Human HLA-B*5801 gene polymorphism detection kit
A technology of genetic polymorphism and detection kit, which is applied in the fields of biotechnology and medicine, can solve the problems that whole blood samples cannot be directly detected, and the detection effect of low-concentration genomic DNA is not ideal, so as to achieve true and reliable results and avoid false positives and false negative results, time-consuming effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Prepare the human HLA-B*5801 gene detection kit of the present invention, comprising the following steps:
[0093] 1. Primer and probe synthesis:
[0094] Design and synthesize specific primers SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, specific probes SEQ ID NO:4 and SEQ ID NO:5, and at the 5' end of SEQ ID NO:4 Label the FAM fluorescent group, label the NFQ-MGB non-luminescent quenching group at the 3' end, label the VIC fluorescent group at the 5' end of SEQ ID NO:5, and label the NFQ-MGB non-luminescent quenching group at the 3' end. The primers and probes were prepared into 100 μM stock solutions for storage.
[0095]2. Prepare the internal standard system: design and synthesize a pair of internal standard primers for the human gene GAPDH, the primer pair sequences are SEQ ID NO:6 and SEQ ID NO:7; design and synthesize internal standard probes, the The probe is SEQ ID NO:8. The ROX fluorescent group is labeled at the 5' end of SEQ ID NO:8, and the BHQ2 non-luminesce...
Embodiment 2
[0105] The human HLA-B*5801 gene detection kit prepared in Example 1 was used to detect the samples to be tested.
[0106] In this example, 10 samples of EDTA anticoagulated whole blood were collected, treated with a blood treatment agent, and then tested with the human HLA-B*5801 gene detection kit prepared in Example 1.
[0107] 1. Blood Sample Processing
[0108] Take 10 μl of whole blood, add 90 μl of blood treatment agent, shake and mix for 1 min.
[0109] 2. Fluorescent quantitative PCR detection of samples
[0110] Add 2 μl of the sample in step 1 to 23 μl of the HLA-B*5801 detection reaction system of the kit in Example 1, so that the total volume of the reaction system is 25 μl, and put it into a fluorescent quantitative PCR instrument, and set it as follows Amplification reaction after PCR reaction procedure:
[0111] 37℃10min;
[0112] 95°C for 5 minutes;
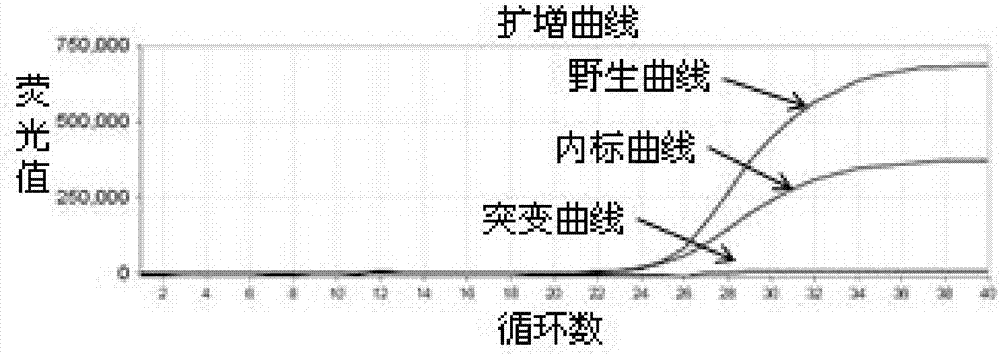
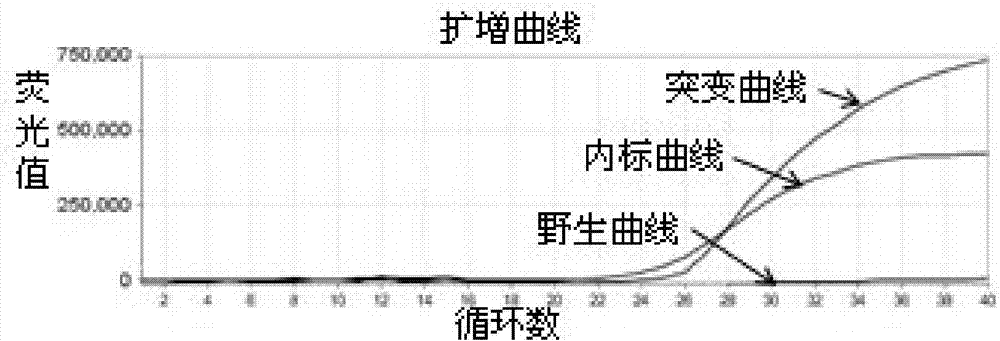
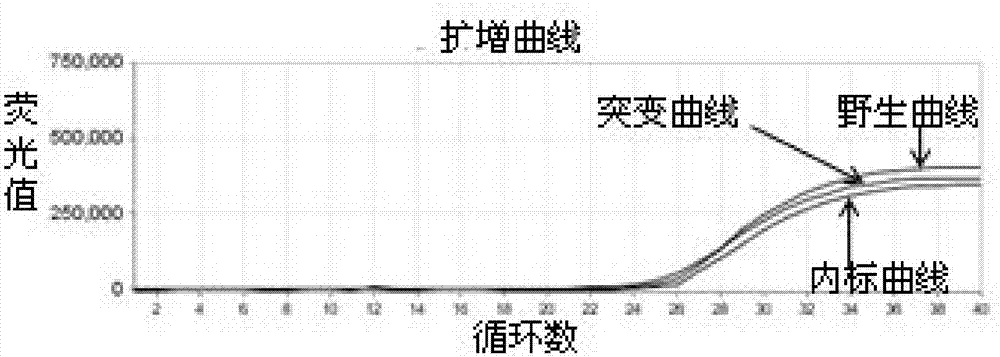
[0113] 95°C for 15s, 60°C for 60s, 40 cycles; after each cycle, the fluorescence signals of FAM, VIC and ...
Embodiment 3
[0118] Take the remaining whole blood sample whose test result is heterozygous mutation in Example 2, extract genomic DNA, measure the DNA concentration, dilute the DNA to 10ng / μl, 1ng / μl and 0.1ng / μl in sequence, and evaluate the detection performance of the kit .
[0119] 1. Genomic DNA Extraction
[0120] Take 300 μl of whole blood, add 900 μl of cell lysate CL, mix by inverting, and let stand for 5 minutes;
[0121] Centrifuge at 10,000rpm (11,500×g) for 1min, suck off the supernatant, leave the cell pellet, add 200μl buffer GS to the cell pellet collected by centrifugation, shake until thoroughly mixed;
[0122] Add 20μl Proteinase K solution and mix well;
[0123] Add 200μl buffer GB, mix well by inversion, place at 56°C for 10min, invert and mix several times during this time, the solution should become clear (if the solution is not completely clear, please prolong the lysis time until the solution is clear);
[0124] Add 200 μl of absolute ethanol, and mix thoroughl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com