Human immunodeficiency virus type 1 one-step fluorescence quantitative RT-PCR detection kit
A technology of human immunodeficiency and detection kits, which is applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc. It can solve the problems of loss of re-amplification ability, etc., and achieve easy operation and high reliability , The effect of preventing multiple operation pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 A one-step fluorescent quantitative PCR detection kit for human immunodeficiency virus type 1.
[0027] 1. Extraction of Human Immunodeficiency Virus Type 1 (HIV-1) RNA
[0028] Nucleic acid was extracted from HIV-1 positive serum samples using column purification reagents (silica membrane adsorption method) from Shanghai Xingyao Medical Technology Development Co., Ltd.
[0029] 2. Reverse transcription and fluorescent quantitative PCR amplification (25ul system per person)
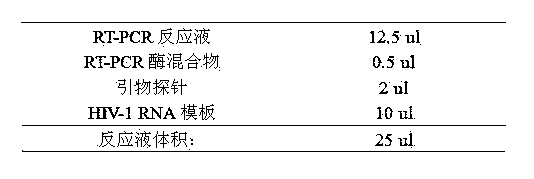
[0030] a. Preparation of one-step fluorescent quantitative RT-PCR reaction solution:
[0031]
[0032] b. One-step fluorescent quantitative RT-PCR reaction procedure:
[0033] [1] 37°C 10 min
[0034] [2] 50°C for 15 minutes
[0035] [3] 95℃ for 2 minutes
[0036] [4] 94°C 10s
[0037] [5] 60°C 45s
[0038] [6] Go to [4] ,45 cycles
[0039] In the fifth step, the fluorescence signals of FAM and JOE channels are collected,
[0040] [7] End.
[0041] 3. Detection:
[0042] ...
Embodiment 2
[0045] Example 2 clinical testing.
[0046] Using the above method to detect 130 clinical samples, including 32 cases of human immunodeficiency virus type 1 patients, the positive rate of detection was 24.6%, and the accuracy rate was 100%. Accurate quantitative analysis is far superior to traditional methods such as ELISA. The detection method and kit of the present invention have strong specificity, high sensitivity, simple operation and high repeatability, can perform rapid qualitative and quantitative detection of human immunodeficiency virus type 1 (HIV-1) RNA, and can replace the conventional Traditional ELISA diagnostic method. Nucleic acid was extracted from HIV-1 positive serum samples using column purification reagents (silica membrane adsorption method) from Shanghai Xingyao Medical Technology Development Co., Ltd. to ensure the purity of the template. At the same time, a one-step fluorescence quantitative RT-PCR technique was used. After the nucleic acid extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com