Lupus anticoagulant detection kit
A detection kit, lupus anticoagulant technology, applied in the biological field, can solve the problems of insufficient anti-heparin interference ability, increase manual operation steps, and stability needs to be improved, so as to prolong the coagulation time, reduce the detection cost and reduce the burden Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
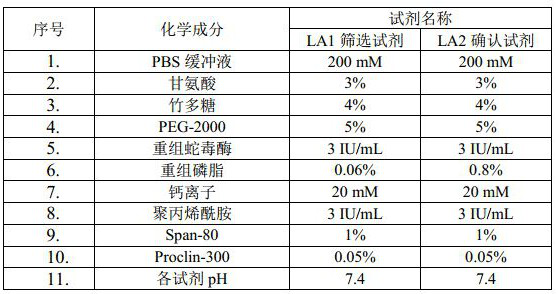
[0043] A lupus anticoagulant detection kit, including LA1 screening reagent and LA2 confirmation reagent; the specific components are shown in the following table:
[0044] Table 1. Reagent components of the kit of Example 1
[0045]
[0046] Note: PBS buffer can be replaced by any one or more of Tris buffer, Hepes buffer, MES buffer, MOPS buffer, citrate buffer; Glycine, polyethylene glycol 2000, Span-80 can be replaced by Any one or more of Tween-20, BSA, HSA, gelatin, trehalose, glucose, β-cyclodextrin and mannitol; Proclin-300 can be replaced with sodium benzoate, sodium azide, gentamicin and Any one or more of nitrites; calcium ions are provided by anhydrous calcium chloride; " / " means no such ingredient.
Embodiment 2
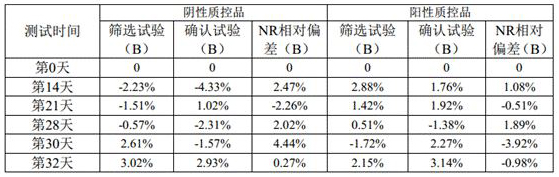
[0048] A lupus anticoagulant detection kit, including LA1 screening reagent and LA2 confirmation reagent; the specific components are shown in the following table:
[0049] Table 2. Reagent components of the kit of Example 2
[0050]
[0051] Note: Calcium ions are provided by calcium chloride; " / " means that this ingredient is not included.
Embodiment 3
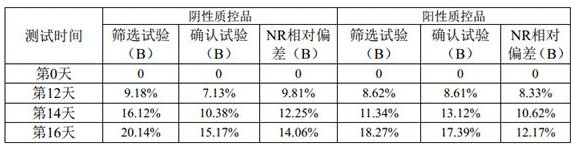
[0053] A lupus anticoagulant detection kit, including LA1 screening reagent and LA2 confirmation reagent; the specific components are shown in the following table:
[0054] Table 3. Reagent components of the kit of Example 3
[0055]
[0056] Note: Calcium ions are provided by anhydrous calcium chloride; " / " means that this ingredient is not included.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com