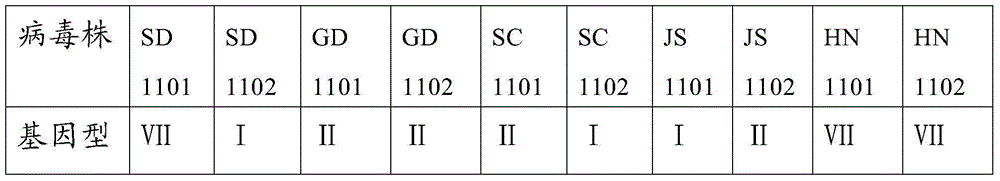
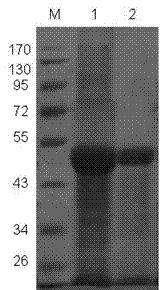
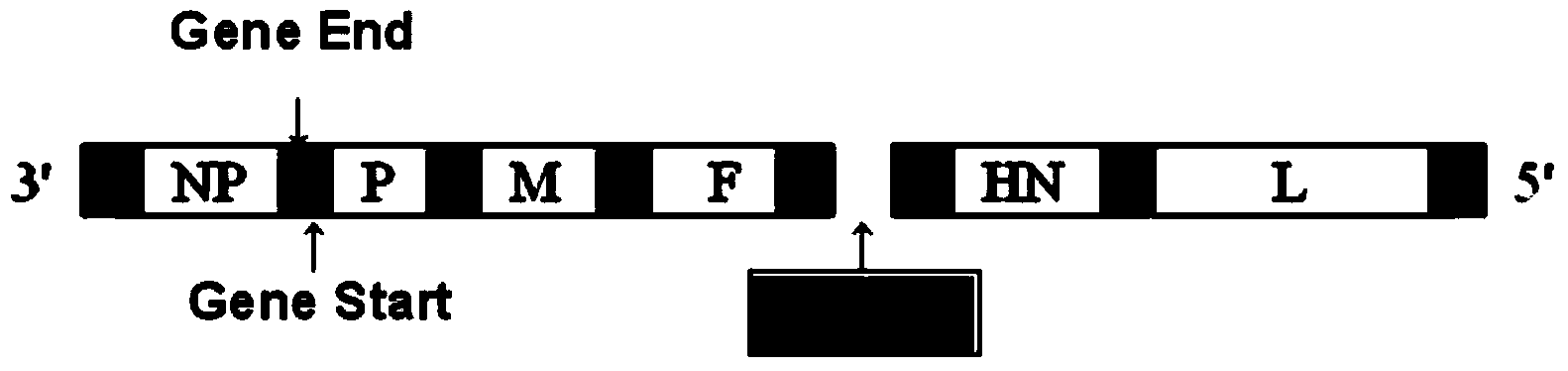
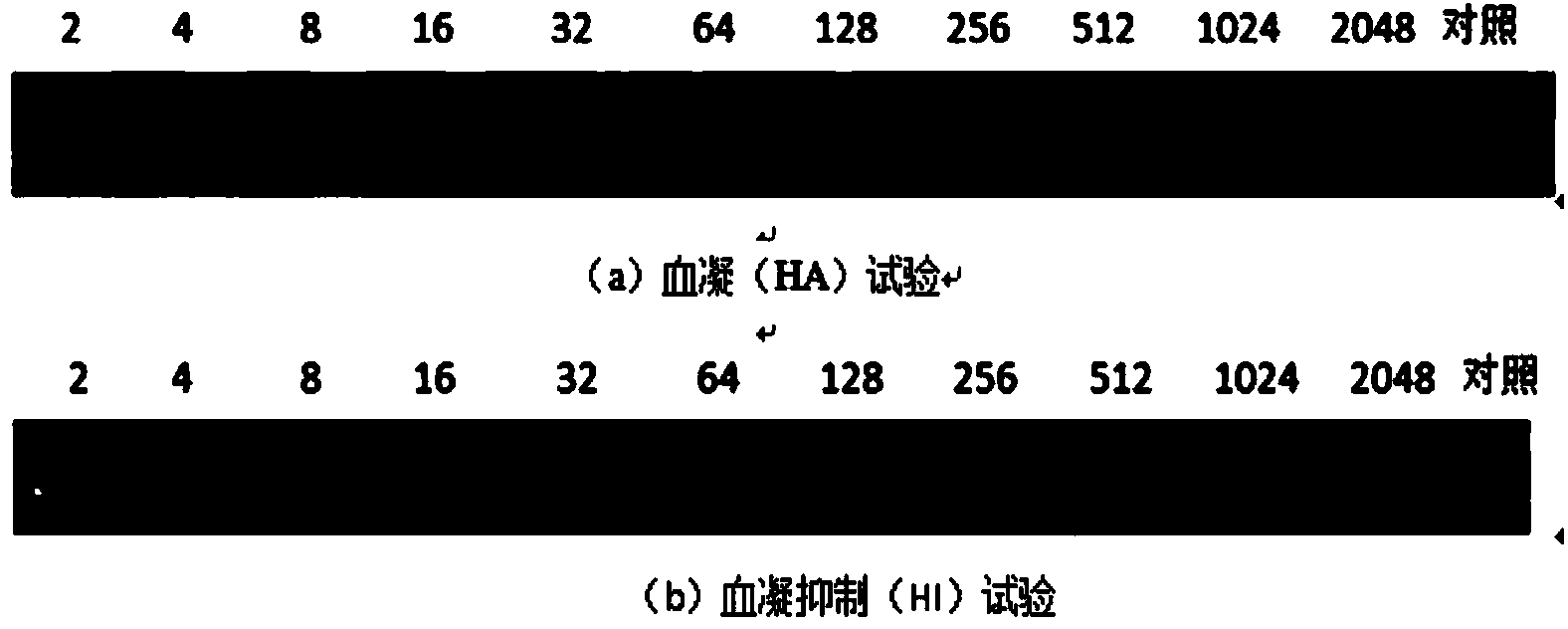
Patents
Literature
89 results about "Newcastle disease vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The vaccine contains the LaSota strain of Newcastle disease virus. The virus has been propagated in fertile eggs from specific pathogen free flocks. The immunizing capability of the vaccine has been proven by the master seed immunogenicity test. The vaccine offers proven immunity with mild reactions.
Gene VII-type newcastle disease virus strain, vaccine composition thereof and preparing method and application of vaccine composition
The invention discloses a gene VII-type newcastle disease virus strain with good immunogenicity and a low virulent strain subjected to passage attenuation through the newcastle disease virus strain. A newcastle disease virus strain F gene comprises a nucleotide sequence of a protein sequence shown by substantially-coded SEQ ID NO.2, and a virus strain HN gene comprises a nucleotide sequence of a protein sequence shown by substantially-coded SEQ ID NO.6. The newcastle disease virus strain is high in toxicity, and the growth rate on a chicken embryo is high; compared with a conventional newcastle disease virus strain, the gene VII-type newcastle disease virus strain has the advantages of being good in safety, high in immune protection capacity and immune efficacy and the like.
Owner:PU LIKE BIO ENG
Preparation method of inactivated newcastle disease vaccine
ActiveCN103110942AReduce in quantityEnhance immune functionViral antigen ingredientsAntiviralsOil phaseAntibody titer
The invention relates to a preparation method of an inactivated newcastle disease vaccine. The preparation method comprises the steps of virus liquid inactivation, preparation of a compound traditional Chinese medicine extract, preparation of an aqueous phase, preparation of an oil phase and emulsification. According to the preparation method, the compound traditional Chinese medicine extract serving as an immunopotentiator is added to the inactivated newcastle disease vaccine so as to well cause cellular immunologic response and enhance the immunologic function of an organism, so that an antibody is generated in advance, and the antibody titer is obviously improved.
Owner:山东滨州沃华生物工程有限公司
Traditional Chinese medicine astragalus polysaccharide immunopotentiator
ActiveCN101884788AGood effectNo side effectsAntibody medical ingredientsCellular immunityNewcastle disease vaccine
The invention relates to a traditional Chinese medicine immunopotentiator (an astragalus polysaccharide immunopotentiator for short) prepared from astragalus extracts and sulfated epimedium polysaccharides, which belongs to the field of immunological adjuvants of livestock and poultry. 1,000ml of the liquid medicine is prepared from 40g of astragalus and 120g of epimedium herb. The preparation method of the immunopotentiator comprises the following steps of: decocting the astragalus with water for three times, then merging the obtained filter liquor and condensing the filter liquor into 500ml of astragalus solution; extracting the epimedium polysaccharides by using the epimedium herb water decoction and ethanol precipitate method, then decorating the polysaccharides by using the chlorosulfonic acid-pyridine method, and preparing the polysaccharides into 500ml of sulfated epimedium polysaccharide solution after carrying out distilled water dialysis on the polysaccharides; and mixing the astragalus solution and the sulfated epimedium polysaccharide solution, and then filtering, sub-packaging and sterilizing to obtain the traditional Chinese medicine astragalus polysaccharide immunopotentiator. The immune experiment shows that the astragalus polysaccharide immunopotentiator has the advantage of obviously improving the proliferation of peripheral blood lymphocyte of chicken and enhancing the cellular immunity, obviously improving the potency of a serum antibody, promoting the lymphocyte proliferation, enhancing the cellular immunity and humoral immunity, and improving the immune response of a vaccine by coordinately immunizing chickling by using the Newcastle disease vaccine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Production of novel Newcastle disease virus strains from cDNAs and improved live attenuated Newcastle disease vaccines
InactiveUS7244558B1SsRNA viruses negative-senseViral antigen ingredientsFowlNewcastle disease virus NDV
Owner:UNIV OF MARYLAND OFFICE OF TECH COMMLIZATION +2
Heat-resisting and freeze-drying protector for live vaccine and preparation method thereof as well as live newcastle disease vaccine
InactiveCN101947321ALittle change in activityImprove protectionViral antigen ingredientsAntiviralsVitamin CFreeze-drying
The invention discloses a heat-resisting and freeze-drying protector for a live vaccine and a preparation method thereof as well as a live newcastle disease vaccine adopting the freeze-drying protector. The heat-resisting and freeze-drying protector for the live vaccine comprises the following compositions in percentage by weight: 10-15 percent of trehalose, 1-2 percent of polyvinylpyrrolidone (PVP), 1-2 percent of alum, 1-2 percent of glutamine, 2-10 percent of sorbitol, 0.1-0.5 percent of potassium dihydrogen phosphate, 0.5-1 percent of vitamin C and the balance of water. Compared with the traditional vaccine, the heat-resisting and freeze-drying protector for the live vaccine has the advantages of good stability, long preservation time, low preservation condition, little activity damage on the live vaccine, good dissolubility, simple raw material and the like.
Owner:河南后羿生物工程股份有限公司
Traditional Chinese medicine compound preparation for enhancing livestock immunity and preparation method
ActiveCN103169773AImprove immunityImmunological disordersAntibody medical ingredientsCodonopsisAnimal body
The invention relates to a traditional Chinese medicine compound preparation for enhancing livestock immunity, which comprises 1-5 parts of Radix Astragali, 1-5 parts of Radix Codonopsis and 1-5 parts of ligustrum lucidum, and the application forms can be a traditional Chinese medicine powder, an oral liquid, a particulate agent and an injection. The traditional Chinese medicine compound preparation for enhancing livestock immunity can be used for enhancing the livestock immunity functions of chicken, ducks, gooses, pigs and sheep; and especially, can be used for enhancing the immunization effect of vaccines such as newcastle disease vaccine or hog cholera vaccine. The traditional Chinese medicine compound preparation before and after the animal immunization and the vaccine are simultaneously used, the immunization function of animal body can be enhanced, and the immunization effect of the vaccine can be enhanced.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Applications of BHK-21 cell full-suspension culture technology in production of newcastle disease vaccine
The invention relates to applications of a BHK-21 cell full-suspension culture technology in production of a newcastle disease vaccine. A process of producing the newcastle disease vaccine by BHK-21 cell full-suspension culturing includes steps as follows: (1) a step of viral strain seed selection, namely a step of inoculating a monolayer BHK-21 cell with a newcastle disease vaccine virus seed cultured by a chick embryo, adding a virus maintenance medium, culturing to obtain a newcastle disease vaccine adapted to the BHK-21 cell, and performing system identification; (2) a step of domestication and seed selection of a suspension cell strain, namely a step of domesticating a full-suspension BHK21 cell used for culturing of the newcastle disease vaccine virus and establishing a basic seed; (3) a step of subjecting the suspension cell to enlarged cultivation; (4) a step of virus inoculation and harvest, namely a step of inoculating the newcastle disease vaccine virus adapted to the BHK-21 cell and harvesting a virus solution; and (5) a step of measuring the viral titer of the multiplicated newcastle disease vaccine virus and preparing the vaccine. According to the applications, culturing and production with chick embryos of the newcastle disease vaccine are changed into to large-scale culture and production with mammalian cells of the newcastle disease vaccine, the process of producing the newcastle disease vaccine is simplified, the production cost is reduced, and the yield and quality of the vaccine are largely improved.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Recombinant newcastle disease vaccine candidate rAI4-penton to express avian adenovirus penton protein and construction method thereof
InactiveCN108728419ASuitable for mass productionImprove reproductive performanceFermentationDsDNA virusesEmbryoAvian adenovirus
The invention relates to recombinant newcastle disease vaccine candidate rAI4-penton to express avian adenovirus penton protein and a construction method thereof. newcastle viral strain rAI4-penton iscollected under CGMCC No: 15492. The construction method includes: using a reconstructed reverse genetics operating platform of a newcastle attenuated strain to penton gene sequence of avian adenovirus into genome full-length transcription vector pNDV / rAI4 of the strain AI4 so as to obtain recombinant newcastle viral genome full-length cDNA clone pNDV / rAI4-penton, and performing transfecting to obtain recombinant viral rAI4-penton that successfully express penton protein. The recombinant virus rAI4-penton has high breeding titer on chicken embryos, can stabilize the penton protein even aftercontinuous passage, is suitable for large-scale production of vaccines, and is suitable for making vaccines.
Owner:YANGZHOU UNIV
Recombination Newcastle vaccine strain rAI4-S1 for expressing infectious bronchitis virus S1 protein and generating method thereof
InactiveCN103468651AMicroorganism based processesViruses/bacteriophagesInfectious bronchitisInfectious bronchitis virus
The invention discloses a recombination Newcastle vaccine strain rAI4-S1 for expressing infectious bronchitis virus S1 protein and a generating method of the recombination Newcastle vaccine strain rAI4-S1. The conservation number of the recombination Newcastle vaccine strain rAI4-S1 is CGMCC No: 8054. According to the generating method of the recombination Newcastle vaccine strain rAI4-S1, a built reverse genetic manipulation platform of a gene VII type NDV attenuated virus AI4 strain is utilized, the sequence revealed in the SEQ ID NO.7 is inserted into an AI4 strain genome full-length transcription carrier pNDV / AI4, and then the recombination Newcastle vaccine virus genome full-length cDNA clonal pNDV / AI4-S1 containing infectious bronchitis virus S1 genes is obtained. According to a recombination virus obtained through transfection, a chick embryo has high breeding titer, the S1 protein still can be expressed after continuous passage, and the generating method is suitable for large-scale production of vaccines and can be used for processing the vaccines.
Owner:YANGZHOU UNIV
Newcastle disease virus-like particles, preparation method and application thereof
ActiveCN106754765AClose to morphological structureClose to immunogenicitySsRNA viruses negative-senseViral antigen ingredientsWAS PROTEINHN Protein
Owner:JILIN UNIV
Preparation method of chitosan newcastle disease vaccine nanoparticles
InactiveCN102327228AGreat application potentialImprove stabilityPowder deliveryViral antigen ingredientsSide effectRelease time
The invention discloses a preparation method of chitosan newcastle disease vaccine nanoparticles, and relates to the preparation method of the chitosan newcastle disease vaccine nanoparticles. The method is that: 1, the chitosan is dispersed in the sterilized distilled water, under the magnetic stirring, the acetic acid is added to be stirred, filtered and carried out standing until the bubbles are eliminated, i.e. the chitosan solution is obtained; 2, the chitosan solution is taken and is dripped with the newcastle disease L-series virus liquid to be stirred and mixed uniformly, then the cross-linking agent is dripped, then the solution A is obtained after being stirred under normal temperature and aseptic conditions; and 3, the solution A undergoes centrifugal treatment, the sediment is taken out, is washed by sterile water, and then is vacuum freezed and dried, i.e. the chitosan newcastle disease vaccine nanoparticles are obtained. The vaccine nanoparticles has strong stability and long medicine release time, under the premise of maintaining the drug effect, the dosage is reduced, the toxic reaction side effect is reduced or avoided, and the immune protection period can be prolonged.
Owner:HEILONGJIANG UNIV
Establishment of hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody
InactiveCN103773736AImmunoglobulins against virusesMicroorganism based processesMolecular ImmunologyHN Protein
The invention relates to establishment of a hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody, which belongs to the technical field of molecular immunology and virology. The purified HN protein expressed by duck NDV isolate SDO3 pronucleus is used as immunogen, one hybridoma cell strain capable of secreting duck NDV-resisting isolate monoclonal antibody is researched, and the preservation number is CCTCC C2013173. The NDV specificity monoclonal antibody secreted by the hybridoma cell strain cannot be specially combined with the NDV strain but can be specifically combined with NDV clinical wild strain, so that the NDV specificity monoclonal antibody can be used as an identification reagent to be used for identifying the NDV vaccine virus and clinical wild strain, and the specificity is strong; the sensitivity is 1: 212 HAU; the hybridoma cell strain has the advantage of high sensitivity.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Application of paecilomyces cicadae cordyceps polysaccharides in preparation of chicken immunity enhancement reagent
ActiveCN105688206AIngredient safetyNo side effectsSsRNA viruses negative-senseAnthropod material medical ingredientsDiseaseSide effect
Owner:ZHEJIANG SUB TROPICS CROP INST
Newcastle disease virus strain and application thereof in preparation of Newcastle disease vaccine
ActiveCN104164410AMake up for the shortcoming of too longProduce quicklyViral antigen ingredientsMicroorganism based processesMaternal antibodyAdjuvant
The invention discloses a Newcastle disease virus strain and an application thereof in preparation of a Newcastle disease vaccine. A preparation method of the Newcastle disease virus strain comprises the following step: culturing recombinant plasmids pBrClone30-GM-CSF, pBL-N plasmids, pBL-P plasmids and pBL-L plasmid cotransfection mammalian cells together so as to obtain the Newcastle disease virus strain, wherein the recombinant plasmids pBrClone30-GM-CSF are of the plasmids of DNA molecules shown in No.2703 to No.18408 nucleotides from the 5' tail end of a sequence 3 with a sequence table. According to the Newcastle disease virus strain, molecular adjuvants GM-CSF are led into the genomes of the existing Newcastle disease vaccine, so that the immune effect is effectively improved and the immune blank period is greatly shortened. Meanwhile, the Newcastle disease virus strain can be used for resisting a maternal antibody so as to improve the immune protective rate. Thus, the Newcastle disease virus strain has an important value for preventing and treating Newcastle diseases.
Owner:JIANGSU KANIONREAL BIOMEDICAL TECH CO LTD
Triad inactivated vaccine for newcastle disease and bird flu (H9 subtype) and escherichia coli disease and preparation method thereof
The invention relates to triad inactivated vaccine for newcastle disease, bird flu (H9 subtype) and escherichia coli disease and a preparation method thereof. The vaccine is an effective coping strategy aiming at a prevalence situation of mixed infection of the newcastle disease, the H9 subtype bird flu and the escherichia coli, and two kinds of disease can be controlled at the same by means of immunization at one time. According to the vaccine, a La Sota strain of a newcastle disease virus, a LG1 strain of an H9 subtype bird flu virus, a QL1 strain (O1) of bird source escherichia coli, QL2 strain (O2) of the bird source escherichia coli, QL78 strain (O78) of the bird source escherichia coli, and QL36 strain (O36) of the bird source escherichia coli serve as vaccine reserve virus strains, and the vaccine is formed through antigen liquid preparation, inactivation, concentration and mixing according to a certain proportion. The vaccine and the preparation method are related with the La Sota strain of the newcastle disease virus, the H9 subtype bird flu virus and the bird source escherichia coli which are all domestic prevalence virus strains, and current prevalence disease can be effectively prevented.
Owner:QILU ANIMAL HEALTH PROD
Chicken subunit four-combination vaccine and preparation and application thereof
ActiveCN103007271AReduce the number of immunizationsReduce stressViral antigen ingredientsAntiviralsNewcastle disease vaccineAvian virus
The invention aims to prepare a chicken subunit four-combination vaccine which is capable of simultaneously preventing newcastle disease, infectious bronchitis, avian influenza, infectious bursal disease and particularly super-strong virus infection. The vaccine comprises infectious bursal disease VP2 protein with the sequence of SEQ ID NO:1, newcastle disease virus, infectious bronchitis virus and avian influenza virus. The four-combination vaccine can be applied to vaccine immunity of chickens of 21 days, can be used for simultaneously preventing the newcastle disease, the infectious bronchitis, the avian influenza and the infectious bursal disease, has immune effects similar to those of a single newcastle disease vaccine, the inactivated vaccine of the infectious bronchitis, the inactivated vaccine of the avian influenza and single infectious bursal disease vaccine, and fulfills the aims of reducing immunization frequency and lowering stress.
Owner:QINGDAO VLAND BIOTECH INC +1
Escape mutants of newcastle disease virus as marker vaccines
InactiveCN1744915AViral antigen ingredientsInactivation/attenuationBinding siteNewcastle disease virus NDV
A vaccine against Newcastle Disease contains one or more mutant immunogens of the NDW strain. The mutant immunogen lacks the antigenic binding site on the F glycoprotein which is recognized by the monoclonal antibody mAb 54. Reagent kits and assay methods help to distinguish vaccinated members of a poultry flock from those that may have been infected with wild-type Newcastle Disease virus.
Owner:ZOETIS W LLC
Traditional chinese medicine mulberry leaves polysaccharide and eucommia polysaccharide immunostimulant and application thereof
ActiveUS20160317655A1Good effectNo side effectSsRNA viruses negative-senseSsRNA viruses positive-senseYolkHumoral immune reaction
A traditional Chinese medicine immunopotentiator prepared from mulberry leaves polysaccharide and eucommia polysaccharide. The immunopotentiator can stimulate proliferation of chicken lymphocytes in vitro. When used together with newcastle disease vaccine to immunize chickens, the immunopotentiator can increase serum antibody titer, promote proliferation of lymphocytes, and enhance cellular immunity and humoral immunity of the chickens. When used together with porcine productive and respiratory syndrome vaccine to immunize piglets, the immunopotentiator can increase the serum antibody titer. When used together with the porcine productive and respiratory syndrome vaccine to immunize layers, the immunopotentiator can increase porcine productive and respiratory syndrome virus yolk antibody titer and improve immune effects of the vaccine.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Virus strain capable of being used for preparing VII type newcastle disease vaccine and encoding gene thereof
ActiveCN105886477ASsRNA viruses negative-senseViral antigen ingredientsFowlNewcastle disease virus NDV
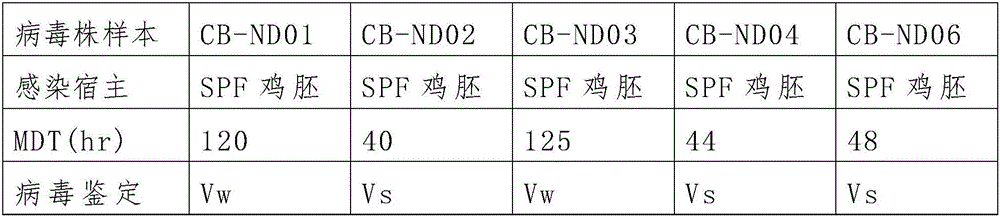
The invention provides a virus strain capable of being used for preparing a VII type newcastle disease vaccine and an encoding gene thereof. The virus strain is Genotype VII Newcastle disease virus (VII NDV) / CB-ND02 with the preservation number of CCTCC NO:V201548, VII NDV / CB-ND04 with the preservation number of CCTCC NO:V201555, or VII NDV / CB-ND06 with the preservation number of CCTCC NO:V201556. The virus strain can effectively improve immunity of a host to newcastle disease viruses and produce antibody immune sustaining force in a poultry body, is applicable to research, development and preparation of a newcastle disease vaccine and also can be used for manufacturing an inactivated or attenuated type vaccine.
Owner:GUONIAN IND CO LTD
Preparation method of anti-neweastle disease virus specific transfer factor and oral liquid, and use thereof
InactiveCN103599130AImprove specific immune effectFree from infectionViral antigen ingredientsPharmaceutical delivery mechanismDiseaseSpecific immunity
The invention provides a preparation method of an anti-neweastle disease virus specific transfer factor. The method comprises the following steps: selecting a healthy chicken as an experiment chicken to carry out supplementary immunization of a newcastle disease inactivated vaccine; monitoring the level of an immune antibody of the newcastle disease vaccine inside the experiment chicken body; taking splenic organs of the experiment chicken in a sterile condition when the level of the antibody achieves 28-29; and processing the splenic organs of the chicken, so as to obtain the anti-neweastle disease virus specific transfer factor. By adopting the anti-neweastle disease virus specific transfer factor disclosed by the invention, the specific immune effect of the chicken body on a neweastle disease virus antigen can be improved; normal cells of the body can be prevented from being infected by the virus. Thus, the transfer factor disclosed by the invention can play the roles in preventing the neweastle disease virus infection and protecting normal cells of the body from being damaged by the neweastle disease virus, so as to reduce the morbidity; meanwhile, the chicken infected by the neweastle disease virus can be treated by using the transfer factor. Therefore, the clinical symptoms can be effectively improved; the recovery rate is increased; the mortality is reduced.
Owner:SHANDONG SINDER TECH
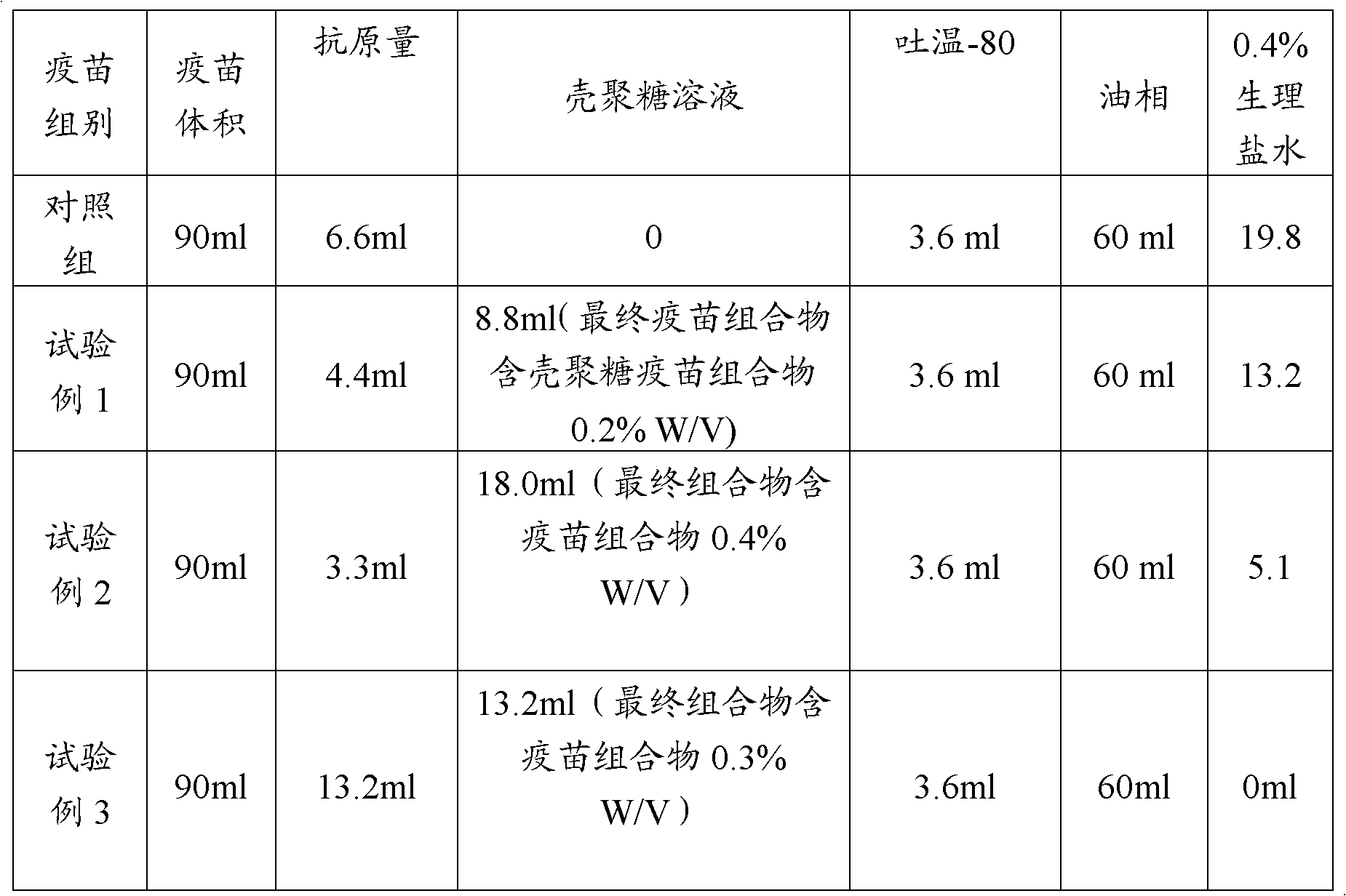
Application of chitosan in avian vaccine composition preparation
InactiveCN102973936AImprove the immunityLess antigenViral antigen ingredientsAntiviralsOil phaseVaccine antigen
The invention relates to an application of chitosan in avian vaccine composition preparation. An avian vaccine composition comprises newcastle disease inactivation virus or avian influenza inactivation virus and other avian vaccine antigens, and a chitosan solution, wherein the avian vaccine comprises a water phase and an oil phase, the chitosan solution is in the water phase, and a concentration of the chitosan solution is 0.1-0.5% W / V, most preferably 0.2-0.5% W / V. According to the avian vaccine composition, the amount of the antigen is low so as to reduce side reactions of the avian vaccine and reduce avian vaccine cost, such that the avian vaccine composition such as newcastle disease vaccines or avian influenza vaccines and the like are suitable for industrial production.
Owner:PU LIKE BIO ENG
Inactivated vaccine prepared by Newcastle disease virus heat stable strain and preparation method thereof
ActiveCN108379575AImprove thermal stabilityOvercome stabilitySsRNA viruses negative-senseViral antigen ingredientsCold chainOil adjuvant
The invention discloses an inactivated vaccine prepared by a Newcastle disease virus heat stable strain and a preparation method thereof. The vaccine takes the Newcastle disease virus heat stable strain as a vaccine virus seed. The preparation method comprises the following steps: preparing viral allantoic fluid of the Newcastle disease virus heat stable strain, diluting the viral allantoic fluidwith optimized dilution buffer, and preparing and inspecting beta-propiolactone and oil-adjuvant inactivated vaccine. The heat stability of the vaccine is obviously higher than that of the other conventional inactivated vaccines, and the inactivated vaccine also has excellent immunogenicity, solves the problems of relatively poor stability, high dependency level on a cold chain system and the likein the conventional common Newcastle disease virus vaccine, and has wide application prospects.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Chinese medicinal herba epimedii and propolis flavone immunopotentiator
ActiveCN101869590AGood effectNo pollution in the processAnthropod material medical ingredientsImmunological disordersPropolisCellular immunity
The invention relates to a Chinese medicinal herb epimedii and propolis flavone immunopotentiator, which is called the herb epimedii and propolis flavone immunopotentiator for short and belongs to the field of immunologic adjuvant for livestock and poultry. Each 1,000 ml of liquid medicine is prepared from 15 grams of herb epimedii and 5 grams of propolis flavone by the following steps of: adding water, decocting the herb epimedii twice, merging percolate and concentrating the percolate to prepare 870 ml of herb epimedii extract; dissolving propolis, which is processed with rice wine, with ethanol, filtering and concentrating the dissolved propolis to prepare 130 ml of propolis; and mixing the 130 ml of propolis and the herb epimedii extract to prepare the product. Immunity tests prove that the herb epimedii and propolis flavone immunopotentiator can remarkably stimulate the multiplication of lymphocytes of chicken in vitro, remarkably improve the antibody tilter of serum when used together with Newcastle vaccine for chicken, promote the multiplication of the lymphocytes, enhance the cellular immunity and humoral immunity of the chicken and improve the immune response of vaccines.
Owner:NANJING AGRICULTURAL UNIVERSITY
Breeding method for white feather hybrid broilers
The invention discloses a breeding method for white feather hybrid broilers. The breeding method comprises the following parts: 1, temperature and humidity control: controlling the temperature to be 35-37 DEG C when the white feather hybrid broilers are 1-7 days old, decreasing the temperature for 1-2 DEG C every week afterwards, and controlling the temperature to be 25-30 DEG C when the white feather hybrid broilers are 6-10 weeks old; controlling relative humidity to be 60-70 percent before the white feather hybrid broilers are 20 days old, and controlling the relative humidity to be 50-60 percent from that the white feather hybrid broilers are 20 days old until slaughtered; 2, nutritional requirements: feeding with 20 percent of complete pellet feed protein when the white feather hybrid broilers are 1-25 days old, and feeding with 17 percent of complete pellet feed protein from that the white feather hybrid broilers are 25 days old until slaughtered; 3, vaccine epidemic prevention: performing the subcutaneous injection of Newcastle disease vaccine, infectious bronchitis bivalent attenuated nasal drip and eye drop vaccine and avian influenza H9, the injection of bursa fabricius attenuated vaccine and the subcutaneous injection of avian influenza H5 inactivated vaccine, and enabling the white feather hybrid broilers to have Newcastle disease IV-series attenuated vaccine drinking water or performing the intramuscular injection of Newcastle disease IV-series attenuated vaccine. The white feather hybrid broilers bred by using the technical method have the advantages that the growth rate is 7-10 days higher than that of the original breed, the disease resistance is strong, the usage amount of a medicine is reduced, the feed conversion rate is high, and can be reduced by 0.1-0.2 percent, the survival rate of the white feather hybrid broilers is 2-3 percent point higher than that of the original breed, and the like. The white feather hybrid broilers are red in crown and bright in feather from the appearance, naturally yellow in feet, skin and mouth, and tender in meat quality, suitable for flavor cooking in all regions.
Owner:CHAOHU ZHENKANG POULTRY FOOD CO LTD
Live vaccine (CC gene stock) of cytokine adjuvant in animal immunopotentiator for treating chicken new castle disease
An animal immunopotentiator, cytokine adjuvant-chicken Newcastle disease vaccine (cc strain) is prepared by coordination of the cytokine (thymopeptide or transfer factor) with low-virulent immunoactivator of Newcastle disease.
Owner:金扩世 +1
Chimeric Newcastle disease virus vector H9 living vaccine candidate strain capable of overcoming influence of Newcastle disease maternal antibody in young chickens and construction method of candidate strain
InactiveCN107435041AHigh reproductive titerSuitable for mass productionSsRNA viruses negative-senseMicroorganism based processesNewcastle disease virus NDVFull length cdna
The invention relates to a chimeric Newcastle disease virus vector H9 living vaccine candidate strain capable of overcoming influence of Newcastle disease maternal antibody in young chickens and a construction method of the candidate strain. Preservation number of the Newcastle disease vaccine strain is CGMCC No.13797. According to the construction method, by virtue of a reverse genetics manipulation platform which is established and is capable of achieving efficient replication of chimeric virus AI4-T4FHN with the presence of the Newcastle antibody, HA sequence of an avian influenza H9N2 strain is interpolated into an AI4-T4FHN strain genome whole-length transcription vector pNDV / rAI4-T4FHN, so that recombinant Newcastle disease virus genome whole-length cDNA cloning is achieved. The recombinant virus AI4-T4FHN-H9, which is obtained from transfection, has a relatively high propagation tiler on a chicken embryo, and stable expression of HA protein can be still guaranteed after continuous passage; and meanwhile, effective replication in a chicken body at a Newcastle disease high maternal antibody level can be implemented; therefore, the candidate strain is applicable to large-scale production of vaccines and is suitable for producing vaccines.
Owner:YANGZHOU UNIV
Method for preparing vaccine by Newcastle disease virus cultured by using chick embryo continuous cell line and bioreactor
ActiveCN103386127AAvoid influenceAvoid the adverse effects of greater stressViral antigen ingredientsMicroorganism based processesNewcastle disease virus NDVEmbryo
The invention provides a method for preparing a vaccine by Newcastle disease virus cultured by using a chick embryo continuous cell line and a bioreactor, and belongs to the technical field of animal biological products. The method comprises the following steps: 1) performing passage and culture on a master cell; 2) multiplying a cell-adapted virus seed; 3) culturing the cell for multiplying virus in a suspending manner; 4) culturing and harvesting the virus; 5) preparing a chick Newcastle disease live vaccine; and 6) preparing the chick Newcastle disease live vaccine. The method adopted for preparing vaccines is simple and stable in production process, high in virus content, small in differences among batches, is easy to operate and control the quality, and can remarkably improve the yield and quality of vaccines; the produced chick Newcastle disease vaccines with high safety and high immune efficacy have a complete immune protection effect on the attack of virulent Newcastle disease virus.
Owner:浙江美保龙生物技术有限公司
Chimeric Newcastle disease virus vector H7 (avian influenza) live vaccine candidate strain capable of overcoming effect of Newcastle disease virus maternal antibody of chick and a construction method thereof
InactiveCN107384875AHigh reproductive titerSuitable for mass productionSsRNA viruses negative-senseMicroorganism based processesHemagglutininMaternal antibody
The invention relates to a chimeric Newcastle disease virus vector H7 (avian influenza) live vaccine candidate strain capable of overcoming an effect of a Newcastle disease virus maternal antibody of a chick and a construction method thereof. The preservation number of the Newcastle disease vaccine candidate strain is CGMCC No.13798. The construction method includes: making use of a reverse genetic manipulation platform of a chimeric Newcastle disease virus rAI4-TFHN which is capable of being replicated in the existence of a Newcastle disease antibody, inserting an HA (hemagglutinin) sequence of a subtype H7N9 (avian influenza) virus strain into a full-length transcription vector of an AI4-T4FHN strain genome so as to obtain a full-length cDNA (complementary deoxyribonucleic acid) clone pNDV / rAI4-T4FHN-H7 of a recombinant Newcastle disease virus genome containing the subtype HA gene of the H7N9 (avian influenza) virus. The recombinant virus AI4-T4FHN-H7 obtained through transfection is high in propagation titer in chick embryos, still capable of expressing HA protein stably after continuous passage and applicable to large-scale production of vaccine and can be used for manufacturing the vaccine.
Owner:YANGZHOU UNIV
Preparation method and application of atractylodes macrocephalaon polysaccharide-bacillus fermentation liquor
InactiveCN105112328AEnhance immune functionImprove disease resistanceBacteriaAnimal feeding stuffAtractylis carduusBiotechnology
The invention relates to a preparation method and application of atractylodes macrocephalaon polysaccharide-bacillus fermentation liquor. The preparation method includes the steps of inoculating activated bacillus subtilis to an atractylodes macrocephalaon polysaccharide liquid medium, and performing ferment culturing to obtain the atractylodes macrocephalaon polysaccharide-bacillus fermentation liquor. The atractylodes macrocephalaon polysaccharide-bacillus fermentation liquor is capable of enhancing animal immunity and improving animal disease resistance and vaccine protection rate, has more remarkable effect especially while being used with Newcastle disease vaccines and thus is highly worthy of popularization in preventing and controlling animal infectious diseases.
Owner:SHANDONG ENKANG PHARMA
Application of maggot extract in preparing medicament that enhances immunity of inactivated vaccine for pigeon Newcastle disease
ActiveCN103385891AImprove immunityHigh protection rateAnthropod material medical ingredientsImmunological disordersAntiviral drugSupersonic waves
The invention discloses an application of maggot extract in preparing medicament that enhances immunity of inactivated vaccine for pigeon Newcastle disease. According to the invention, live maggots are induced through supersonic wave, bodies are crashed to obtain extract as an additive to pigeon forge or drinking water, and immunization effect of pigeon Newcastle disease vaccine is improved, therefore immunity against pigeon pest is enhanced. Through the immunopotentiation of the maggot, protection rate of Newcastle disease vaccine is improved, use of antiviral drug is greatly reduce, which is beneficial for production of nuisance-free foodstuff and the effective rate is up to 100%.
Owner:江苏欧克动物药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com