Shared neoantigens
An antigen peptide, resistance technology, applied in the field of treatment of neoplasia, can solve the problems of unsatisfactory immunogen, immunosuppressive effect and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
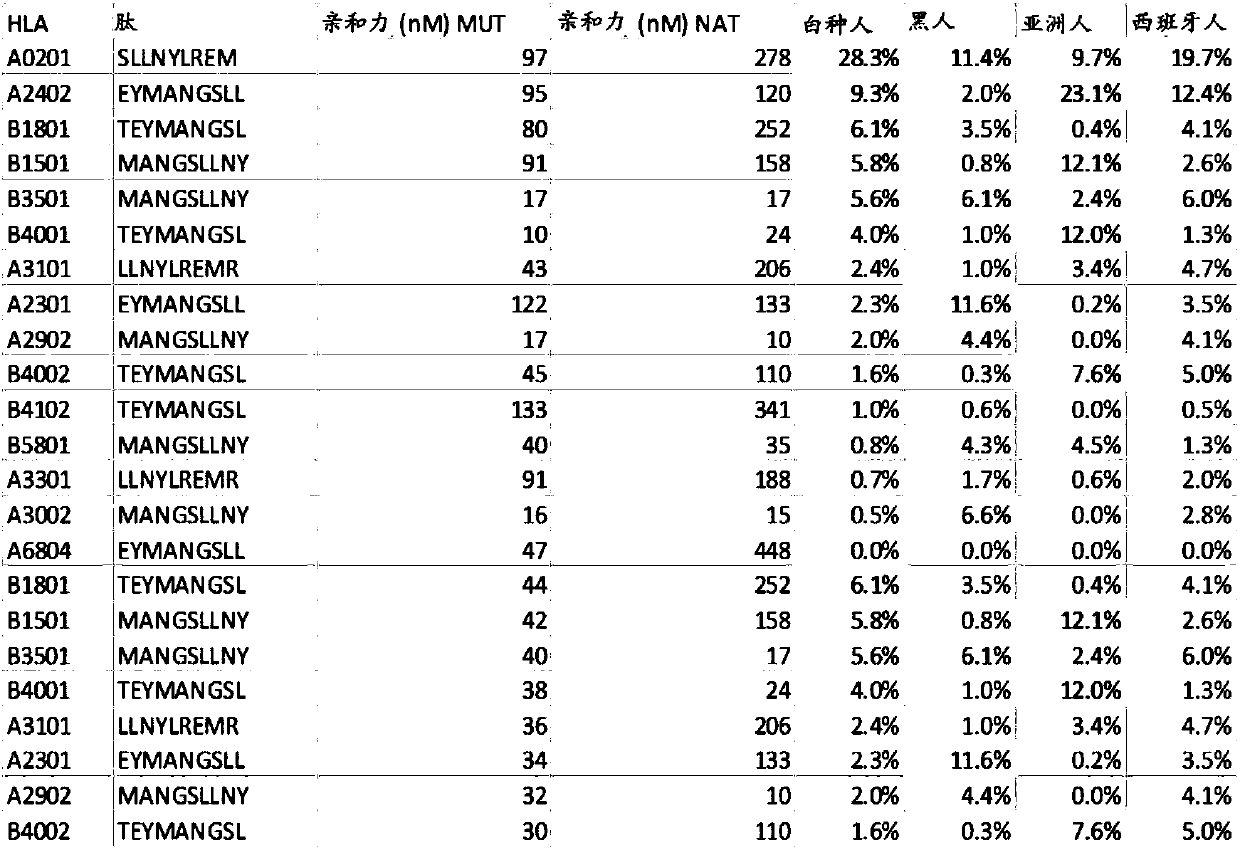
[0761] sPDL1 generates immunogenic epitopes across multiple HLA alleles. PDL1 (CD274) is a transmembrane protein that interacts with PD1 (CD279; PDCD1) on T cells and may be involved in various forms of innate immune suppression, such as during pregnancy. Expression of PDL1 is also used by tumor cells as a way to evade host immune responses. sPDL1 is an alternate spliced form of the PDL1 gene. This alternative splicing form is due to a lack of splicing at the end of exon 4, which reads into the fourth intron. The transcript terminates within intron 4. The translation product is in frame of 18 amino acids until a stop codon is encountered.
[0762] The translated product lacks the transmembrane domain typically found in PDL1 and is thus secreted. It also contains cysteines in new ORFs translated from this intron and appears to dimerize in mediators. This secreted form appears to block the binding between PDL1 and PD1.
[0763] Applicants analyzed the predicted binding p...
example 2
[0769] The androgen receptor generates immunogenic epitopes across multiple HLA alleles. The androgen receptor was identified as another alternatively spliced form of the messenger that results in a new ORF that can be expressed specifically in tumor cells. Alternative splicing results in at least two isoforms, resulting in cryptic exons that are new ORFs.
[0770] Among these alternative transcripts, AR-V1 and AR-V7 were consistently present in hormone-resistant prostate cancer samples.
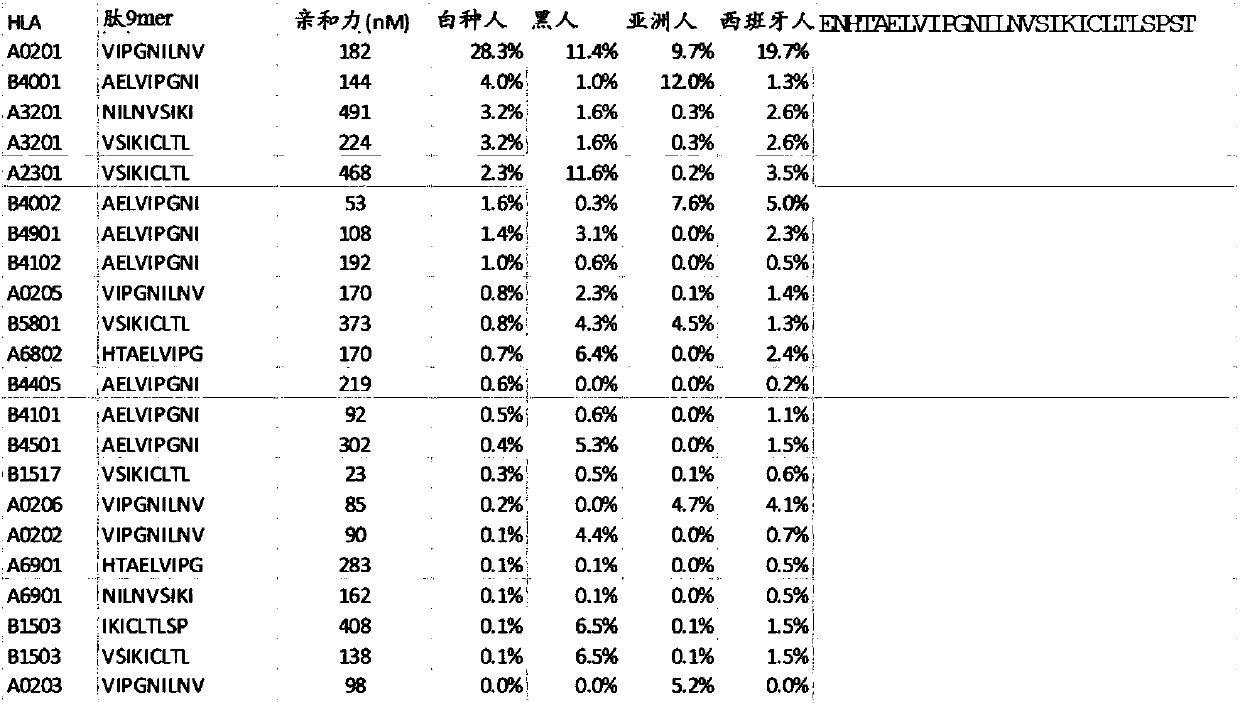
[0771] The immunogenic potential of these novel ORFs is unknown, and applicants therefore performed predicted binding analyzes across multiple common HLA alleles. These are shown for the HLA A allele (Table 2).
[0772] Table 2
[0773]
[0774] Likewise, for sPDL1, a number of peptides are predicted to be immunogenic binders, and these peptides can be applied across large subgroups of patients. These immunogenic peptides can be used across multiple patients.
example 3
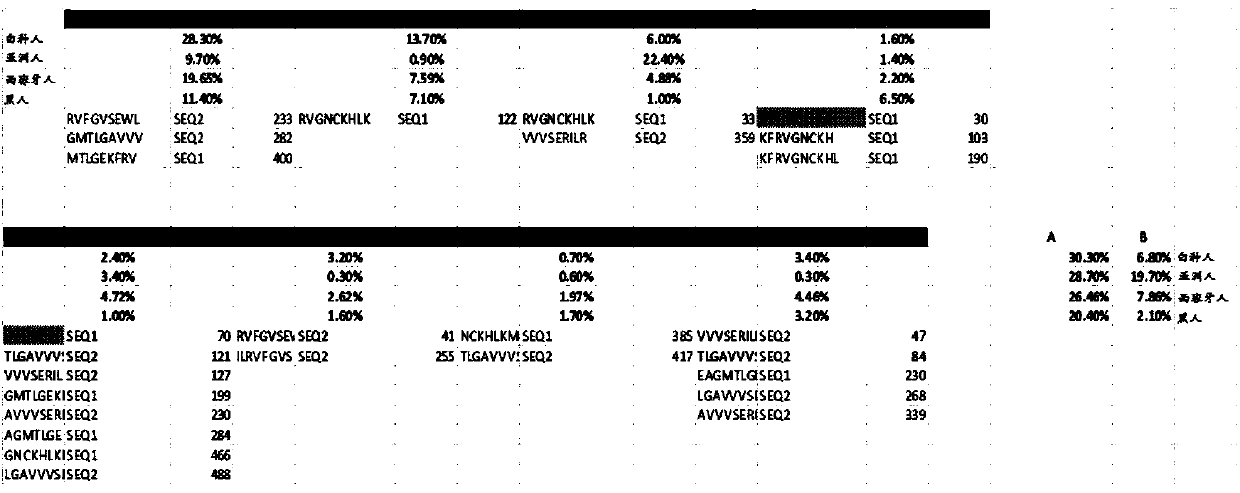
[0776] Drug resistance mutations. Treatment with various chemotherapeutic agents, especially with targeted therapies such as tyrosine kinase inhibitors, often leads to new mutations in the target molecule that resist therapeutic activity. Multiple strategies to overcome this resistance are being evaluated, including the development of second-generation therapies that are not affected by these mutations, and treatment with a variety of agents, including those that act downstream of the resistance mutation.
[0777] Applicants have evaluated the immunogenic potential of mutant peptides resulting from these resistance mutations and found that for some, predicted immunogenic peptides were generated that could bind a range of HLA alleles. Two specific examples and data for a series of resistant variants are provided below.
[0778] BTK / C481S
[0779] A very common mutation for ibrutinib, a molecule that targets Bruton's tyrosine kinase (BTK) and is used in CLL and certain lymph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com