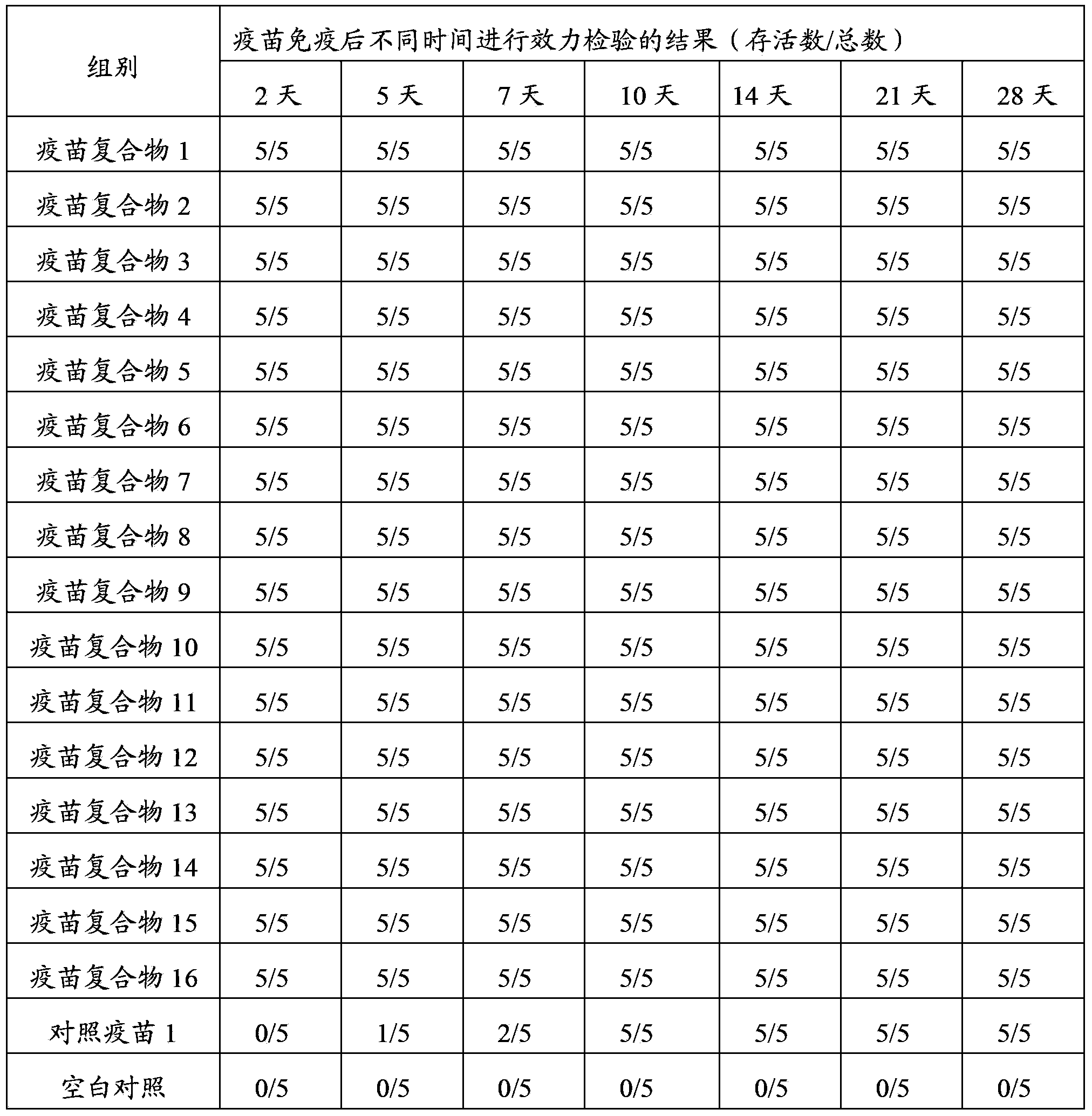
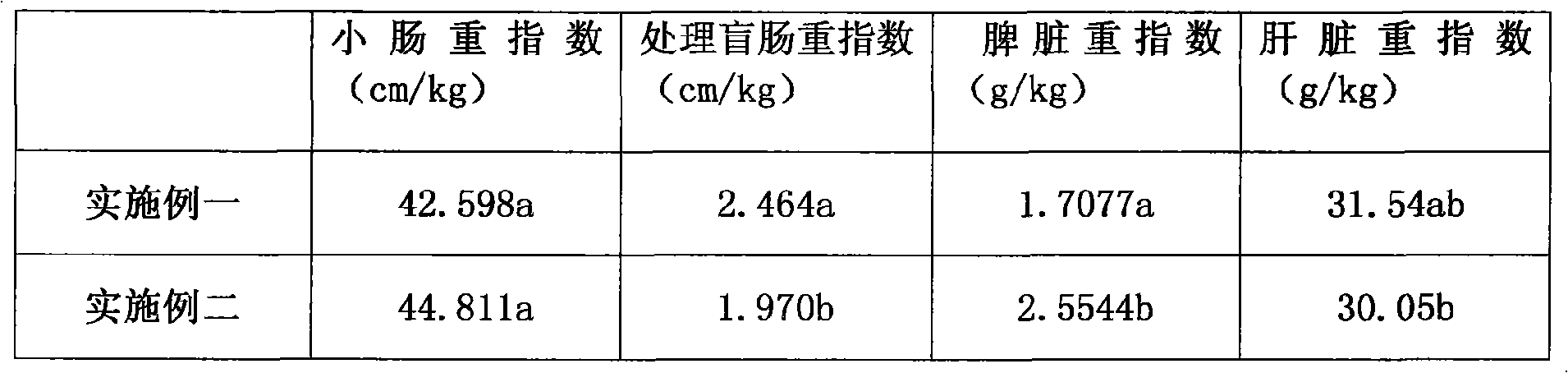
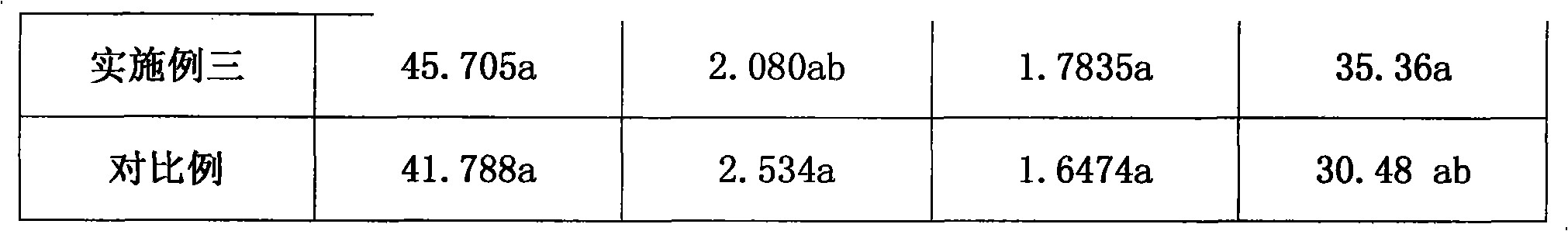
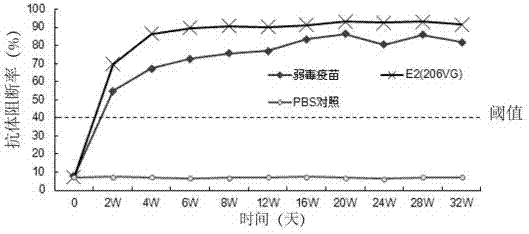
Patents
Literature
75 results about "Maternal antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Maternal Antibodies: Clinical Significance, Mechanism of Interference with Immune Responses, and Possible Vaccination Strategies. ... Maternal antibody levels in children of vaccinated mothers are lower and decline earlier than in children from naturally infected mothers.
Segregated early weaning method of piglet
ActiveCN101406166AReduce feeding costsIncrease the number of births per yearFood processingAnimal feeding stuffPig farmsMaternal antibody
The invention discloses a method for segregated early weaning of piglets, which comprises the steps of medicine adding, segregation, early transfer and the feeding of early weaning by piglet feed, wherein the medicine adding is performed before and after the delivery of a sow, the advanced medicine adding is used to kill off microorganisms which is expected to be killed, and the medicine adding is also performed from the birth of a suckling piglet to the weaning; the segregation adopts that the parturient sow is transferred into a clean farrowing house for delivery from the pregnancy, and the piglet at the age of between 10 and 14 days is transferred into a clean and comfortable breeding house from the farrowing house; when the level of a maternal antibody of the piglet at the age of between 10 and 14 days is in higher state, the piglet is moved away from the sow and is transferred into a cleaner environment; and the piglet feed for segregated early weaning adopts four stages to feed. The piglet has early weaning segregation time, high survival rate, and quick growth speed, the segregated early weaning of the piglet can save feeding cost of the sow, improve the yearly parity number of the sow, improve the utilization rate of the farrowing house, and reduce the probability that the piglet is infected with sow diseases, and a segregated early weaning technology of the piglet is suitable for the application of modern pig farms.
Owner:MUYUAN FOODS CO LTD
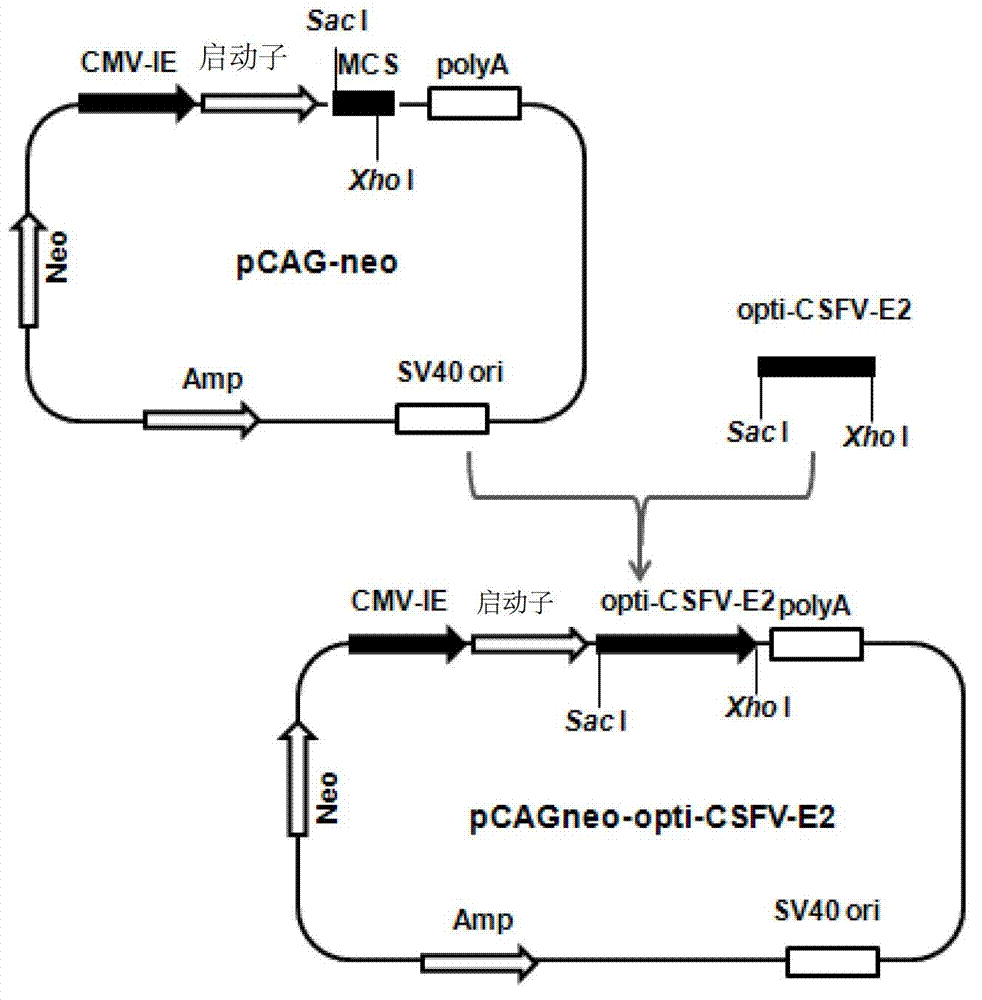
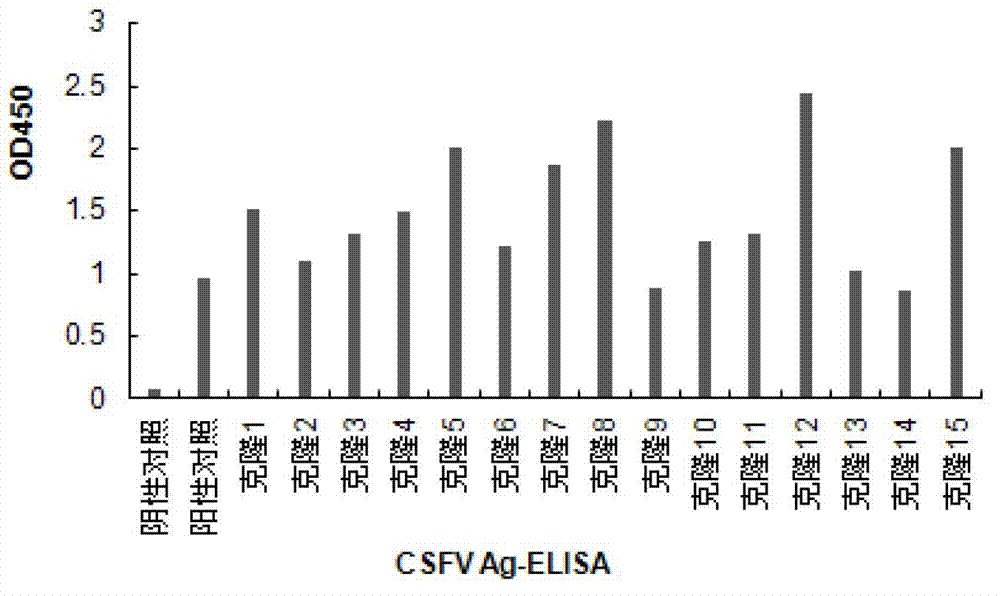
Recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the same in preparation of subunit vaccines and diagnosis reagents of classical swine fever
ActiveCN103751774AStable in natureFight infectionMicroorganism based processesAntiviralsMaternal antibodyAntigen
The present invention discloses a strain of a recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the recombinant cell line in preparation of subunit vaccines and diagnosis reagents of classical swine fever, wherein specifically the recombinant cell line is BCSFV-E2, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7719. The classical swine fever subunit vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no influence of the maternal antibody on immunization of swine, and can induce and produce high level classical swine fever virus neutralization antibodies after the swine is immunized. In addition, the present invention further discloses a method for constructing the recombinant mammalian cell line, a method for preparing the classical swine fever subunit vaccine, and applications of the antigen expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents.
Owner:HARBIN WEIKE BIOTECH DEV +1
Goose parvovirus and applications thereof
ActiveCN103525771AImproving immunogenicityRaise antibody levelsEgg immunoglobulinsMicroorganism based processesMaternal antibodyDisease
The invention provides goose parvovirus (GPV) and applications thereof, and belongs to the technical field of biology. The invention provides a goose parvovirus GPV-YZ strain as well as applications thereof in preparing medicaments for preventing and treating the gosling plague disease. The microorganism preservation number is CGMCC NO.8160. The invention also provides a gosling plague disease viral vaccine as well as an anti-gosling plague yolk antibody. The goose parvovirus YZ strain provided by the invention has favorable immunogenicity for the present epidemic goose parvovirus, can resist toxicity attacking of the GPV-AV240 strain, the GPV-GD strain as well as the toxic strain of the goose parvovirus, and has the protection capability against the GPV-AV240 strain and the GPV-GD strain. After breeding geese are immunized by the gosling plague disease viral vaccine provided by the invention, young geese can be protected by maternal antibodies, and can be prevented from being infected by gosling plague viruses. The anti-gosling plague yolk antibody provided by the invention can prevent and treat gosling disease.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
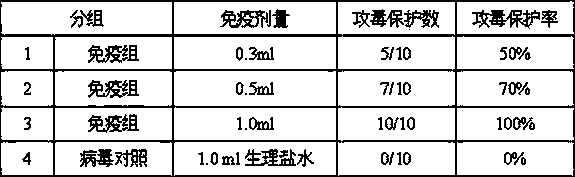
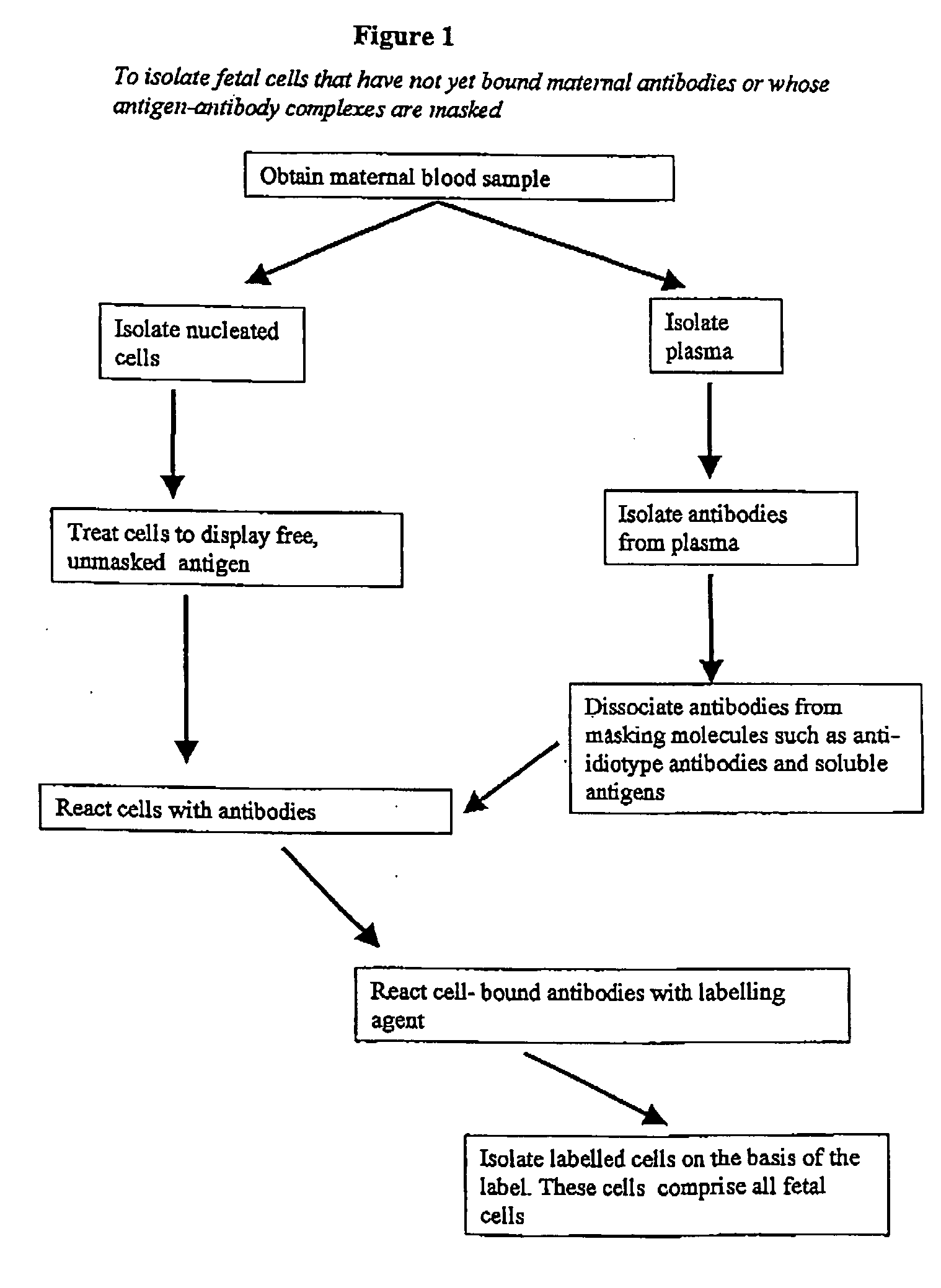
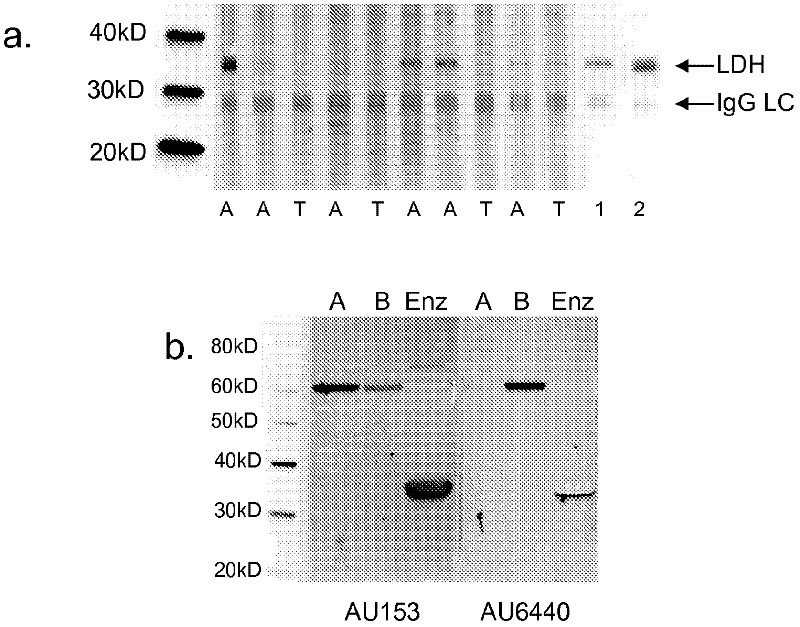
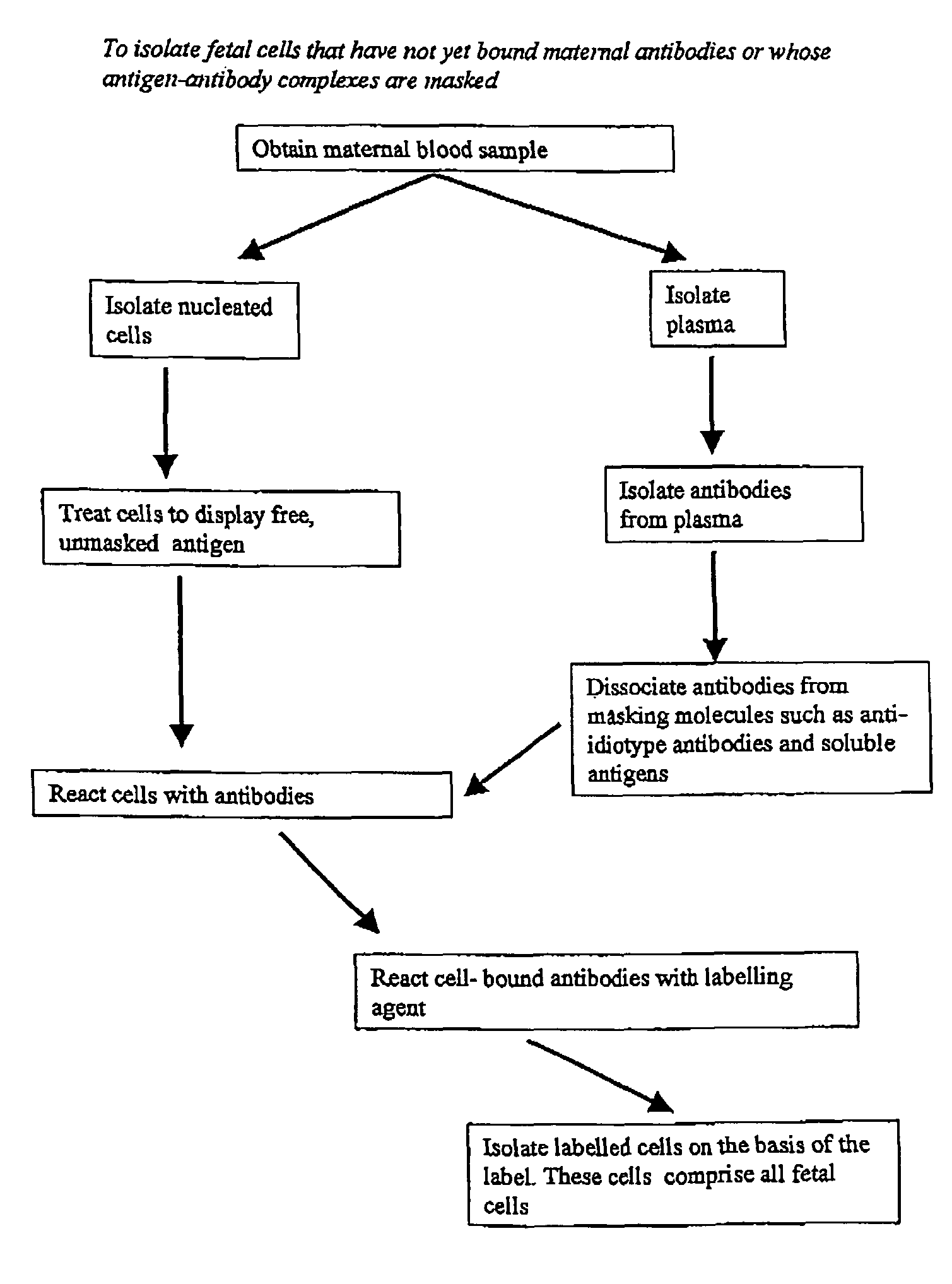
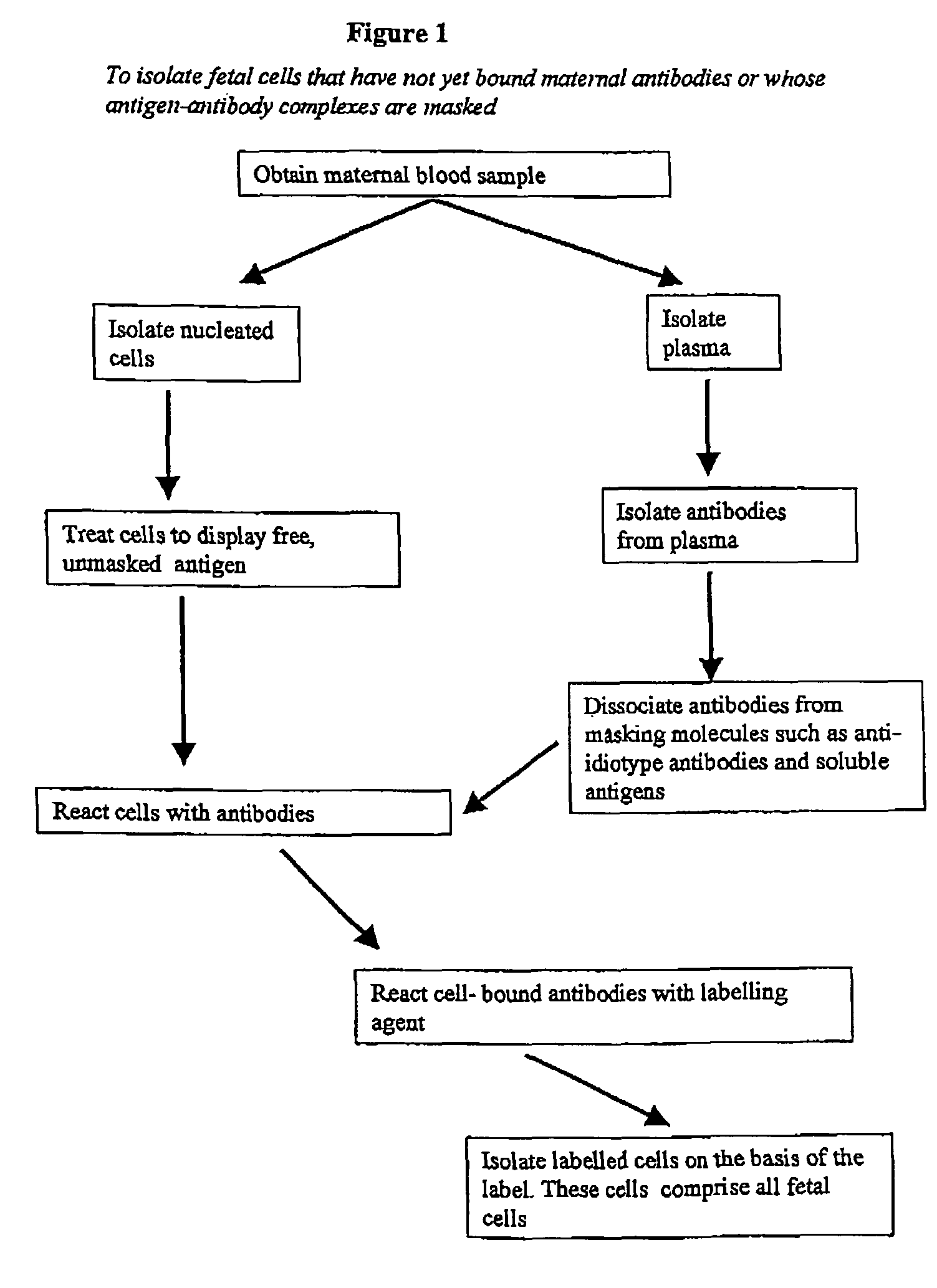
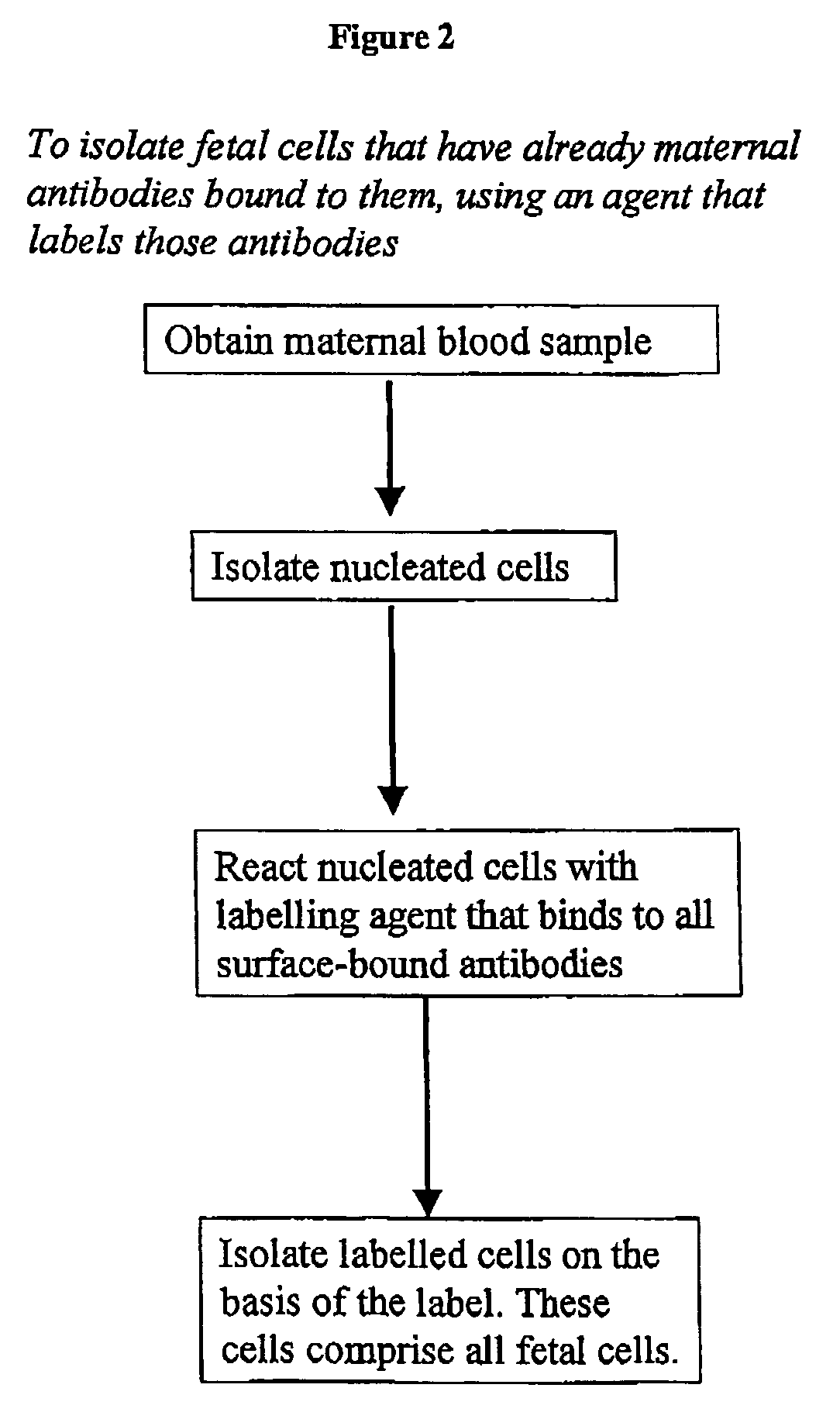
Maternal antibodies as fetal cell markers to identify and enrich fetal cells from maternal blood
InactiveUS20050287604A1Increase the number ofMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementAntigenMaternal antibody
The present invention provides methods for identifying and / or enriching fetal cells from maternal blood, using as fetal cell markers the antibodies that the mother produces against paternally inherited fetal antigens. The fetal cell-maternal antibody complexes are identified and isolated using labelled agents that bind to the maternal antibodies. The present invention also provides fetal cells, isolated by use of said maternal antibodies, as a source of fetal DNA for prenatal genetic diagnosis of the fetus.
Owner:GENETIC TECHNOLOGIES LIMTIED
Preparation method and application of immunization preparation for controlling novel duck reovirus
InactiveCN102492661APrevent Hemorrhagic Necrotizing HepatitisEasy to manufactureViral antigen ingredientsMicroorganism based processesMaternal antibodyYolk
The invention discloses a novel duck reovirus strain NDRV-JM85 with a preservation number of CCTCC NO: V201127. The novel duck reovirus strain NDRV-JM85 is used as a virus seed to inoculate an allantoic cavity of a muscovy duck embryo and obtain a virus-containing allantoic fluid, or inoculate an MDEF cell to obtain cytotoxin when a CPE reaches 75%. The virus liquid is inactivated by formaldehyde and added with a white oil adjuvant, and the mixture is mixed thoroughly to from a safe and effective oil emulsion inactivated vaccine for novel duck reovirus. The vaccine is used to immunize a laying hen three times, with an immunization interval of 21 days and 1.0 ml for each hen at each time. Eggs laid 21-63 days after the three immunizations are gained, and extracted yolk and a proper amount of disinfected normal saline is prepared into a hyperimmune antibody preparation for the novel duck reovirus. A duckling without an NDRV maternal antibody is injected with the antibody preparation at 1-2 days old for 0.5ml / duckling, or a duckling with a maternal antibody is injected with the antibody preparation at 10 days old for 1.0 ml / duckling, so as to prevent and control the novel duck reovirus. The invention applies to large-scale production under a GMP condition.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +1
Specific chicken CpG ODN and its application
ActiveCN1810823AHigh attack protection rateImprove the level ofSugar derivativesGenetic material ingredientsMaternal antibodyBird flu
The present invention relates to CpG DNA, and is especially specific chicken CpG ODN and its use as the H5-subtype bird flu DNA vaccine adjuvant. The CpG ODN sequence is 5í»-TTGGAACGTTCCTTTCCAACGTTGGTTTGGAACGTTCCTT-3í». The H5-subtype bird flu DNA vaccine with the CpG ODN adjuvant is taken by chicken through oral taking or nose dropping to immunize, and can raise the protection rate against the challenge virus and the intestinal mucous membrane IgA antibody level of chicken obviously. The DNA vaccine of the present invention has the advantages of safety, no interference on the monitoring of bird flu epidemic situation, capacity of overcoming the interference of maternal antibody, use convenience, etc.
Owner:YANGZHOU UNIV
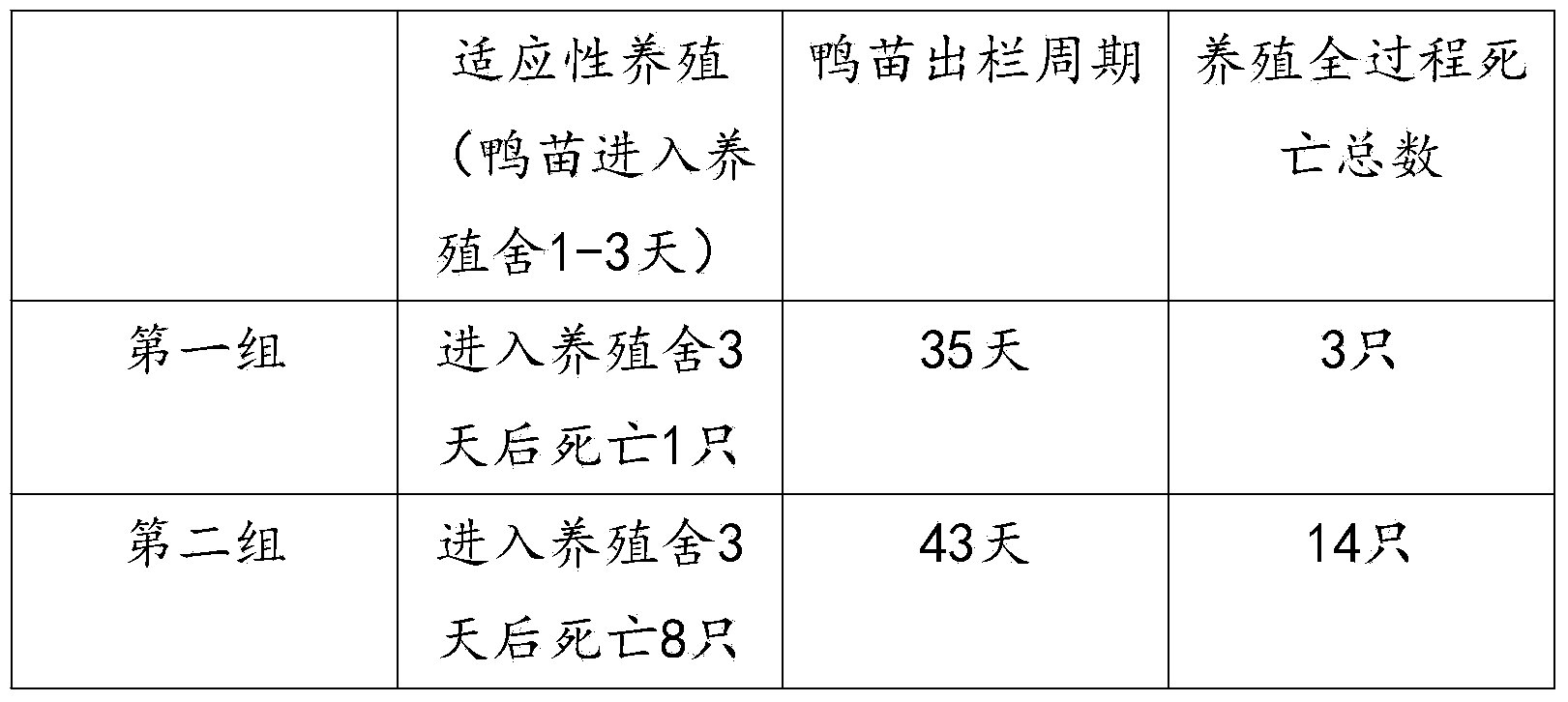
Meat duck online raising method
InactiveCN104137808AImprove the immunityReduce mortalityAnimal husbandryMaternal antibodyMortality rate
The invention provides a meat duck online raising method, and relates to the field of commercial duck raising. The method includes the following steps that according to a place where a farm is located, ducklings adapting to the local growing environment are purchased; when the ducklings just enter the farm, adaptive raising for 1 to 3 days needs to be conducted; after adaptive raising, the maternal antibody conditions of the ducklings are detected, accordingly, immunization of an avian influenza vaccine and feed preparation are conducted, and primary immunization time is determined according to a maternal antibody fading rule; after being vaccinated, the ducklings are placed in a mesh raising house to be raised, suspended meshes are horizontally arranged in the raising house, and meat ducks are raised on the meshes until being slaughtered. The temperature and the humidity of the environment where the ducklings are located are adjusted according to a steam fumigation method, essential oil separated out from traditional Chinese medicine is contained in steam, stress of the ducklings to the new environment can be reduced, immunity of the ducklings is improved, and therefore the death rate of the ducklings is decreased when the ducklings enter the new environment; compound feed is mixed with water extract of traditional Chinese medicine auxiliary materials, so that the purposes that immunity of the ducks is improved and the slaughter period is shortened are achieved.
Owner:安徽诺阳禽业有限公司
Nasal immune vaccine for porcine epizootic diarrhea virus and preparation method of immune vaccine
ActiveCN104667269AEffective immune protectionPreventing the Harm of Epidemic DiarrheaPharmaceutical delivery mechanismAntiviralsMaternal antibodyEpidemic diarrhea
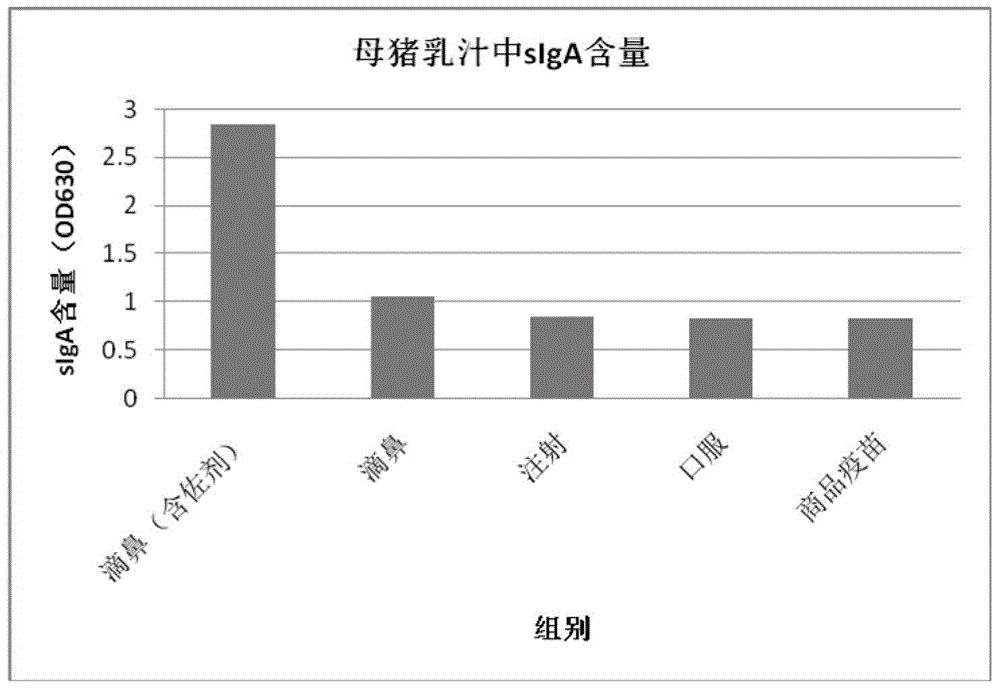
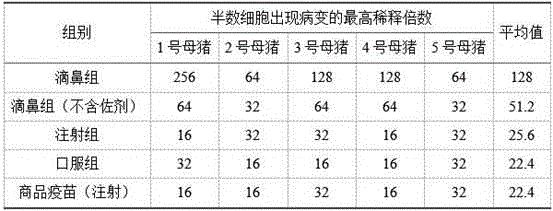
The invention discloses a nasal immune vaccine for a porcine epizootic diarrhea virus (PEDV) and a preparation method of the nasal immune vaccine. According to the preparation method, the nasal immune vaccine is prepared by mixing a vaccine concentrate for the porcine epizootic diarrhea virus, an adjuvant solution and a sodium azide solution at a specific ratio, wherein the adjuvant solution contains a TLR agonist, an agonist for an NOD sample receptor, carbomer and PVPK. Compared with an existing related vaccine, the nasal immune vaccine for the PEDV prepared by the method is high in titer and good in immune effect. The vaccine disclosed by the invention is subjected to vaccine immunization through a nasal mucosa tissue; meanwhile, mucosal immunization and system immunization of the organism are stimulated at the same time; a lot of sIgA is generated in sow milk; and a maternal antibody is transferred to piglets through milk so that the piglets obtain effective immune protection and the harms caused by the epidemic diarrhea of the piglets are prevented.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
Feeding and management method for female yak and yak calf for producing traceable white yak calf meat
The invention discloses a feeding and management method for a female yak and a yak calf for producing traceable white yak calf meat. The steps include that a pregnant female yak is fed and managed in a mode of pasturing, a warm shed and supplementary feeding within three months before calving; after calving, healthy male yak calves with the birth weight larger than 12.5 kg are selected; the calves must suck beestings within 0.5-1 hour after birth and obtain maternal antibody as soon as possible, in the first 0-7 days, the beestings is mainly fed, in the day 7, paratyphoid immunization is conducted on the calves, and from the day 8 before the calves leave the shed, a feeding and management mode of full-breast-feeding and pasturing along with the female yak is practiced; simultaneously the female yak after giving birth to the calves is fed and managed in a mode of pasturing and supplementary feeding, after the calves are fed for 24-26 weeks, healthy bull calves with weight larger than 80 kilograms leave the shed and are butchered to produce the traceable white yak calf meat. The yak calves fed in a standard mode is tender in meat texture, and the meat is rich in amino acid, vitamin and the like required by human bodies and easy to digest and has Tibet plateau characteristics.
Owner:SICHUAN ACAD OF GRASSLAND SCI
Methods of diagnosing and treating autism
The present invention provides diagnostic methods for determining the risk of developing an autism spectrum disorder (ASD) in a fetus or child by detecting in a biological sample from the mother antibodies that bind to one or more biomarkers selected from the group consisting of lactate dehydrogenase (LDH), guanine deaminase (GDA), collapsin response mediator protein 1 (CRMP1), stress-induced phosphoprotein 1 (STIP1), alpha subunit of the barbed-end actin binding protein Cap Z (CAPZA2), Y Box Binding Protein 1 (YBX1), eukaryotic translation and elongation factor 1A1 (EEF1A1), microtubule-associated protein Tau (MAPT), dihydropyrimidinase-like protein 2 (DPYSL2), dynamin 1-like protein (DNM1L), radixin (RDX), moesin (MSN), and ezrin (EZR). The invention further provides methods of preventing or reducing the risk of a fetus or child developing an ASD by administering to the mother an agent that blocks the binding of maternal antibodies to the one or more fetal biomarkers listed above or by removing from the mother antibodies that bind to the one or more fetal biomarkers.
Owner:RGT UNIV OF CALIFORNIA
Maternal antibodies as fetal cell markers to identify and enrich fetal cells from maternal blood
InactiveUS7785898B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMaternal antibodyAntigen
The present invention provides methods for identifying and / or enriching fetal cells from maternal blood, using as fetal cell markers the antibodies that the mother produces against paternally inherited fetal antigens. The fetal cell-maternal antibody complexes are identified and isolated using labelled agents that bind to the maternal antibodies. The present invention also provides fetal cells, isolated by use of said maternal antibodies, as a source of fetal DNA for prenatal genetic diagnosis of the fetus.
Owner:GENETIC TECHNOLOGIES LIMTIED
Muscovy duck parvovirus inactivation vaccine and application thereof
ActiveCN103830724ALow toxicityRaise antibody levelsAntiviralsAntibody medical ingredientsMaternal antibodyTGE VACCINE
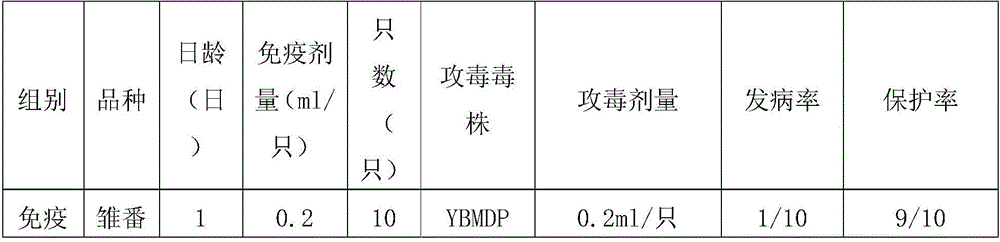
The invention provides a Muscovy duck parvovirus inactivation vaccine which comprises an antigen and a vaccine adjuvant, wherein the antigen is inactivated Muscovy duck parvovirus, and the collection number of the Muscovy duck parvovirus is CGMCC No.8504. The Muscovy duck parvovirus inactivation vaccine provided by the invention is prepared by the steps of screening a Muscovy duck parvovirus YBMDP strain with low toxicity and high immunogenicity; inoculating and collecting the virus liquid; performing ultrafiltration concentration and formaldehyde solution inactivation; adding an oil adjuvant and mixing and emulsifying to obtain the vaccine. The vaccine provided by the invention can improve the antibody level of Muscovy duck, guarantee the maternal antibody level of the filial generation and prevent the duckling parvovirus infection caused by the Muscovy duck parvovirus; the vaccine has the advantages of high efficiency and good safety.
Owner:YEBIO BIOENG OF QINGDAO
Recombinant fowl pos virus vaccine rFPV 1218AIH5/H9 and its construction process and use
InactiveCN101066448AMaintain and enhance immunogenicityImprove practicalityAntiviralsAntibody medical ingredientsMaternal antibodyFowl
The present invention relates to recombinant fowl pos virus vaccine constructing technology, and is especially recombinant fowl pos virus vaccine rFPV-1218AIH5 / H9 and its construction process and use. Through setting the H5 subtype-containing AIV HA gene segment and H9 subtype-containing AIV HA gene segment separately in the downstream of respective promoter, back-to-back connecting serially, inserting into fowl pos virus expressing vector p12-18 containing FPV copying non-essential segment FPV12-18 and reporter gene LacZ to constitute transfer vector; transfecting wt-FPV-infecting chick embryo fibroblast with the transfer vector; and homogeneous recombination, virus vaccine with co-expressed H5 and H9 subtype AIV HA gene and free from interference of maternal antibody is obtained.
Owner:YANGZHOU UNIV
Newcastle disease virus strain and application thereof in preparation of Newcastle disease vaccine
ActiveCN104164410AMake up for the shortcoming of too longProduce quicklyViral antigen ingredientsMicroorganism based processesMaternal antibodyAdjuvant
The invention discloses a Newcastle disease virus strain and an application thereof in preparation of a Newcastle disease vaccine. A preparation method of the Newcastle disease virus strain comprises the following step: culturing recombinant plasmids pBrClone30-GM-CSF, pBL-N plasmids, pBL-P plasmids and pBL-L plasmid cotransfection mammalian cells together so as to obtain the Newcastle disease virus strain, wherein the recombinant plasmids pBrClone30-GM-CSF are of the plasmids of DNA molecules shown in No.2703 to No.18408 nucleotides from the 5' tail end of a sequence 3 with a sequence table. According to the Newcastle disease virus strain, molecular adjuvants GM-CSF are led into the genomes of the existing Newcastle disease vaccine, so that the immune effect is effectively improved and the immune blank period is greatly shortened. Meanwhile, the Newcastle disease virus strain can be used for resisting a maternal antibody so as to improve the immune protective rate. Thus, the Newcastle disease virus strain has an important value for preventing and treating Newcastle diseases.
Owner:JIANGSU KANIONREAL BIOMEDICAL TECH CO LTD
Blocking test paper for swine fever antibody
InactiveCN110058019ABeneficial positive effectQuick checkVirus peptidesBiological material analysisMaternal antibodySwine Fever Virus
The invention discloses a blocking test paper for swine fever antibody. The test paper is composed of a support plate, an antigen pad, a gold standard pad, a detection membrane and an absorbent pad, wherein the antigen pad absorbs the detection antigen of swine fever virus, the gold standard pad absorbs the colloidal gold labeled anti-swine fever virus monoclonal antibody mAb1, the detection membrane contains "|" blots of a detection line T and a quality control line C, the detection line T is the blot of an anti-swine fever monoclonal antibody mAb2 or an anti-swine fever polyclonal antibody pAb1, and the quality control line C is the blot of an anti-mouse IgG antibody pAb2 or a staphylococcus aureus SPA. The test paper provided by the invention realizes the rapid detection of neutralizingantibody of swine fever virus, can realize real-time monitoring of the maternal antibody and immune antibody level of the swine fever as well as the immune evaluation of the vaccine immune effect, and is simple in operation and applicable for everyone, which can well meet the needs of different levels of personnel, is easy to promote and apply in a wide range, and has broad market prospects and large economic and social benefits.
Owner:HENAN ACAD OF AGRI SCI
Biological product for treating gosling plague and preparing method thereof
InactiveCN106237328AImprove securityImprove immunityViral antigen ingredientsAntiviralsMaternal antibodyPathogenic microorganism
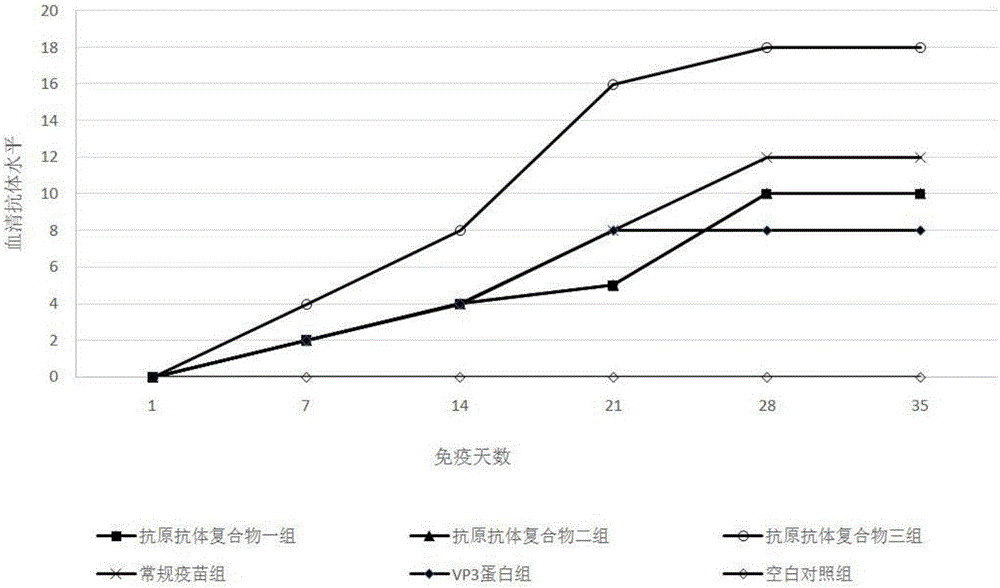
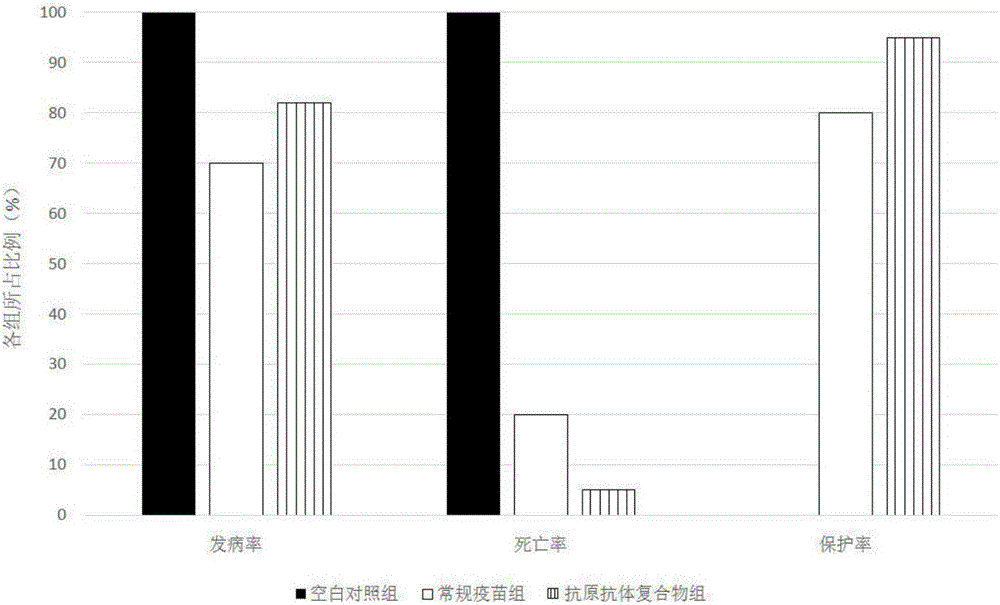
The invention provides a biological product for treating gosling plague and a preparing method thereof. The technical scheme is based on the good controlling effect of an antigen-antibody compound on a virus, a combination scheme with VP3 protein as an antigen and an egg yolk antibody as an antibody is determined according to the characteristics of a gosling plague virus, and on this basis, the specific preparing methods of the VP3 protein and the egg yolk antibody are further designed. According to the technical scheme, on the basis of the original gosling-plague egg yolk antibody, the gosling-plague VP3 protein antigen is added, and a compound is prepared. The gosling-plague antigen-antibody compound can resist persistent infection caused by pathogenic microorganism, excess using of antibiotics is avoided, the interference of a maternal antibody is broken through, and an intense stress reaction caused by live vaccine is reduced; as the used antigen is not a complete virus antigen, but is the antigen prepared through the VP3 protein of the gosling plague, the danger that full viruses are dispersed outwards can be reduced, and the biological product is safer, more reliable and more effective.
Owner:TIANJIN RINGPU BIO TECH
Trivalent vaccine with maternal anitbody transfer via the milk
Owner:INTERVET INT BV
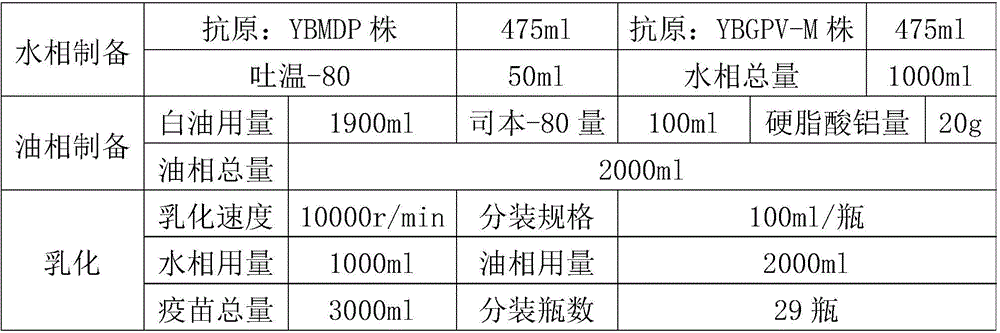
Muscovy duck parvovirus and gosling plague bivalent vaccine
ActiveCN105920596AImproving immunogenicityImprove securityViral antigen ingredientsAntiviralsMaternal antibodyImmunogenicity
The invention provides a Muscovy duck parvovirus and gosling plague bivalent vaccine. The antigens used by the vaccine is inactivated Muscovy duck parvoviruses and Muscovy duck-source gosling plague viruses, the preservation number of the Muscovy duck parvoviruses is CGMCC No. 8504, and the preservation number of the Muscovy duck-source gosling plague viruses is CCTCC No. V201620. A preparation method of the Muscovy duck parvovirus and gosling plague bivalent vaccine includes: the Muscovy duck parvovirus YBMDP strains and Muscovy duck-source gosling plague virus YBGPV-M strains which are high in virus content and good in immunogenicity are screened, infected embryos and allantoic fluid are collected after duck embryo inoculation, and oil emulsion adjuvant is added for emulsification and mixing to obtain the vaccine after homogenization, ultrafiltration and concentration, and formaldehyde solution inactivation. The prepared vaccine can immunize breeding Muscovy ducks and increase the level of two types of antibodies of the breeding Muscovy ducks at the same time, guarantee the offspring maternal antibody level of the breeding Muscovy ducks, and prevent the young Muscovy duck parvovirus diseases caused by the Muscovy duck parvoviruses and gosling plague virus infection caused by the Muscovy duck-source gosling plague viruses.
Owner:YEBIO BIOENG OF QINGDAO
BI-Specific Diabodies For Masking And Targeting Vaccines
InactiveUS20140079704A1Promote chemotaxisImprove responseSsRNA viruses positive-senseVectorsAntigenMaternal antibody
The present invention describes compositions and methods for priming protective immunity in the presence of pre-existing maternal antibody. In some embodiments, the invention contemplates simultaneously masking vaccines to avoid antibody neutralization while targeting those vaccines to specific cell types in order to elicit an enhanced immune response. In other embodiments, vectors that recruit and activate specific antigen-presenting cells may further enhance the efficacy of those immune responses.
Owner:TEXAS A&M UNIVERSITY
Infectious bursal disease live vaccine and production method thereof
ActiveCN103316334AImprove immunityBacterial antigen ingredientsMicroorganism based processesMaternal antibodyVaccine Production
The present invention relates to an infectious bursal disease live vaccine production method, which screens virulent strains with good immunogenicity, wider antigen spectrum and immune synergy from studies of characteristics of different infectious bursal disease virus such as immunogenicity, antigen spectrum, pathogenicity of bursa of Fabricius and capacity of breaking through maternal antibody, carries out development of the infectious bursal disease trivalent live vaccine, and develops a live vaccine--an infectious bursal disease trivalent live vaccine which has good immunogenicity, can break through higher level maternal antibody, has long immune duration more than 3 months, and has good protection to the various virulent strains.
Owner:北京中海生物科技有限公司
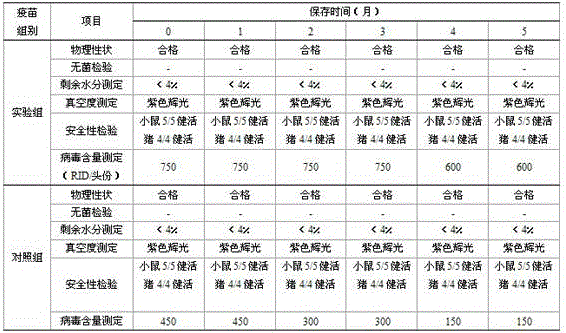
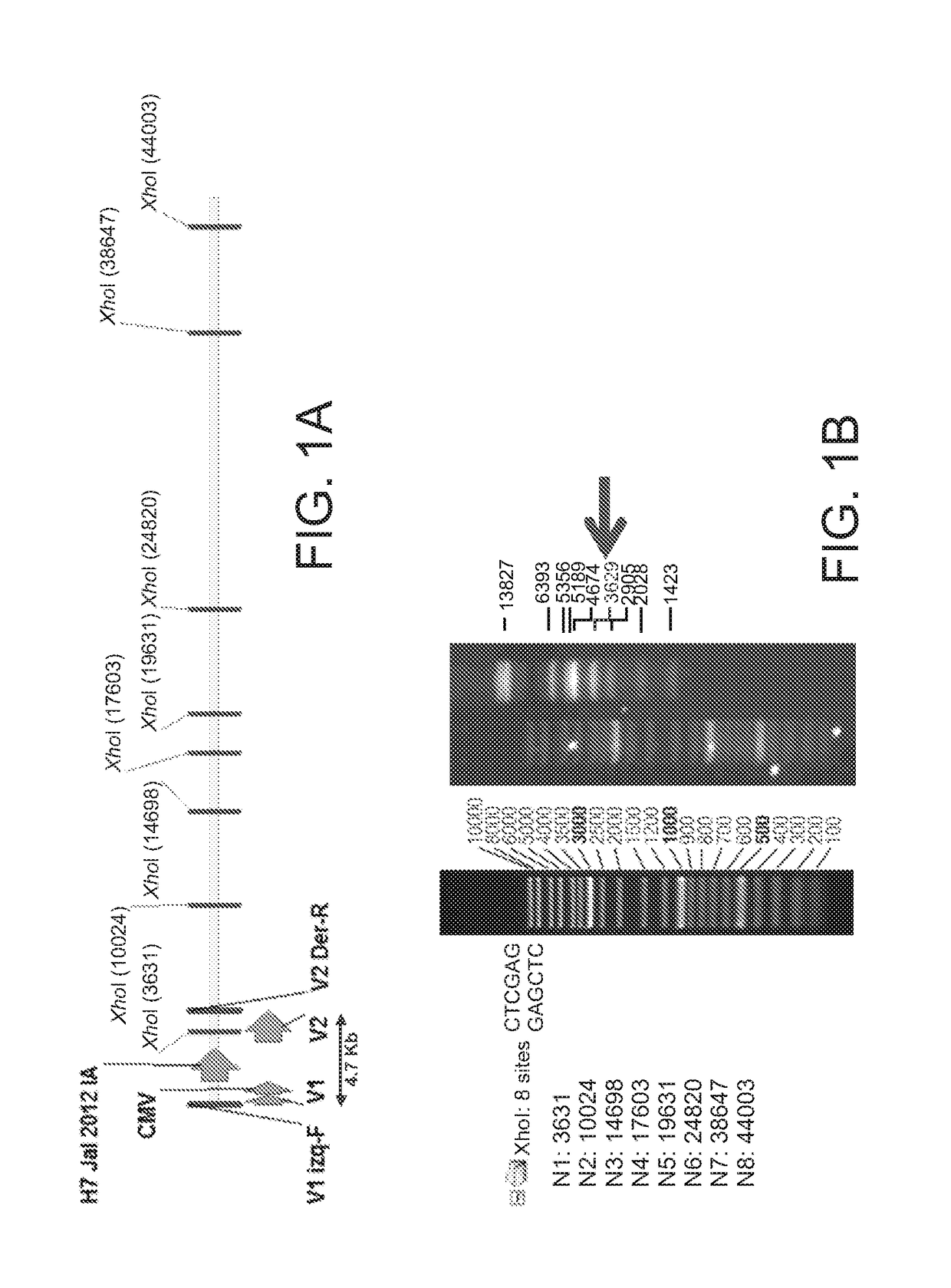
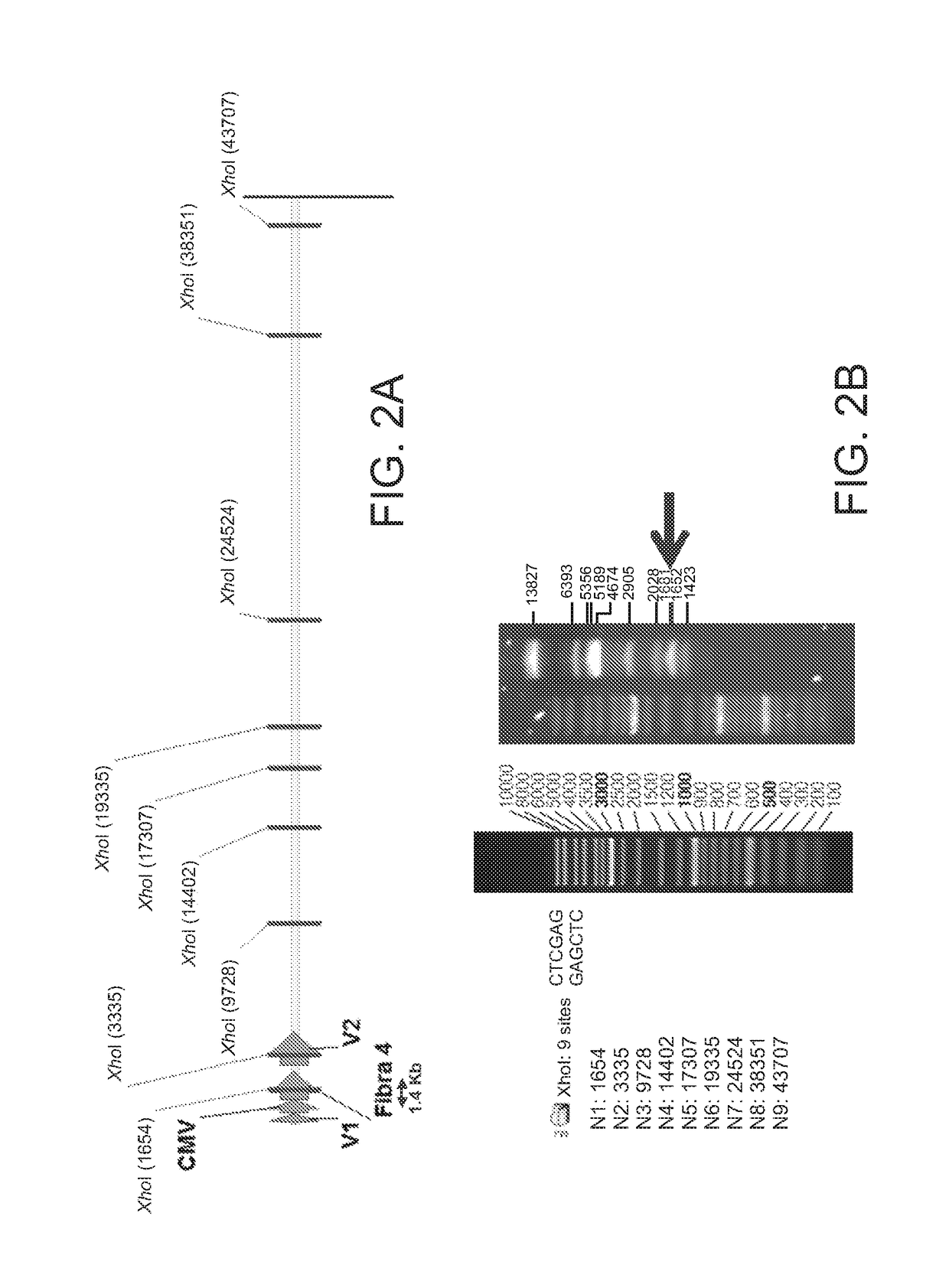
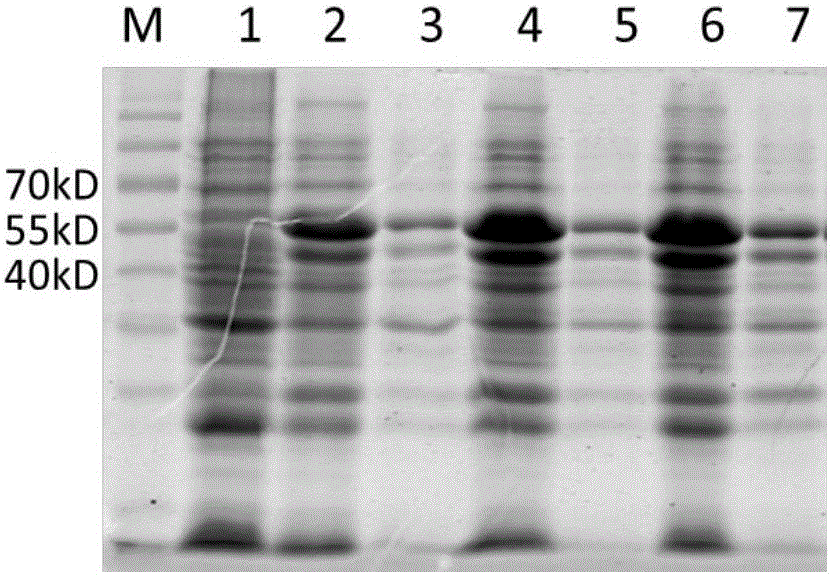
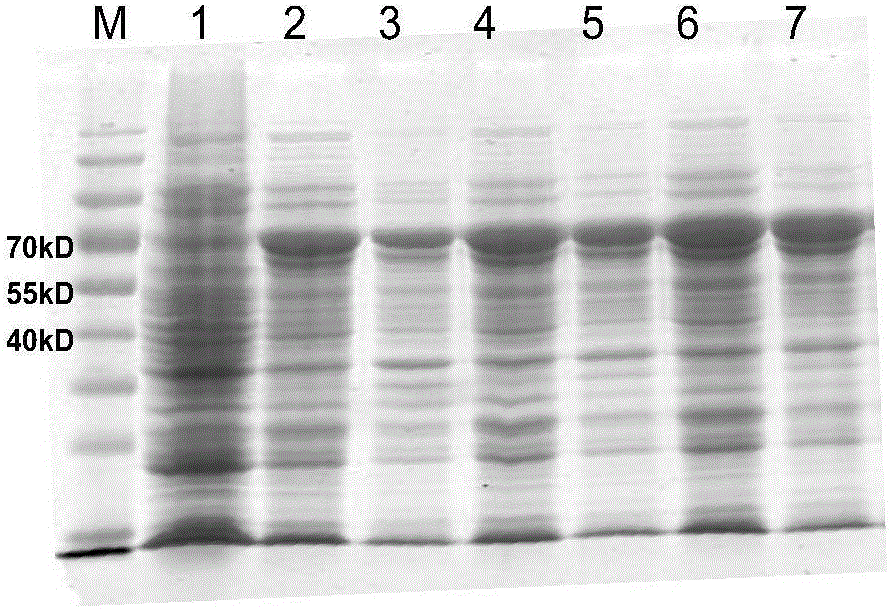
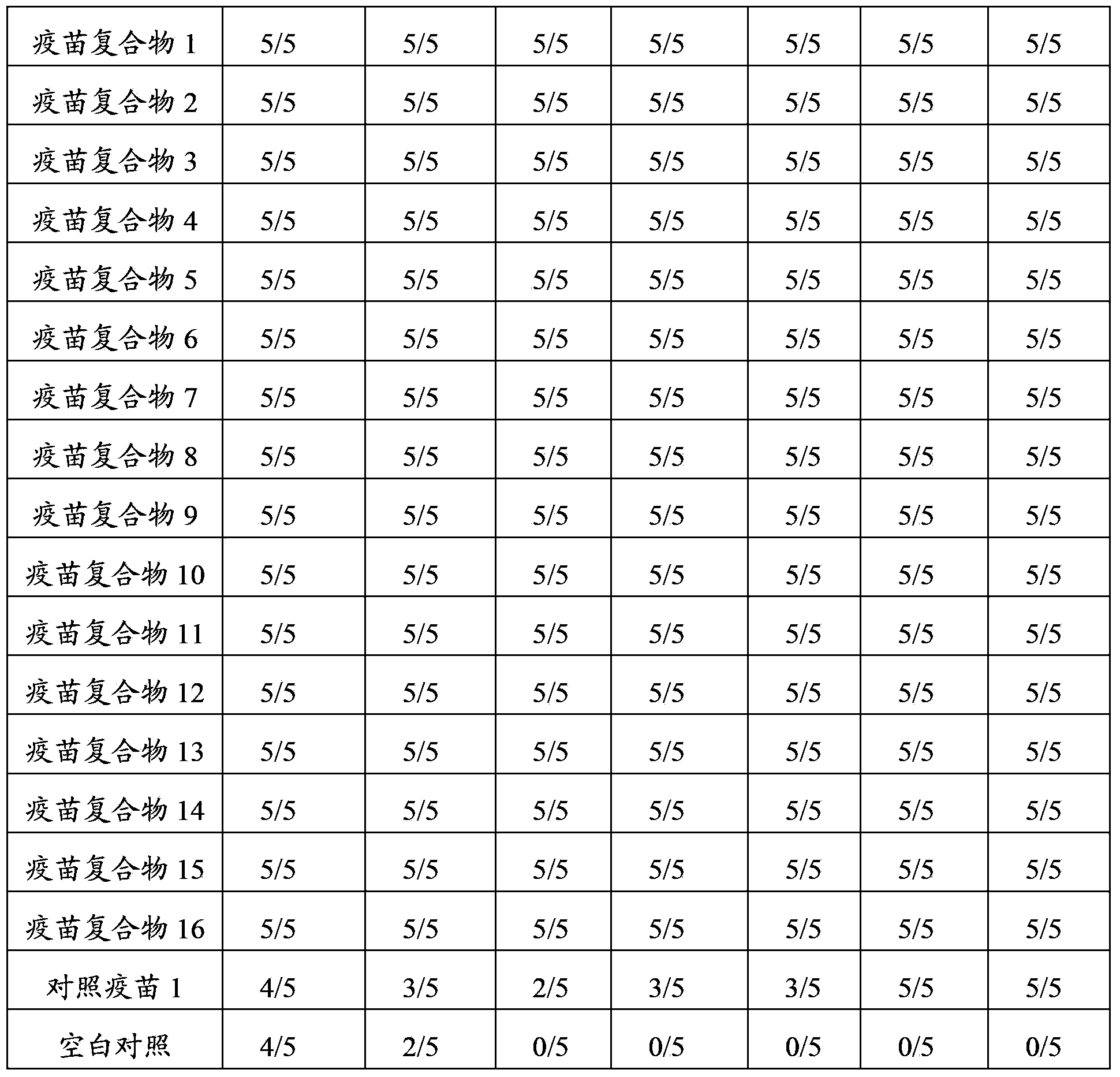
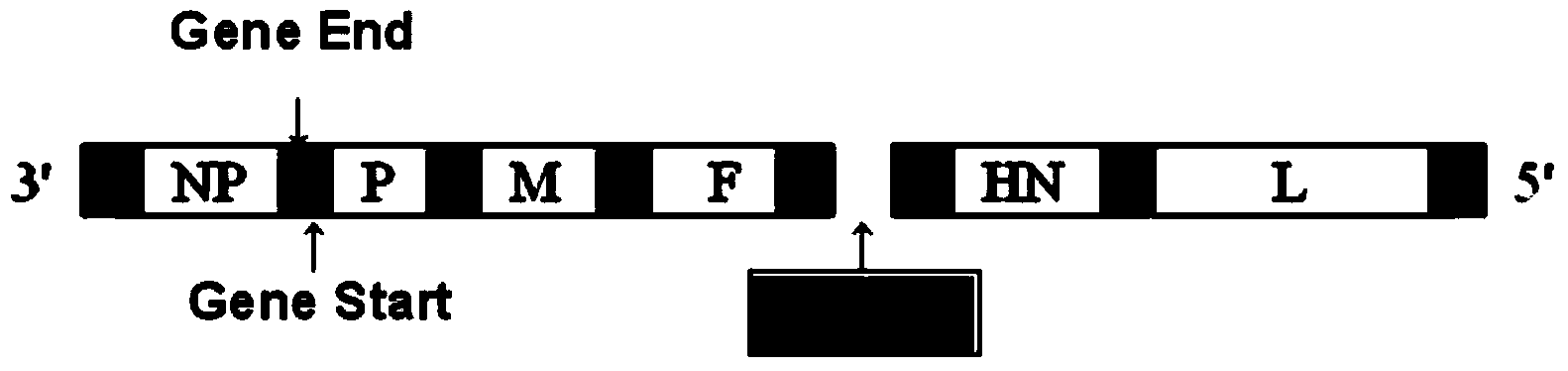
Mosaic chimeric viral vaccine{[s]] particle
Owner:TEXAS A&M UNIVERSITY
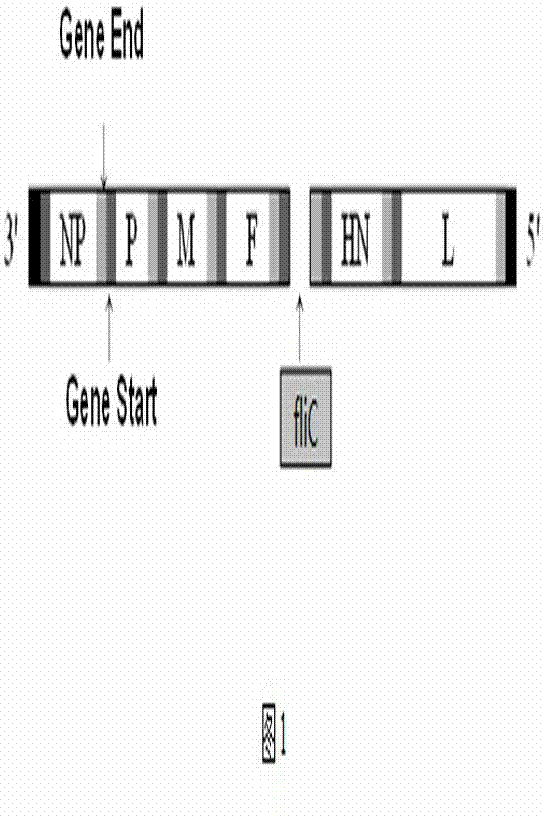
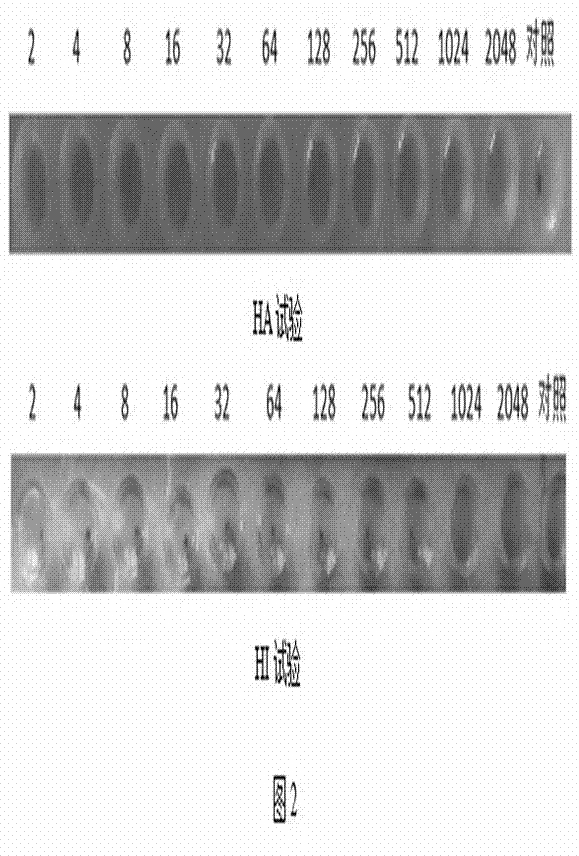
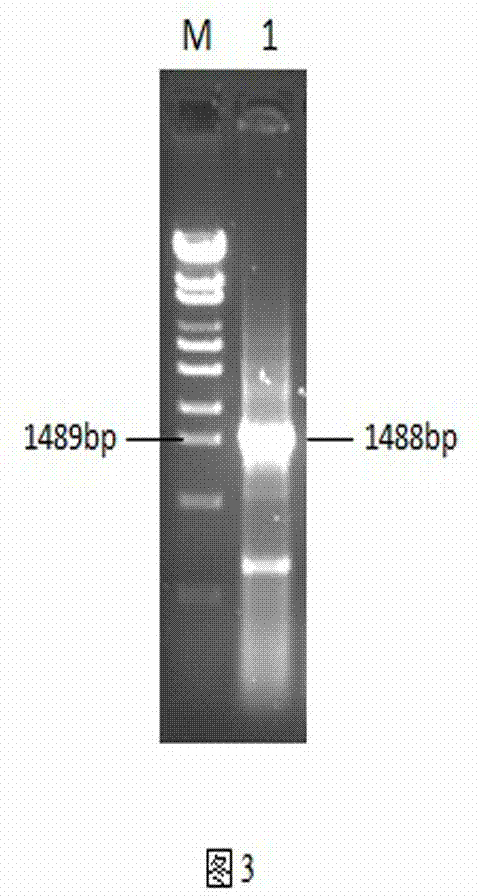
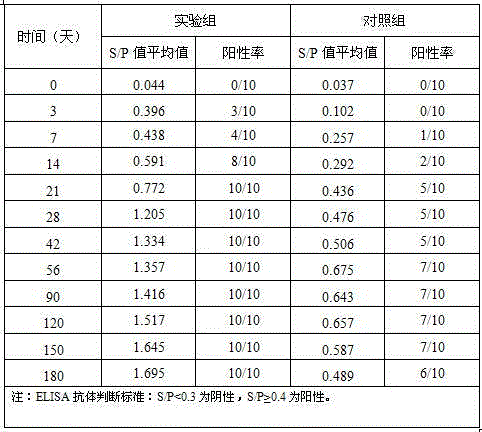
Preparation of Newcastle disease virus strain rClone30-fliC and application of the Newcastle disease virus strain rClone30-fliC in prevention and treatment of Newcastle disease
ActiveCN104328136AMake up for the shortcoming of producing antibodies for too longProduce quicklyViral antigen ingredientsMicroorganism based processesMaternal antibodyAdjuvant
The invention discloses a Newcastle disease virus strain rClone30-fliC and an application thereof in prevention and treatment of Newcastle disease. The Newcastle disease virus strain rClone30-fliC and a preparation method thereof are disclosed. The preparation method comprises a step of co-transfecting a constructed recombinant plasmid pBrClone30-fliC and corresponding auxiliary plasmids pBL-N, pBL-P and pBL-L to a BHK-21 cell which can stably express a T7 polymerase with rescue cultivation to obtain the Newcastle disease virus strain. In the invention, by means of introducing a molecular adjuvant fliC to a conventional Newcastle disease live vaccine genome, immunoreactions of an inoculated chicken is enhanced and an HI antibody is quickly generated beyond expectation so that an early-stage immune effect is achieved and an immune blanking period is reduced. Meanwhile, the Newcastle disease virus strain rClone30-fliC is free of interference of a maternal antibody and an immune protective effect is increased. The invention has an important value on prevention and treatment of the Newcastle disease.
Owner:JIANGSU KANIONREAL BIOMEDICAL TECH CO LTD
Preparation method of classical swine fever spleen-lymph-sourced compound living vaccine
InactiveCN104888213AEnhance humoral immunityBreak through interferenceOrganic active ingredientsAnthropod material medical ingredientsMucosal Immune ResponsesMaternal antibody
The invention relates to a preparation method of a classical swine fever spleen-lymph-sourced compound living vaccine and belongs to the technical field of veterinary biological products. The preparation method of the classical swine fever spleen-lymph-sourced compound living vaccine comprises the following process steps: selecting a domestic rabbit to be inoculated; inoculating; measuring the temperature and observing; harvesting; preparing virus suspension and inspecting; carrying out mixed adsorption on the harvested full-suspension virus bulk and chicken anti-hog cholera virus egg yolk antibody; and adding freezing and drying protecting agent containing an immunopotentiator into antigen antibody compound obtained through adsorption, carrying out split charging, freezing, and carrying out vacuum drying, so that the classical swine fever spleen-lymph-sourced compound living vaccine is obtained. By adopting the preparation method of the classical swine fever spleen-lymph-sourced compound living vaccine, an antibody acquiring route is simple, animal welfare can be improved, manpower and material resources are saved, production cost is greatly reduced, humoral immunity of the living vaccine can be greatly improved, and high neutralizing antibodies can be produced; and the classical swine fever spleen-lymph-sourced compound living vaccine can be applied to piglet immunization, maternal antibody interference can be avoided, no interference is produced to other vaccines, especially a mycoplasma hyopneumoniae vaccine and the like, after the classical swine fever spleen-lymph-sourced compound living vaccine is applied to piglets, and strong mucosal immune response can be induced, so that protection rate is improved.
Owner:浙江美保龙生物技术有限公司
Vaccine in the form of a recombinant sero type 9 avian adenovirus vector
ActiveUS20170232096A1Adequate responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenMaternal antibody
A recombinant vaccine comprising a serotype 9 fowl adenovirus vector (FAdV-9) having at least one exogenous nucleotide sequence inserted encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region, and a pharmaceutically acceptable vehicle, adjuvant and / or excipient, wherein the at least one exogenous nucleotide sequence encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region is located between the 491 and 2782 nucleotides.The vector of this vaccine is stable for industrial scale production. Likewise, even when administering this vaccine in combination with a vaccine against Marek's disease, both vaccines produce an adequate immune response which is not affected by interference between each other. In the same way, effectiveness of the recombinant vaccine is not affected by maternal antibodies, and is capable of inducing both an early and lasting protective response, even with only one application.
Owner:GRUPO IND PECUARIO S A DE CV
DHAV (Duck Hepatitis A Virus) III type complex live vaccine and preparation method thereof
ActiveCN106563125AImprove securityWide range of securitySsRNA viruses positive-senseViral antigen ingredientsDuck hepatitis A virusMaternal antibody
The invention discloses a DHAV (Duck Hepatitis A Virus) III type complex live vaccine and a preparation method thereof. The method firstly discloses the DHAV III type complex live vaccine, which comprises antigen-antibody complex and pharmaceutically acceptable freeze-drying protective agents, wherein the antigen-antibody complex is prepared from DHAV III type chick embryo weakening strain JS57 virus liquid and duck anti-DHAV III type egg yolk antibodies. The invention further discloses a preparation method of the DHAV III type complex live vaccine. The preparation method comprises the following steps of mixing the freeze-drying protective agents with the antigen-antibody complex prepared from the DHAV III type chick embryo weakening strain JS57 virus liquid and the duck anti-DHAV III type egg yolk antibodies; performing freeze vacuum drying; and obtaining the DHAV III type complex live vaccine. The preparation method provided by the invention has the advantages that the antibody obtaining path is simple; the production cost is low; the prepared DHAV III type complex live vaccine can break through the maternal antibody interference to generate high neutralizing antibody valence; and the production rate on ducklings is improved.
Owner:哈药集团生物疫苗有限公司
Muscovy duck parvovirus subunit vaccine
InactiveCN106139140ARaise antibody levelsAvoid infectionViral antigen ingredientsAntiviralsMaternal antibodyViral infection
The invention aims to provide a muscovy duck parvovirus subunit vaccine. The muscovy duck parvovirus subunit vaccine comprises an antigen and a vaccine adjuvant, wherein the used antigen is muscovy duck parvovirus VP protein; and the amino acid sequence of the muscovy duck parvovirus VP protein is SEQ ID NO: 1. After muscovy ducks are inoculated with the vaccine prepared by the invention, the antibody level of the muscovy ducks can be improved, the maternal antibody level of filial generation is guaranteed, and young muscovy duck goose parvovirus infection caused by muscovy duck source goose parvovirus is prevented. If young muscovy ducks at the age of one day are inoculated with the vaccine, the young muscovy duck goose parvovirus infection caused by muscovy duck source goose parvovirus can be prevented effectively.
Owner:青岛真源生物技术有限公司
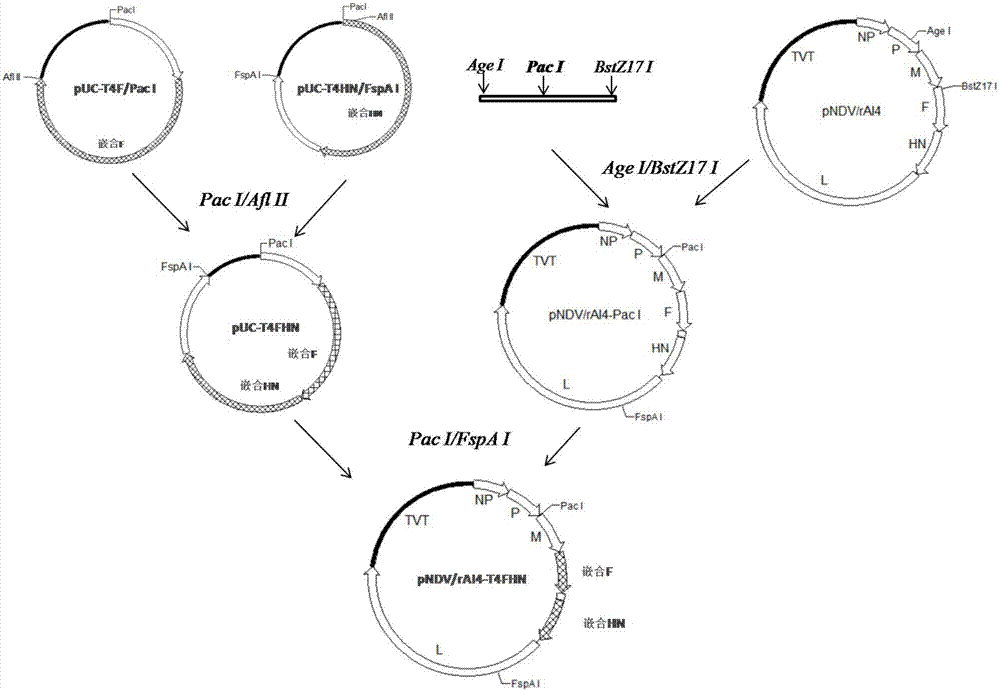
Chimeric Newcastle disease virus vaccine vector candidate strain capable of overcoming influence of maternal antibody of Newcastle disease and construction method thereof
InactiveCN107254450ASuitable for mass productionEfficient replicationSsRNA viruses negative-senseMicroorganism based processesMaternal antibodyAvian paramyxovirus
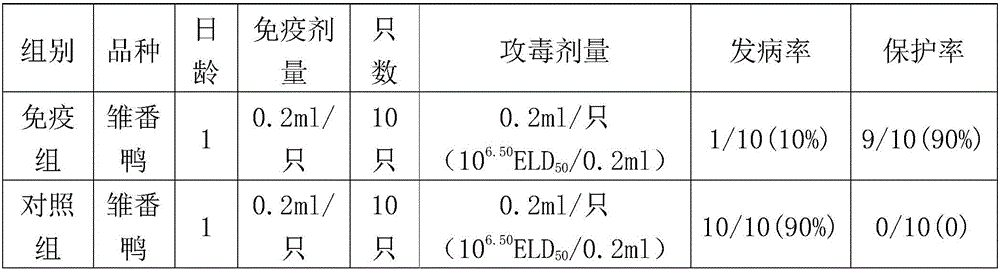
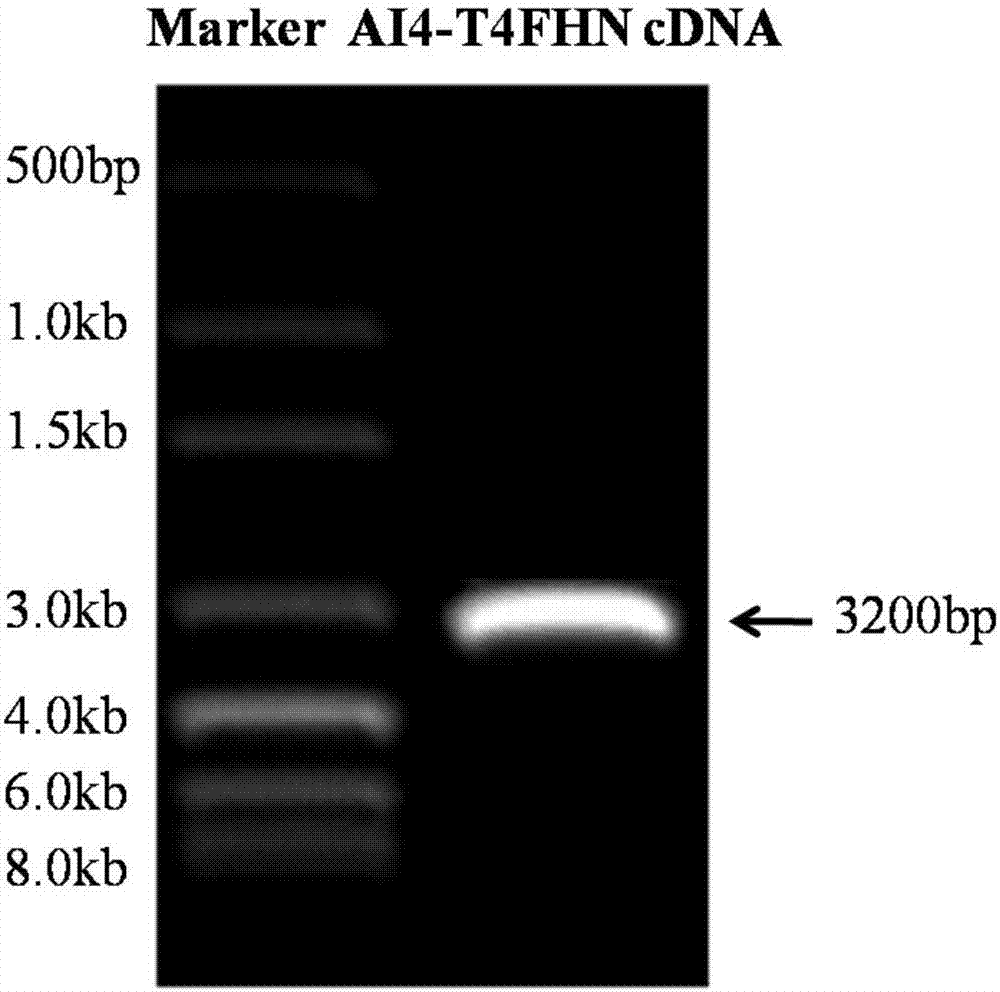
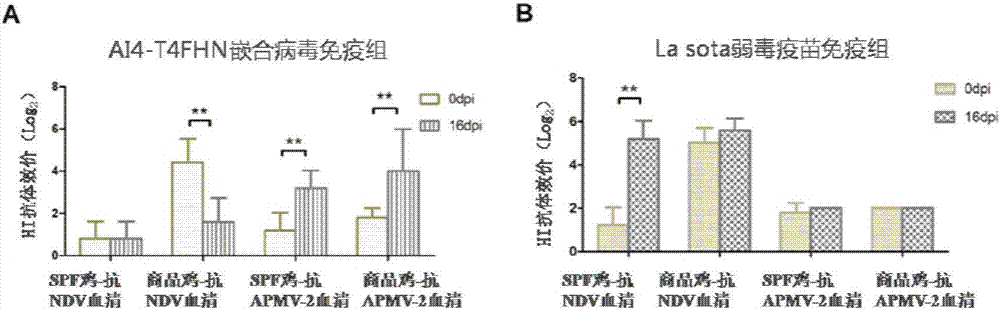
The invention belongs to the technical field of application of reverse genetics and gene replacement, and particularly relates to a chimeric Newcastle disease virus vaccine vector candidate strain capable of overcoming the influence of a maternal antibody of a Newcastle disease and a construction method of the chimeric Newcastle disease virus vaccine vector candidate strain. The candidate strain is avian paramyxovirus type-1 AI4-T4FHN, and the preservation number is CGMCC No:12987. An established reverse genetics manipulation platform for a Newcastle disease virus gene type-VII attenuated virus strain AI4 is utilized to replace the corresponding part of a genome of the strain AI4 through the sequence of an extracellular region of envelope proteins F and HN of an avian paramyxovirus type-2, and the recombinant virus AI4-T4FHN is obtained through saving. The cross reaction capacity of the avian paramyxovirus type-2 and the Newcastle disease virus are very weak, and the avian paramyxovirus type-2 is not pathogenic for a chicken. The chimeric virus has a high reproductive capacity similar to that of a female parent virus, and meanwhile, the chimeric virus is effectively replicated in the chicken body with the maternal antibody of the Newcastle disease at high level, and is a novel vaccine carrier.
Owner:YANGZHOU UNIV
New method of replacing serum antibody monitoring by H5 subtype avian influenza egg yolk antibody
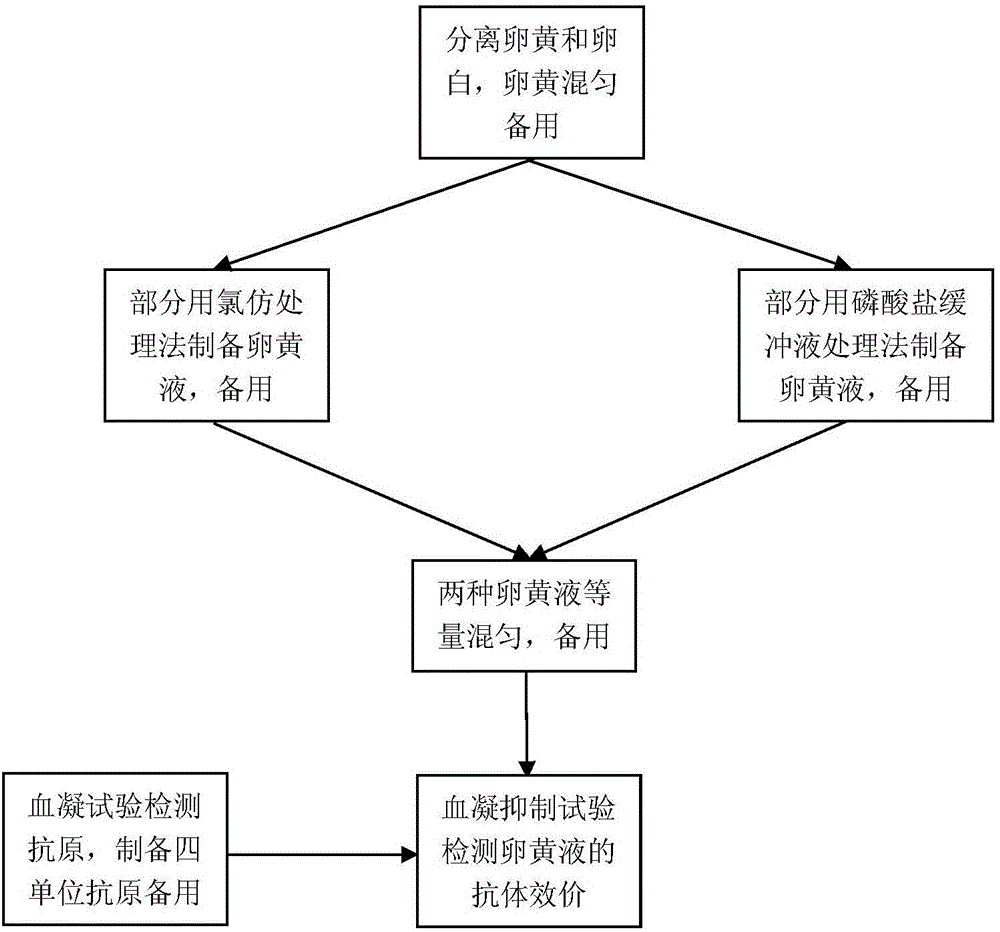
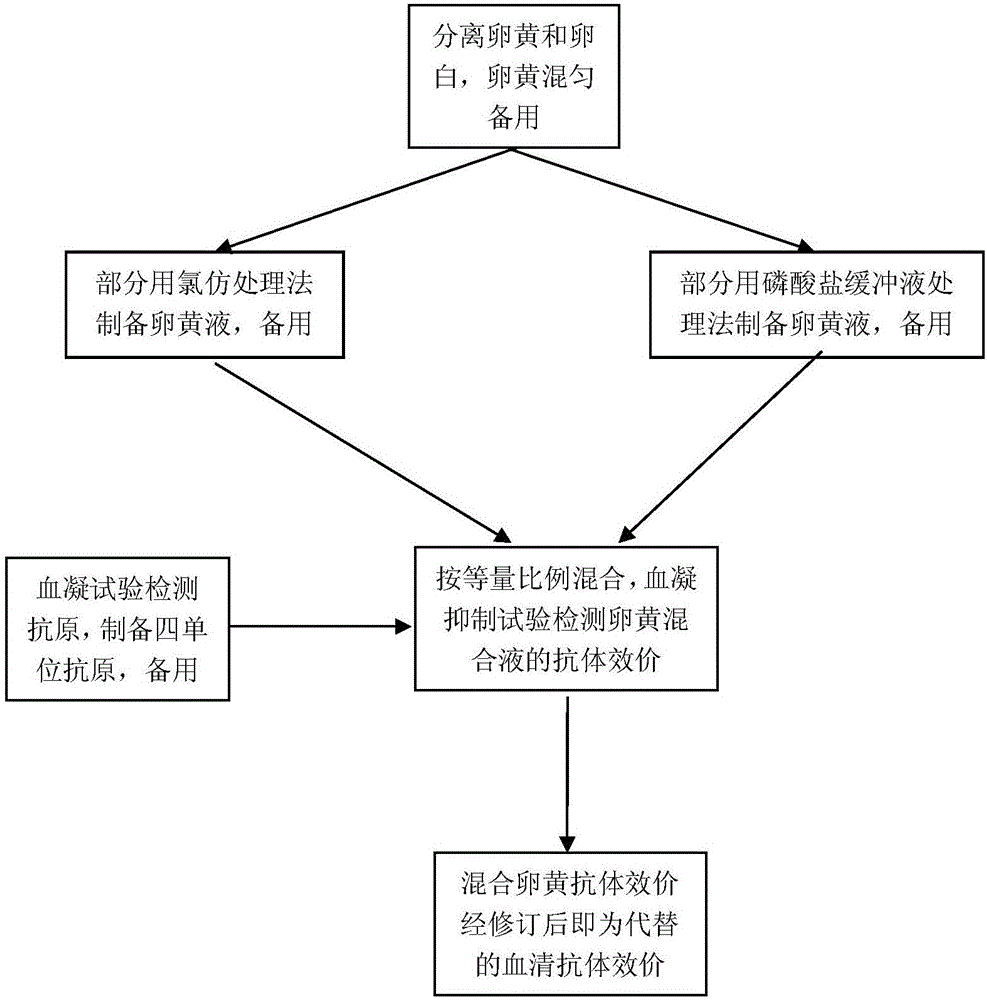
InactiveCN106749639AEasy to monitorMonitoring Operational SecurityEgg immunoglobulinsMaterial analysisMaternal antibodyFowl
The invention belongs to the technical field of egg yolk antibody monitoring, and in particular relates to a new method of replacing serum antibody monitoring by a H5 subtype avian influenza egg yolk antibody. The invention provides a method of replacing a serum sample by use of an egg yolk sample, and of replacing the serum antibody monitoring by use of an egg yolk solution extracted through a phosphate buffer solution and the egg yolk solution extracted through chloroform to perform the antibody monitoring. The method is characterized in that the blood sampling is unnecessary in the antibody monitoring process, the antibody monitoring process only needs to collect the eggs, cannot produce stress on the poultry and cannot influence the health and production performance of the poultry; the mixed solution H5 subtype avian influence antibody valence of two egg yolk treating solution can replace the serum antibody valence after being modified in the antibody monitoring process, the H5 subtype avian influence antibody is mainly used for the H5 subtype avian influenza antibody monitoring of the laying fowl and the breeding poultry, and further can be used for evaluating the nestling epidemic disease maternal antibody level so as to determine the first immune time.
Owner:LINYI UNIVERSITY
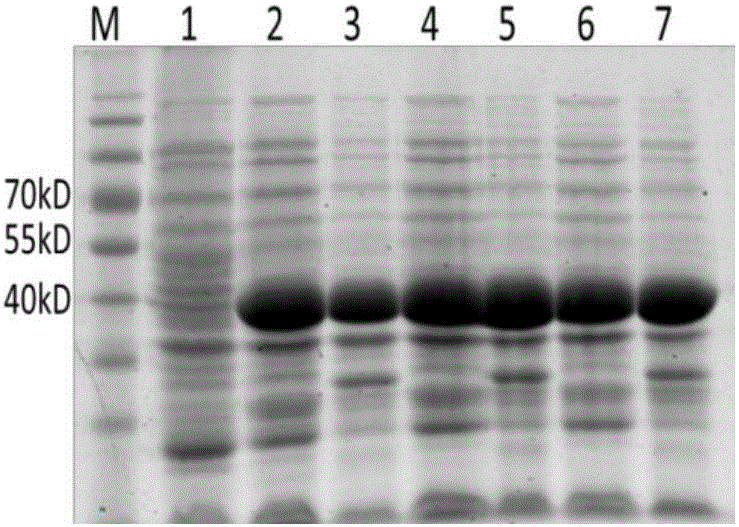
Detection kit for chicken infectious bronchitis indirect ELISA antibody
InactiveCN105092839ASimple indirect ELISA methodSpecific indirect ELISA methodMaterial analysisMaternal antibodyInfectious bronchitis virus Antibody
The invention discloses a detection kit for chicken infectious bronchitis indirect ELISA antibody, belonging to the field of biotechnology. According to the invention, genetically engineered bacterium capable of steadily expressing partial nucleoprotein of chicken infectious bronchitis virus is utilized for inducible expression of recombined N160 protein which is subjected to affinity chromatographic purification, and the purified recombined N160 protein is taken as a coating antigen, so as to establish a simple and convenient and specific indirect ELISA method. The invention further provides a specific, sensitive, quick and convenient-to-operate kit for detecting the antibody of the chicken infectious bronchitis virus, which can be used for quickly diagnosing whether the chicken flocks are infected by the chicken infectious bronchitis virus and monitoring the immune chicken serum antibody level and the chicken maternal antibody level in actual production, so as to evaluate the degree of risk that the chicken flocks are infected by the infectious bronchitis virus and provide reference for the formulation of an infectious bronchitis immune procedure.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Vaccine composition, and preparation method and application thereof
InactiveCN104288766AImmunity builds up fastProtectAntiviralsAntibody ingredientsMaternal antibodyAntigen
The invention provides a vaccine composition, comprising an immune amount of muscovy duck Parvovirus antigen and an immune amount of muscovy duck parvovirus antibody. The vaccine composition can be applied to immunization of young muscovy duck, also can be directly applied to immunization of muscovy duck embryo; the immunization effect is not affected by maternal antibody, and quickly produces immunity, and ideal protection can be produced of the in the second day after immunization; attacked by strong virus, the immune ducks 5 / 5 have no morbidity, and control ducks 5 / 5 are dead.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
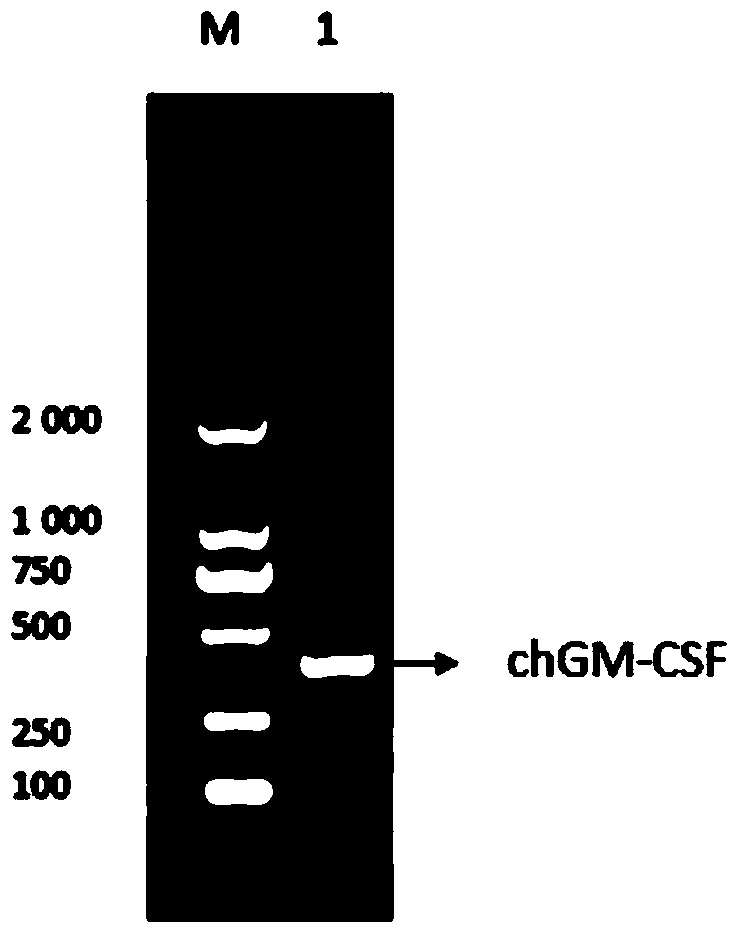
![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000003_0000.png)
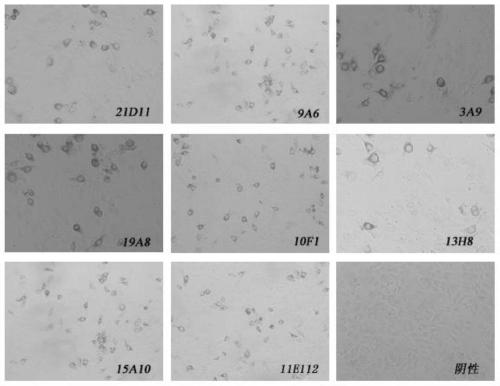
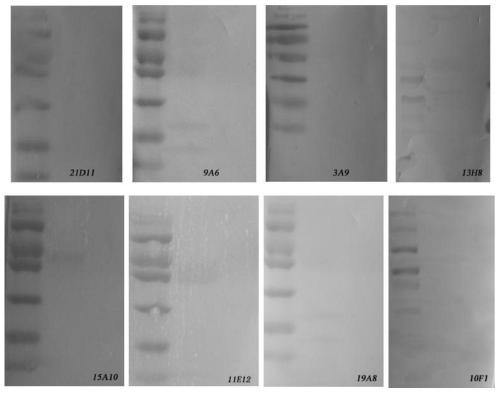
![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000003_0001.png)
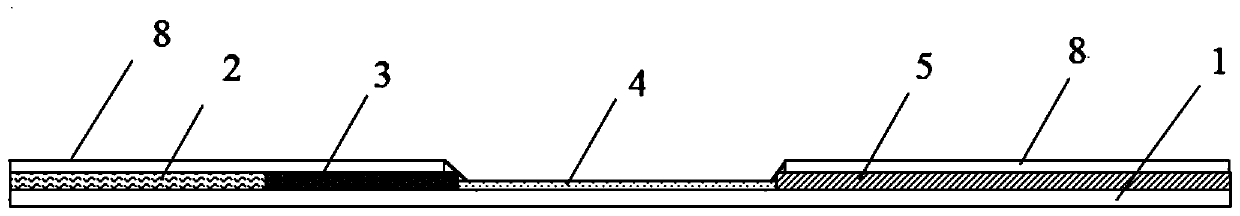
![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000007_0000.png)