Blocking test paper for swine fever antibody
A swine fever antibody and detection test strip technology, which is applied in the field of livestock disease antibody detection equipment, can solve the problems of inability to realize a large number of clinical samples, real-time detection, cumbersome operation, high cost, etc., and achieve easy large-scale promotion and application, broad market prospects, highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] (1) Preparation of swine fever virus immune antigen
[0056] Pig kidney cells (PK-15) were inoculated with Shimen strain, a standard virulent strain of classical swine fever virus, and the virus culture medium was collected for 48-60 hours, centrifuged at 3000 r / min at 4°C for 30 minutes at a low speed to remove impurities, and the virus culture medium was subjected to ultrafiltration with a 50kDa ultrafiltration membrane. Concentrate by filtration, carry out gel filtration chromatography purification to classical swine fever virus with Sepherose4 Fast Flow agarose column, measure half cell culture infection amount (TCID) of classical classical swine fever virus 50 ) up to 10 -7 Above, fluorescent quantitative PCR assay purified virus copy number up to 10 10 above.
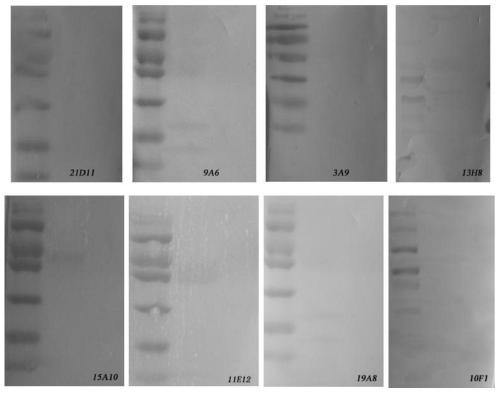
[0057] (2) Preparation of Anti-CSFV Monoclonal Antibody
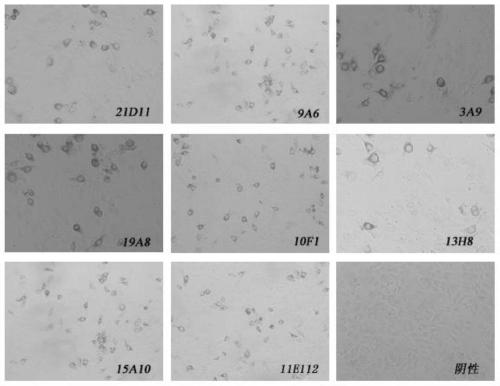
[0058] (2.1) Establishment of hybridoma cell lines
[0059] Mix the same amount of classical swine fever virus immune antigen with Freund's immu...
Embodiment 1
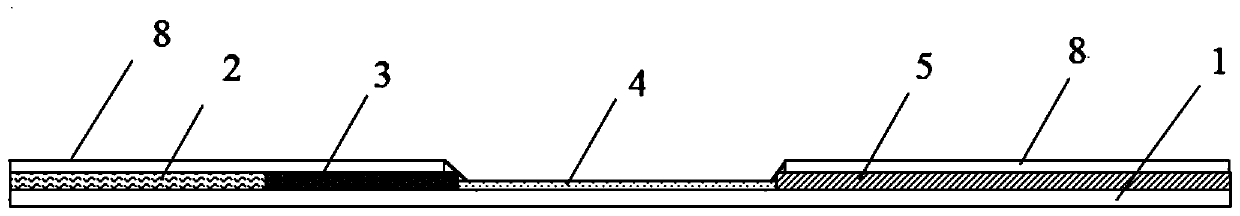
[0131] Example 1, monitoring the maternal antibody level of swine fever, collecting animal serum, taking 100 μL serum sample and adding 300 μL PBS or normal saline to dilute, preparing the serum solution to be tested, and using the swine fever antibody blocking test paper to detect according to the operation method (16) And the results are judged, and the maternal antibody level of swine fever is detected. There are two red bands (test line and quality control line) "||" on the test paper, and the color intensity of the test line T is equivalent to that of the blank control, which is negative for CSF antibody, indicating that the serum sample to be tested does not contain the maternal source of CSF Antibody; two red bands "||" appear, but the detection line T is significantly weaker than that of the blank control, which is weakly positive for CSF antibody, indicating that the serum sample to be tested contains a low level of maternal antibody to CSF; only one red band appears ...
Embodiment 2
[0132] Example 2: Evaluation of the immunization effect of the classical swine fever vaccine. 2 to 3 weeks after immunization with the inactivated classical swine fever vaccine, the serum of the immunized animal was collected, and 100 μL of the serum sample was added to 300 μL of PBS or normal saline for dilution or 1:2, 1:4, 1 : 8 ... Doubling dilution, prepare the serum solution to be tested, carry out detection and result judgment by (16) operating method with the swine fever antibody blocking detection test paper, detect the swine fever immune antibody level. Two red bands (test line and quality control line) "||" appear on the test paper, and the color intensity of the test line T is equivalent to that of the blank control, which is negative for swine fever antibody, indicating that the serum sample to be tested does not contain swine fever neutralizer Antibody; two red bands "||" appear, but the detection line T is significantly weaker than that of the blank control, whic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com