Patents
Literature
711 results about "Whole blood sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Whole blood samples are composed of several basic components: red blood cells, white blood cells, platelets and plasma. In addition, whole blood also contains an abundance of small vesicles (exosomes) containing RNA and proteins that serve as intercellular messengers.
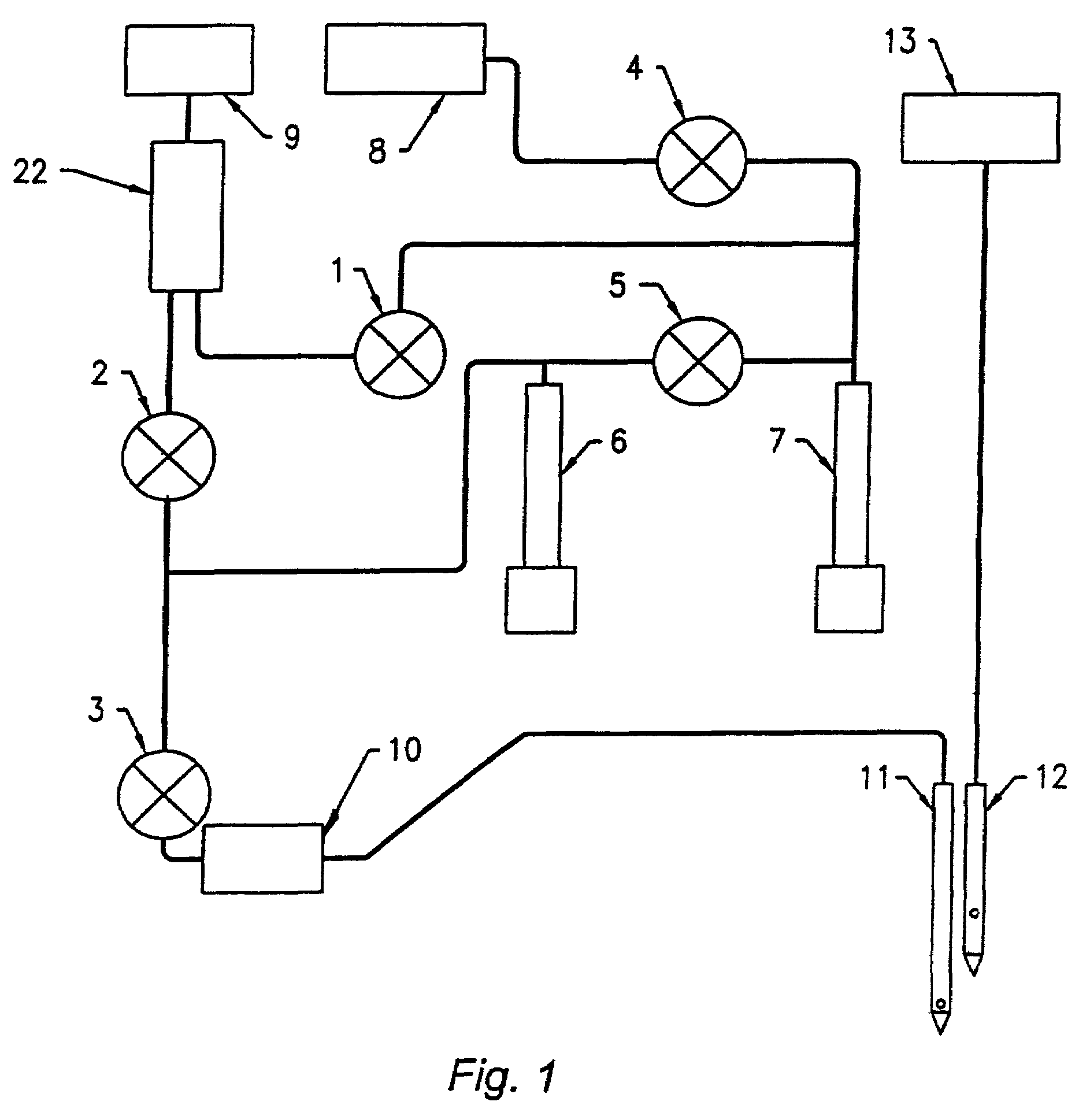
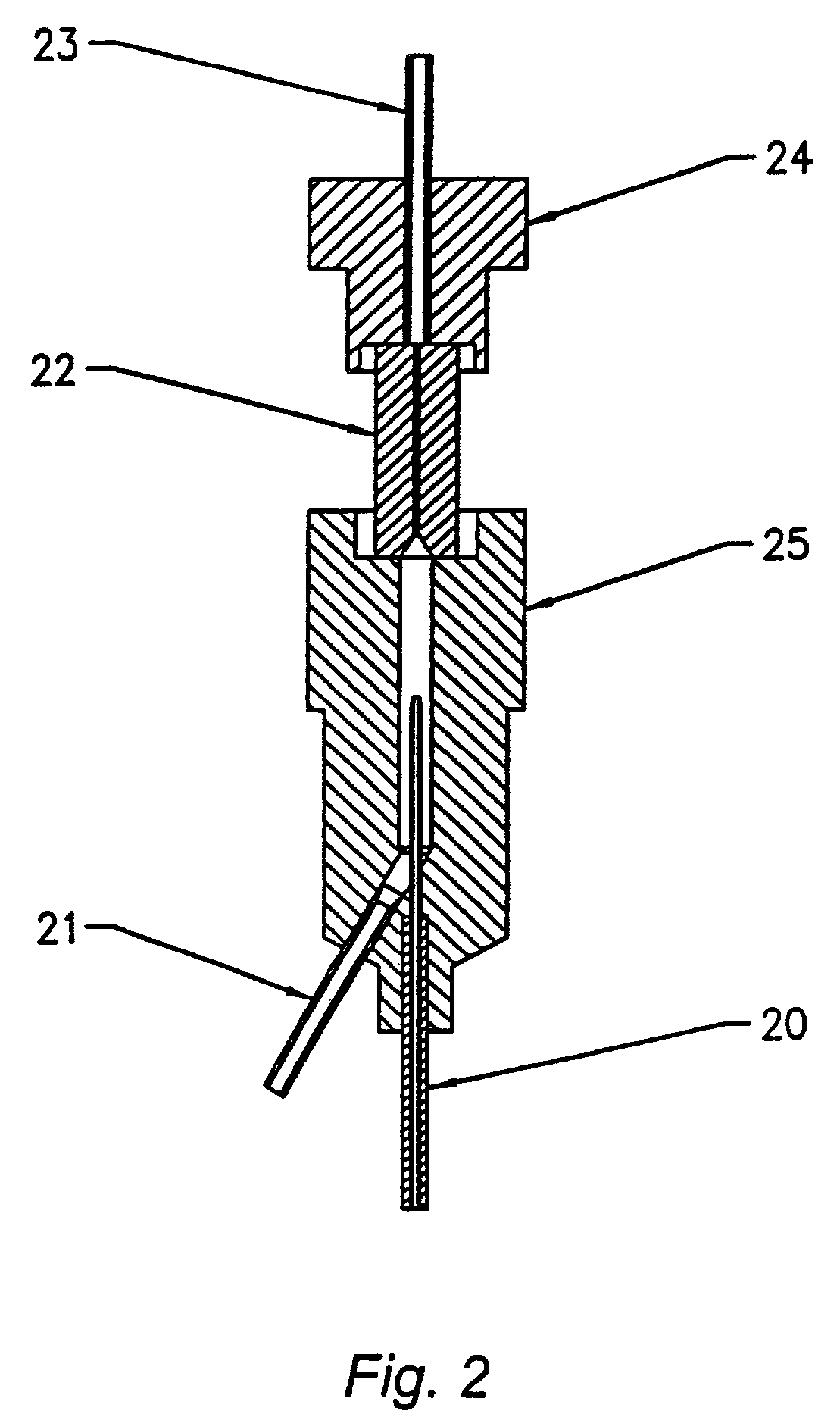
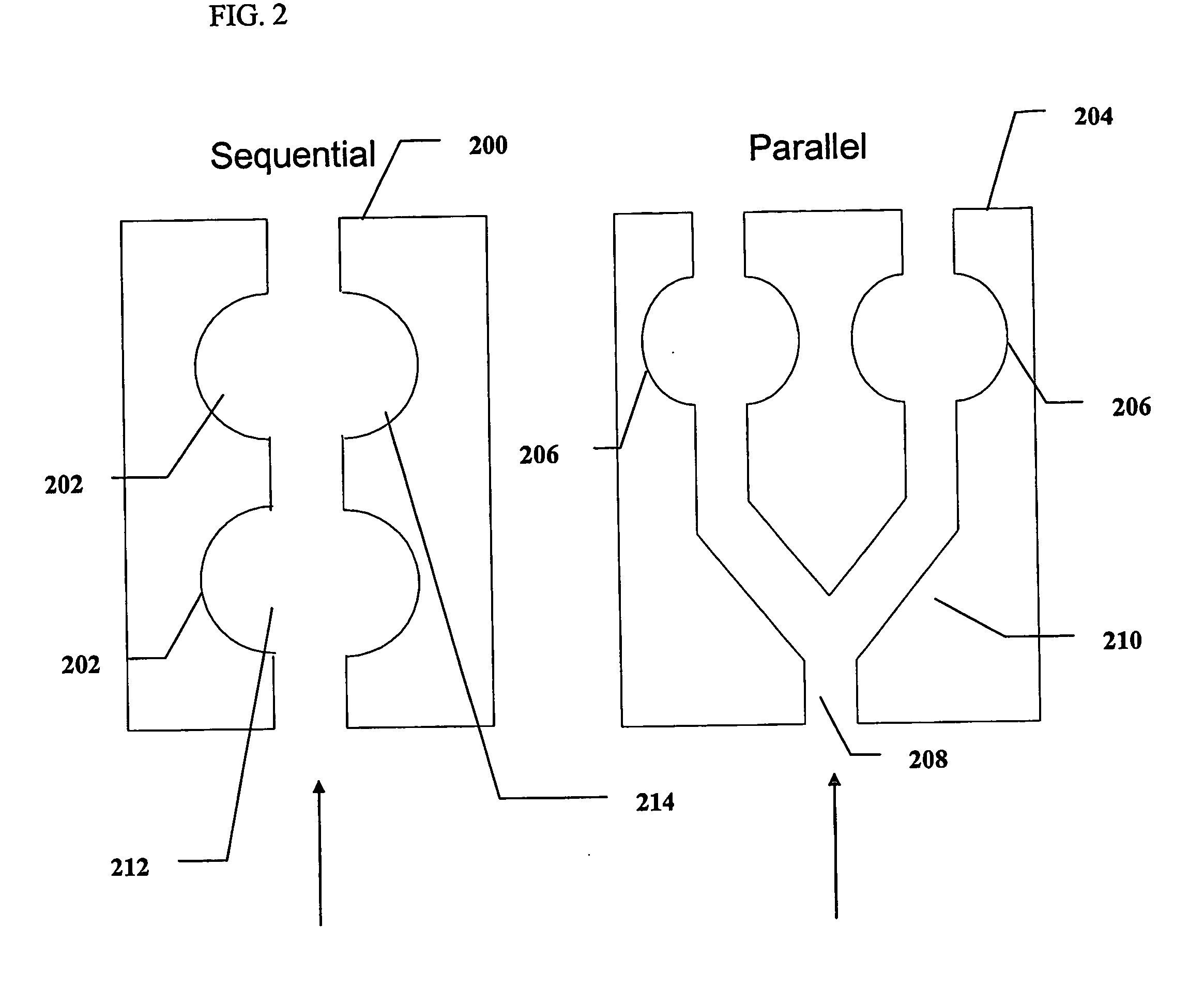
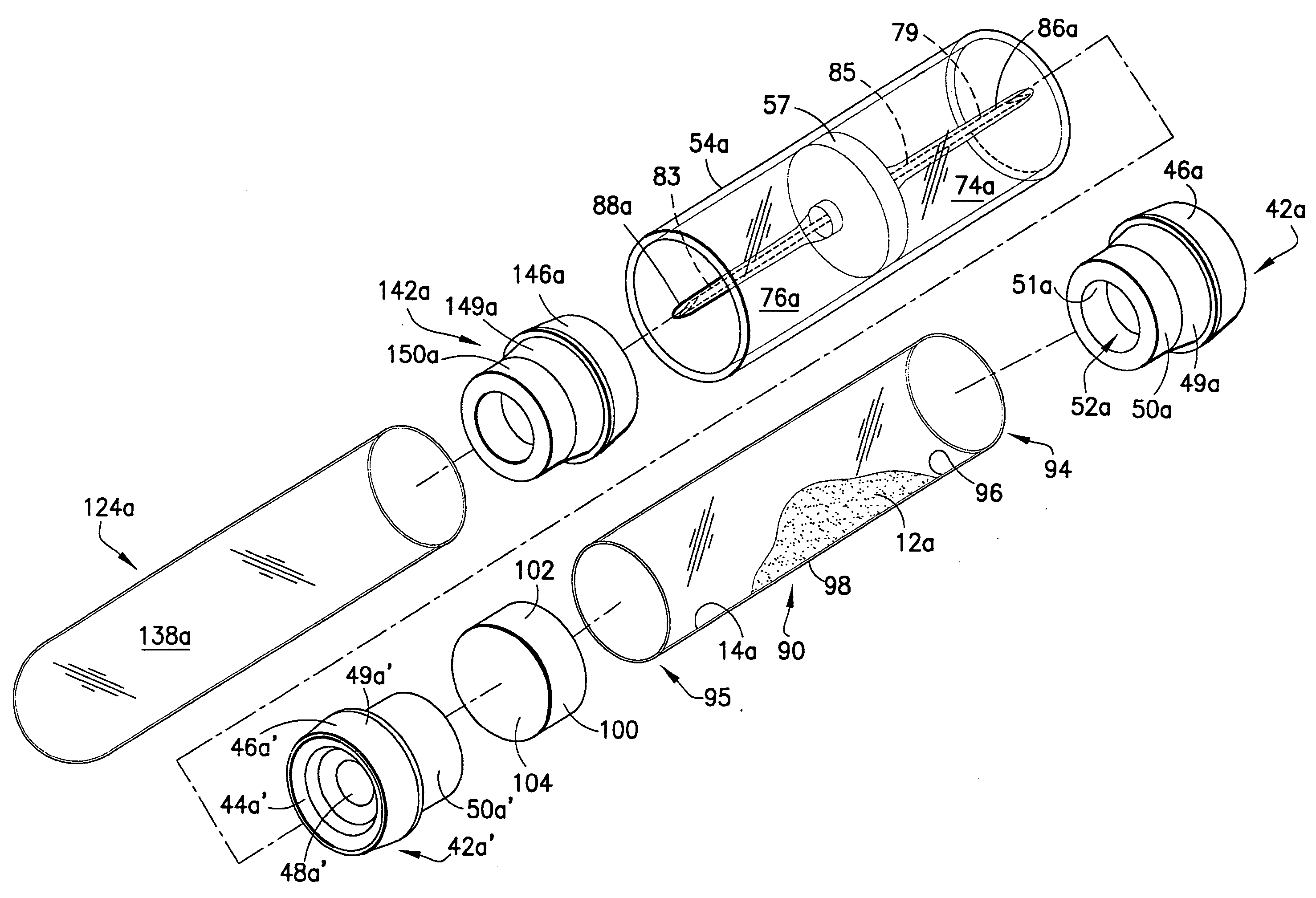
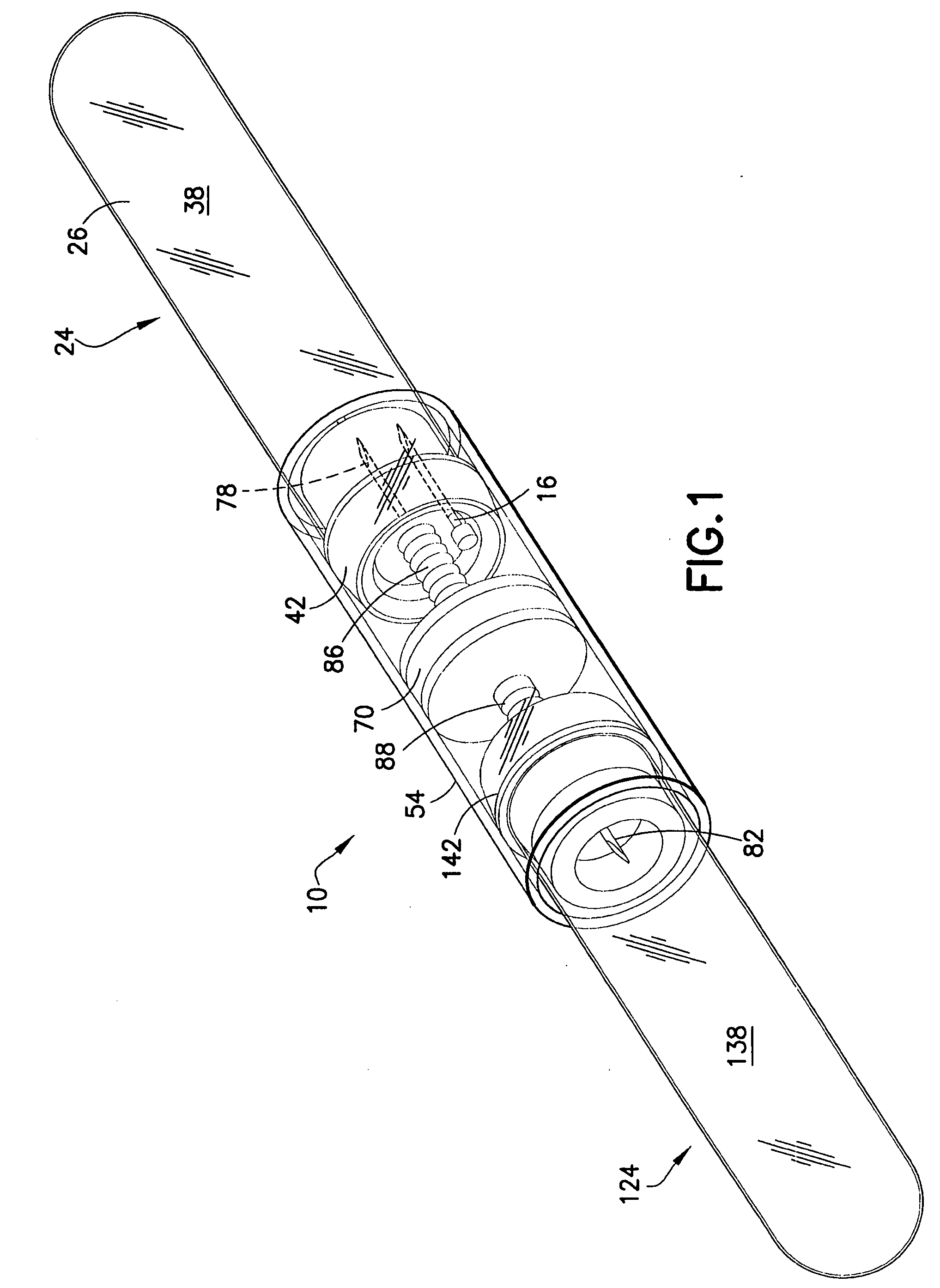
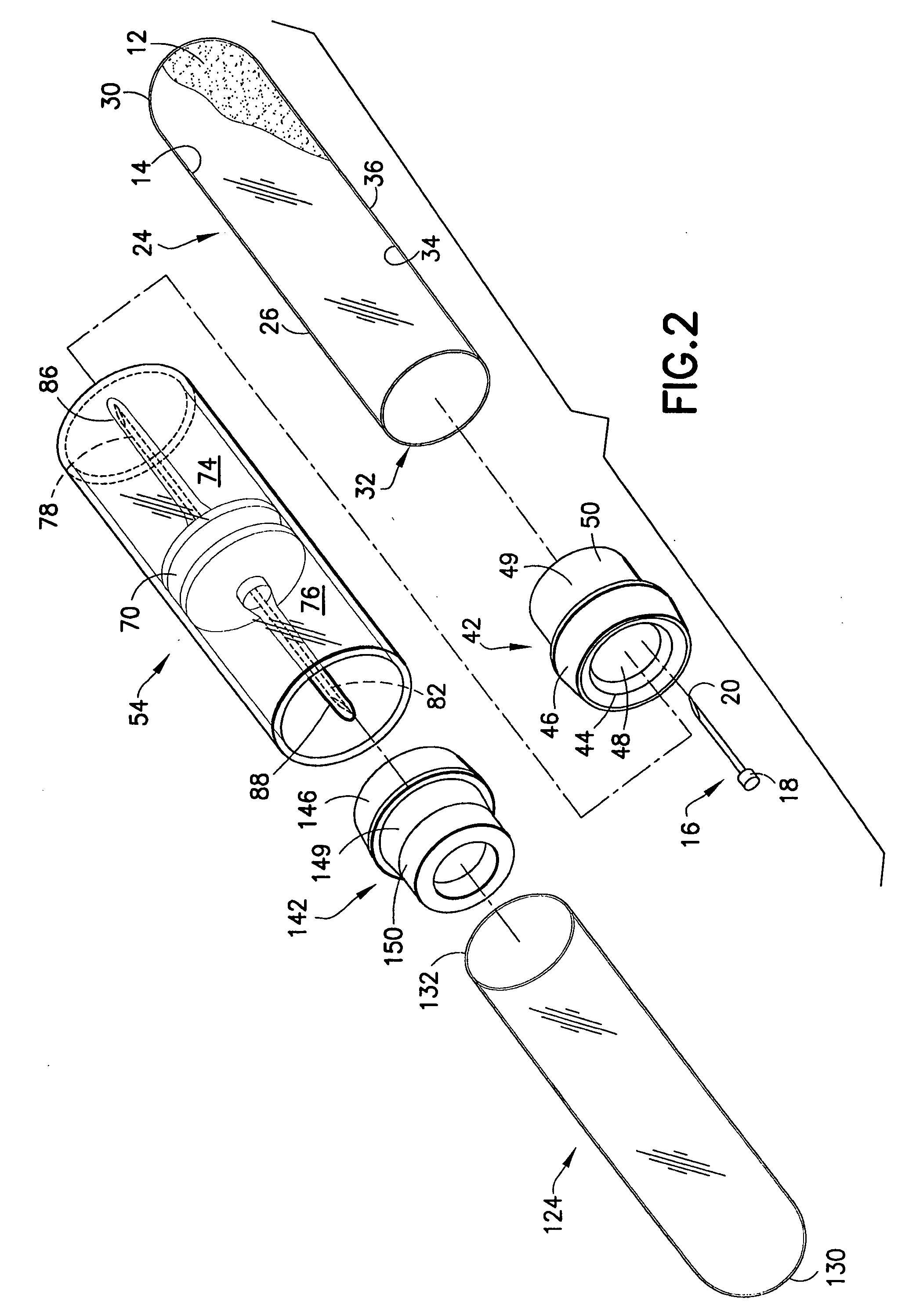
Method and device for sampling and analyzing interstitial fluid and whole blood samples
InactiveUS20070017805A1Less riskLess invasiveSurgeryVaccination/ovulation diagnosticsAnalyteWhole blood sample
Owner:LIFESCAN IP HLDG LLC
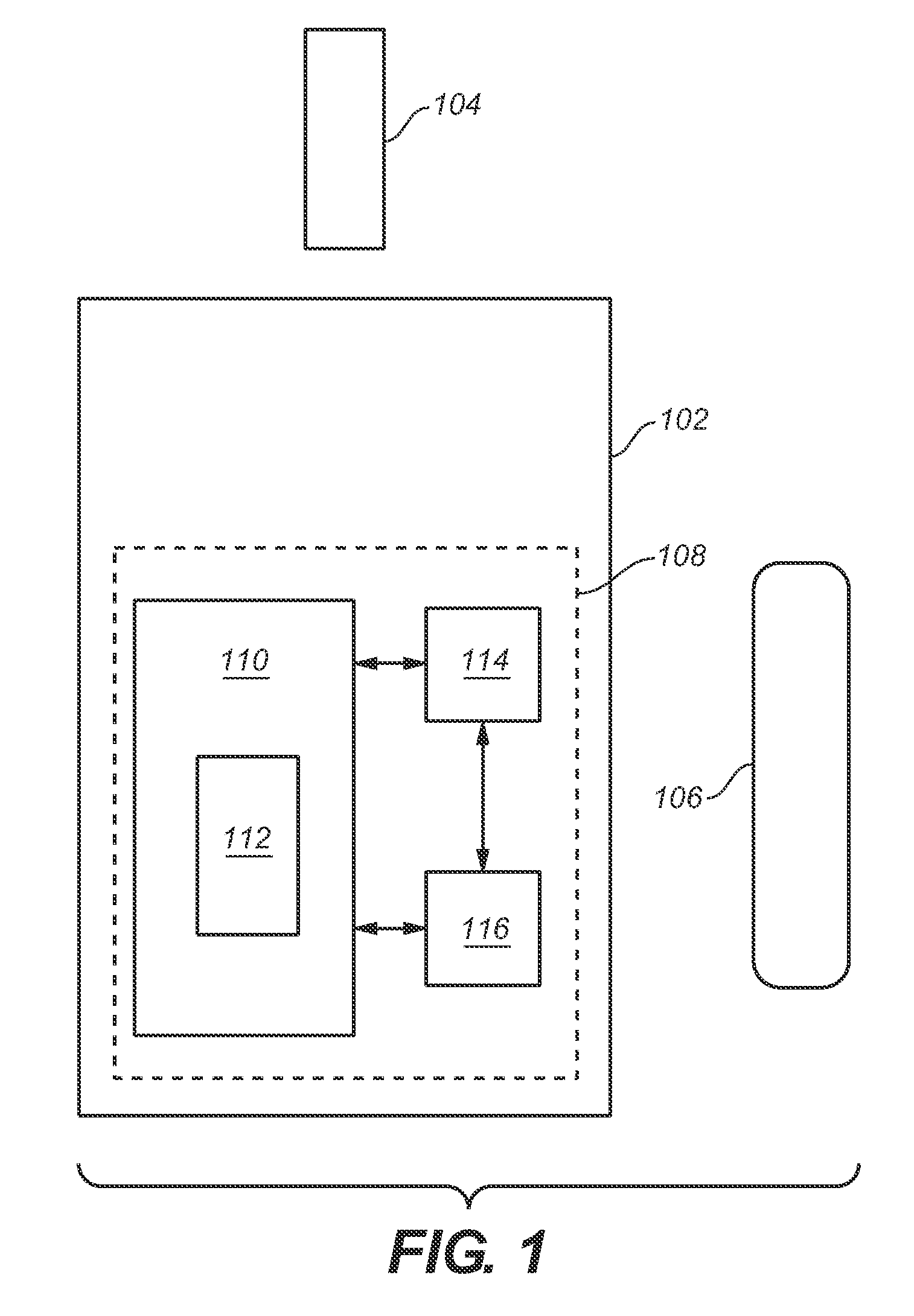
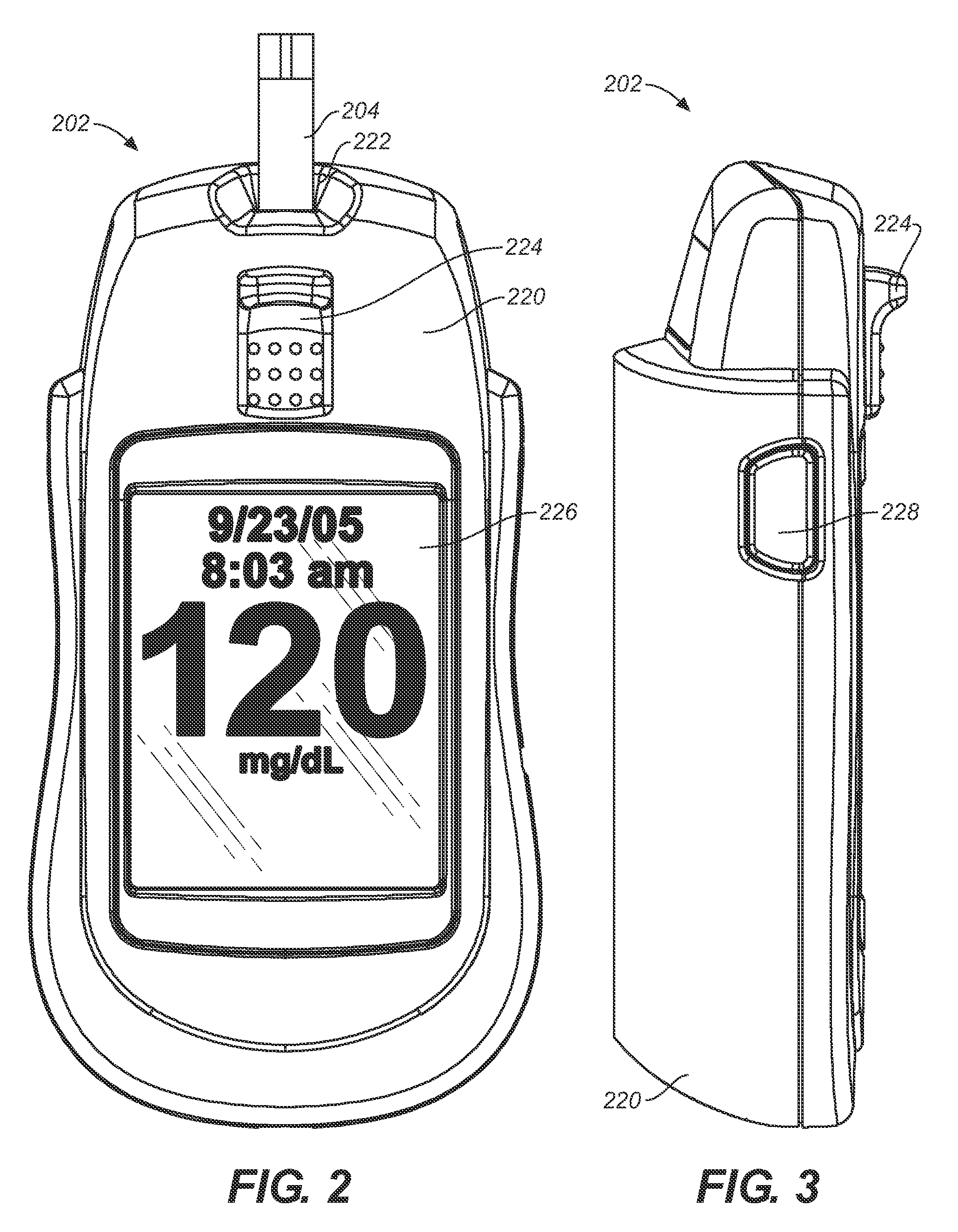
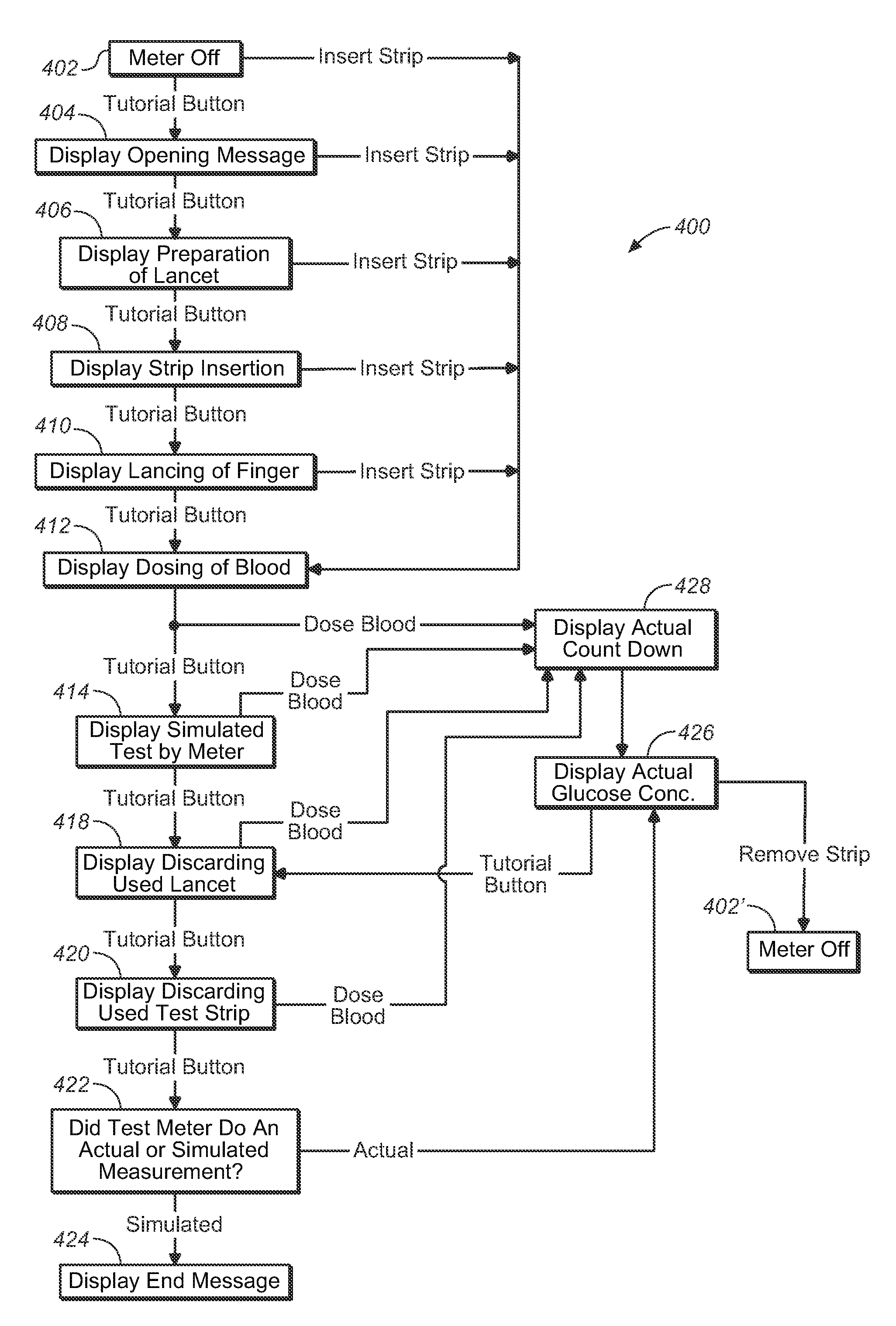
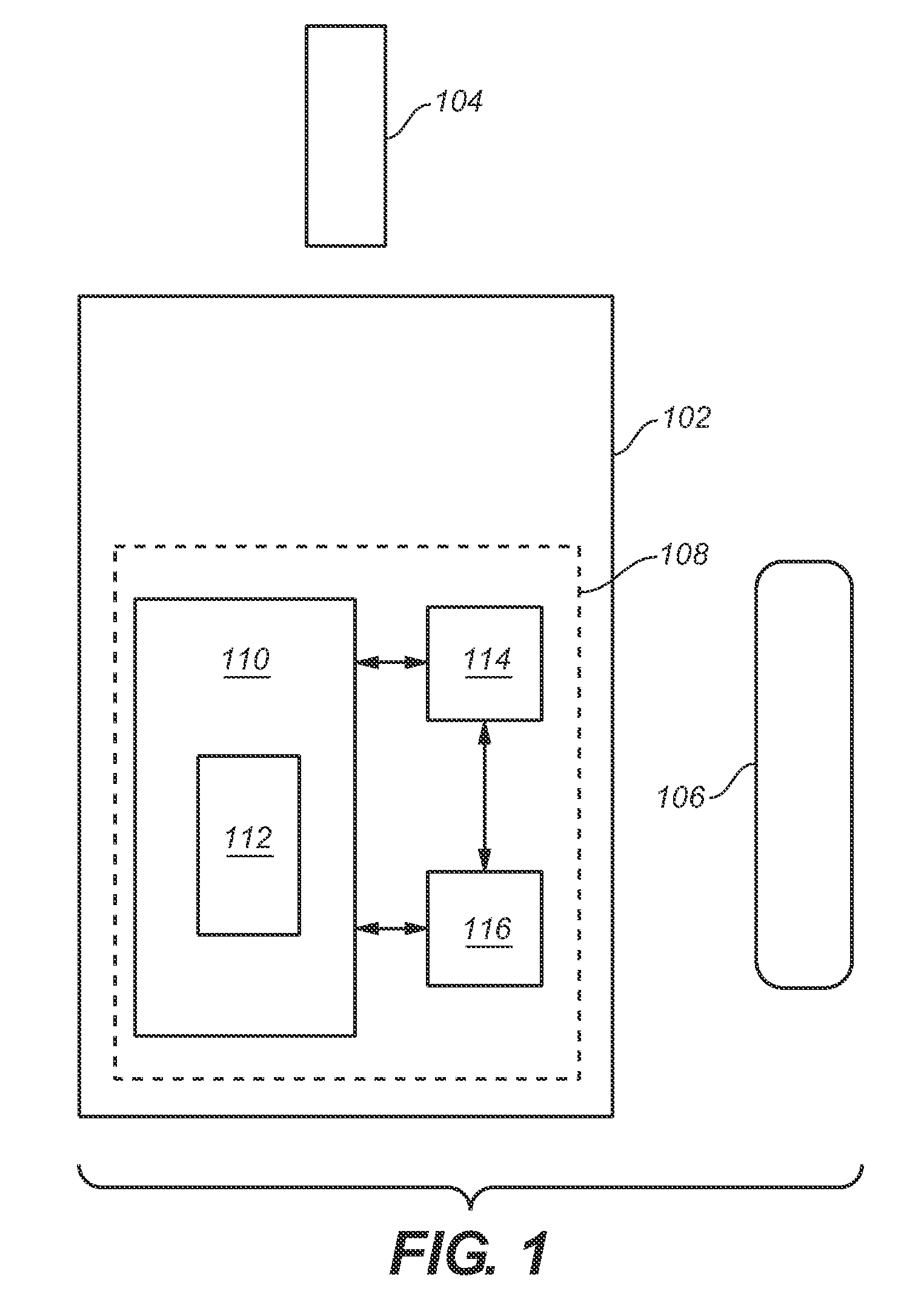
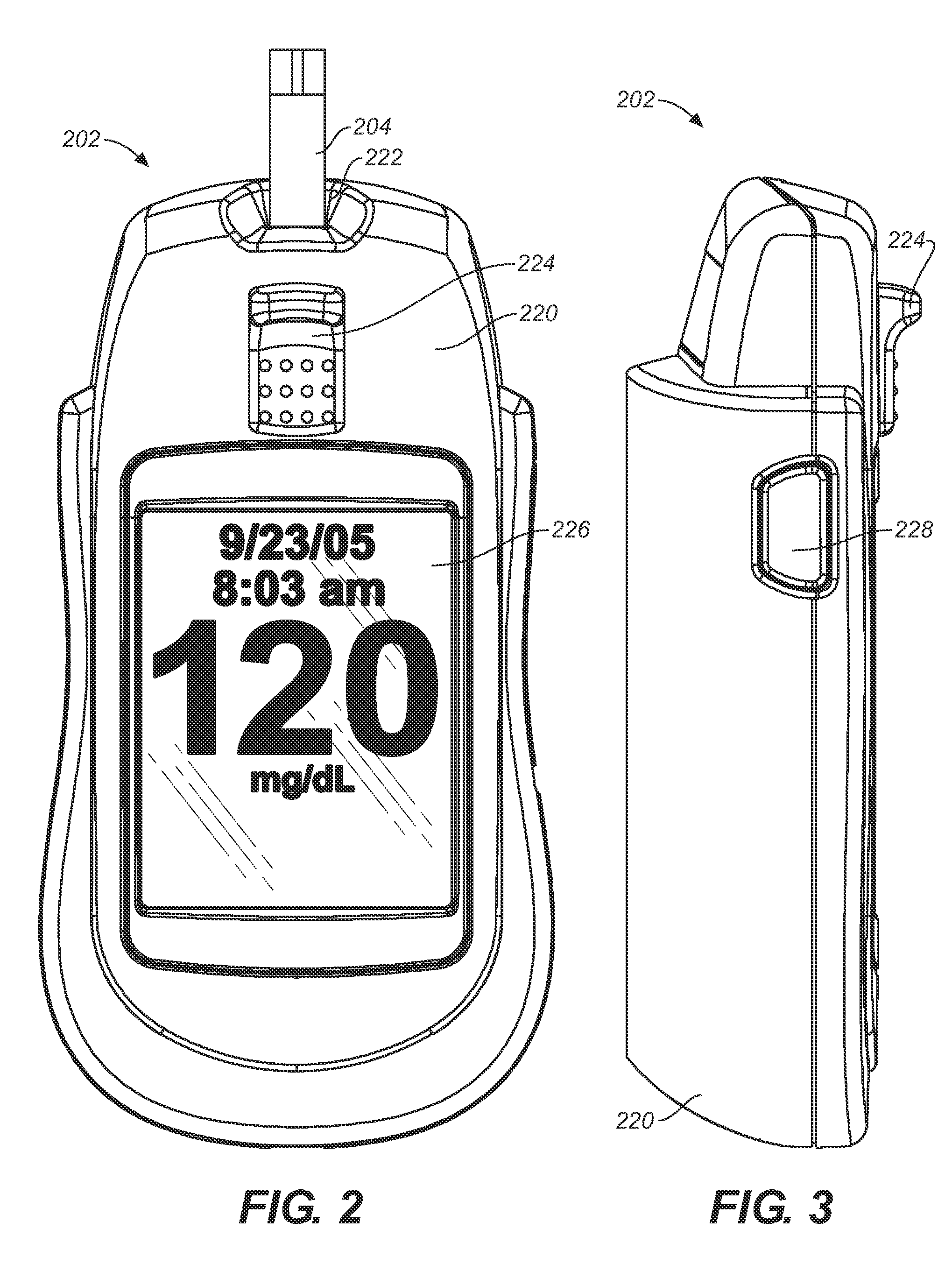
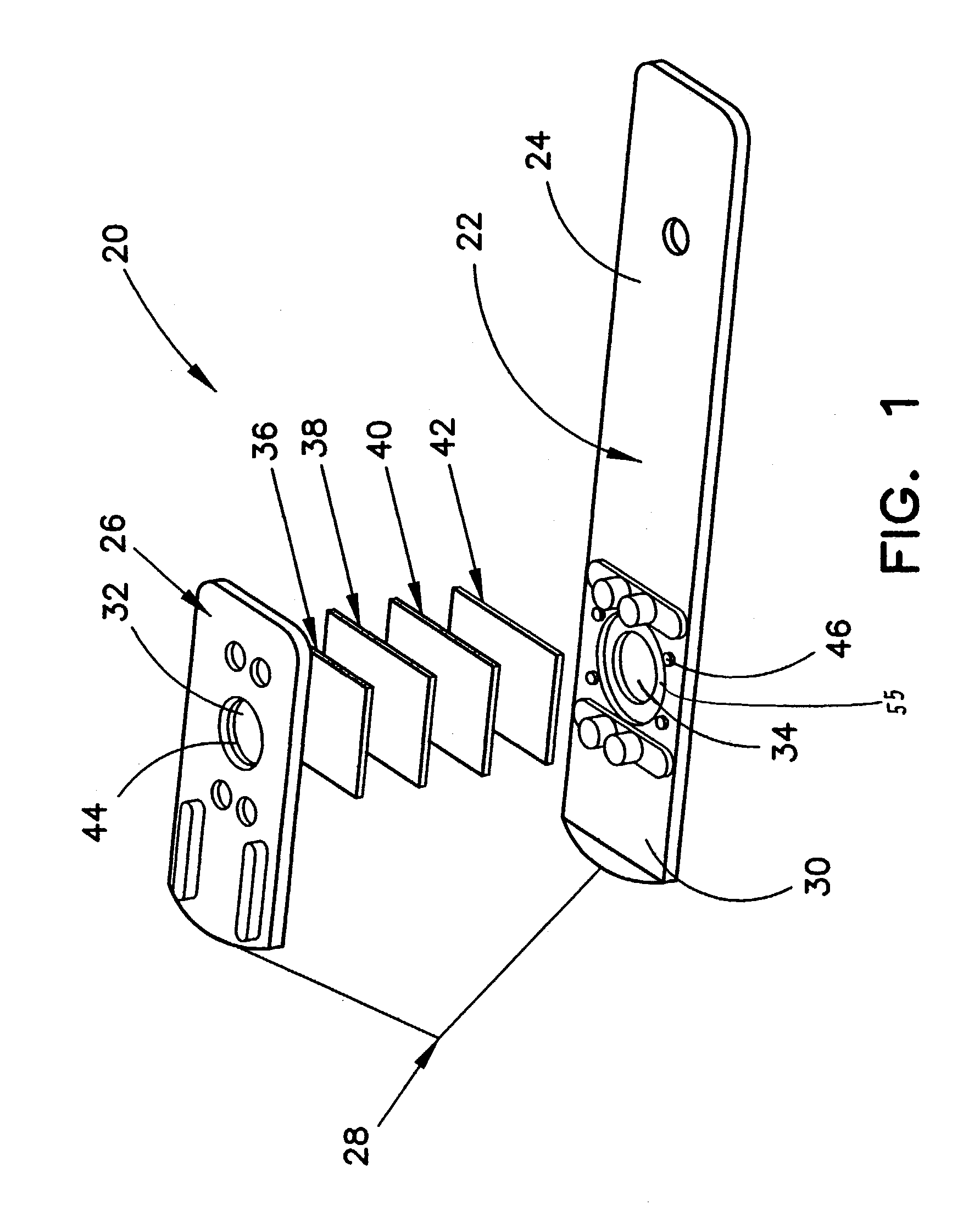
Analytical meter with display-based tutorial module
An analytical meter for determining an analyte (e.g., glucose) in a bodily fluid sample (for example a whole blood sample) includes a display-based tutorial module. In addition, the display-based tutorial module includes a user interface (with a visual display), a memory unit and a microprocessor unit. The memory unit is configured for storing a tutorial, with the tutorial having a plurality of chapters, each containing one or more tutorial images depicting use of the analytical meter. The microprocessor is unit configured for controlling and coordinating at least the user interface and the memory unit. Moreover, the user interface, microprocessor unit and memory unit are operatively linked and configured for event-driven chapter-based display of the tutorial images to a user on the visual display.
Owner:JOHNSON & JOHNSON KK
Event-driven method for tutoring a user in the determination of an analyte in a bodily fluid sample
InactiveUS20080057484A1Eliminates potentialCatheterDiagnostic recording/measuringAnalyteDisplay device
A method for tutoring a user in use of a kit for determining an analyte (such as glucose) in a bodily fluid sample (for example a whole blood sample) includes activating an analytical meter of the kit, with the analytical meter including a display-based tutorial module. In addition, the display-based tutorial module includes a user interface with a visual display, a memory unit storing a tutorial, and a microprocessor unit configured for controlling and coordinating the user interface and the memory unit. Moreover, the tutorial stored in the memory unit has chapters with each of the chapters containing one or more tutorial images depicting use of the kit. In addition, the user interface, microprocessor unit and memory unit are operatively linked and configured for event-driven chapter-based display of the tutorial images to a user on the visual display. The method also includes tutoring the user on use of the kit by displaying the tutorial images in an event-driven chapter-based manner.
Owner:JOHNSON & JOHNSON KK
Method and device for sampling and analyzing interstitial fluid and whole blood samples
InactiveUS20050010137A1Less riskLess invasiveSurgeryVaccination/ovulation diagnosticsAnalyteGlucose polymers
The invention disclosed in this application is a method and device for combining the sampling and analyzing of sub-dermal fluid samples, e.g., interstitial fluid or whole blood, in a device suitable for hospital bedside and home use. It is applicable to any analyte that exists in a usefully representative concentration in the fluid, and is especially suited to the monitoring of glucose.
Owner:HODGES ALASTAIR +2
Apparatus and method for separation of particles suspended in a liquid from the liquid in which they are suspended
InactiveUS20100078384A1Improve concentrationAvoid turbulenceSemi-permeable membranesLiquid separation by electricityUltrasonic sensorCompressibility
A method for separating, or removing, particulate material, e.g., blood cells, from a sample of fluid, e.g., whole blood of a patient, in which the particulate material is suspended. In the case of separating blood cells from blood plasma or blood serum, the resulting samples of blood plasma or blood serum can be used for in vitro diagnostic applications. In normal practice, a whole blood sample of a patient are provided and then introduced into an apparatus that contains a flow channel. An acoustic field, which contains acoustic standing waves from external ultrasonic transducers, is located within the flow channel. Laminar flow is maintained in the flow channel. Blood cells and platelets are separated from blood plasma or blood serum at the end of the flow channel and collected. The method described herein allows fluid components to differentially migrate to areas of preferred acoustic interaction. The parameters that affect separation of particles are size, density, compressibility of the particles, and the fluid surrounding the particles.
Owner:ABBOTT LAB INC
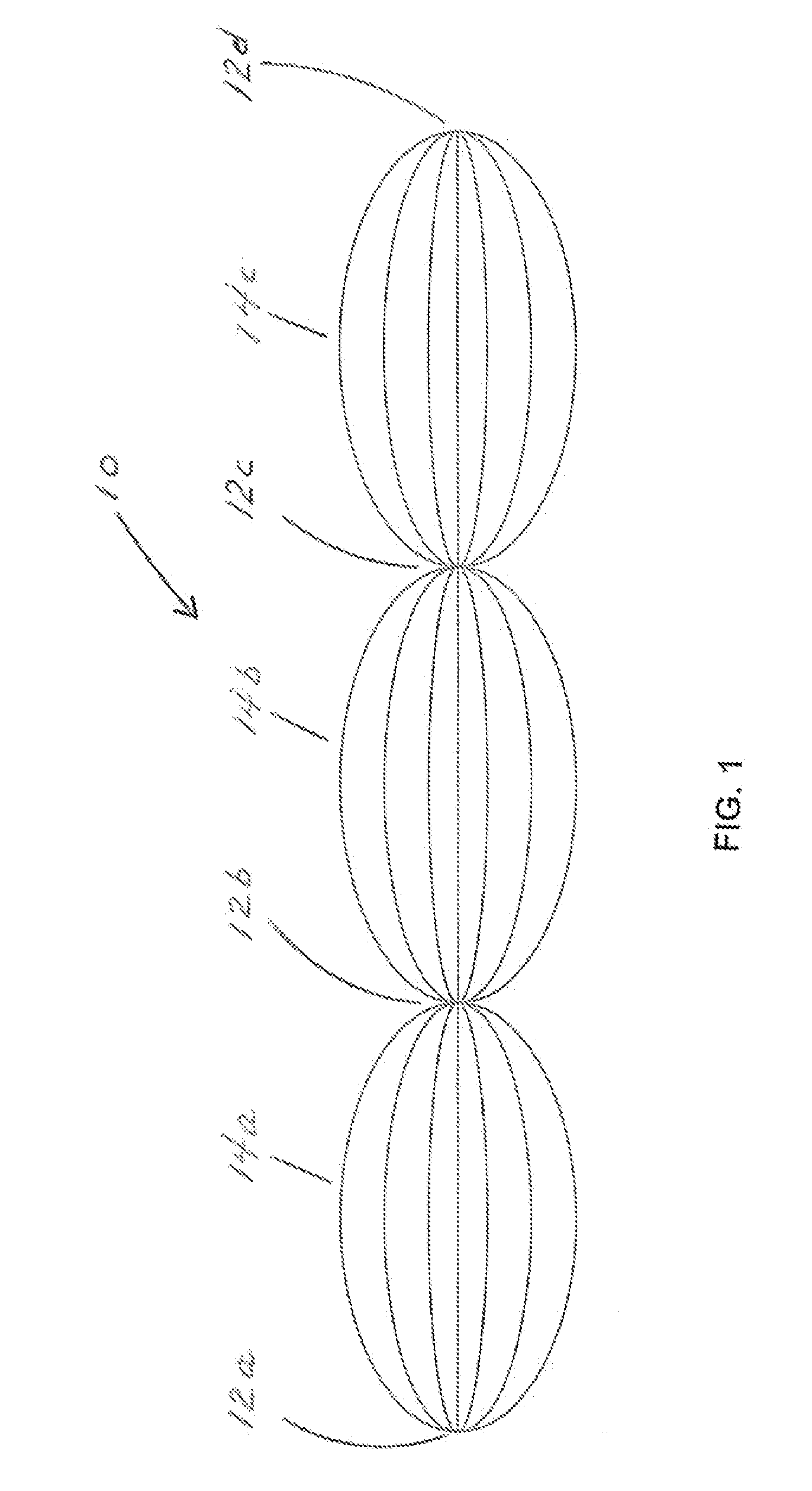
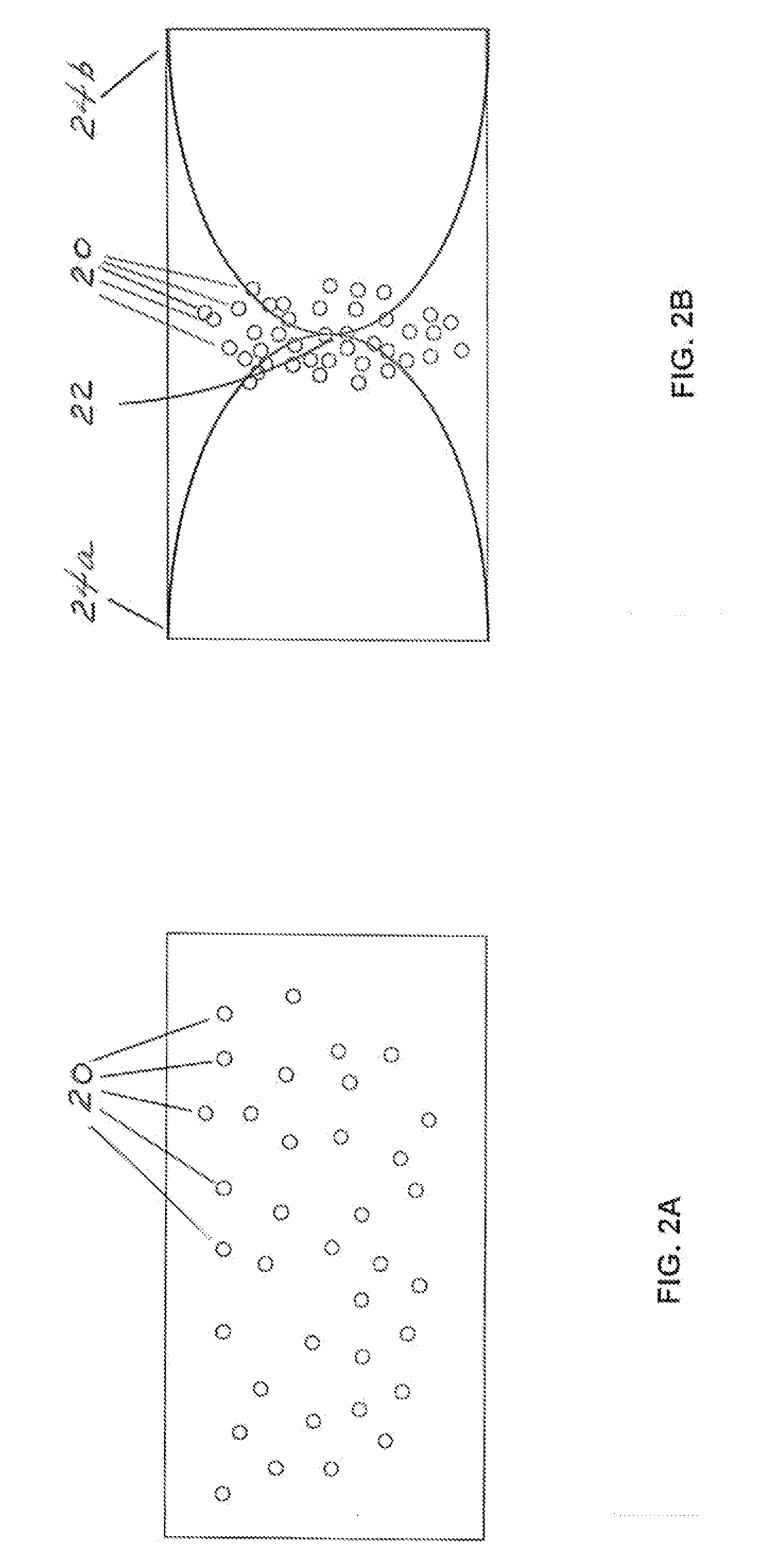
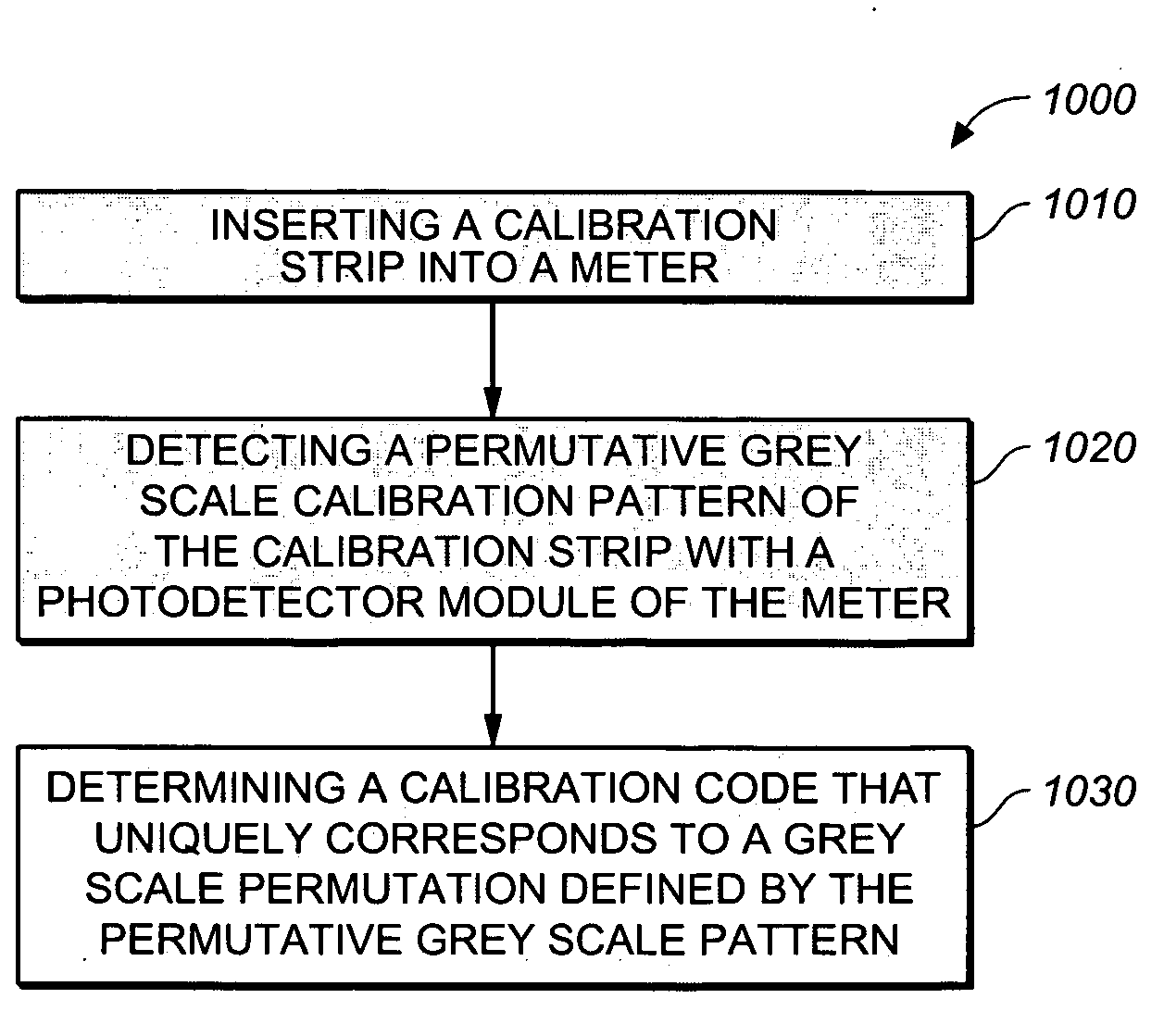
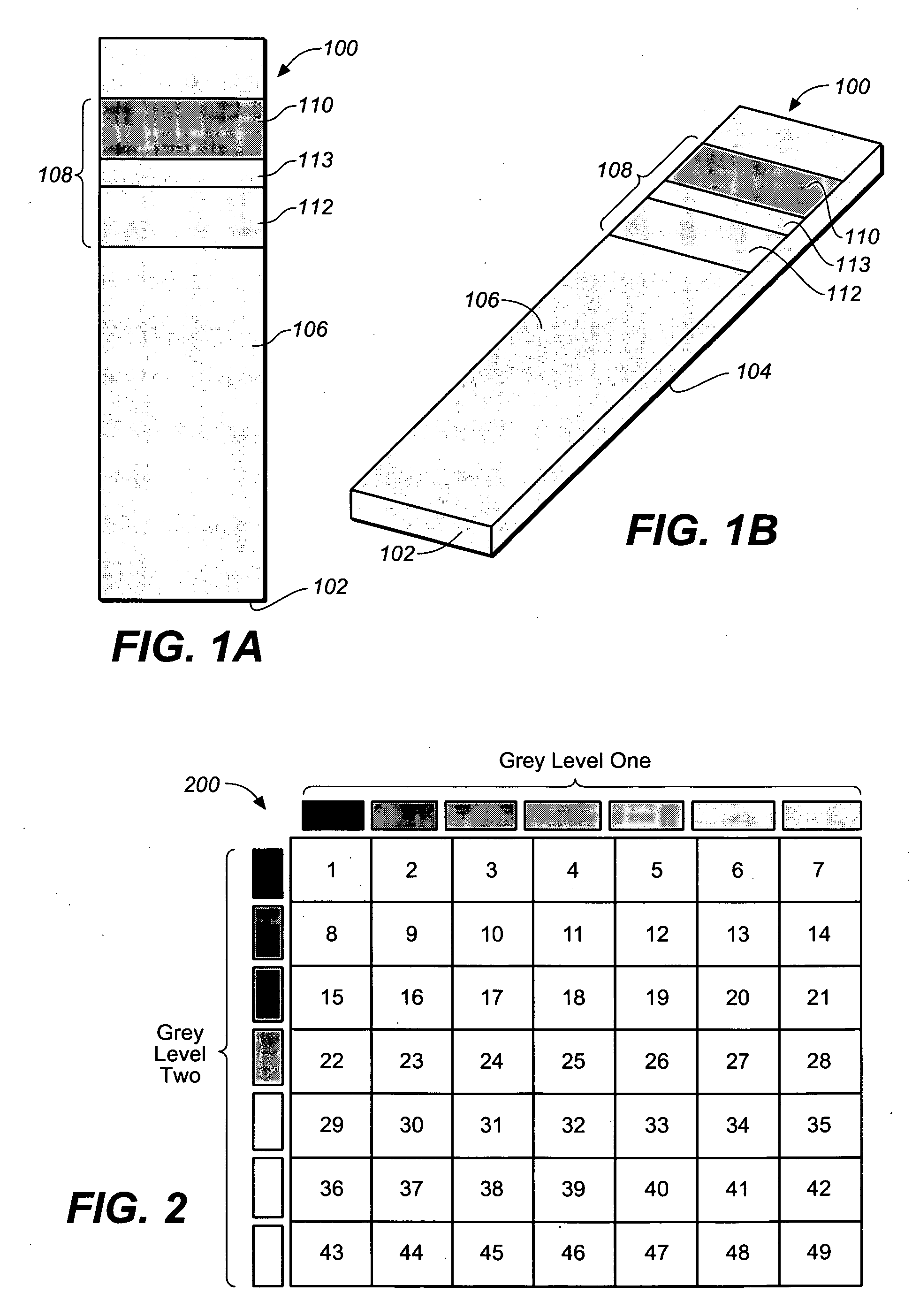
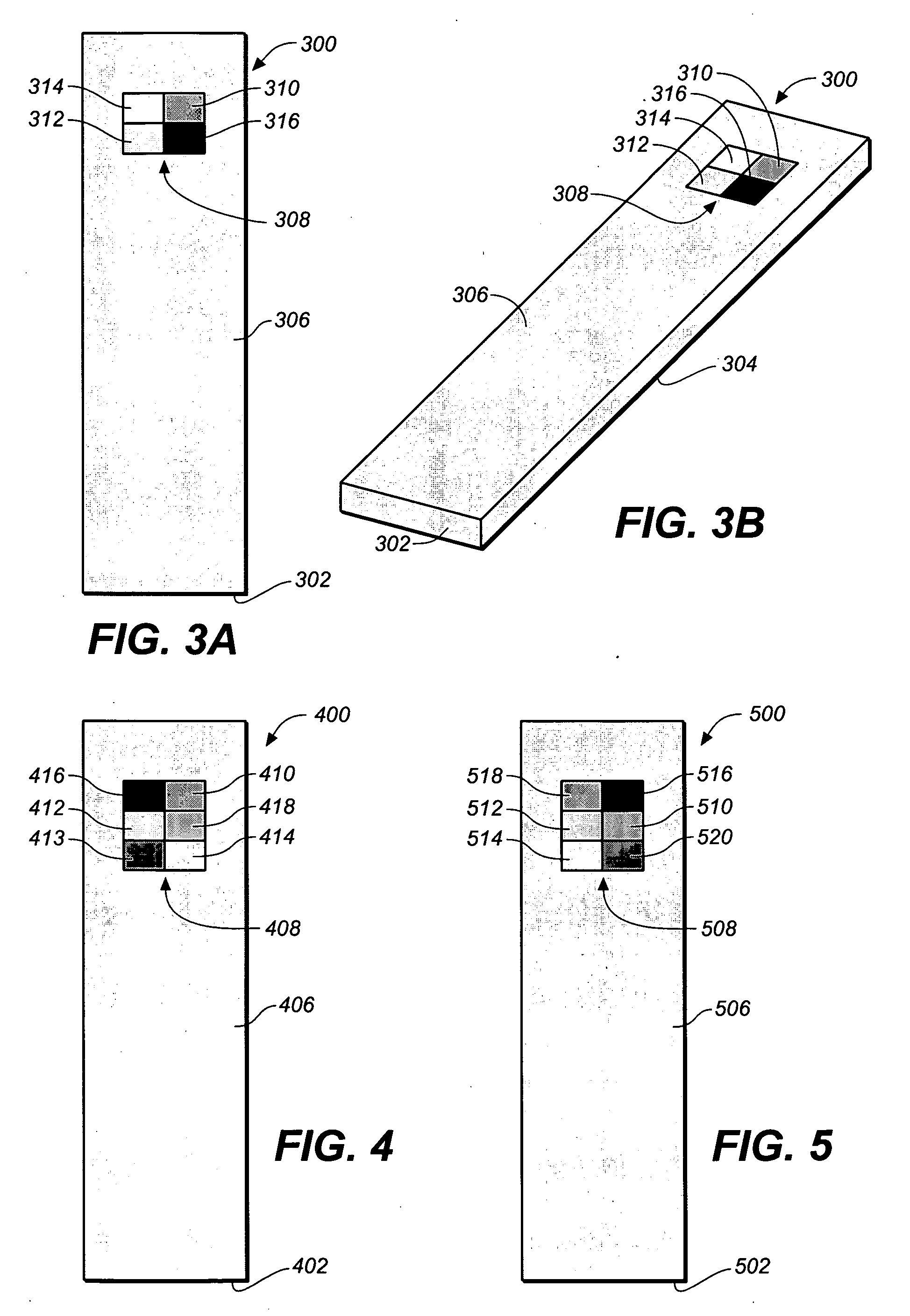
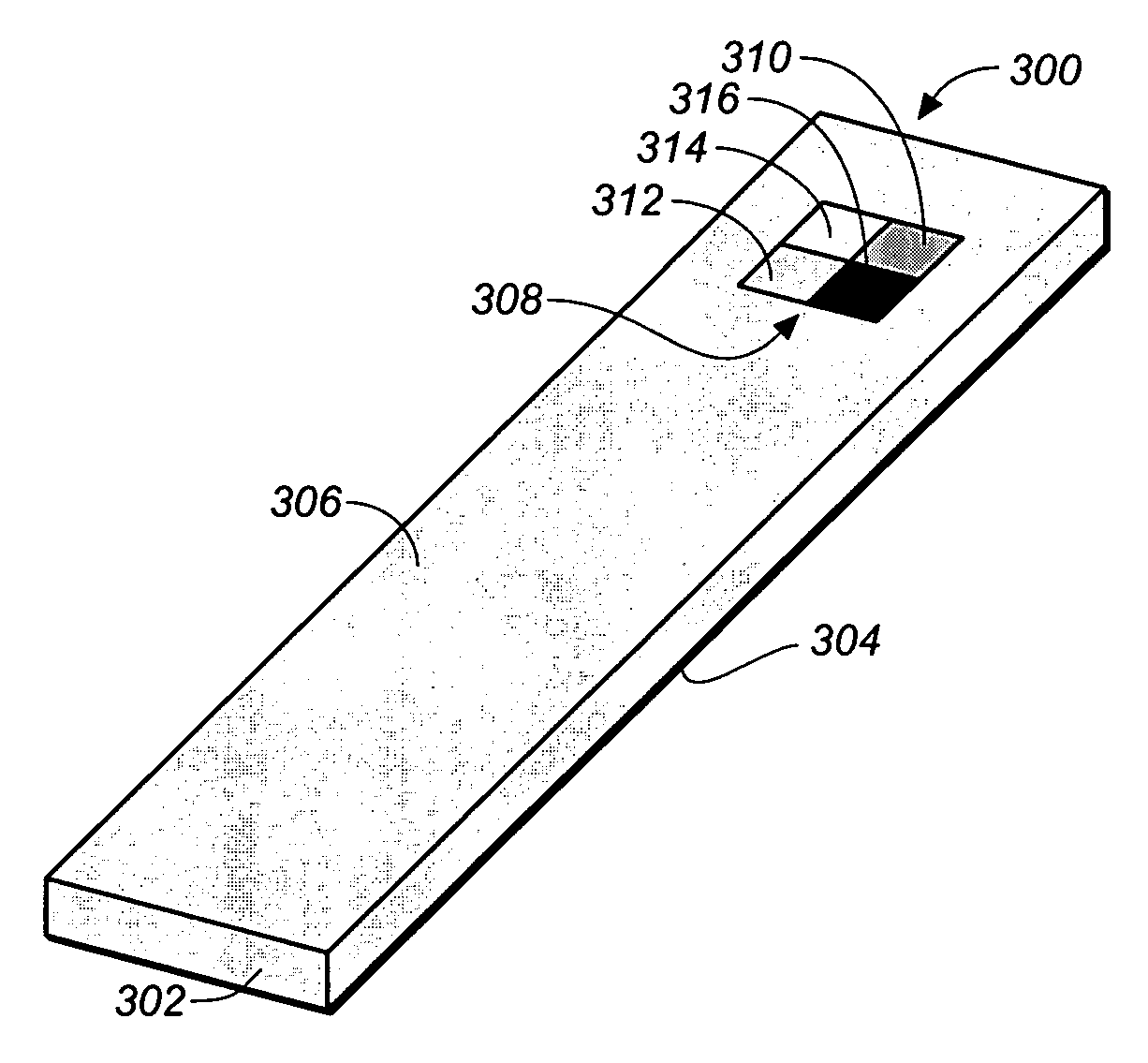
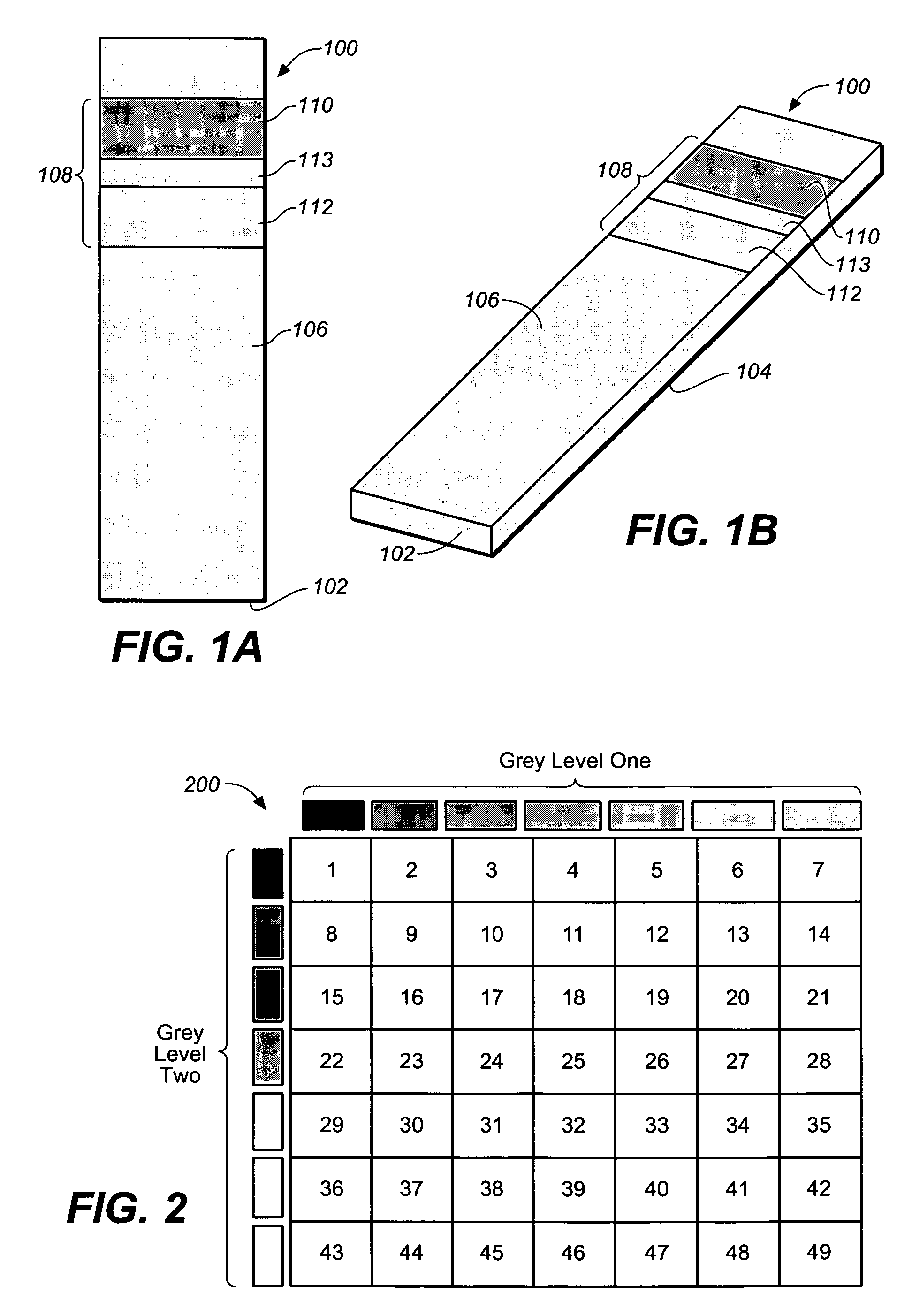
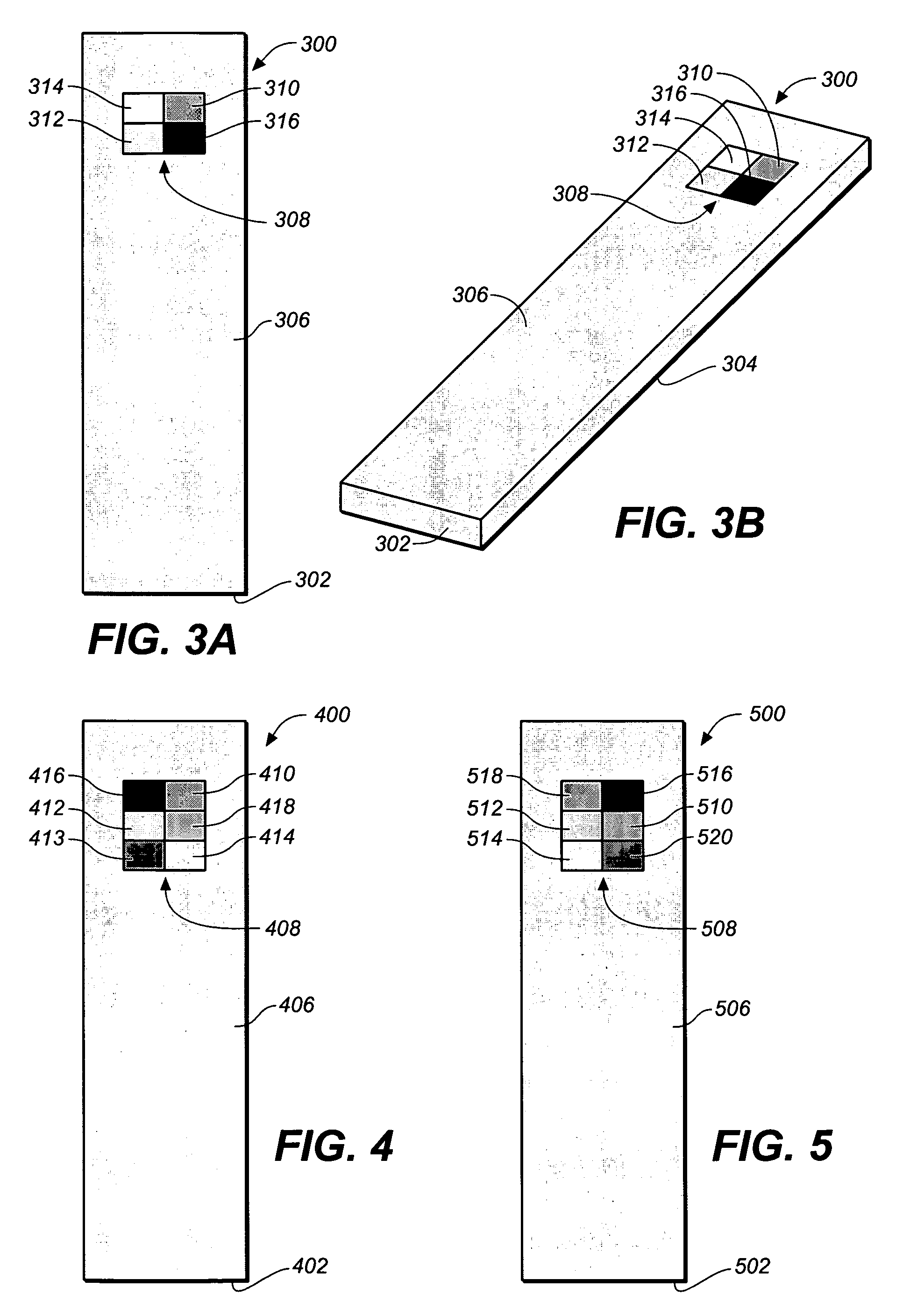
Test strip with permutative grey scale calibration pattern
A test strip for the determination of an analyte, such as glucose, in a body fluid sample (for example, a whole blood sample) includes a substrate with a working surface for receiving the body fluid sample and a reverse surface that is in opposition to the working surface. The test strip also includes a permutative grey scale calibration pattern disposed on either of the working and reverse surfaces with the permutative grey scale calibration pattern including more than one grey scale region. Moreover, the scale regions of the test strip define a grey scale permutation that uniquely corresponds to a calibration code of the test strip.
Owner:LIFESCAN IP HLDG LLC
Method and device for sampling and analyzing interstitial fluid and whole blood samples
The invention disclosed in this application is a method and device for combining the sampling and analyzing of sub-dermal fluid samples, e.g., interstitial fluid or whole blood, in a device suitable for hospital bedside and home use. It is applicable to any analyte that exists in a usefully representative concentration in the fluid, and is especially suited to the monitoring of glucose.
Owner:LIFESCAN INC
Method of selectively determining glycated hemoglobin
InactiveUS7235378B2Easily and accurately determinedEliminate the effects ofMicrobiological testing/measurementBiological testingProteinase activityHemoglobin F
Owner:ARKRAY INC
Kit for the determination of an analyte in a bodily fluid sample that includes a meter with a display-based tutorial module
A kit for determining an analyte (e.g., glucose) in a bodily fluid sample (for example a whole blood sample) includes a meter and analytical test strip(s). In addition, the meter includes a display-based tutorial module with a memory unit, a microprocessor unit and a user interface that includes a visual display. The memory unit is configured for storing a tutorial, while the microprocessor unit is configured for controlling and coordinating the user interface and the memory unit. In addition, the tutorial stored in the memory unit has chapters, with each of the chapters containing one or more tutorial images depicting use of the kit. Moreover, the analytical test strip is configured for the application of a bodily fluid sample thereon and for insertion in the meter for subsequent determination of the analyte. Furthermore, the user interface, microprocessor unit and memory unit are operatively linked and configured for event-driven chapter-based display of the tutorial images to a user on the visual display.
Owner:JOHNSON & JOHNSON KK
Test strip with permutative grey scale calibration pattern
A test strip for the determination of an analyte, such as glucose, in a body fluid sample (for example, a whole blood sample) includes a substrate with a working surface for receiving the body fluid sample and a reverse surface that is in opposition to the working surface. The test strip also includes a permutative grey scale calibration pattern disposed on either of the working and reverse surfaces with the permutative grey scale calibration pattern including more than one grey scale region. Moreover, the scale regions of the test strip define a grey scale permutation that uniquely corresponds to a calibration code of the test strip.
Owner:LIFESCAN IP HLDG LLC
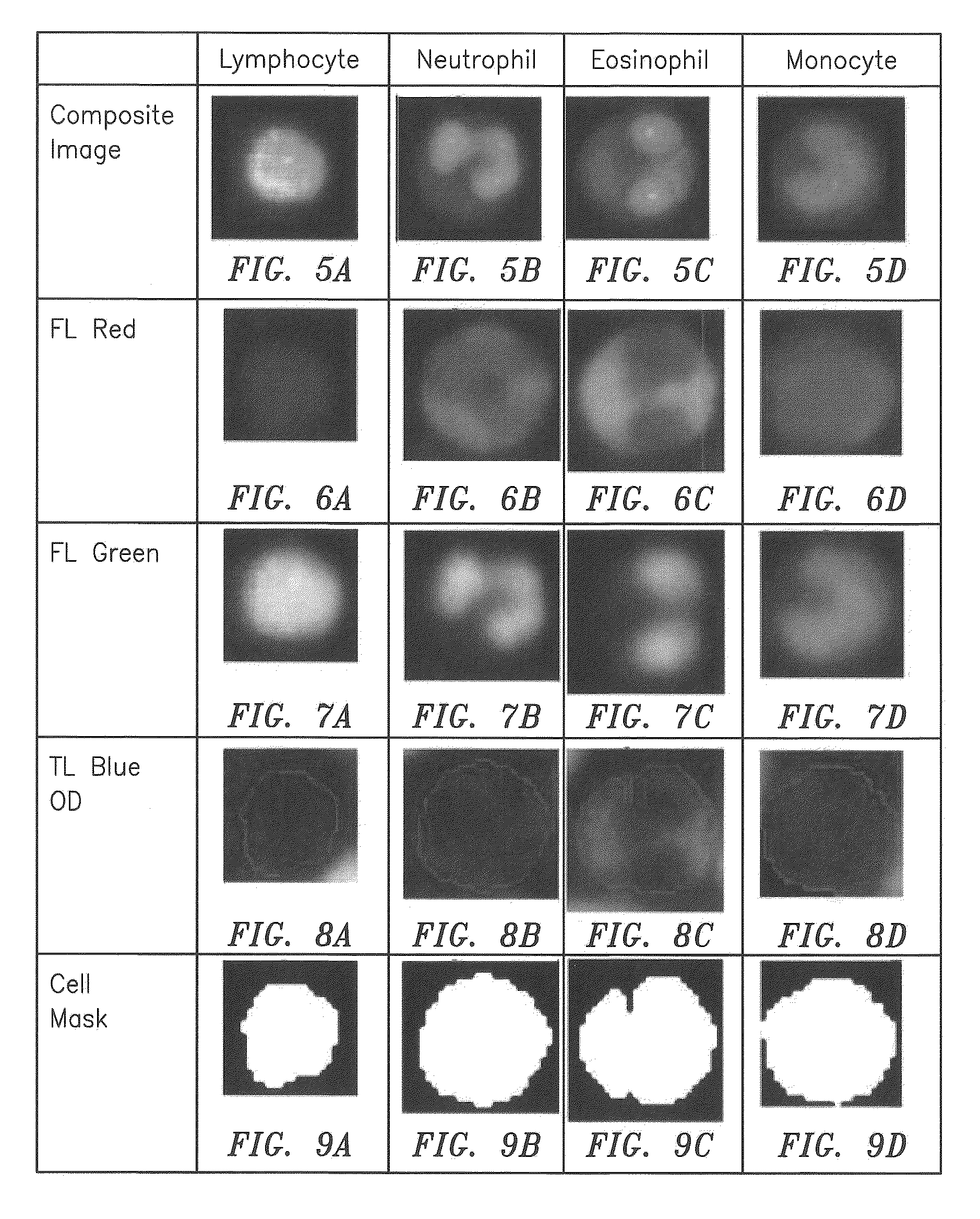
Method and apparatus for automated whole blood sample analyses from microscopy images
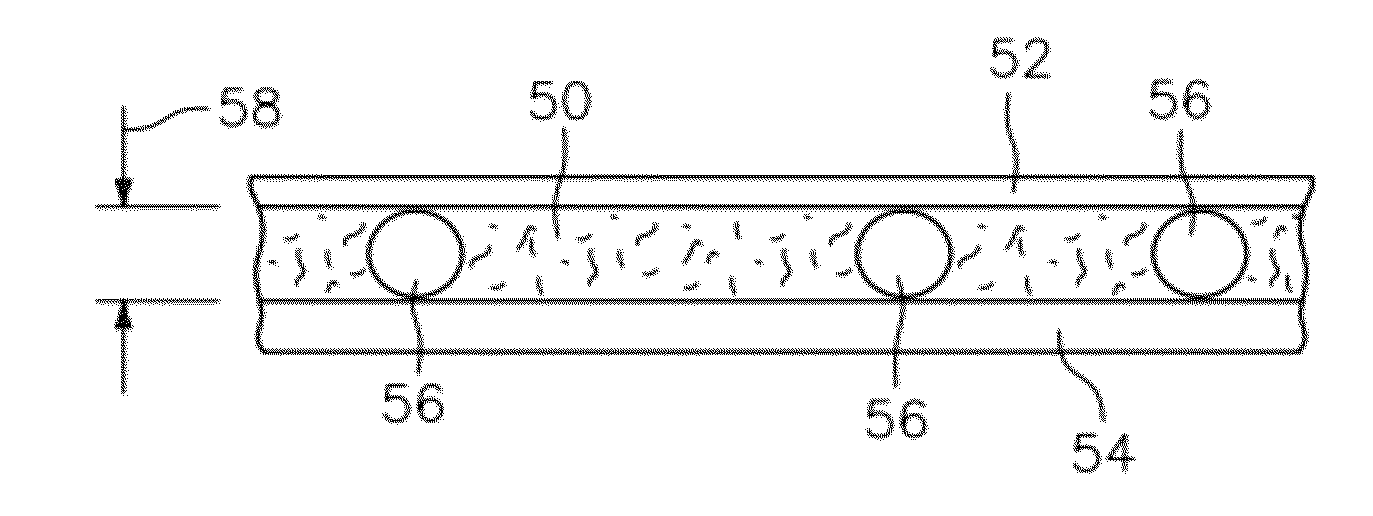
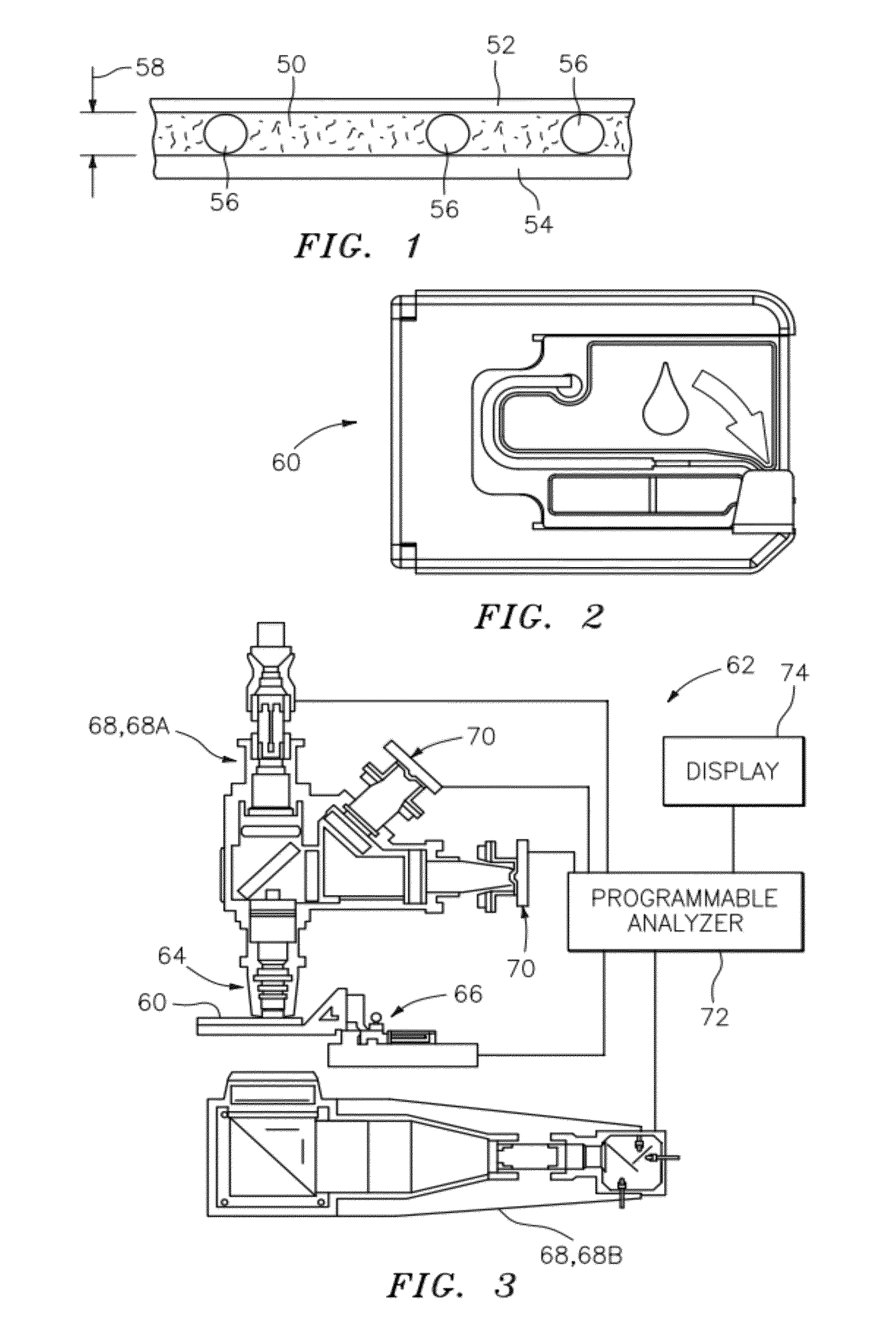
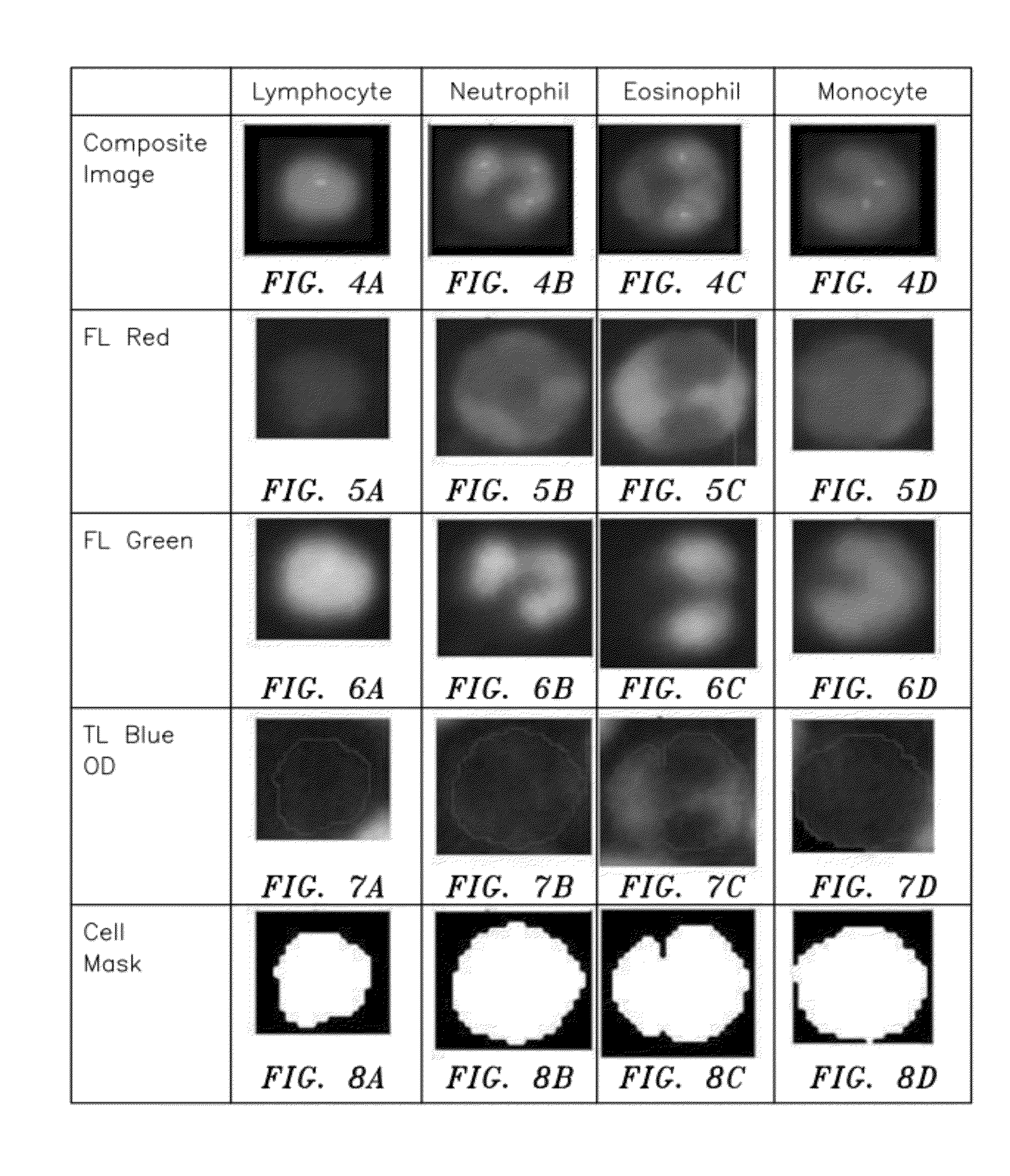
ActiveUS20120034647A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBlood gas analysisWhite blood cell
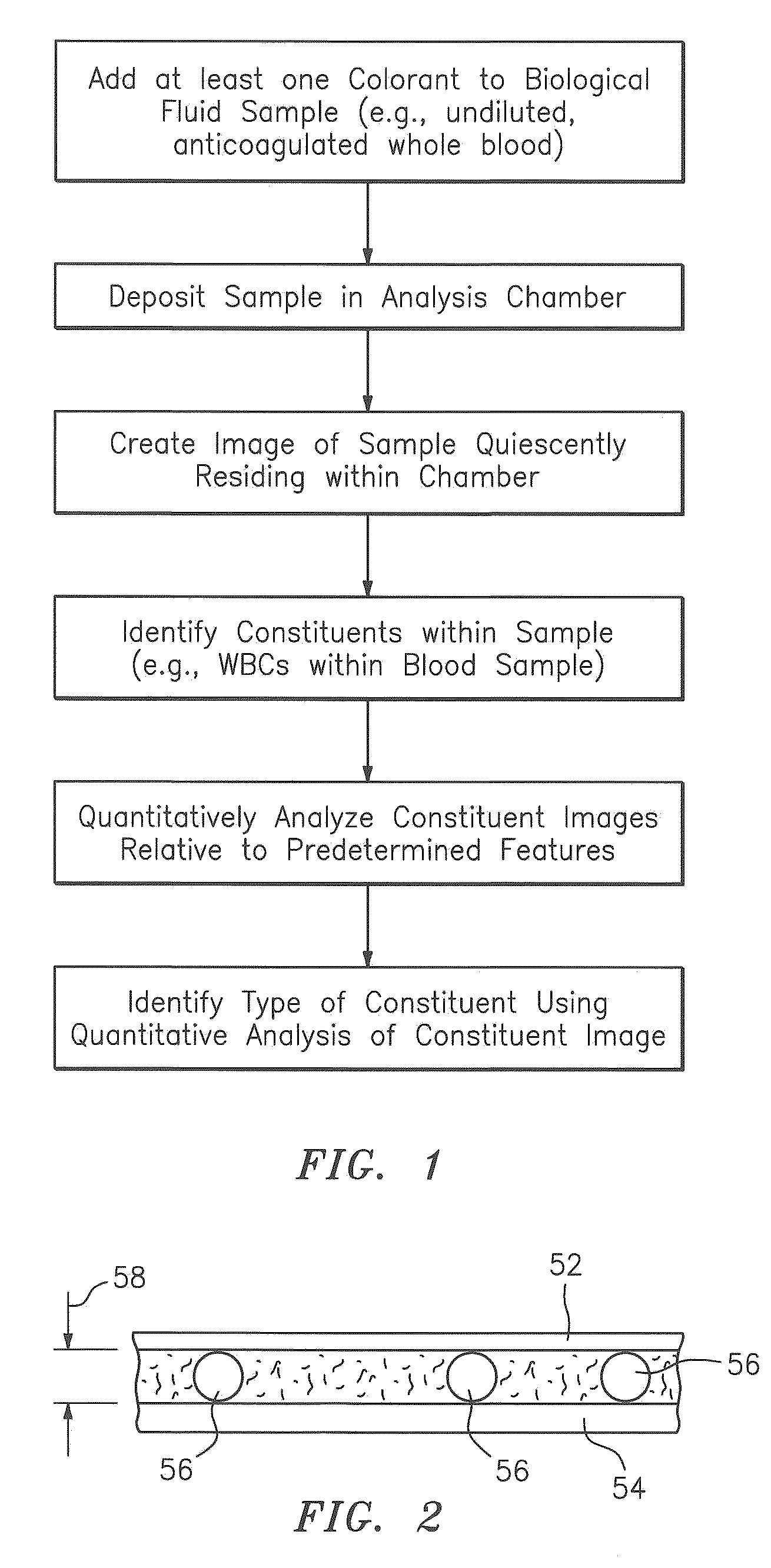
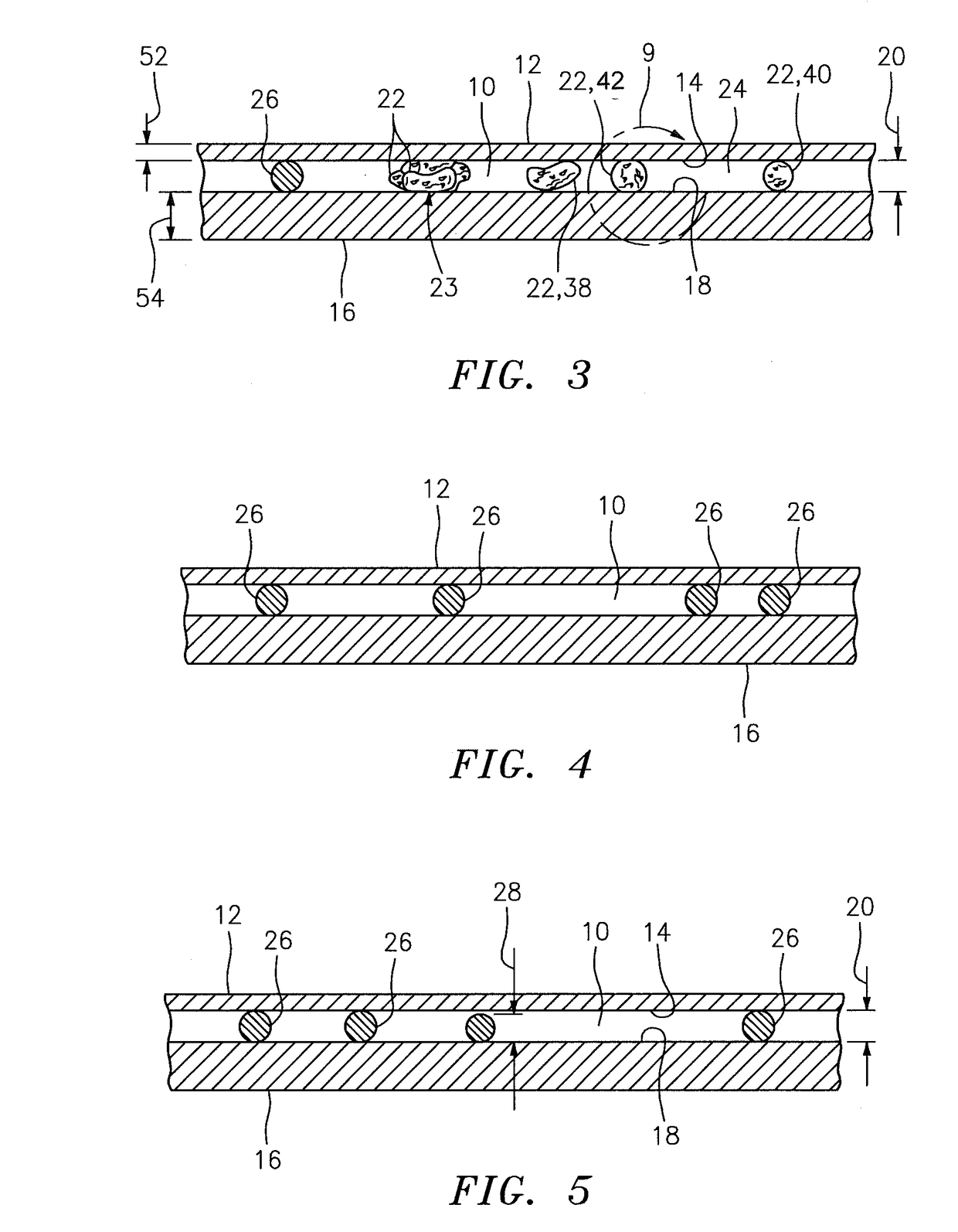
A method and apparatus for identifying one or more target constituents (e.g., white blood cells) within a biological sample is provided. The method includes the steps of: a) adding at least one colorant to the sample; b) disposing the sample into a chamber defined by at least one transparent panel; c) creating at least one image of the sample quiescently residing within the chamber; d) identifying target constituents within the sample image; e) quantitatively analyzing at least some of the identified target constituents within the image relative to one or more predetermined quantitatively determinable features; and f) identifying at least one type of target constituent within the identified target constituents using the quantitatively determinable features.
Owner:ABBOTT POINT CARE
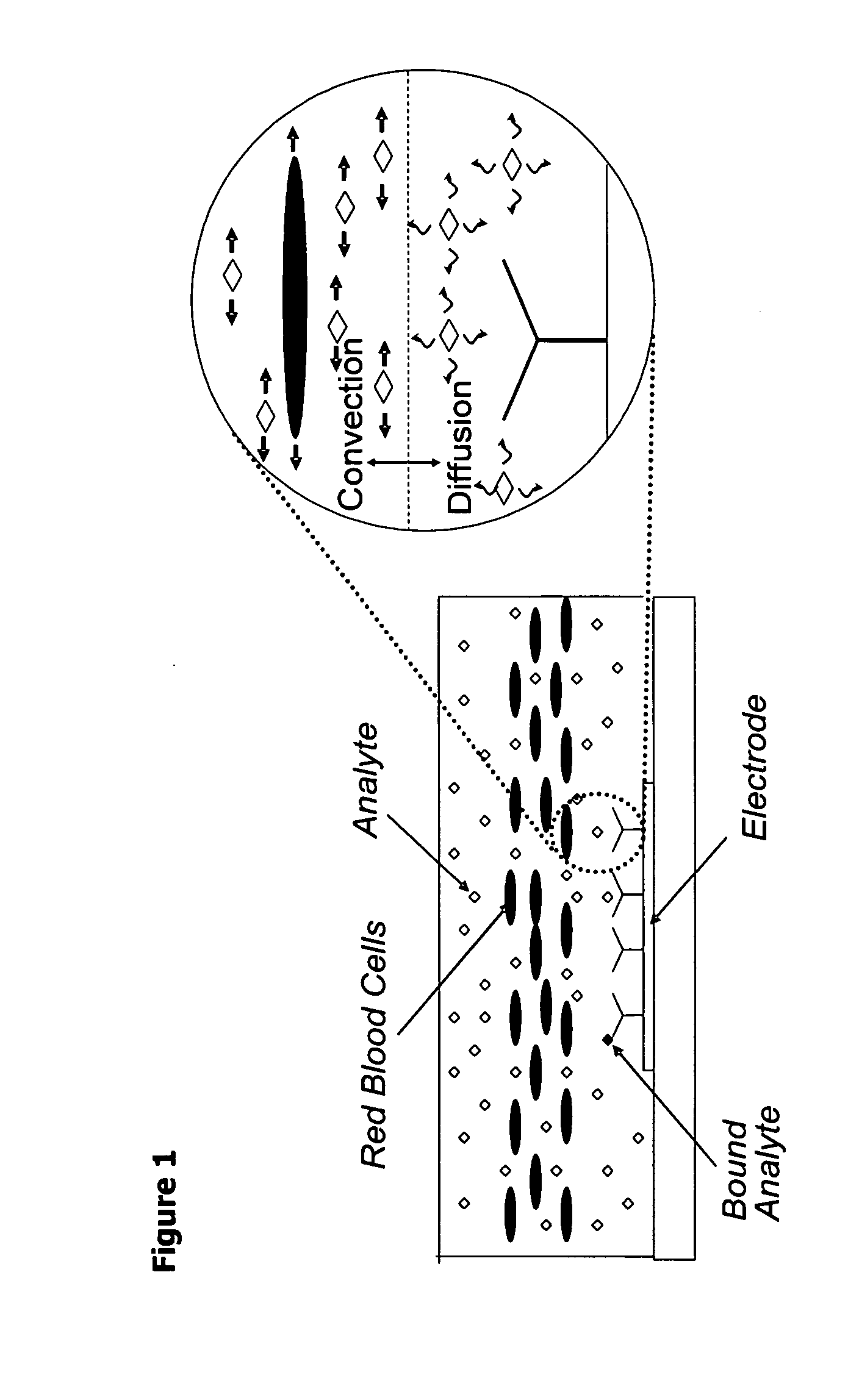
Methods and apparatuses for conducting assays
ActiveUS20060019319A1Bioreactor/fermenter combinationsBiological substance pretreatmentsParticulatesAnalyte
Disclosed are methods for conducting assays of samples, such as whole blood, that may contain cells or other particulate matter. Also disclosed are systems, devices, equipment, kits and reagents for use in such methods. One advantage of certain disclosed methods and systems is the ability to rapidly measure the concentration of an analyte of interest in blood plasma from a whole blood sample without blood separation and hematocrit correction.
Owner:MESO SCALE TECH LLC
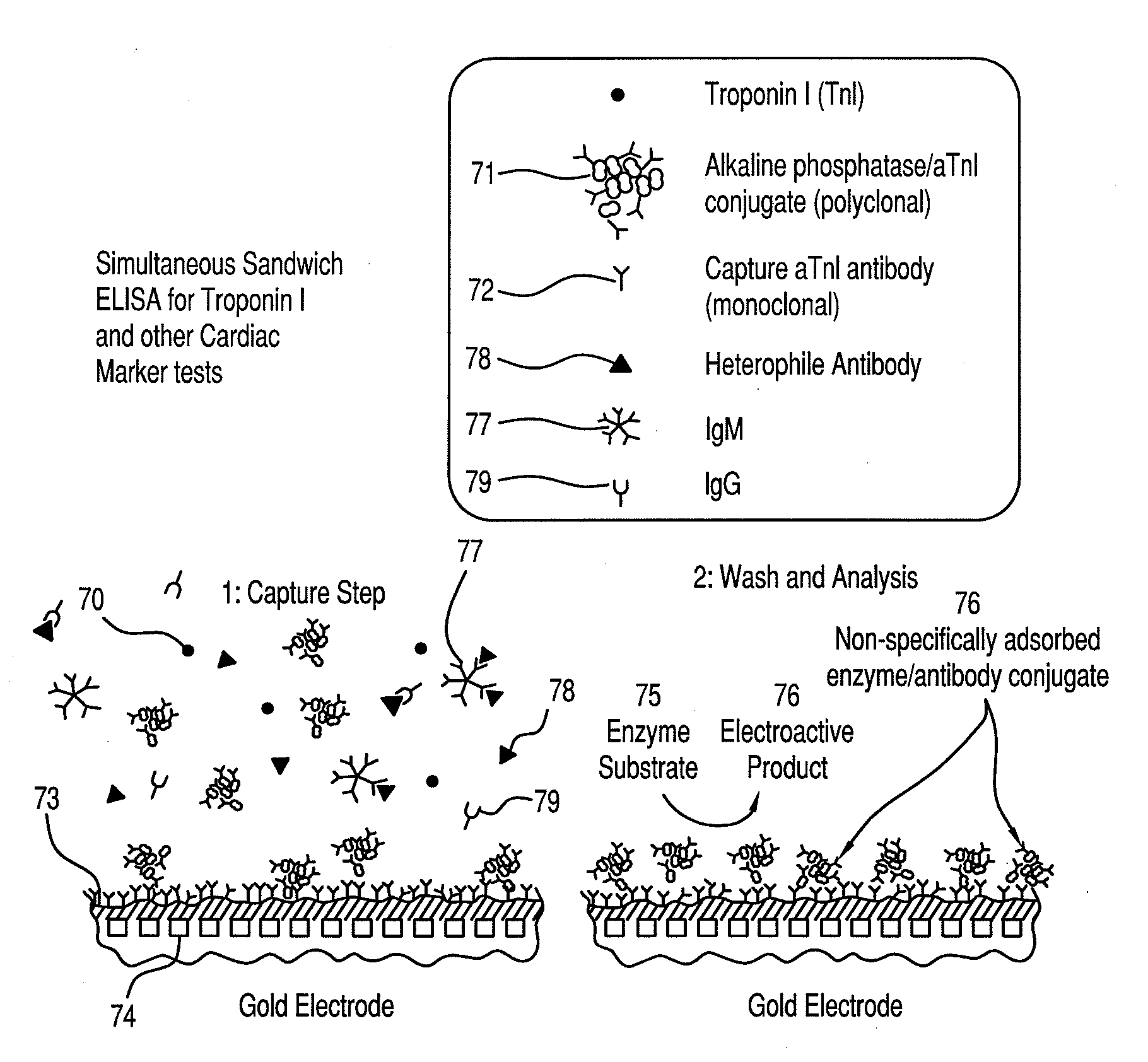
Amelioration of heterophile antibody immunosensor interference
ActiveUS20100248273A1Reduce distractionsBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteWhole blood sample
The invention is directed to methods and devices for reducing interference from heterophile antibodies in an analyte immunoassay. In one embodiment, the invention is to a method comprising the steps of (a) amending a biological sample such as a whole blood sample with non-human IgM or fragments thereof by dissolving into said sample a dry reagent to yield a non-human IgM concentration of at least about 20 μg / mL or equivalent fragment concentration; and (b) performing an electrochemical immunoassay on the amended sample to determine the concentration of said analyte in said sample. Preferably, the sample is amended with IgG or fragments thereof in addition to the IgM of fragments thereof.
Owner:ABBOTT POINT CARE
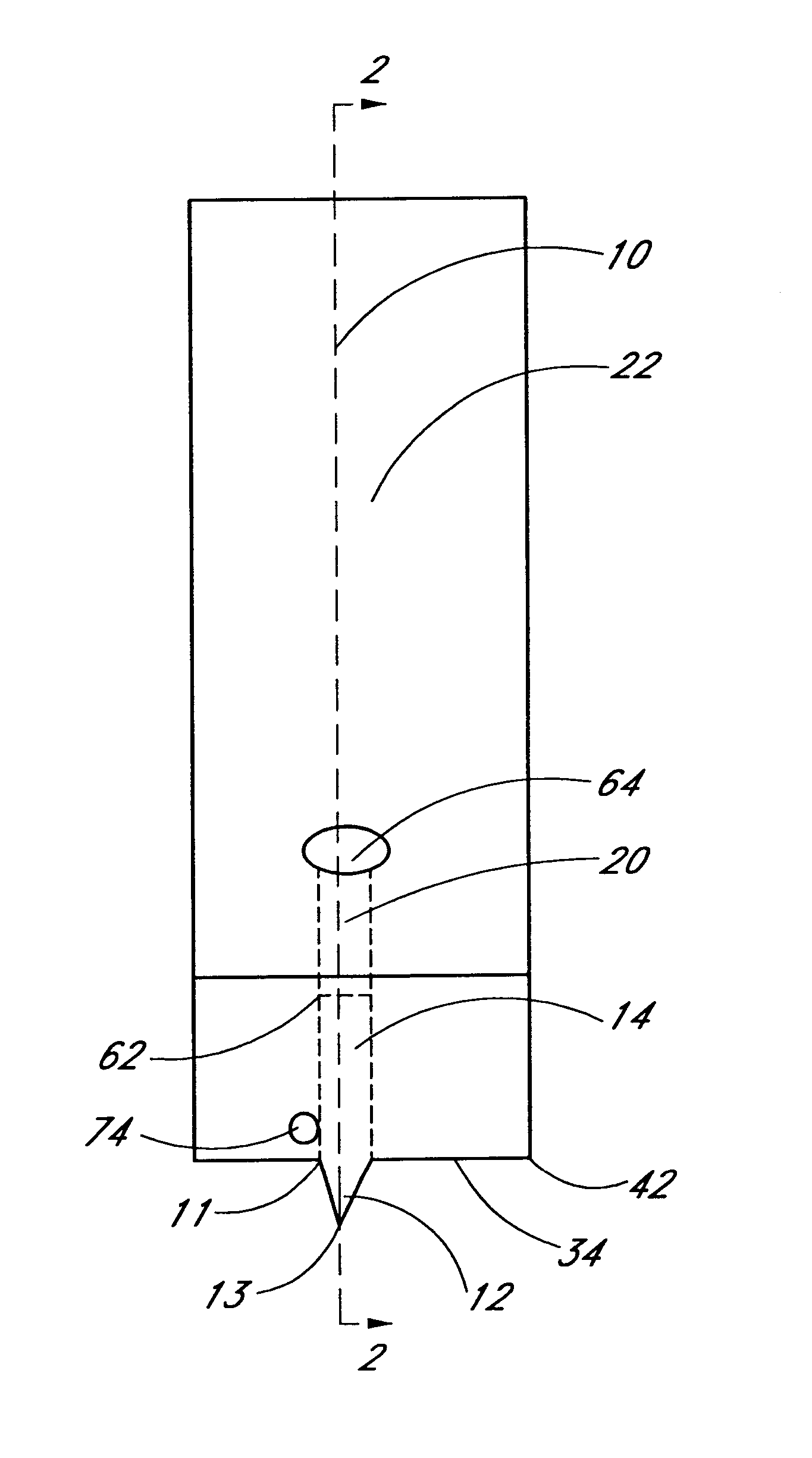
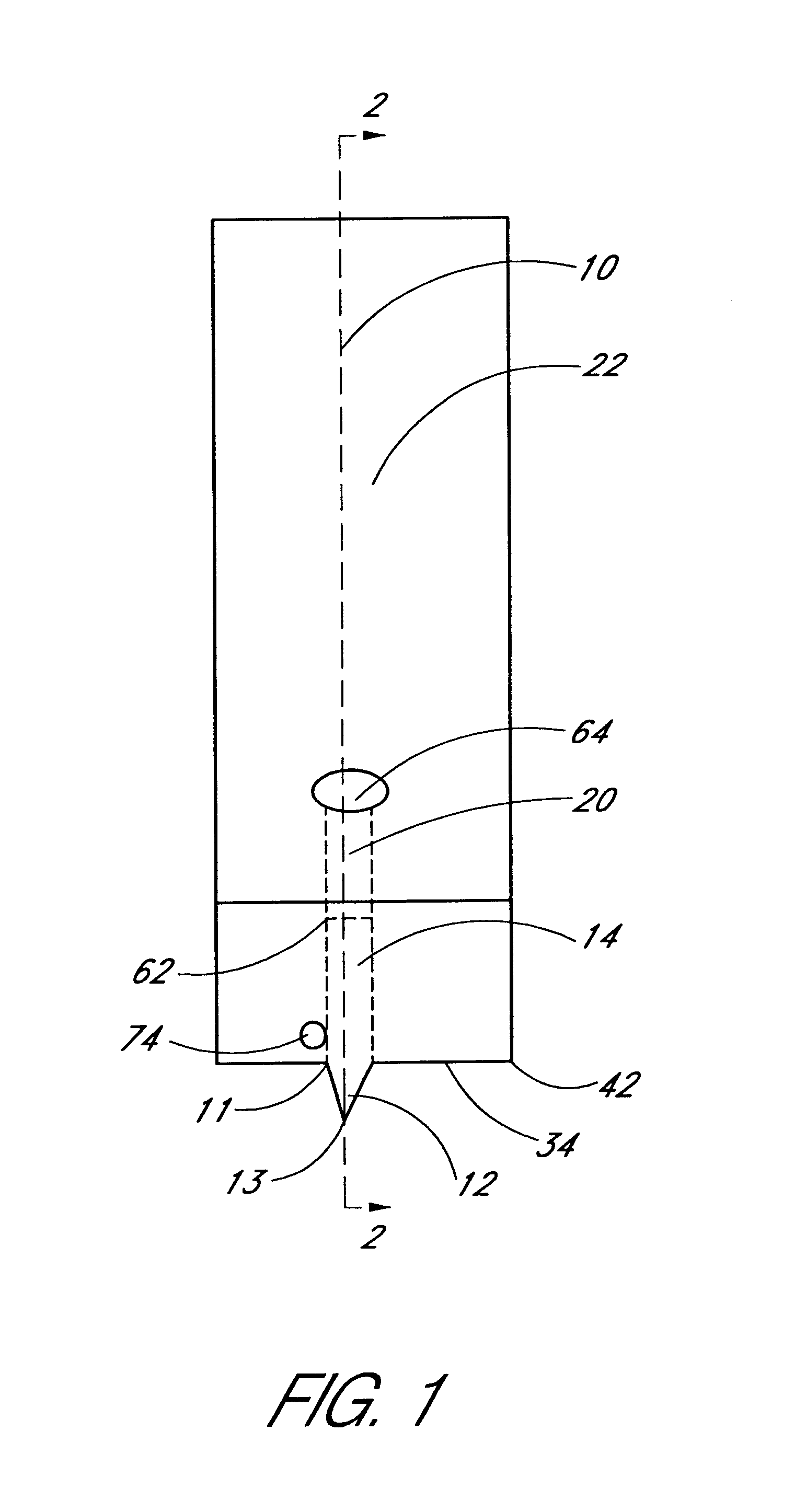
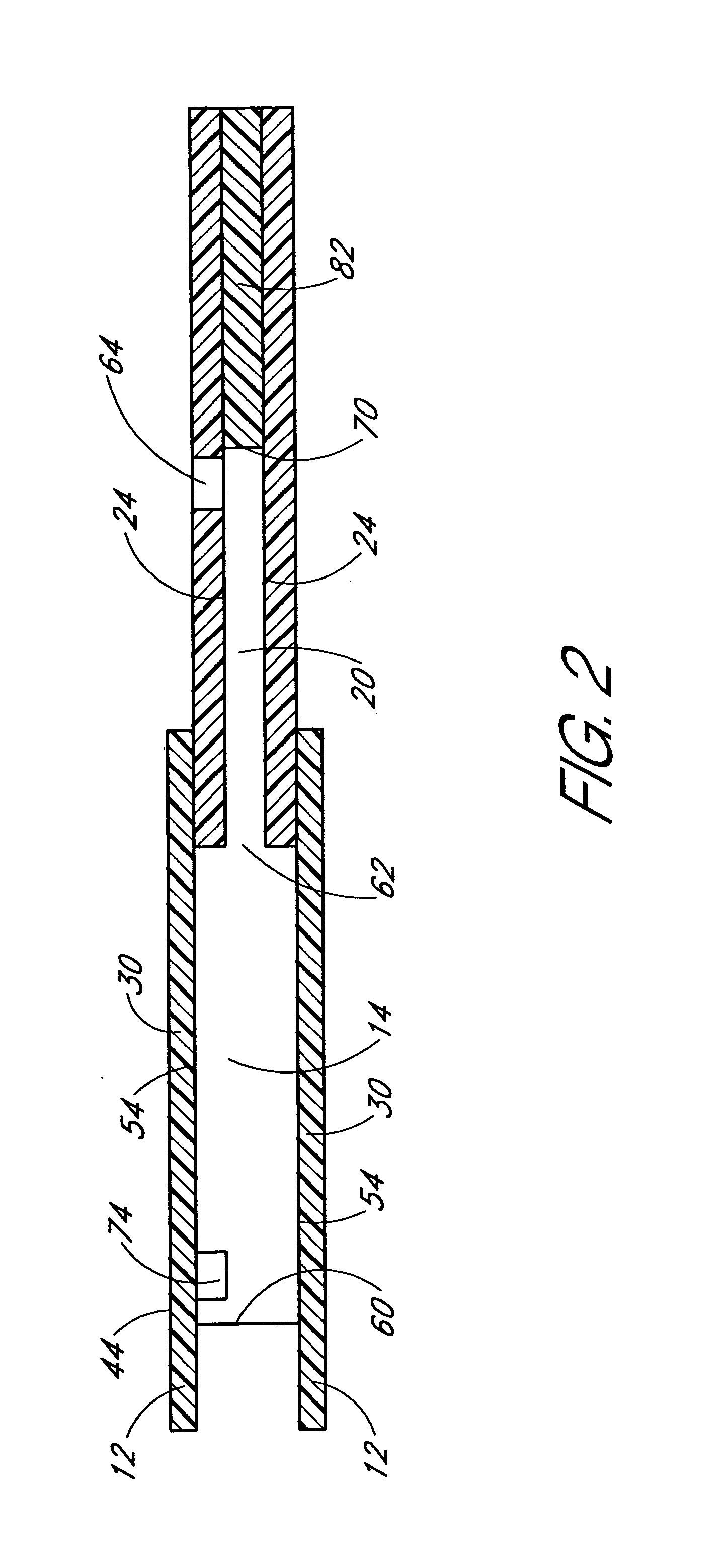
Method for determining at least one hemoglobin related parameter of a whole blood sample
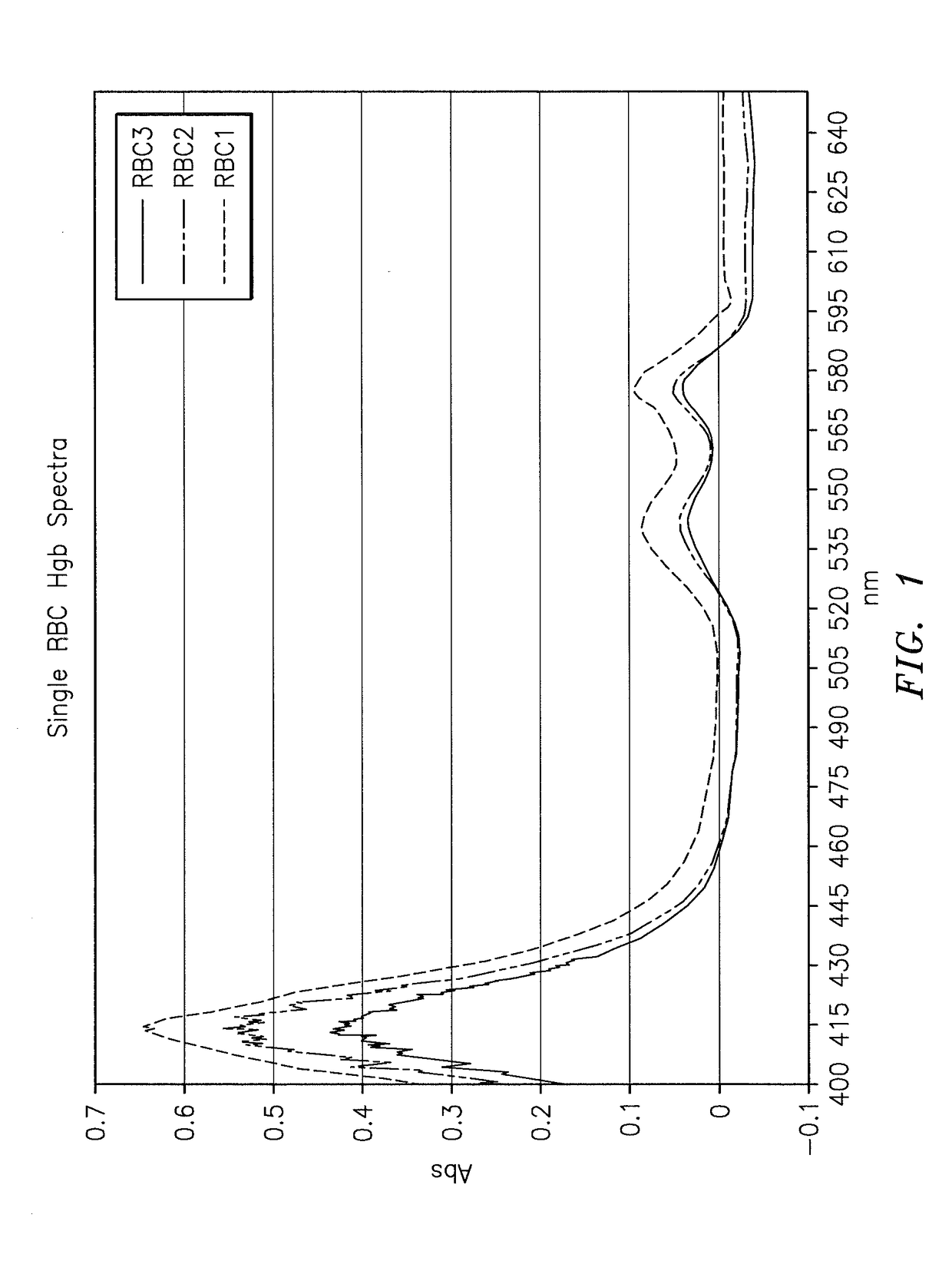
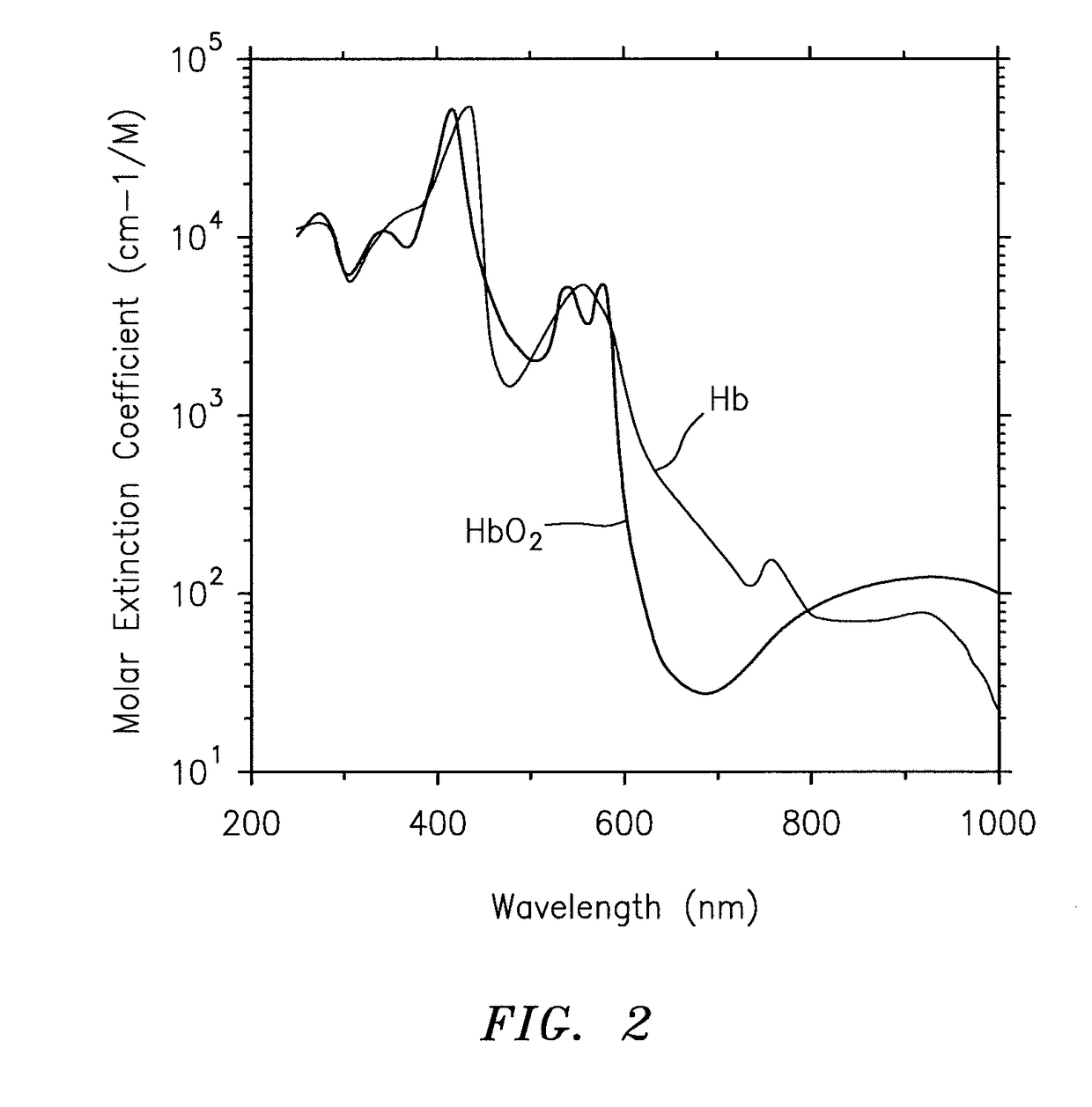
A method and apparatus for determining at least one hemoglobin related parameter of a whole blood sample is provided. The method includes the steps of: a) depositing the sample into an analysis chamber adapted to quiescently hold the sample for analysis, the chamber defined by an interior surface of a first panel, and an interior surface of a second panel, and the chamber has a height extending between the interior surfaces of the panels, wherein the chamber is configured to increase the oxygenation state of the sample to a substantially oxygenated state within a predetermined amount of time after entry into the chamber; b) imaging the at least one red blood cell contacting the interior surfaces, and producing image signals; c) determining an optical density of at least a portion of the imaged red blood cell contacting both interior surfaces; and d) determining the at least one hemoglobin related parameter of the red blood cell contacting the interior surfaces, using the determined optical density and a molar extinction coefficient for oxygenated hemoglobin.
Owner:ABBOTT POINT CARE
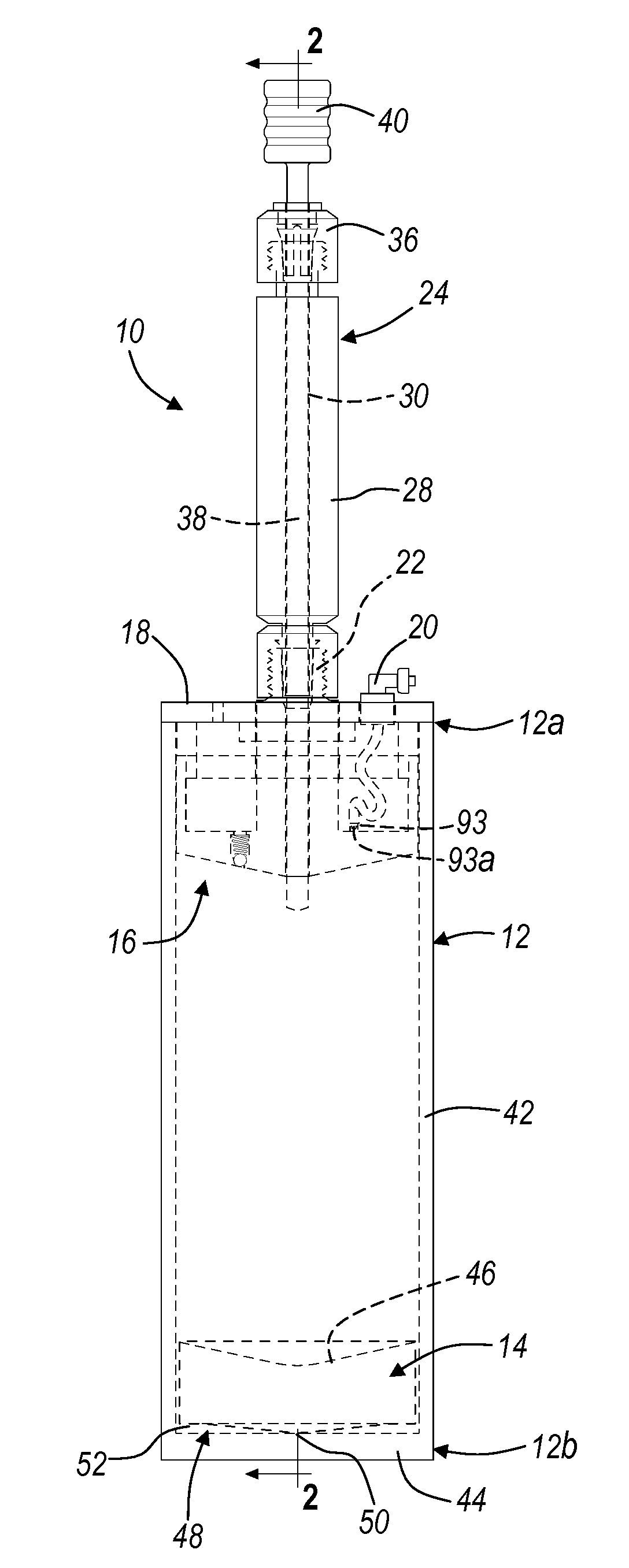
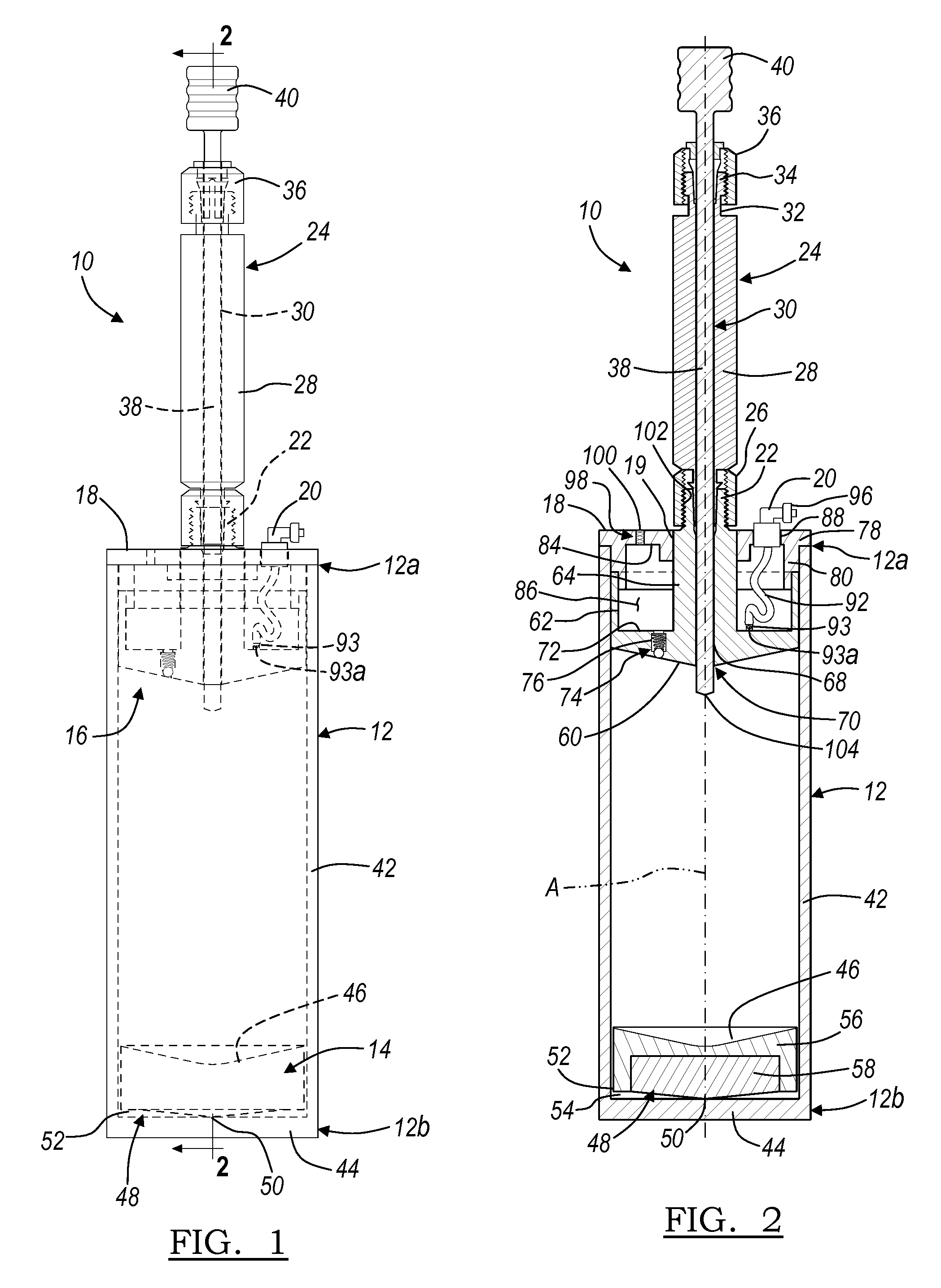
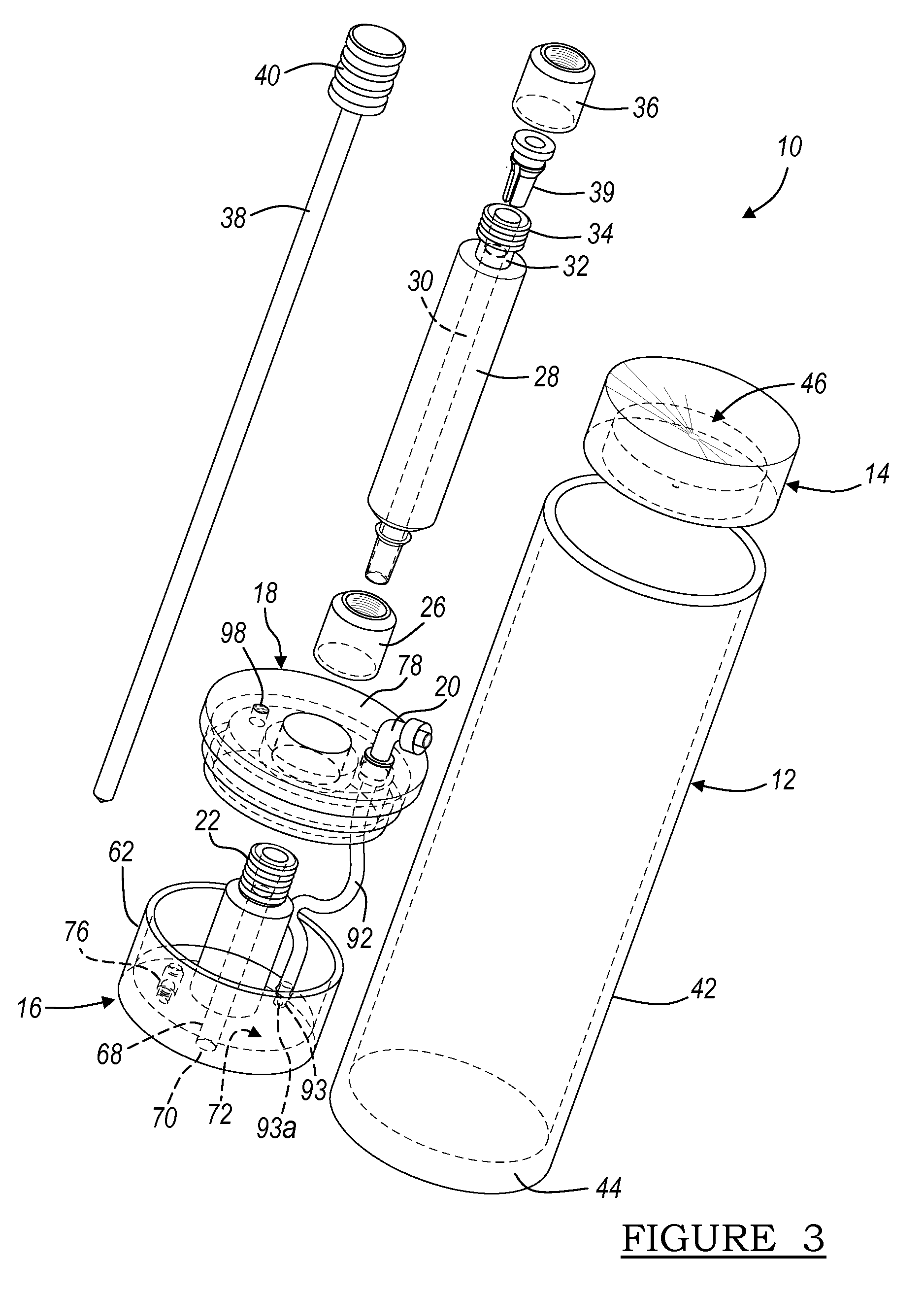
Apparatus And Method For Separating And Concentrating Fluids Containing Multiple Components
An apparatus is disclosed that allows for separating and collecting a fraction of a sample. The apparatus, when used with a centrifuge, allows for the creation of at least three fractions in the apparatus. It also provides for a new method of extracting the buffy coat phase from a whole blood sample. A buoy system that may include a first buoy portion and a second buoy member operably interconnected may be used to form at least three fractions from a sample during a substantially single centrifugation process. Therefore, the separation of various fractions may be substantially quick and efficient.
Owner:BIOMET MFG CORP
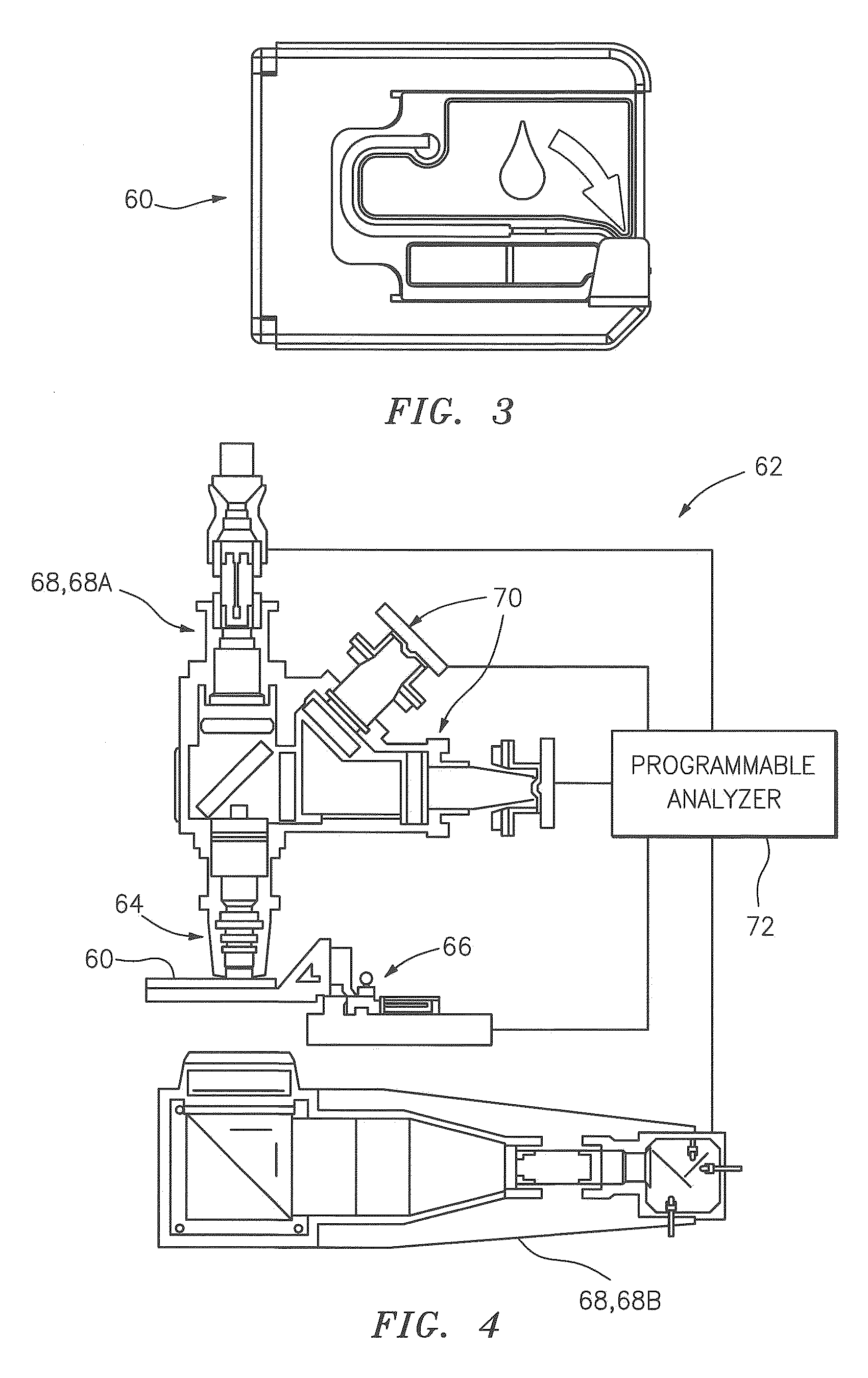
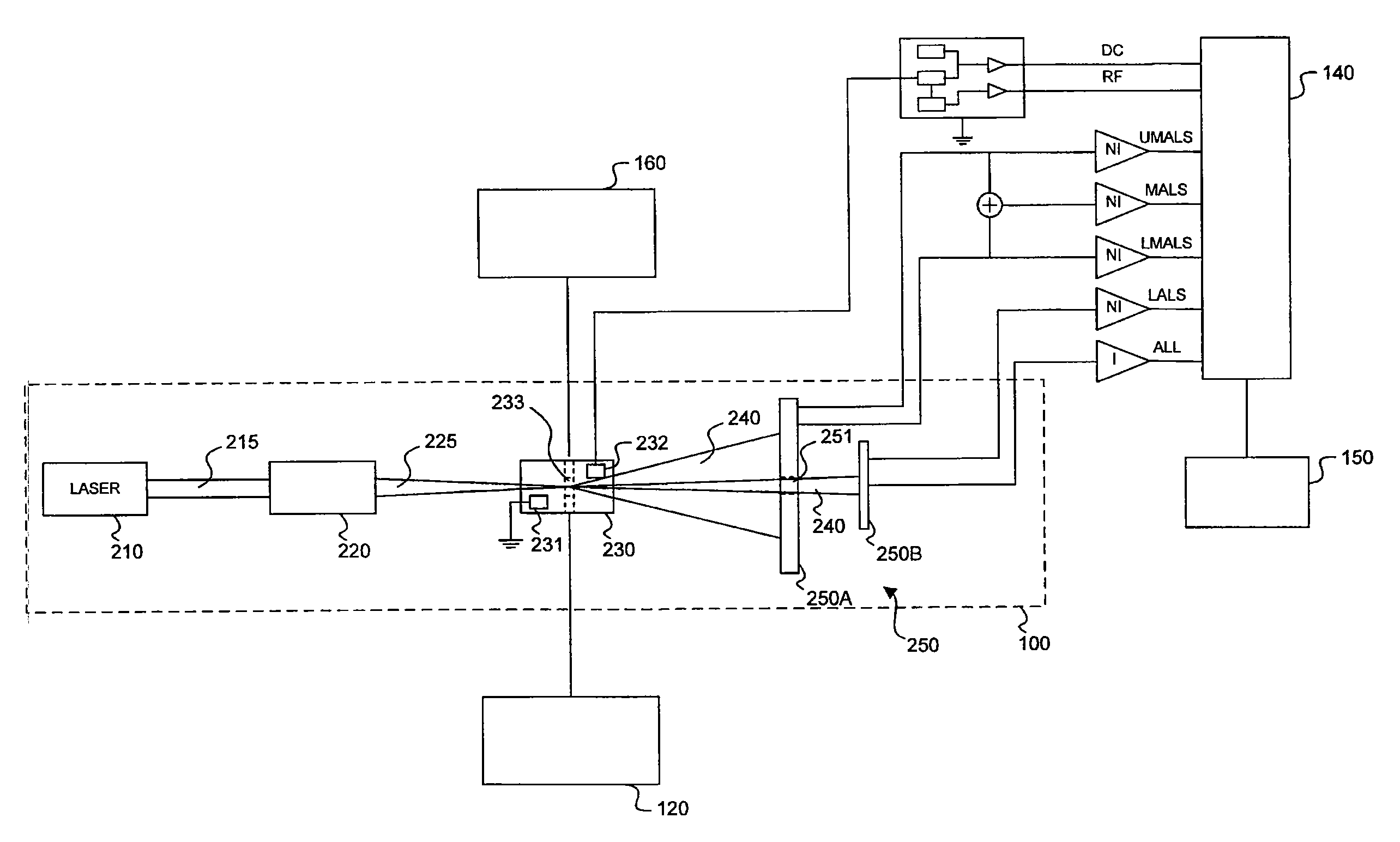
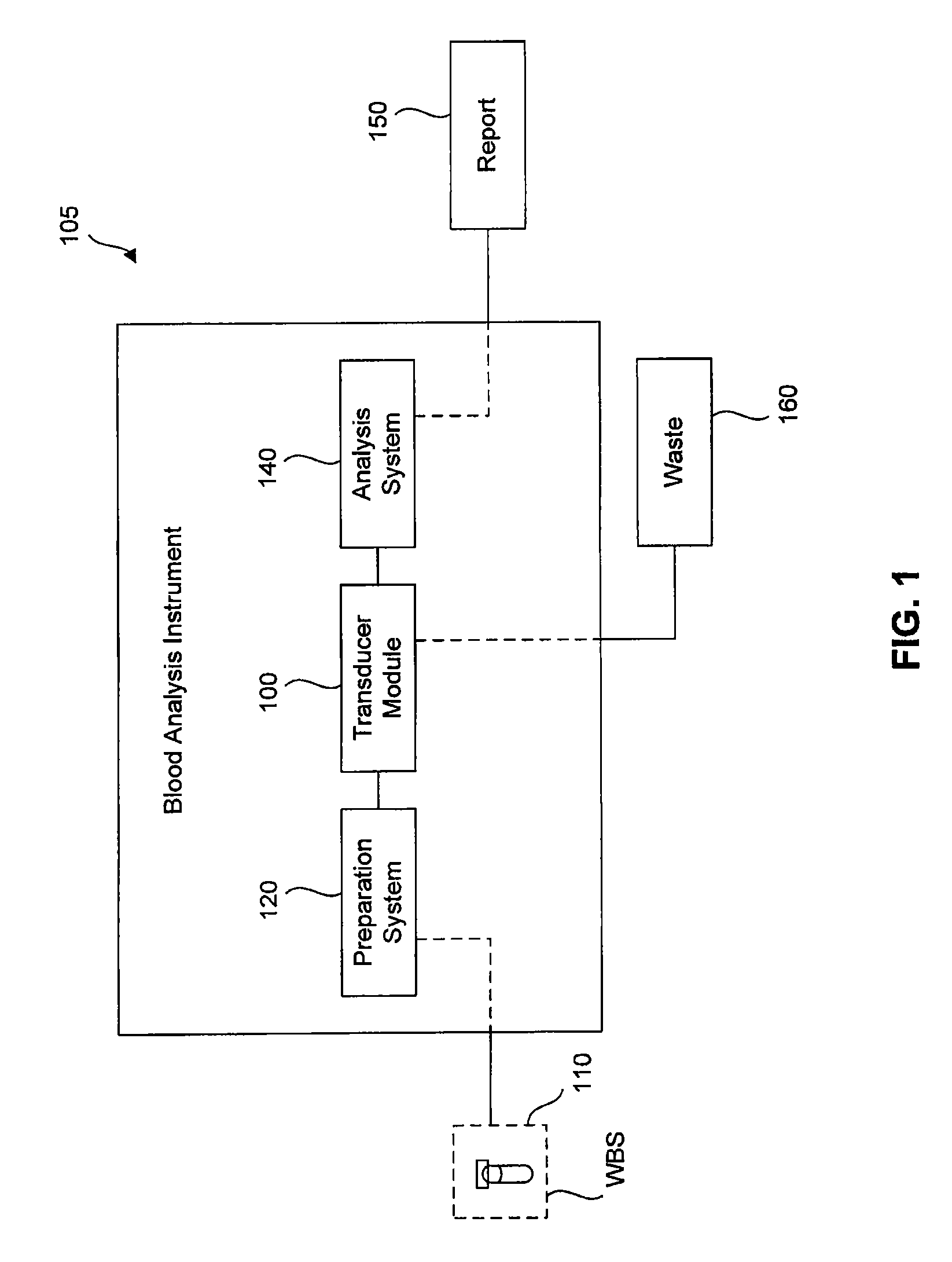
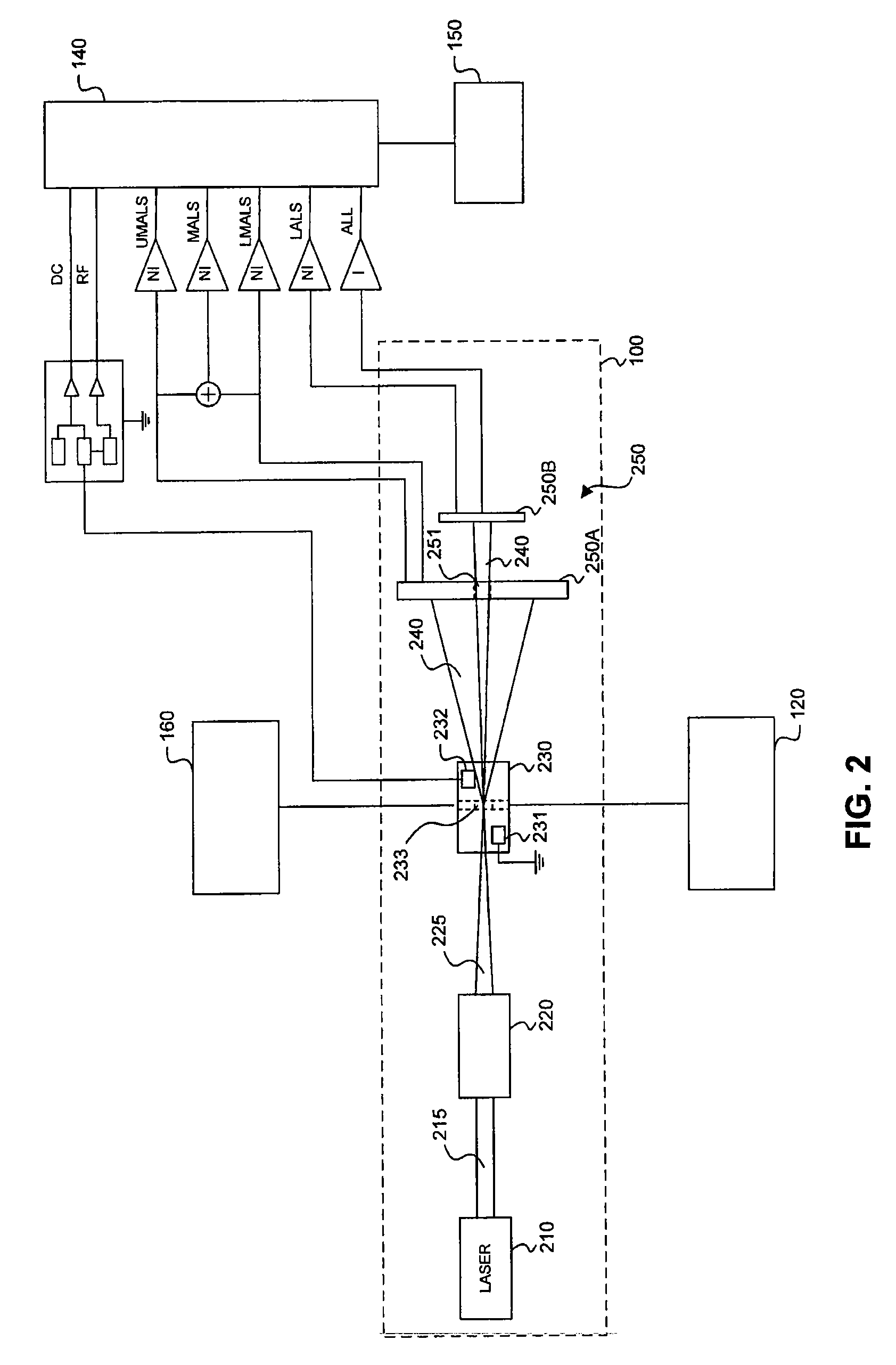
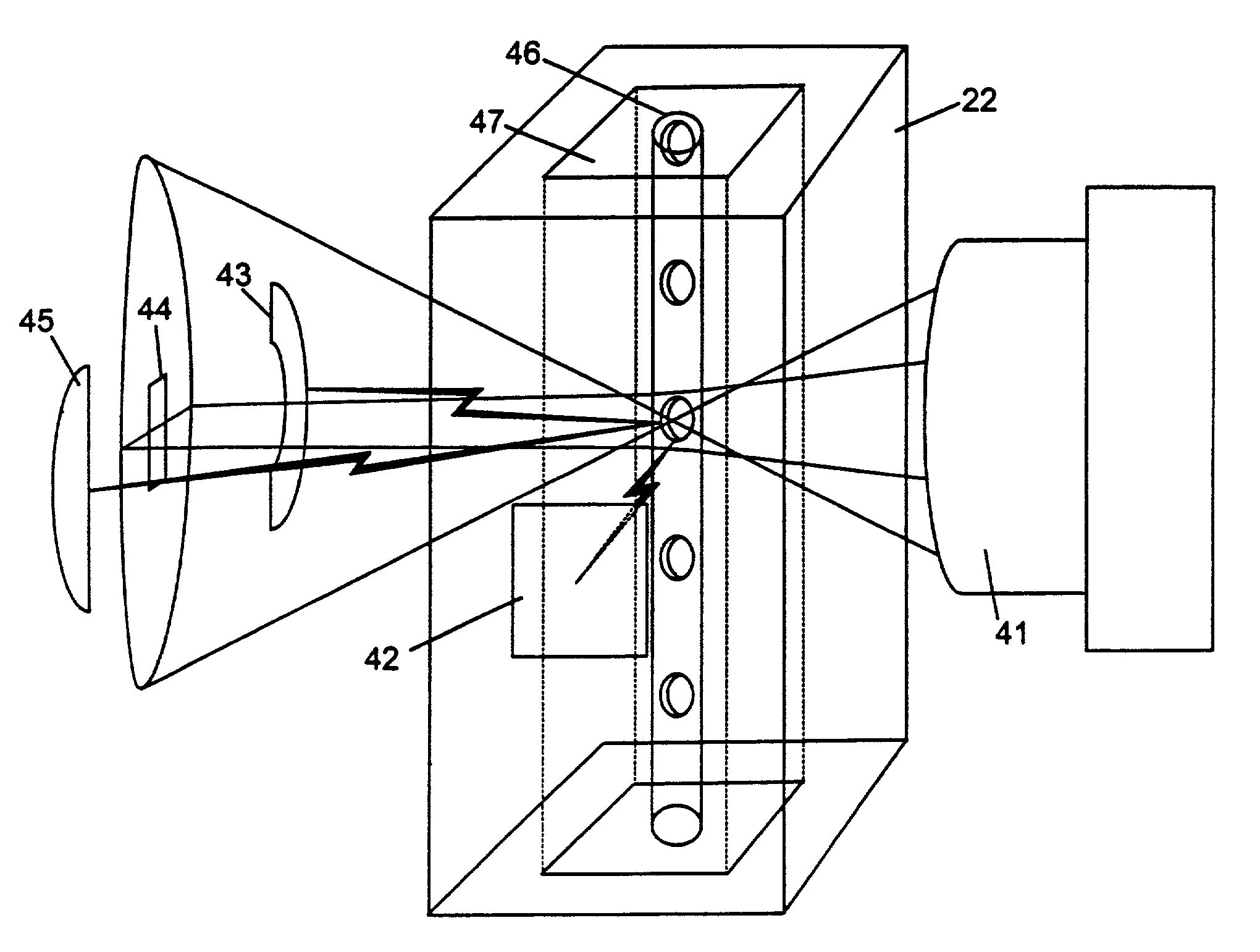
Transducer module
ActiveUS8094299B2Material analysis by optical meansBiological particle analysisPresent methodTransducer
Transducer modules for use in a blood analysis instrument and methods for analyzing a blood sample. The transducer modules presented generally include a light source, a focus-alignment system, a flow cell, and a light scatter detection system. Electrodes within the flow cell allow for the measurement of the DC impedance and RF conductivity of cells passing through a cell-interrogation zone in the flow cell. Light scatter from the cells passing through the cell-interrogation zone is measured by the light scatter detection system. The light scatter detection system measures the light scatter parameters of upper median light scatter, lower median angle light scatter, low angle light scatter, and axial light loss. The presented methods for analyzing a blood sample generally include aspirating a whole blood sample into a blood analysis instrument, preparing the blood sample for analysis, passing the blood sample through a flow cell in a transducer system, and measuring axial light loss, multiple angles of light scatter, DC impedance and / or RF conductivity.
Owner:BECKMAN COULTER INC
Optical method and apparatus for red blood cell differentiation on a cell-by-cell basis, and simultaneous analysis of white blood cell differentiation
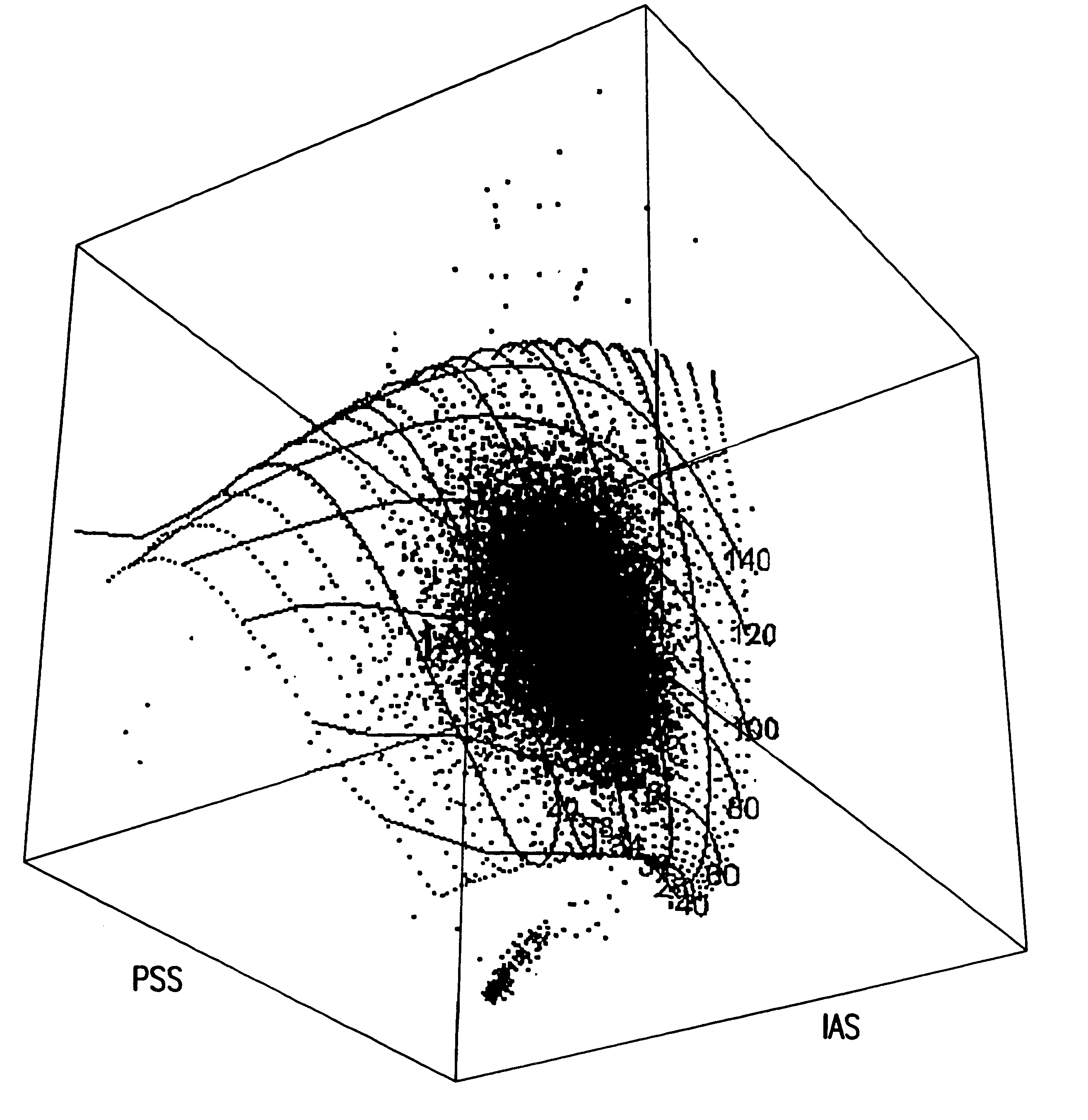
InactiveUS6630990B2Accurate methodMaterial analysis by observing effect on chemical indicatorPhase-affecting property measurementsWhite blood cellForward angle
Methods and apparatus are disclosed for determining the volume, hemoglobin concentration, maturity and cell shape of mammalian red blood cells in a whole blood sample and simultaneously monitoring system standardization. Methods for distinguishing red blood cells from other cellular particles, prior to the red blood cell analysis are also disclosed. Red blood cells are passed through a beam of light in single file at a selected wavelength, obtaining an initial cytogram by means of the resultant magnitude of one light loss signal and one forward angle light scatter signal at a selected angular interval and a third side angle light scatter or two forward angle light scatter signals at a selected angular intervals and a third side-angle light scatter signal, projecting the cytogram, point by point, onto a pre-calibrated 3-dimensional surface containing grid lines of volume and hemoglobin concentration and determining accurate values of cell volume and hemoglobin concentration.
Owner:ABBOTT LAB INC
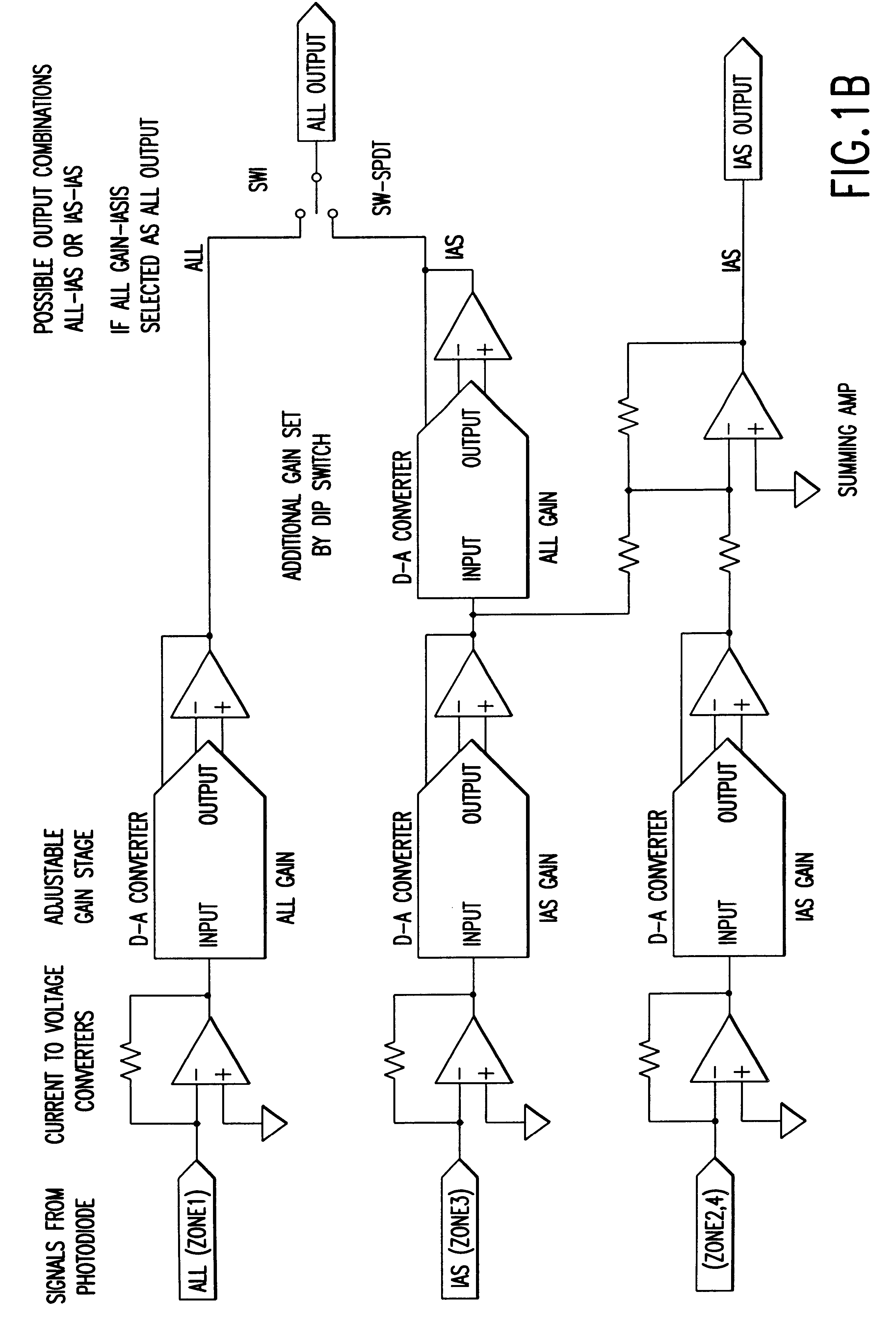
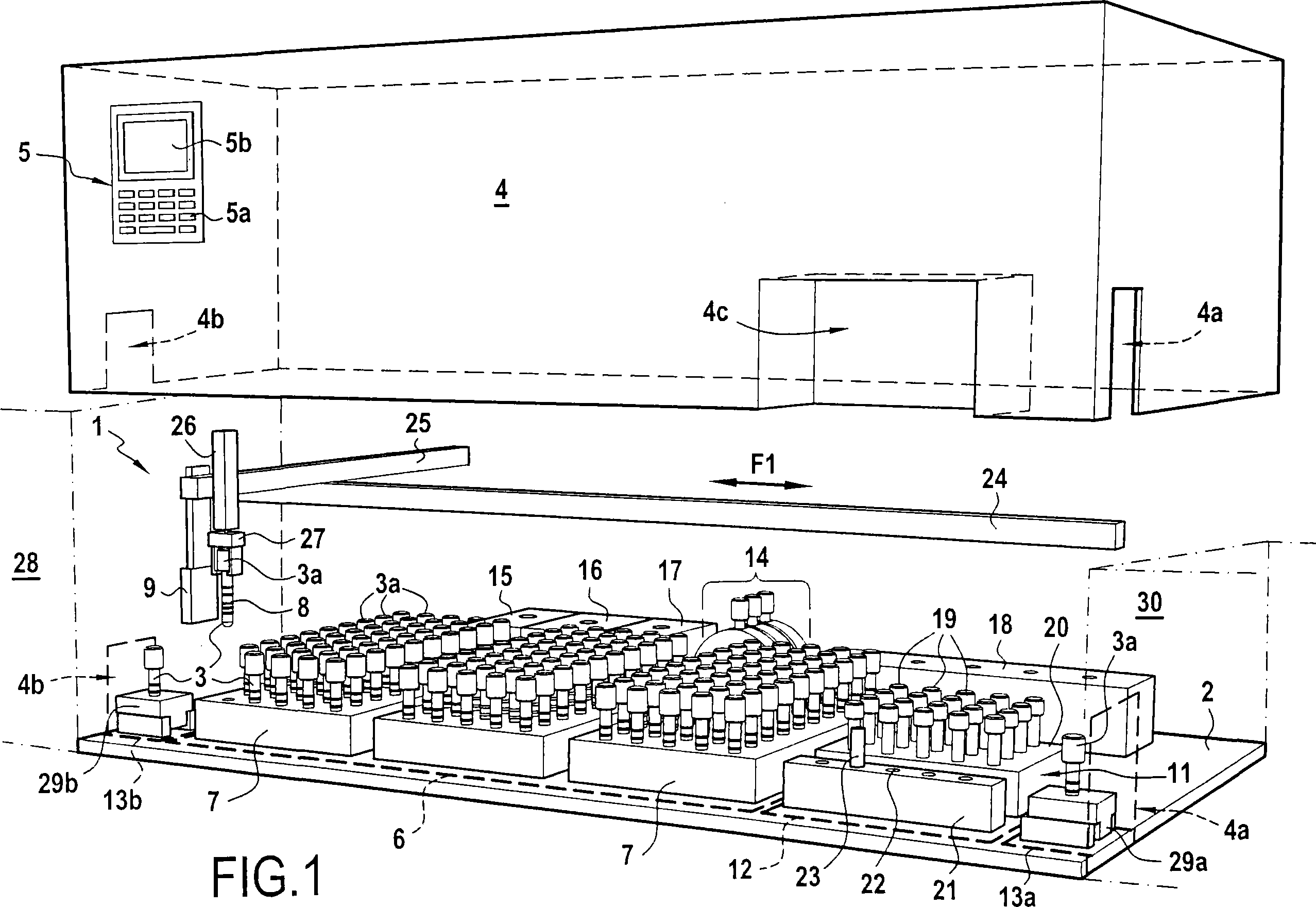
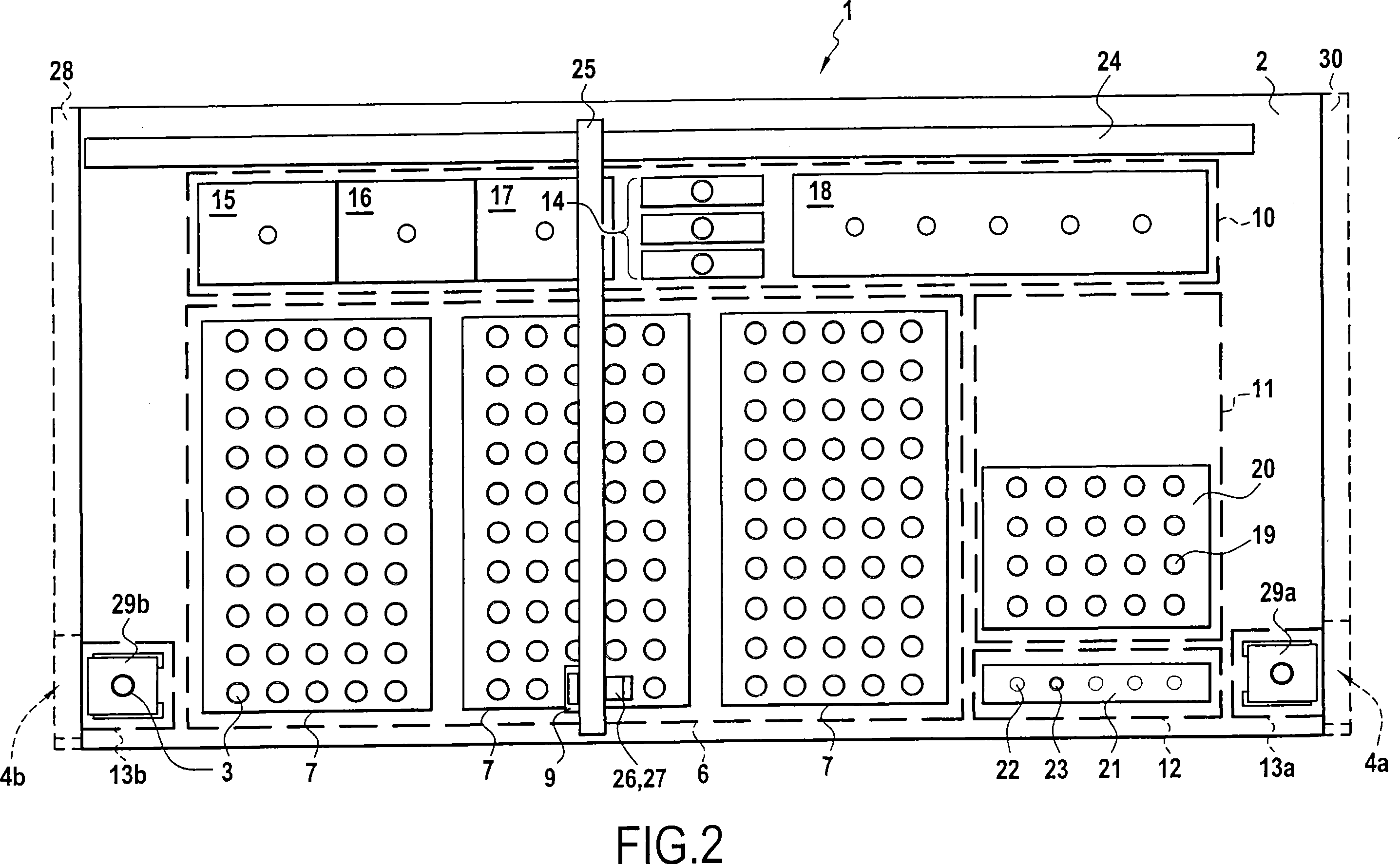
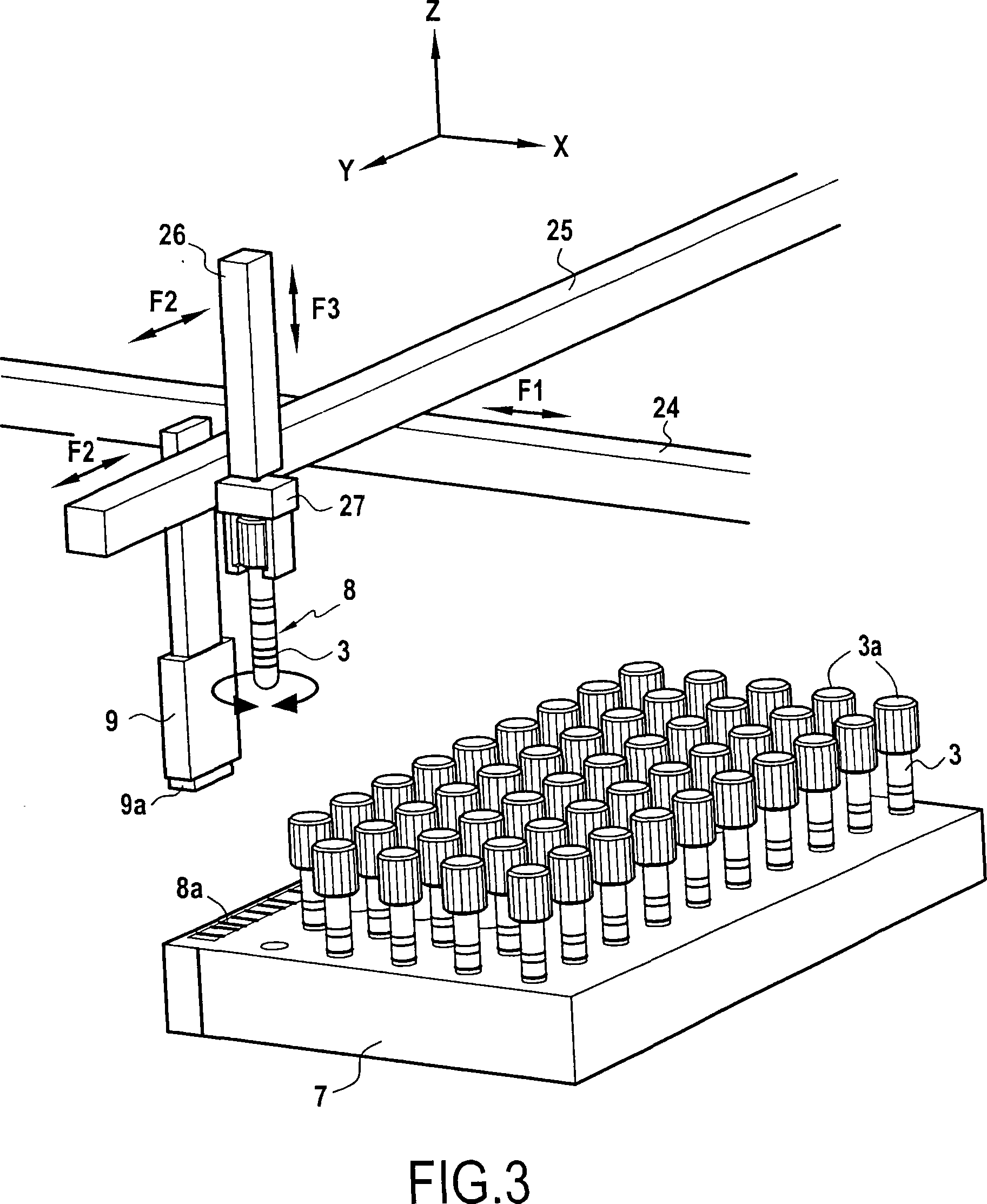
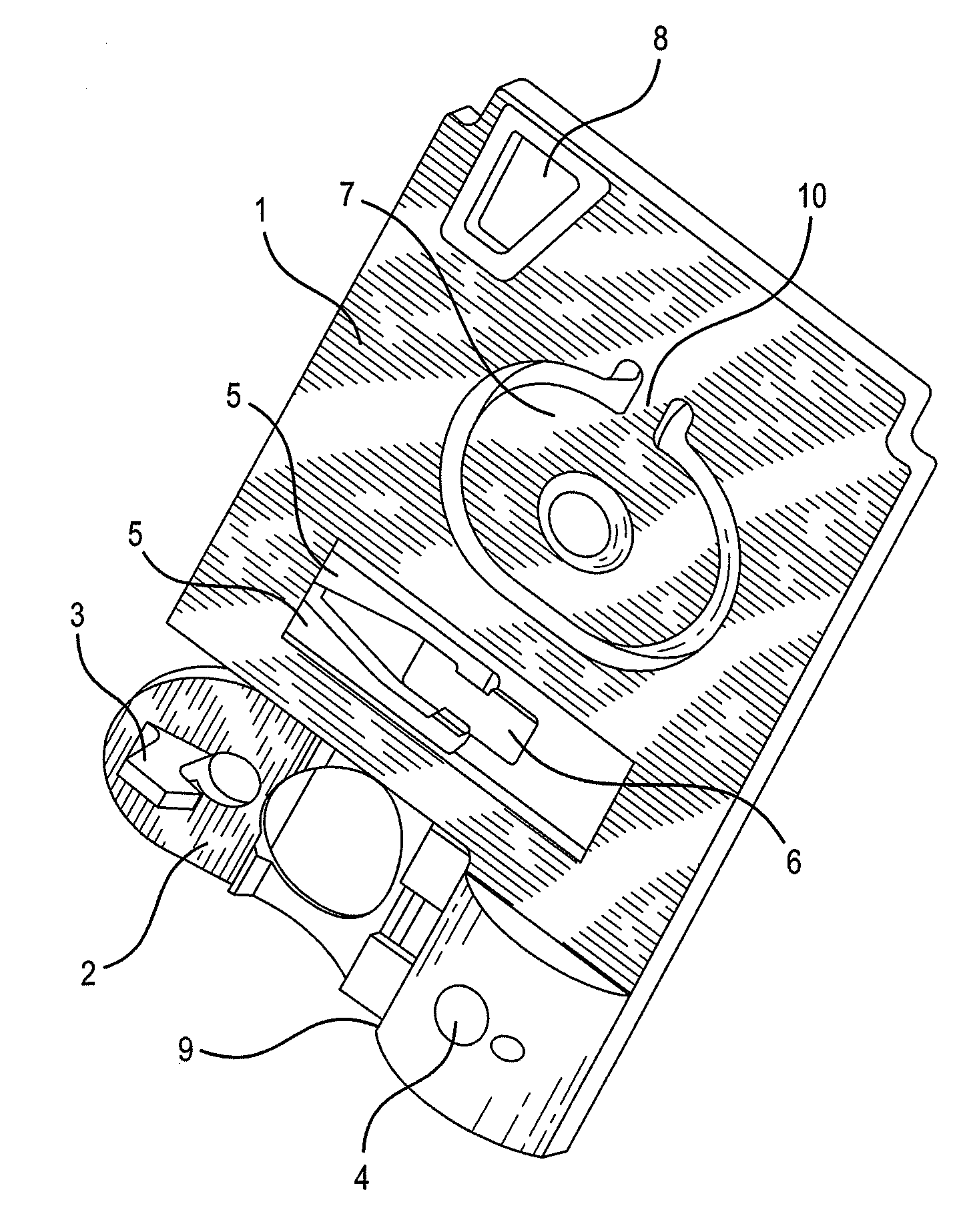
Automated method for preparing whole blood sample analysis and automated device therefor
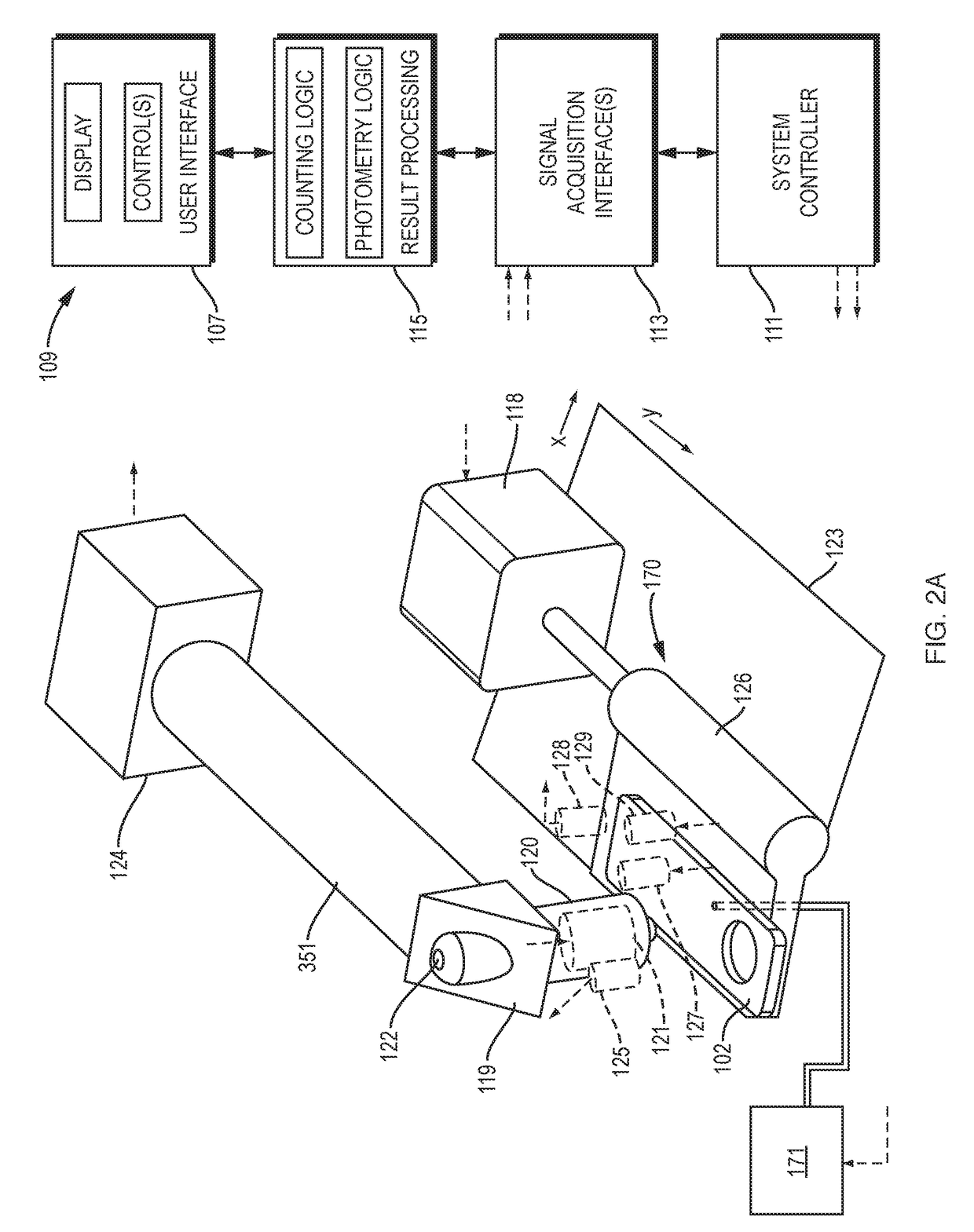
The invention concerns a method for preparing total blood sample analyses and a device (1) for implementing said method, said samples being preserved in tubes (3) comprising at least one means for identifying (8) the sample, said device comprising: at least one compartment forming the storage zone (6, 11, 12) for said tubes (3, 19, 23) before and after analysis, and at least one means for reading (9) said identifying means (8) of said tubes; and at least one zone for preparing (10) said blood samples prior to analysis including means (14, 15, 16, 17, 18) for verifying and / or processing said tubes (3, 19, 23) containing said samples, in particular at least one means for stirring (14) said tubes, and at least one zone for accessing (13a, 13b) at least one automatic analyzer (28, 30) on total blood, said access zone enabling one said tube (3, 19, 23) to be placed in said analyzer, and robotized gripping and displacing means (24, 25, 26, 27) controlled by a control automaton (5) and adapted to grip and set down said tubes (3, 19, 23) individually in said storage zone (6, 11, 12) and transport same in at least three directions XYZ between said storage zone, said preparation zone (10) and said access zone (13a, 13b) to said analyzer, said analyzer (28, 30) being preferably connected to and / or controlled by said control automaton (5).
Owner:HORIBA ABX SAS
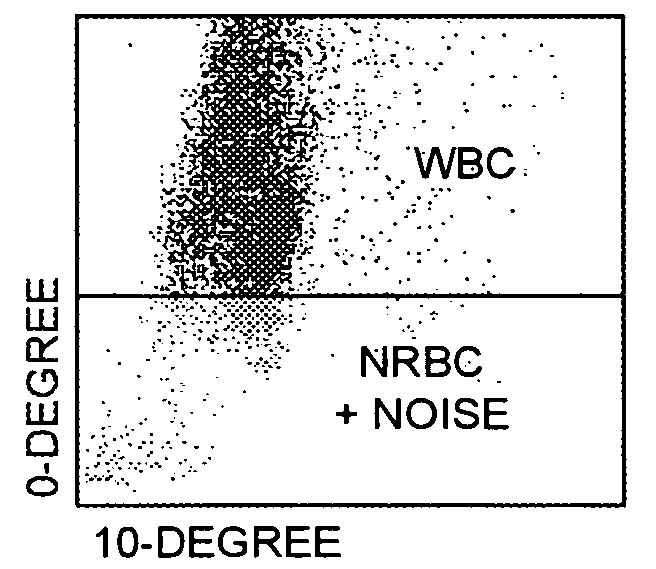
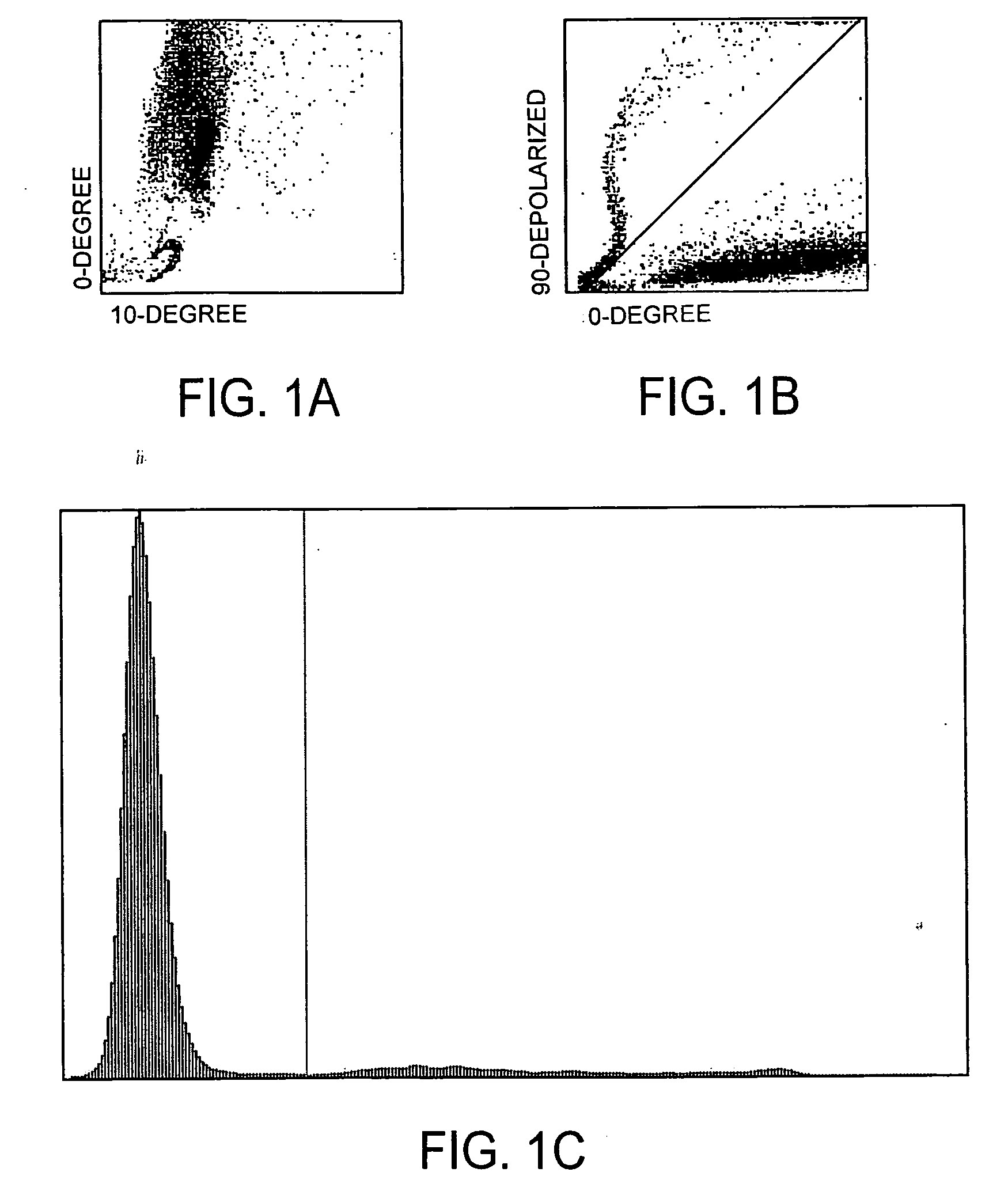
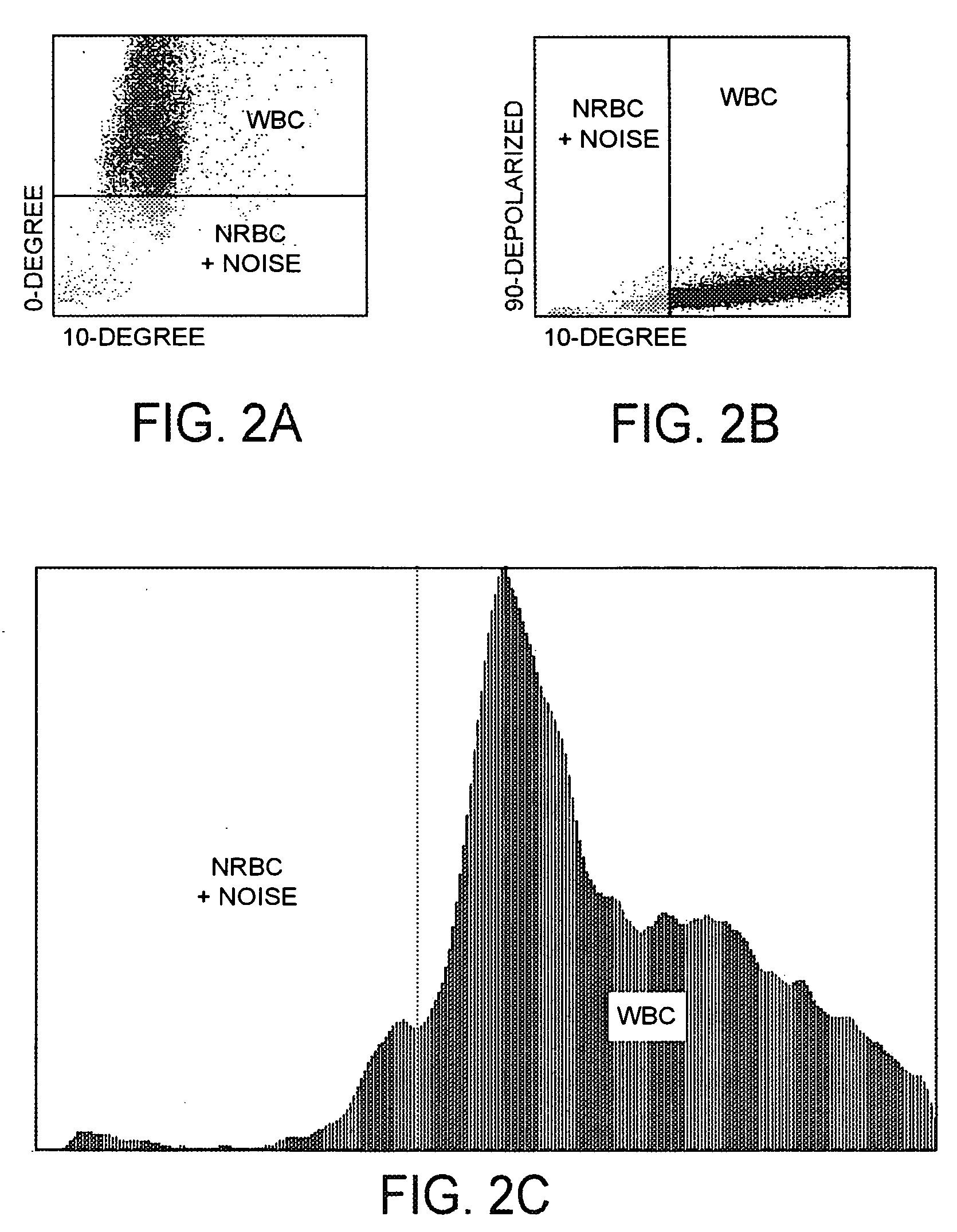
Method for determination of nucleated red blood cells and leukocytes in a whole blood sample in an automated hematology analyzer
ActiveUS20080153170A1Reduce distractionsInterfere with accurateSamplingBiological particle analysisLipid formationRed blood cell
A method for enumerating white blood cells and nucleated red blood cells. The method comprises the steps of:(b) providing a lysed sample of whole blood;(b) introducing the lysed sample to a light-scattering multi-angle depolarizing flow cytometer;(c) removing depolarizing interference, e.g., lipid droplets and other measured particles;(d) differentiating nucleated red blood cells and noise from white blood cells in the absence of depolarizing interference;(e) differentiating nucleated red blood cells from noise in the absence of depolarizing interference and white blood cells; and(f) differentiating possible platelet clumps.
Owner:ABBOTT LAB INC
Reducing leukocyte interference in non-competitive immunoassays
ActiveUS20110117580A1Reducing and eliminating leukocyte interferenceReducing leukocyte interferenceBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteWhite blood cell
The invention is directed to methods and devices for reducing interference from leukocytes in an analyte immunoassay, and in particular in non-competitive immunoassays. In one embodiment, the invention is to a method comprising the steps of (a) amending a biological sample such as a whole blood sample with sacrificial beads; and (b) performing a non-competitive immunoassay on the amended sample to determine the concentration of said analyte in said sample. Preferably, the sample is amended with IgG-coated sacrificial beads.
Owner:ABBOTT POINT CARE
Method of selectively determining glycated hemoglobin
InactiveUS20030162242A1Easily and accurately determinedEliminate the effects ofMicrobiological testing/measurementBiological testingProteinase activityHemoglobin F
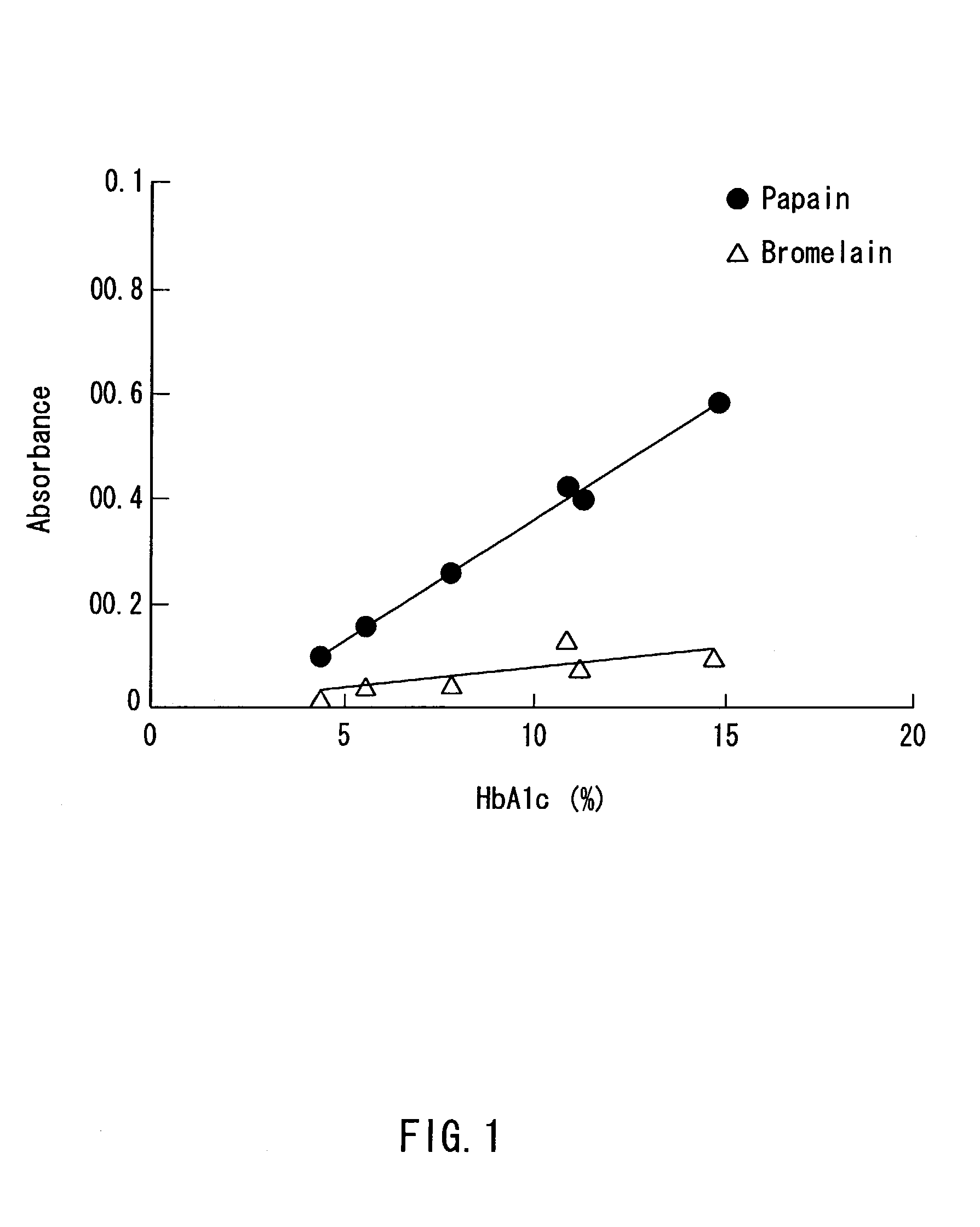
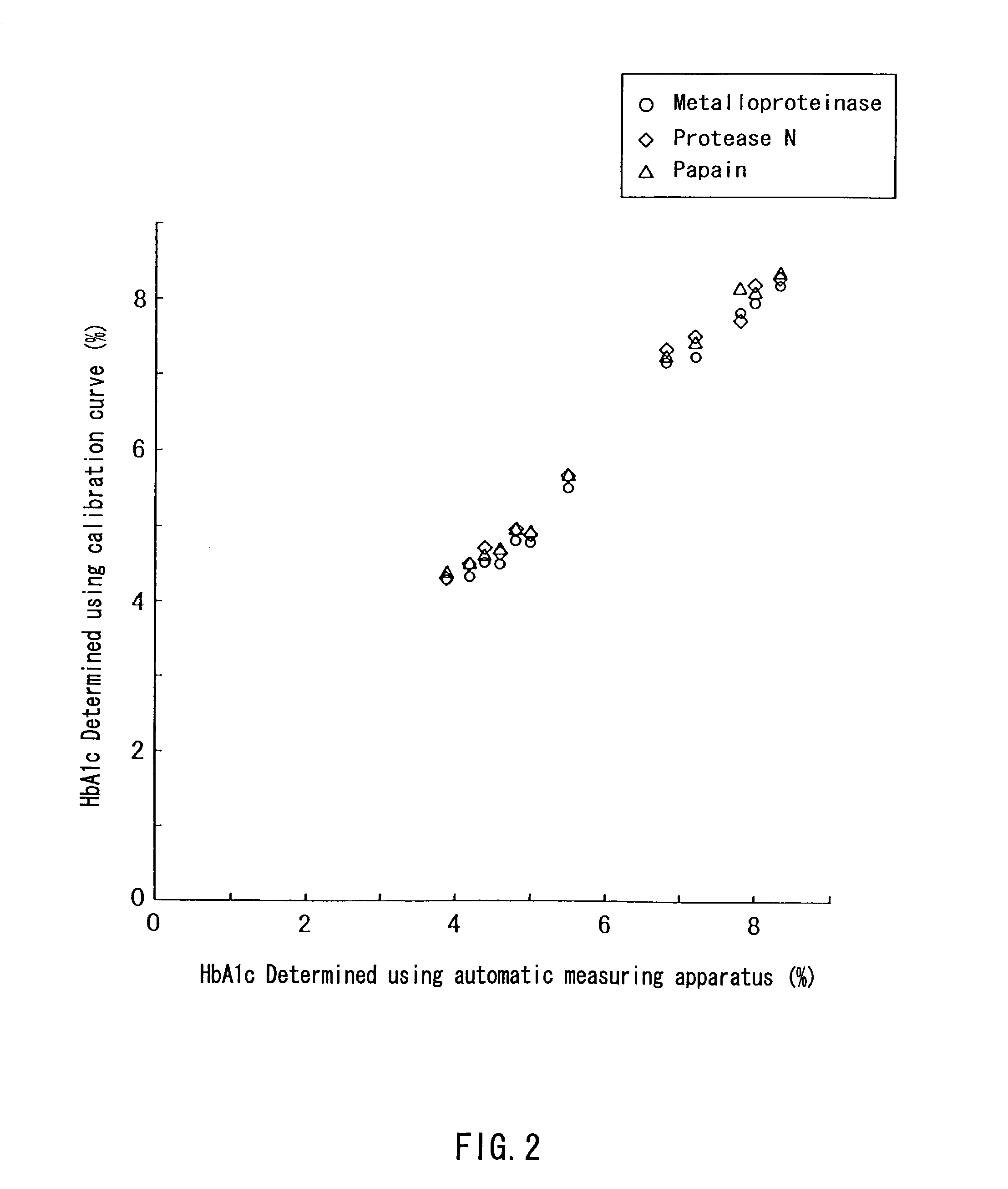
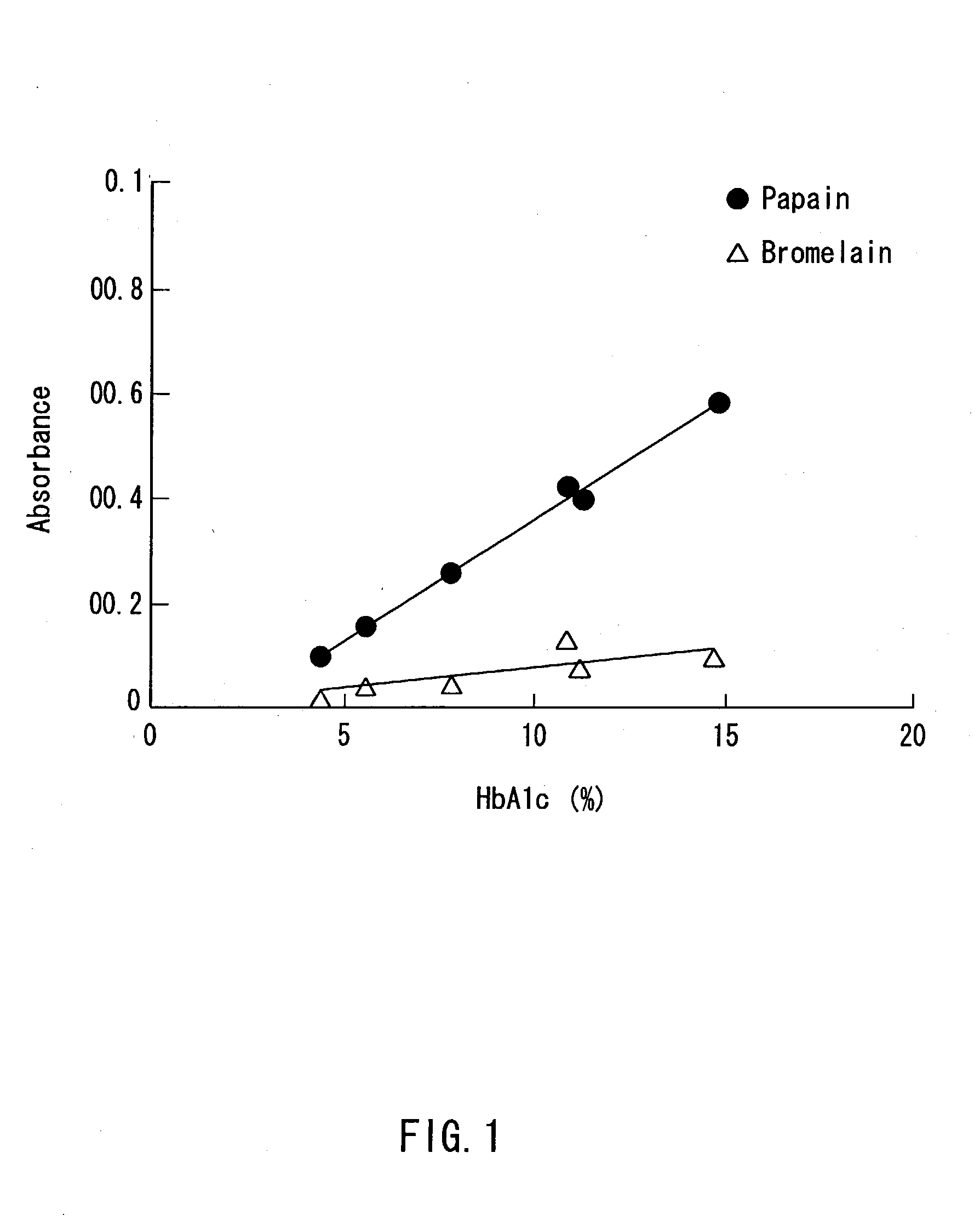
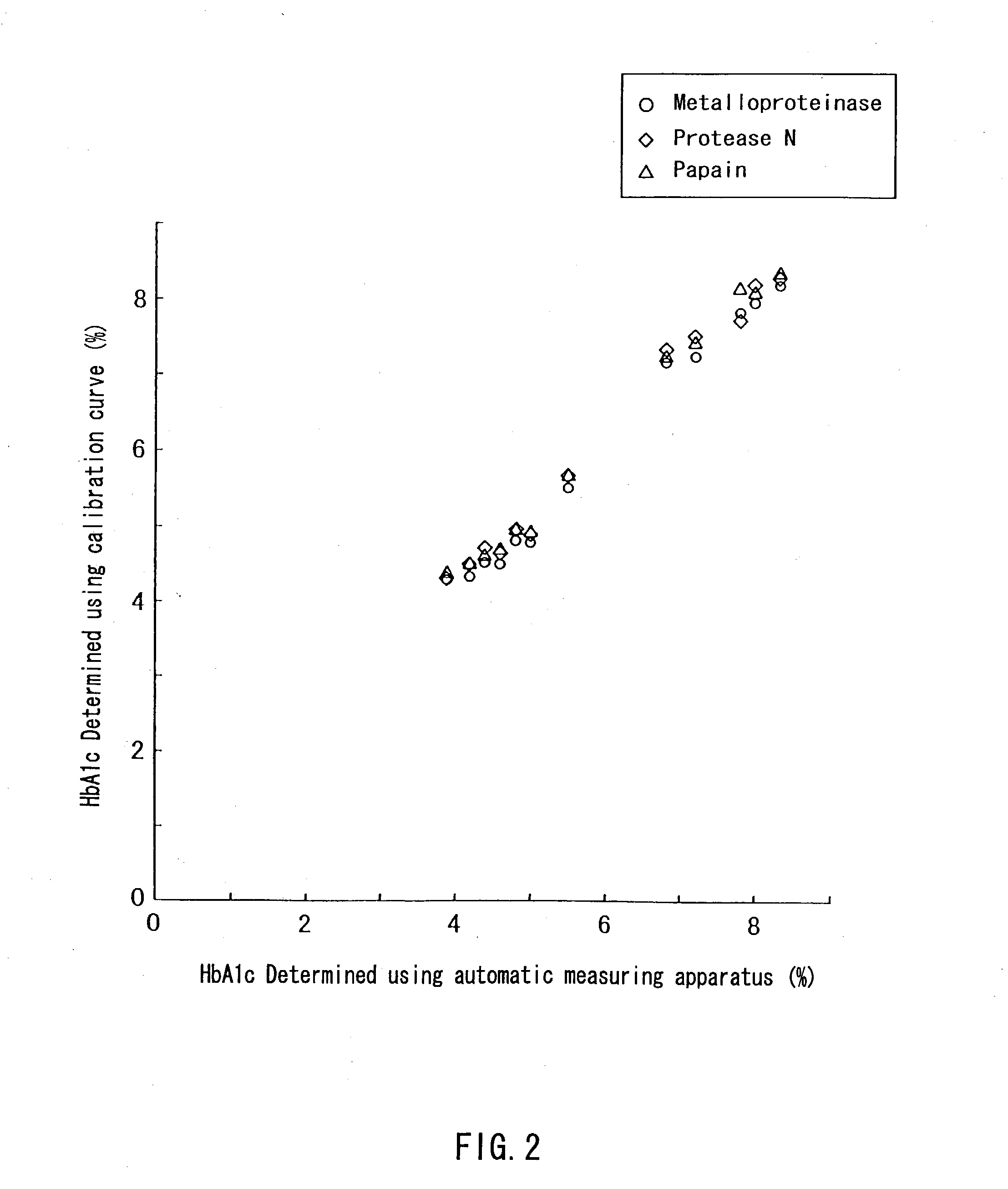
A method of determining glycated hemoglobin is provided, by which a ratio of the glycated hemoglobin in a sample can be determined accurately and easily. The ratio of glycated hemoglobin can be determined by degrading a glycated hemoglobin in a whole blood sample selectively with a protease to give a glycated hemoglobin degradation product`; causing a redox reaction between a glycation site of the glycated hemoglobin degradation product and a fructosyl amino acid oxidoreductase; and determining this redox reaction. Further, as shown in FIG. 1, in a whole blood sample, there is a correlation between the ratio of the glycated hemoglobin determined by this method and an HbA1c concentration. Thus, without determining the glycated alpha-amino group as a characteristic structure of HbA1c, an amount of HbA1c can be determined accurately and easily from the determined ratio of the glycated hemoglobin.
Owner:ARKRAY INC
Consumable tube for use with a flow cytometry-based hematology system
InactiveUS7064823B2Minimizes reagent wasteReduces systemWithdrawing sample devicesPreparing sample for investigationMedicine.hematologyFlow cell
The present invention is a flow cytometry-based hematology system useful in the analysis of biological samples, particularly whole blood or blood-derived samples. The system is capable of determining at least a complete blood count (CBC), a five-part white blood cell differential, and a reticulocyte count from a whole blood sample. The system preferably uses a laser diode that emits a thin beam to illuminate cells in a flow cell and a lensless optical detection system to measure one or more of axial light loss, low-angle forward scattered light, high-angle forward scattered light, right angle scattered light, and time-of-flight measurements produced by the cells. The lensless optical detection system contains no optical components, other than photoreactive elements, and does not include any moving parts. Finally, the system uses a unique system of consumable reagent tubes that act as reaction chambers, mixing chambers, and waste chambers for the blood sample analyses. The consumable tubes incorporate reference particles, which act as internal standards to ensure that the dilutions made during processing of the samples have been carried out correctly, and to ensure that the instrument is working properly. The present invention also relates to methods for using the system.
Owner:IDEXX LABORATORIES
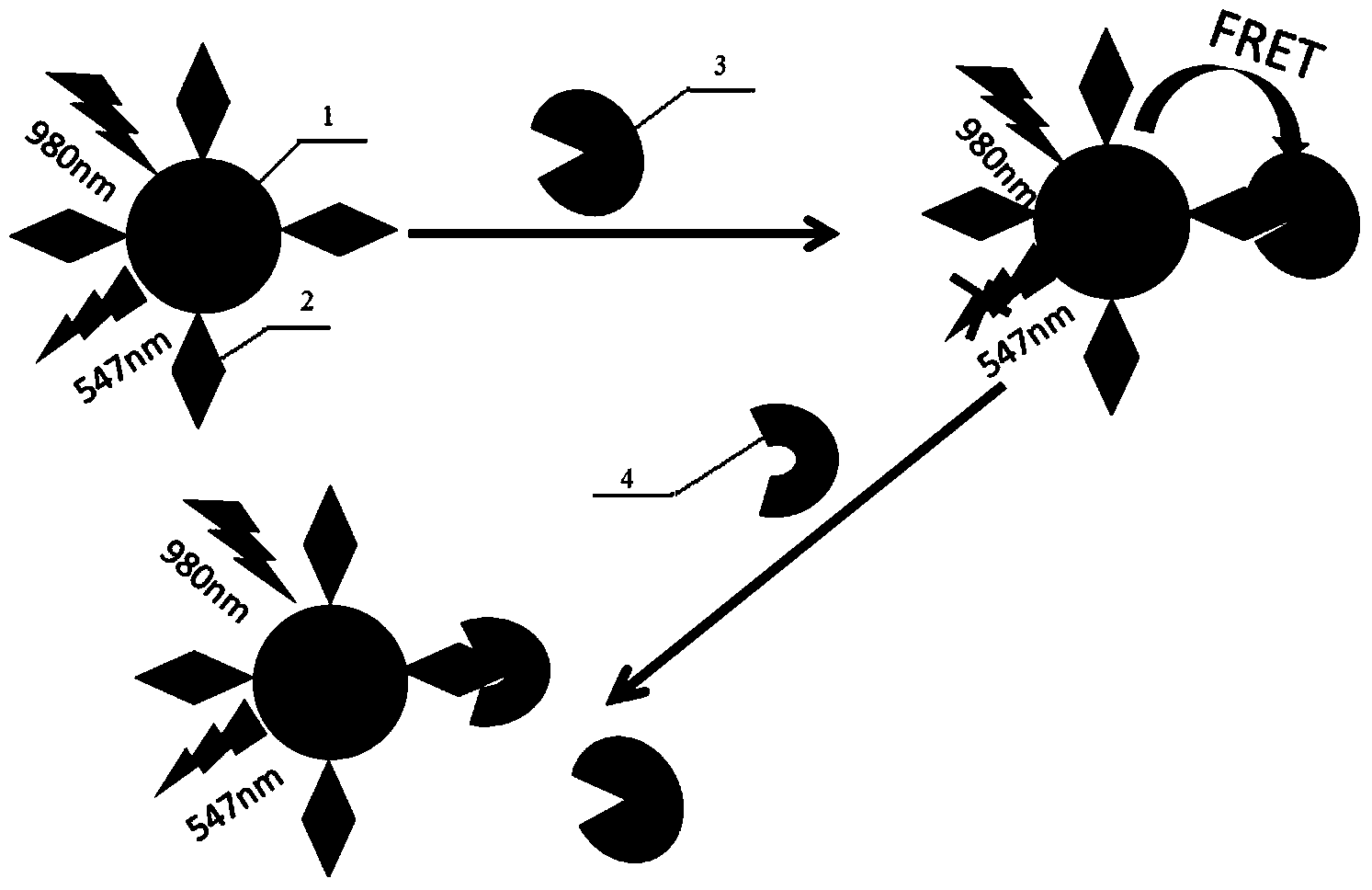
Method for detecting upconversion fluorescence resonance energy transfer by using carbon nanomaterial as receptor
InactiveCN103487418ACarbon source is easy to getEasy to prepareFluorescence/phosphorescenceLuminescent compositionsSurface markerQuenching
The invention discloses a method for detecting fluorescence resonance energy transfer by using a water-soluble upconversion fluorescence nanomaterial as a fluorescence donor and using a carbon nanomaterial as a fluorescence receptor. The method comprises the following specific steps: (1) preparing the water-soluble upconversion fluorescence nanomaterial and performing surface marker to obtain a fluorescence donor solution; (2) preparing the carbon nanomaterial to obtain a fluorescence receptor solution; (3) mixing the fluorescence donor solution and the fluorescence receptor solution for incubation and measuring the fluorescence intensity to obtain a fluorescence quenching curve; (4) mixing certain-concentration fluorescence donor solution and certain-concentration fluorescence receptor solution for incubation, adding a target object with different concentrations for continuous incubation, measuring the fluorescence intensity and drawing a standard curve; (5) calculating the concentration of the target object in an actual sample according to the standard curve. According to the method, interference of the background fluorescence of a biological sample can be avoided, detection to serum or the target object in a whole blood sample can be directly realized, the washing and separation processes are not needed, the detection speed is high, and the cost is low.
Owner:GUANGZHOU IMPROVE MEDICAL TECH CO LTD
Photometric determination of coagulation time in undiluted whole blood
InactiveUS20060110283A1Easy to implementEasy to operateMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceAgglutinationBlood coagulations
A device, system and method for photometric detection of coagulation in whole blood. The present invention is easy to implement and operate. Furthermore, the present invention has the advantage of being considered to fulfill the desired standard of using photometry for measuring blood coagulation. Also, a photometric coagulation test device for whole blood specimens according to the present invention provides medical accuracy to the home user and, at the same time, is simple to construct. The present invention is also useful for detecting and determining blood agglutination, for example as the results of a serological reaction with an antibody.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
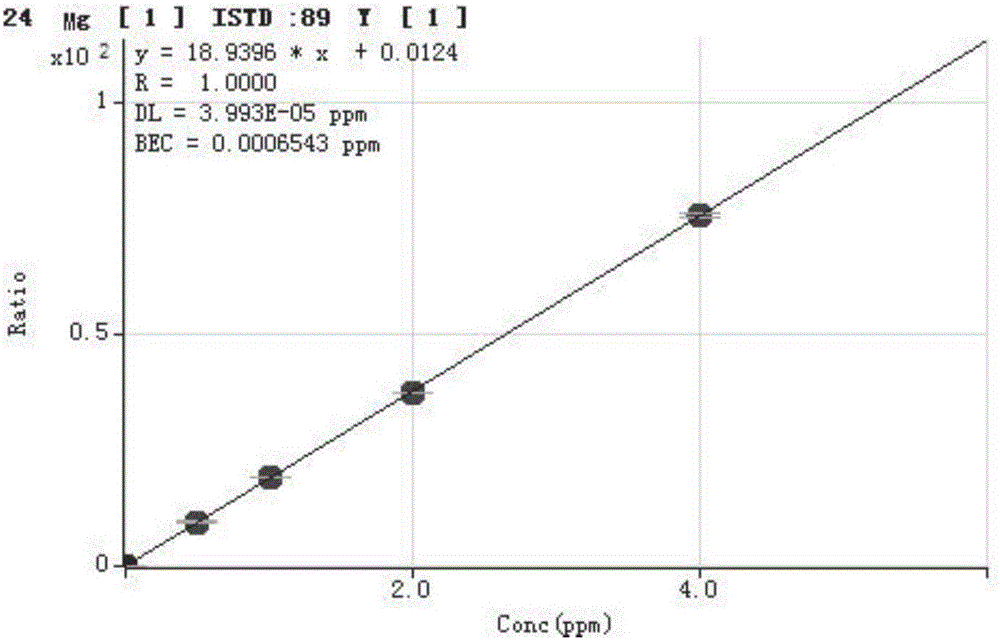
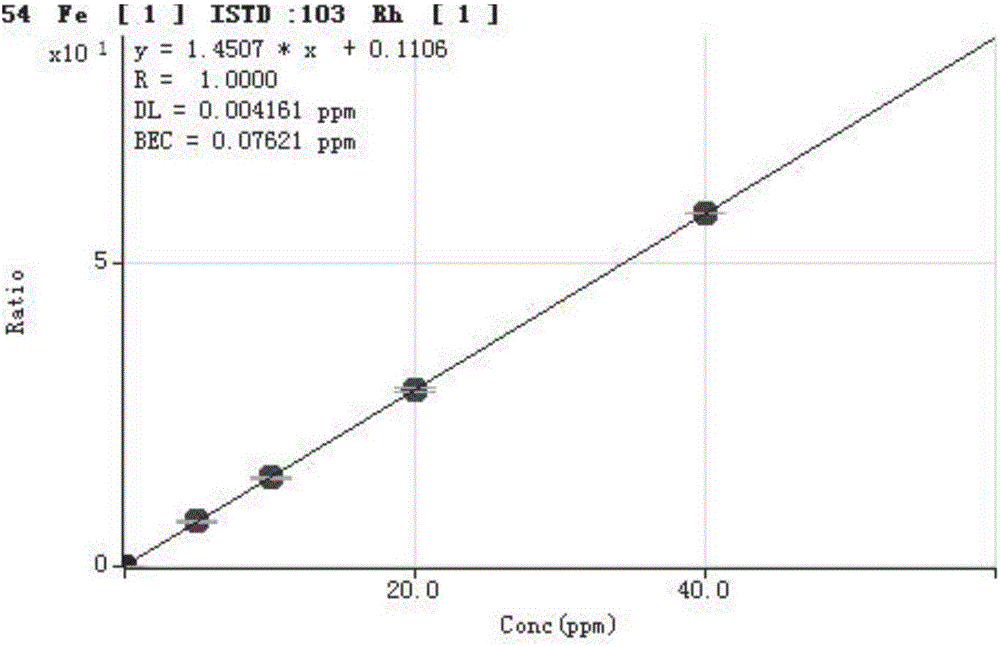
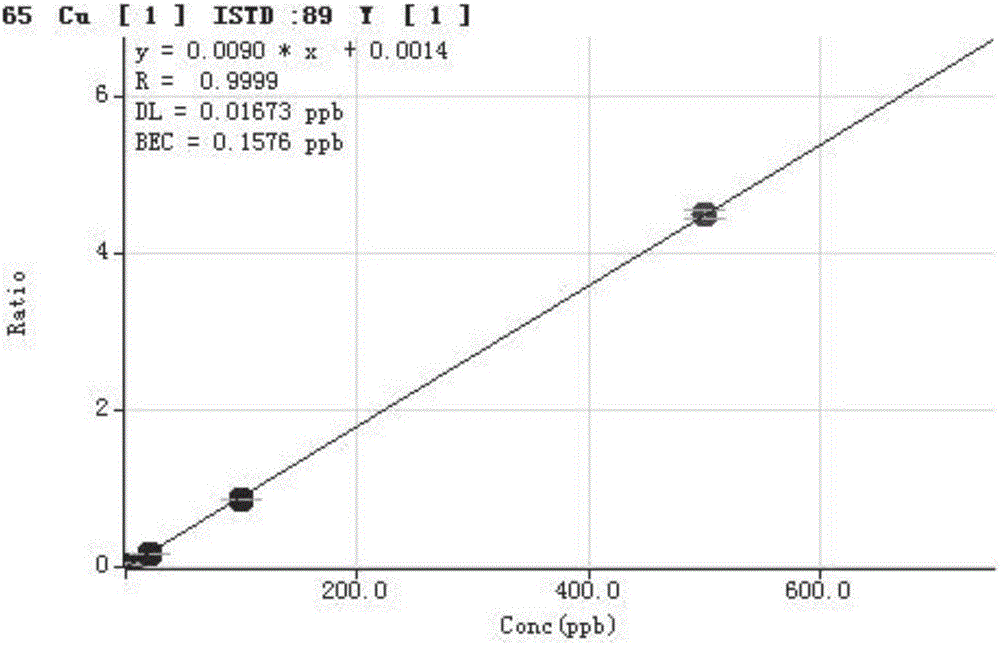
Method for detecting trace elements in human body whole blood through inductively coupled plasma mass spectrometry method
InactiveCN105784831AReduce dosageThe pre-processing process is simplePreparing sample for investigationMaterial analysis by electric/magnetic meansTrace elementManganese
The invention provides a method for detecting trace elements in human body whole blood through an inductively coupled plasma mass spectrometry method and belongs to the technical field of biotechnical detection. The method comprises the following steps: (1) pre-treating a whole blood sample; (2) detecting a mixed element standard solution and a to-be-detected sample solution by an inductively coupled plasma mass spectrometer; and (3) performing quantitative analysis on a detection result and calculating the content of to-be-detected elements in the human body whole blood sample. The method provided by the invention can be used for simultaneously detecting the content of trace elements, such as magnesium, manganese, calcium, iron, copper, zinc and lead, in the human body whole blood; the sample dosage is less; the pretreatment is simple; the cost is low; the whole detection process is short in time and high in efficiency; the detection sensitivity and precision andthe detection stability are high.
Owner:GUANGZHOU SHENGXIN BIOTECH CO LTD
Plasma on demand tube
ActiveUS20050139547A1Avoid easy cloggingEasy to useCapsClosure lidsEvacuated blood collection tubeMedicine
A device for separating plasma from whole blood is provided having an evacuated primary collection chamber capable of fluid communication through a porous filter to an evacuated secondary collection chamber. An agglutinating agent is provided within the primary collection chamber so as to aggregate blood cells within a whole blood sample. The porous filter has a pore size which is small enough to capture the aggregated blood cells therein, yet large enough to permit plasma to transfer therethrough under pressures associated with conventional evacuated blood collection tubes. The primary and secondary collection chambers may be provided in separate containers or tubes, with transfer occurring therebetween through a transfer device including the porous filter therein.
Owner:BECTON DICKINSON & CO
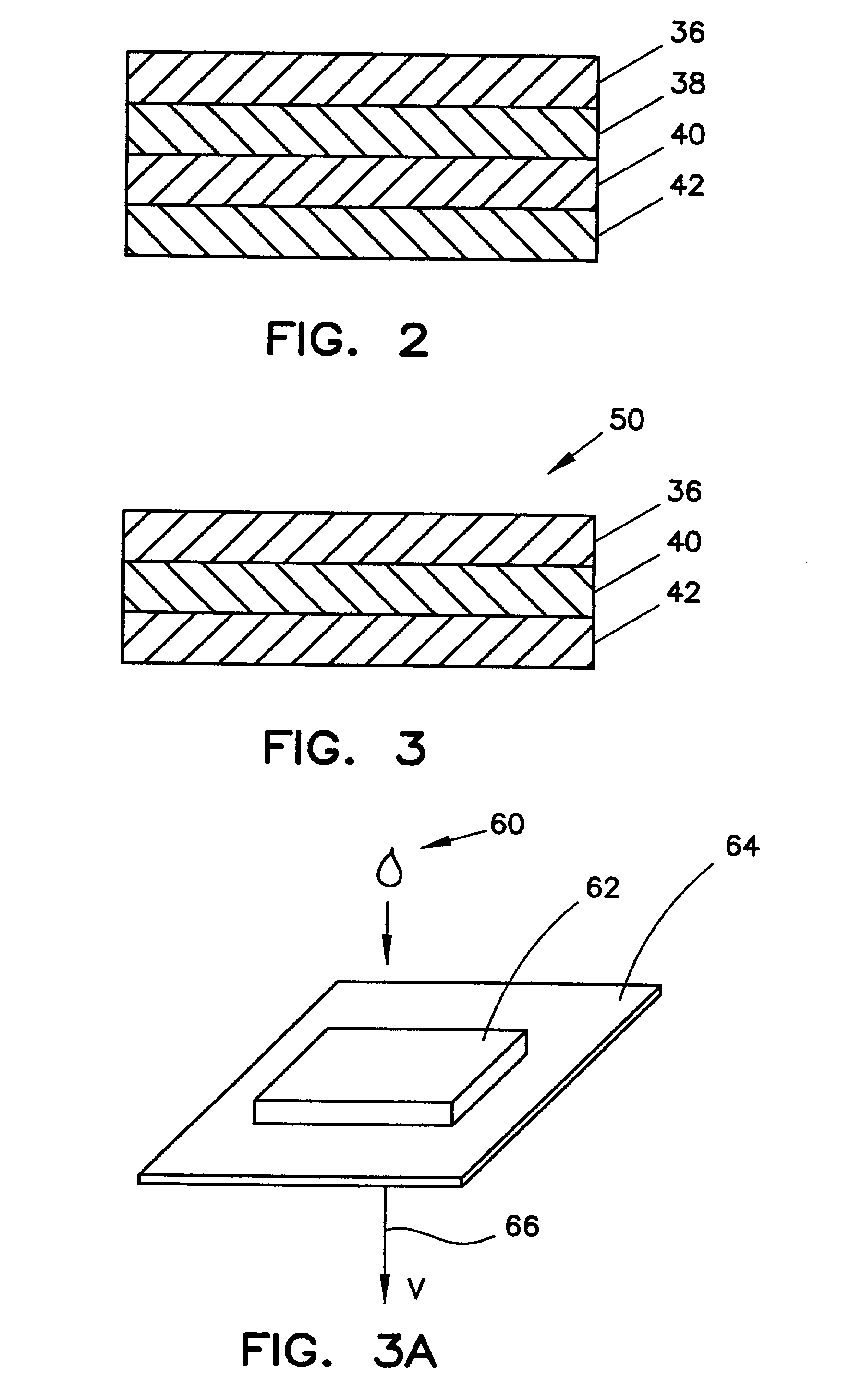
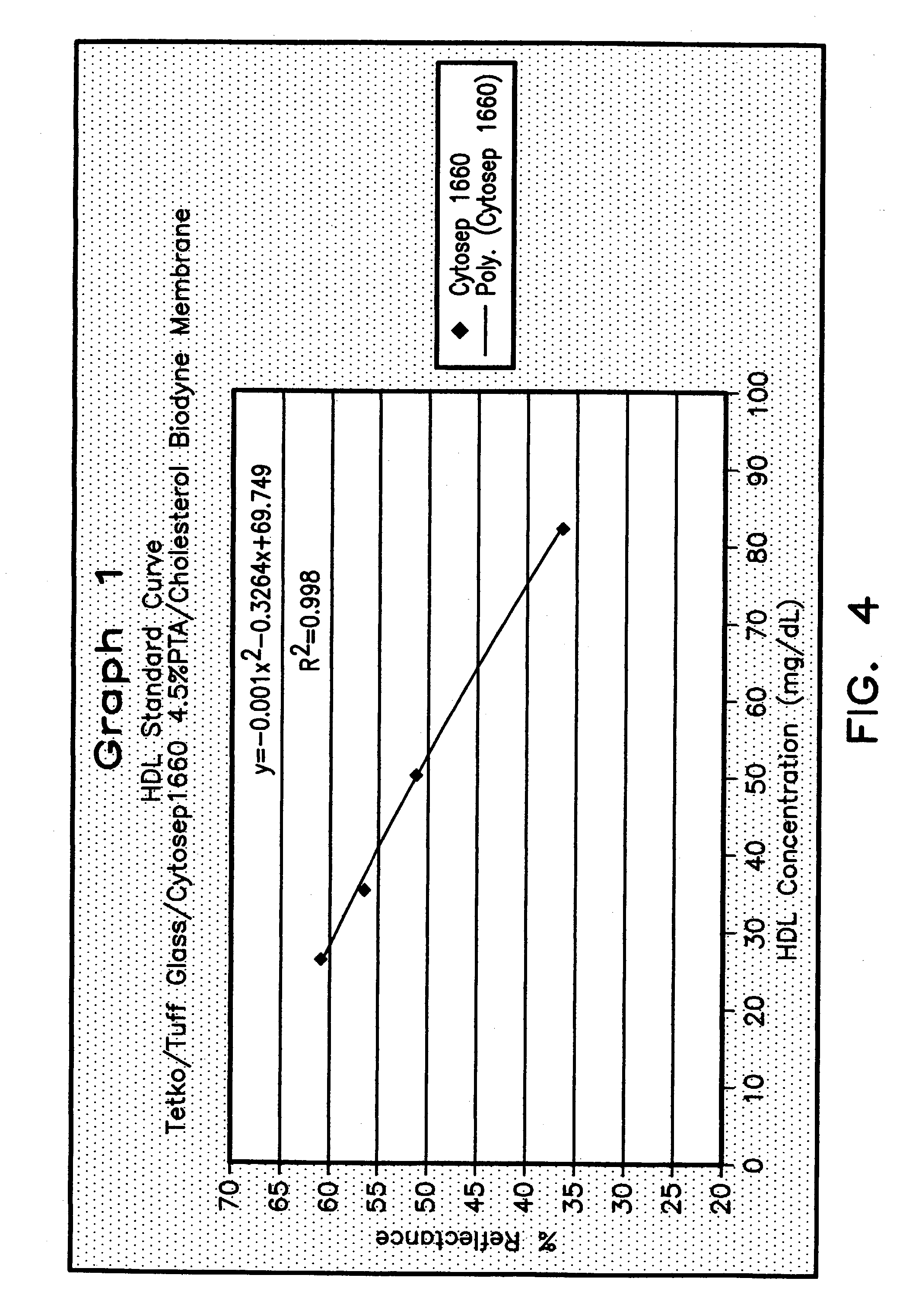
Method for determining HDL concentration from whole blood or plasma
ActiveUS7087397B2Avoid flowEasy and fast assemblyAnalysis using chemical indicatorsMicrobiological testing/measurementReaction layerCholesterol
A multilayer test strip and method of using the test strip for determining concentration of HDL cholesterol in a whole blood sample. The inventive test strip includes a two-stage blood separation mechanism, including a first glass fiber matrix which separates most of the blood cells and an adjacent, second matrix preferably also containing glass fibers that separates the remainder of the blood cells. The second layer also precipates and retains non-HDL cholesterol, thereby providing plasma that is substantially free of red blood cells and substantially free of non-HDL cholesterol to a reaction layer. Precipitation and retention on non-HDLs takes place by a vertical or dead-end filtration in a single layer. The reaction layer produces a color, the intensity of which is proportional to the concentration of HDL cholesterol in the blood sample which is applied to the test strip. Advantageously, the inventive test strip is a vertical flow device, which can be made more compact and operates more efficiently than a lateral flow device.
Owner:POLYMER TECH SYST
Automated microscopic cell analysis
InactiveUS20170328924A1Eliminate Bubble ProblemsSolve insufficient capacityReagent containersPreparing sample for investigationWhite blood cellRed blood cell
Disclosed in one aspect is a method for performing a complete blood count (CBC) on a sample of whole blood by metering a predetermined amount of the whole blood and mixing it with a predetermined amount of diluent and stain and transferring a portion thereof to an imaging chamber of fixed dimensions and utilizing an automated microscope with digital camera and cell counting and recognition software to count every white blood cell and red blood corpuscle and platelet in the sample diluent / stain mixture to determine the number of red cells, white cells, and platelets per unit volume, and analyzing the white cells with cell recognition software to classify them.
Owner:MEDICA CORP
Sensor device for and a method of sensing particles
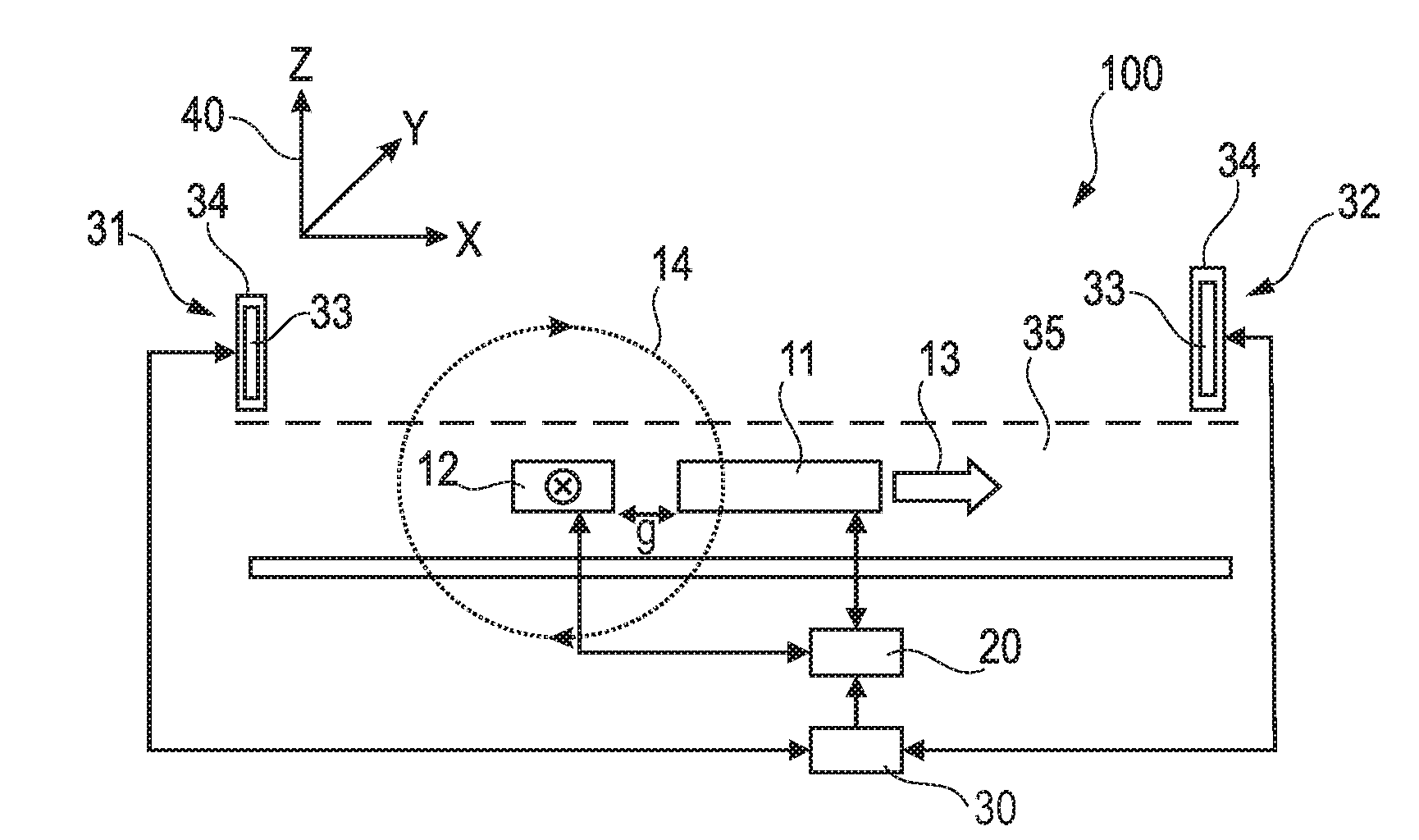
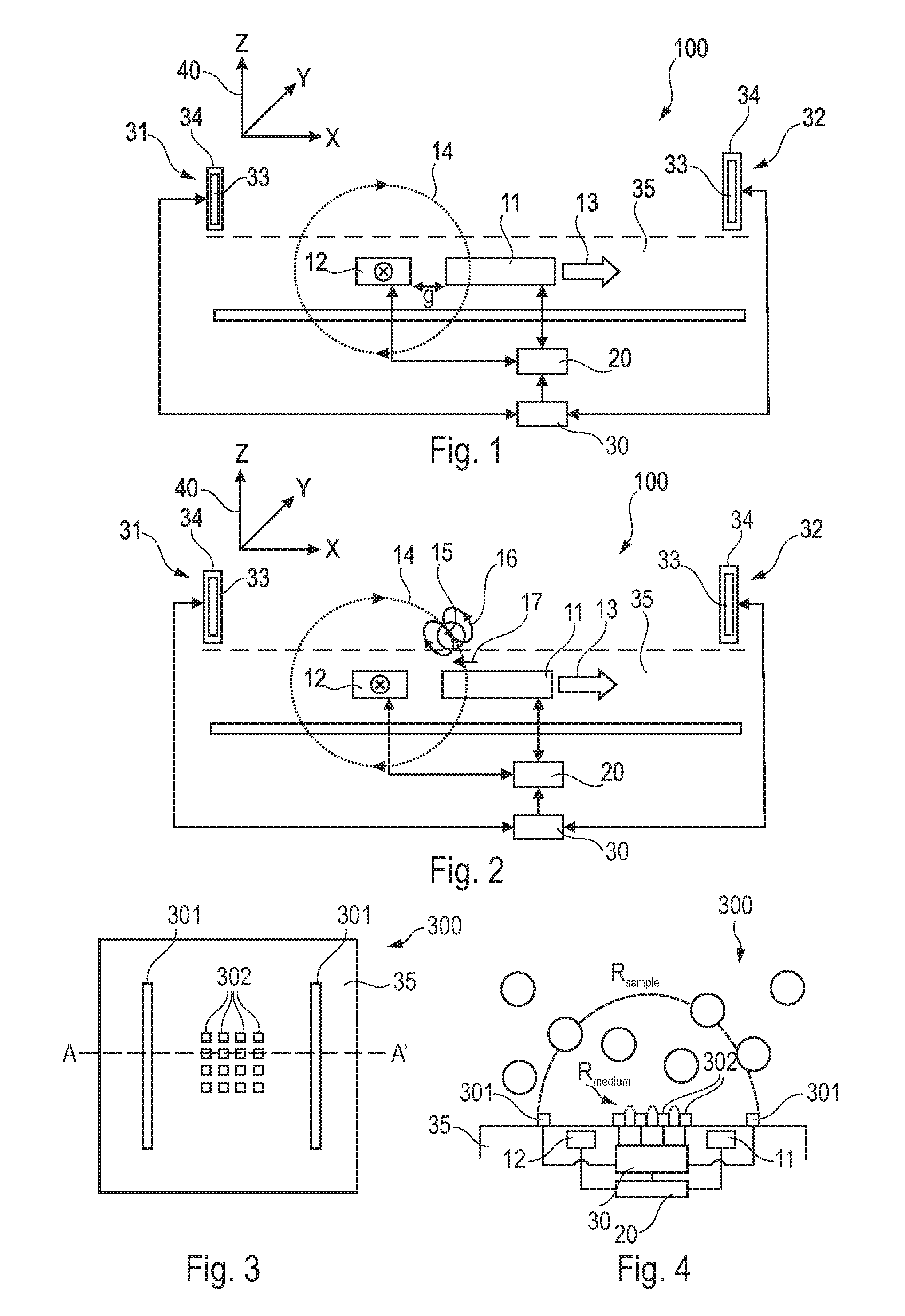
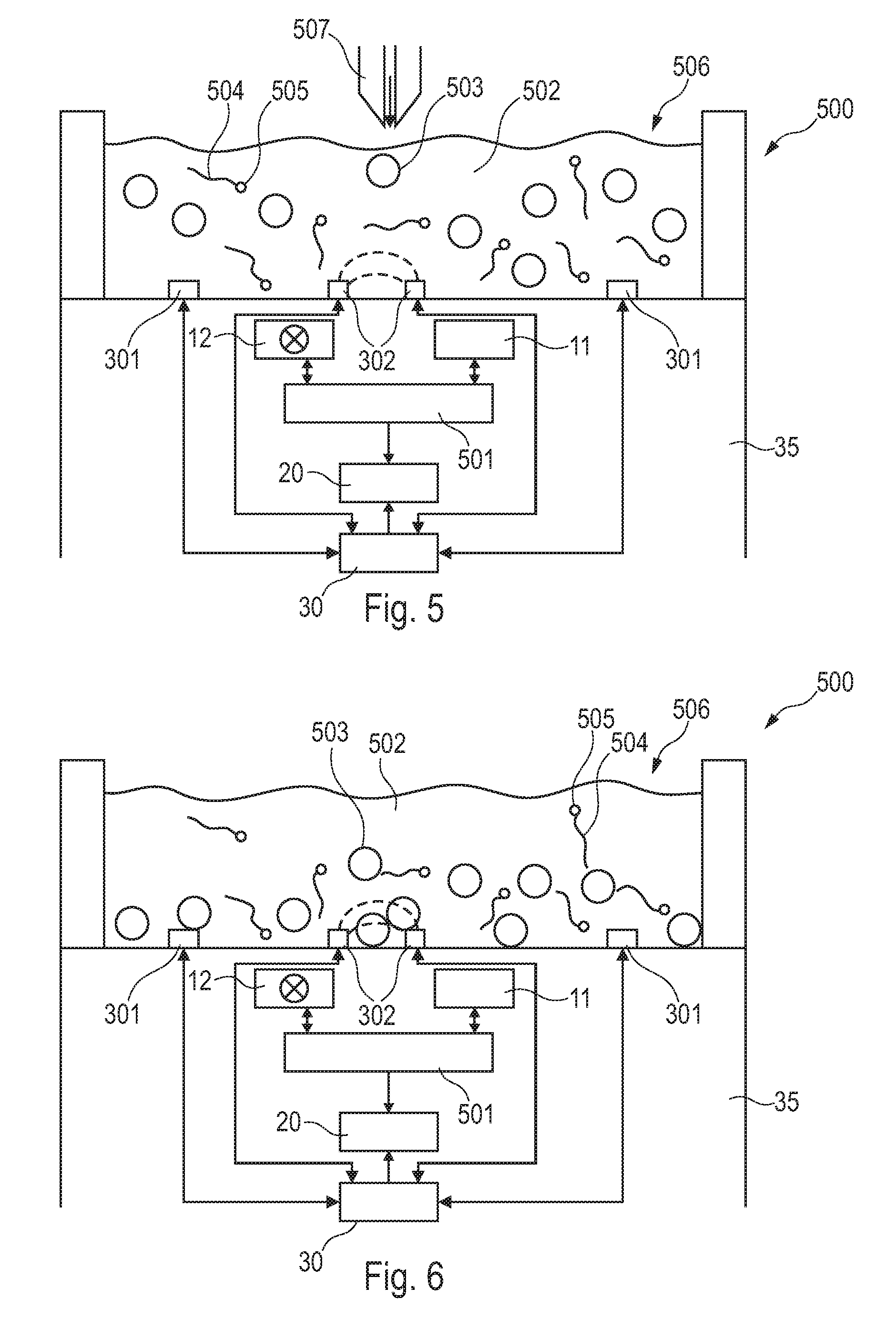
InactiveUS20090314066A1Simplifies cartridgeReduce and minimize durationResistance/reactance/impedenceIndividual particle analysisImmuno assayRed blood cell
A GMR based sensor device (100) for sensing first particles (504, 505) e.g. magnetic beads for immuno assay of a sample comprising the first particles (504, 505) and second particles (503) e.g. red blood cells, the sensor device (100) comprising a detection unit (11, 12) adapted to detect a signal which depends on a quantity of the first particles (504, 505) and which depends on a quantity of the second particles (503)″ based on a measurement performed with the sample comprising the first particles (504, 505) and the second particles (503), an estimation unit (30) for estimating information indicative of the quantity of the second particles (503) e.g. haematocrit based on an impedance measurement, and a determining unit (20) adapted for determining the quantity of the first particles (504, 505) based on the detected signal under consideration of the estimated information. The advantage of this arrangement is that whole blood samples may be used.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method and apparatus for compressing imaging data of whole blood sample analyses
A method and apparatus for analyzing white blood cells (WBCs) within a whole blood sample quiescently residing within a chamber is provided. The chamber is defined by at least one transparent panel, and the whole blood sample includes at least one colorant operable to differentially identify at least one WBC type from another WBC type within the sample. The method includes the steps of: a) creating at least one image of the sample quiescently residing within the chamber; b) identifying portions of the sample image, with each portion representing a single WBC; c) compressing the sample image portions using a first compression algorithm; and d) one of compressing a remainder of the sample image not included in the portions using a second compression algorithm, or discarding the remainder.
Owner:ABBOTT POINT CARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com