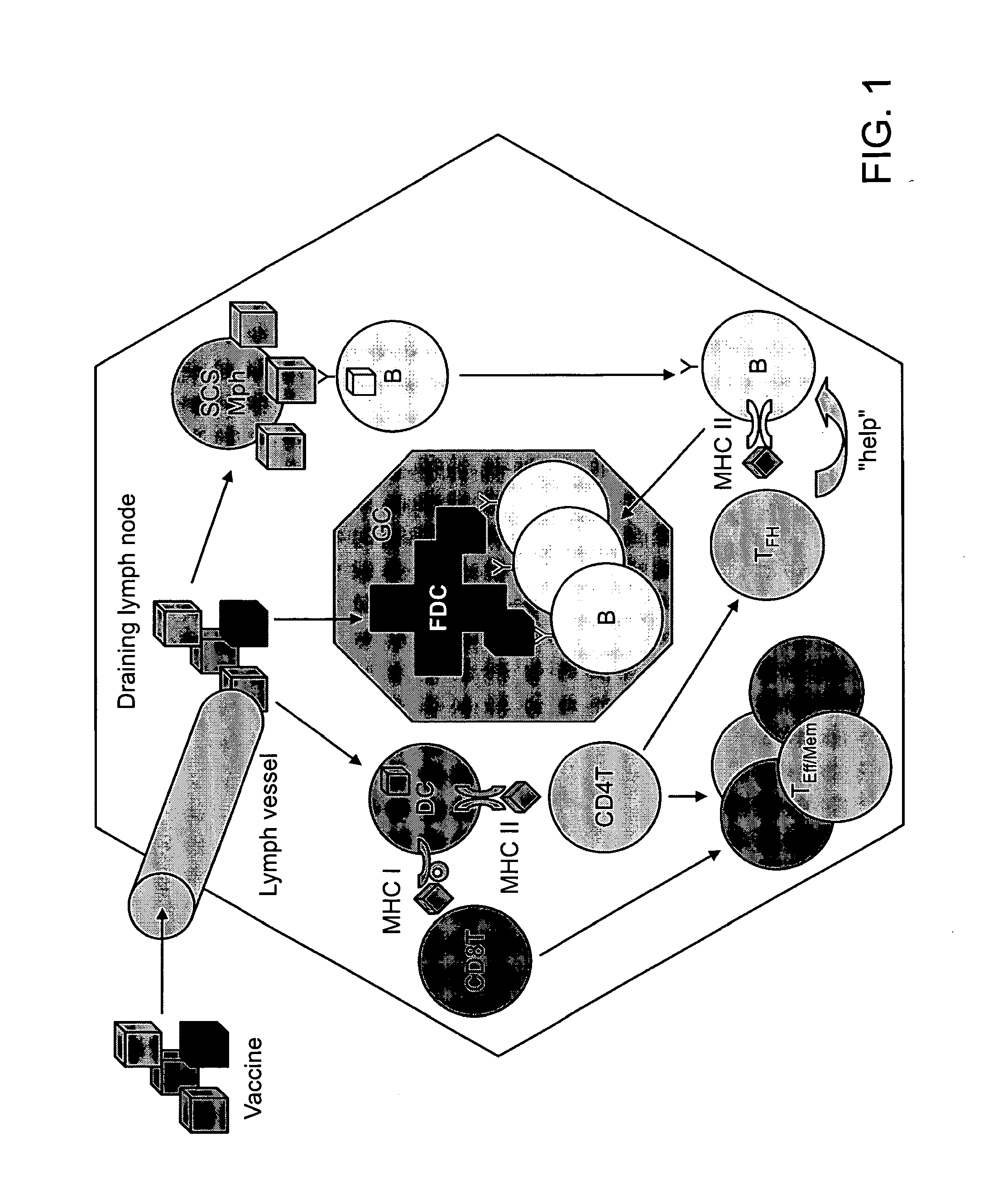
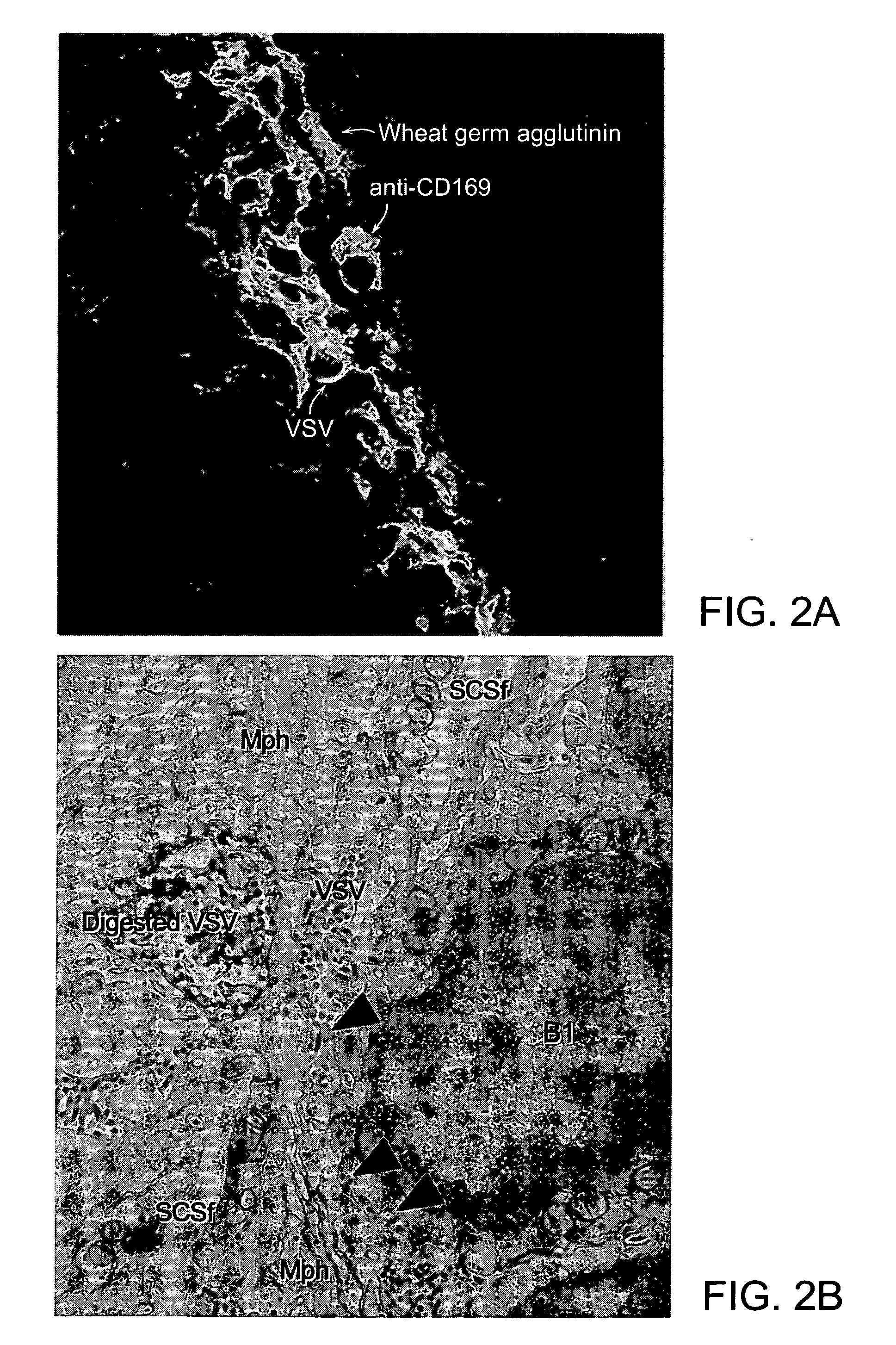
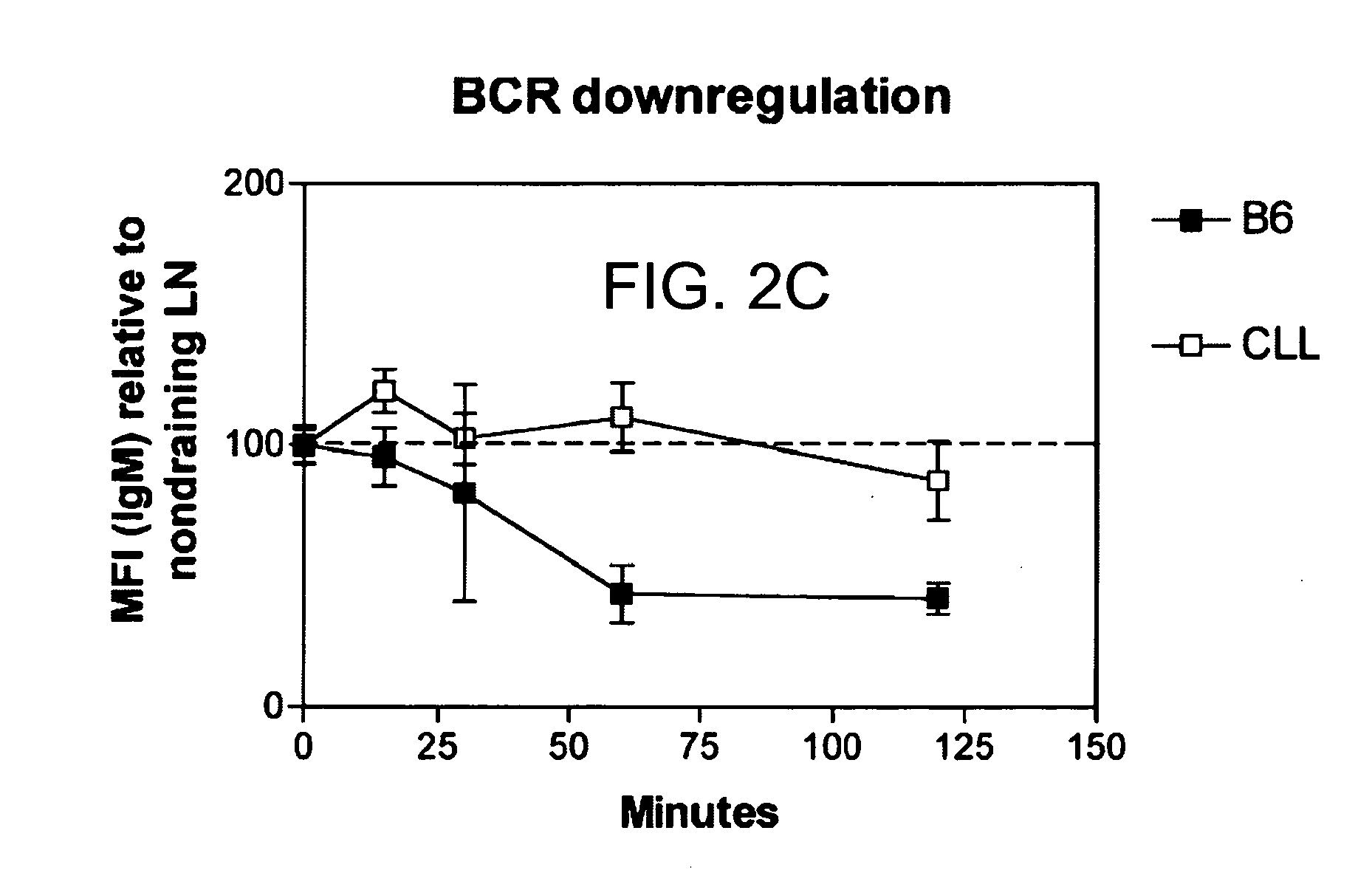
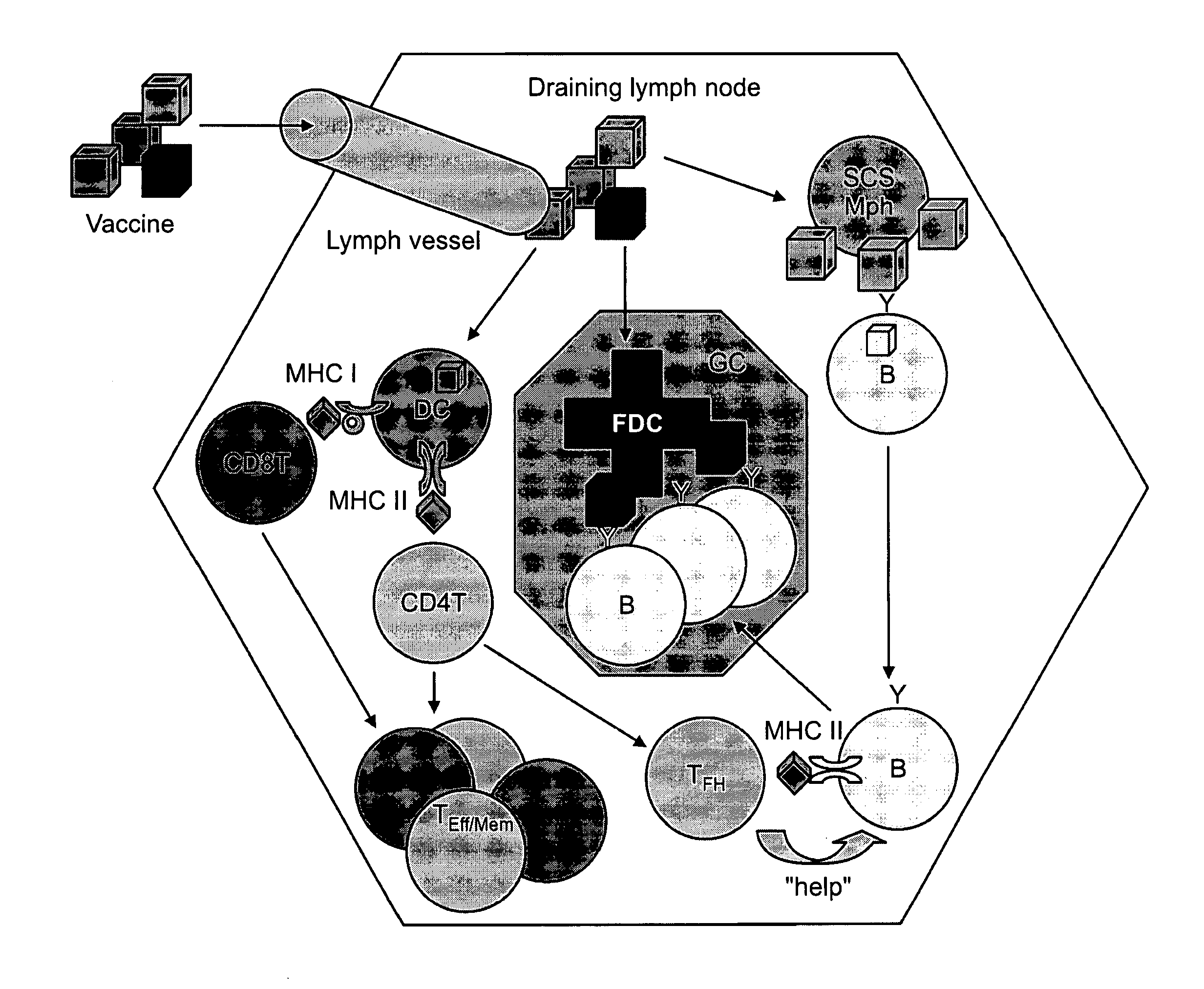
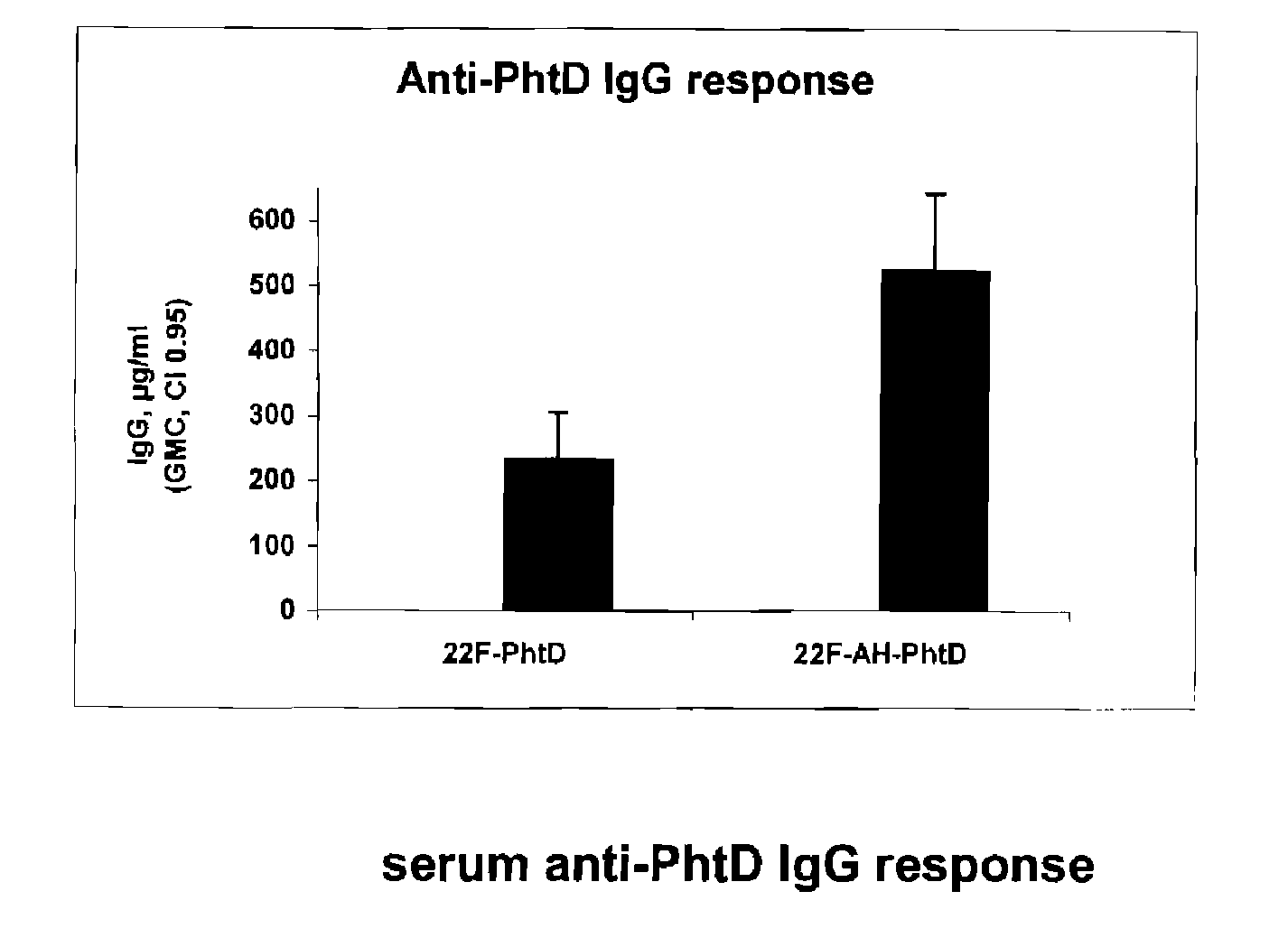
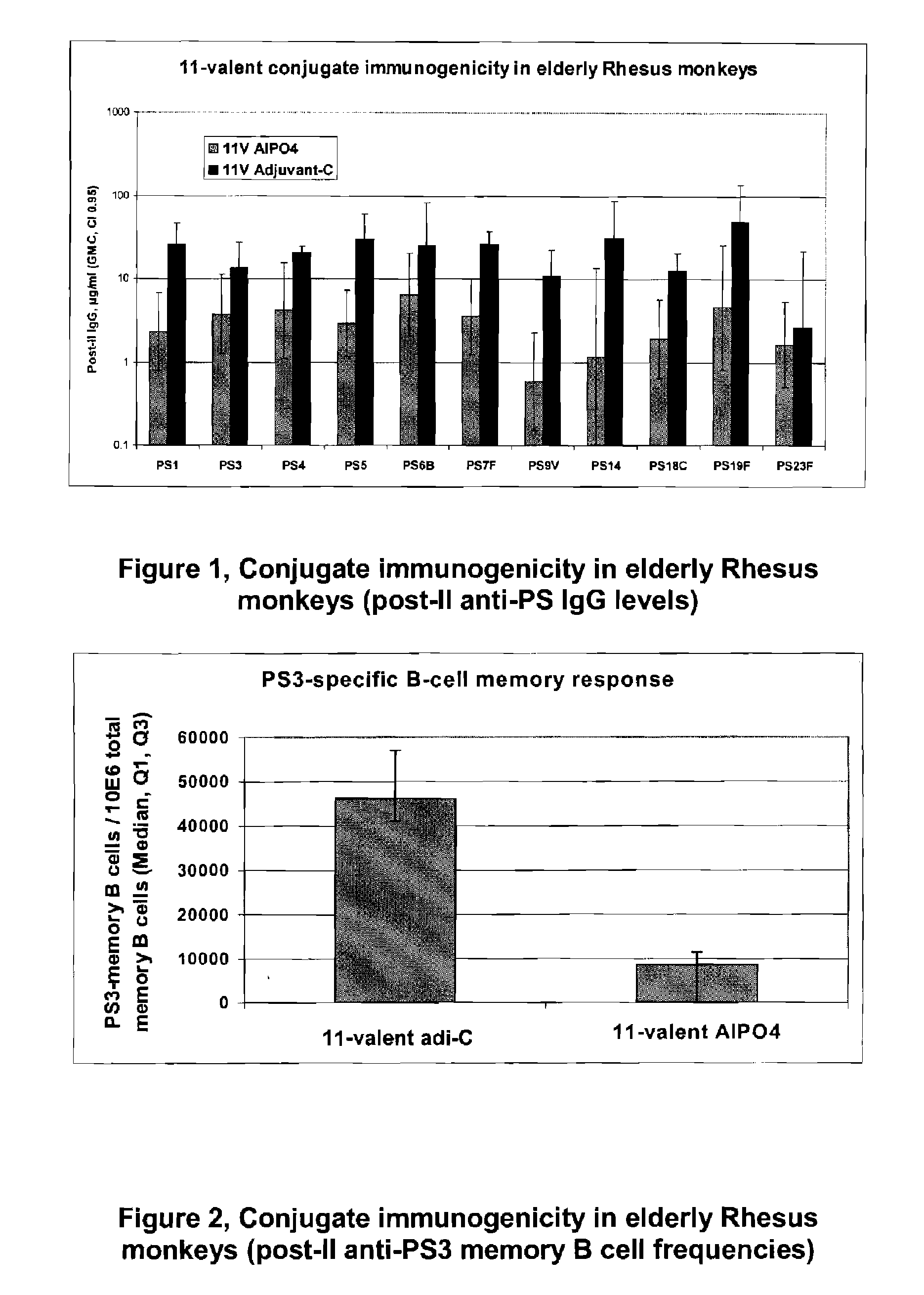
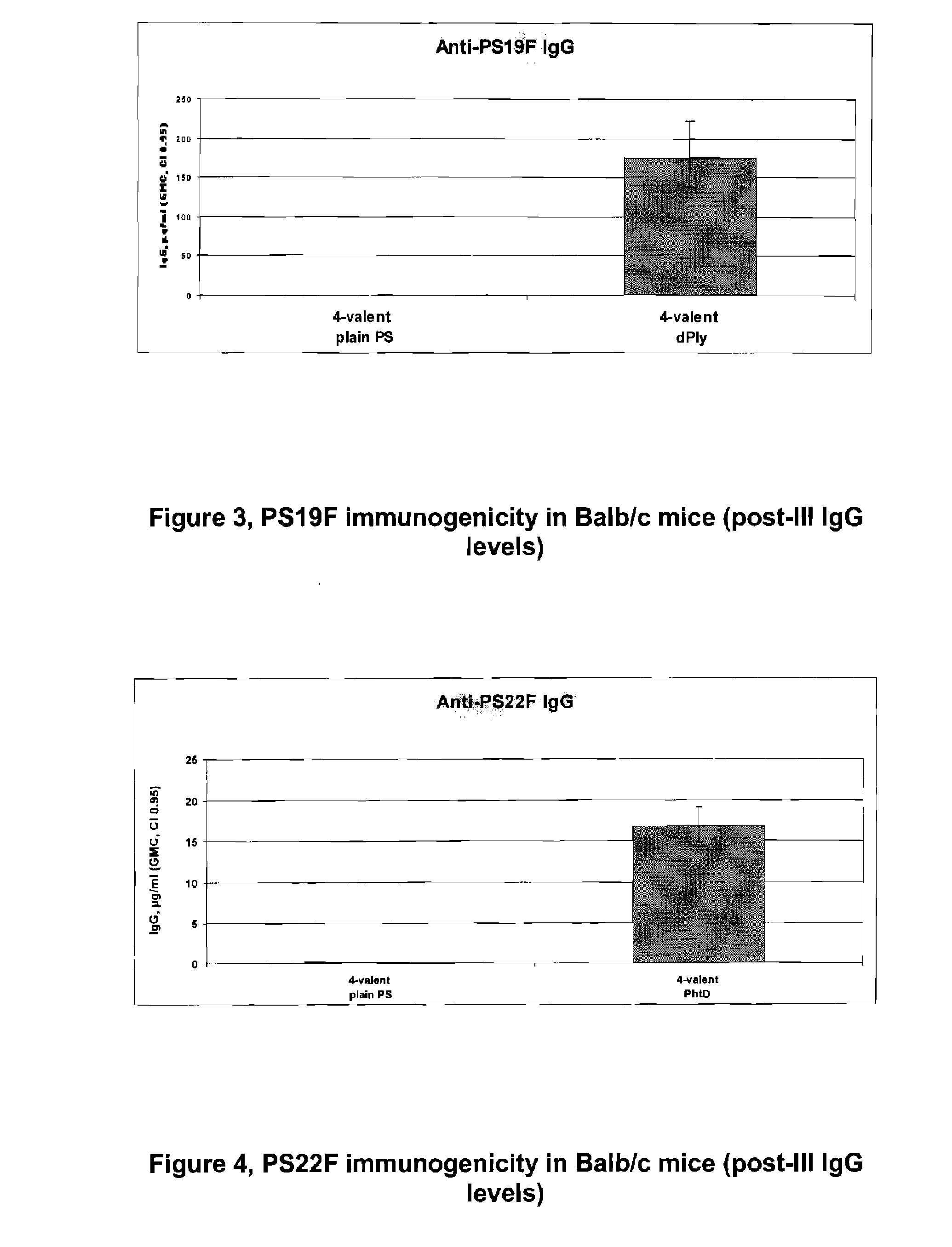
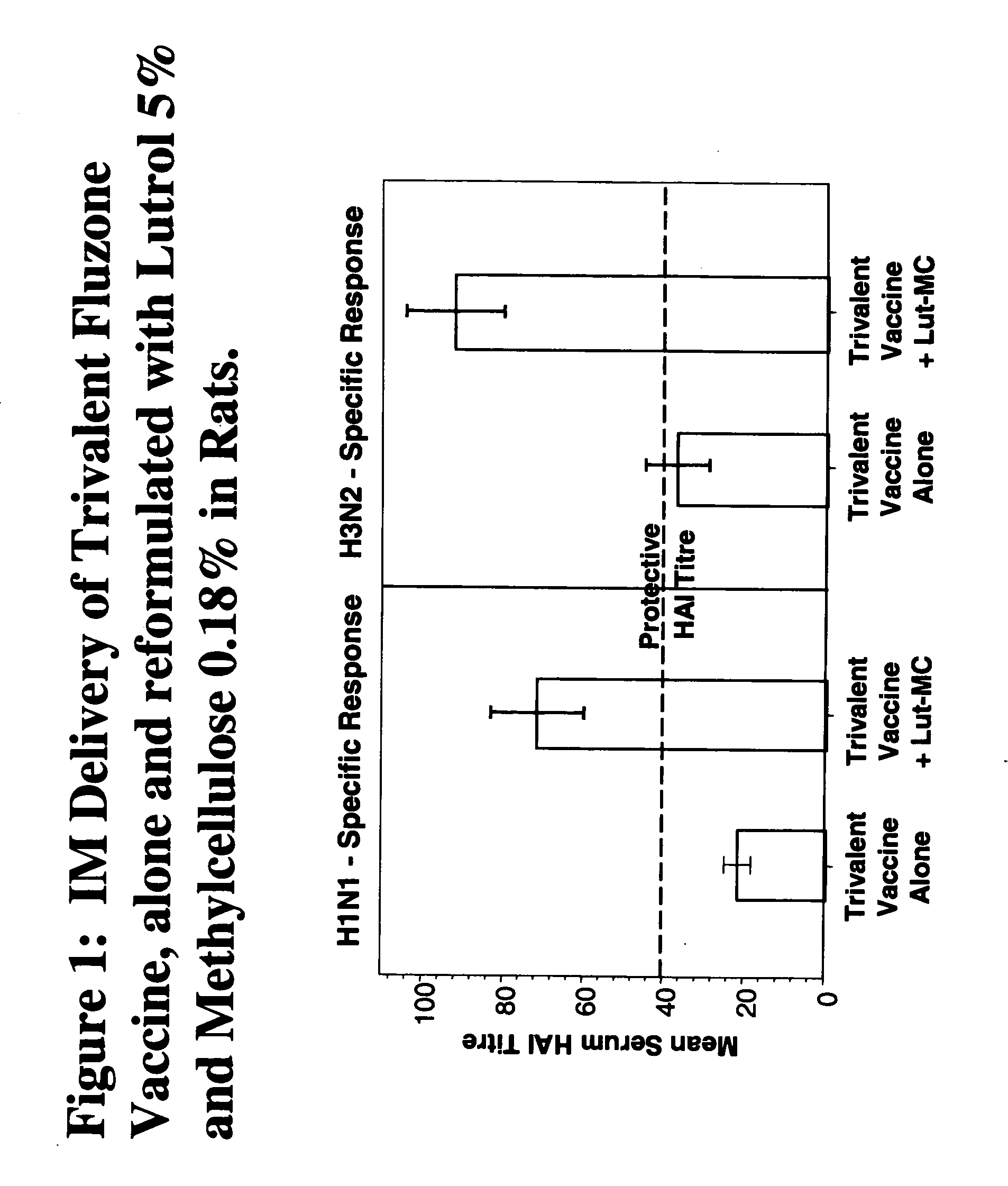
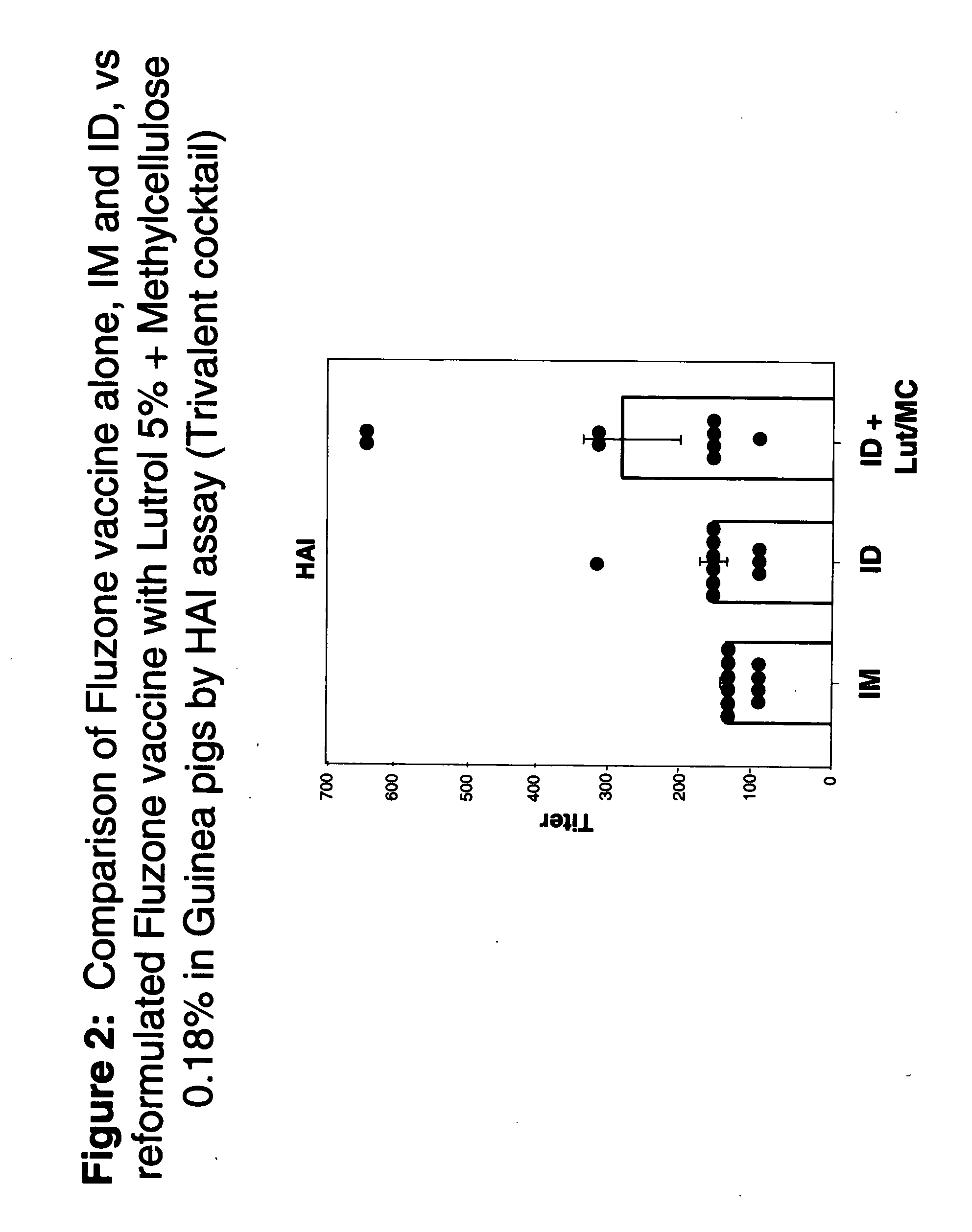
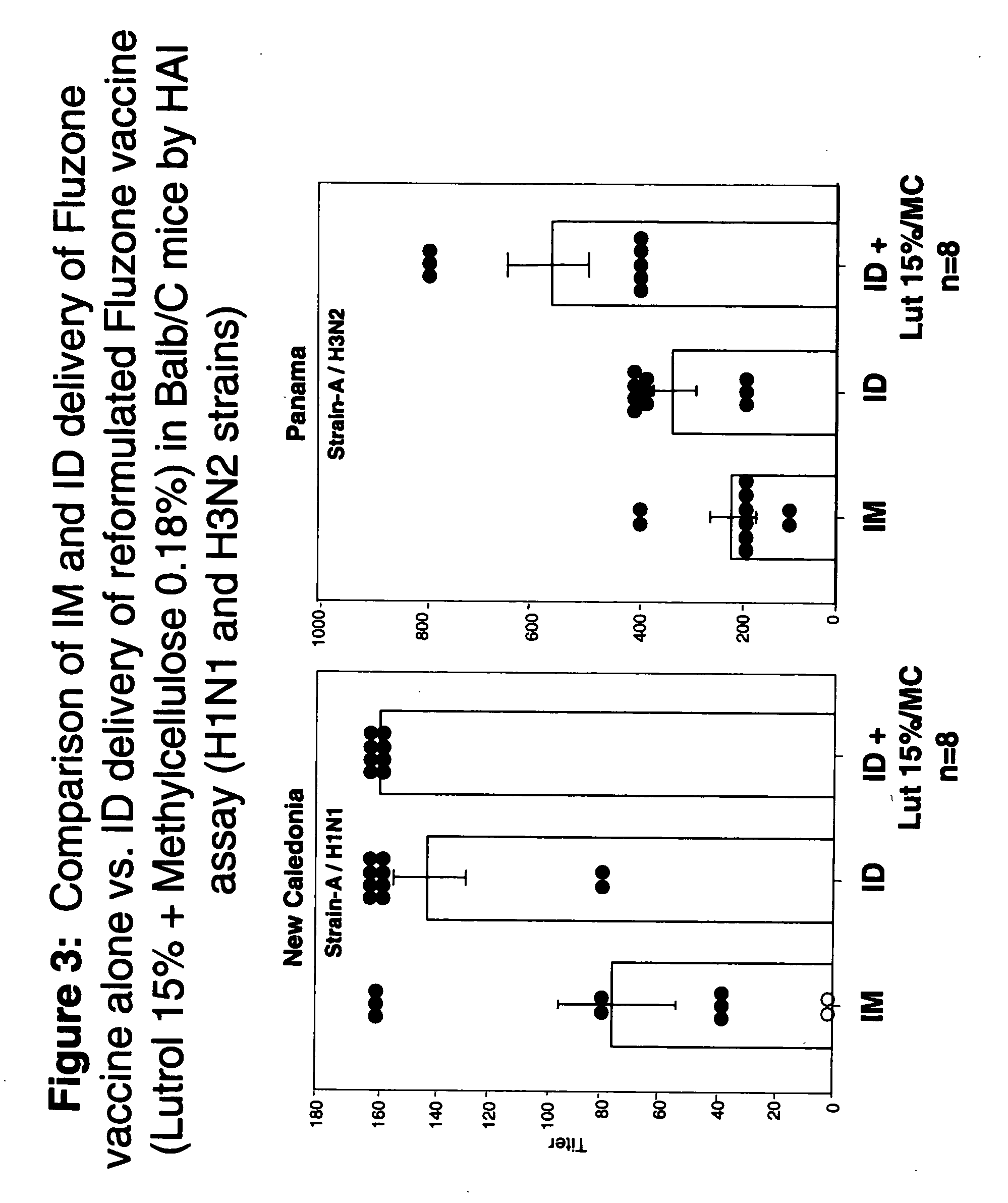
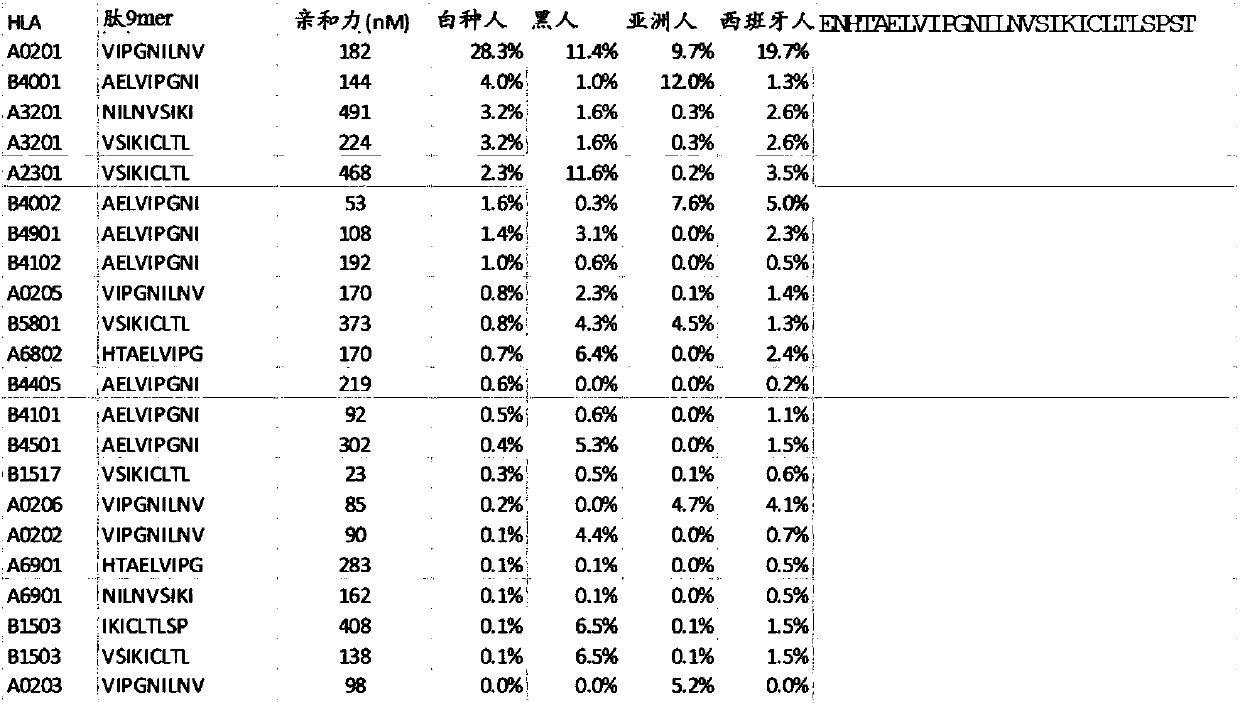
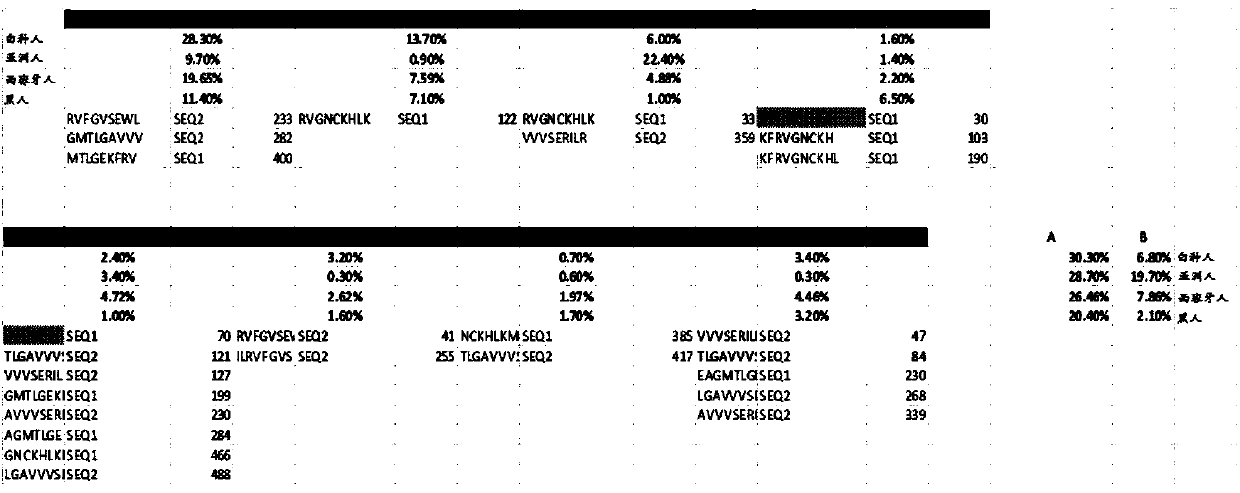
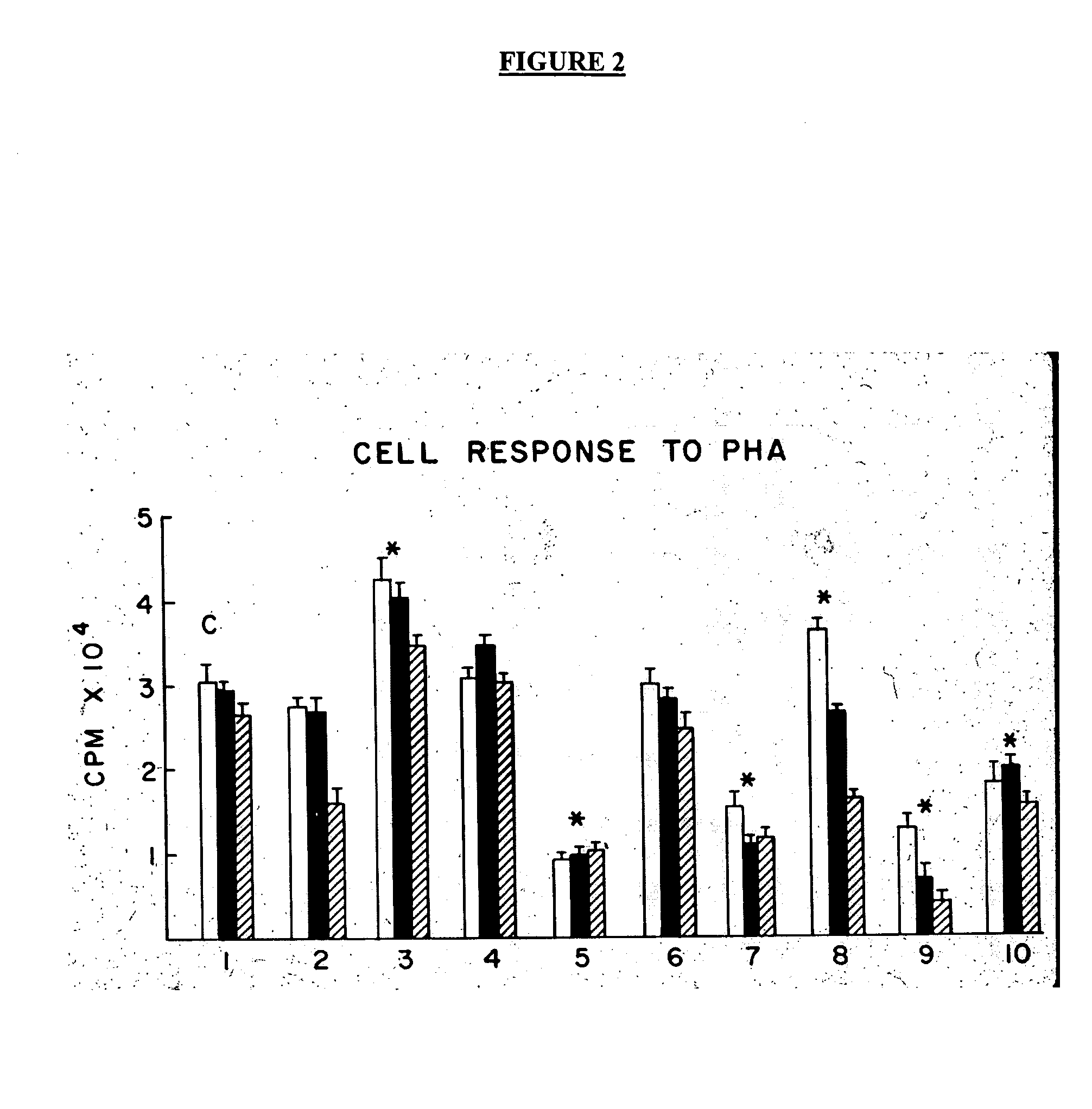
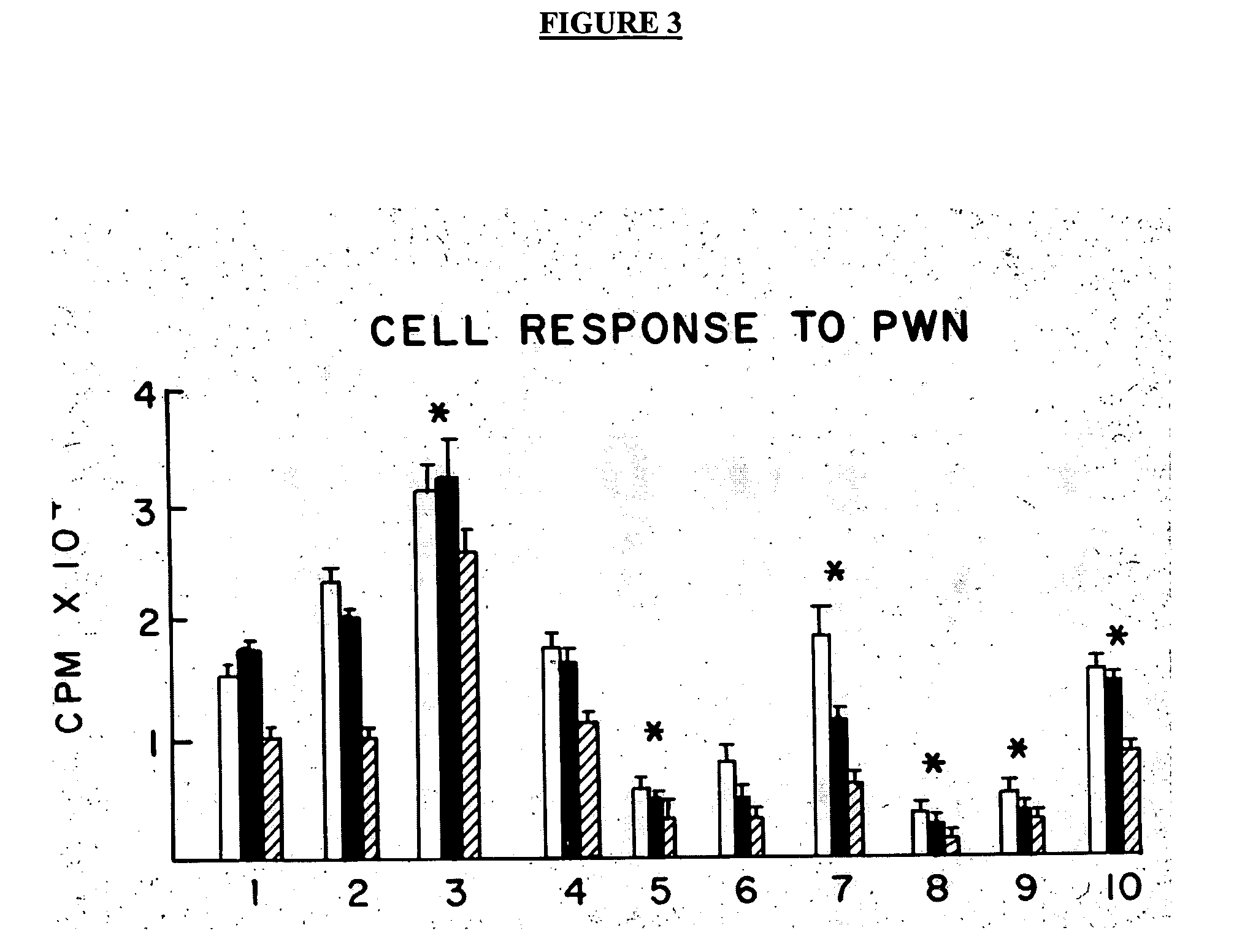
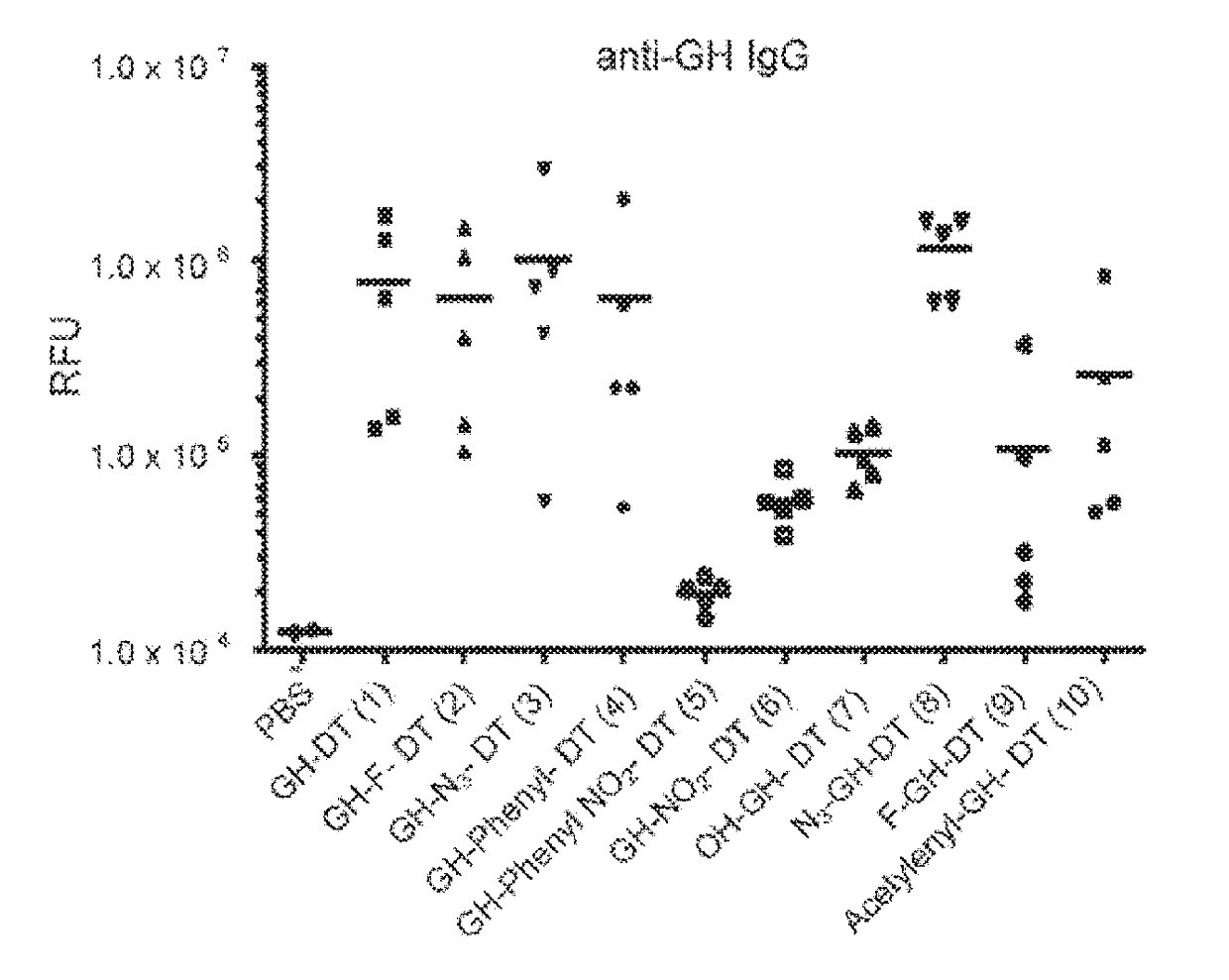
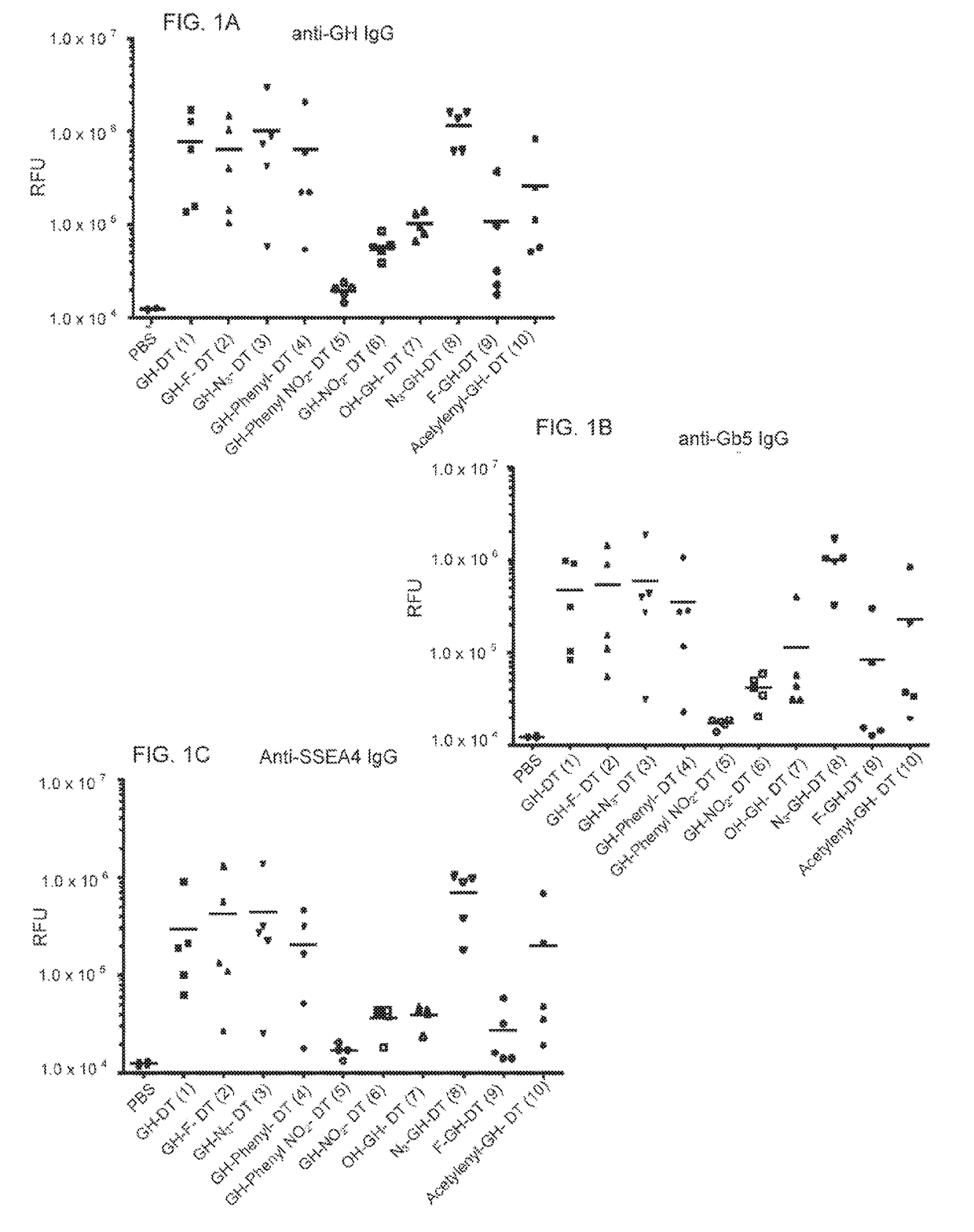
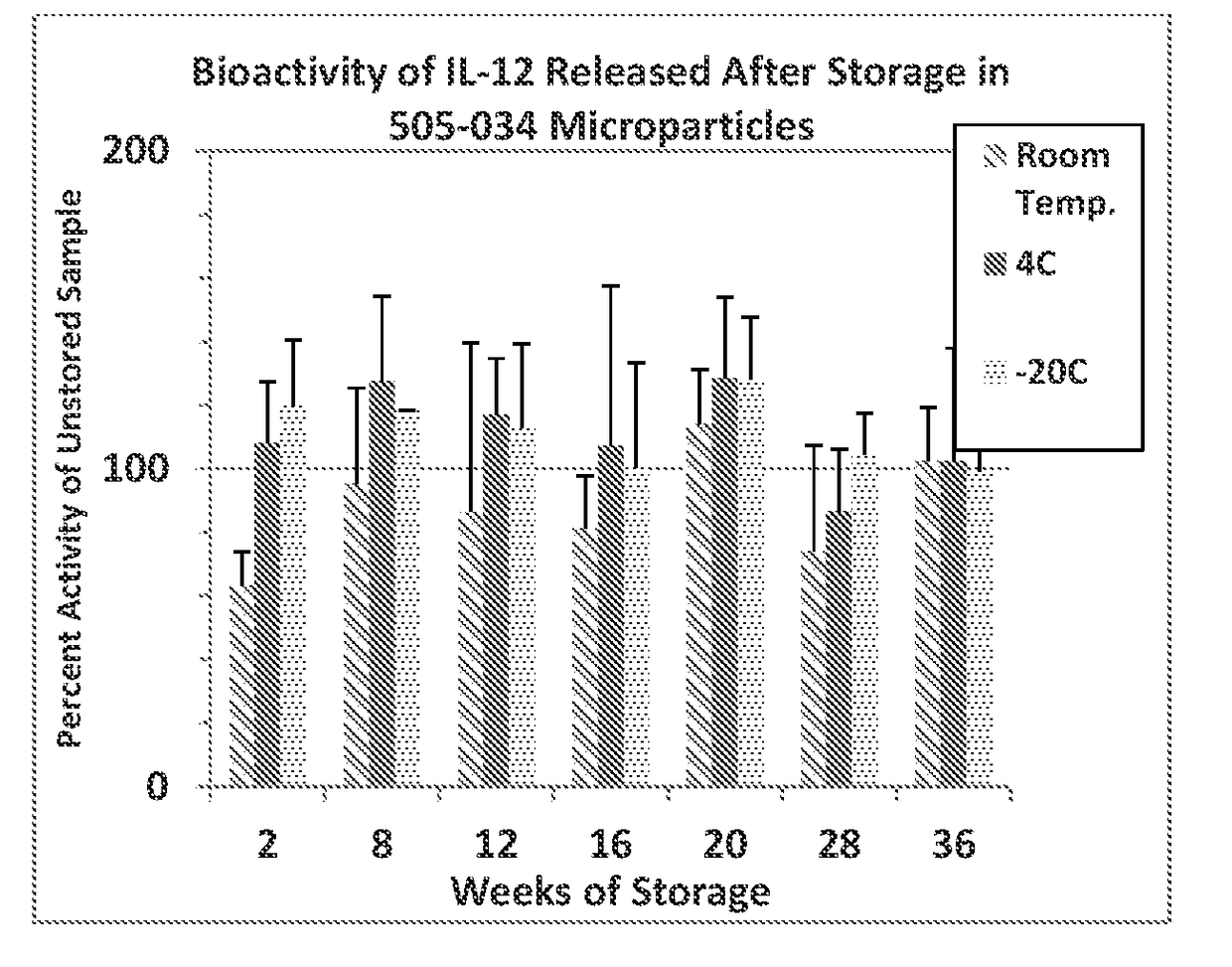
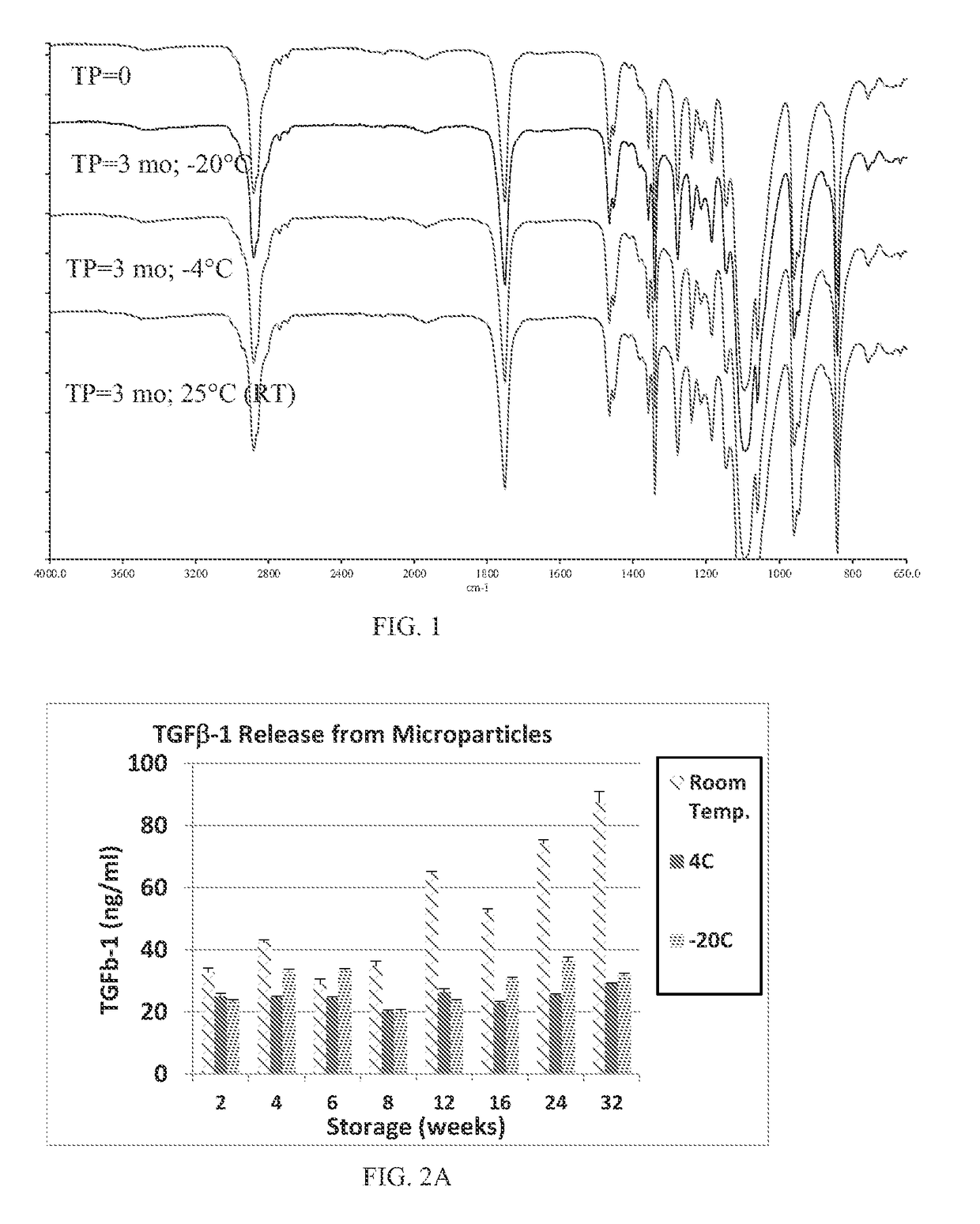
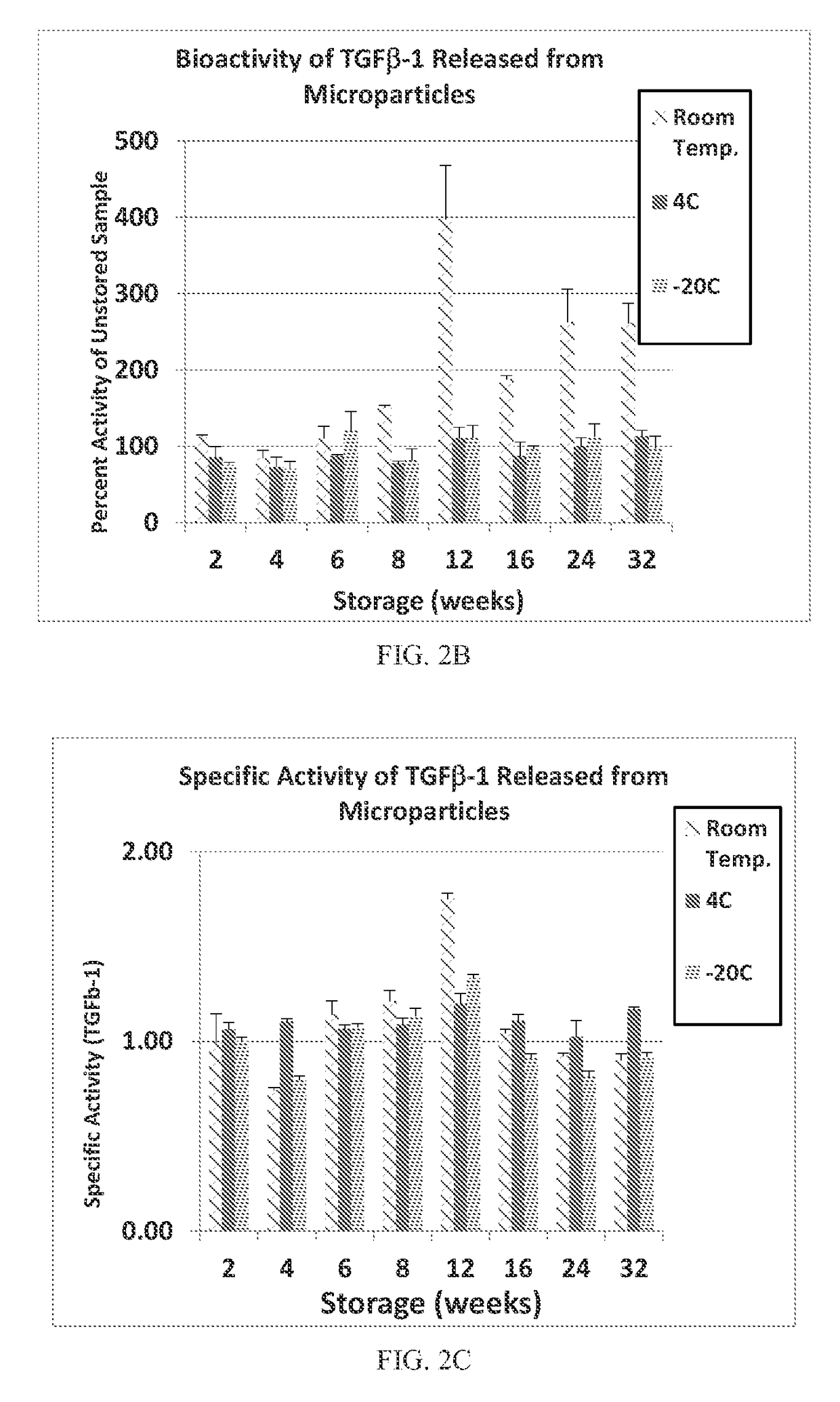
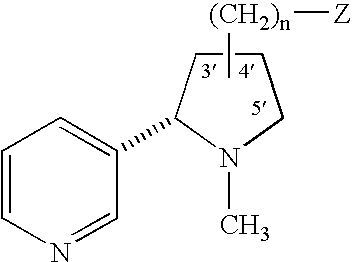
Patents
Literature
206results about "Antigen/adjuvant combination ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
Vaccine Nanotechnology
ActiveUS20130236533A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +3
Pneumococcal Polysaccharide Conjugate Vaccine
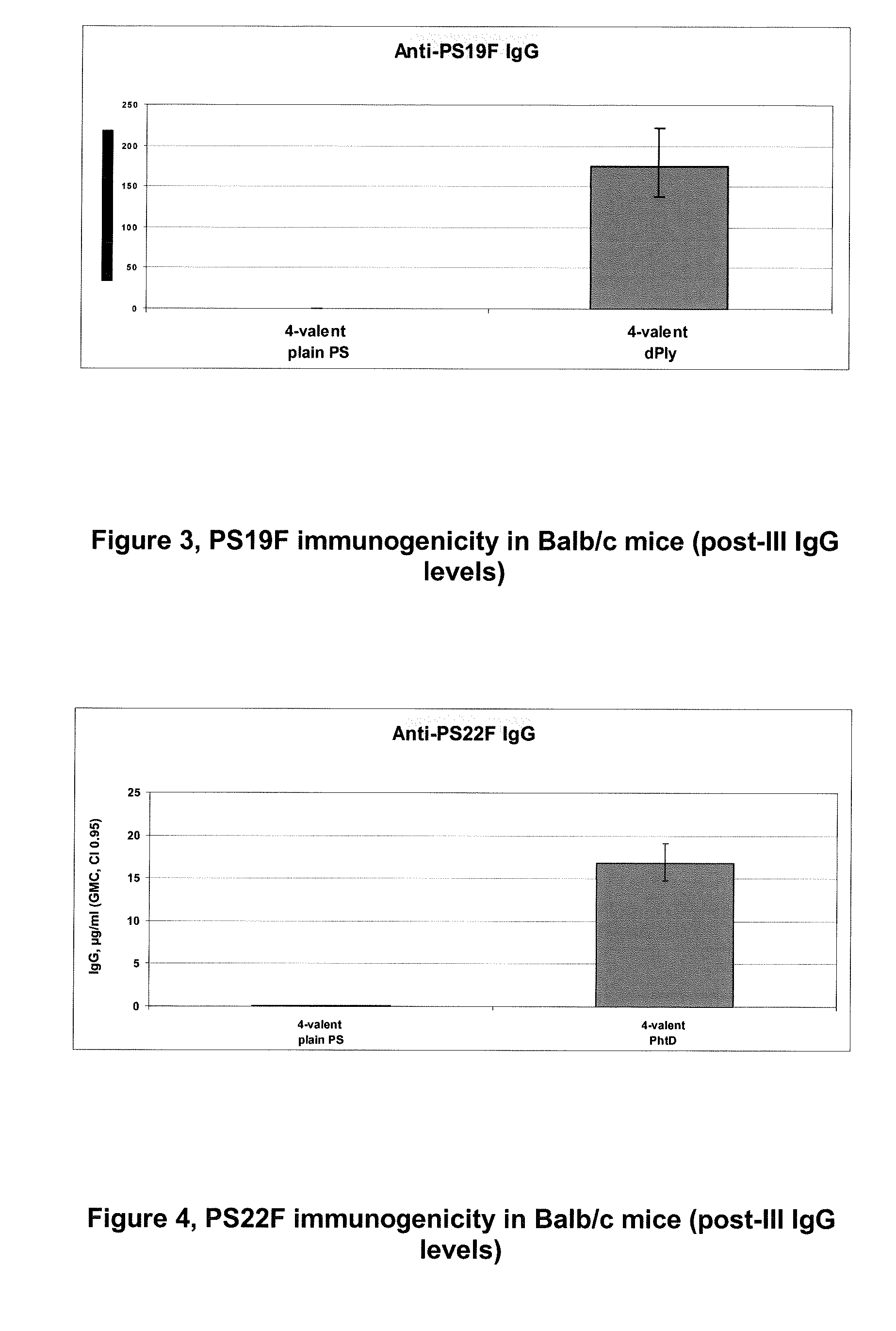
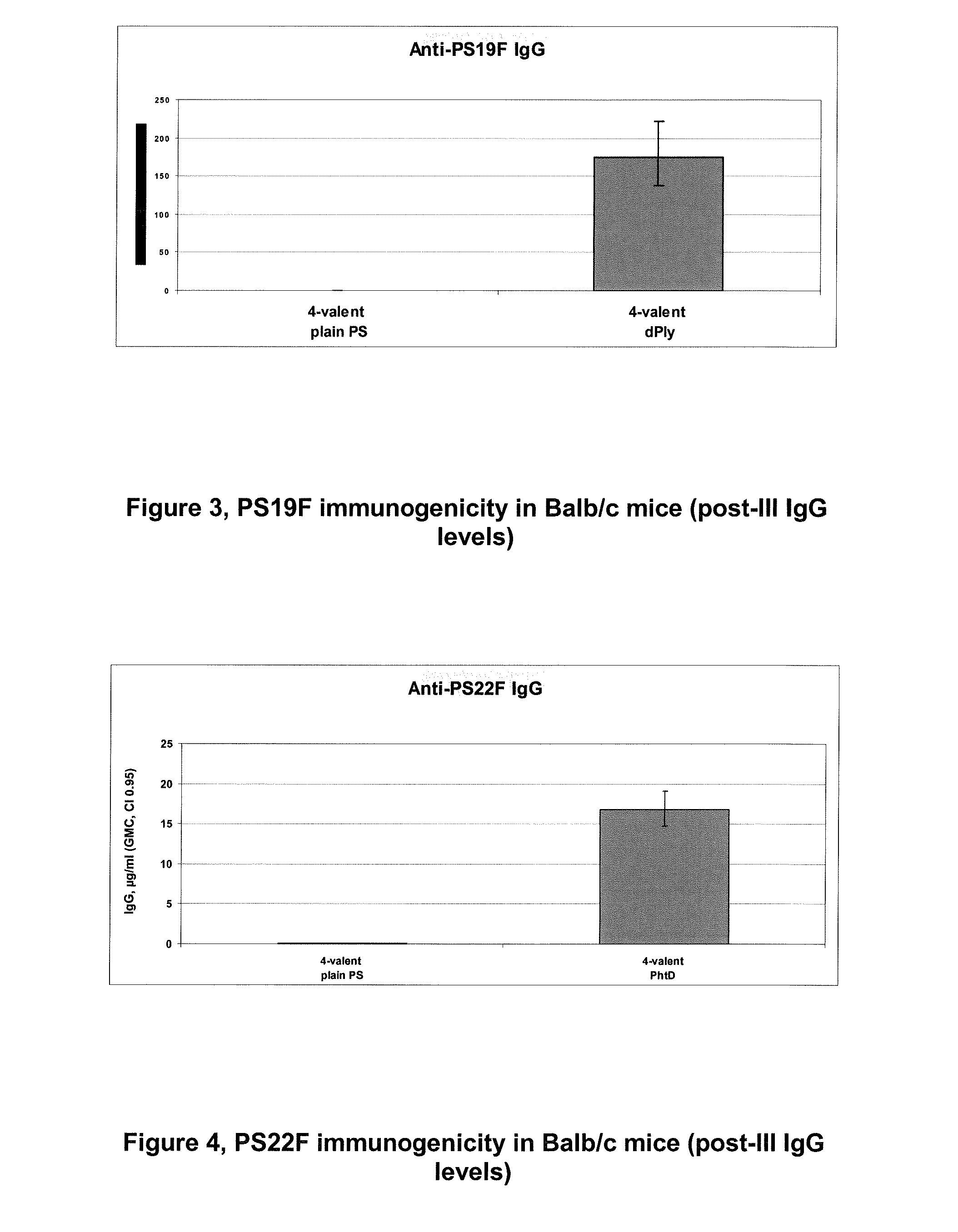
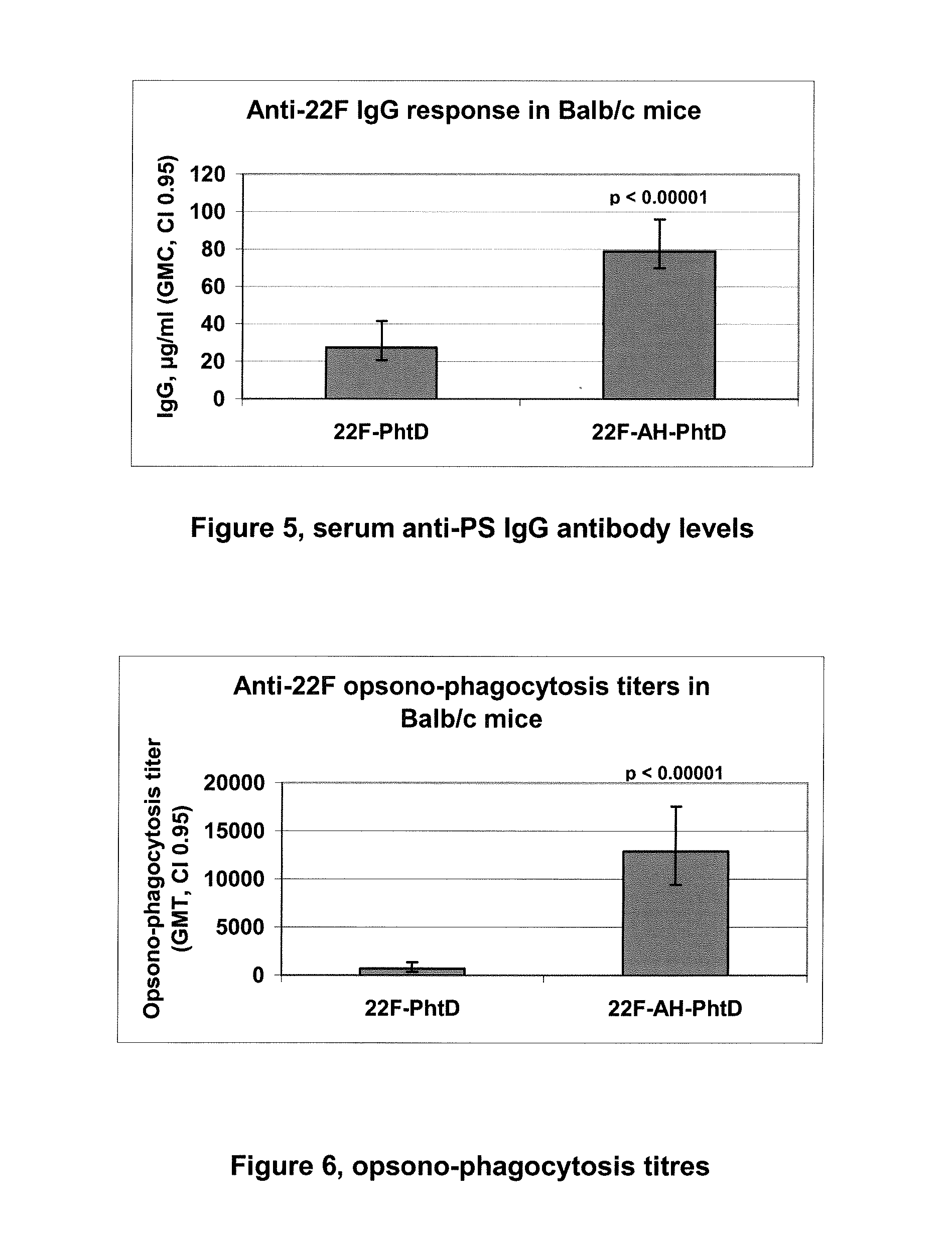
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Screening assays for compounds that inhibit membrane fusion-associated events
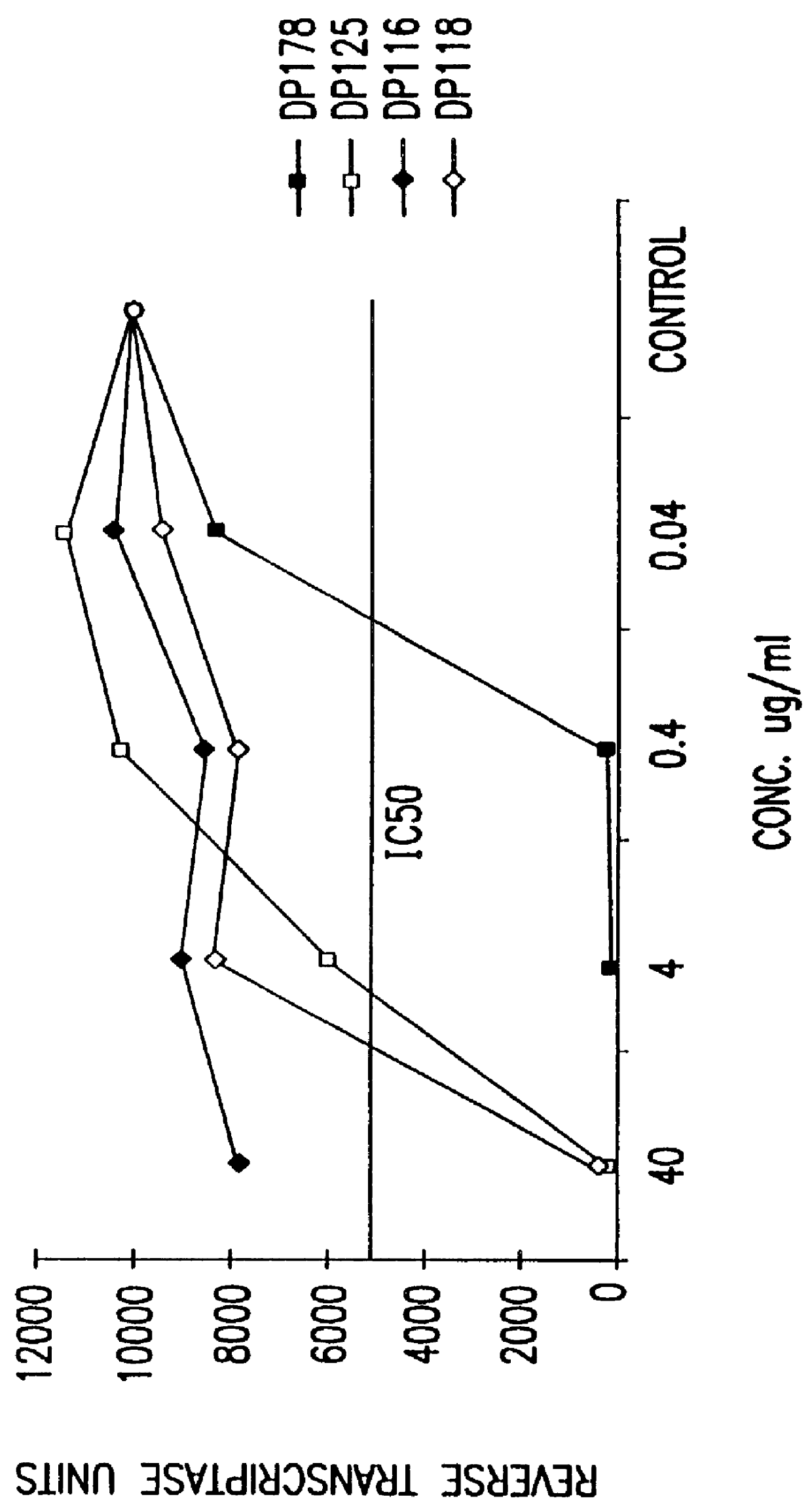
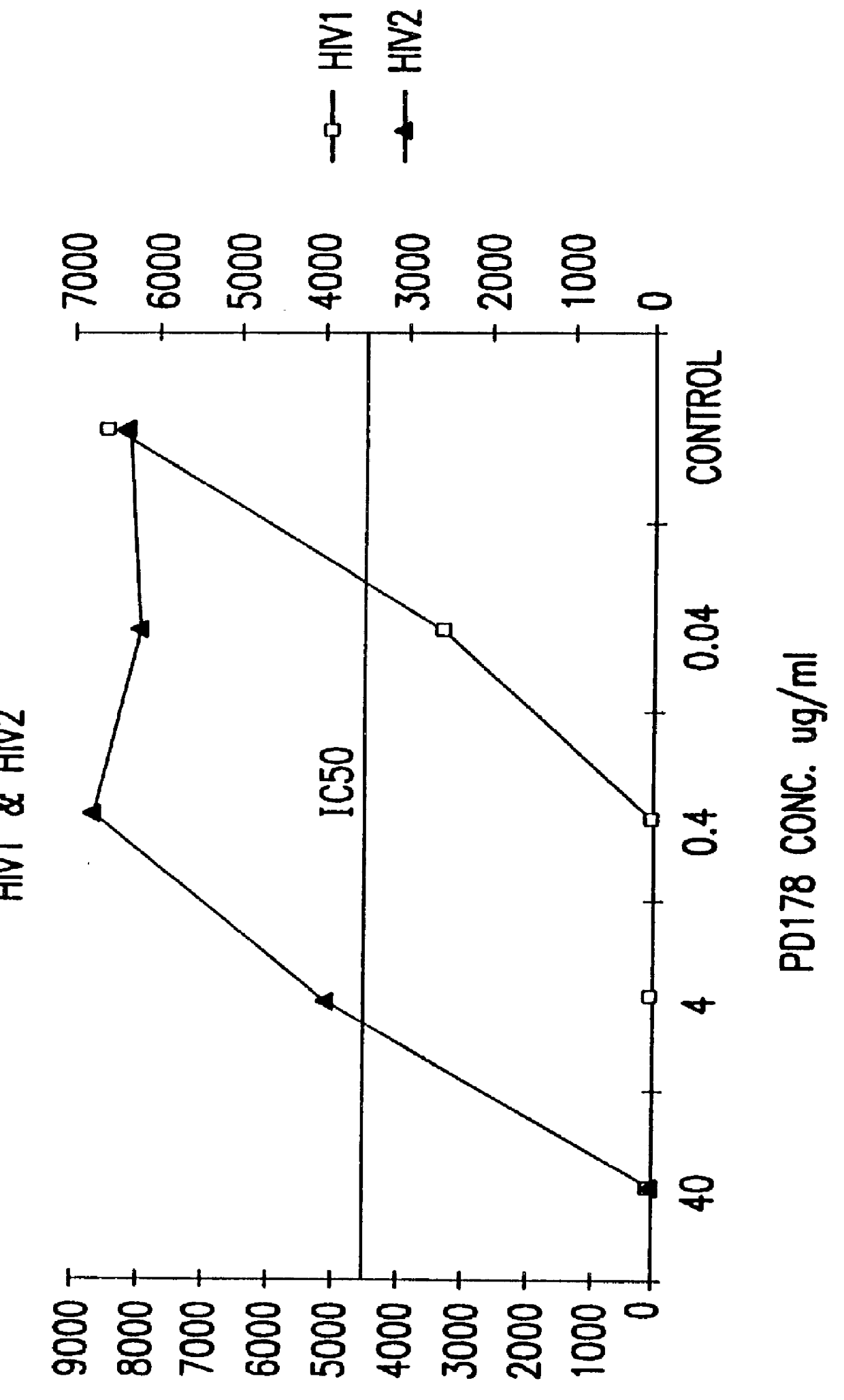
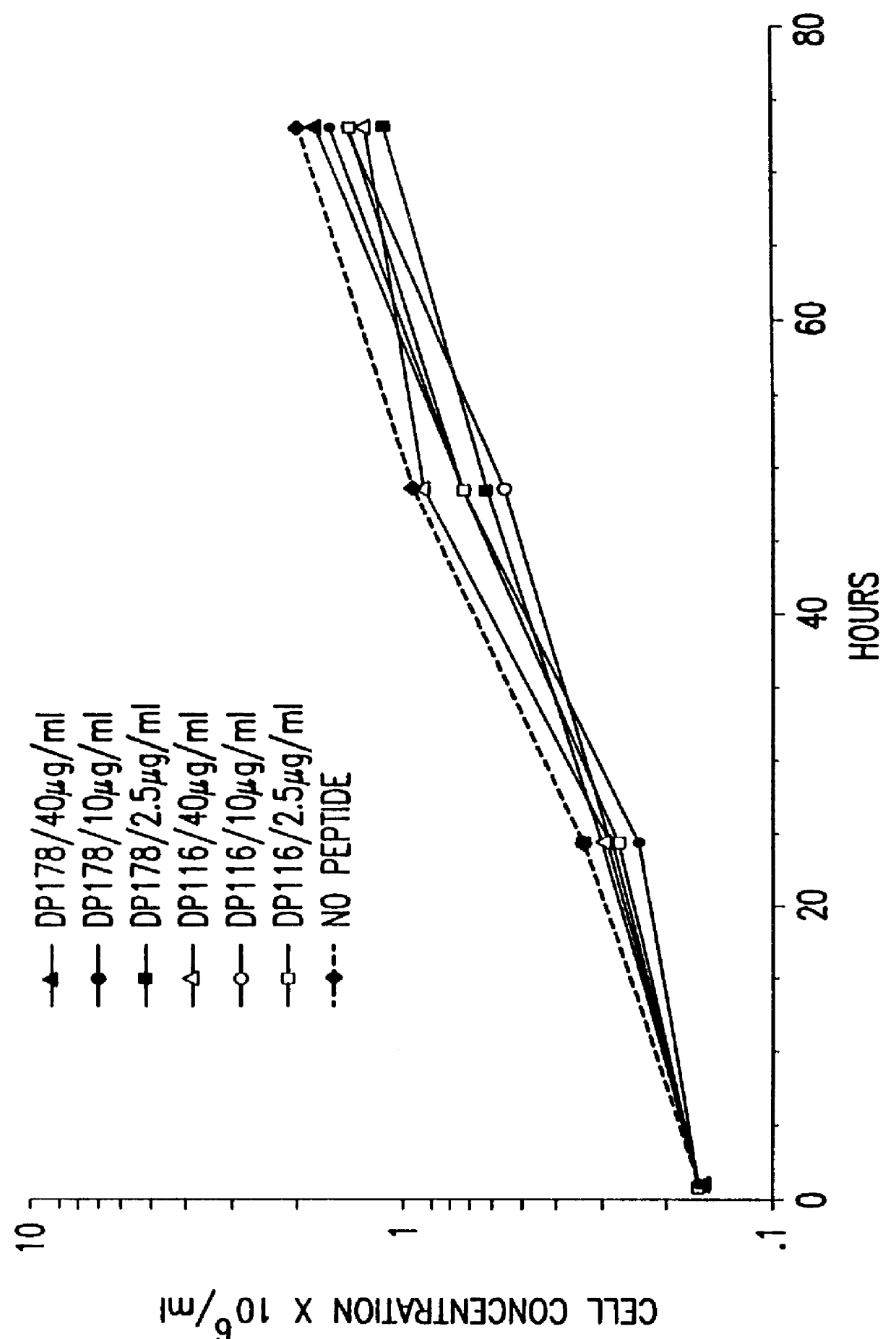
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Altered strain of the modified vaccinia virus ankara (mva)
InactiveUS20030013190A1Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsAdjuvantVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Vaccine Comprising Streptococcus Pneumoniae Capsular Polysaccharide Conjugates
InactiveUS20090017059A1Antibacterial agentsSenses disorderStreptococcus pneumoniae capsular polysaccharideStreptococcus mitis
The present invention discloses an immunogenic composition comprising S. pneumoniae capsular saccharide conjugates from serotypes 19A and 19F wherein 19A is conjugated to a first bacterial toxoid and 19F is conjugated to a second bacterial toxoid. Vaccines, methods of making vaccines and uses of the vaccines are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions with enhanced immunogenicity
InactiveUS20060121055A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideAdjuvantVaccine Immunogenicity
The present invention relates to immunogenic compositions containing an immunogen and a specific combination of two or more traditional excipients. The excipients in the composition act in combination and enhance immune responses to the immunogen from a subject. The combination of excipients may be used as adjuvant in immunogenic compositions, regardless of route or target of delivery. The compositions can be administered, for example, intradermally, epidermally, transdermally, junctionally, nasally, or subcutaneously.
Owner:BECTON DICKINSON & CO
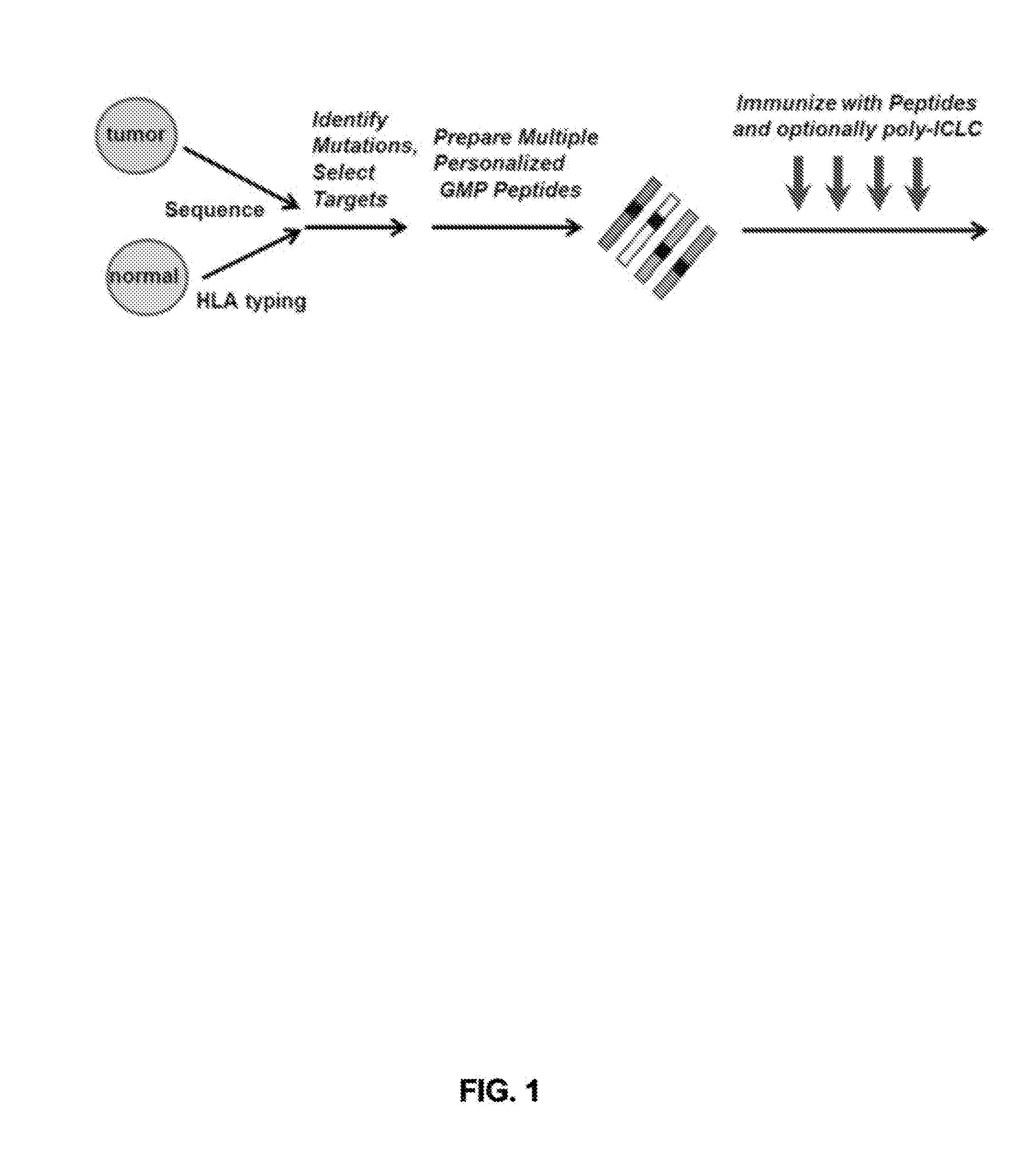
Shared neoantigens
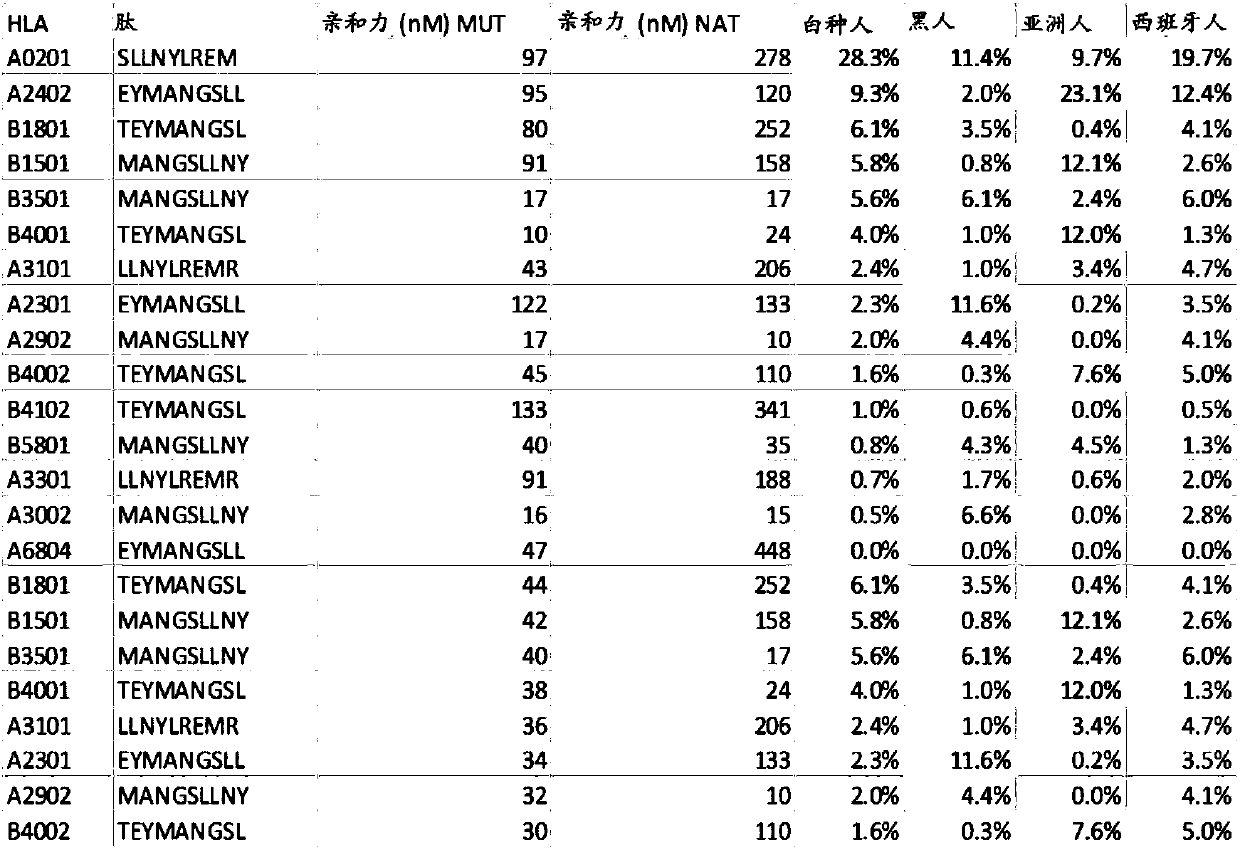
ActiveCN108025048ATumor rejection antigen precursorsPeptide/protein ingredientsPharmaceutical drugPharmaceutical medicine
Disclosed herein in one aspect is a pharmaceutical composition comprising a plurality of neoantigenic peptides and a pharmaceutically acceptable carrier, each neoantigenic peptide comprising a tumor-specific neoepitope capable of binding to an HLA protein in a subject, each tumor-specific neoepitope comprising a tumor-specific mutation present in a tumor, wherein (a) the composition comprises neoantigenic peptides comprising tumor-specific mutations present in at least 1% of subjects in a population of subjects suffering from cancer; (b) the composition comprises neoantigenic peptides comprising tumor-specific neoepitopes which bind to HLA proteins present in at least 5% of subjects in the population; and (c) the composition comprises at least one neoantigenic peptide capable of elicitingan immune response against a tumor present in at least 5% of the subjects in the population of subjects suffering from cancer.
Owner:THE BROAD INST INC +2
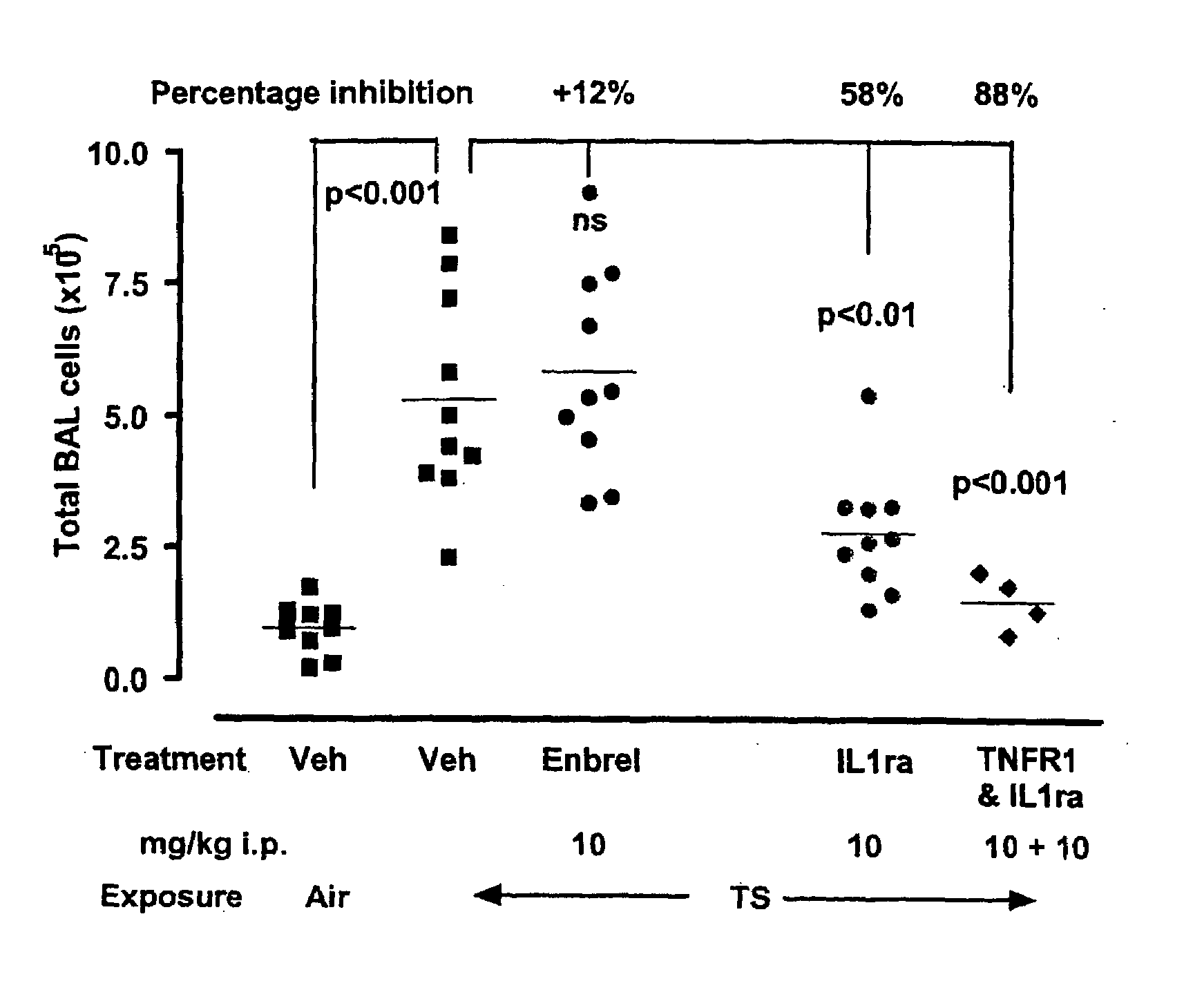
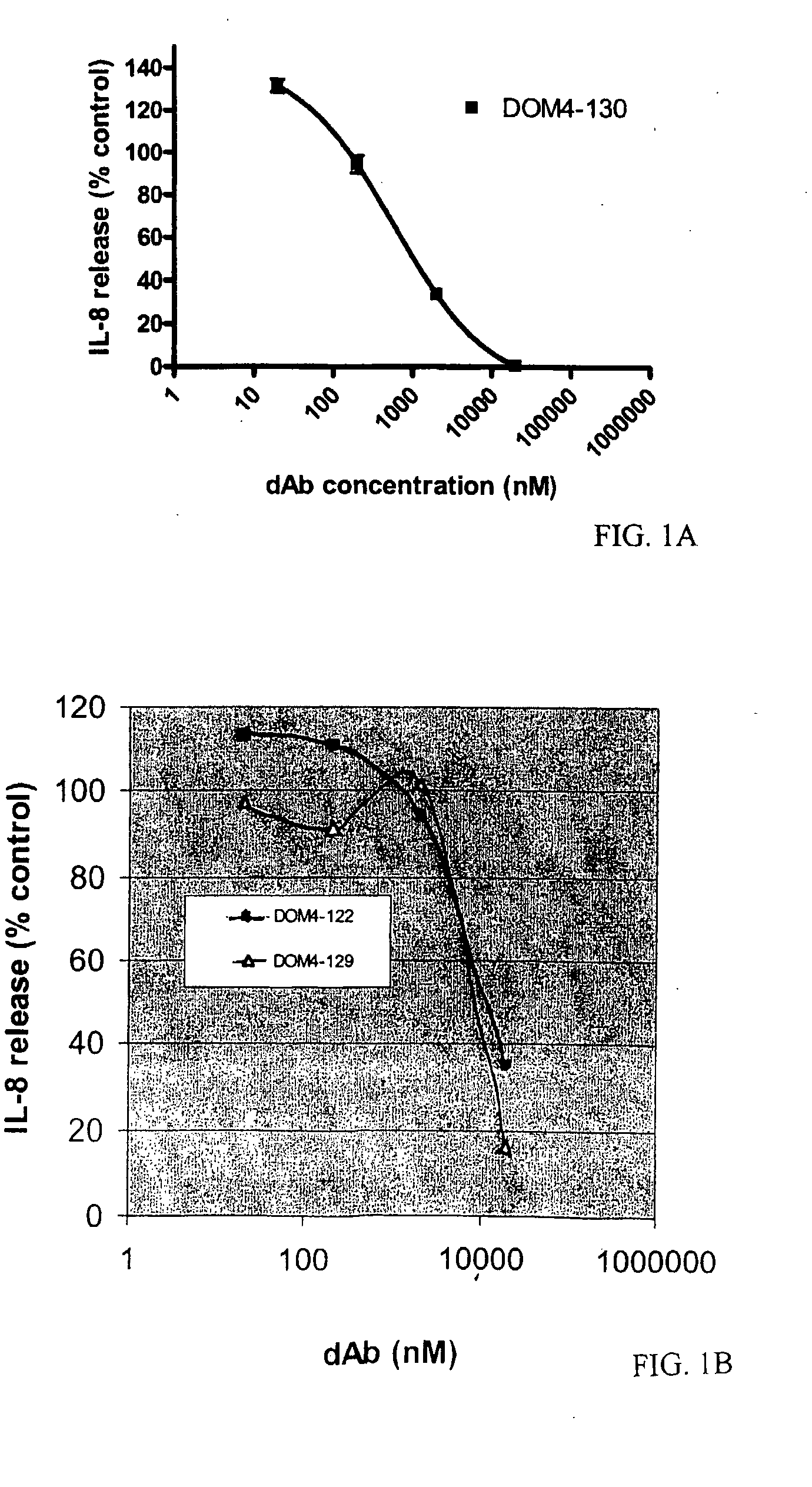
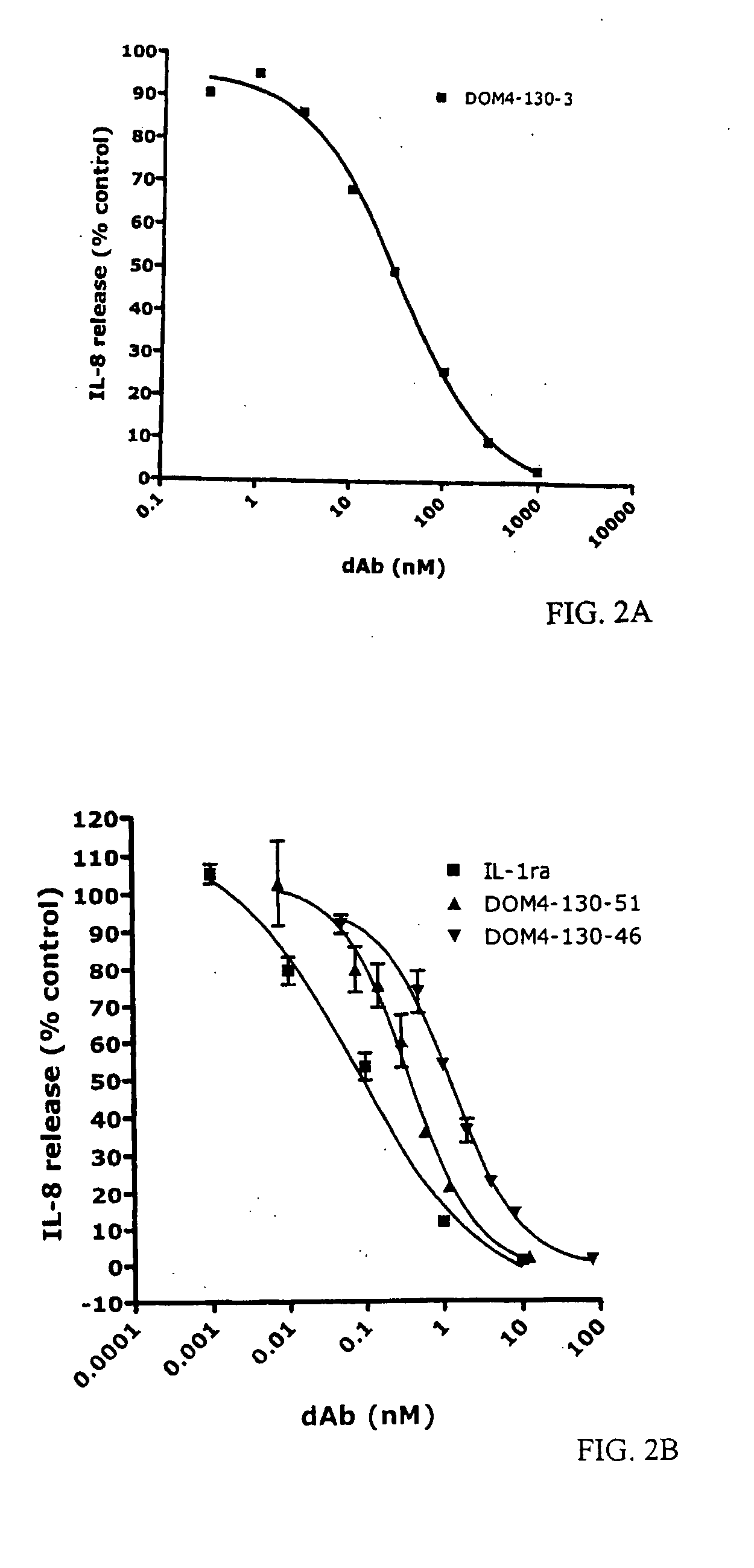
Anti-IL-1R1 Single Domain Antibodies And Therapeutic Uses
InactiveUS20090191217A1Extended half-lifeAntibody mimetics/scaffoldsMedical devicesDiseaseLung Inflammations
Disclosed is the use of an antagonist of Interleukin 1 receptor type 1 (IL-1R1) for the manufacture of a medicament treating, preventing or suppressing lung inflammation or a respiratory disease. In some embodiments of the described invention, the medicament is for local administration to pulmonary tissue. Also disclosed are methods for treating lung inflammation or a respiratory disease.
Owner:ARGENTA DISCOVERY LTD +1
Vaccine Nanotechnology
ActiveUS20130287857A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides vaccine nanocarriers capable of stimulating an immune response in T cells and / or B cells, in some embodiments, comprising at least one immunomodulatory agent, and optionally comprising at last one targeting moiety and optionally at least one immunostimulatory agent. The invention provides pharmaceutical compositions comprising inventive vaccine nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive vaccine nanocarriers and pharmaceutical compositions thereof. The invention provides methods of prophylaxis and / or treatment of diseases, disorders, and conditions comprising administering at least one inventive vaccine nanocarrier to a subject in need thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +3
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
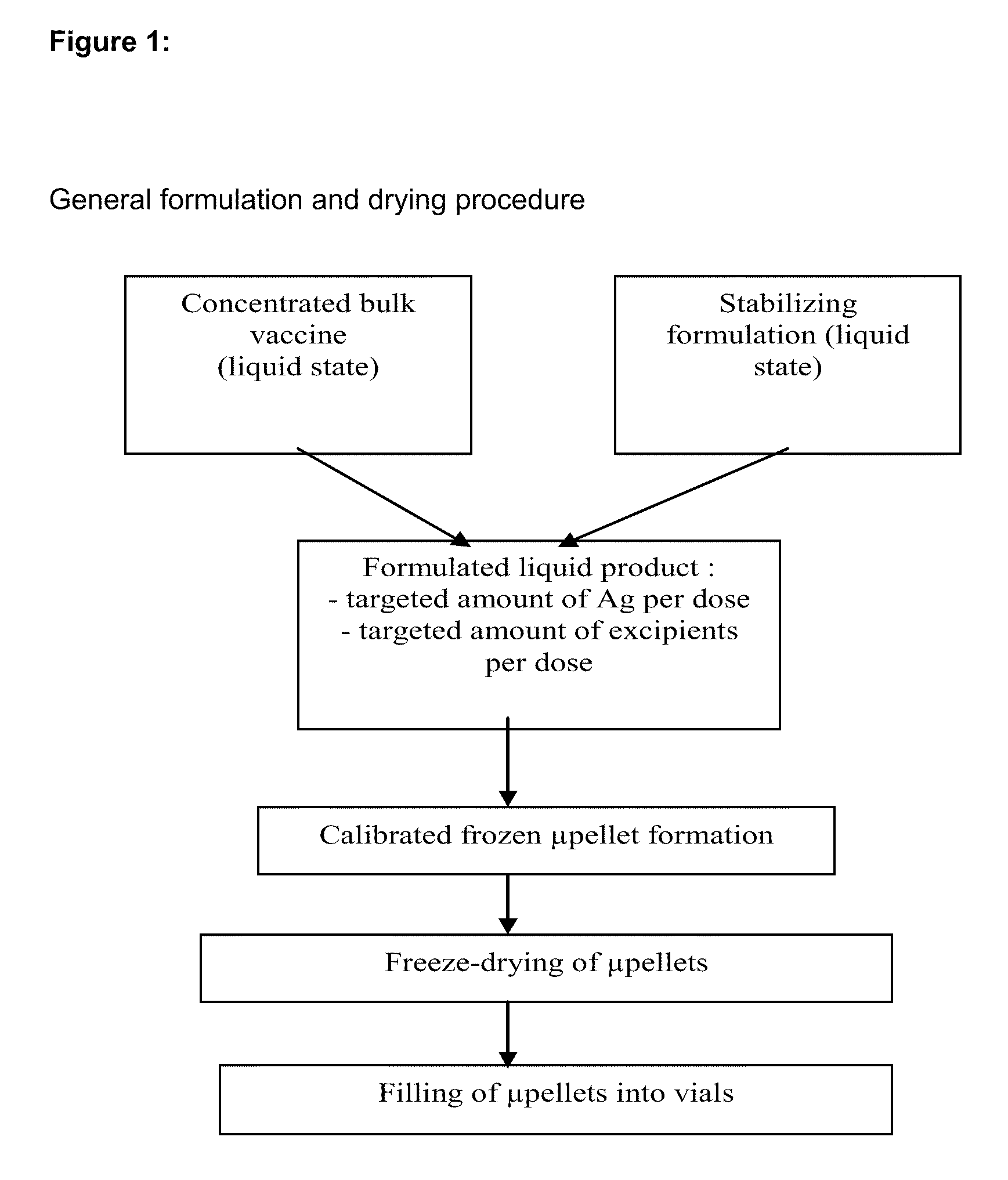
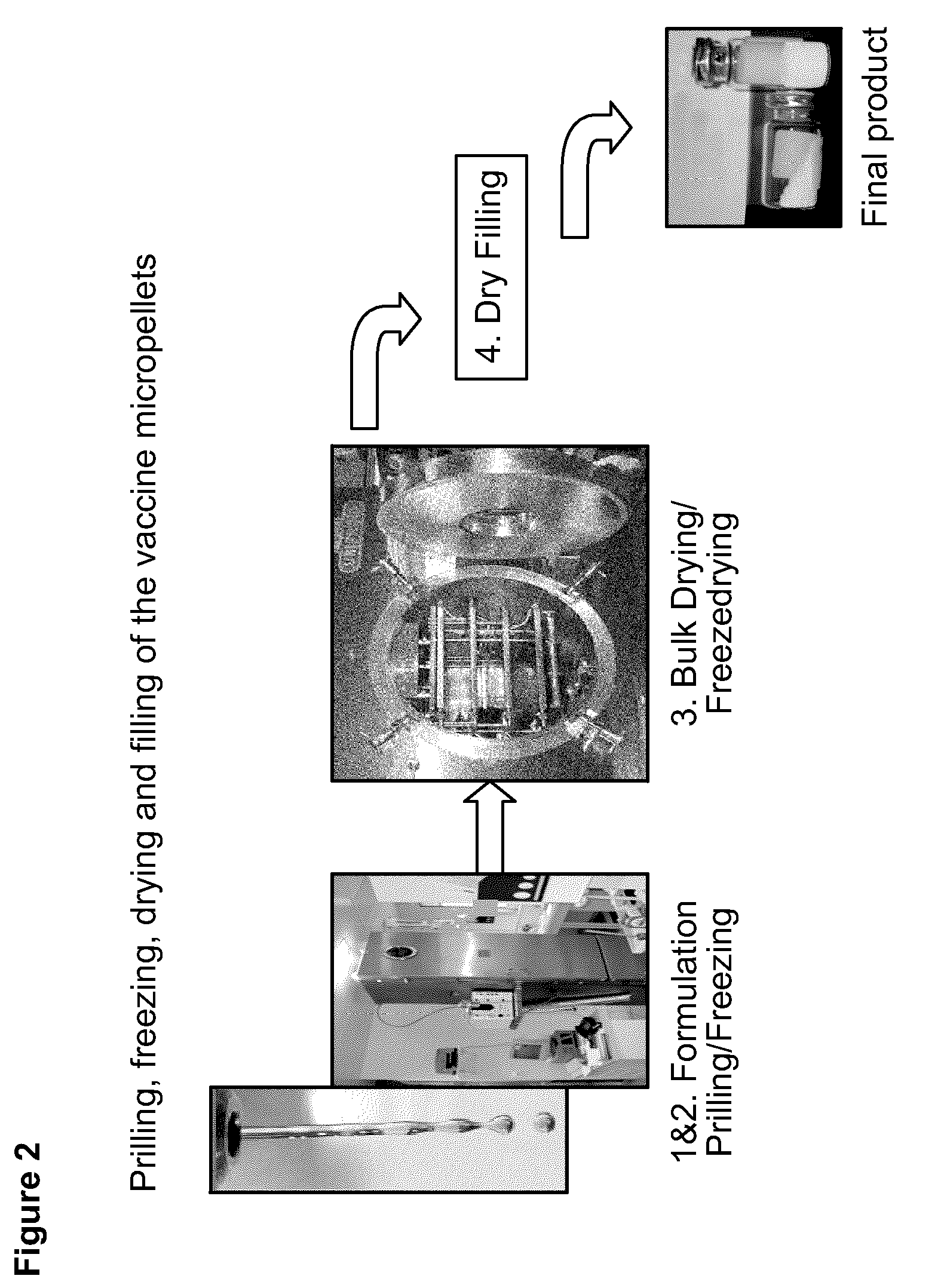
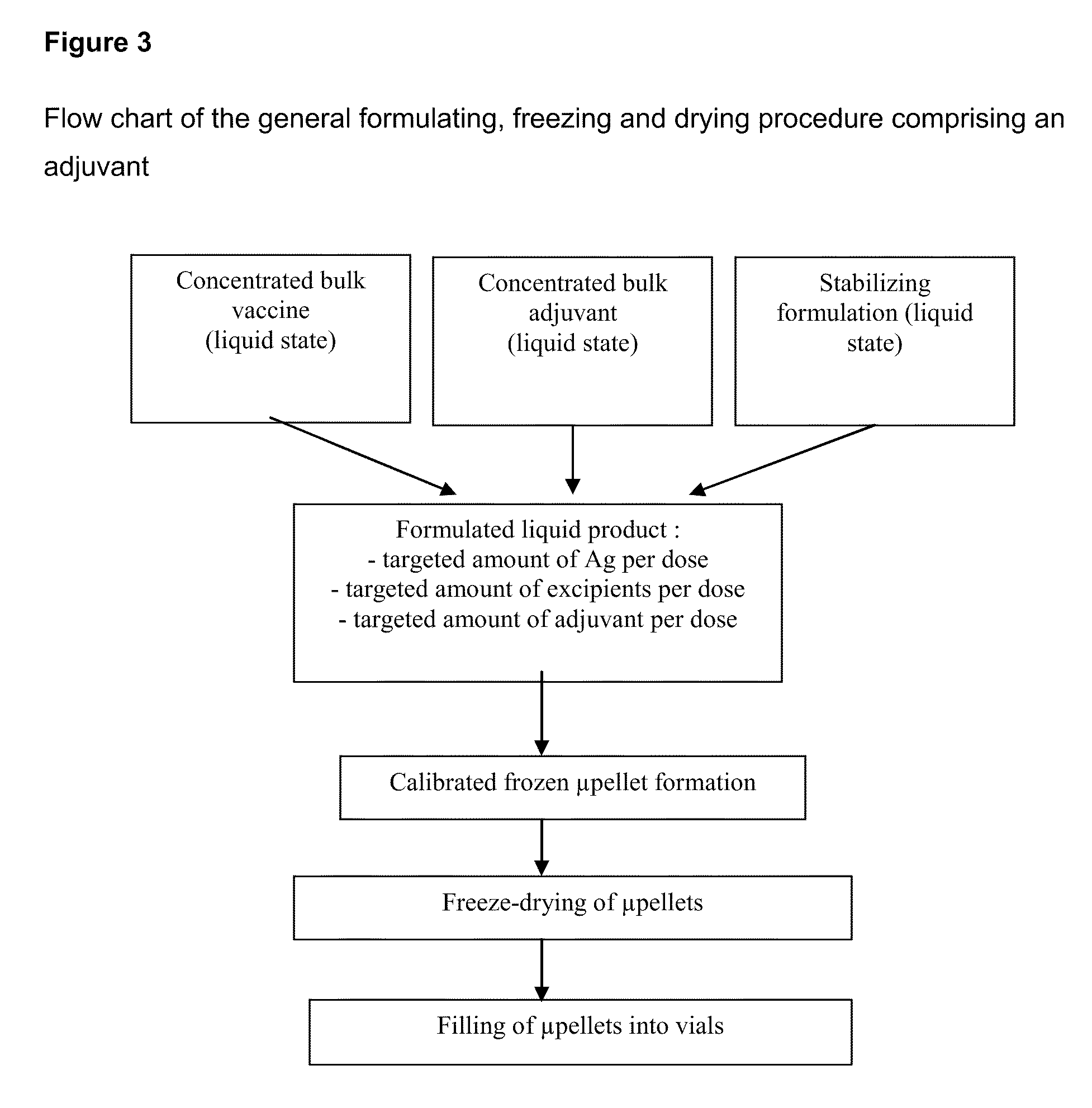
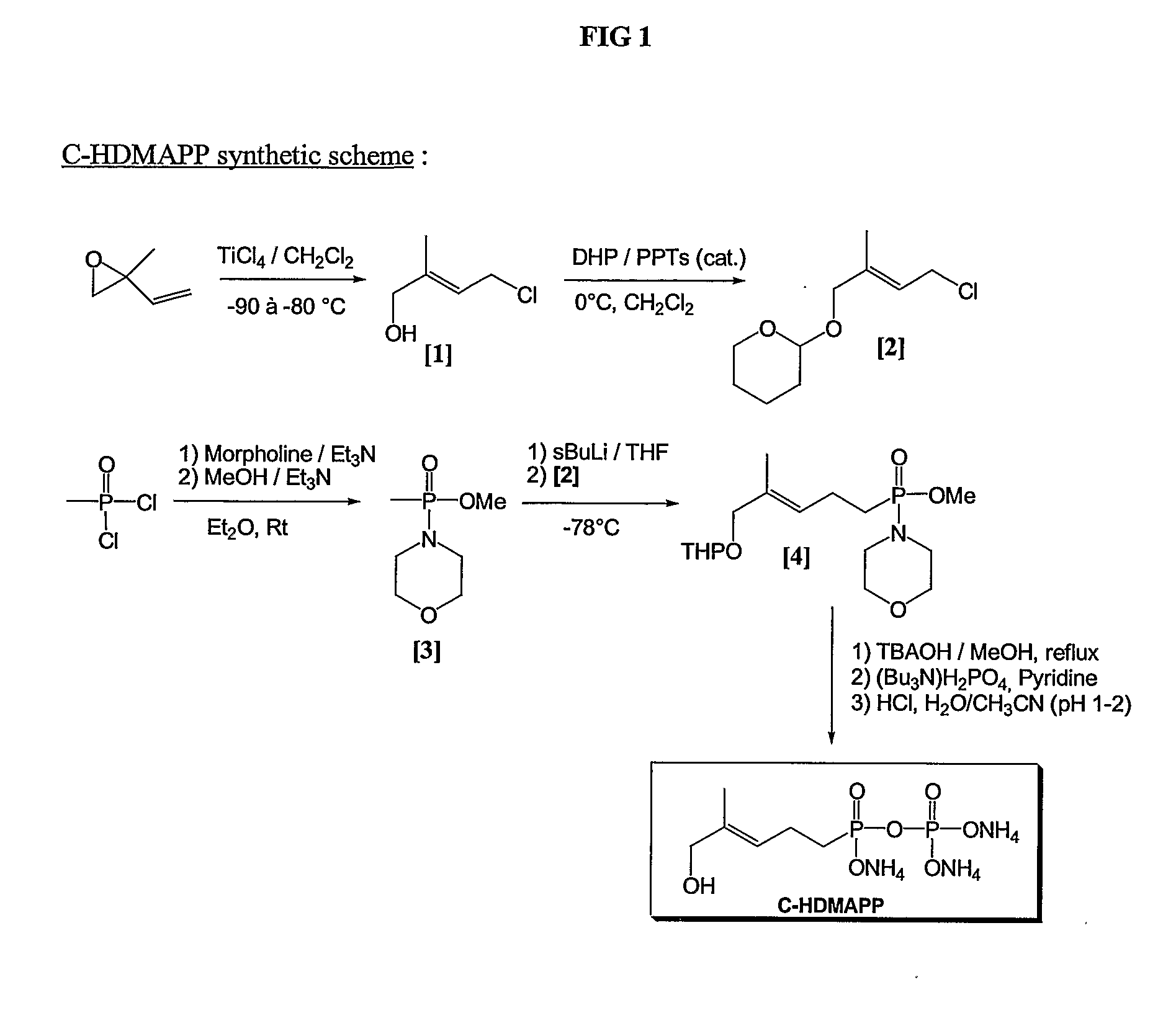
Process for Stabilizing an Adjuvant Containing Vaccine Composition
The present invention relates to a process for stabilizing an adjuvant containing vaccine composition, an adjuvanted vaccine composition in dry form and in particular a process for stabilizing an influenza vaccine composition, particularly an adjuvanted influenza vaccine composition in dry form.
Owner:SANOFI PASTEUR SA
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
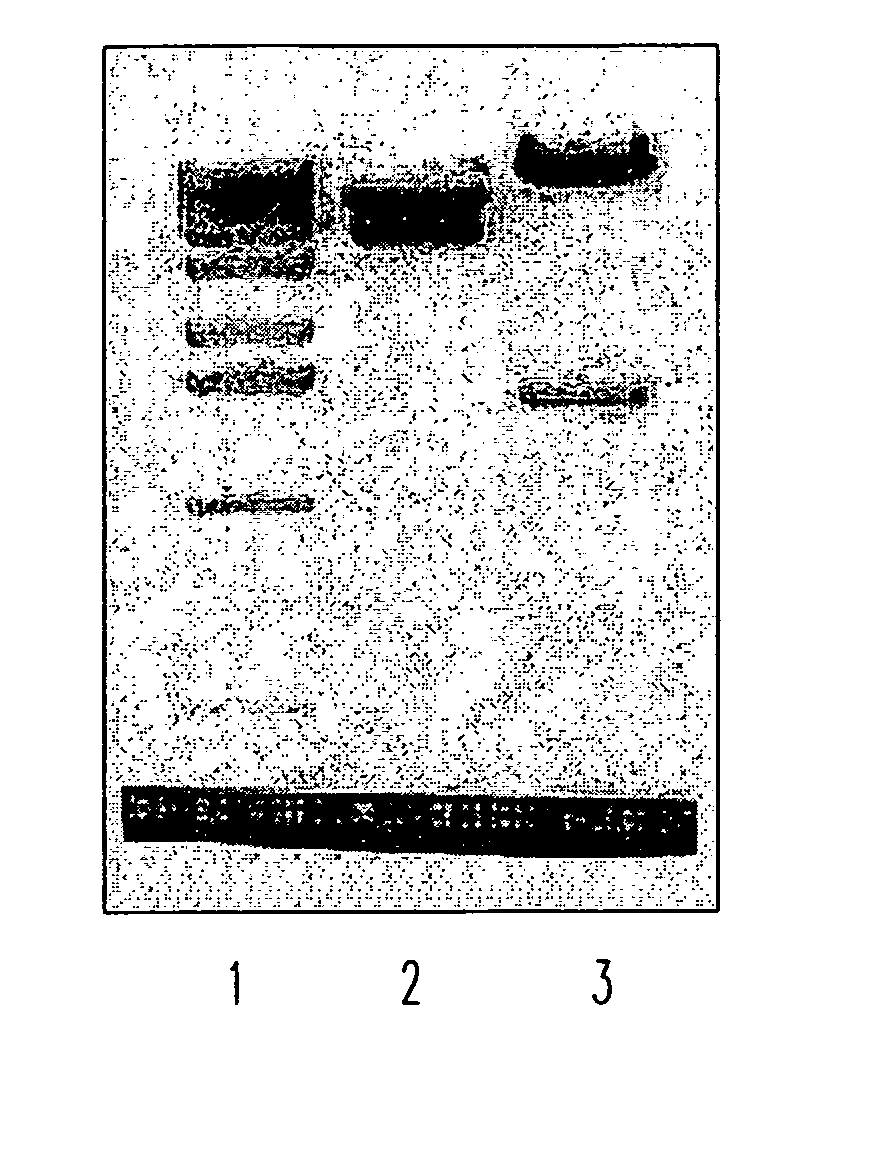
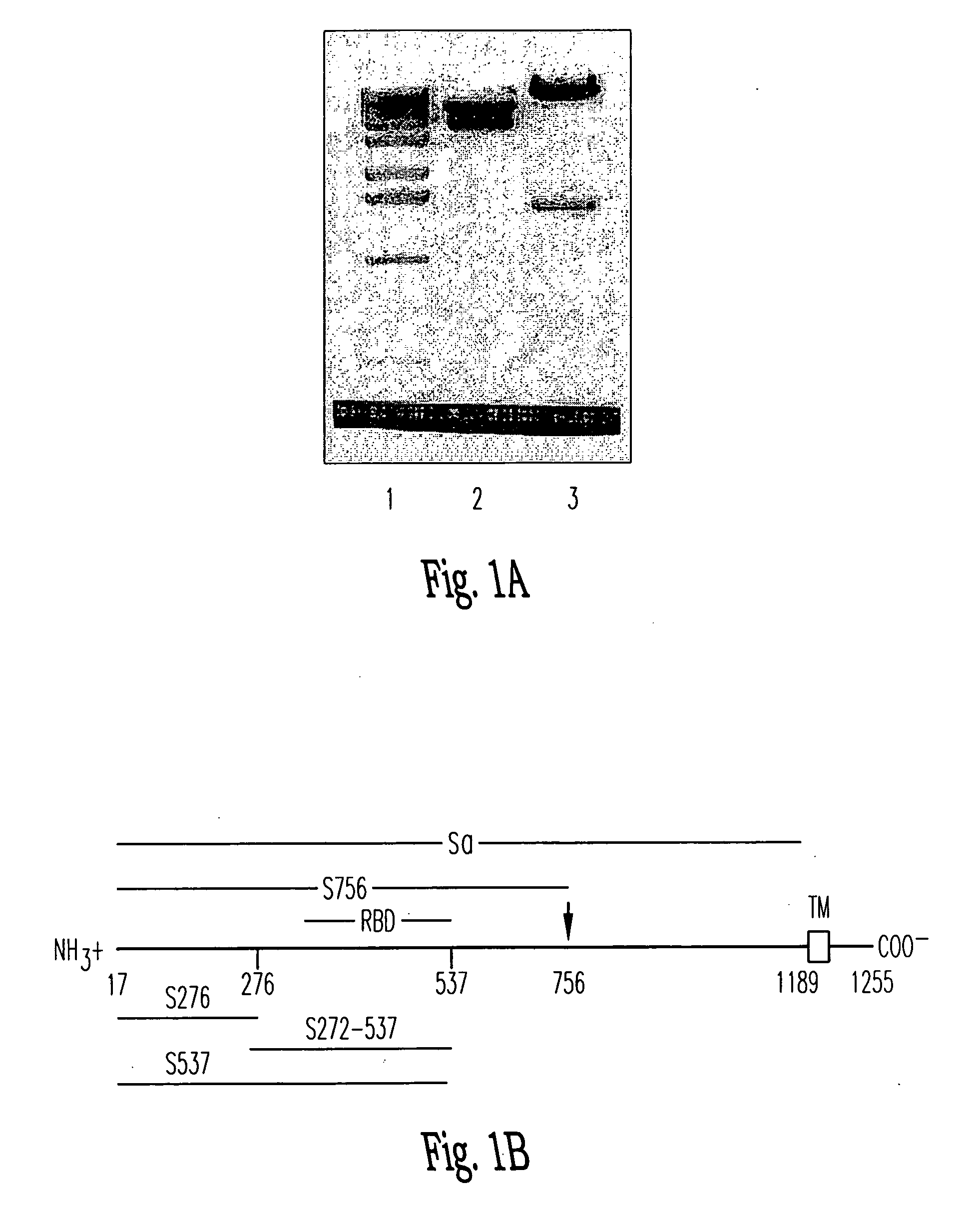
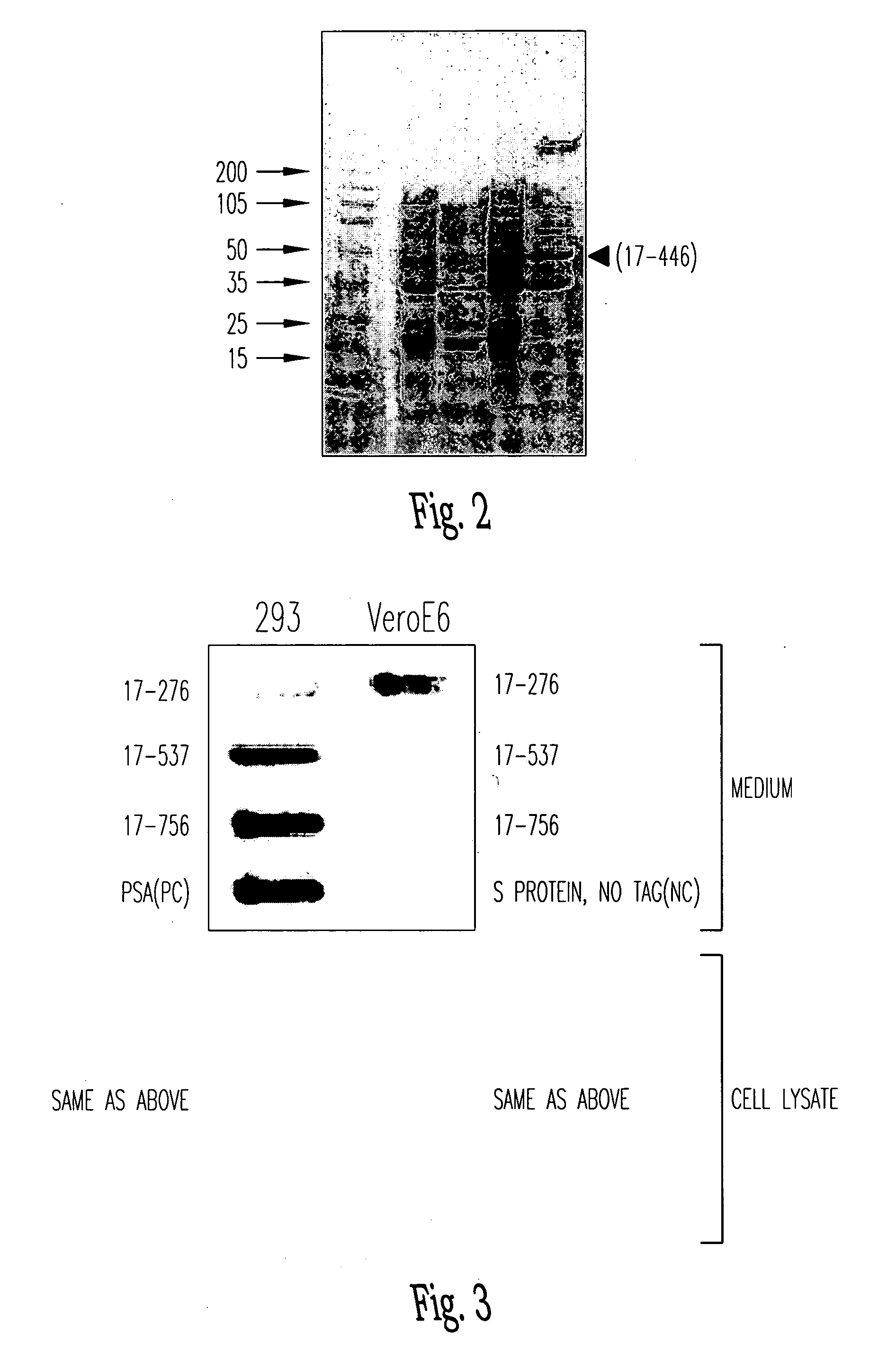
Soluble fragments of the SARS-CoV spike glycoprotein
InactiveUS20060240515A1Easy to produceImprove resistance to degradationSsRNA viruses positive-senseAntibody mimetics/scaffoldsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF US SEC THE DEPT OF
Altered strain of the modified vaccinia virus ankara (MVA)
InactiveUS6682743B2Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsVector systemVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
Novel glycan conjugates and use thereof
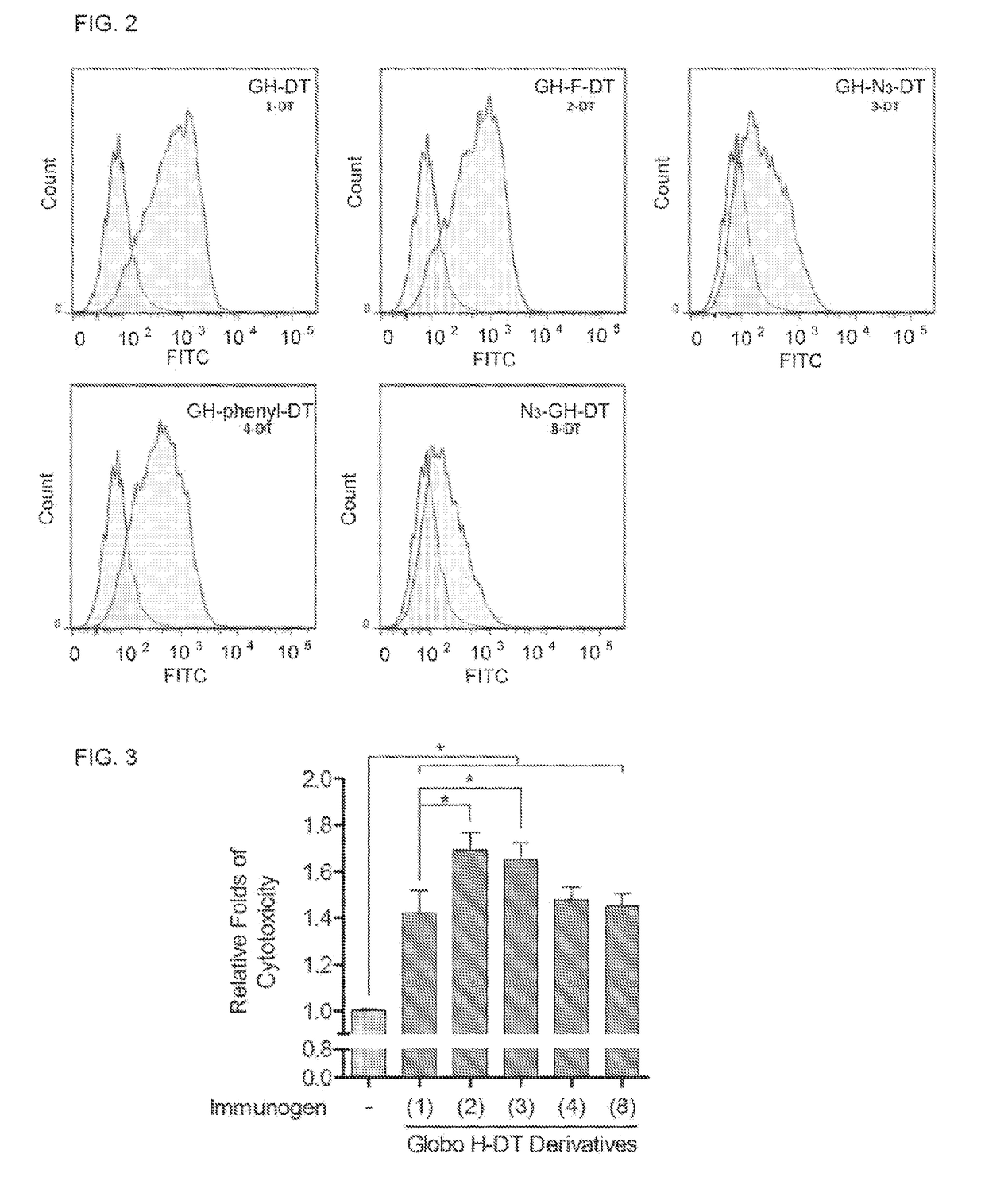
ActiveUS20170275389A1Shrink tumorElimination of malignant cellBiological material analysisDepsipeptidesAdjuvantMedicine
This disclosure includes an immunogenic composition containing (a) a glycan conjugate including a carrier and one or more glycans, wherein each of the one or more glycans is conjugated with the carrier through a linker, and optionally (b) an adjuvant. The one or more glycan is each a Globo H derivative.
Owner:ACAD SINIC
Methods and compositions for improving immune responses
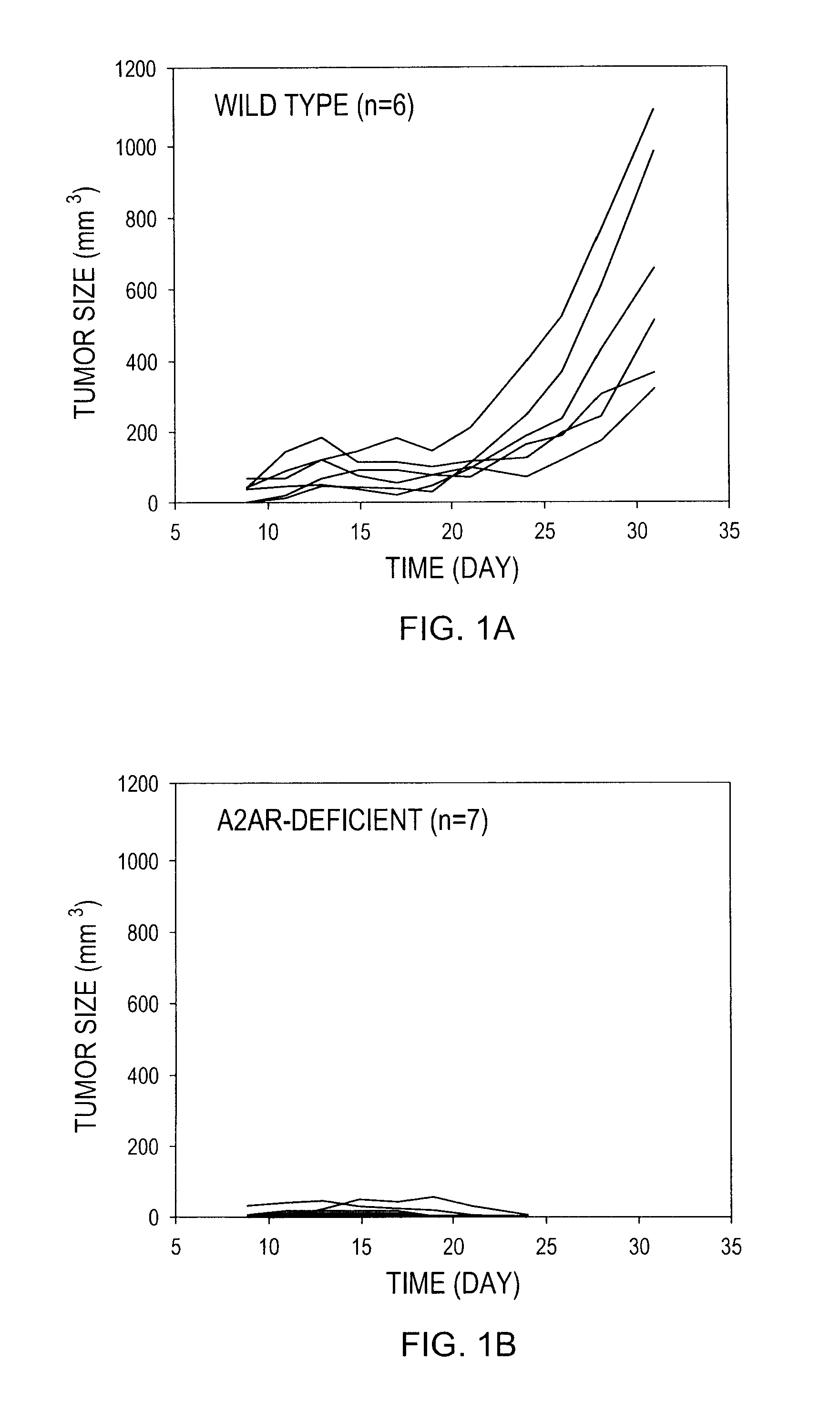
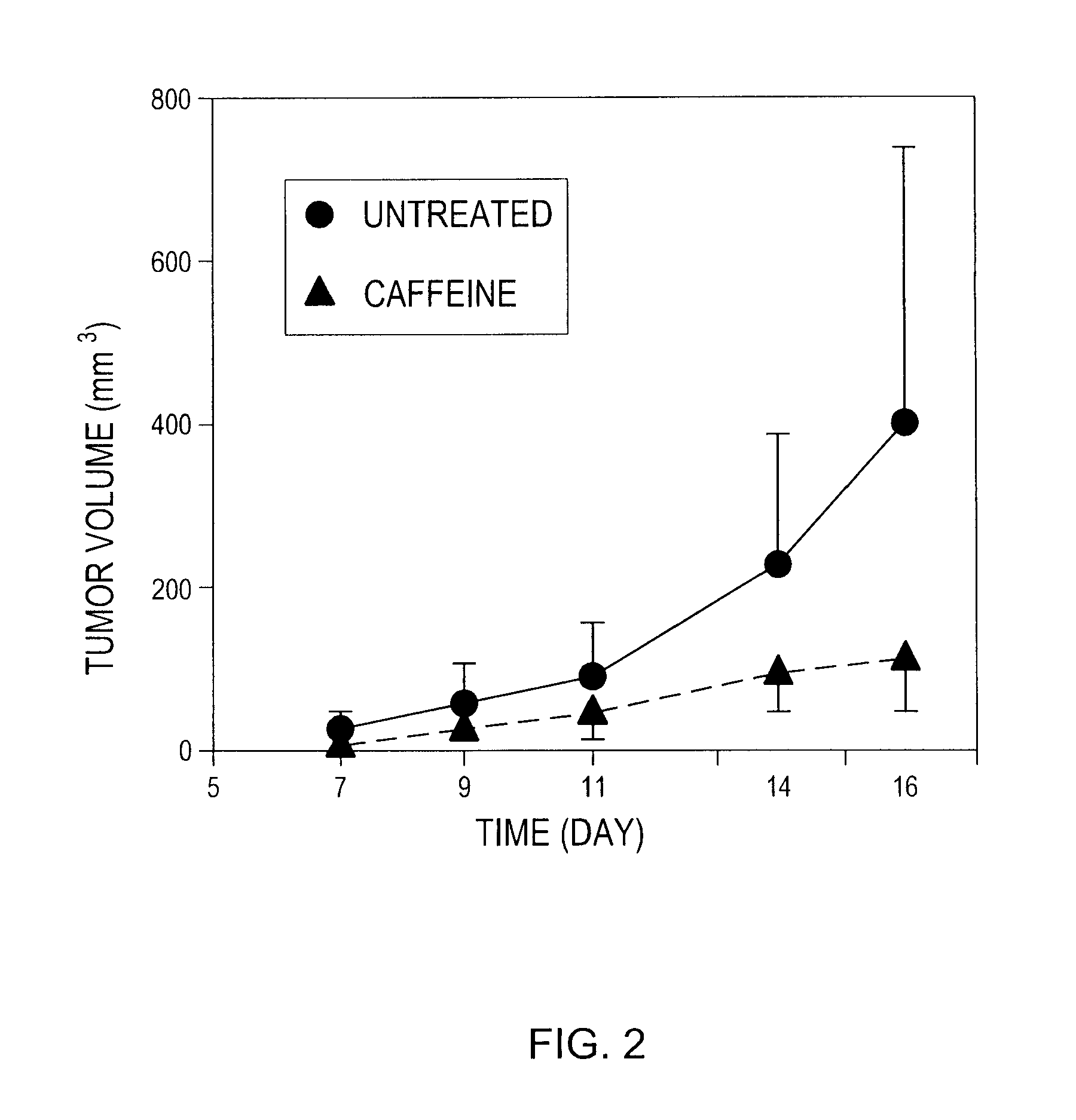
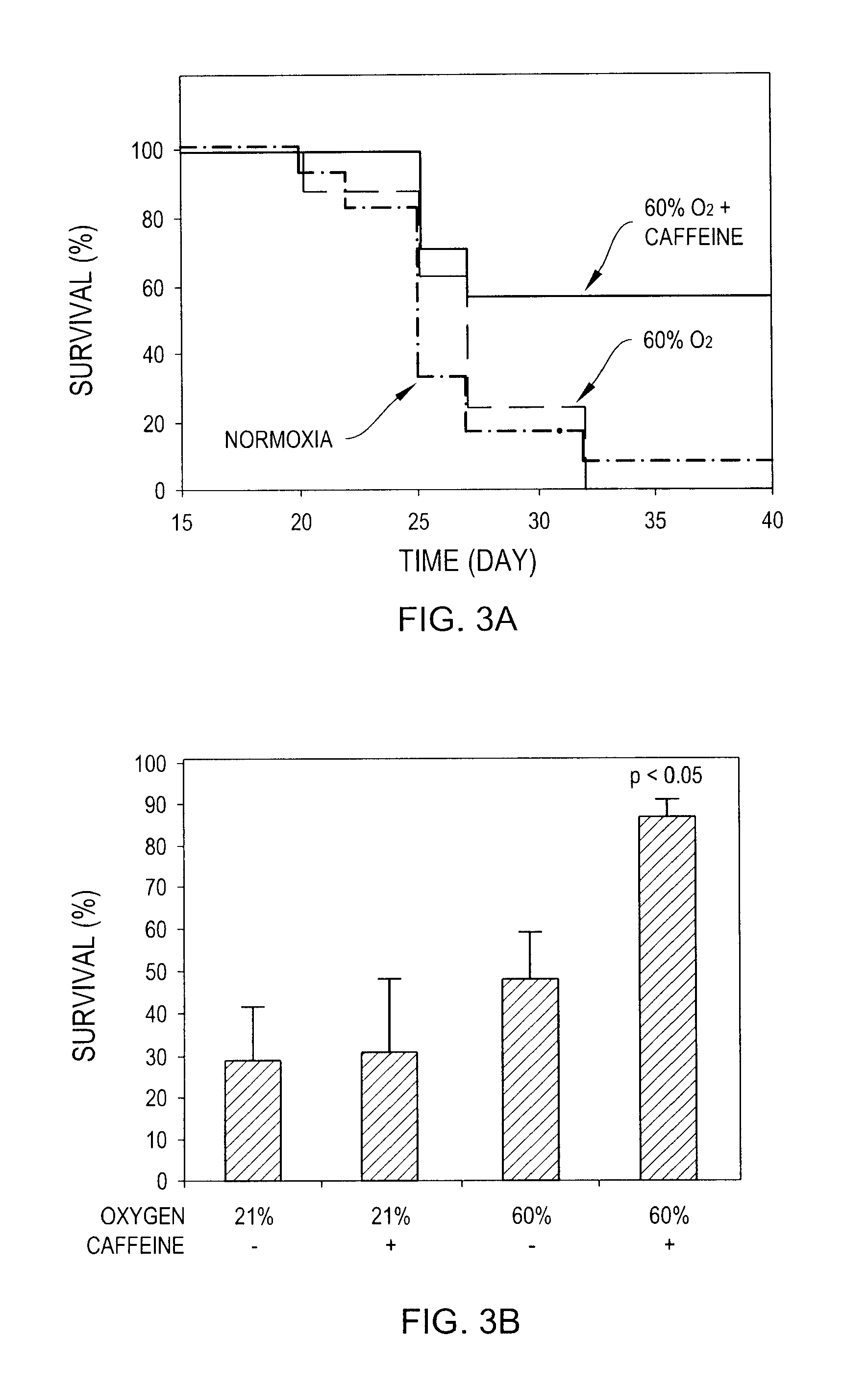
InactiveUS20100178299A1Enhance immune responsePreventing and reducing physiological down-regulation of immune responseMicrobiological testing/measurementSnake antigen ingredientsAbnormal tissue growthAdenosine
The present invention relates to compositions and methods for enhancing an immune response, for example to a vaccine, by combining the administration of oxygen (O2 gas), an adenosine pathway antagonist and / or an HIF-1α antagonist, and / or inhibitors of enzymes that produce or generate adenosine with the administration of the vaccine to the patient.The present invention also relates to methods of inducing or enhancing immune responses, methods of treating subjects having a tumor, methods of ablating or killing tumor cells and methods of disrupting the blood supply to a tumor, comprising administering oxygen alone or in combination with therapeutic agents that prevent inhibition of anti-tumor T cells. Tumor defense-resistant immune cells, anti-viral immune cells, and methods of their production are also disclosed.
Owner:NORTHEASTERN UNIV
Vaccine for treatment and prevention of herpes simplex virus infection
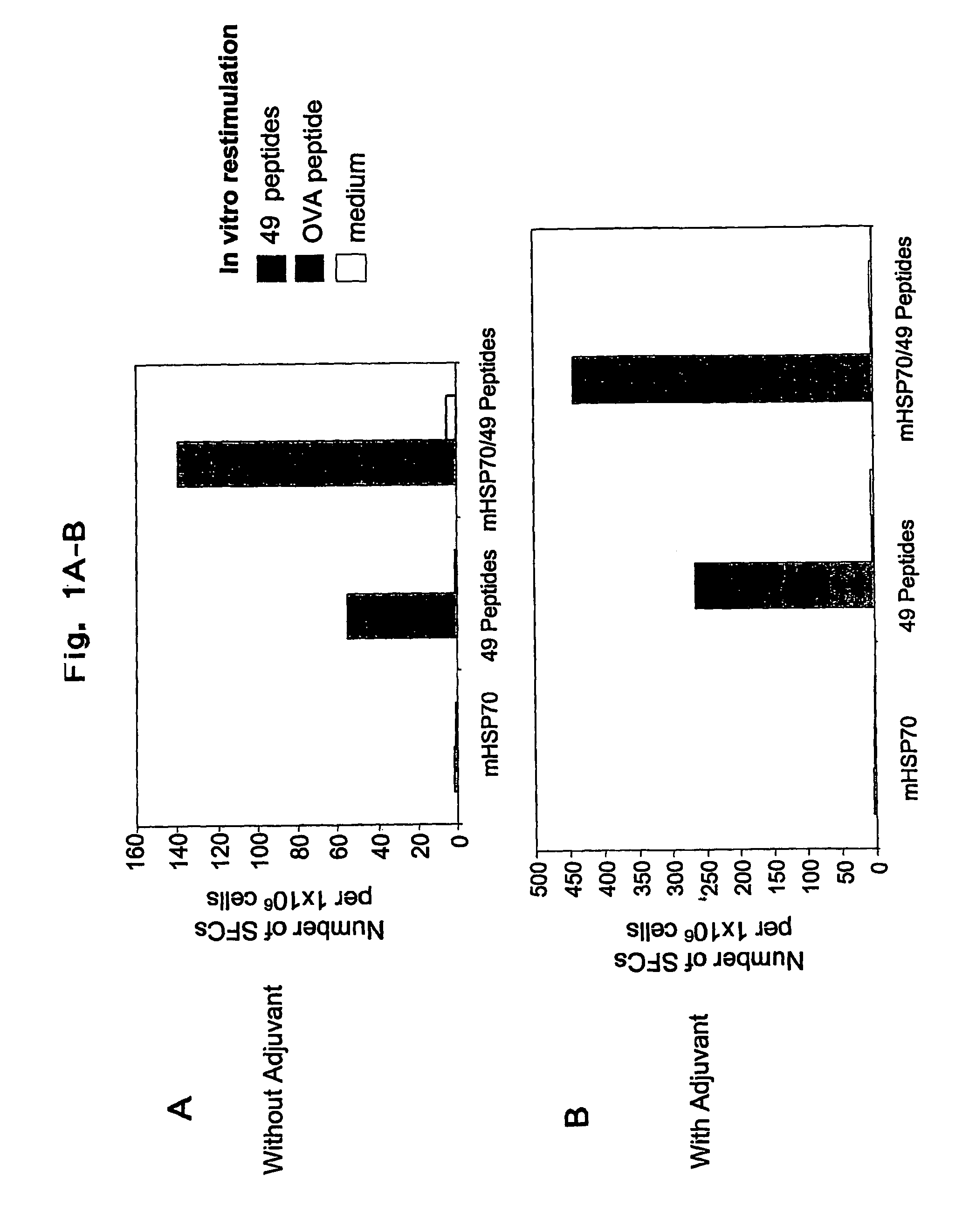
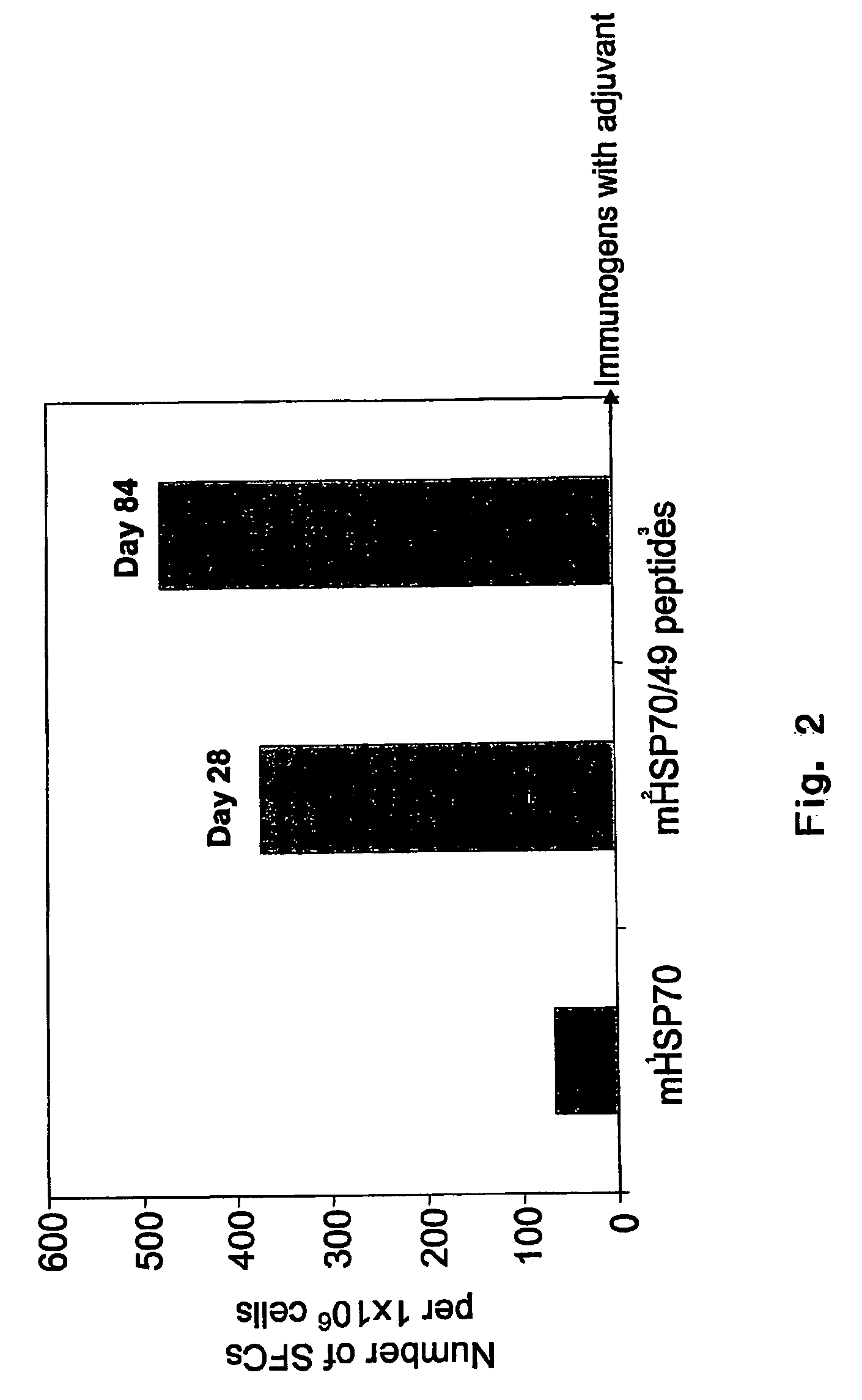
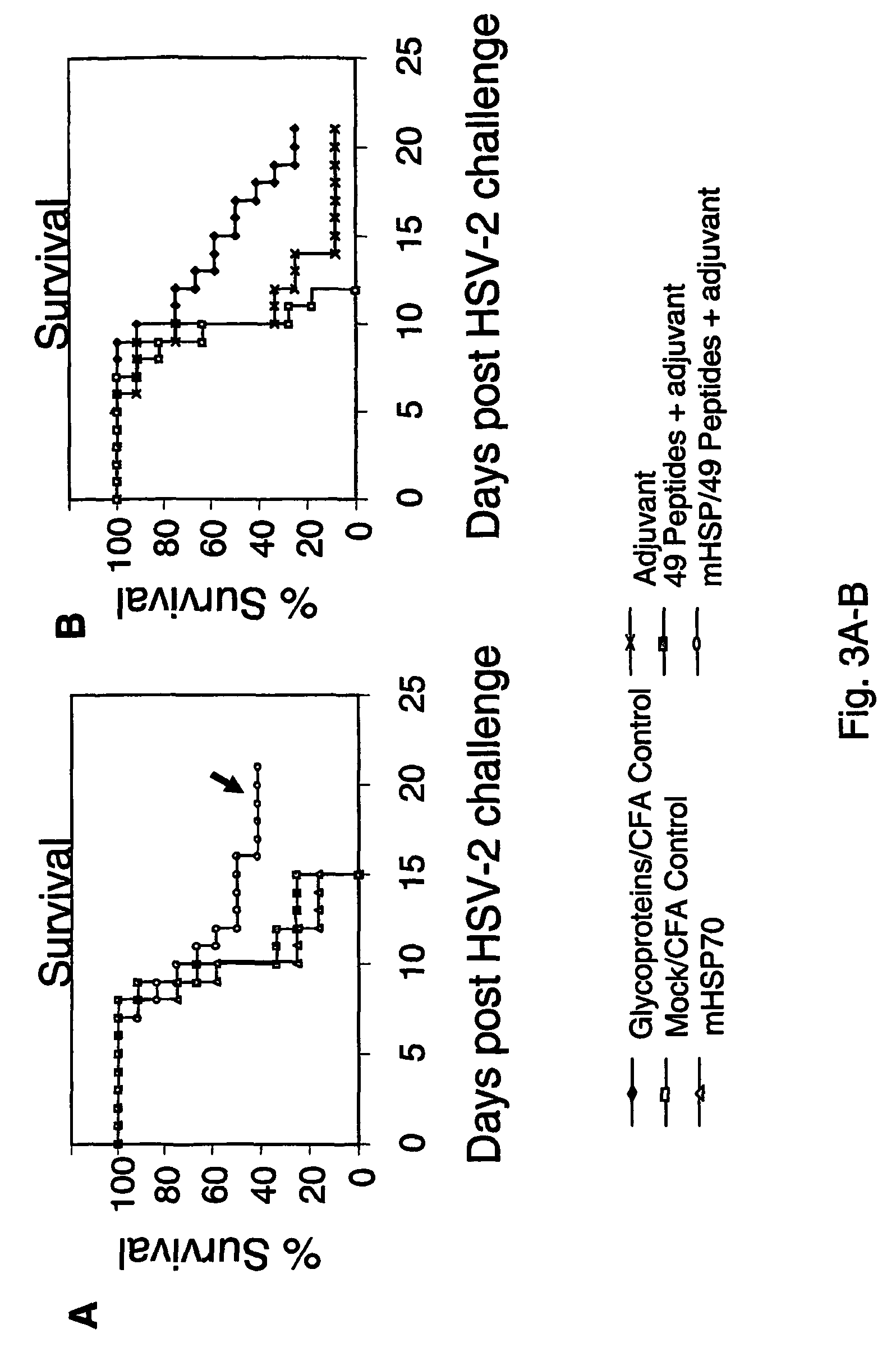
The present invention relates to methods and compositions for the prevention and treatment of herpes virus infections. The invention provides antigenic peptides, and pharmaceutical compositions comprising complexes of antigenic peptides and adjuvants that can activate an immune response against herpes viruses. The invention also provides methods of making the antigenic peptides and complexes of antigenic peptides and adjuvants. Methods of use of the pharmaceutical compositions are also provided.
Owner:AGENUS INC
Vaccines for Mycoplasma bovis and methods of use
The invention of novel, effective vaccines against Mycoplasma bovis for use in cattle is described. These vaccines demonstrate no undesirable side effects and protect against M. bovis related disease, such as contagious mastitis, respiratory pneumonia, joint infections, keratoconjunctivitis and middle ear infections. The novel vaccines also lessen the effect M. bovis infections on milk production, weight gain and animal health. Methods of diagnosing characterizing and treating M. bovis infections as specific biotypes are also disclosed. Vaccine compositions made in accordance with the invention may be either of the attenuated or inactivated variety. Vaccines may also include antigens from other pathogens so as to provide a protective immunogenic response to diseases other than those caused by M. bovis.
Owner:BIOMUNE
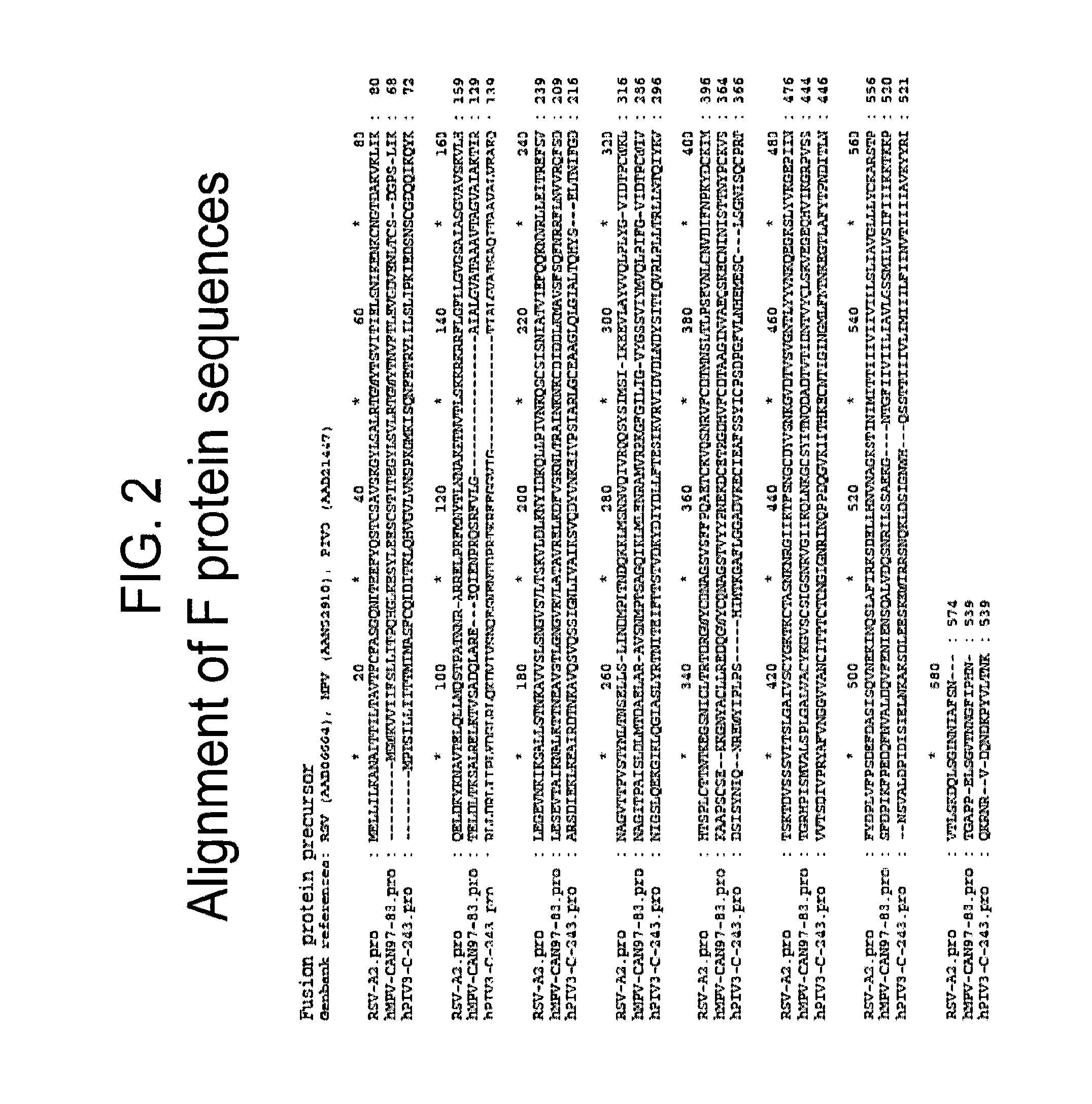
Vaccine
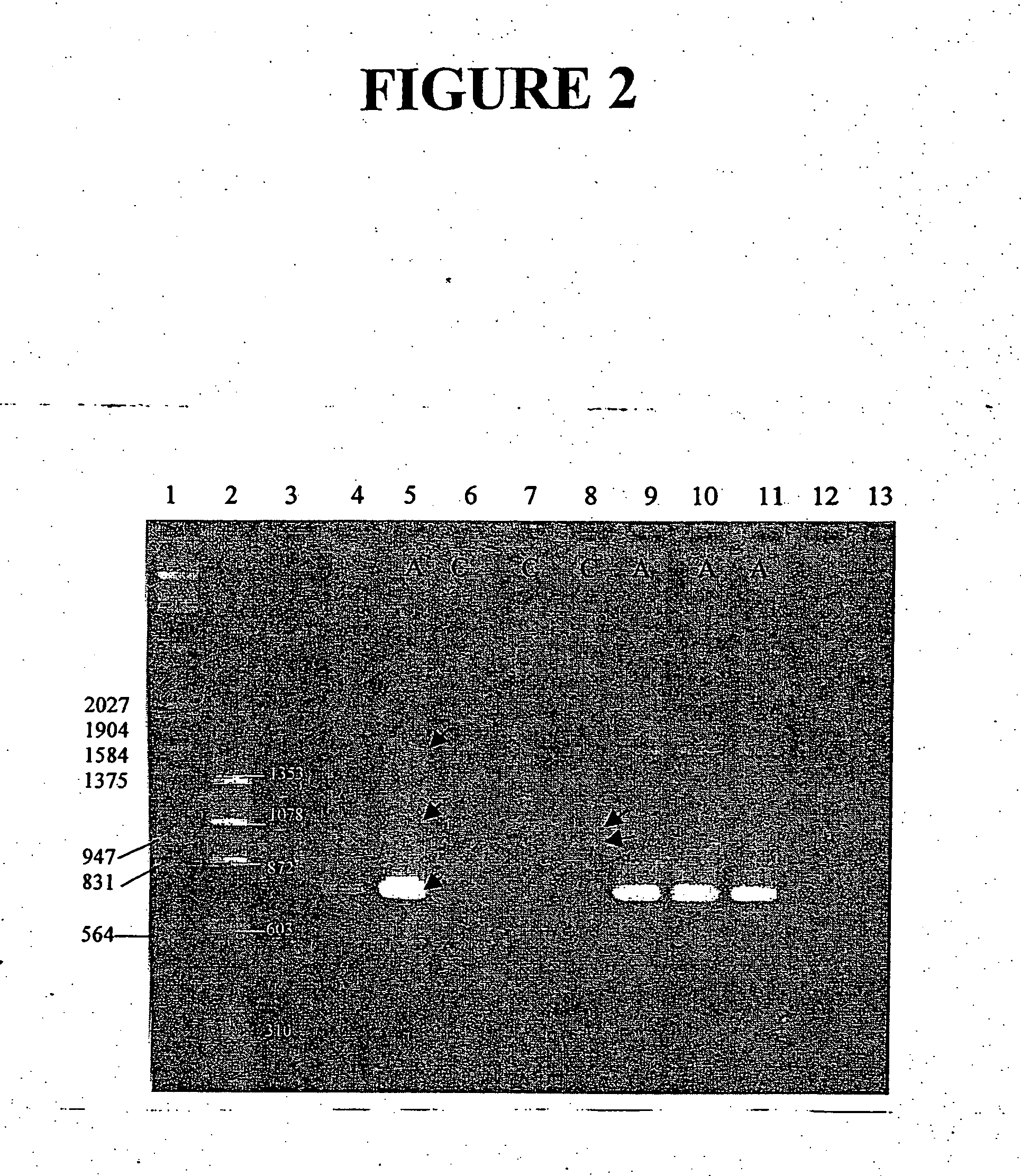
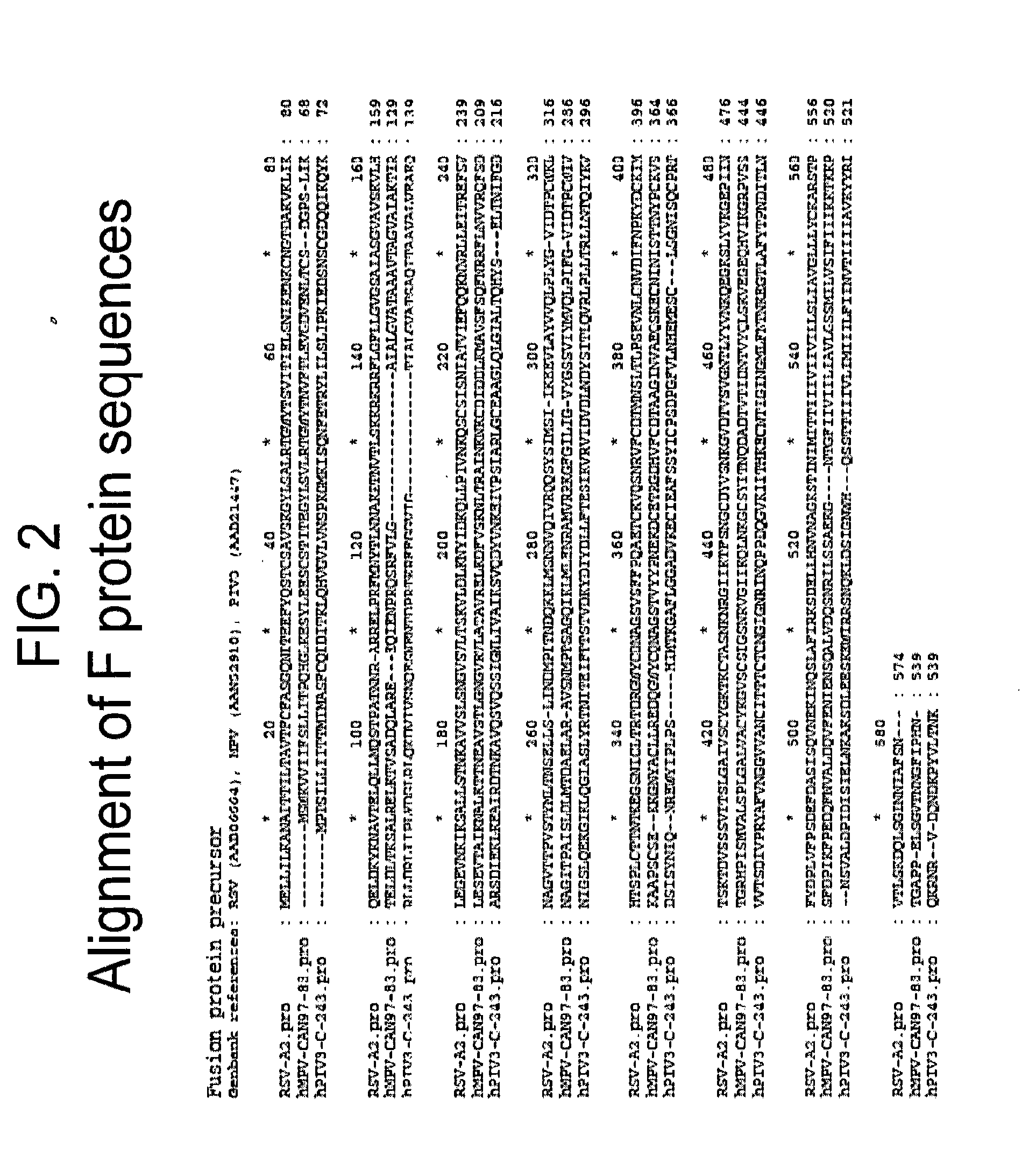
The present disclosure provides immunogenic compositions that include at least two paramyxovirus F protein antigens selected from human metapnuemovirus (hMPV), paarainfluenza virus (PIV) and respiratory syncytial virus (RSV). The antigens of the disclosed compositions are recombinant F protein polypeptides, which have been modified to stabilize the trimeric prefusion conformation. Nucleic acids encoding the antigens, as well as methods for their production and use are also provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
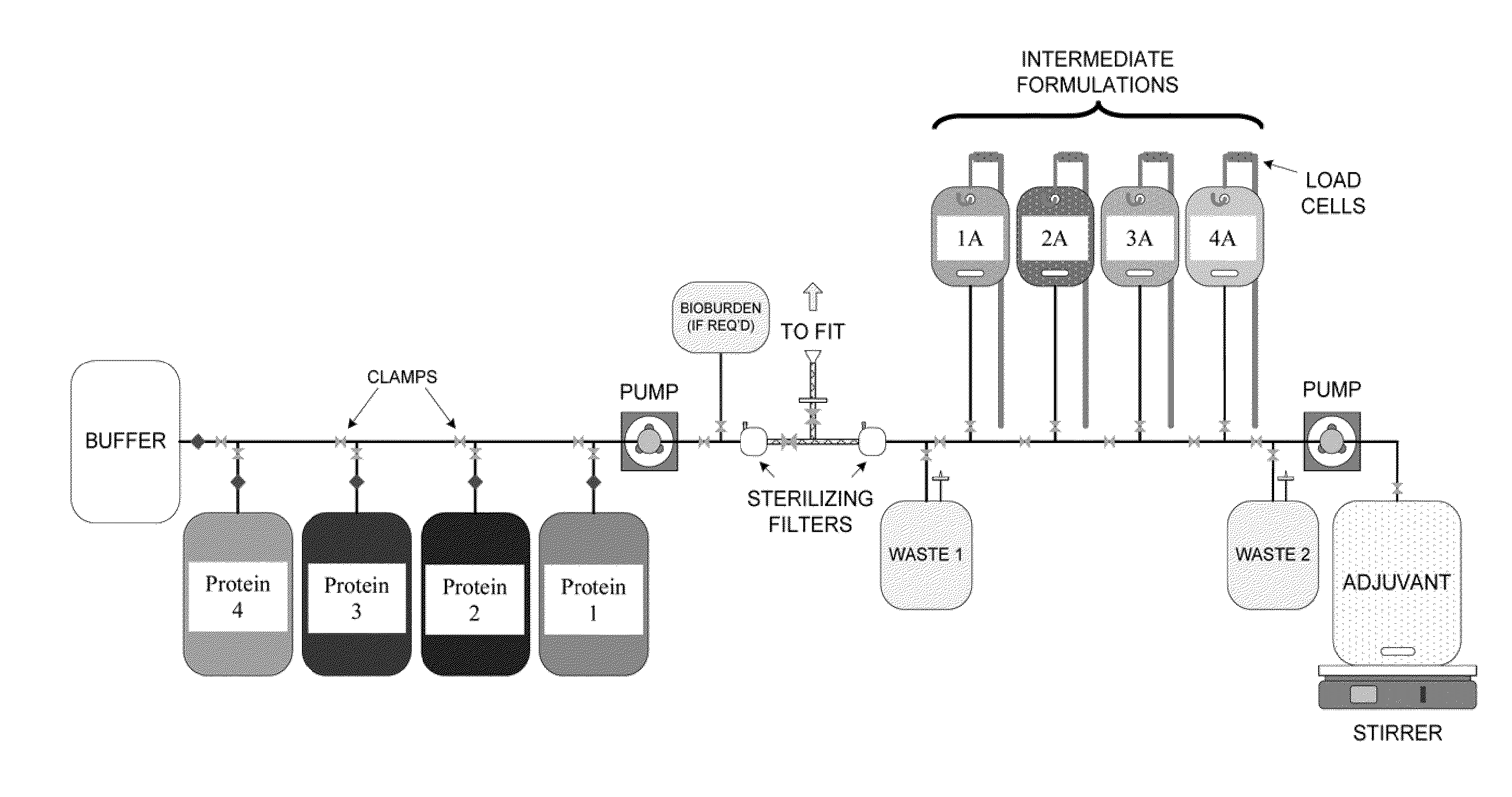
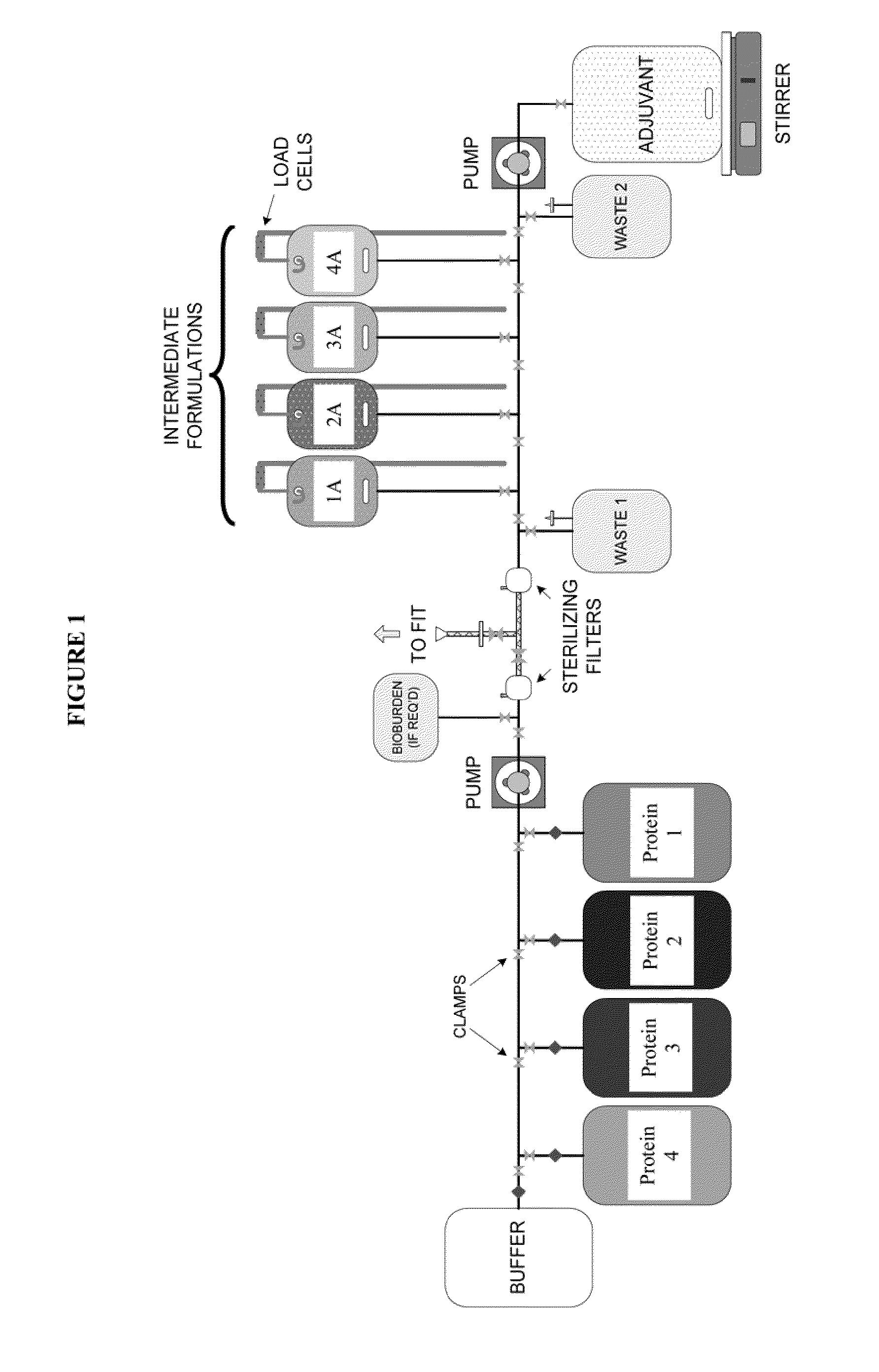
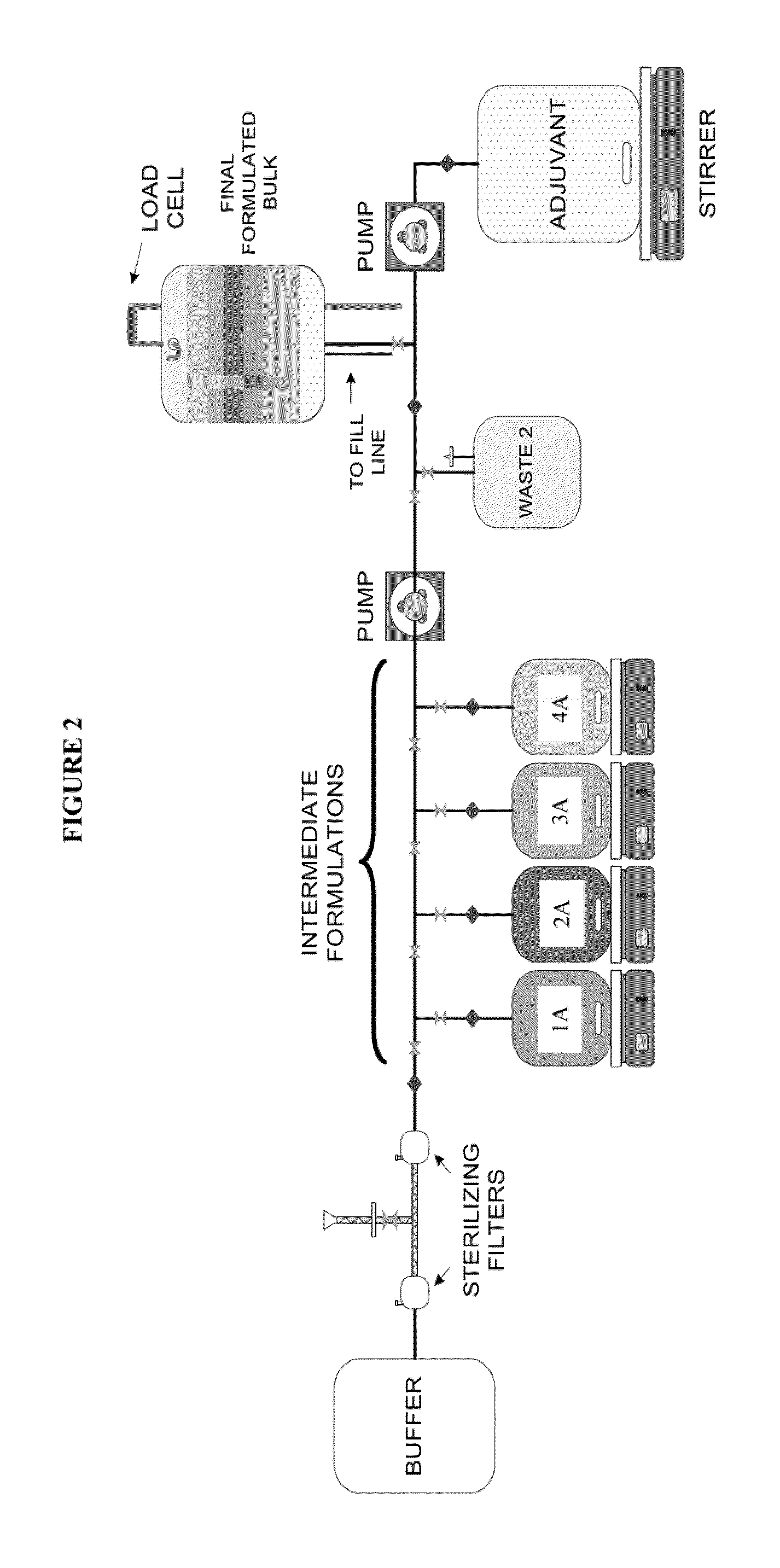
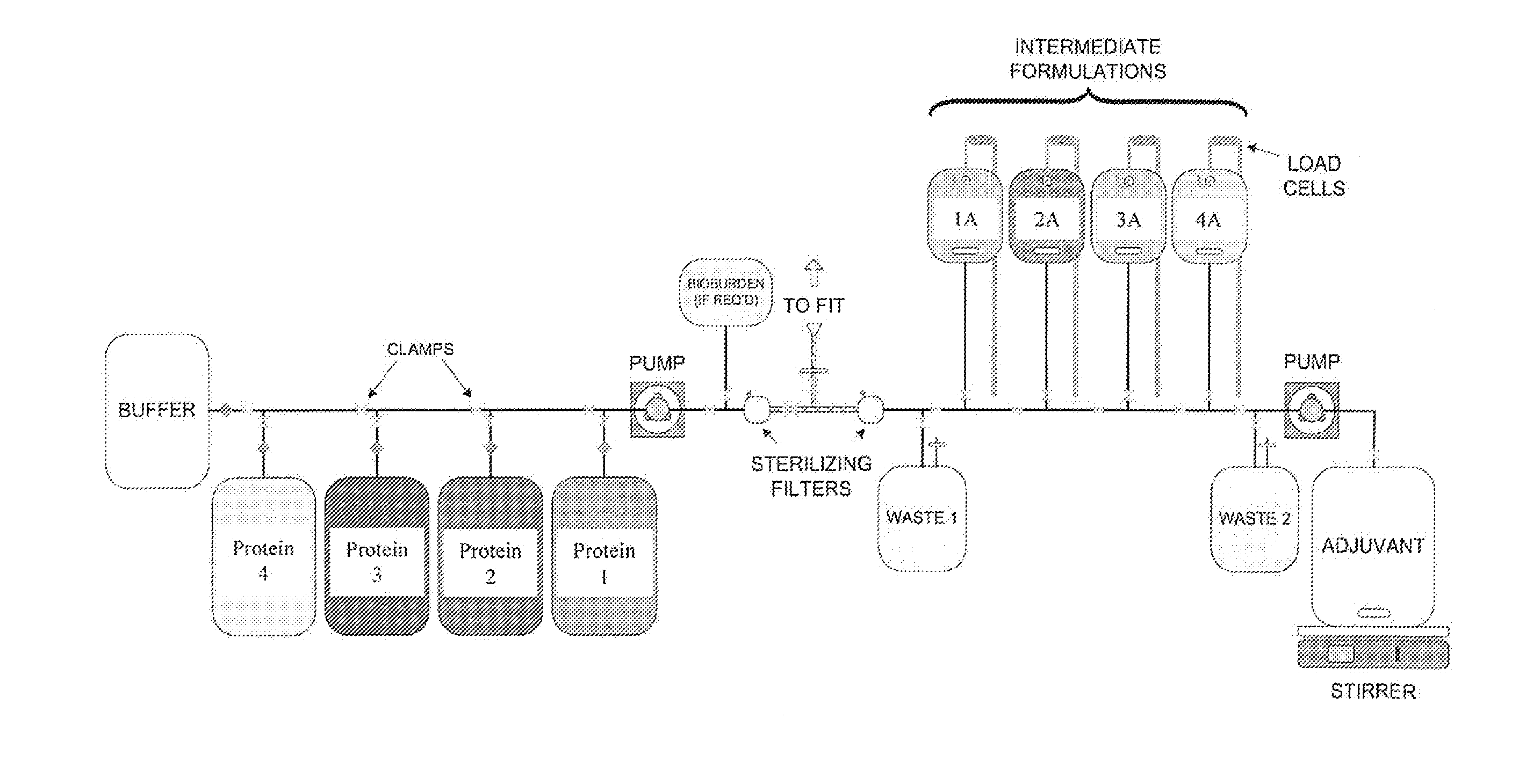
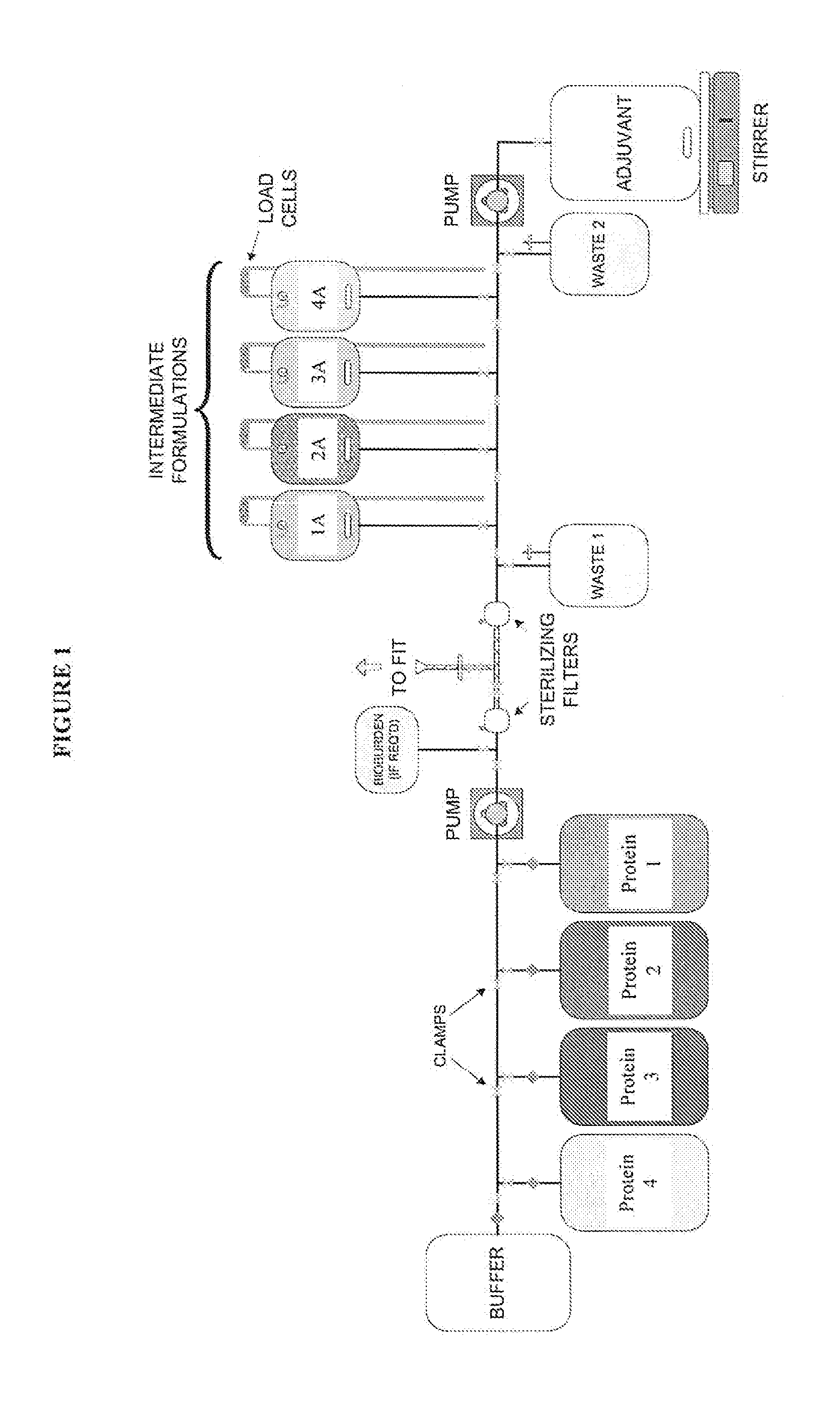
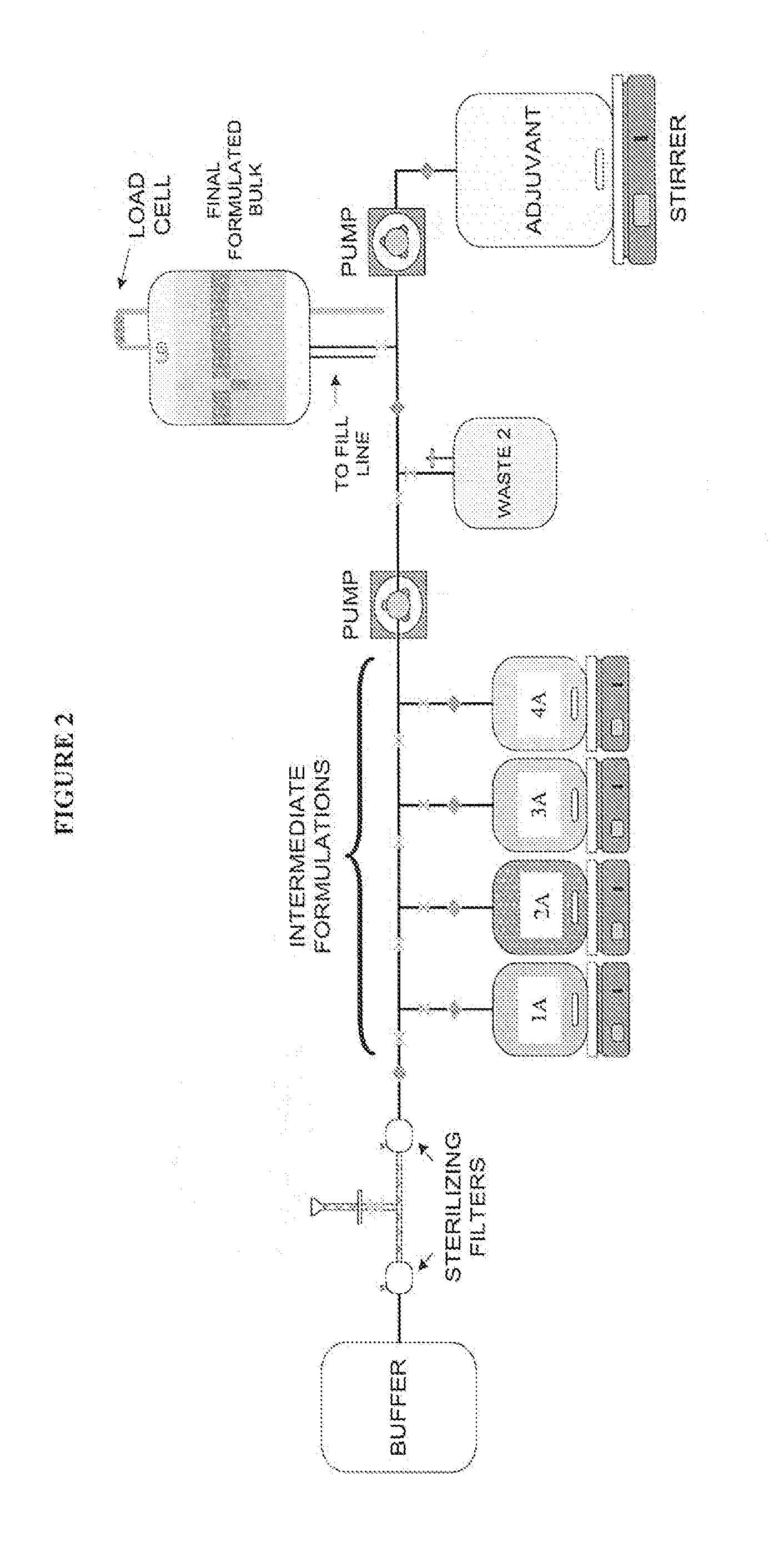
System and process for producing multi-component biopharmaceuticals
A sterile, closed, disposable system for formulating biopharmaceutical compositions containing multiple active agents is described herein.
Owner:SANOFI PASTEUR LTD
Formulations for neoplasia vaccines
InactiveUS20160310584A1Efficient transferHigh expressionOrganic active ingredientsCancer antigen ingredientsImmunogenicityPathology
Owner:THE BROAD INST INC
Type III bacterial strains for use in medicine
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN
Compositions for stabilizing and delivering proteins
ActiveUS20170273909A1Good storage stabilityEffective shieldingPowder deliveryPeptide/protein ingredientsProtein compositionNanoparticle
Owner:THERAPYX +1
Methods for decreasing the toxic effects of nicotine on fetuses in pregnant women
InactiveUS7547712B2Reduce adverse effectsReduce weightBiocideNervous disorderPoisonous effectsNicotine
Owner:NABI BIOPHARMLS +1
Vaccine
The present disclosure provides immunogenic compositions that include at least two paramyxovirus F protein antigens selected from human metapnuemovirus (hMPV), paarainfluenza virus (PIV) and respiratory syncytial virus (RSV). The antigens of the disclosed compositions are recombinant F protein polypeptides, which have been modified to stabilize the trimeric prefusion conformation. Nucleic acids encoding the antigens, as well as methods for their production and use are also provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Mucosal immunogenic substances comprising a polyinosinic acid - polycytidilic acid based adjuvant
InactiveUS20070166239A1Enhance immune responseImprove responseSsRNA viruses negative-senseOrganic active ingredientsMucosal Immune ResponsesAdjuvant
The present invention provides a polynucleotide adjuvant composition and methods of use in eliciting an immune response, in particular a mucosal immune response. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine). The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response, in particular a mucosal immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Method of treating cancer
A method of treating cancers by administering to a patient an anti-tumor effective amount ot at least one of a thermostable macromolecular antigen complex or a fragment of such a complex which is interspecific for microorganisms of the Mycobacteria, Nocardia and Corynebacteria group and which exhibits after immunoelectrophoresis an immunoelectrophoretic precipitation pattern corresponding to that of the antigen complex 60 of Mycobacteria bovis Calmette Guerin Bacillus strain. Preferably, the patient is also treated with an additional therapeutic agent which is specific against the patient's cancer such as a tumoral antigen or a non-proliferative tumor cell.
Owner:ANDA BIOLOGICALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com