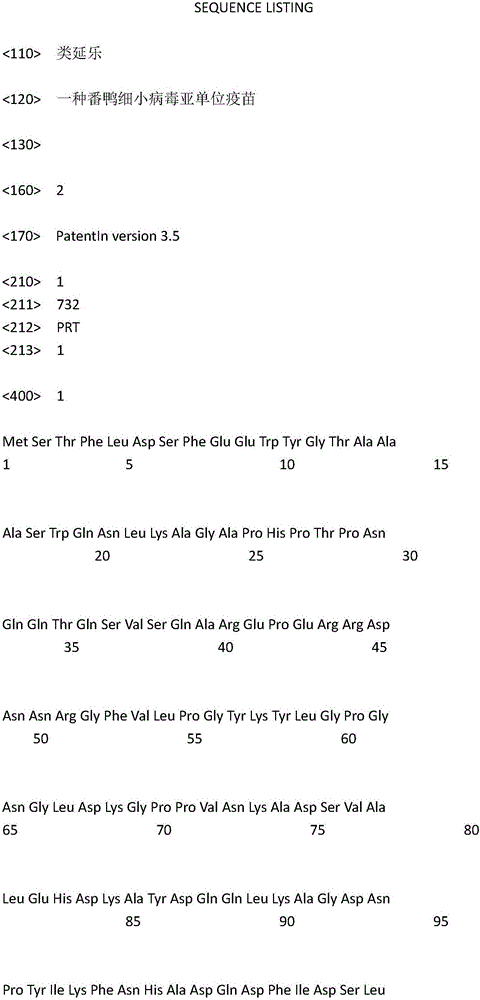Muscovy duck parvovirus subunit vaccine
A technology of Muscovy duck parvovirus and subunit vaccine, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of virus spread, parvovirus difference, harm, etc., and achieve the prevention of muscovy duck and gosling plague virus infection , the effect of increasing the antibody level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
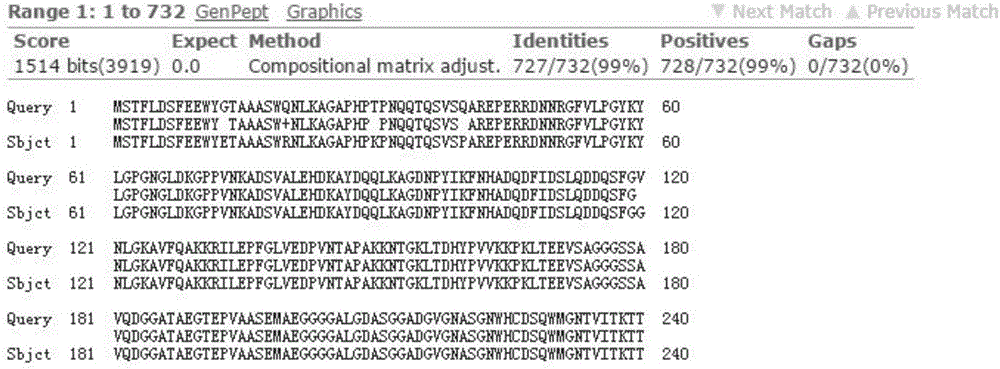
[0014] Embodiment 1, the screening of Muscovy duck parvovirus VP protein
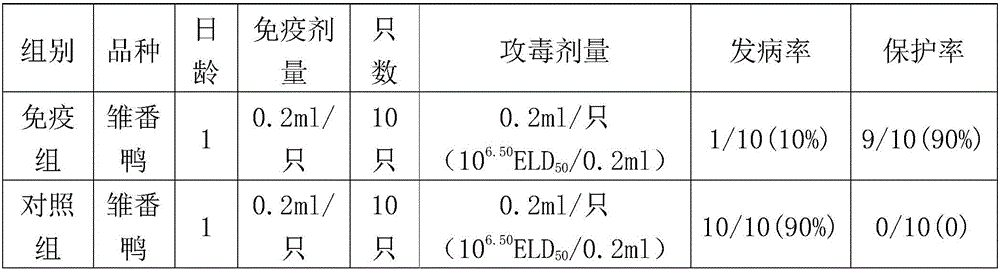
[0015] In 2014, typical symptoms of Muscovy duck parvovirus disease appeared in young Muscovy ducks in a certain duck farm. However, the infected ducks had been vaccinated against Muscovy duck parvovirus before. Therefore, it was suspected that the pathogenic bacteria had a genetic mutation. The immune effect of the vaccine is not good. In order to isolate the source of the disease, the liver, spleen and pancreas of the dying duck were aseptically collected, homogenized with sterile saline to make a suspension, centrifuged to remove the bacteria, and then inoculated with 11-day-old Muscovy duck embryos through the allantoic cavity, and hatched for 168 After 24 hours, the allantoic fluid and embryo body tissue of the dead embryos were collected, homogenized, frozen and thawed repeatedly, and the supernatant was frozen for storage. After the harvested virus liquid was purified, the virus content, immunog...
Embodiment 2
[0018] The construction of the recombinant plasmid that embodiment 2 expresses VP gene
[0019] According to the VP gene sequence and the multiple cloning restriction site of the expression vector PET32a (+), DNAStar software was used to design primers to amplify the full sequence of the VP gene, wherein the 5' end of the forward primer added a BamHI restriction site; A NotI restriction site was added to the 3' end of the reverse primer. The GPV201401 virus was used as a template for PCR amplification, and the target gene was recovered with a DNA purification and recovery kit. Use the pET32a(+) plasmid to obtain the pET32a-VP recombinant plasmid, transfer the recombinant plasmid into expressive Escherichia coli host bacteria, pick a single clone, identify positive clones by enzyme digestion, shake the bacteria, and sequence.
Embodiment 3
[0020] Example 3. Expression, purification, renaturation and SDS-PAGE identification of recombinant fusion protein
[0021] The single cloned recombinant plasmid with correct sequencing results was transformed into expressive Rosetta host bacteria to construct recombinant genetically engineered bacteria. The recombinant genetically engineered bacteria were inoculated into LB bacterial medium containing ampicillin (100mg / L), cultured overnight at 37°C with shaking (200r / min), and the next day, 1ml of the culture was taken out and inoculated into 120ml of LB medium, 37 After culturing at ℃ for 2.5 hours with shaking (200r / min), the bacterial solution was shaken to a translucent and semi-turbid state, and the OD600 was measured by absorbance photometry=0.6~0.8. Before induction, 10ml was taken as a control before induction, and the shaking temperature was changed to Add α-lactose (final concentration: 1.0mmol / L) for induction at 30°C, collect 10ml of bacterial liquid for 1h, 2h, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com