. The invention further discloses vaccine composition containing the adjuvant and an application of the vaccine composition. The adjuvant can make an organism produce effective humoral immunity when used for the inactivated vaccine, cannot cause body temperature rise and side effects and can have better immune response during lower antigen content and single immunity.
Owner:PU LIKE BIO ENG
Vaccine composition for porcine circovirus and swine influenza, preparation method and application
ActiveCN105709220BImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The present invention relates to a vaccine composition for preventing and / or treating porcine circovirus disease and swine influenza and its preparation method and application. Circular virus subunit antigen and nucleic acid capable of being expressed in pigs, and ii) swine influenza virus subunit antigen or nucleic acid encoding swine influenza virus subunit antigen and capable of being expressed in pigs. The present invention also relates to nucleic acid molecules, recombinant plasmids and host cells for simultaneous treatment and / or prevention of two porcine diseases.
Owner:PU LIKE BIO ENG
Application of fullerene derivative to prepare gene transmission vector
ActiveCN102552901BImprove securityNo obvious stimulating effectViral antigen ingredientsGenetic material ingredientsNanoparticleSolvent
The invention provides a fullerene derivative, which is a fullerol shown by a general formula C60OxHy (y is more than 10 and less than or equal to x, and x is more than or equal to y and less than 50) or a fullerene carboxyl derivative shown by a general formula C60(C(COOH)2)n (n is an integral number of between 1 and 4), wherein the diameter range of nano-particles of the fullerene derivative in a solvent is between 1 and 400nm. The invention also provides application of the vaccine adjuvant of the fullerene derivative in the field of vaccines, and the vaccine adjuvant of the fullerene derivative has high safety, can remarkably improve the immunogenicity of the vaccine through different immune ways, and has great application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA +1
Adjuvant for vaccine, vaccine composition containing the adjuvant and application thereof
ActiveCN106421775BLow antigen contentStimulate immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantTGE VACCINE
The invention provides an adjuvant for vaccines, which includes aluminum gel, chitosan, and lecithin. The invention also discloses a vaccine composition containing the adjuvant and its application. When this adjuvant is used in an inactivated vaccine, it not only enables the body to produce effective humoral immunity without any increase in body temperature or side effects, but it can also produce a better immune response when the antigen content is low and a single immunization is performed.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com