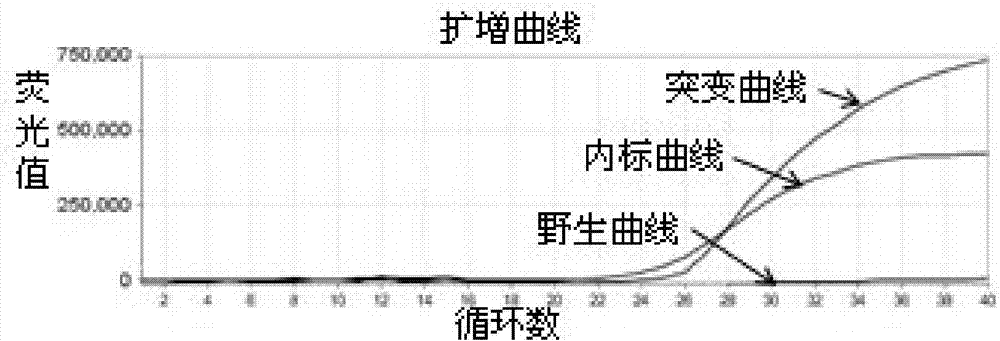
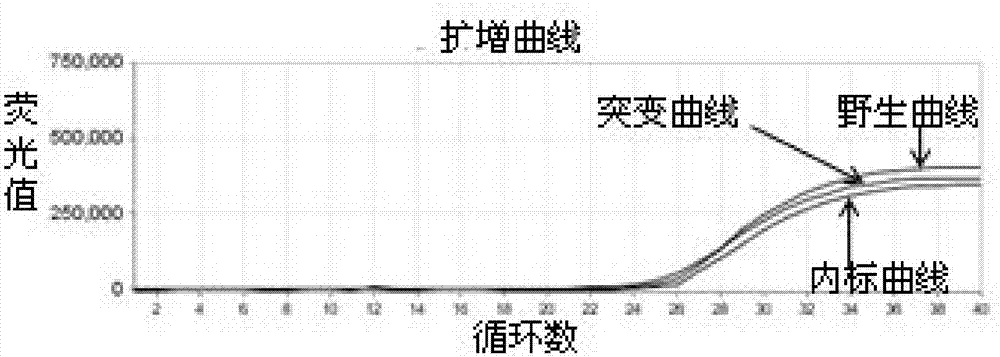
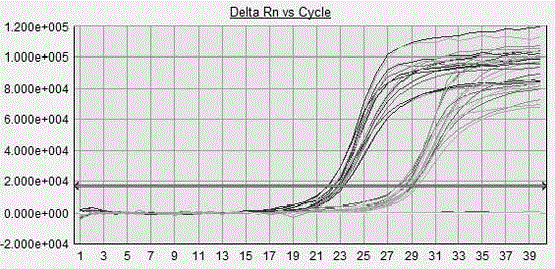
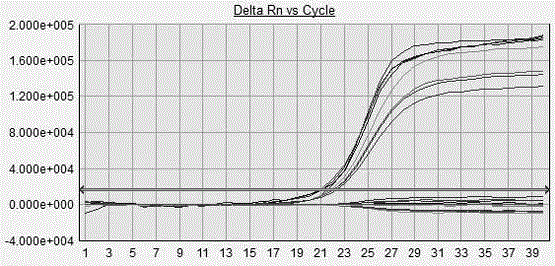
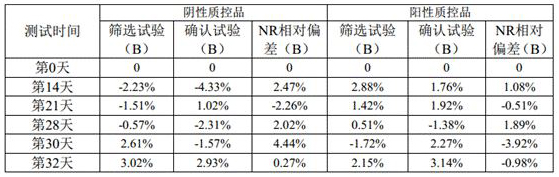
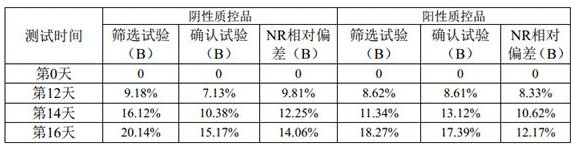
Patents
Literature
33 results about "Weakly positive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
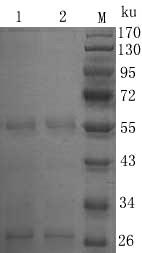
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
Human HLA-B*5801 gene polymorphism detection kit
InactiveCN106929591AAccurate detectionHigh sensitivityMicrobiological testing/measurementInternal standardHLA-B
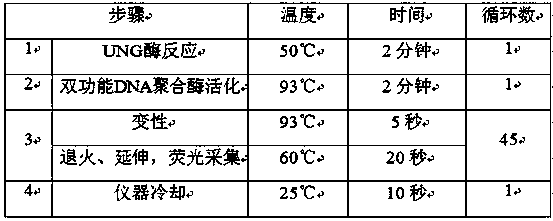
The invention relates to a human HLA-B*5801 gene polymorphism detection kit. The human HLA-B*5801 gene polymorphism detection kit comprises PCR damping liquid, a specific primer, a specific probe, an interior label system, a Taq enzyme, a UNG enzyme, a weakly-positive control group and a blank control group, and also comprises a blood treating agent; a blood sample is simply treated and can be directly subjected to PCR amplification, the DNA extracting process is omitted, and operating time is saved. According to the human HLA-B*5801 gene polymorphism detection kit, the SNP probe is used in cooperation with the technology of the ARMS primer, and it is achieved that two different gene types are detected in one pipe; meanwhile, the interior label system is designed and used for monitoring the quality of the sample, and the weakly-positive control group and the blank control group are designed and used for monitoring the quality of the kit. The human HLA-B*5801 gene polymorphism detection kit for detecting HLA-B*5801 alleles has the advantages of being high in specificity and sensitivity, rapid and easy to operate, safe, objective in result interpretation and the like when being used for detecting HLA-B*5801 alleles.
Owner:WUHAN YZY MEDICAL SCI & TECH
Primer, probe and kit for detecting type-16 HPV (human papillomavirus)
ActiveCN105087827AComprehensive detectionQuick screeningMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus
The invention discloses a primer, probe and kit for detecting type-16 HPV (human papillomavirus). The primer and the probe are suitable for screening the HPV related diseases quickly, accurately and simply, do not cross-react with other viruses, are capable of detecting common high and low risk types of HPVs, two types of HPVs and 14 types of HPVs in total, cover a wide range of high risk types of HPVs and are more accurate and complete in detection than kits in the present market. The kit further comprises negative, positive and weakly positive quality controls and helps further increase detection accurateness.
Owner:北京鑫诺美迪基因检测技术有限公司
Human immunodeficiency virus type 1 one-step fluorescence quantitative RT-PCR detection kit
InactiveCN103966356AQuick analysisAccurate analysisMicrobiological testing/measurementFluorescence/phosphorescenceBioinformaticsHuman immuno deficiency virus
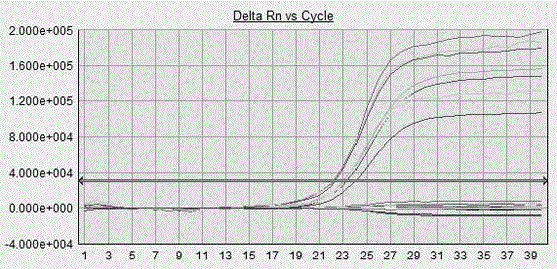
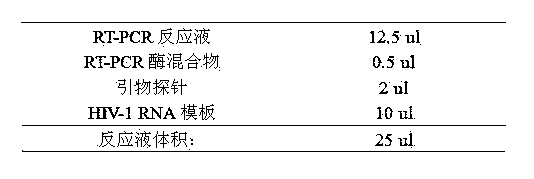
The present invention provides a human immunodeficiency virus type 1 one-step fluorescence quantitative RT-PCR detection kit, which comprises a RT-PCR reaction solution, a RT-PCR enzyme mixture, a primer probe, a negative control, a strong positive control, a weak positive control, and calibration substances No.1-5. According to the present invention, a one-step fluorescence quantitative reaction can be directly performed on the extracted HIV-1RNA, the virus loading of the HIV-1RNA in a sample can be detected, the reference gene is adopted as the internal control, and the UNG enzyme is adopted to prevent pollution; and the kit has characteristics of simple one-step amplification method, short procedure, easy operation, pollution prevention, strong detection result specificity, high sensitivity, clear result and high result reliability, and can be used for detection of the virus loading of the human immunodeficiency virus type 1 (HIV-1) RNA in blood serum.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Positive-serum and negative-serum standard substance of avian influenza virus H5N1 subtype Re-5 strain and preparation method thereof
The invention relates to a positive-serum and negative-serum standard substance of avian influenza virus H5N1 subtype Re-5 strain and a preparation method thereof. The preparation method comprises the following steps of: preparing a standard substance positive serum (strongly positive serum and weakly positive serum): intramuscularly injecting an avian influenza virus H5N1 subtype Re-5 strain oil-emulsion inactivated vaccine in a young or adult SPF (Specific Pathogen Free) chicken, and then collecting the serum of the SPF chicken and carrying out semi-finished product inspection, adding a proper stabilizing agent and then freeze-drying; preparing negative serum: taking the blood of the SPF chicken, separating serum, carrying out semi-finished product inspection, and freeze-drying; and carrying out a series of technical processes, such as finished-product inspection, uniformity inspection, stability inspection, calibration and value determination of the standard substance and the like to obtain the positive-serum and negative-serum standard substance. The standard substance is the fundamental guarantee for the accurate diagnosis of avian influenza virus H5N1 subtype, the immune monitoring of a H5N1 subtype Re-5 strain and the accurate evaluation on the vaccine immune effect of the H5N1 subtype Re-5 strain, thereby improving the prevention and control level of avian influenza. The standard substance is the basic guarantee for the diagnosis of avian influenza virus H5N1 subtype and the evaluation and the quality control of the inspection working of related products.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit stored at 2 to 8 DEG C and used for rapidly detecting hepatis c virus nucleic acid
InactiveCN108977580AImprove stabilityAvoid sex changeMicrobiological testing/measurementMicroorganism based processesReference productSodium azide
The invention discloses a kit stored at 2 to 8 DEG C and used for rapidly detecting hepatis c virus nucleic acid. The kit comprises a qRT-PCR reaction solution, a qRT-PCR initiator, an RNA internal control, a strongly positive quality control product, a weakly positive quality control product, a negative quality control product and a quantitative reference product; and the qRT-PCR reaction solution comprises a qRT-PCR reinforcing agent, a reaction buffer solution, two specific primers for detecting the hepatis c virus nucleic acid, one specific probe for detecting the hepatis c virus nucleic acid, two specific primers for detecting the RNA internal control, one specific probe for detecting the RNA internal control, a bi-directional DNA polymerase, UNG enzyme, dATP, dGTP, dCTP, dUTP and 0.1percent sodium azide. The kit is high in sensitivity (the minimum detection limit of the HCV virus is 10IU / ml), good in specificity, high in precision, accurate in qualification, and wide in linear range, simple in detection method, short in detection time, high in reference value for clinically and rapidly detecting the hepatis c virus nucleic acid, and suitable for popularization and application.
Owner:AUTOBIO DIAGNOSTICS CO LTD
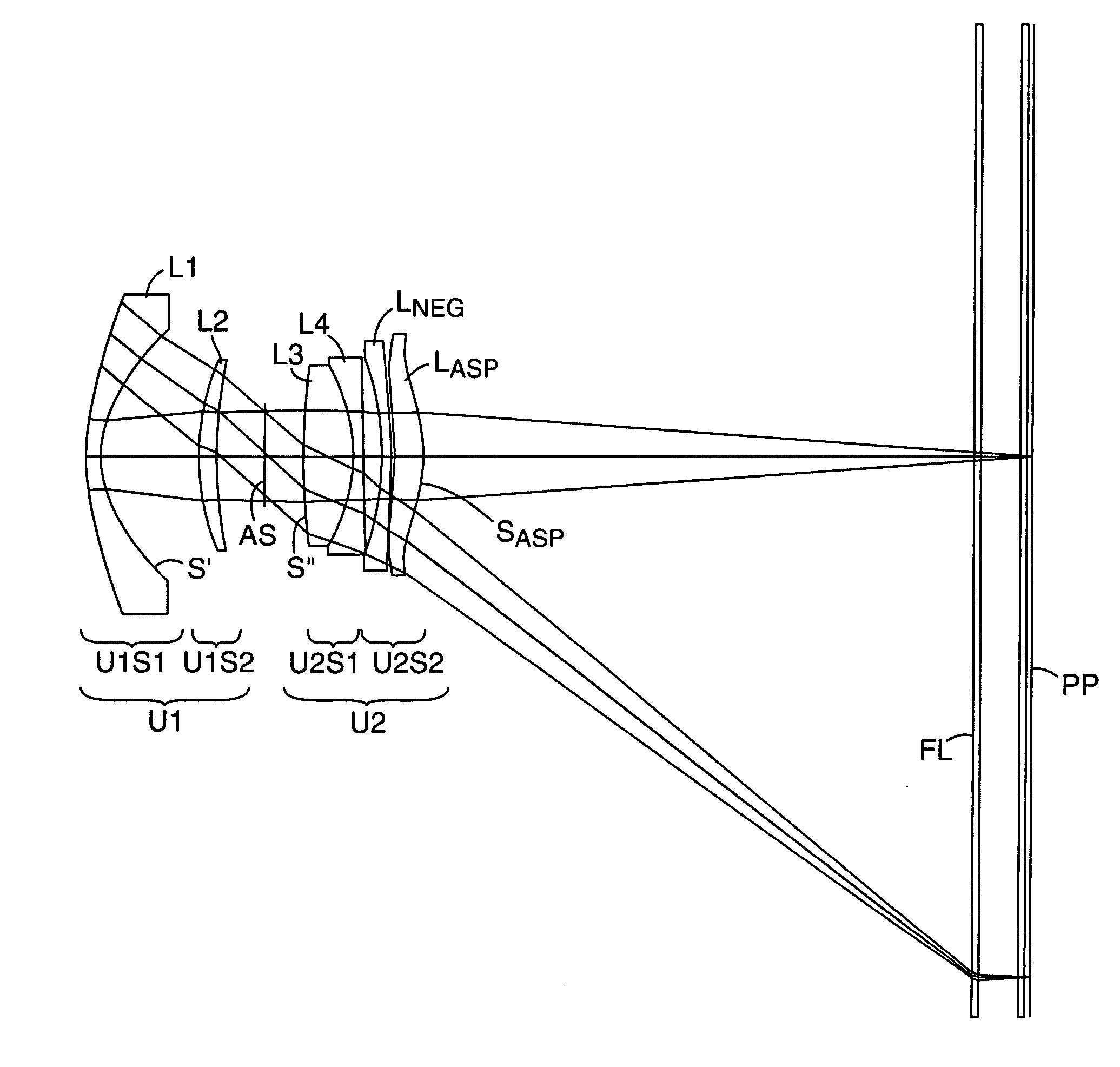
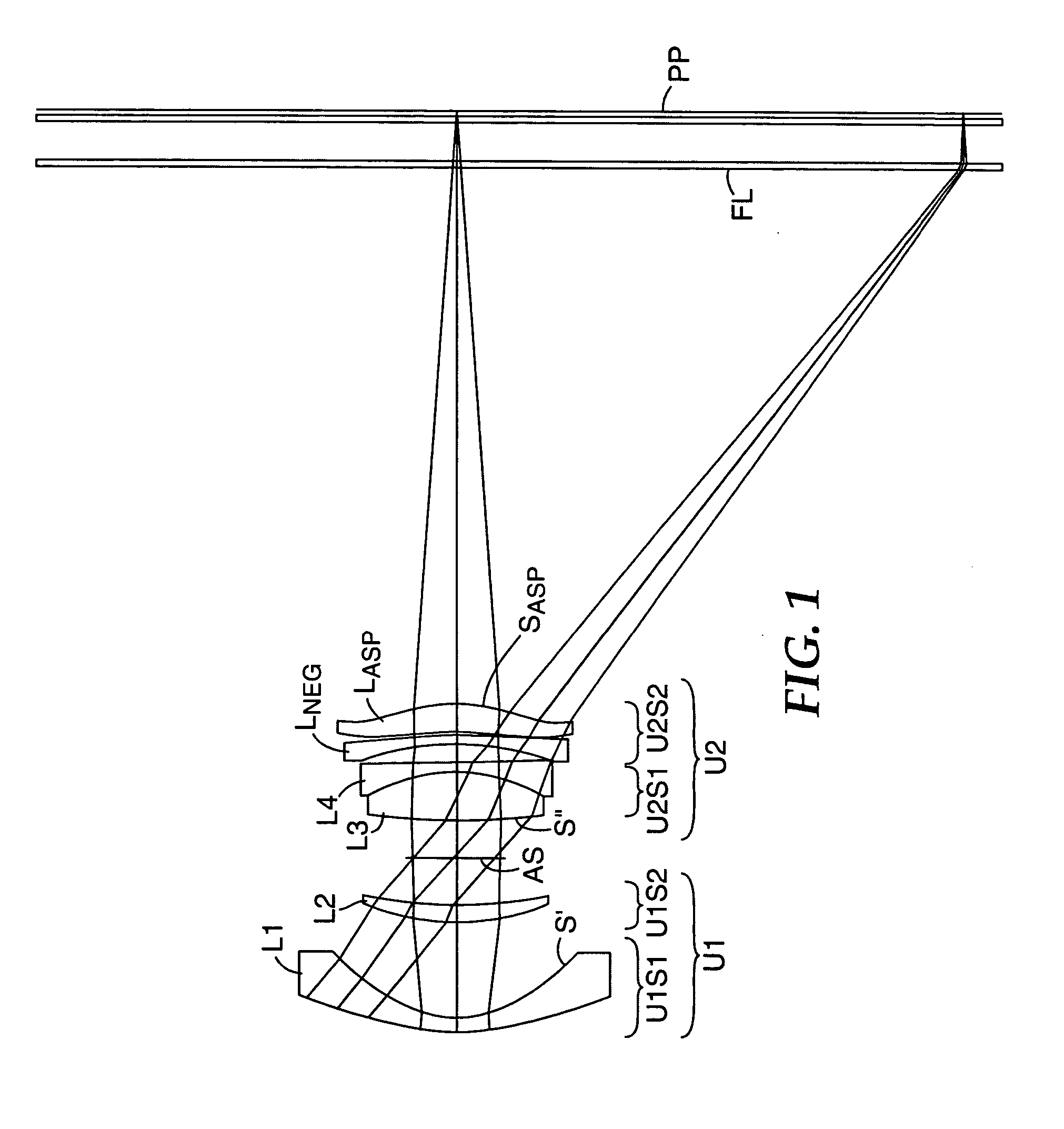
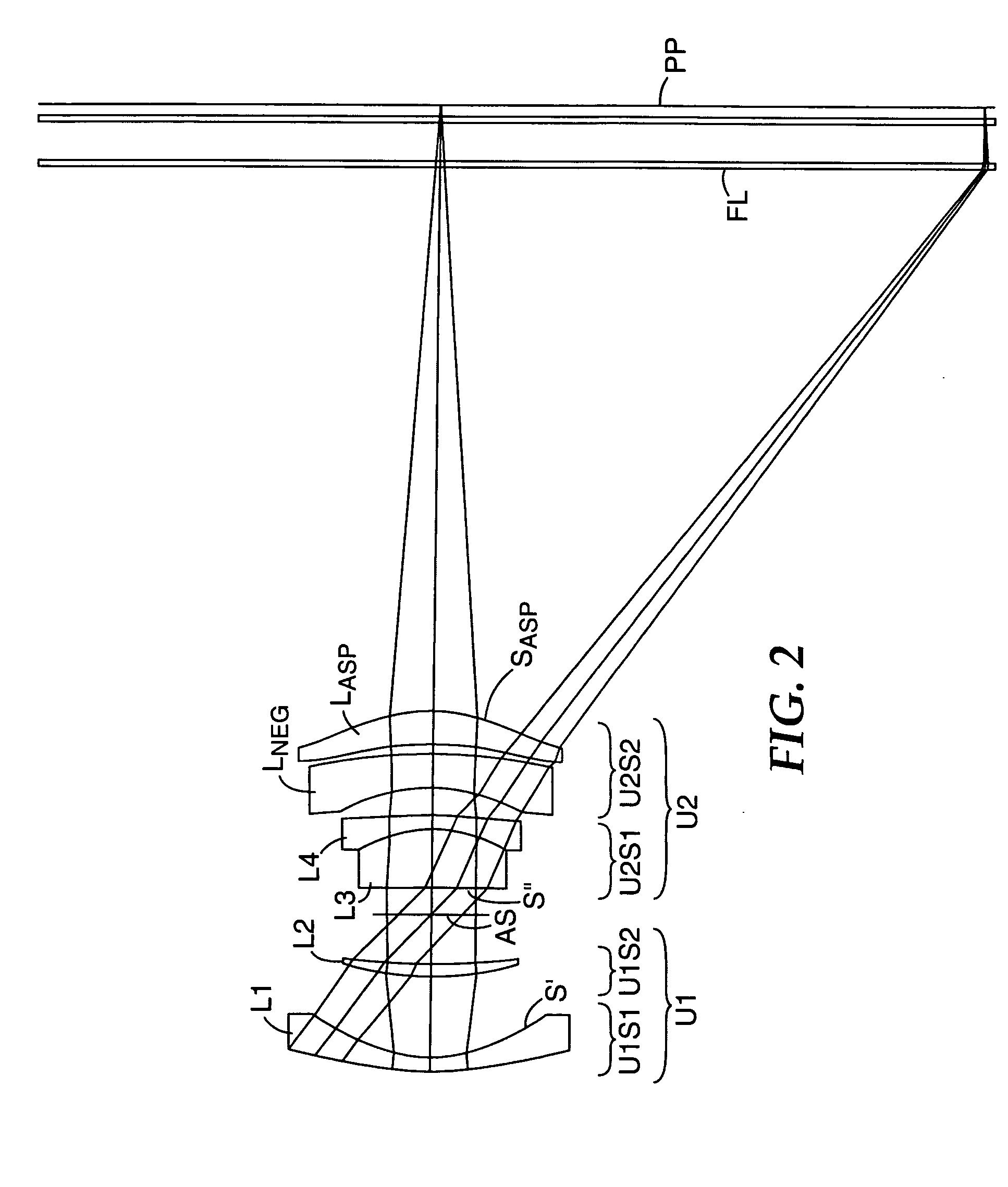
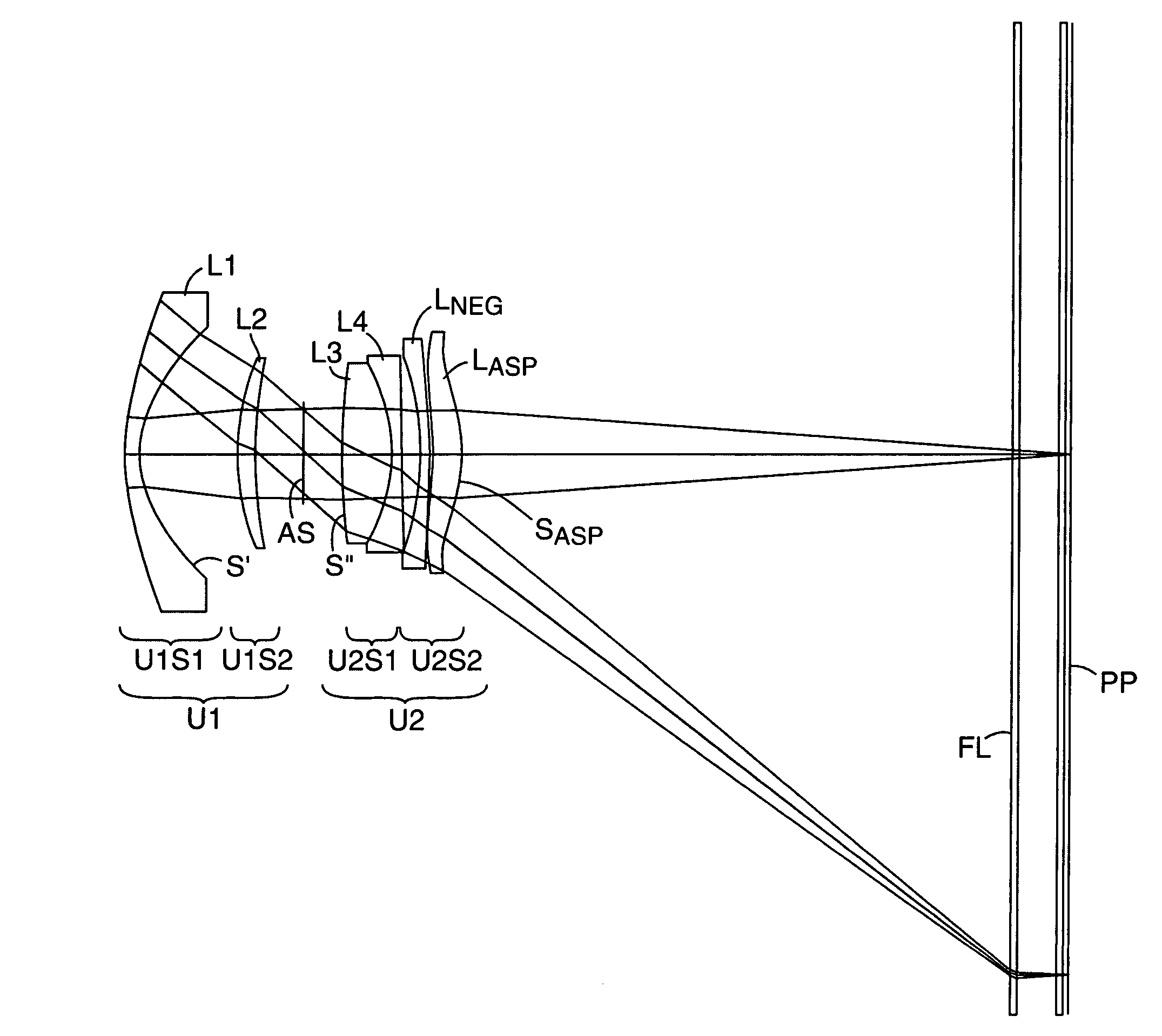
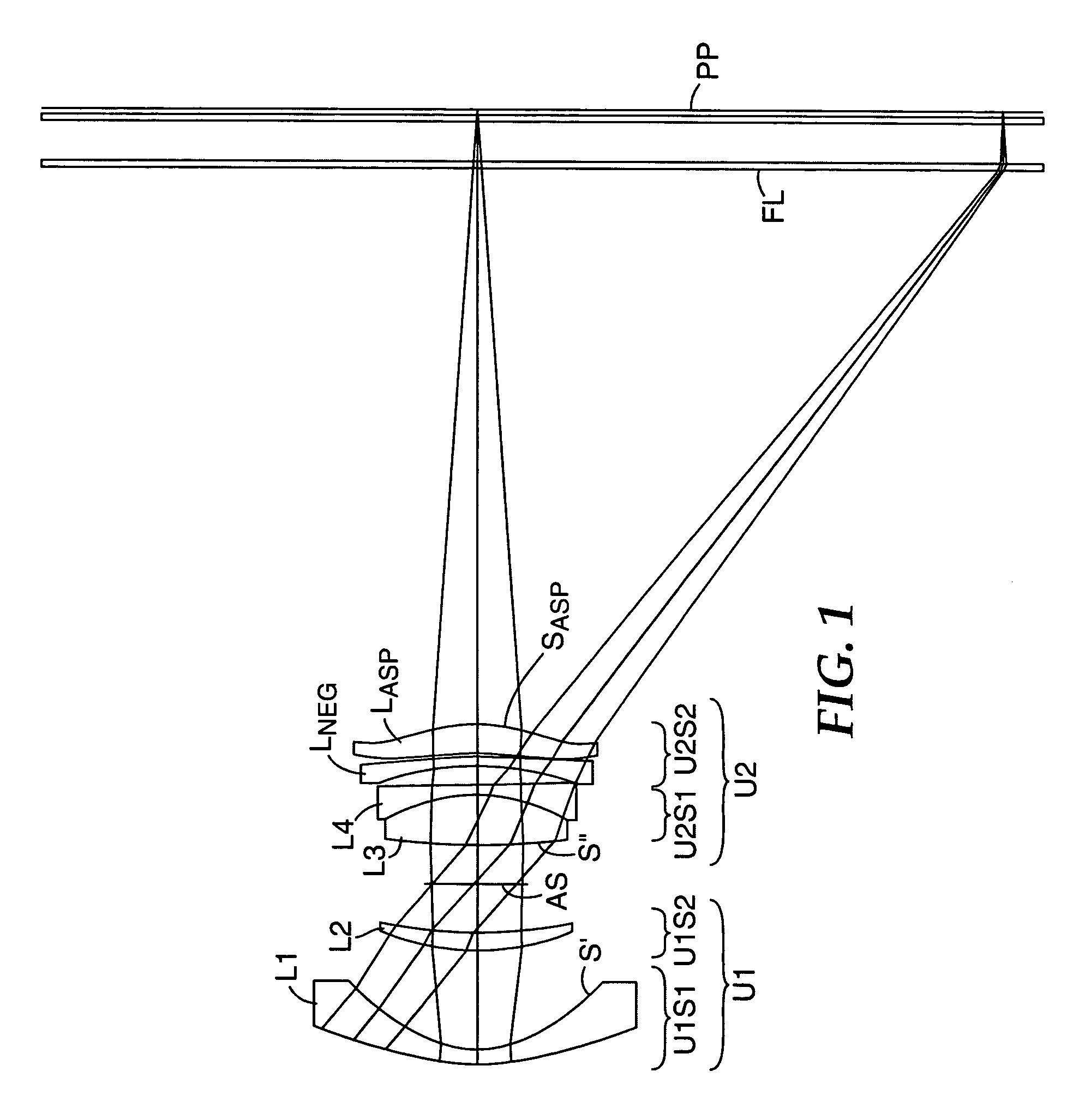
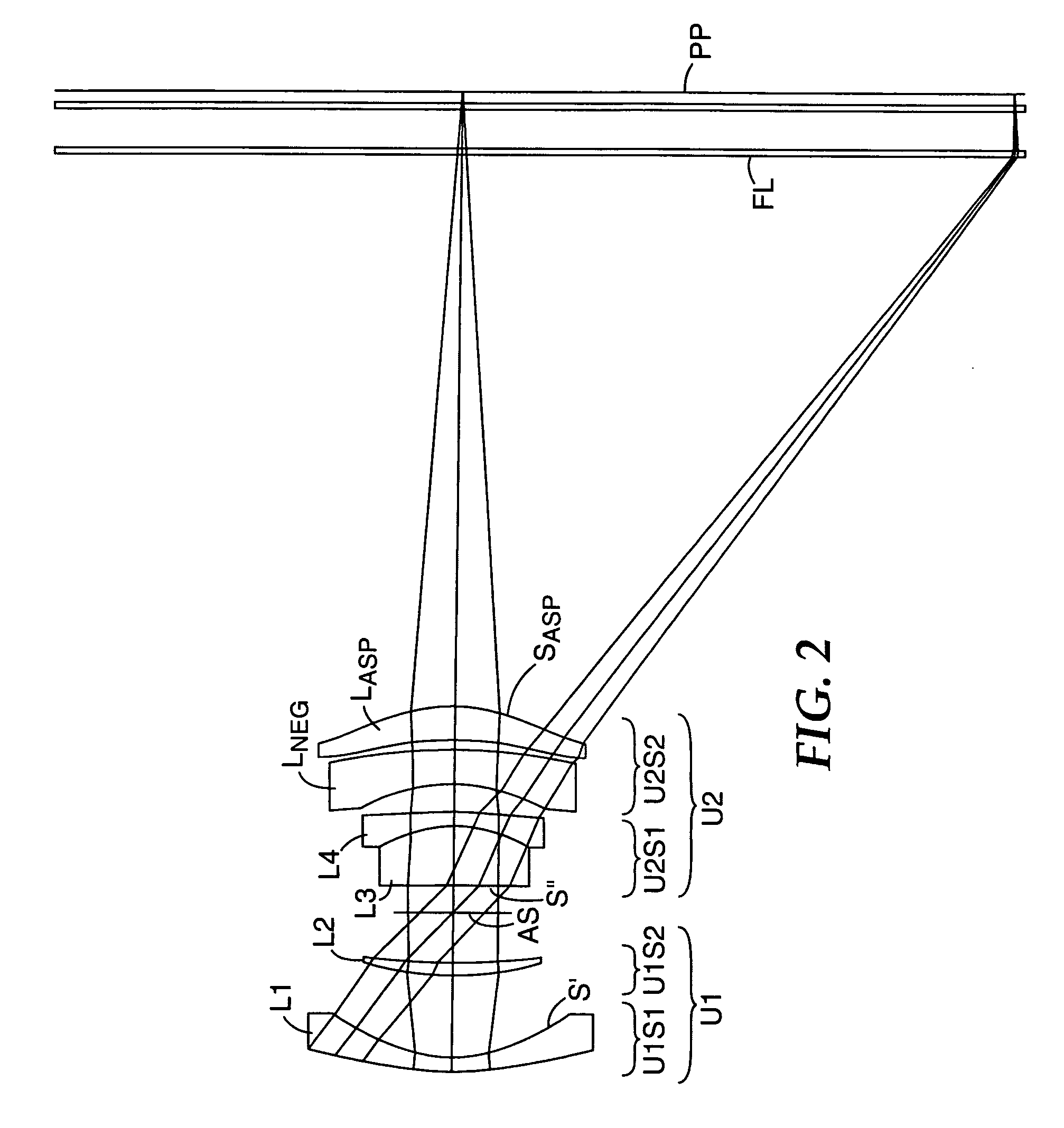
Compact projection lenses for use with large format pixelized panels
Projection lenses for use with pixelized panels (PP) are provided. The projection lenses have first and second lens units (U1,U2), with the first lens unit having a negative or weakly positive power and the second lens unit having a positive power. The second lens unit has first and second subunits (U2S1 and U2S2) which provide positive power followed by negative power. The second subunit (U2S2) of the second unit (U2), in turn, has negative power followed by positive power. In this way, an overall short projection lens (e.g., BRL / f0≦0.9) along with small lens elements (e.g., CAmax / f0≦0.8) can be achieved.
Owner:3M INNOVATIVE PROPERTIES CO
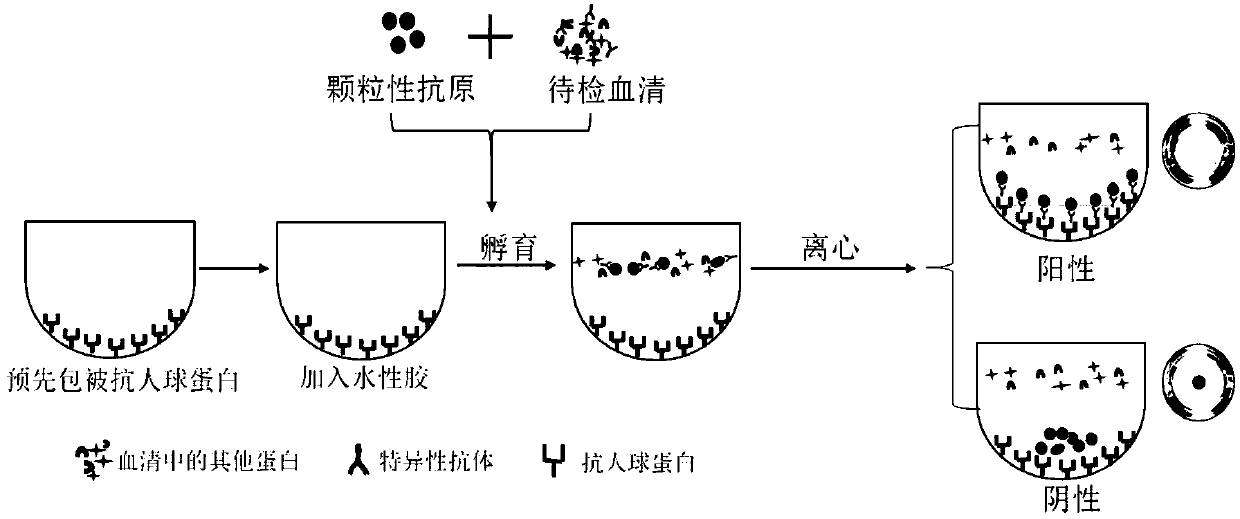
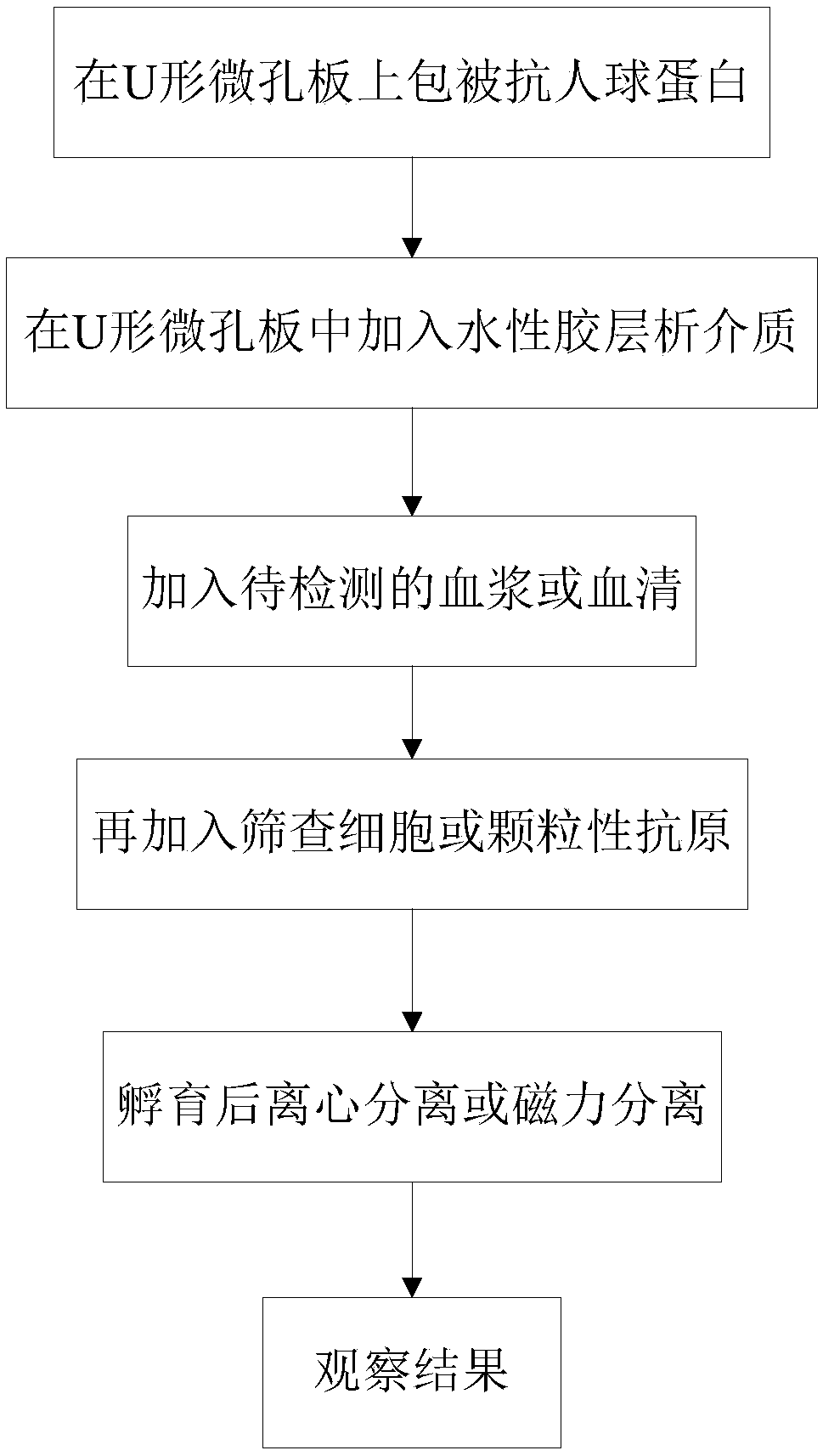
Incomplete antibody detection kit and detection method
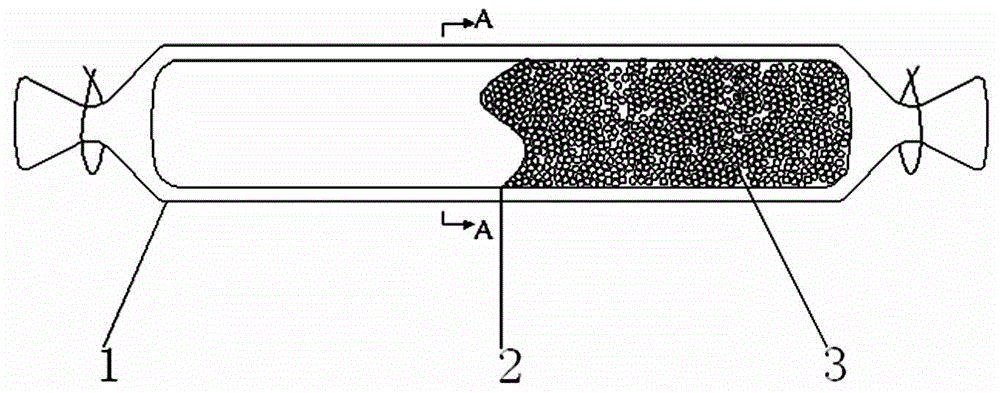
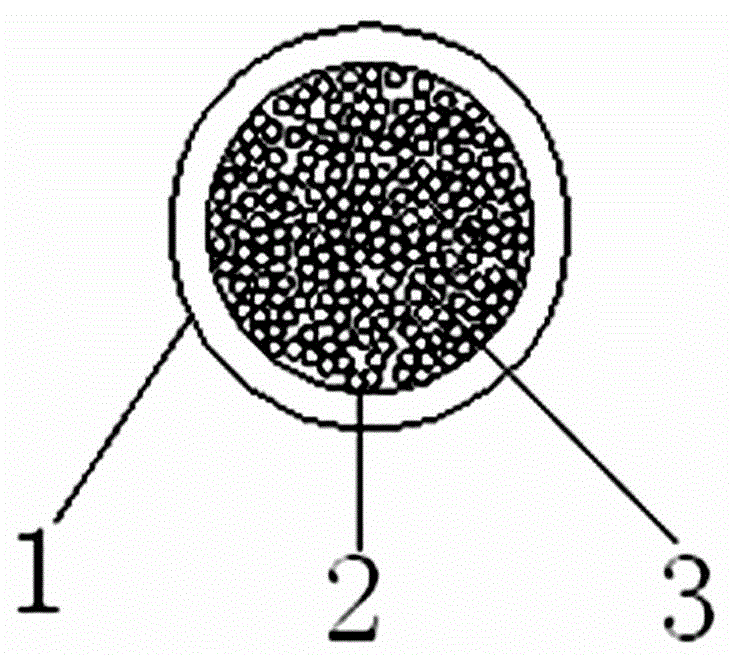
InactiveCN108680756AEasy to operateImprove detection efficiencyBiological testingAntihuman globulinBlood plasma
The invention discloses an incomplete antibody detection kit which comprises a U-shaped micro-pore plate, an aqueous glue chromatography medium and granular antigen, wherein the U-shaped micro-pore plate is wrapped with antihuman globulin; the aqueous glue chromatography medium is used for incubating a mixture of serum or plasma with the granular antigen; the granular antigen is reacted with an incomplete antibody in the serum or plasma to form an antigen-antibody compound. The invention further relates to an incomplete antibody detection method. A granular antigen-antibody compound is separated from free antibodies in a centrifugal or magnetic mode, and a tedious washing process in a conventional method can be replaced. When results are observed, if the incomplete antibody is sensitized on the granular antigen, the incomplete antibody can be captured by the antihuman globulin and is spread at the bottom of the U-shaped micro-pore plate, and a positive result is judged; if sensitization is not resulted, the incomplete antibody is not captured but being focused at the bottom to form a small point, and a negative result is judged; a weakly positive result is judged if a situation falls in between. By adopting the method, operation steps are greatly simplified, the detection efficiency can be improved, results are easy to judge, in addition, a great amount of samples can be detected simultaneously, and automation can be achieved easily.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Traditional Chinese medicine health-care cervical vertebra pillow
InactiveCN103598778AReduce tensionRelief the painSenses disorderNervous disorderCervical spondylosisSemen
The invention provides a traditional Chinese medicine health-care cervical vertebra pillow. The cervical vertebra pillow comprises a pillow sleeve 1 and a pillow inner 2 located in the pillow sleeve 1. The pillow inner 2 comprises, by weight, 20 parts to 25 parts of agastache, 21 parts to 26 parts of mints, 10 parts to 12 parts of schisandra chinensis, 85 parts to 110 pars of semen cassiae and 90 parts to 120 parts of tartary buckwheat. The clinic use proves that according to the clinical symptoms and signs of cervical spondylosis and the corresponding physical examination result, after the traditional Chinese medicine health-care cervical vertebra pillow is used, the corresponding clinical symptoms are obviously relieved, the specialized examination result which is originally positive is converted to be weakly positive or negative, and the average service life is 19.64 days.
Owner:李博文
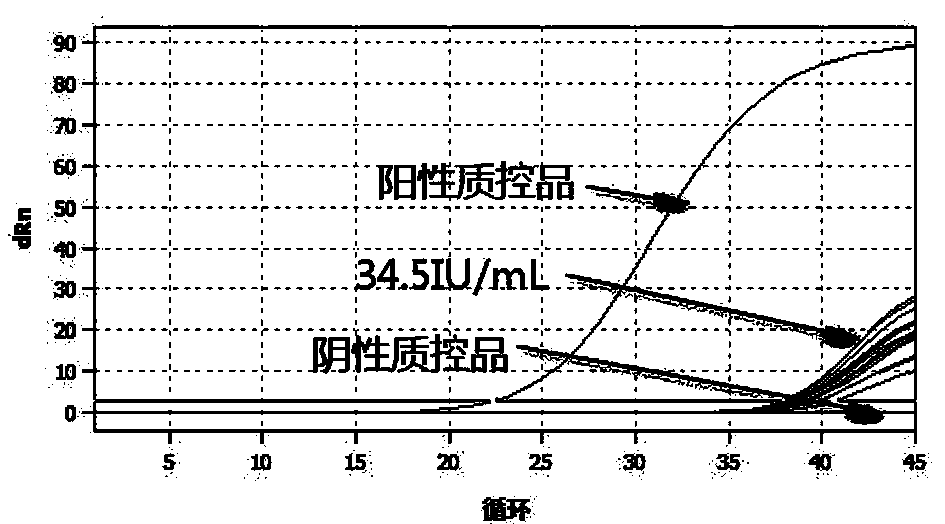
Kit for detecting human cytomegalovirus
InactiveCN109022620AAchieve traceabilityEasy to operateMicrobiological testing/measurementDiseasePositive control
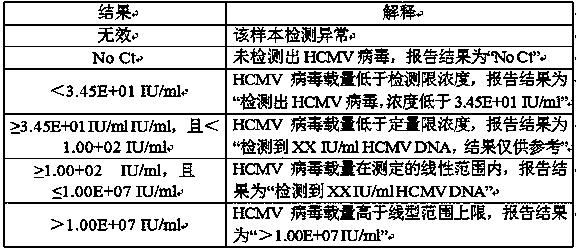
The invention discloses a kit for detecting human cytomegalovirus. The kit comprises specific PCR primers and a fluorescent probe for detecting human cytomegalovirus DNAs, specific primers and a probefor detecting a DNA internal standard, a PCR reaction buffer, a mixed enzyme liquid, theDNA internal standard, a negative control, a strongly positive control, a weakly positive control and quantitative references A, B, C and D; wherein the sequences of the primers and the probes are as shown in SEQ ID No. 1-6. The kit of the invention has the advantages of convenient and quick detection, capacity of quantitatively detecting human cytomegalovirus within 20 min, high sensitivity, the lowest detection limit of 34.5 IU / ml, and good specificity. According to the invention, a pair of primers are designed according to the internal repetitive sequence [mu]l65, so the primers have no cross-interference with other pathogens and have high specificity. The kit of the invention can be traced to the World Health Organization (WHO) standard unit IU / mL; quantitative results have traceability; and the kit has high reference value for clinical diagnosis of diseases related to human cytomegalovirus andis suitable for promotion and application.
Owner:AUTOBIO DIAGNOSTICS CO LTD
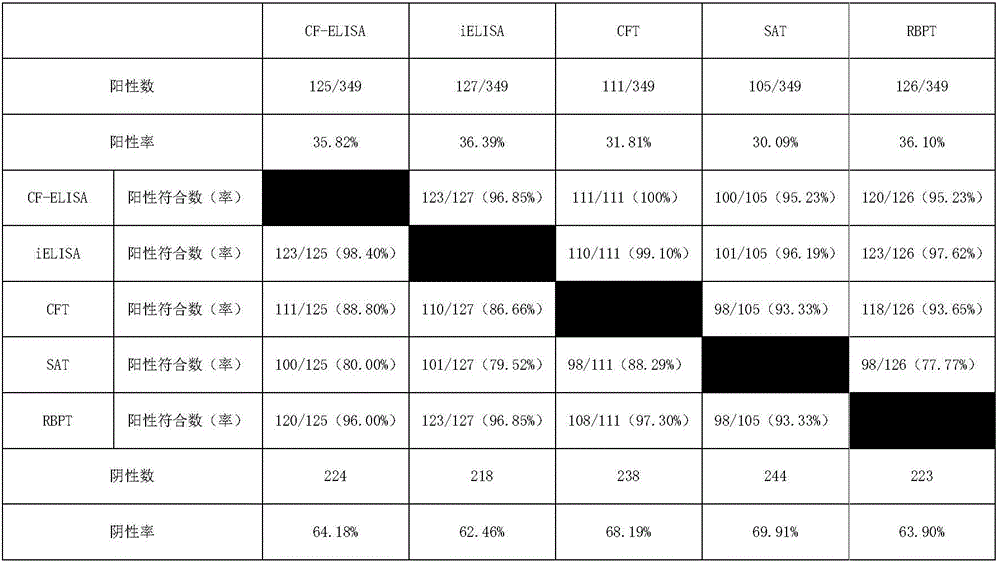
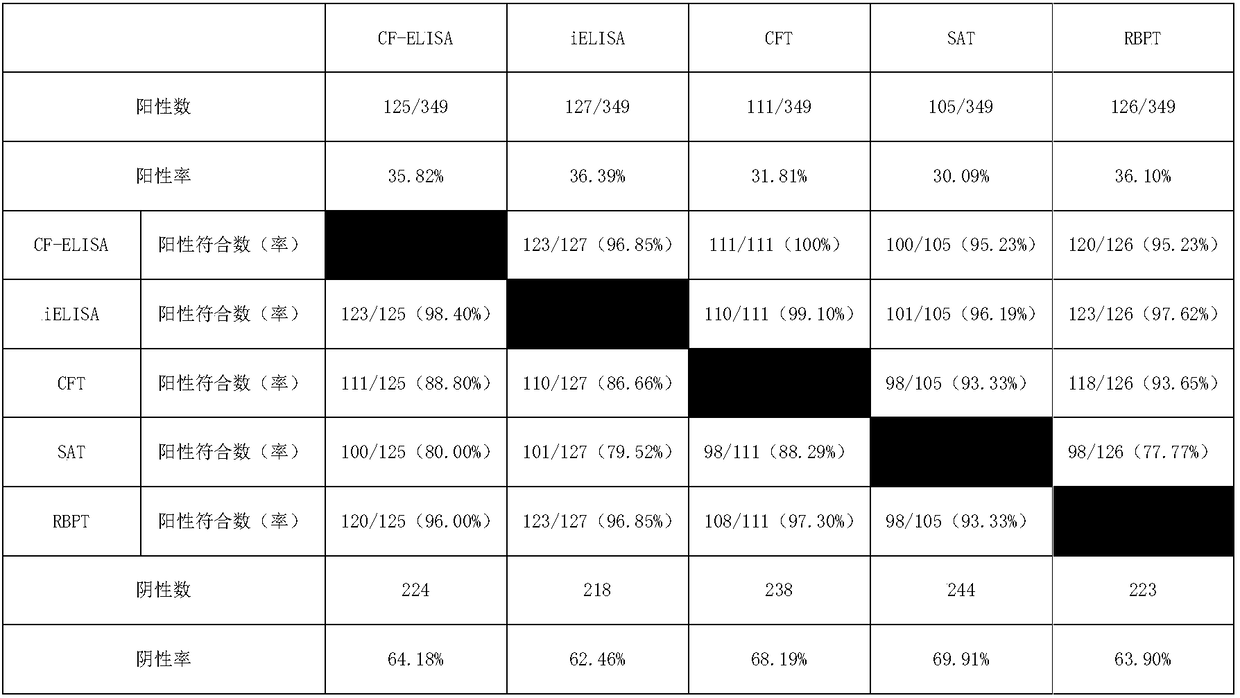
Brucellosis CF-ELISA antibody detection kit
ActiveCN106596958AStrong specificityIncreased sensitivityBiological material analysisPositive controlBiology
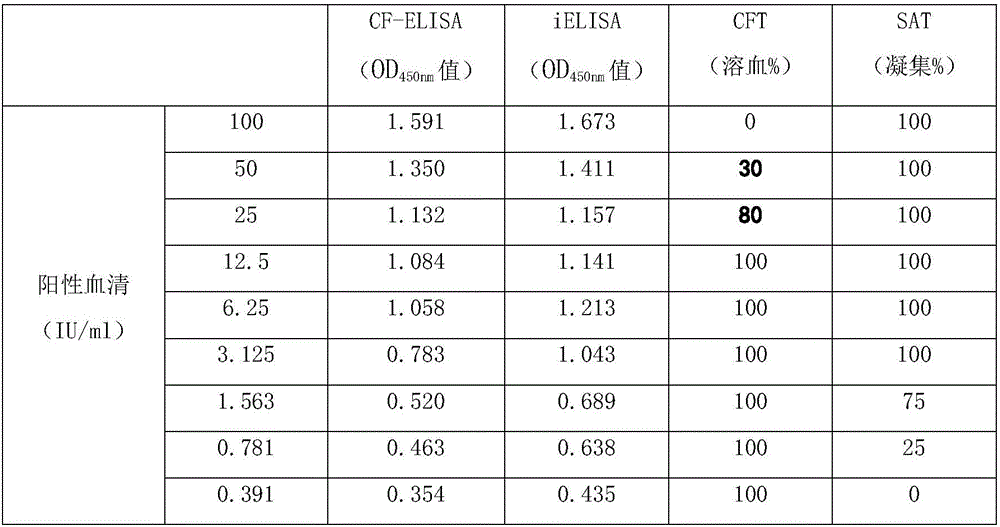
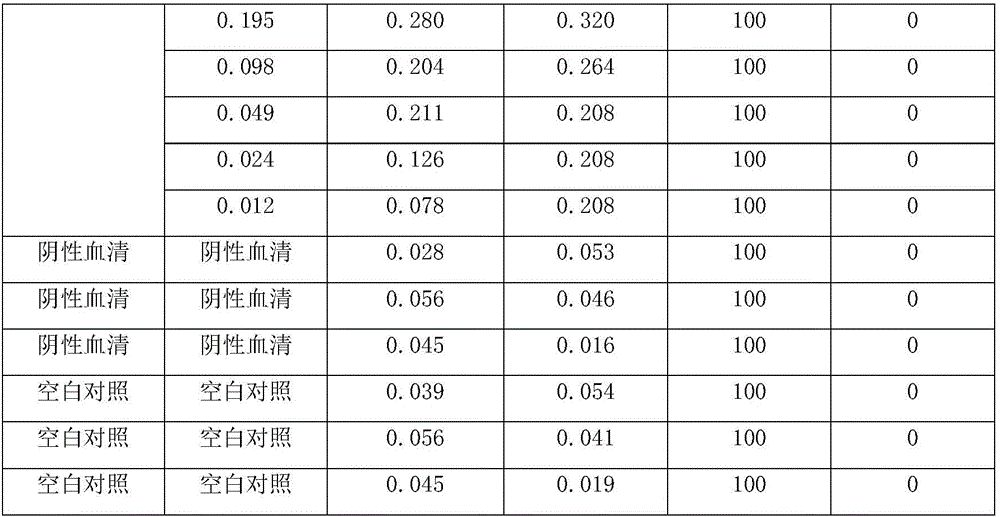
The invention relates to a brucellosis complement fixation-enzyme-linked immunosorbent assay (CF-ELISA) diagnostic kit, and the kit combines a reaction system in a complement fixation test (CFT) and an enzyme marker amplification system in an enzyme-linked immunosorbent assay (ELISA). The brucellosis complement fixation-enzyme-linked immunosorbent assay (CF-ELISA) diagnostic kit has the characteristics of high sensitivity, easy and fast use, high throughput and high degree of standardization compared with a traditional complement fixation test diagnostic reagent, has the characteristics of high specificity and capability of detecting a variety of brucellosis specific antibodies compared with a traditional ELISA diagnostic kit, and is an ideal new brucellosis diagnostic tool. The kit comprises the following main components: a lipopolysaccharide LPS antigen-coated plate, strongly-positive control serum, weakly positive control serum, negative control serum, a guinea pig complement, enzyme labeled guinea pig complement C1q-B monoclonal antibody 60G4, a substrate coloring-developing solution, a stop solution and washing liquid.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
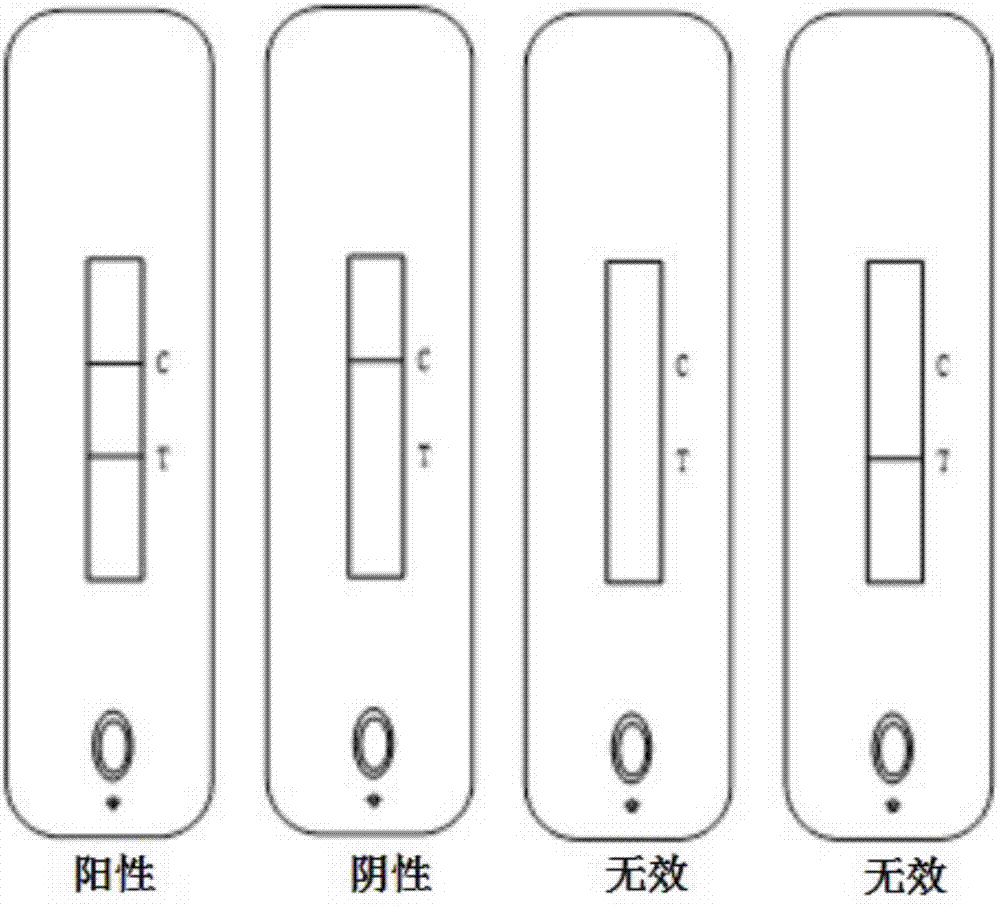
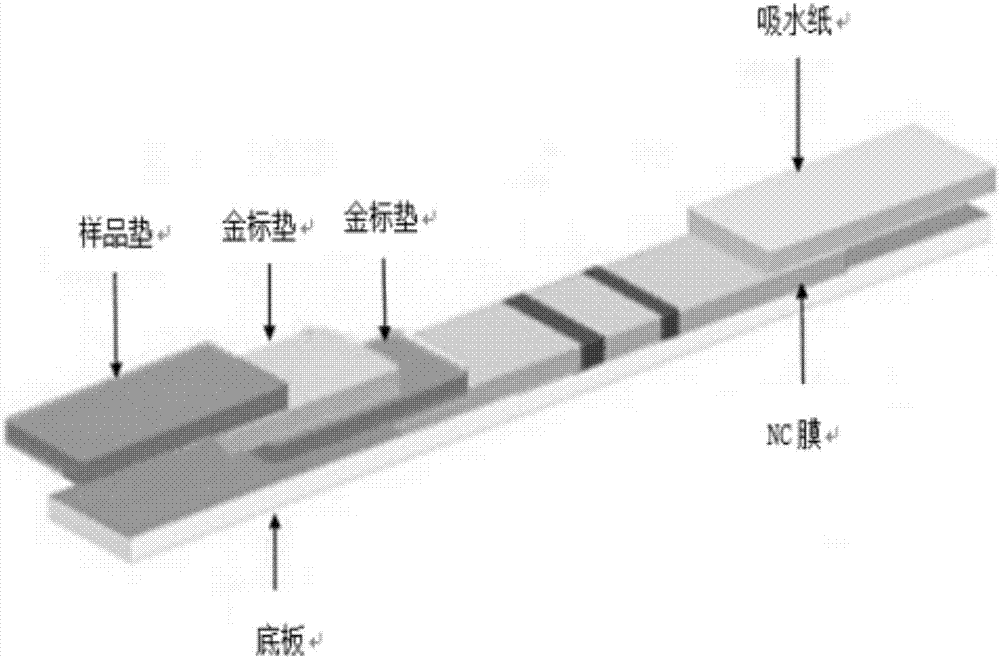
Test strip and kit for detecting HIV antibody
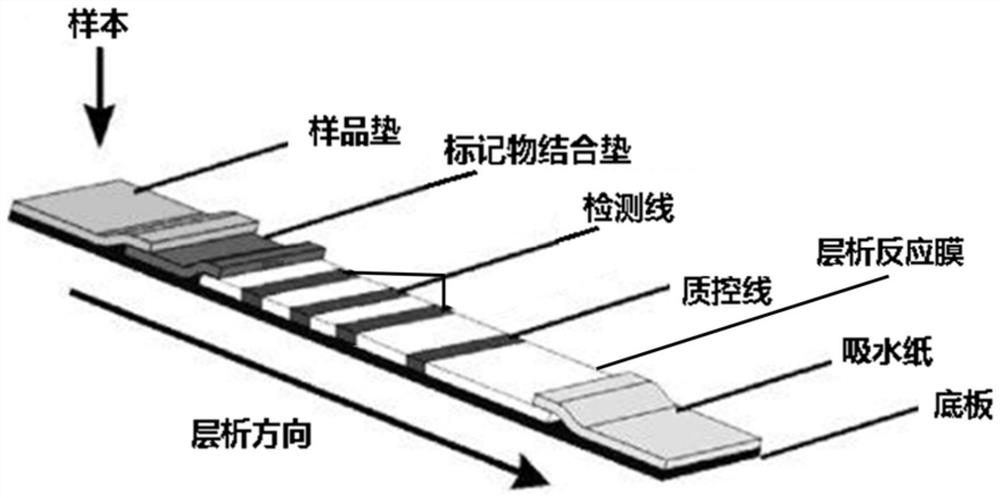
InactiveCN107966561AImprove hydrophilicityImprove protectionMaterial analysisPolyethylene glycolControl line
The invention relates to a test strip and kit for detecting a HIV antibody. The test strip of the invention comprises a base plate and further comprises a sample pad, a marker binding pad, a nitrocellulose membrane and a water absorbing pad which are all pasted onto the base plate and sequentially overlapped, wherein the sample pad is treated with a sample pad treating fluid; the sample pad treating fluid comprises: a PBS buffer with a pH value of 7.2-7.5, polyethylene glycol with a mass fraction of 0.5-1%, casein with a mass fraction of 0.5-1%, Tween-20 with a volume fraction of 0.3 to 1% andpolyvinylpyrrolidone K-30 with a mass fraction of 0.6 to 1%; the marker binding pad is coated with an anti-human IgG antibody labeled by a marker; the nitrocellulose membrane is provided with a testline and a control line, the test line is coated with an HIV antigen, and the control line is coated with an anti-antibody; and the anti-antibody binds to the anti-human IgG antibody. The test strip of the invention can amplify the weakly positive detection result of the HIV antibody so as to reduce false negative and improve detection accuracy.
Owner:德诺杰亿(北京)生物科技有限公司
FPA antibody detection kit for animal brucellosis fluorescence polarization detection method
The invention provides an FPA antibody detection kit for an animal brucellosis fluorescence polarization detection method. The FPA antibody detection kit comprises the following components of 100 ml of brucella FITC labelled antigen, 0.2 ml of animal brucellosis standard negative serum, 0.2 ml of animal brucellosis standard positive serum, 0.2 ml of standard weakly-positive serum and 100 ml of 10-time sample diluent. The FPA antibody detection kit is assembled by using polysaccharide OPS in lipopolysaccharide of smooth type brucellosis as the antigen to mark the fluorescein FITC, preparing positive control serum and standard weakly-positive serum by using inactivated bacterium solution immune healthy cattle or artificially infected healthy cattle, and using healthy non-immune cattle serum as negative control serum and sample diluent. The invention is used for distinguishing mammal brucella infected antibodies and immune antibodies.
Owner:张晓艳
Compact projection lenses for use with large format pixelized panels
Projection lenses for use with pixelized panels (PP) are provided. The projection lenses have first and second lens units (U1,U2), with the first lens unit having a negative or weakly positive power and the second lens unit having a positive power. The second lens unit has first and second subunits (U2S1 and U2S2) which provide positive power followed by negative power. The second subunit (U2S2) of the second unit (U2), in turn, has negative power followed by positive power. In this way, an overall short projection lens (e.g., BRL / f0≦0.9) along with small lens elements (e.g., CAmax / f0≦0.8) can be achieved.
Owner:3M INNOVATIVE PROPERTIES CO
Carcinoembryonic antigen (CEA) rapid simple detection kit and making and application methods thereof
The aim of the invention is to provide a carcinoembryonic antigen (CEA) rapid simple detection kit and making and application methods thereof, the carcino-embryonic antigen rapid simple detection kit is lower in cost than a test strip; by use of specific responses of human carcinoembryonic antigen and anti carcinoembryonic antigen antibody, the carcinoembryonic antigen and the antibody are bonded onto a NC (nitrocellulose) membrane and colloidal gold particles, a colloidal gold and antibody complex is added onto the NC membrane, and whether the CEA is negative or positive is determined by observation of the color. When the CEA content is lower than 2.5uM, a 2.5uM CEA standard product is not significantly different from a 0uM standard product in color development, so when the CEA content is lower than 2.5uM, the CEA is determined to be negative; when the CEA content is in the 2.5-10uM range, the detection point color is significantly shallower than the 0uM spot color, the CEA is determined to be weak positive; when the CEA content is more than 10uM, and no red spot appears, the CEA is determined to be positive.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Fluorescent quantitative PCR detection kit for chlamydia trachomatis
InactiveCN105018573AStrong specificityShort detection timeMicrobiological testing/measurementFluoProbesA-DNA
The invention provides a fluorescent quantitative PCR detection kit for chlamydia trachomatis in clinic samples, used for assisted diagnosis of chlamydia trachomatis. The kit comprises a PCR solution, a DNA polymerase solution, positive quality control, weakly positive quality control, negative quality control, positive quantitative reference, lysate and protease K, wherein the PCR solution contains forward and reverse primers and the fluorescence probe are specific primers and probe designed for the specific sequence of chlamydia trachomatis and are capable of amplifying a target DNA sequence specifically so as to conveniently and quickly detect chlamydia trachomatis infection in clinic samples. The kit has the characteristics of high specificity and high sensitivity.
Owner:兰州安康伯乐生物技术有限公司
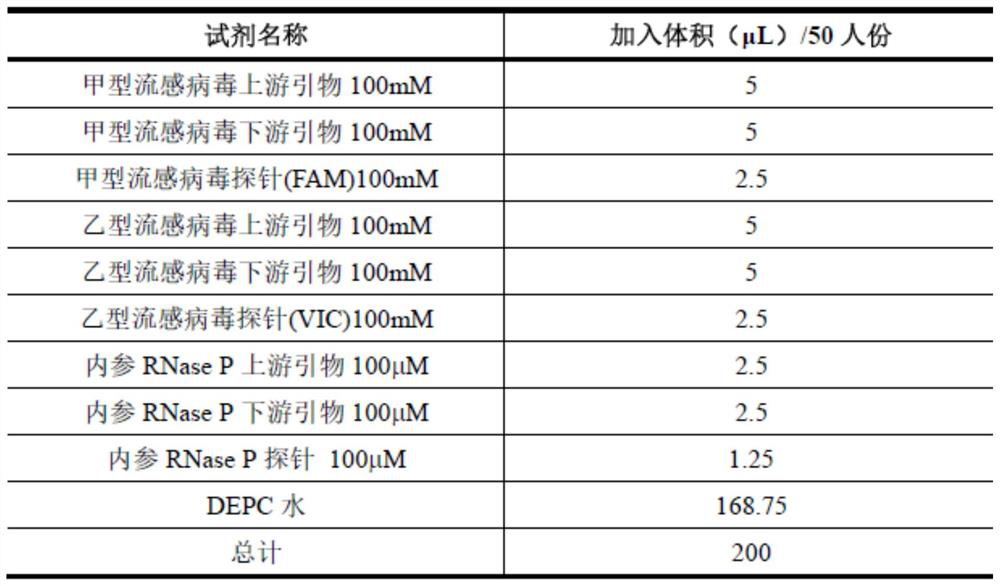
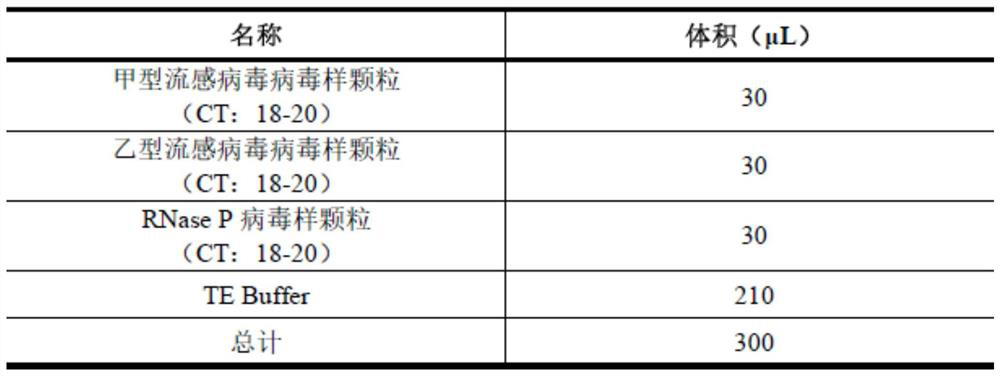
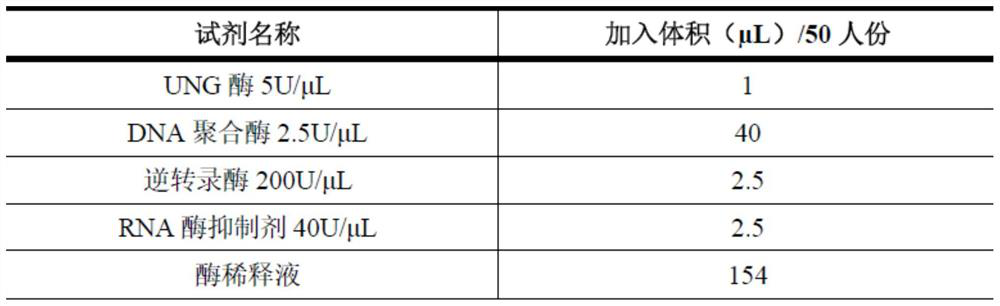
Influenza A and B virus nucleic acid detection kit and use method
InactiveCN112176109AAvoid False Negative Interpretation ResultsMicrobiological testing/measurementNucleic acid detectionRNase P
The invention discloses an influenza A and B virus nucleic acid detection kit. The influenza A and B virus nucleic acid detection kit comprises a nucleic acid amplification reaction solution, an enzyme mixed solution, an influenza A / B virus mixed reaction solution, a positive control solution, a weakly positive control solution and a negative control solution, wherein the influenza A / B virus mixedreaction solution comprises an influenza A virus upstream primer, an influenza A virus downstream primer, an influenza A virus probe, an influenza B virus upstream primer, an influenza B virus downstream primer, an influenza B virus probe, an internal reference RNase P upstream primer, an internal reference RNase P downstream primer, an internal reference RNase P probe and DEPC water, and the internal reference means the detection of the internal reference of the product. The internal reference of the embodiment participates in the process from nucleic acid extraction to RT-PCR, and can be used for monitoring the collection, storage and transportation of samples and the nucleic acid extraction process and amplification process, so that false negative interpretation results are avoided.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
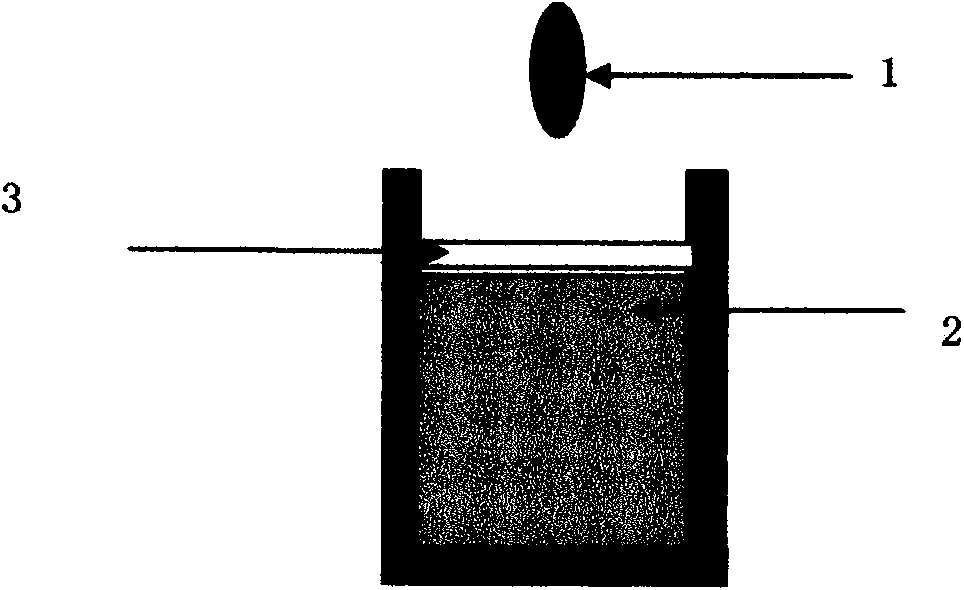
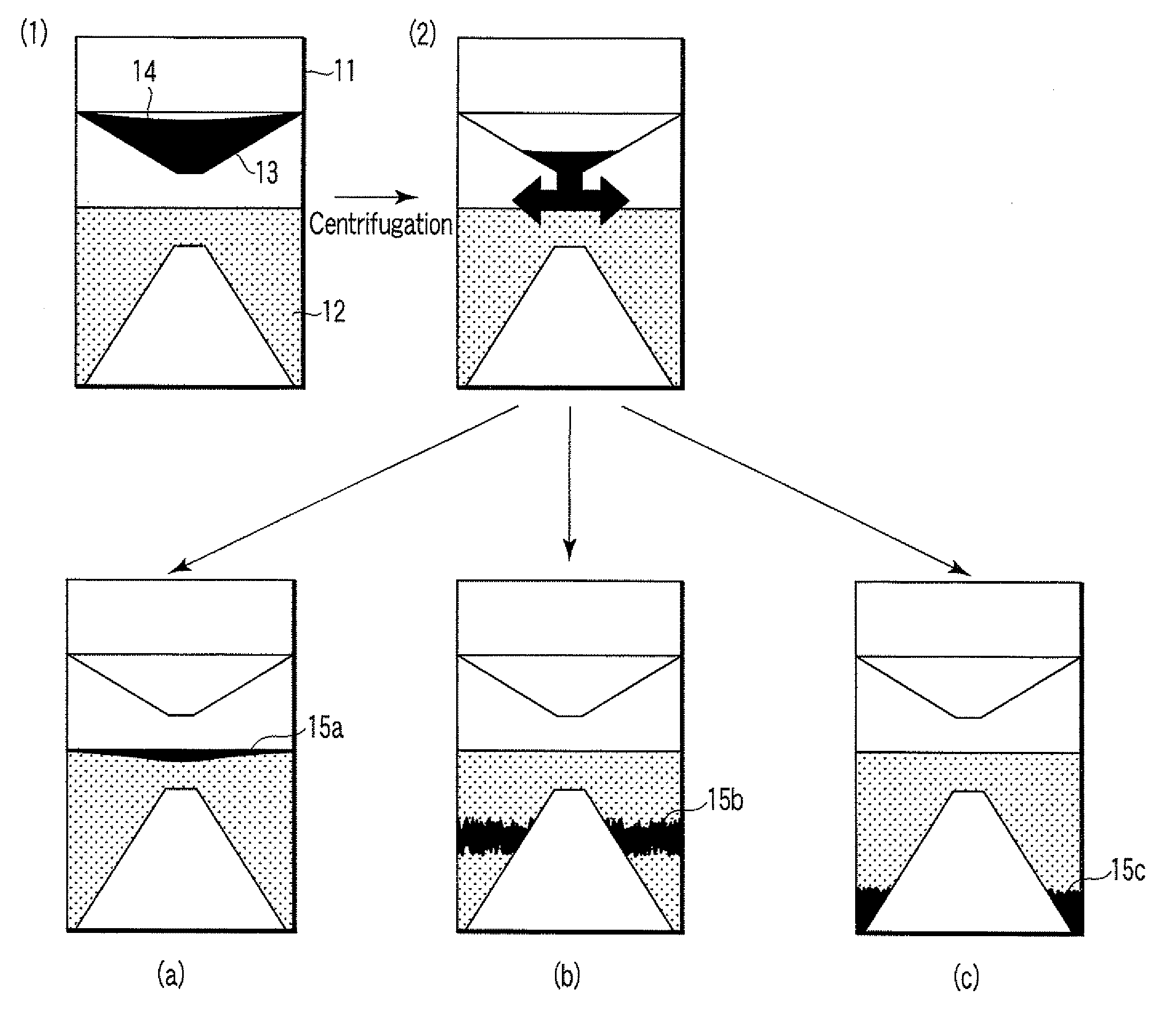
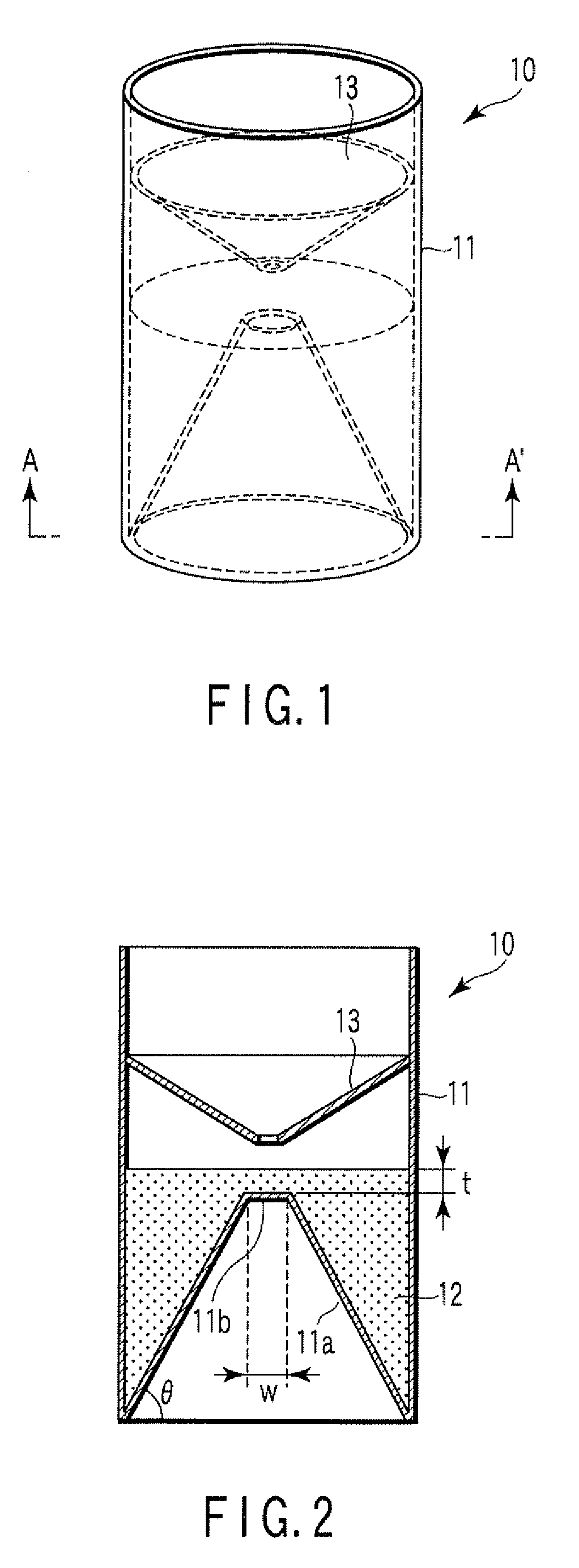
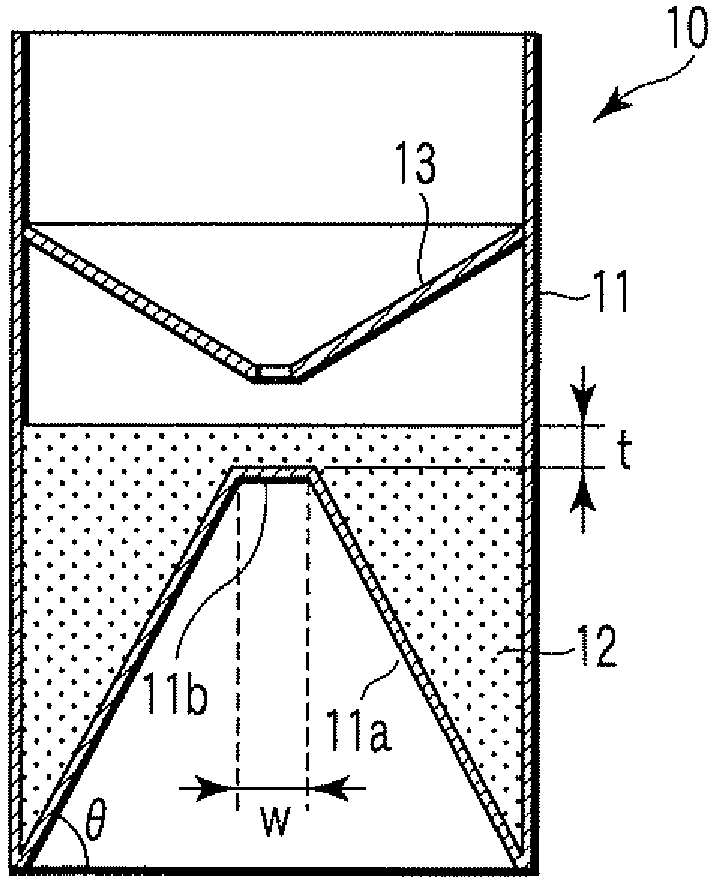
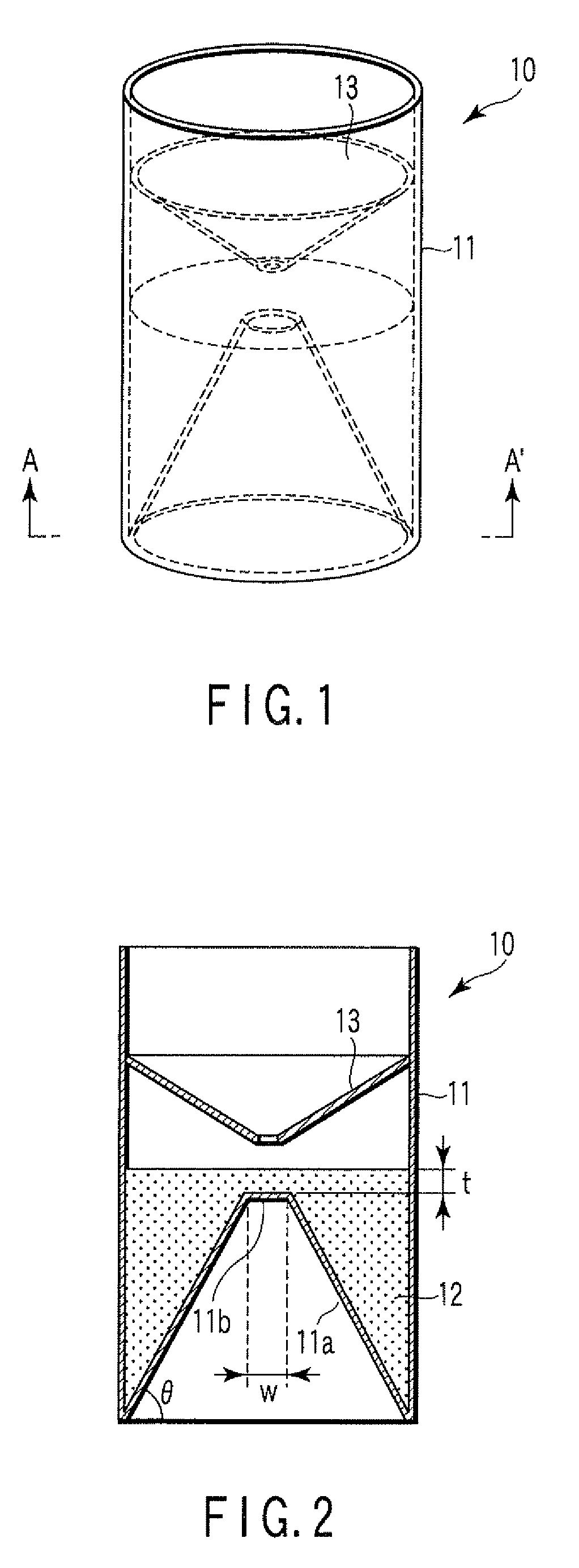
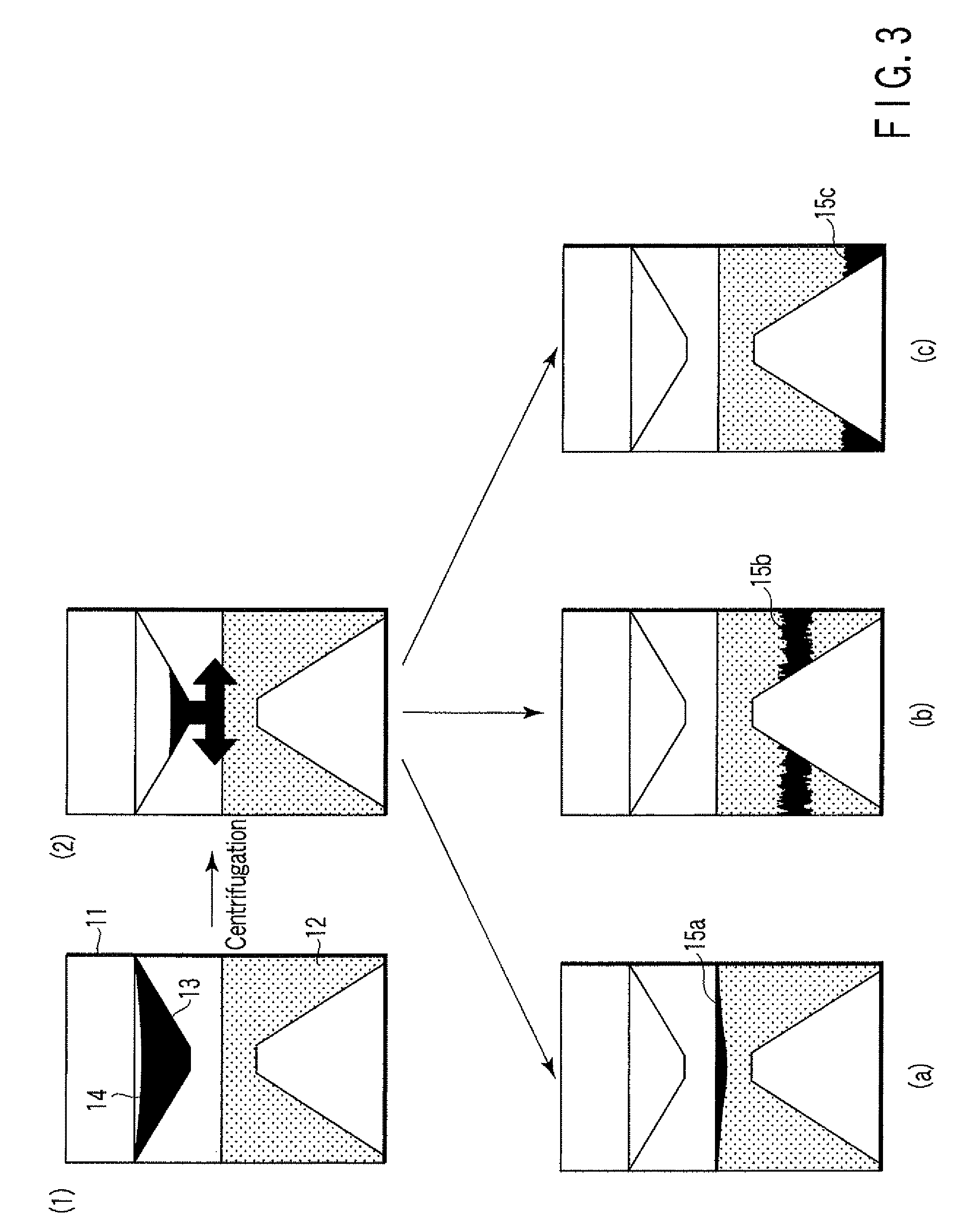
Particle agglutination-evaluating container
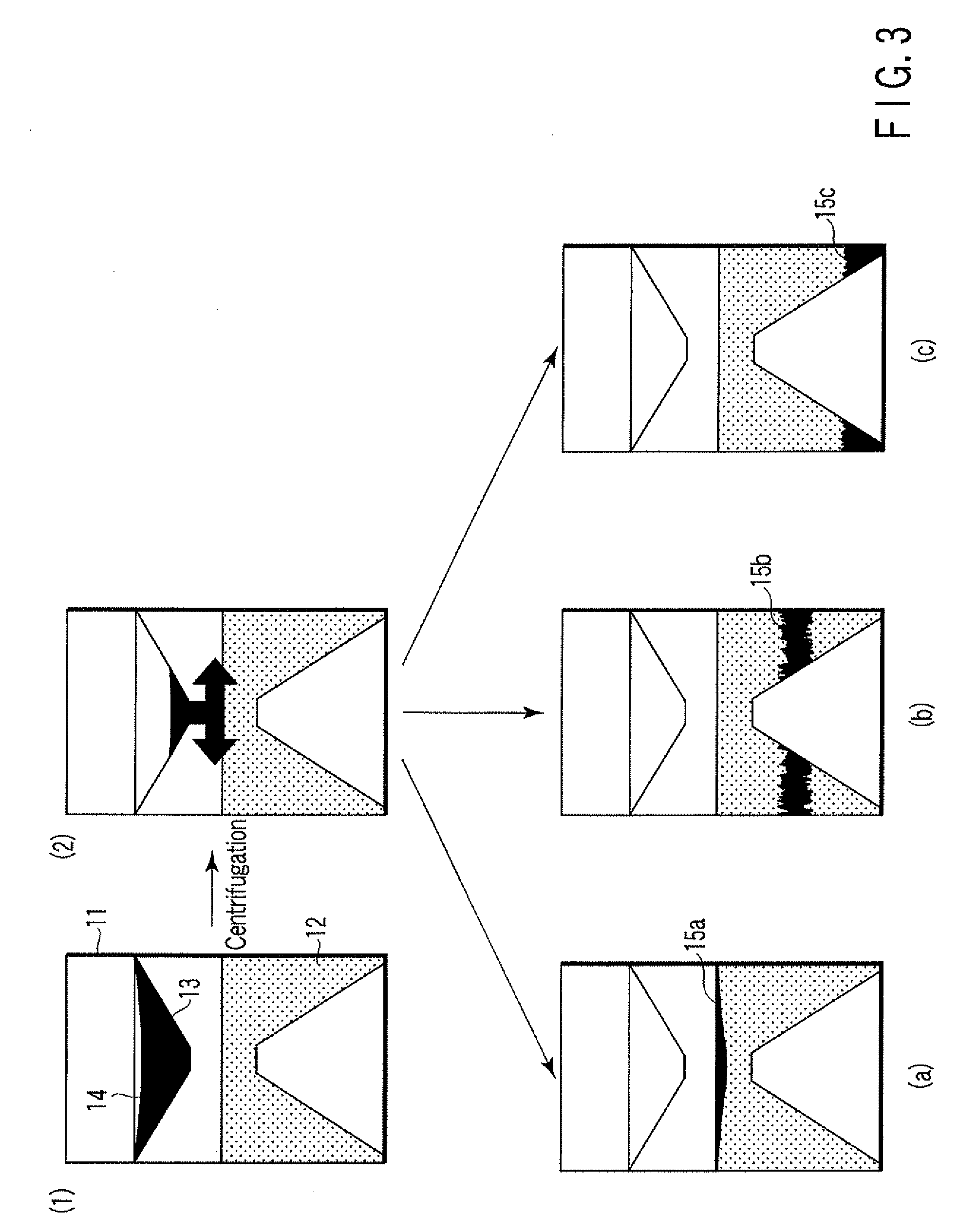
ActiveUS20080213131A1Easily and accuratelyEasy and accurate evaluationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenEngineering
A particle agglutination-evaluating container for immunological analysis, based on an agglutinate formed in an agglutination reaction between an antibody- or antigen-containing sample and agglutination particles, includes a transparent container body having a bottom face including a sloping bottom face and a raised bottom face extended in a horizontal direction at the top of the sloping bottom face to form an obtuse angle with the sloping bottom face, and a fluidal separation layer containing insoluble particles which are filled in the container body and where the agglutination particles are separated according to the agglutination degree, wherein agglutination is evaluated by observation from the bottom of the container body, where the agglutination reaction is judged positive when the agglutination particles are observed through the raised bottom face, negative when observed at the bottom end of the sloping bottom face, and weakly positive when observed in the middle of the sloping bottom face.
Owner:BECKMAN COULTER INC
Mesothelin detection cell quality control piece
PendingCN110531083AStable environmentImprove buffering effectPreparing sample for investigationBiological testingCytoplasmParaffin section
The invention relates to a mesothelin immunohistochemical detection cell quality control piece and a related detection reagent. The cell quality control piece comprises a sectioning area, wherein paraffin sections are adhered to the sectioning area, and the paraffin sections contain four kinds of cell clusters for expressing negative, weak positive, positive and strong positive properties of the mesothelin. The immunohistochemical detection cell quality control piece involved in the invention is mainly used for improving the accuracy and the consistency of immunohistochemical detection results, and has a high application prospect.
Owner:REMEGEN CO LTD
Immunochromatography detection reagent strip, kit containing immunochromatography detection reagent strip and application of immunochromatography detection reagent strip
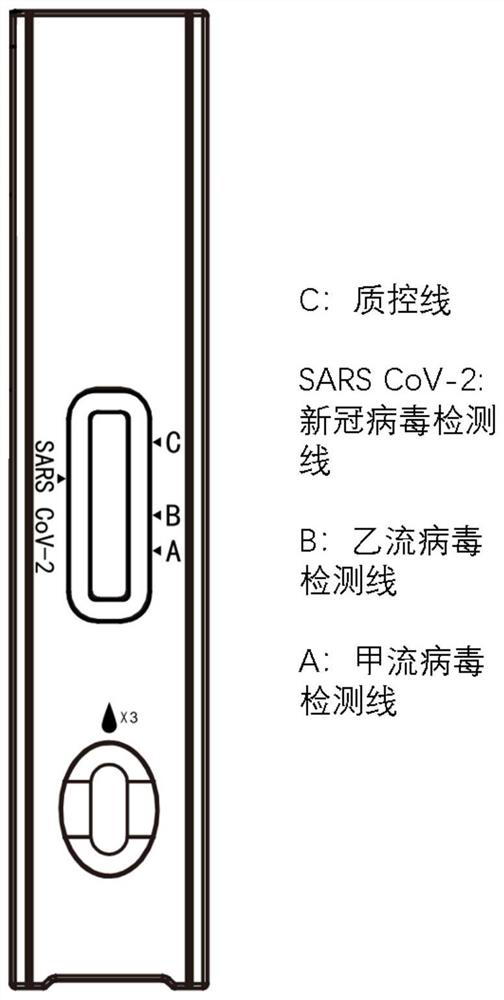
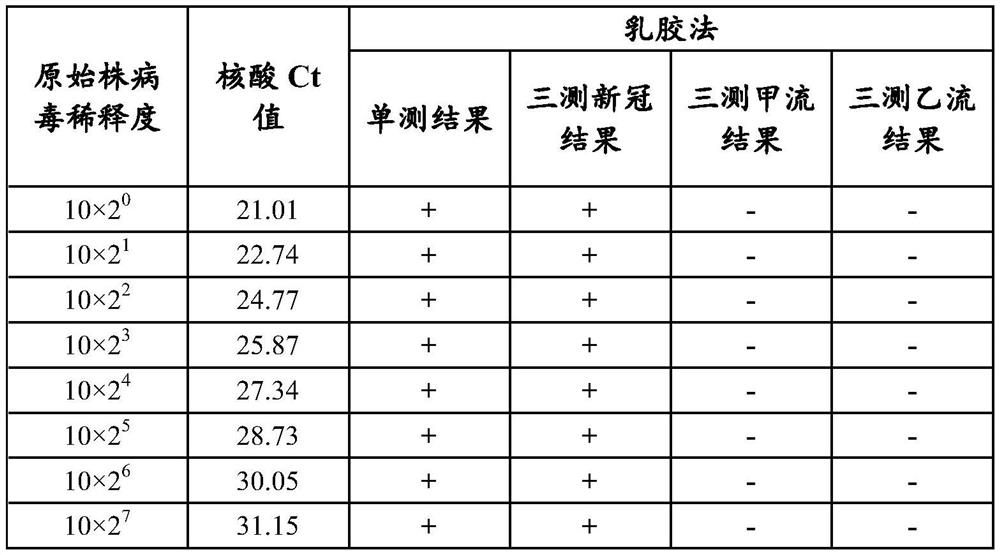
ActiveCN113777299AStrong specificityHigh sensitivityBiological testingImmunoassaysAntigenReagent strip
The invention provides an immunochromatography detection reagent strip, a kit containing the immunochromatography detection reagent strip and application of the immunochromatography detection reagent strip, and relates to the technical field of biological detection. According to the immunochromatography detection reagent strip, an immunochromatography technology is adopted, COVID-19 N protein, influenza A virus NP protein and influenza B virus NP protein serve as detection antigens, and fluorescent microspheres, latex, colloidal gold or colloidal carbon are coupled with specific protein to obtain a specific protein compound; the nasopharyngeal swab, oropharyngeal swab, or saliva of the patient is then detected. The reagent strip has the advantages of high sensitivity, high specificity and quantitative detection, and avoids leak detection of weak positive samples and false detection of false positive samples to the greatest extent. Meanwhile, the immunochromatography used in the invention is rapid in detection, results can be obtained within 15 minutes, and field operation can be realized through simple training.
Owner:HANGZHOU BAOLIN BIOTECHNOLOGY CO LTD
Particle agglutination-evaluating container
ActiveUS7807107B2Easy and accurate evaluationHigh-throughput analysisBioreactor/fermenter combinationsBiological substance pretreatmentsAgglutinationEngineering
Owner:BECKMAN COULTER INC
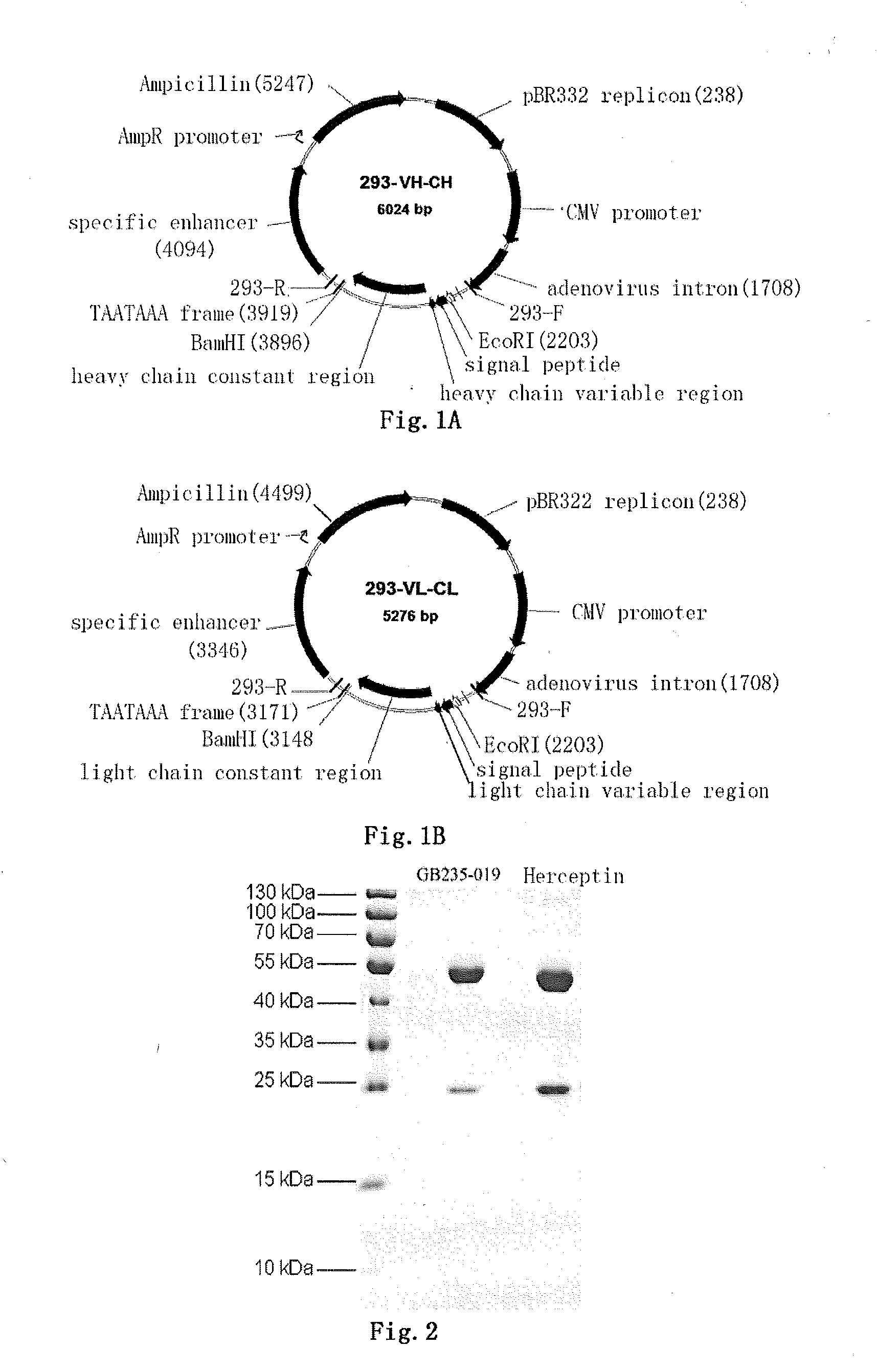
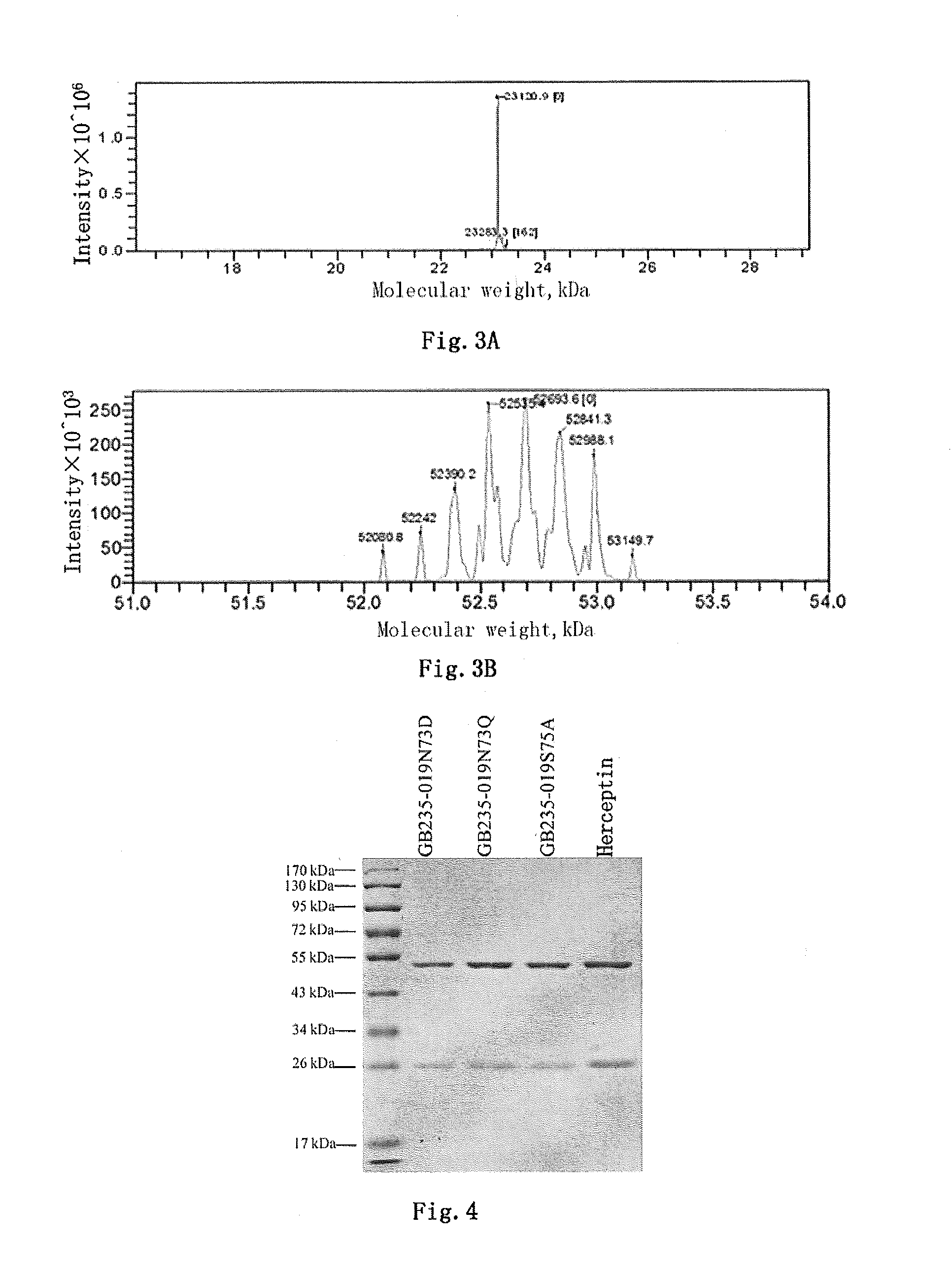
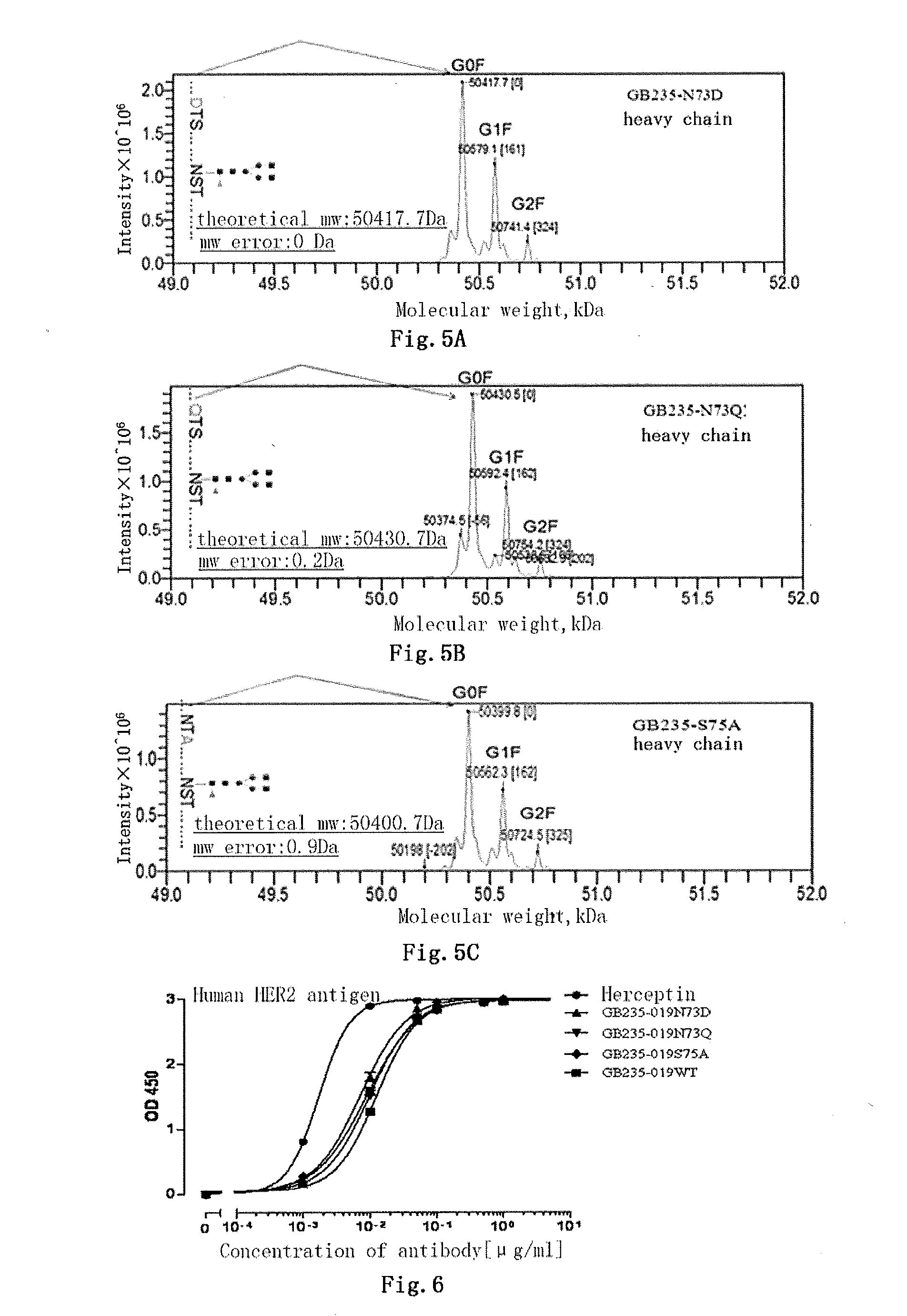
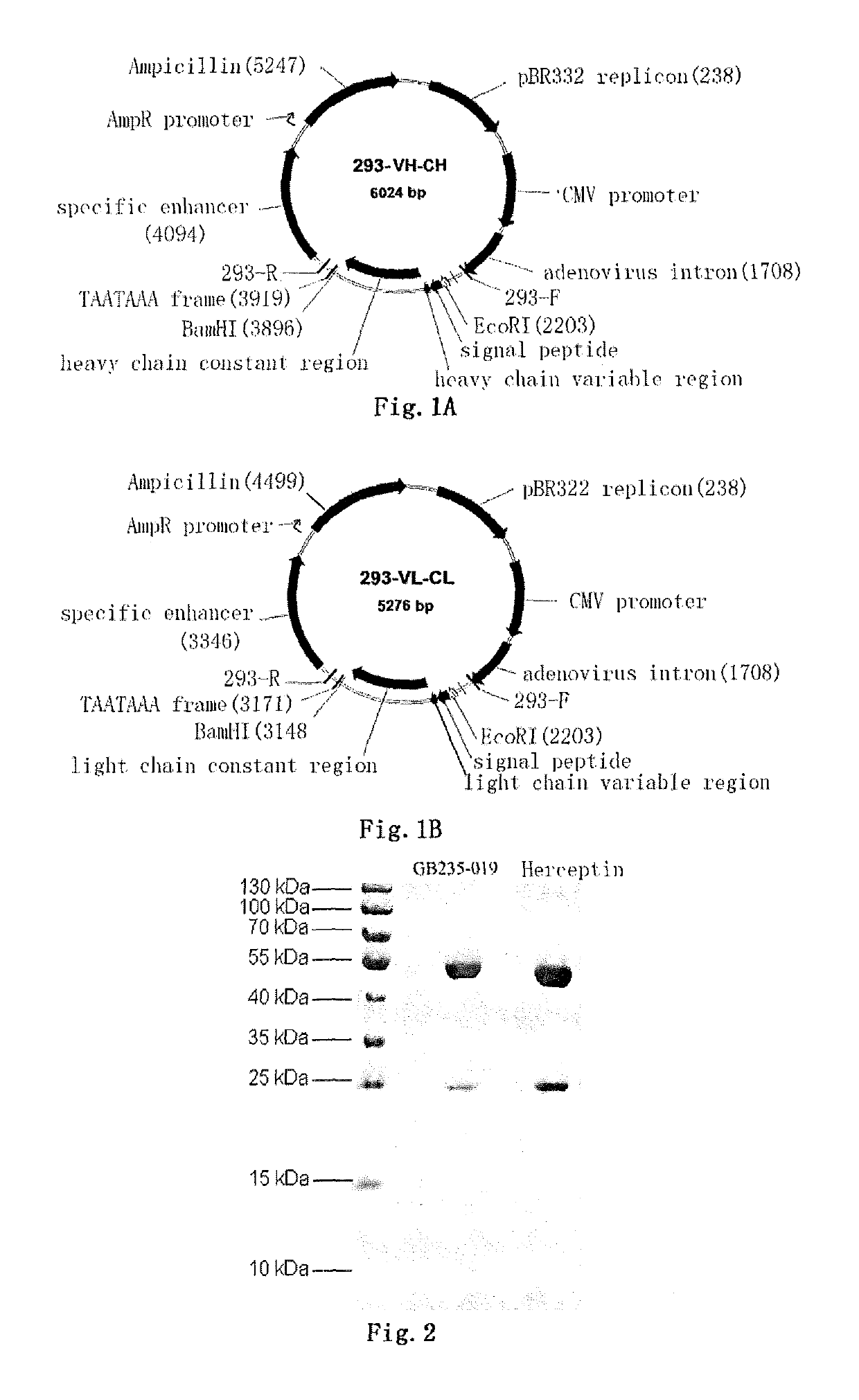
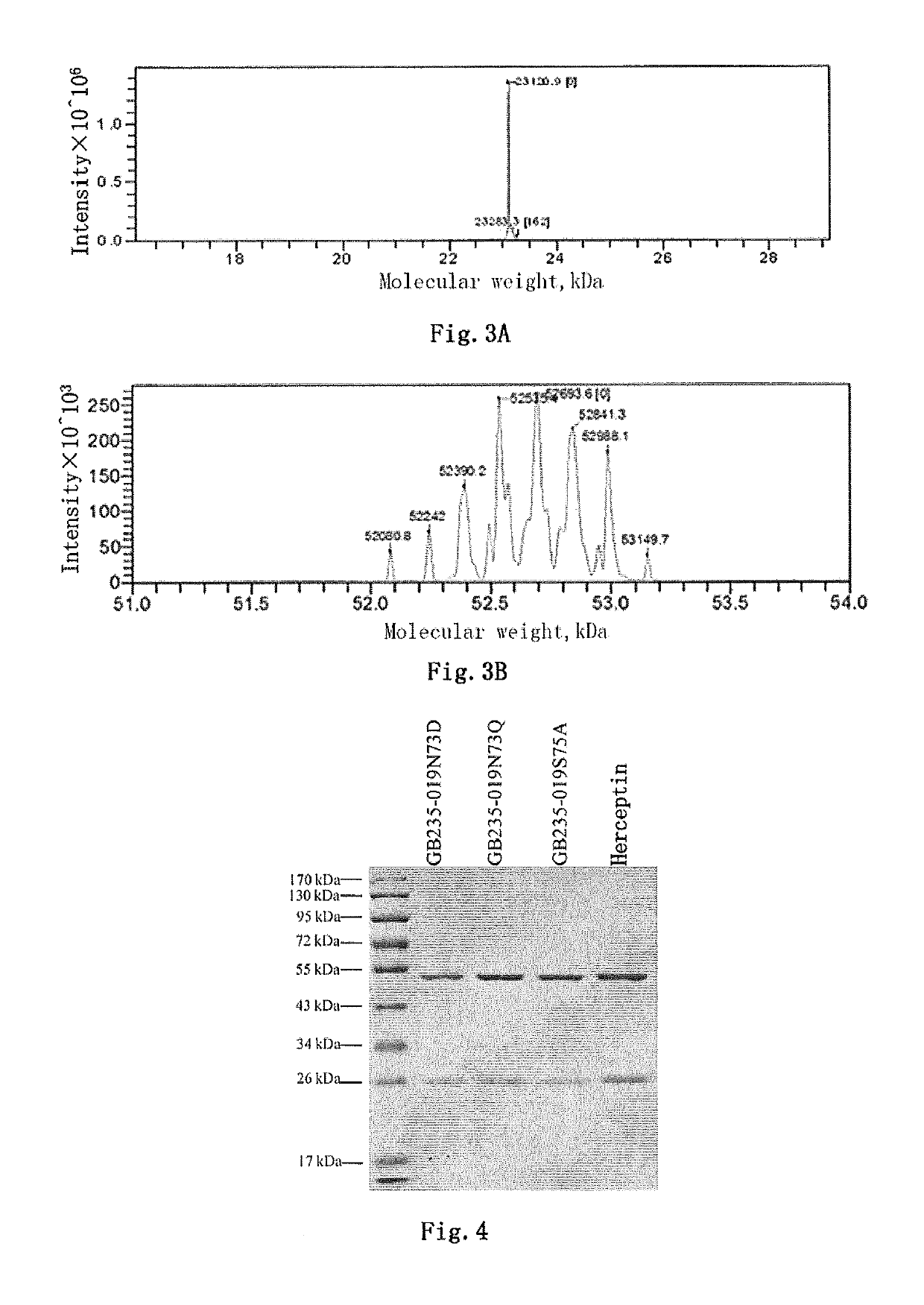
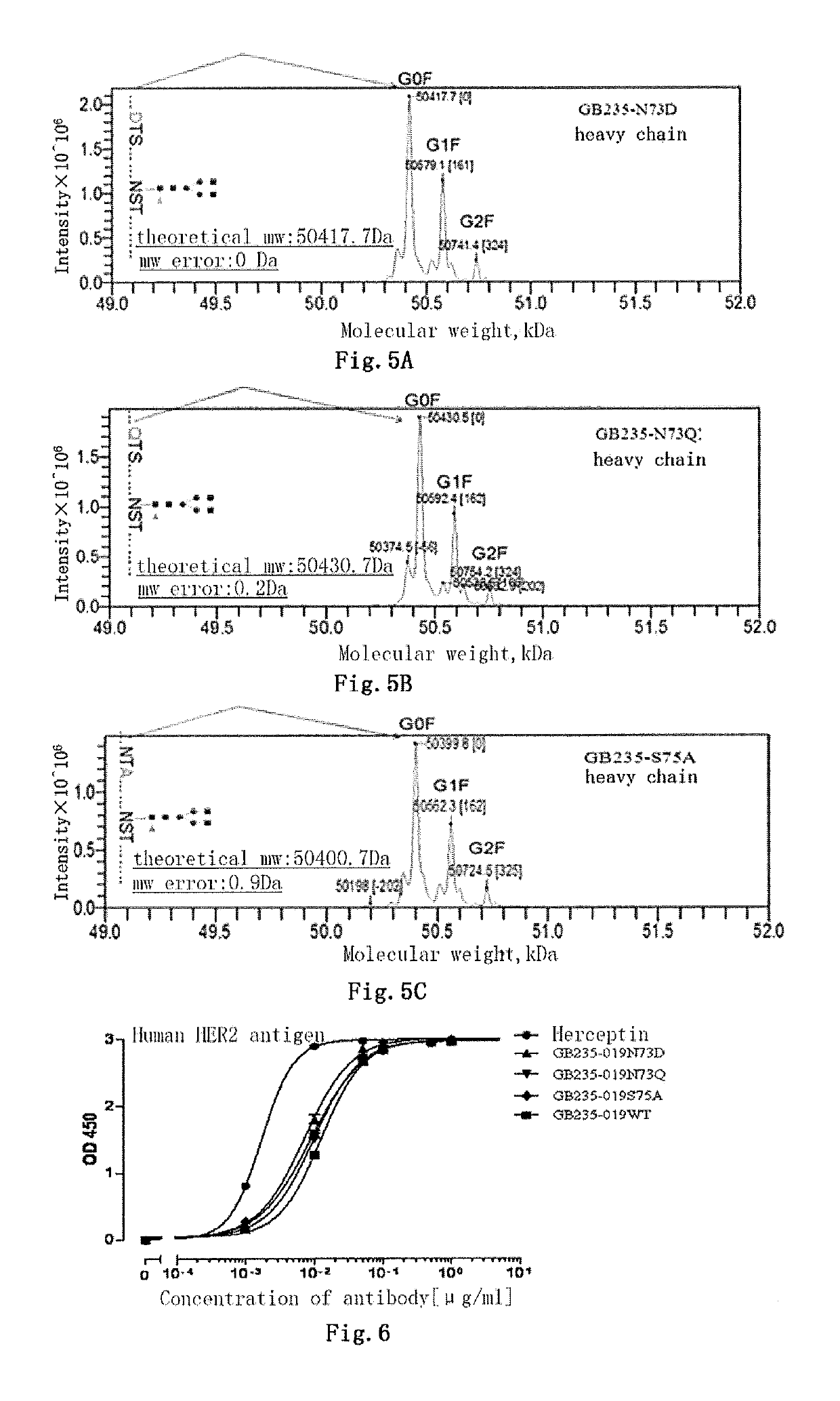
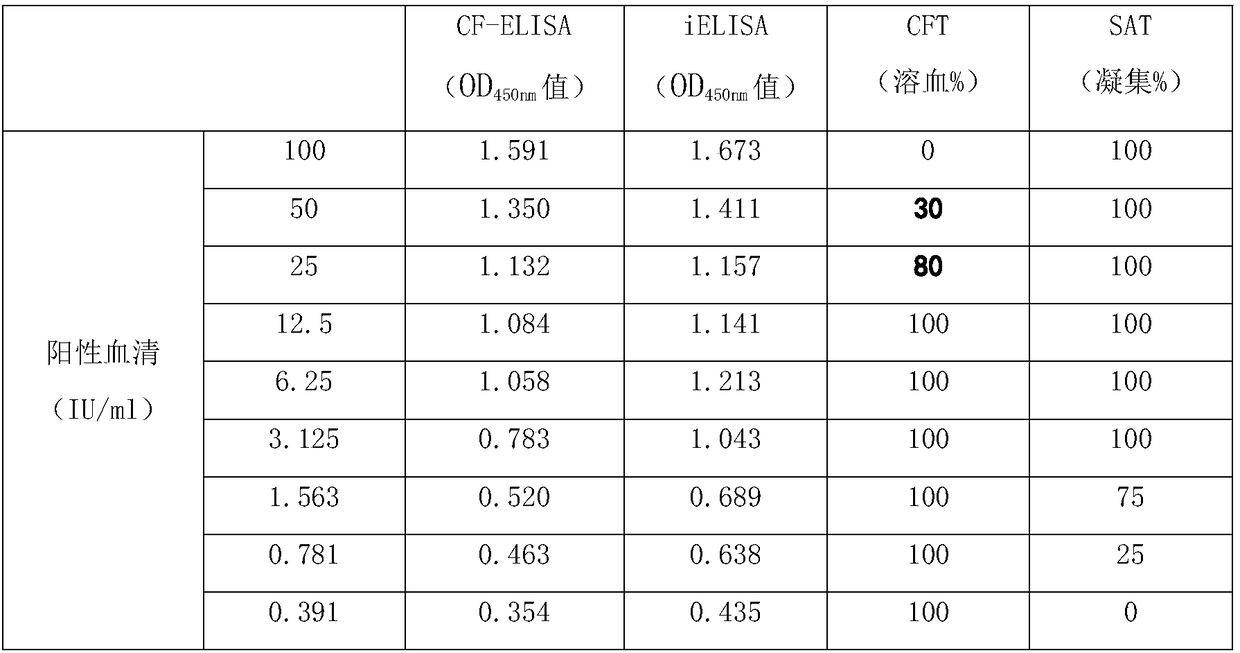
Mutated antibody of fully humanized her2 antibody, and encoding gene and use thereof
ActiveUS20170037146A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenHeavy chain
The present invention provides a mutated antibody of the fully humanized HER2 antibody GB235-019, wherein the amino acid sequence of the heavy chain variable region and the amino acid sequence of the light chain variable region of the mutated antibody are respectively SEQ ID NO: 10, SEQ ID NO: 2; SEQ ID NO: 11, SEQ ID NO: 2; or SEQ ID NO:12, SEQ ID NO:2. The mutated antibody has the ability to specifically bind to human HER2 antigen, similar to the GB235-019 antibody. They can also be used in combination with additional HER2 positive tumor therapeutic agents for treating HER2 positive tumor, weakly positive tumor or negative tumor.
Owner:GENOR BIOPHARMA
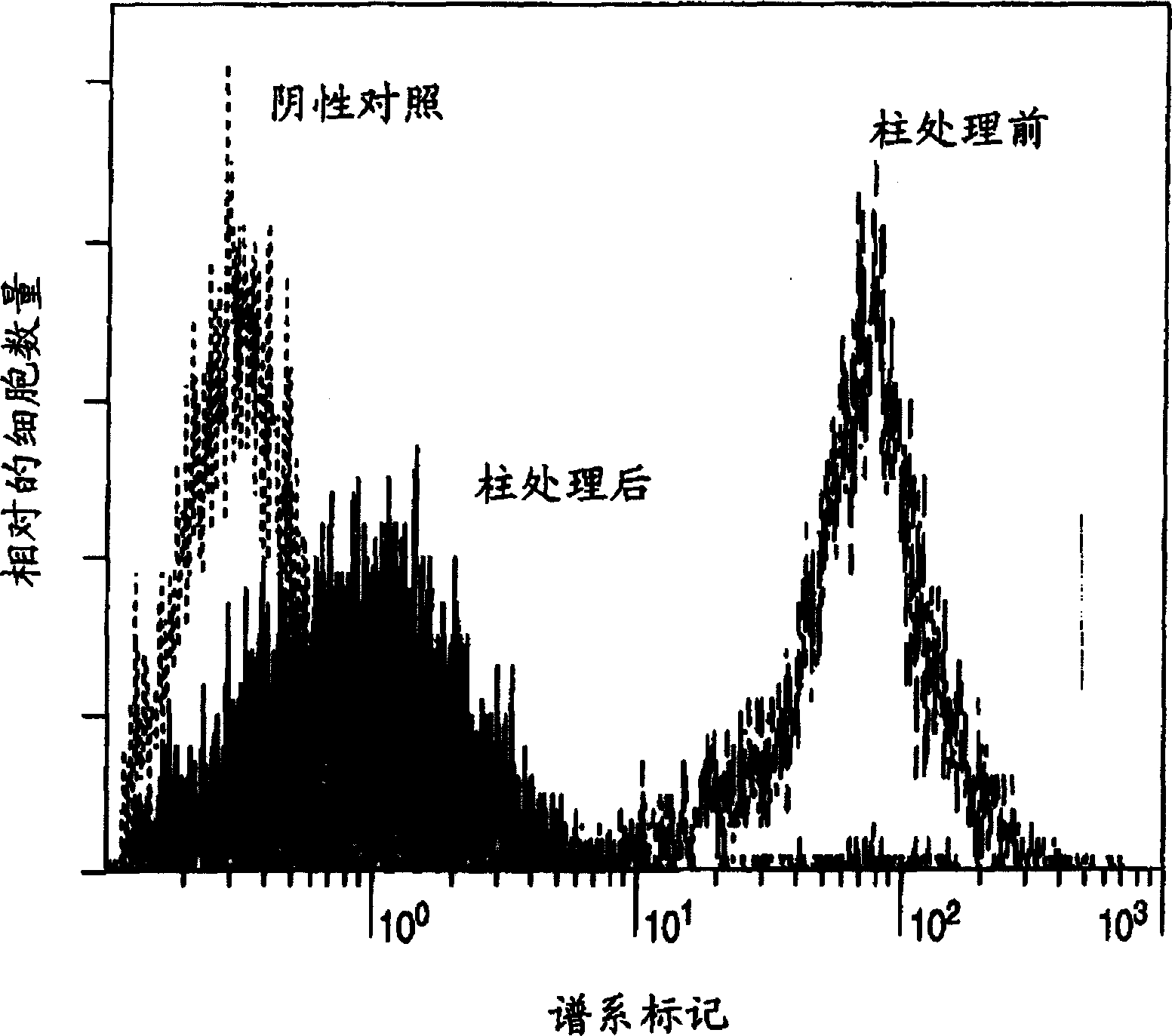
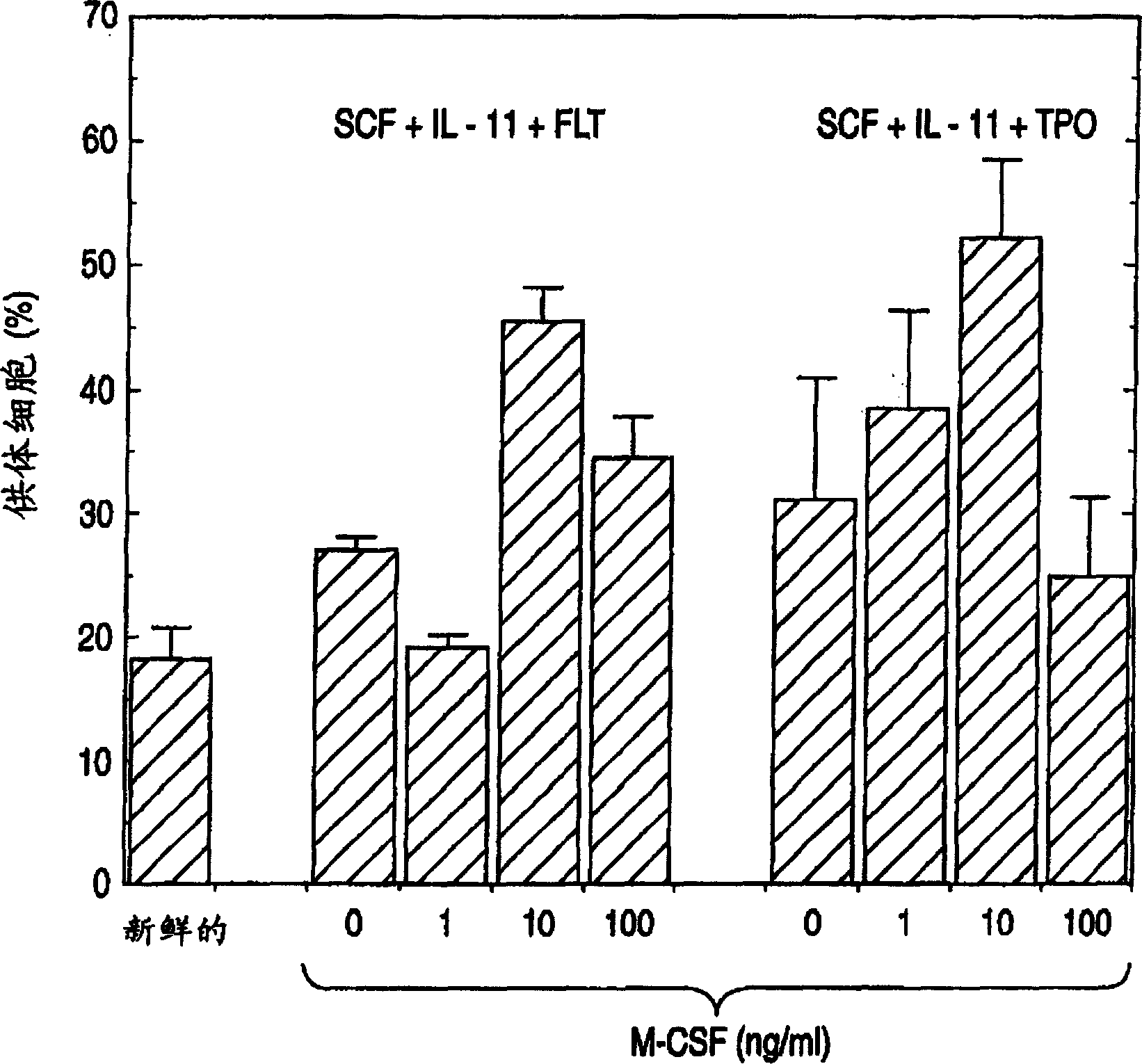
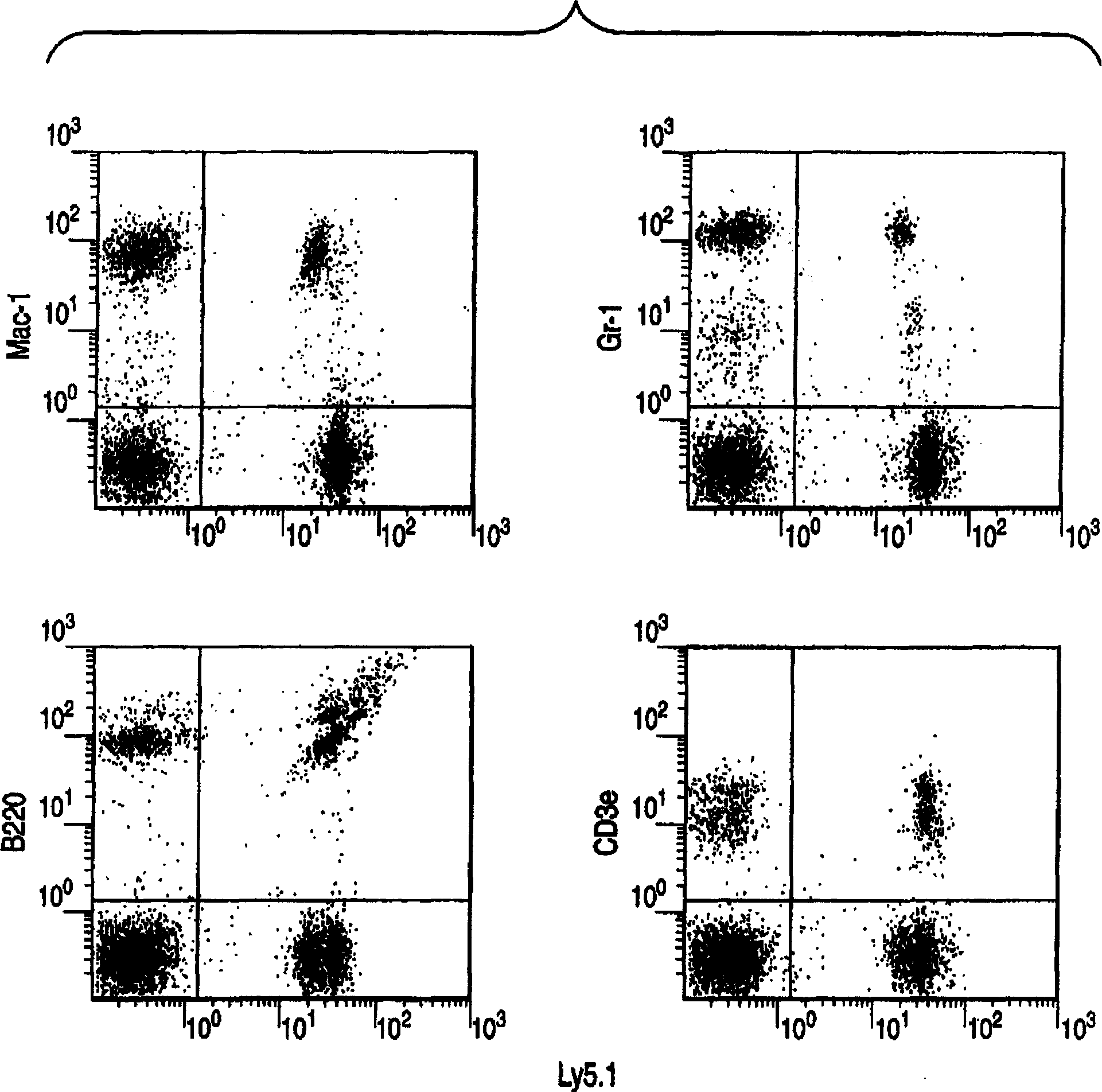
Method for expanding hematopoietic stem cells
A method for culturing Lin-negative or weakly positive cells in the presence of macrophage colony-stimulating factor (M-CSF); a method for growing Lin-negative or weakly positive cells by culturing them in the presence of M-CSF A method for hematopoietic stem cells; a population of hematopoietic stem cells obtained by the proliferation method; a kit containing M-CSF for cultivating Lin-negative or weakly positive cells; and a kit containing M-CSF for proliferating hematopoietic Reagents for stem cells.
Owner:DAIICHI SEIYAKU CO LTD
Mutated antibody of fully humanized HER2 antibody, and encoding gene and use thereof
ActiveUS10253108B2Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenHeavy chain
The present invention provides a mutated antibody of the fully humanized HER2 antibody GB235-019, wherein the amino acid sequence of the heavy chain variable region and the amino acid sequence of the light chain variable region of the mutated antibody are respectively SEQ ID NO: 10, SEQ ID NO: 2; SEQ ID NO: 11, SEQ ID NO: 2; or SEQ ID NO: 12, SEQ ID NO: 2. The mutated antibody has the ability to specifically bind to human HER2 antigen, similar to the GB235-019 antibody. They can also be used in combination with additional HER2 positive tumor therapeutic agents for treating HER2 positive tumor, weakly positive tumor or negative tumor.
Owner:GENOR BIOPHARMA
Brucellosis cf-elisa antibody detection kit
ActiveCN106596958BStrong specificityIncreased sensitivityBiological material analysisPositive controlBrucellosis
The invention relates to a brucellosis complement fixation-enzyme-linked immunosorbent assay (CF-ELISA) diagnostic kit, and the kit combines a reaction system in a complement fixation test (CFT) and an enzyme marker amplification system in an enzyme-linked immunosorbent assay (ELISA). The brucellosis complement fixation-enzyme-linked immunosorbent assay (CF-ELISA) diagnostic kit has the characteristics of high sensitivity, easy and fast use, high throughput and high degree of standardization compared with a traditional complement fixation test diagnostic reagent, has the characteristics of high specificity and capability of detecting a variety of brucellosis specific antibodies compared with a traditional ELISA diagnostic kit, and is an ideal new brucellosis diagnostic tool. The kit comprises the following main components: a lipopolysaccharide LPS antigen-coated plate, strongly-positive control serum, weakly positive control serum, negative control serum, a guinea pig complement, enzyme labeled guinea pig complement C1q-B monoclonal antibody 60G4, a substrate coloring-developing solution, a stop solution and washing liquid.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Hepatitis A virus antibody colloidal gold detection kit
ActiveCN103336119BImprove accuracyChanging the order of detection reactionsMaterial analysisAgainst vector-borne diseasesReagent stripAbsorption filter
Owner:山东康华生物医疗科技股份有限公司
Detection primers, probes and kits for 16 types of hpv virus
ActiveCN105087827BComprehensive detectionQuick screeningMicrobiological testing/measurementMicroorganism based processesHuman papillomavirusLower risk
The invention discloses a primer, probe and kit for detecting type-16 HPV (human papillomavirus). The primer and the probe are suitable for screening the HPV related diseases quickly, accurately and simply, do not cross-react with other viruses, are capable of detecting common high and low risk types of HPVs, two types of HPVs and 14 types of HPVs in total, cover a wide range of high risk types of HPVs and are more accurate and complete in detection than kits in the present market. The kit further comprises negative, positive and weakly positive quality controls and helps further increase detection accurateness.
Owner:北京鑫诺美迪基因检测技术有限公司
Anti-type a foot-and-mouth disease virus neutralizing monoclonal antibody and its application
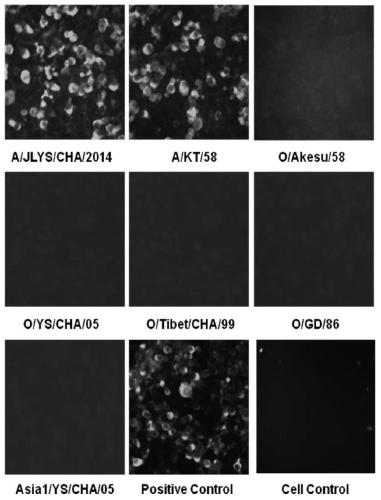
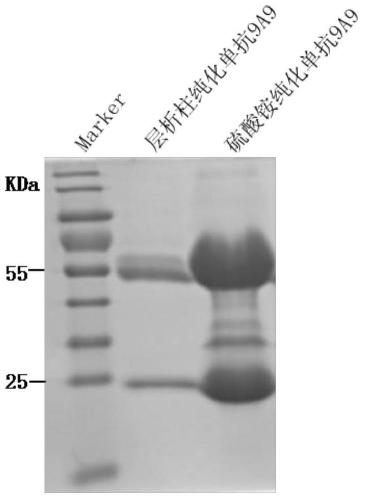
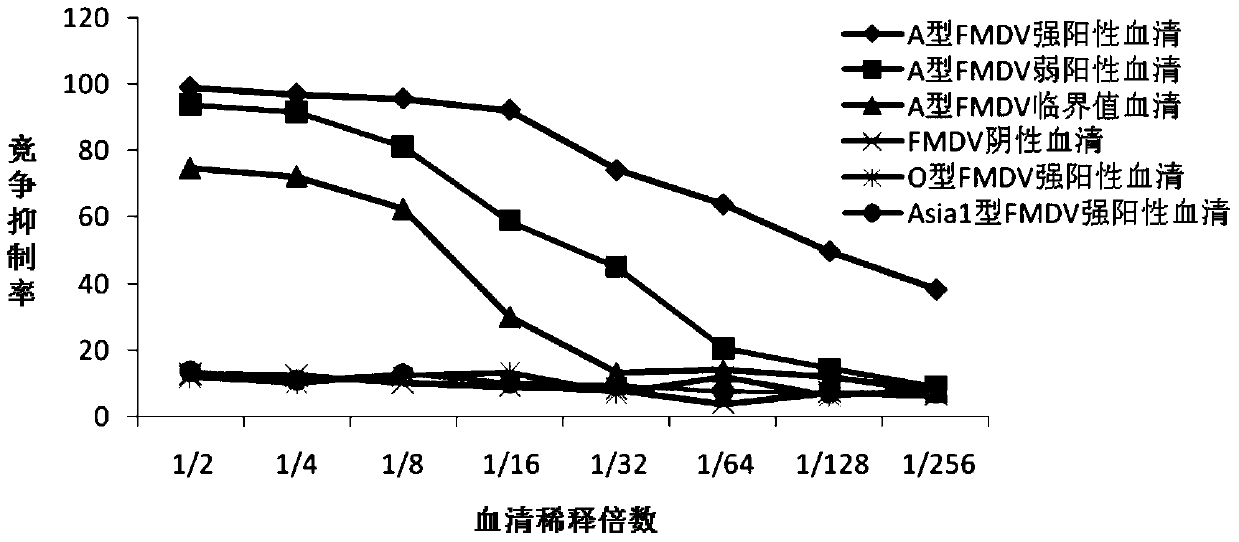
ActiveCN105859880BHigh strengthImprove neutralization abilityImmunoglobulins against virusesAntiviralsSerotypeHybridoma cell
The invention discloses preparation and application of a monoclonal antibody of an A-type foot and mouth disease virus (FMDV) and belongs to the field of prevention and treatment of foot and mouth diseases. According to the preparation and the application, a hybridoma cell line secreting the neutralizing monoclonal antibody of the A-type FMDV is constructed, the microbiological collection number of the hybridoma cell line is CGMCC 12049, and the secreted monoclonal antibody is an A-type FMDV serotype specific monoclonal antibody and has high-strength neutralizing ability; and a detection method for the A-type FMDV antibody is created by using the monoclonal antibody. The monoclonal antibody prepared by the invention shows good competitive ability with strongly-positive, weakly-positive and suspected serum of the A-type FMDV in the detection method for the A-type FMDV antibody and is free of competitiveness with strongly-positive serum of an O-type FMDV, strongly-positive serum of an Asia1-type FMDV and negative serum of an FMDV, so that the monoclonal antibody can be used for specifically discriminating the negative and positive serum of the A-type FMDV and has an important application prospect in aspects such as the diagnosis, prevention and treatment of the A-type foot and mouth diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Lupus anticoagulant detection kit
InactiveCN114778858AHigh sensitivityImprove accuracyDisease diagnosisBiological testingMedicineLiquid state
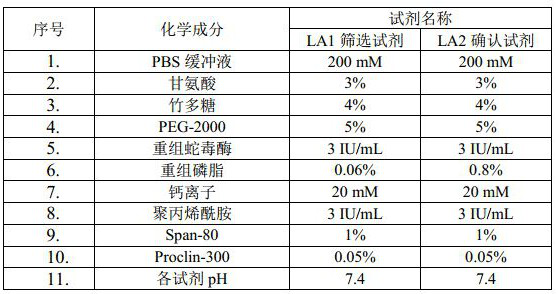
The invention discloses a lupus anticoagulant detection kit. The lupus anticoagulant detection kit comprises an LA1 screening reagent and an LA2 confirmation reagent, the LA1 screening reagent comprises recombinant snake venom enzyme, recombinant phospholipid, calcium ions, a first heparin neutralizer, a first buffer solution and a first auxiliary material, and the LA1 screening reagent is in a liquid state; the LA2 confirmation reagent comprises recombinant snake venom enzyme, recombinant phospholipid, calcium ions, a second heparin neutralizer, a second buffer solution and a second auxiliary material, and the LA2 confirmation reagent is in a liquid state; the content of the recombinant phospholipid in the LA1 screening reagent is lower than that of the recombinant phospholipid in the LA2 confirmation reagent; the recombined snake venom enzyme and the recombined phospholipid are matched for use, the reagent sensitivity is higher, the change amplitude in unit time is remarkably enhanced when a positive or weak positive sample is tested, the detection rate of the positive sample is increased, and false negative is reduced; the kit also comprises a heparin neutralizer, so that the influence of heparin is eliminated, and the accuracy is improved; the reagent is liquid, convenient to use, low in cost and good in stability.
Owner:SHENZHEN DYMIND BIOTECH
Traditional Chinese medicine health-care cervical vertebra pillow
InactiveCN103598778BReduce tensionRelief the painSenses disorderNervous disorderCervical spondylosisSemen
The invention provides a traditional Chinese medicine health-care cervical vertebra pillow. The cervical vertebra pillow comprises a pillow sleeve 1 and a pillow inner 2 located in the pillow sleeve 1. The pillow inner 2 comprises, by weight, 20 parts to 25 parts of agastache, 21 parts to 26 parts of mints, 10 parts to 12 parts of schisandra chinensis, 85 parts to 110 pars of semen cassiae and 90 parts to 120 parts of tartary buckwheat. The clinic use proves that according to the clinical symptoms and signs of cervical spondylosis and the corresponding physical examination result, after the traditional Chinese medicine health-care cervical vertebra pillow is used, the corresponding clinical symptoms are obviously relieved, the specialized examination result which is originally positive is converted to be weakly positive or negative, and the average service life is 19.64 days.
Owner:李博文
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com