Patents
Literature
40 results about "A serotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of generating chimeric adenoviruses and uses for such chimeric aden oviruses
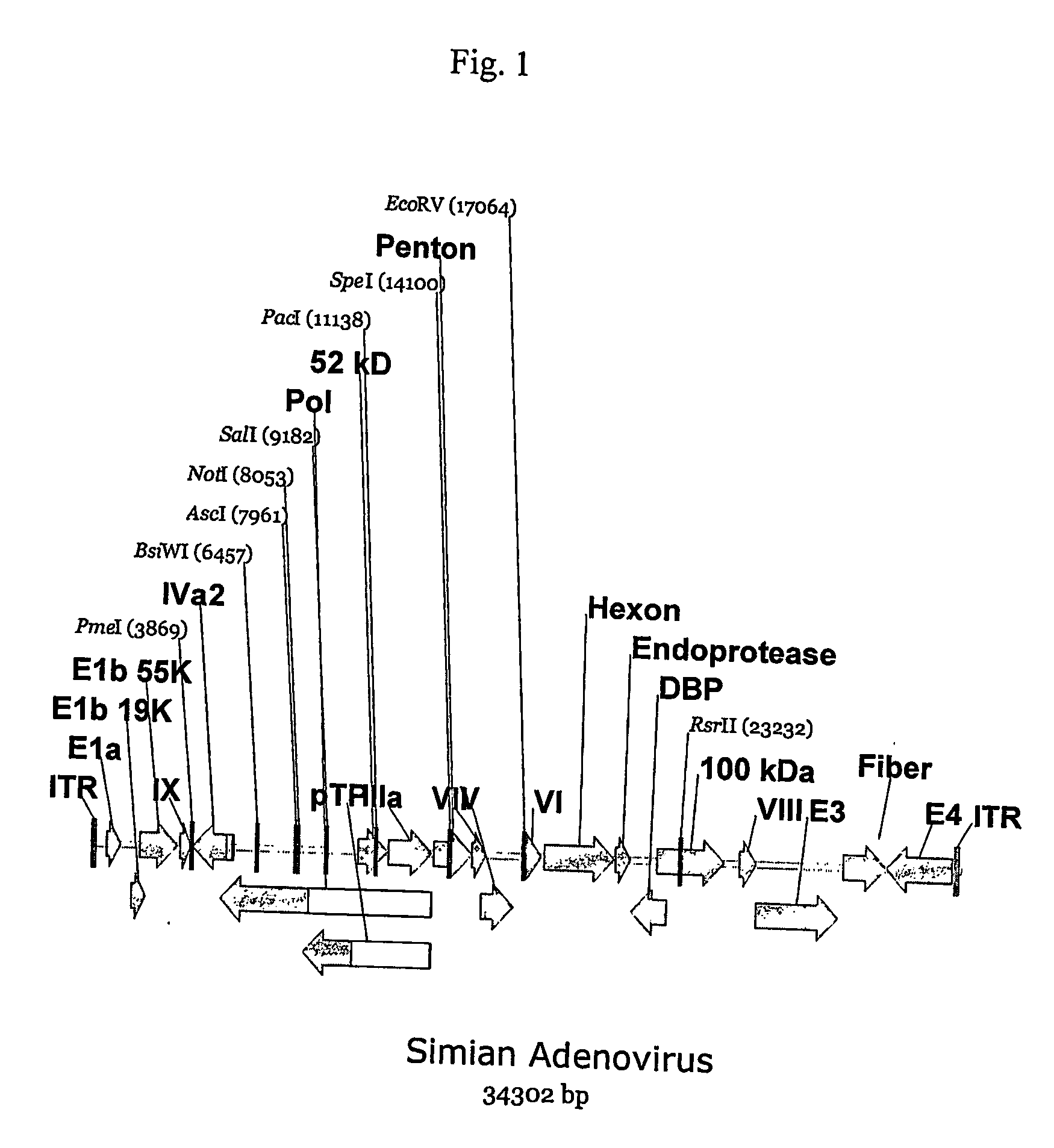
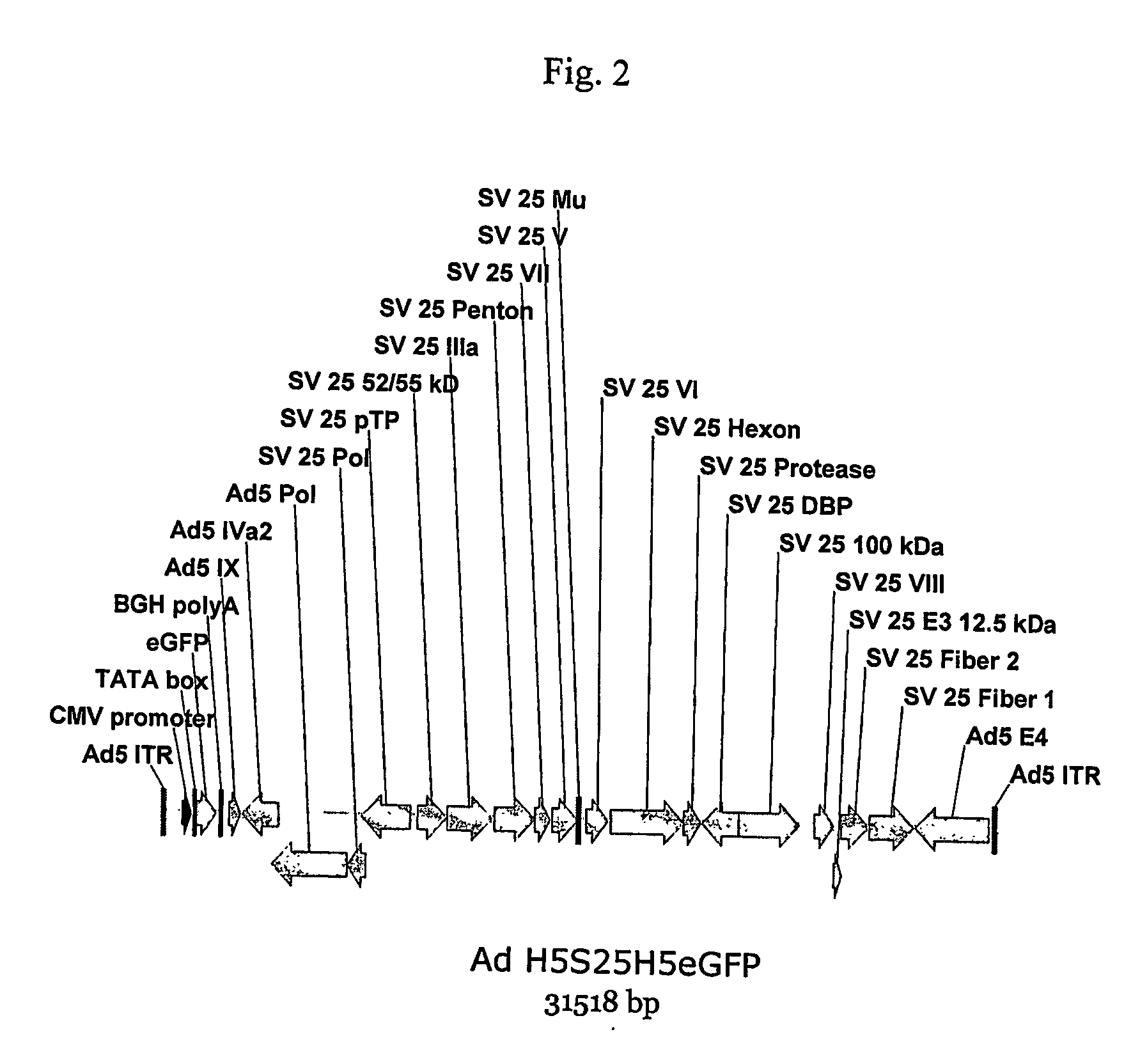
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
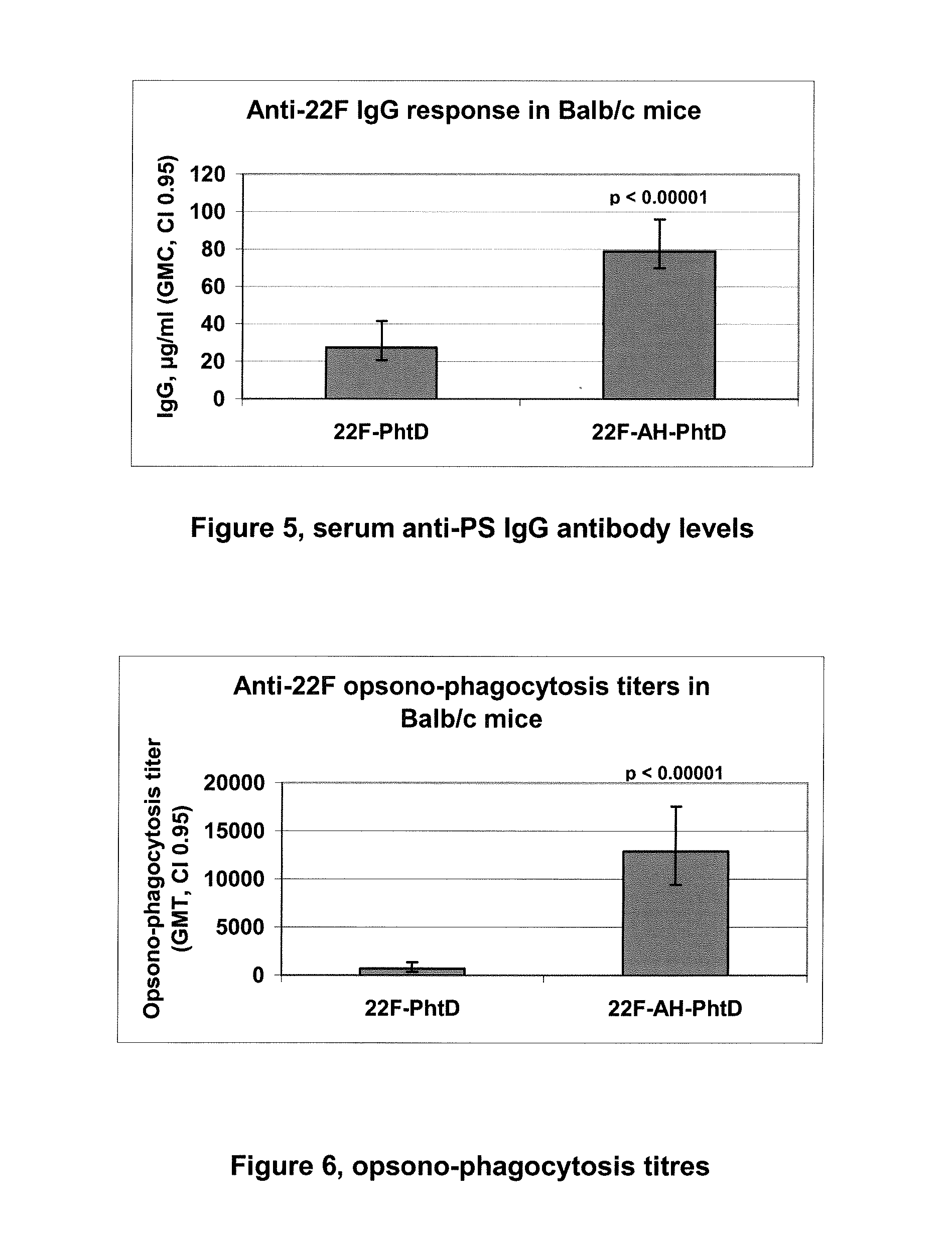
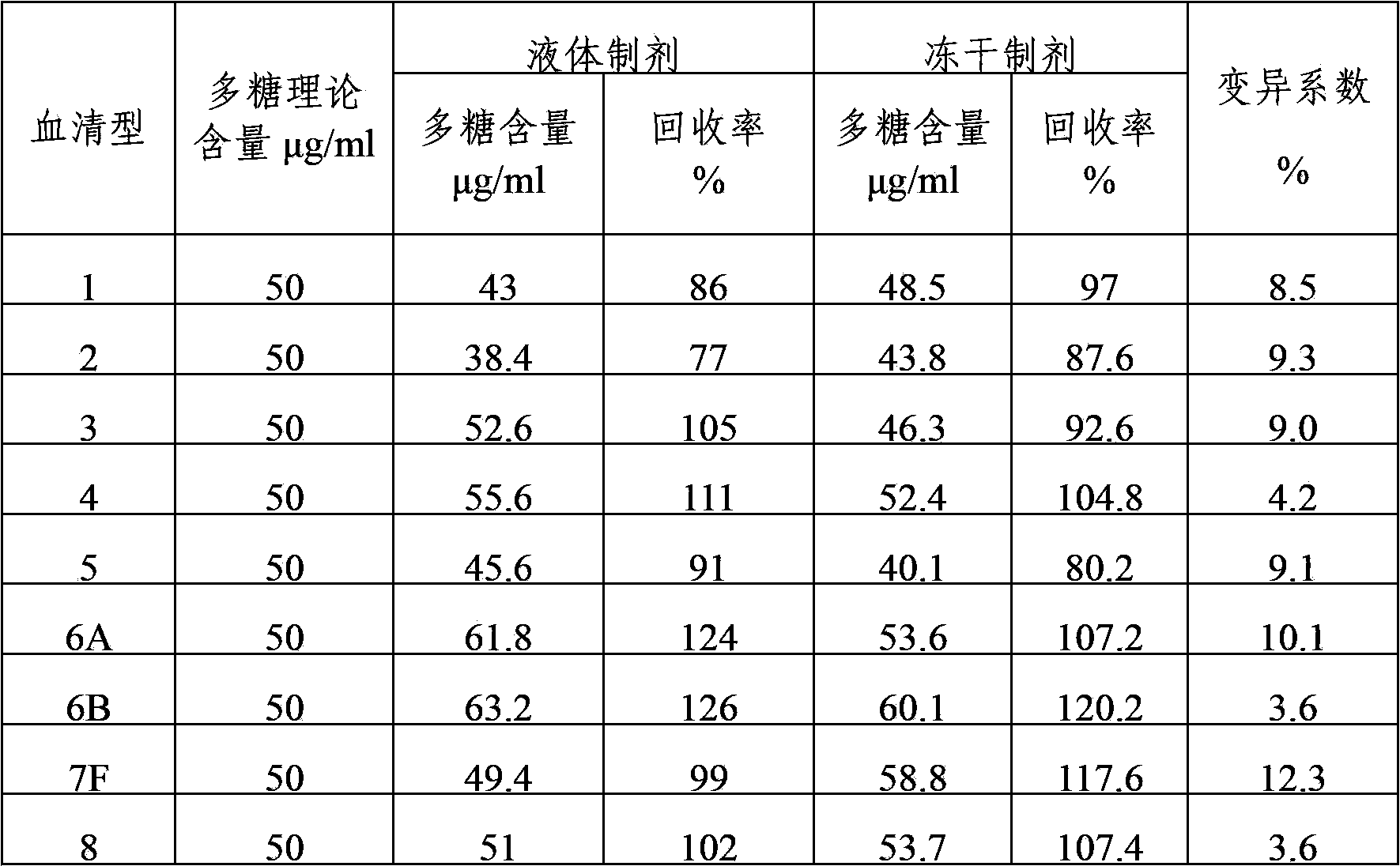
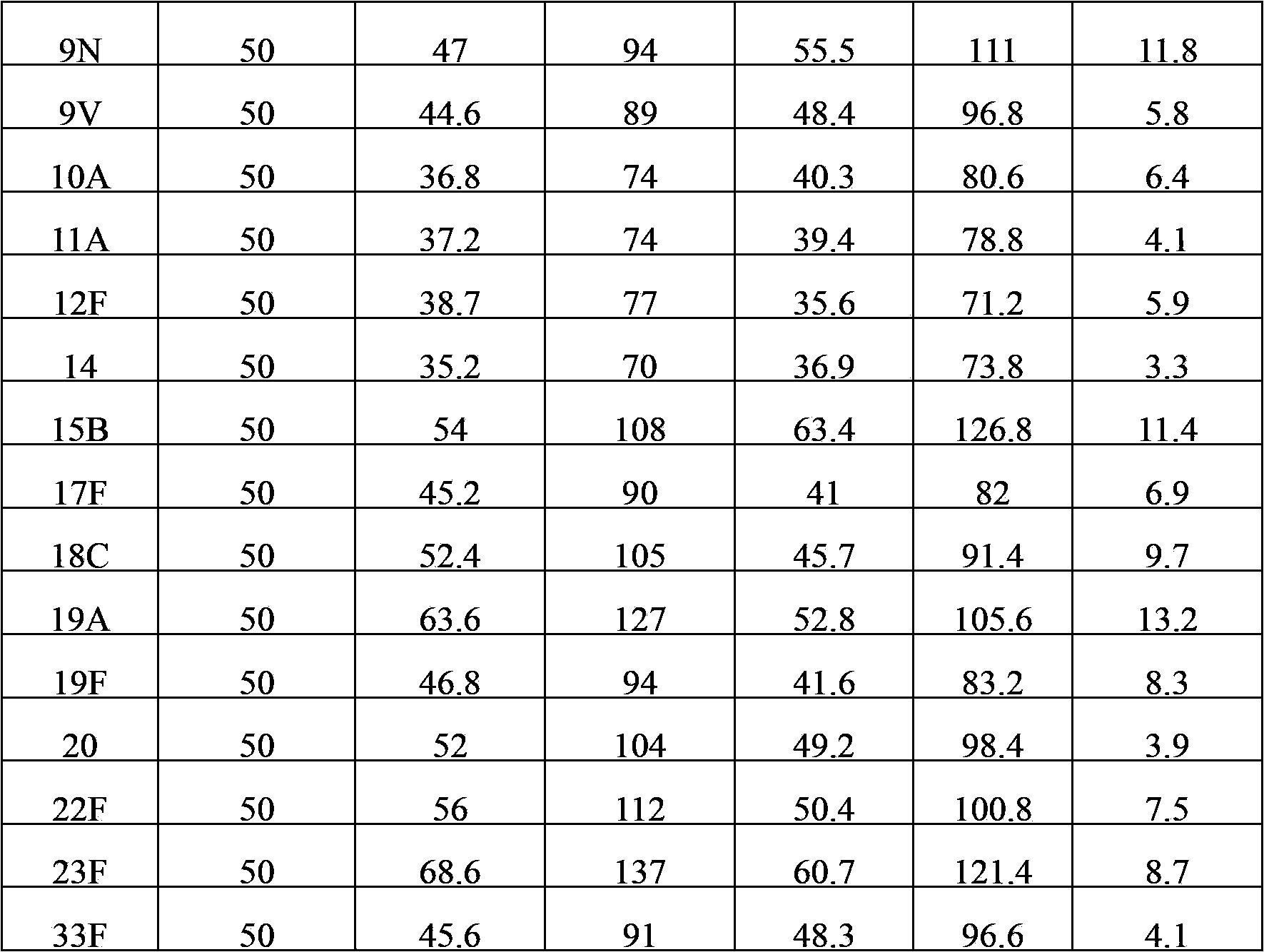
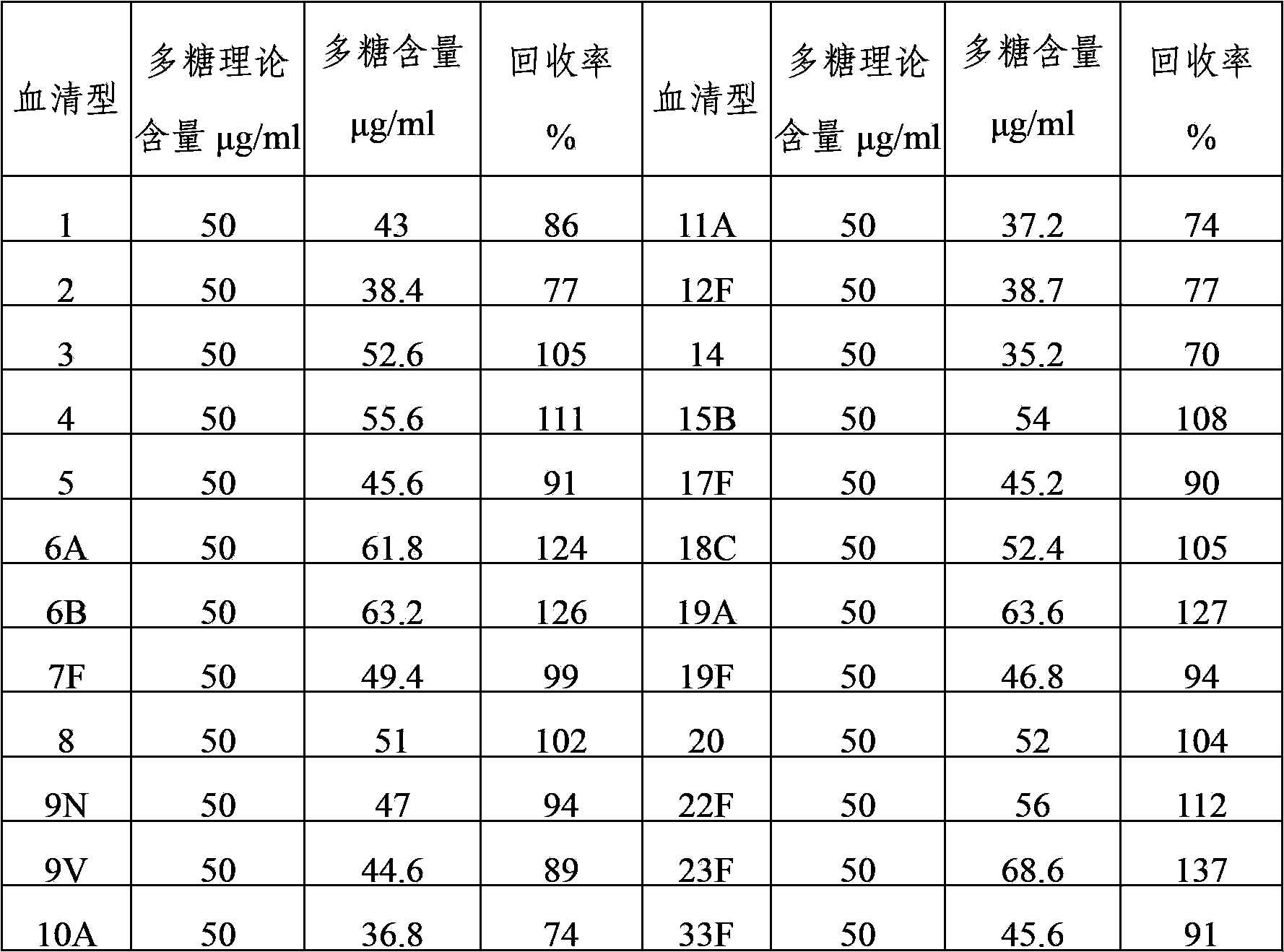
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Adenoviral vector-based vaccines
InactiveUS20060286121A1Increase capacityImprove stabilityAntibody mimetics/scaffoldsViral antigen ingredientsHeterologousAntigen
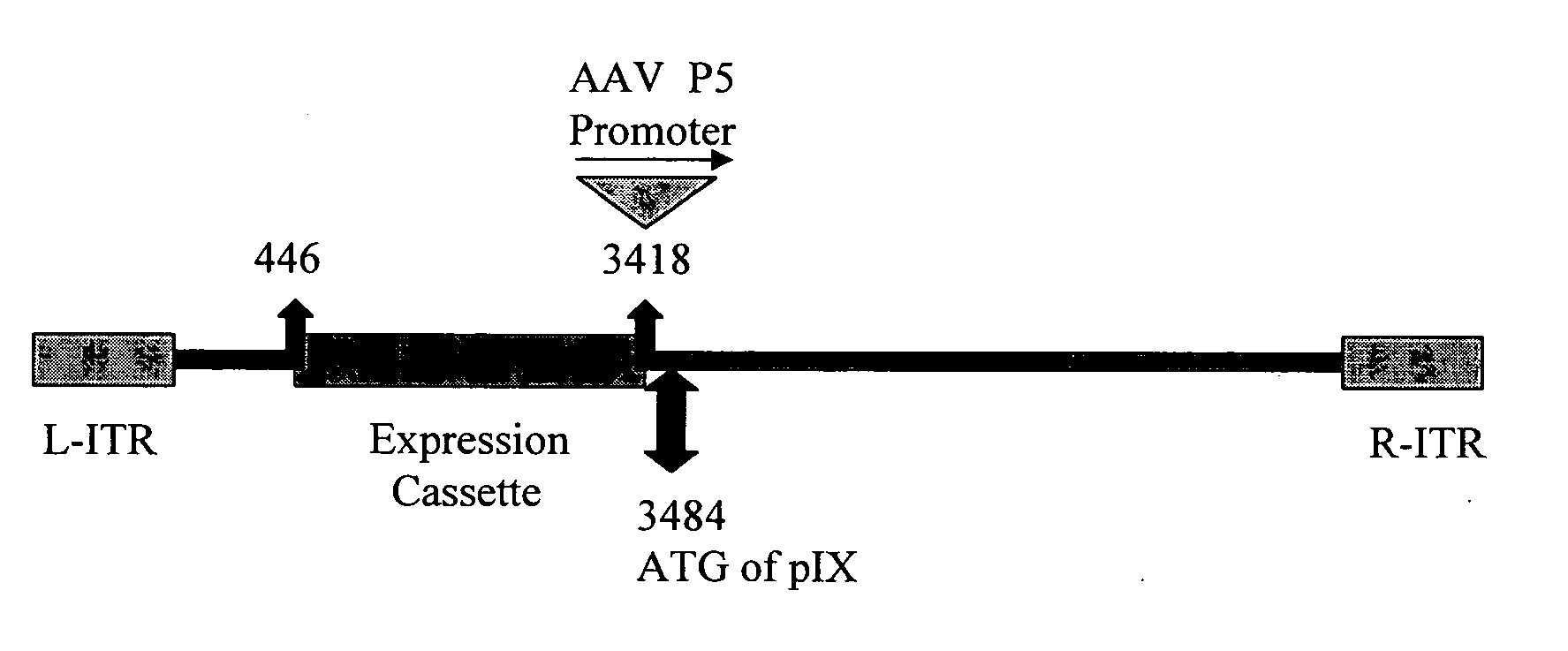
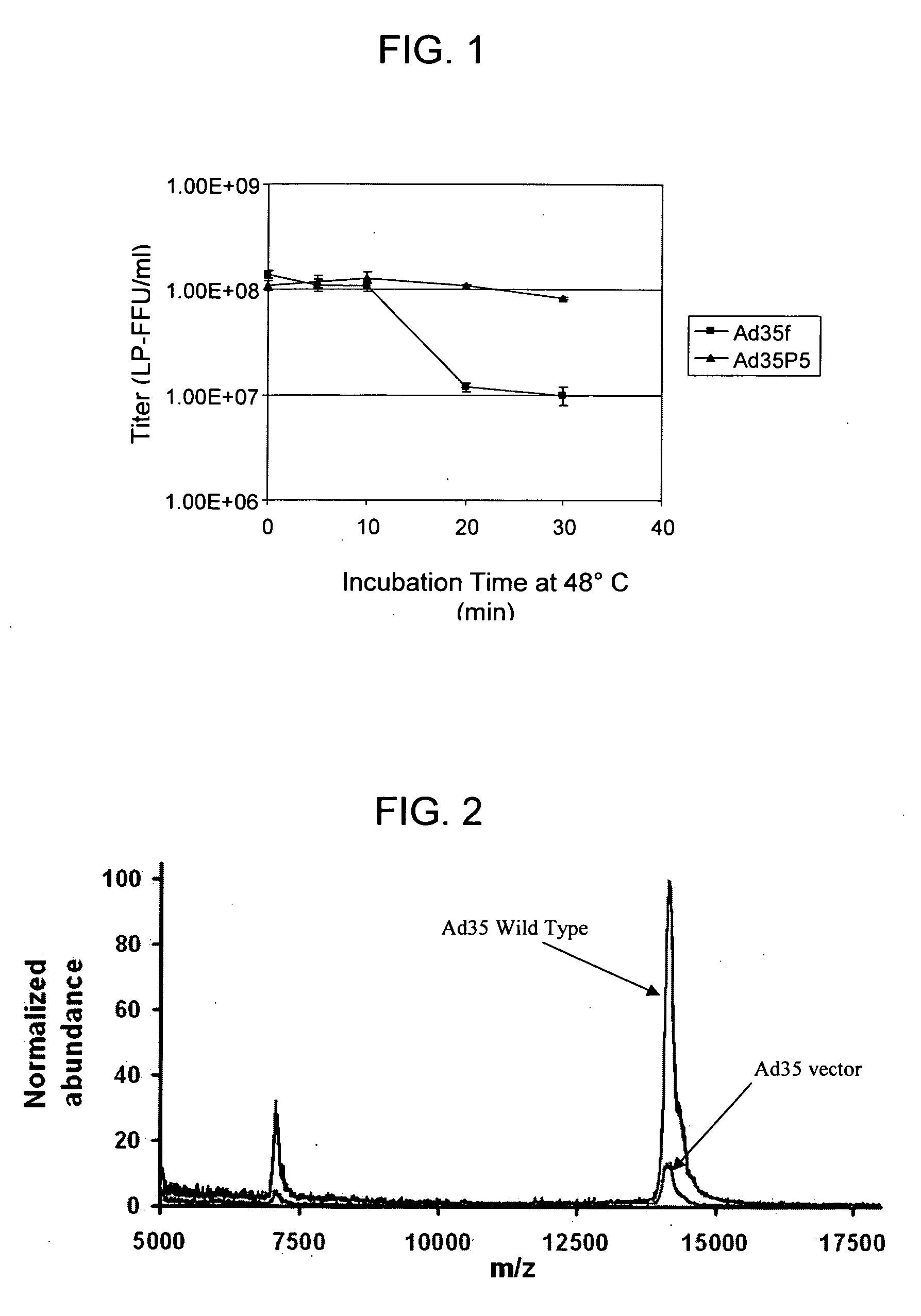
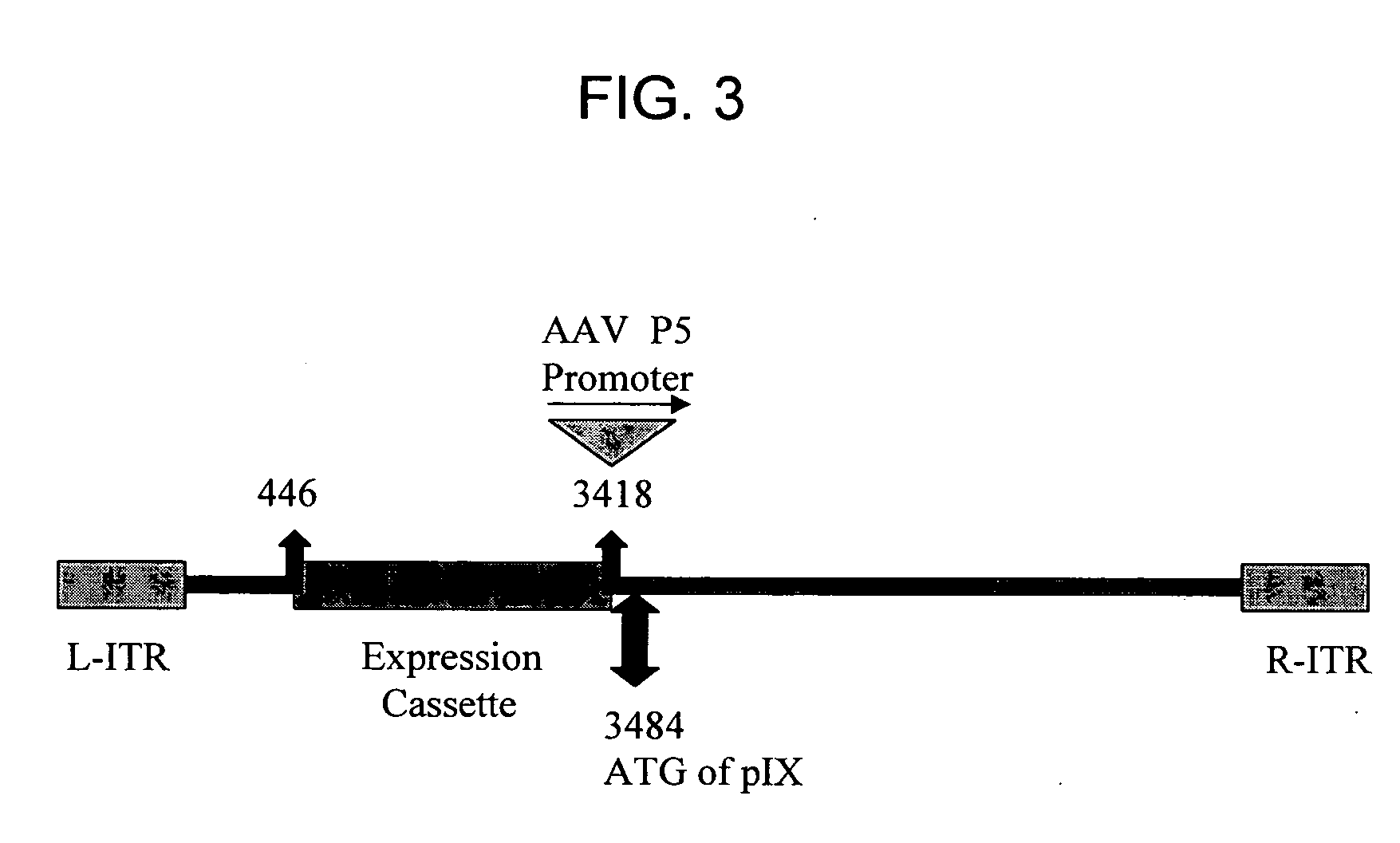
The invention provides a method of inducing an immune response in a mammal. The method comprises administering to the mammal a non-subgroup C adenoviral vector comprising an adenoviral fiber protein having an amino acid sequence comprising about 80% or more identity to an amino acid sequence encoding a subgroup C adenoviral fiber protein. The adenoviral vector further comprises a nucleic acid sequence encoding an antigen which is expressed in the mammal to induce an immune response. The invention further comprises a method of producing an adenoviral vector, and a composition comprising a serotype 41 or a serotype 35 adenoviral vector and a carrier. The invention also provides an adenoviral vector comprising a nucleic acid sequence encoding an adenoviral pIX protein operably linked to a heterologous expression control sequence, as well as a method of enhancing the stability and / or packaging capacity of an adenoviral vector.
Owner:UNITED STATES OF AMERICA +1
Detection card for serotype O foot and mouth disease virus antibody and preparation method of detection card
InactiveCN105606819AQuick checkHigh sensitivityBiological material analysisBiological testingAntigenQuality control
The invention discloses a detection card for a serotype O foot and mouth disease virus antibody and a preparation method of the detection card. The detection card comprises a detection card body assembled in a shell. The detection card body comprises a base plate. A sample pad, a marking pad, a nitrocellulose membrane and water absorption paper are sequentially arranged on the base plate. A serotype O foot and mouth disease antigen coats the nitrocellulose membrane to serve as a detection line, and an anti-chicken antibody produced in goat coats the nitrocellulose membrane to serve as a quality control line. A sample adding hole corresponding to the sample pad is formed in the shell. Window holes corresponding to the detection line and the quality control line on the nitrocellulose membrane are formed in the shell. The detection card is high in sensitivity, small in detection error in a batch and detection error between batches, good in repeatability, stable in performance, capable of fast detecting the serotype O foot and mouth disease virus antibody on site, and high in practical value.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Chimeric proteins that induce effects directed against viruses
InactiveUS7279164B2Improve the level ofSignificant positive effectSsRNA viruses positive-senseAntibody mimetics/scaffoldsMutated proteinImmunity response
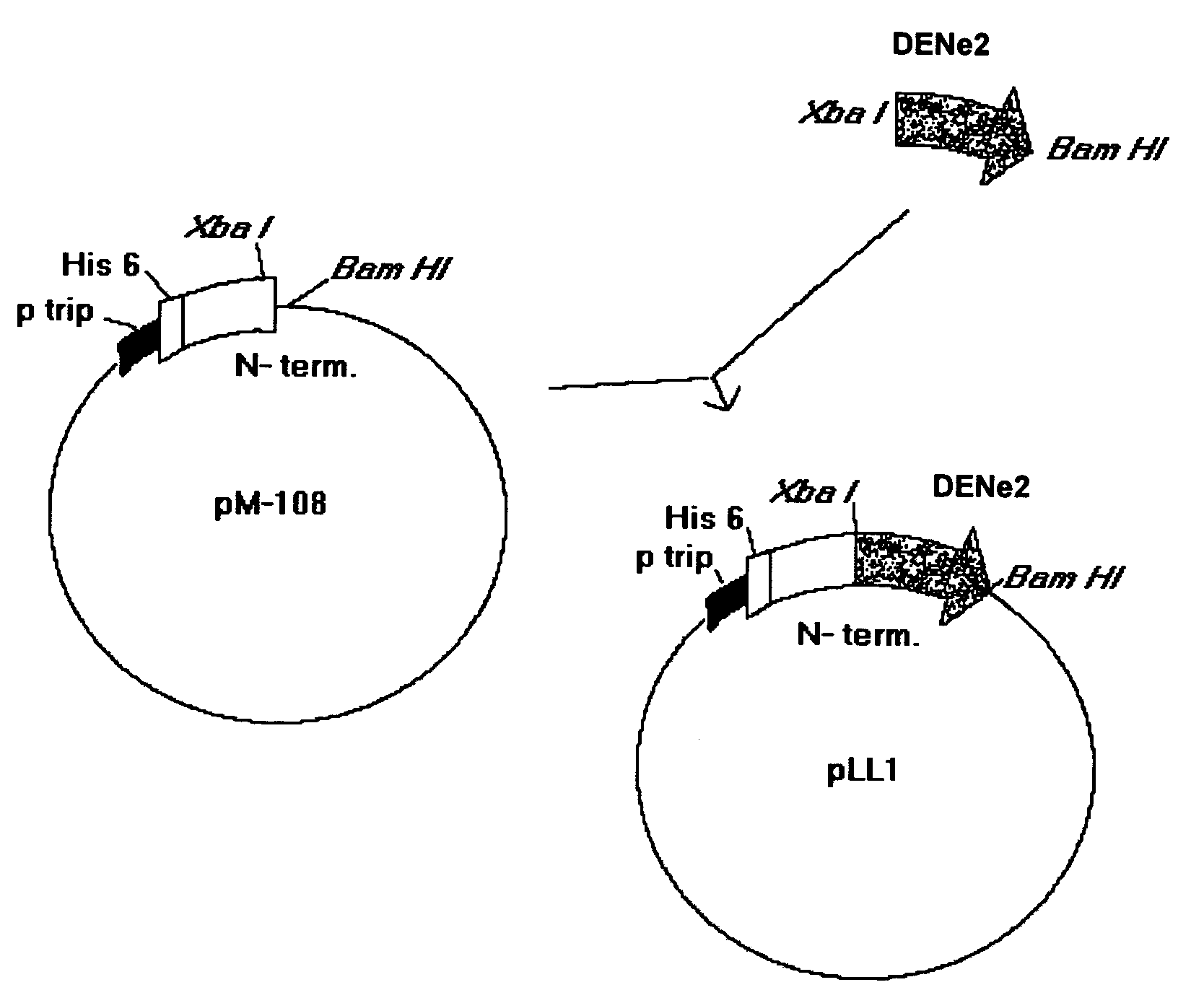
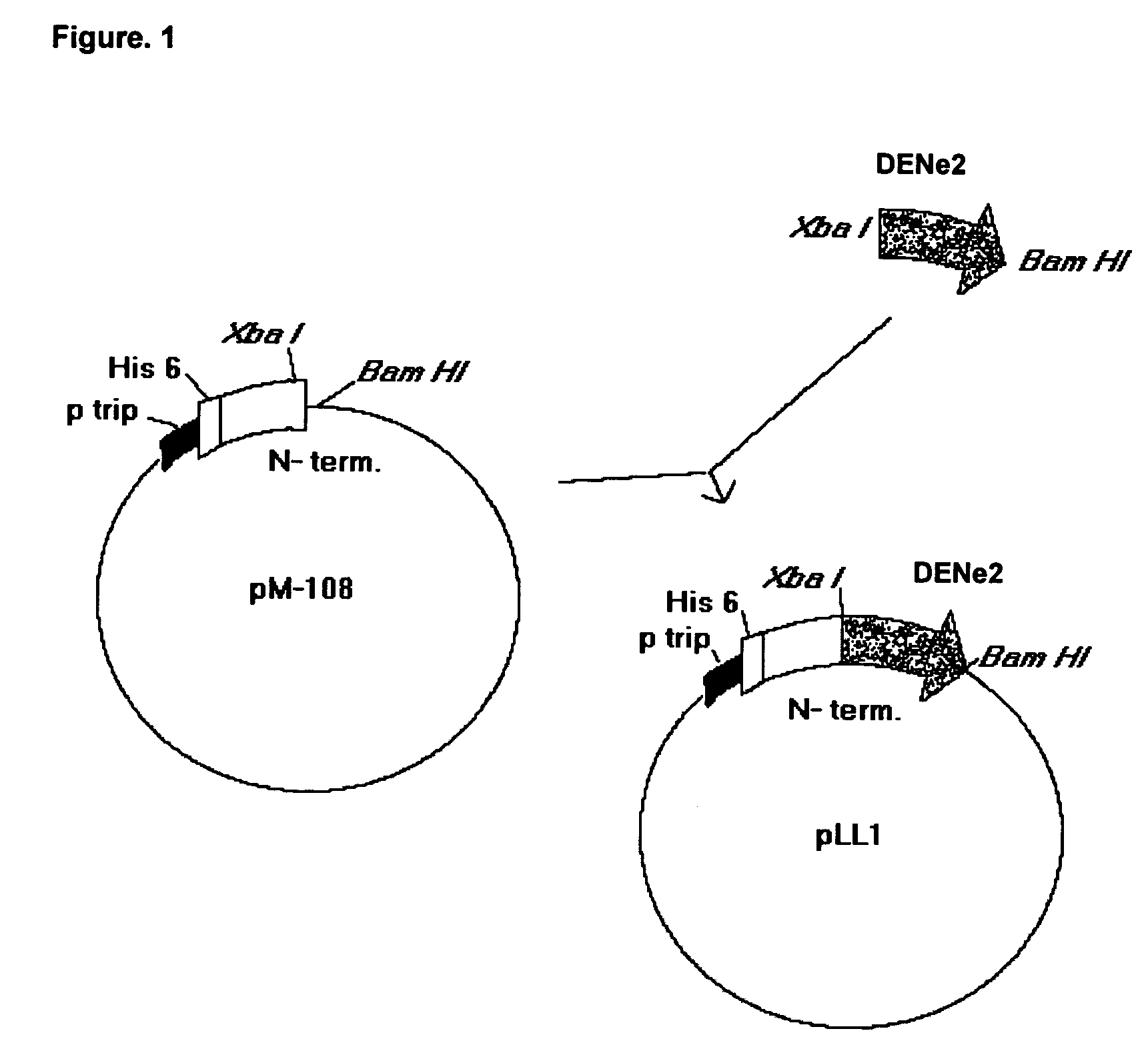
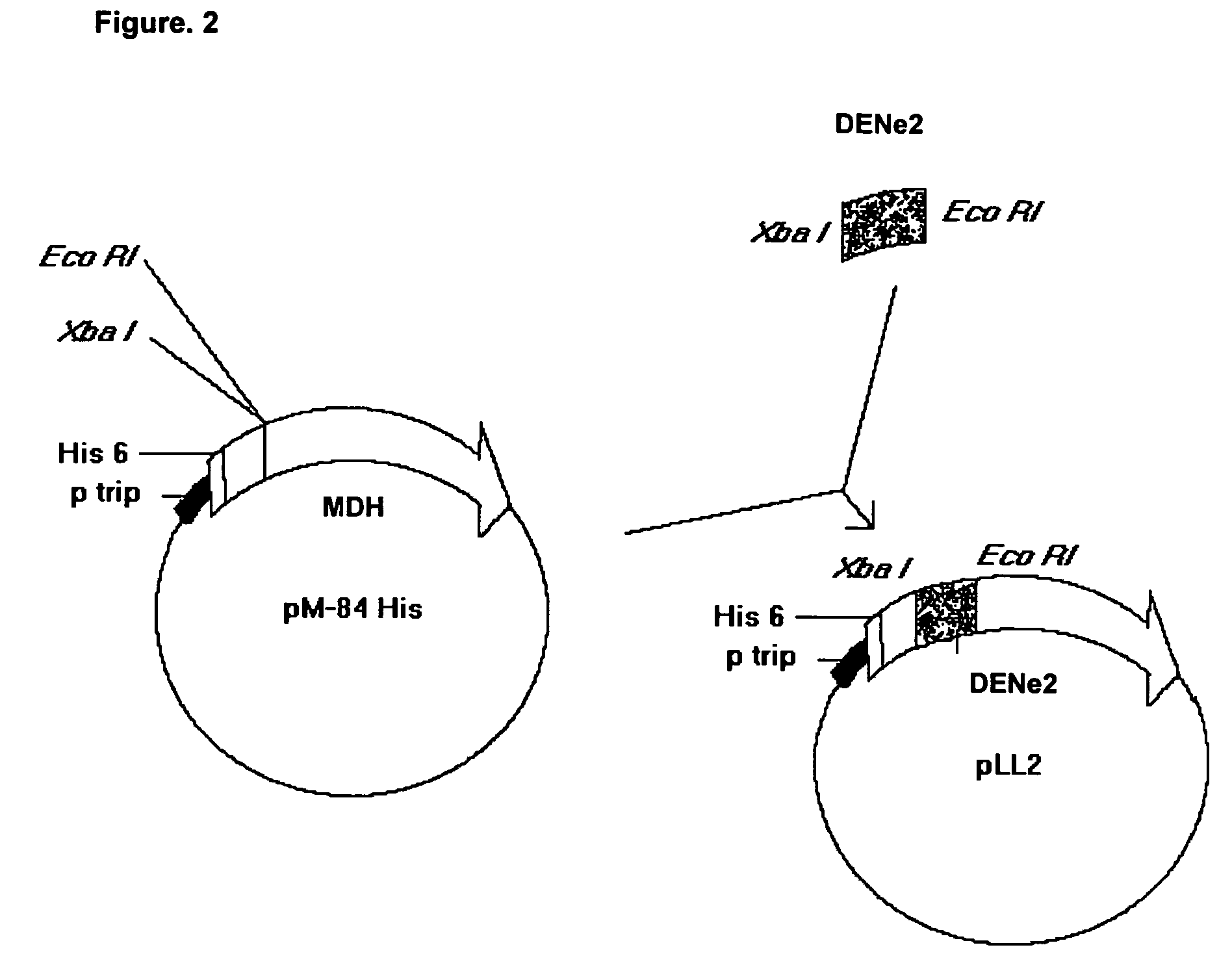
The present invention is related to the obtaining of chimeric chains coding for proteins capable of inducing, in the recipient, a serotype-specific and protective humoral immune response against the infection by the Dengue virus, thus eliminating the effects of the serotype-nonespecific viral immunoenhancement that causes hemorrhagies and clinical complications described for this kind of pathology. These chimeric chains of nucleic acids are composed by the specific combination of fragments belonging to the gene of a mutated protein from Neisseria meningitidis with dehydrogenase activity and fragments that codify for a region of the envelope (E) protein from the Dengue virus which, when inserted to an expression vector, give rise to chimeric proteins with particular properties. The resultant chimeric molecules from this invention are applicable to the pharmaceutical industry for the obtaining of vaccine preparations and diagnostic means of high serotype-specificity to be used in humans.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Haemophilus parasuis disease antibody detecting test strip and preparation method thereof
The invention discloses a haemophilus parasuis disease antibody detecting test strip and a preparation method thereof. The haemophilus parasuis disease antibody detecting test strip consists of a PVC (polyvinyl chloride) lining plate, a nitrocellulose membrane, an absorbing pad, a gold label pad and a sample pad, wherein the PVC lining plate is arranged at the very bottom; the nitrocellulose membrane is arranged on the middle section of the upper part of the PVC lining plate; the absorbing pad is arranged at the left end of the upper part of the nitrocellulose membrane; the gold label pad is arranged at the right end of the upper part of the nitrocellulose membrane; the sample pad is arranged at the right end of the upper part of the gold label pad; an anti-haemophilus parasuis IgG is sprayed at the left end of the nitrocellulose membrane as a quality control line; a purified and renatured His-OppA fusion protein is sprayed at the right end of the nitrocellulose membrane as a detection line; and a colloidal gold-labeled purified and renatured His-OppA fusion protein is sprayed on the gold label pad. The haemophilus parasuis disease antibody detecting test strip can determine whether a haemophilus parasuis disease antibody exists in serum or not according to whether the detection line and the quality control line have color strips or not, and can detect all antibodies generated by a serotype haemophilus parasuis infection simply, conveniently and quickly.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
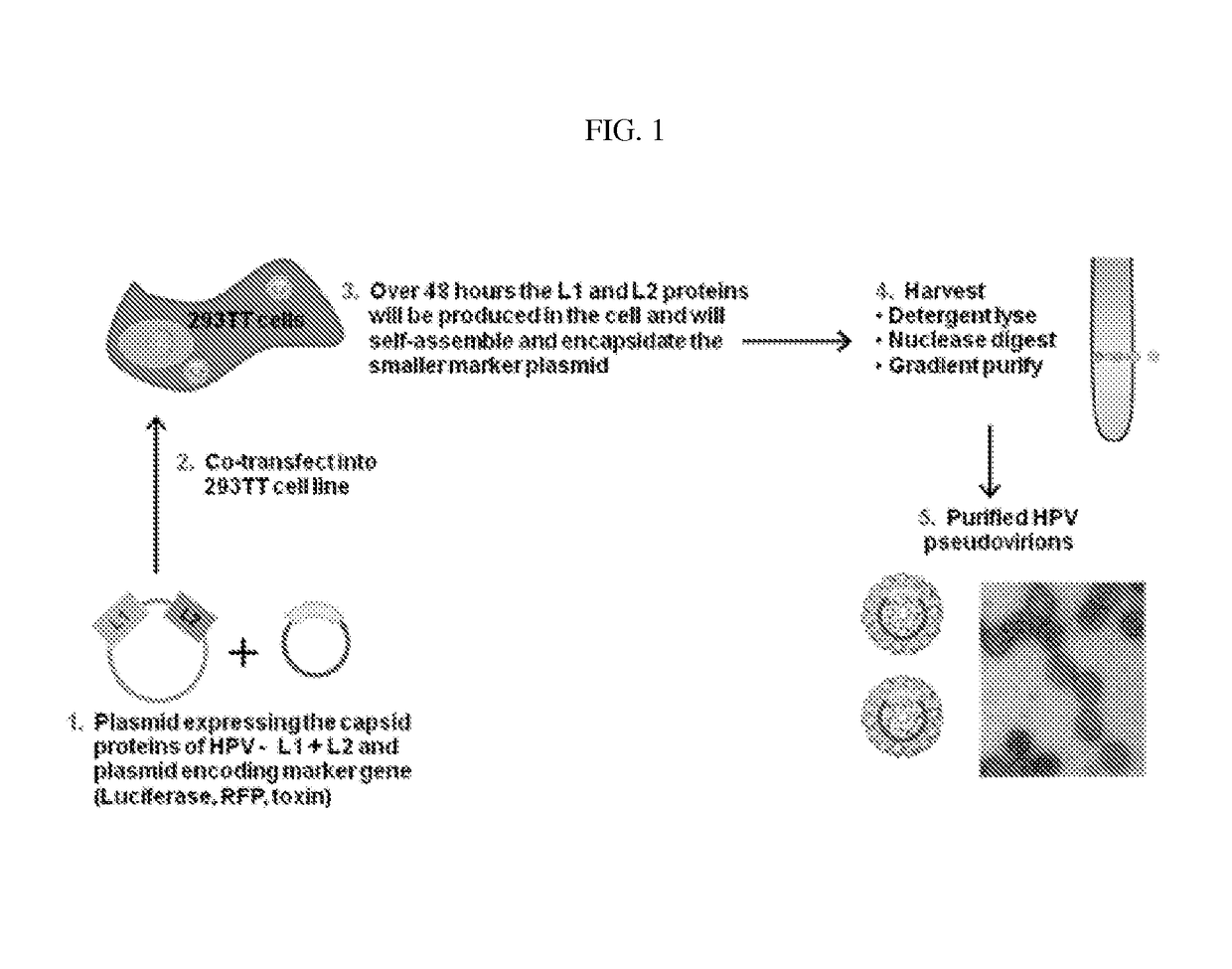
Virion-derived nanospheres for selective delivery of therapeutic and diagnostic agents to cancer cells
ActiveUS9700639B2Easy to detectIncrease contrastPowder deliveryPeptide/protein ingredientsCancer cellDiagnostic agent
The invention relates to methods for producing papilloma-derived nanosphere particles that contain therapeutic, diagnostic, or other agents. The invention also provides nanosphere particle preparations that are useful for selectively delivering therapeutic, diagnostic, and / or other agents to cancer cells of subjects without eliciting a serotype-specific immunogenic response in the subjects.
Owner:AURA BIOSCI
Method of using adenoviral vectors with increased persistence in vivo
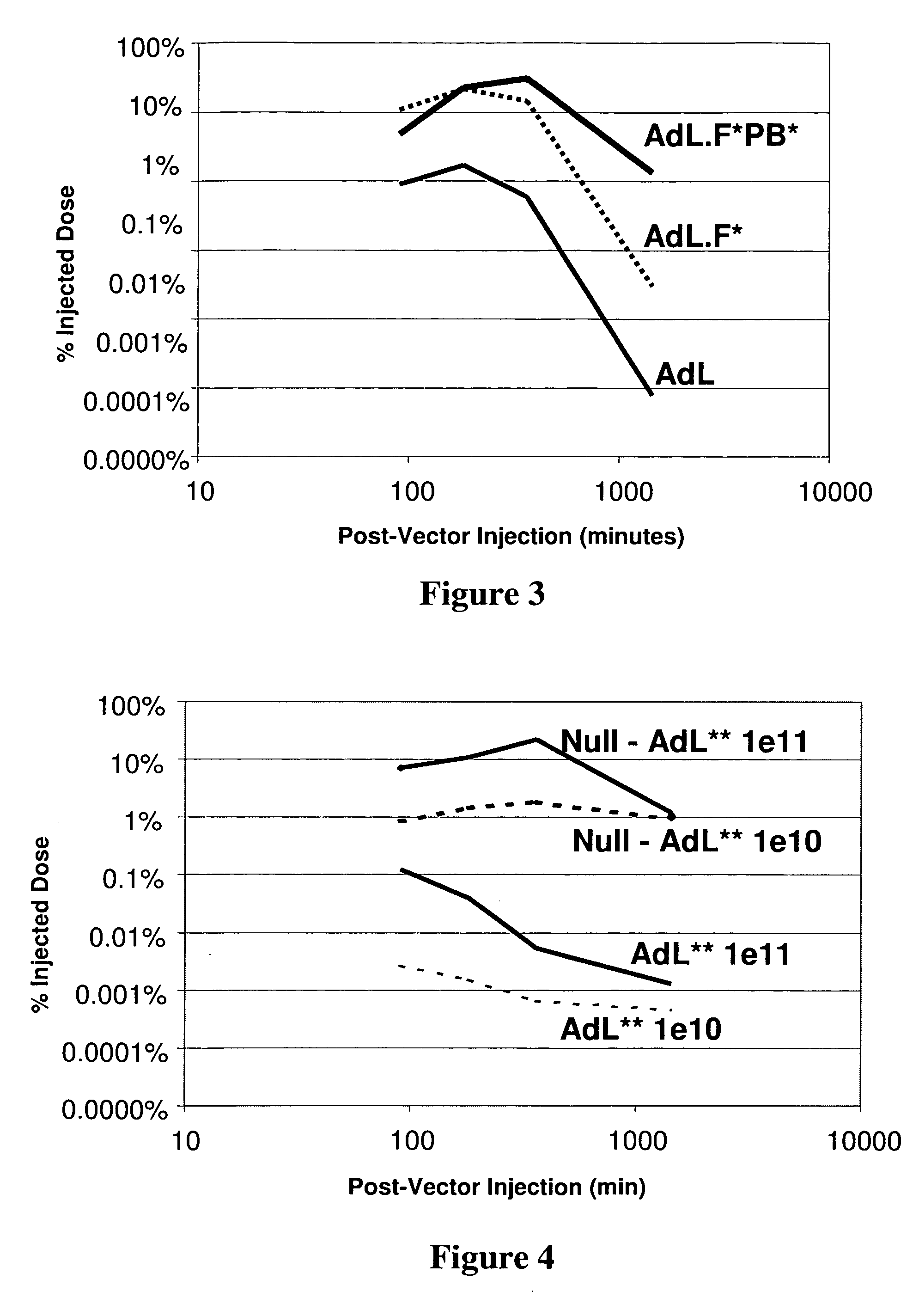
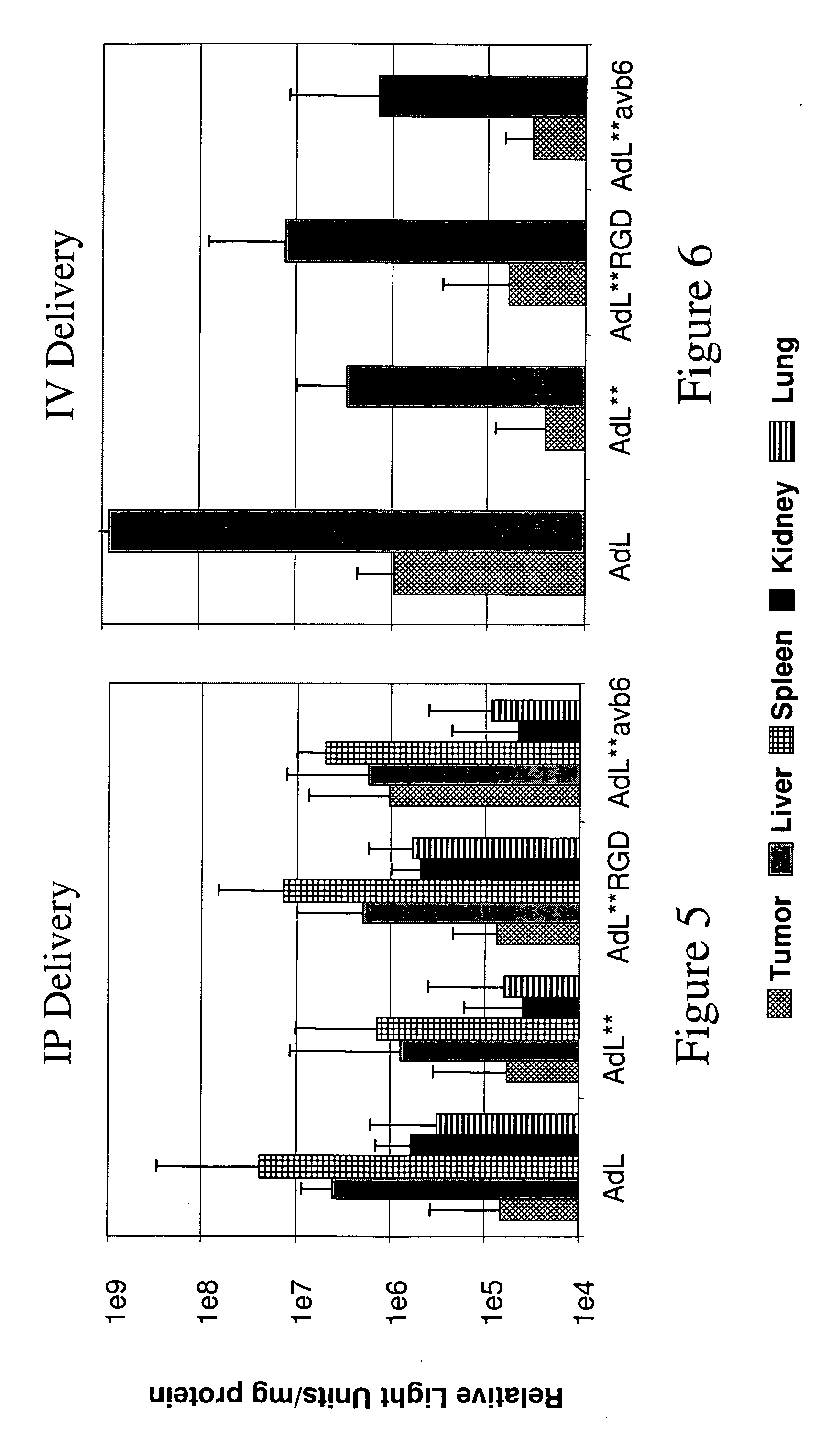
The invention provides a method of expressing an exogenous nucleic acid in a mammal. The method comprises slowly releasing into the bloodstream a dose of replication-deficient or conditionally-replicating adenoviral vector having reduced ability to transduce mesothelial cells and hepatocytes. The normalized average bloodstream concentration of the adenovirus over 24 hours post-administration is at least about 1%. Alternatively, the normalized average bloodstream concentration over 24 hours post-administration is at least about 5-fold greater than the normalized average bloodstream concentration for an equivalent dose of a wild-type adenoviral vector. A method of destroying tumor cells in a mammal also is provided, as is a replication-deficient adenoviral vector comprising a serotype 5 or serotype 35 adenoviral genome with a serotype 41 fiber protein, wherein the replication-deficient adenoviral vector exhibits reduced native binding to integrins.
Owner:GEN VEC INC +1
15-valence pneumococcus conjugate combined vaccine containing 2-type and 12 F serotypes
PendingCN105999254AGrowth assessment positive conversion rateAntibacterial agentsBacterial antigen ingredientsCoccidiaSerotype
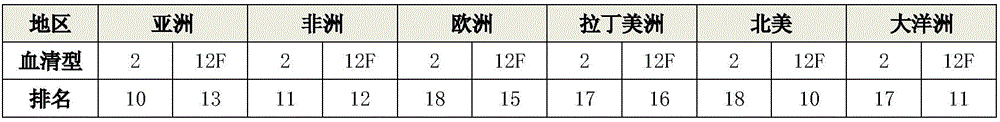
The invention relates to a 15-valence pneumococcus conjugate combined vaccine. The combination particularly contains 2-type and 12 F serotypes, has a wide immunization coverage rate and is used for preventing infectious diseases caused by pneumococcus. The combined vaccine further contains a 1-type serotype, a 3-type serotype, a 4-type serotype, a 5-type serotype, a 6A serotype, a 6B serotype, a 7F serotype, a 9V serotype, a 14-type serotype, a 18 C serotype, a 19 A serotype, a 19 F serotype and a 23 F serotype.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Novel serotype streptococcus mutans and utilization of the same
InactiveUS20090041782A1Efficient detectionMutans </i>is reducedBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenInfective endocarditis
A polynucleotide encoding a polypeptide which lowers the glucose side chain amount in a serotype-specific polysaccharide antigen of Streptococcus mutans; novel Streptococcus mutans strain having the above polynucleotide; an antibody specific to this Streptococcus mutans strain; and a method of detecting the above-described Streptococcus mutans strain occurring in a subject sample. According to this method, it is possible to examine the presence or absence of Streptococcus mutans of an untypable serotype in a subject sample to thereby specify a subject having a high risk of the onset of infective endocarditis.
Owner:SUNTORY HLDG LTD
Recombinant adenovirus with increased safety and anticancer activities, and use thereof
InactiveUS10077430B2High activityImprove securityOrganic active ingredientsSpecial deliveryFiberGene delivery
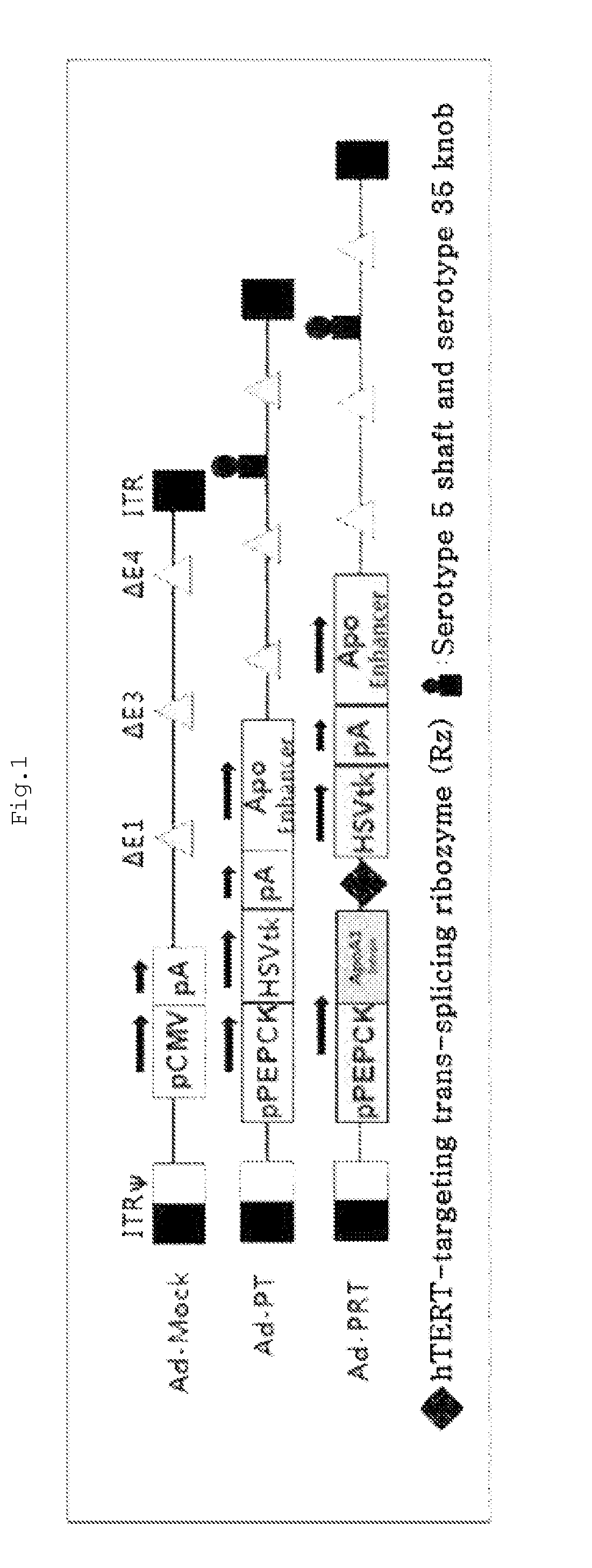
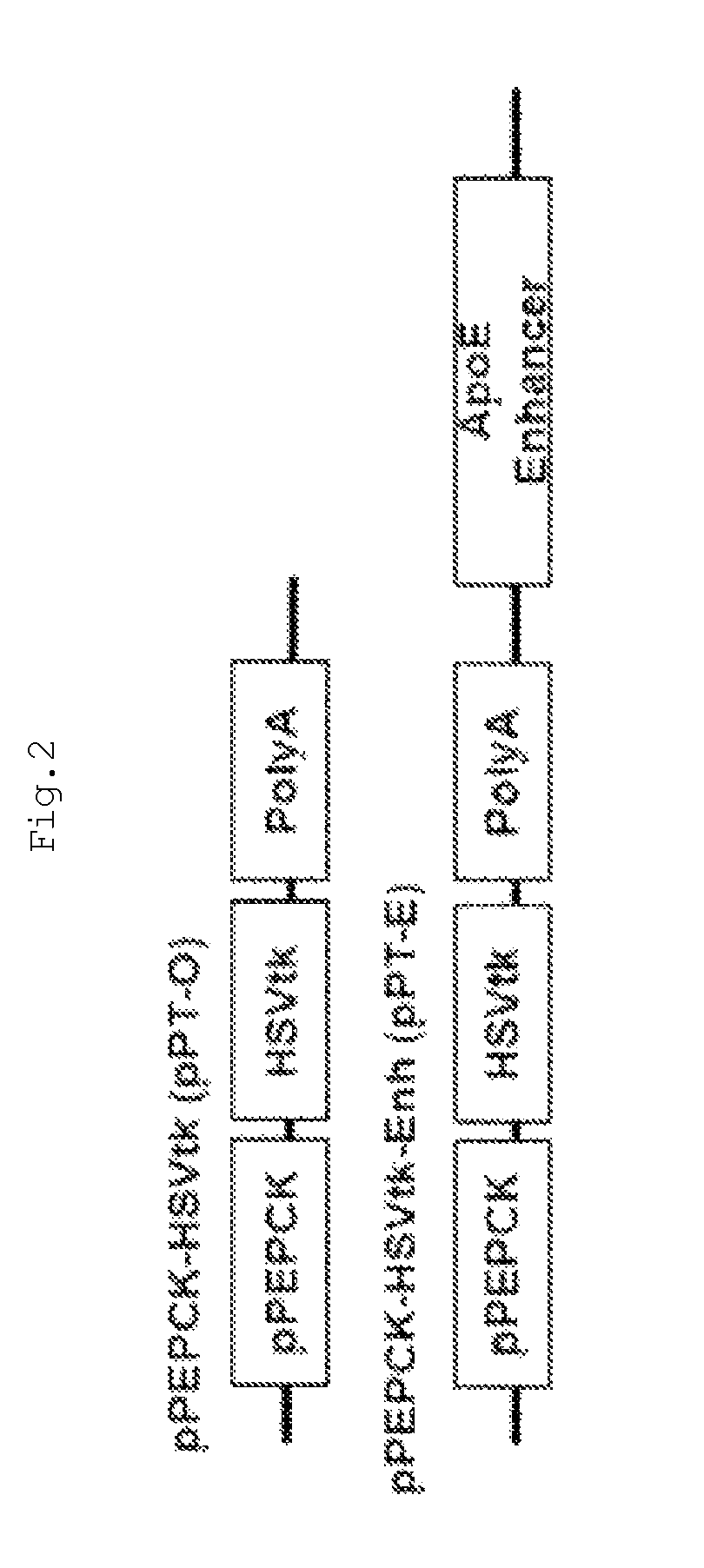
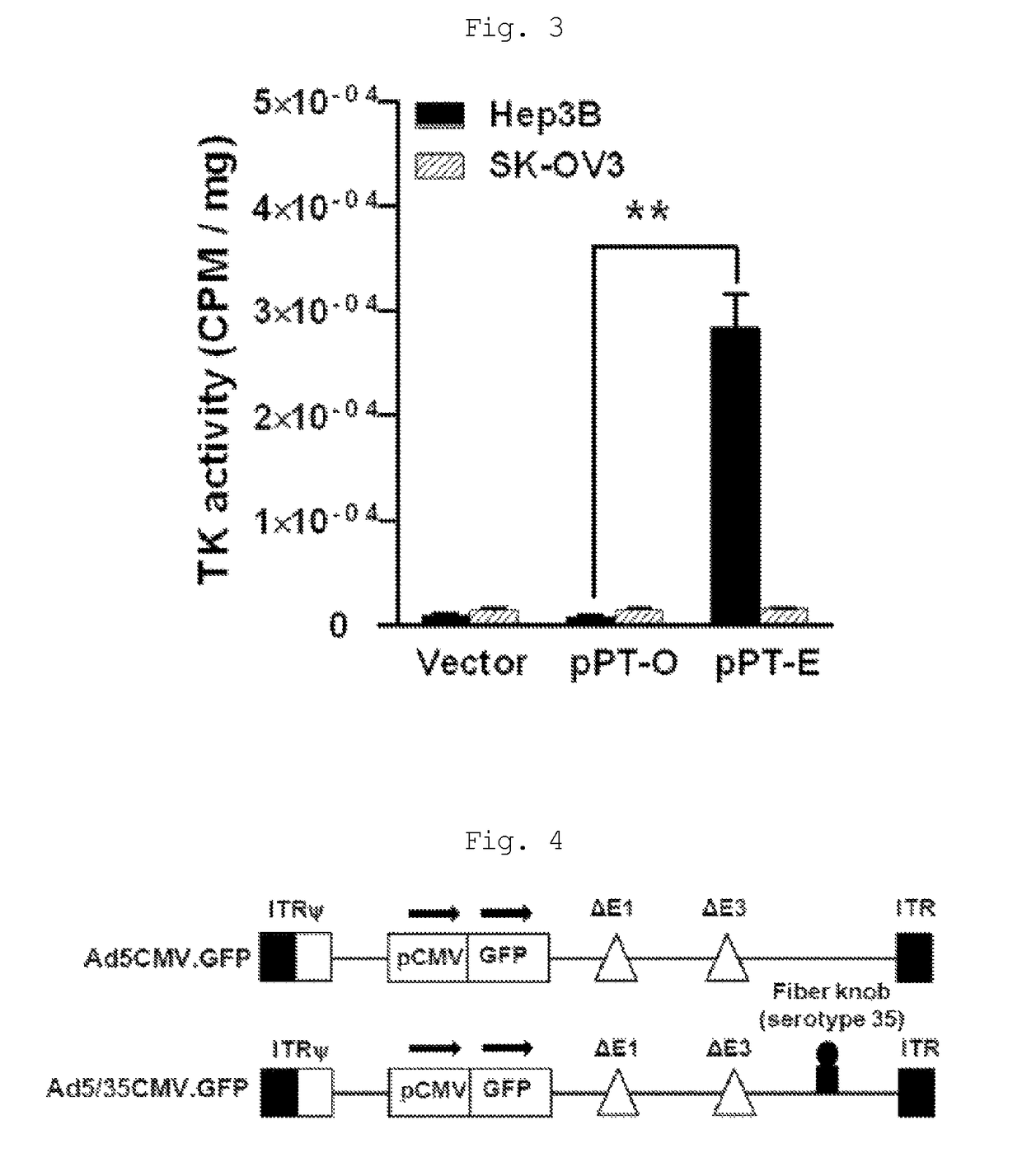
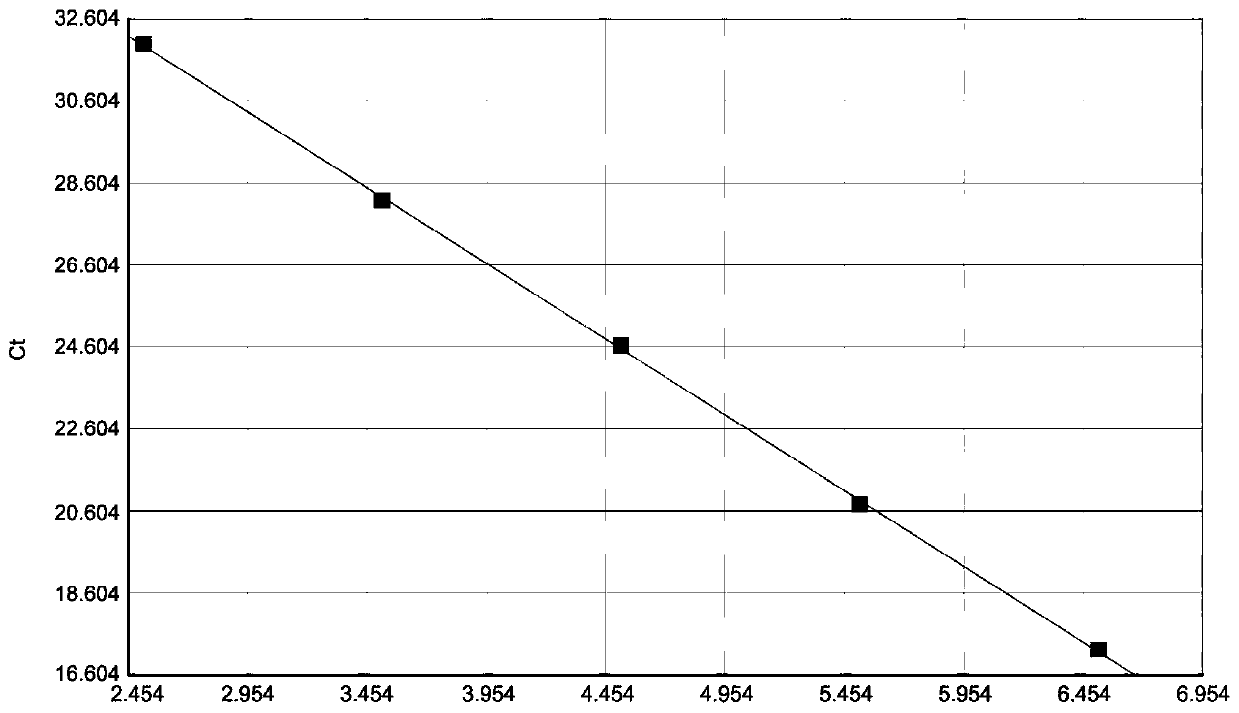
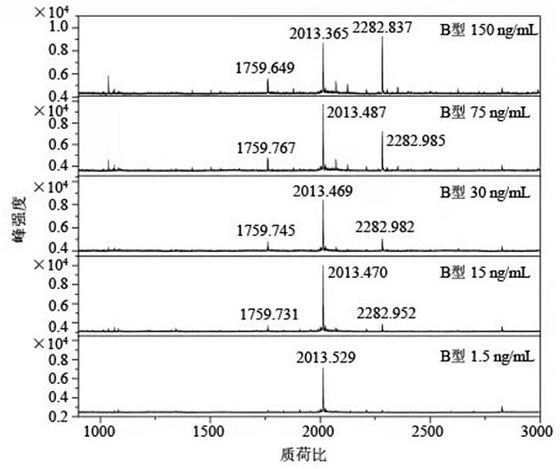
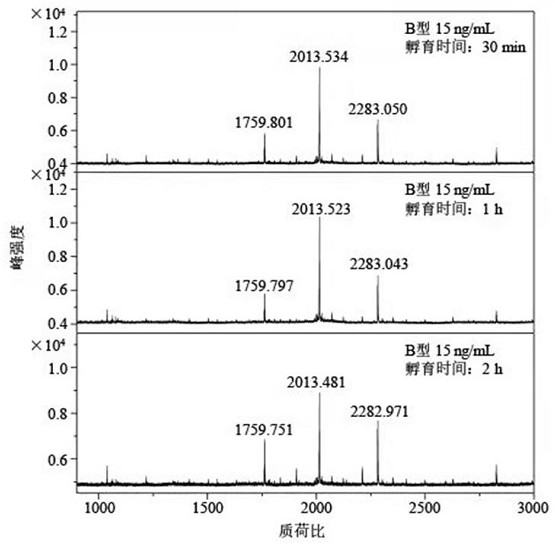
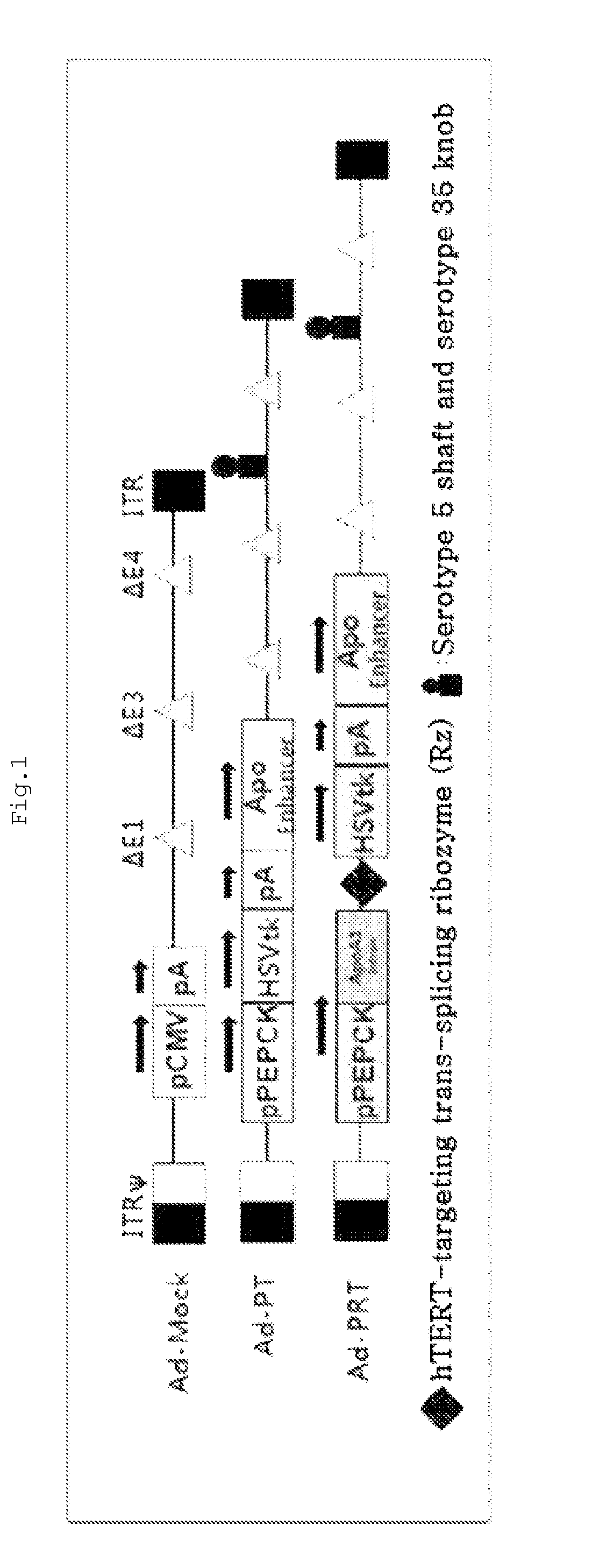
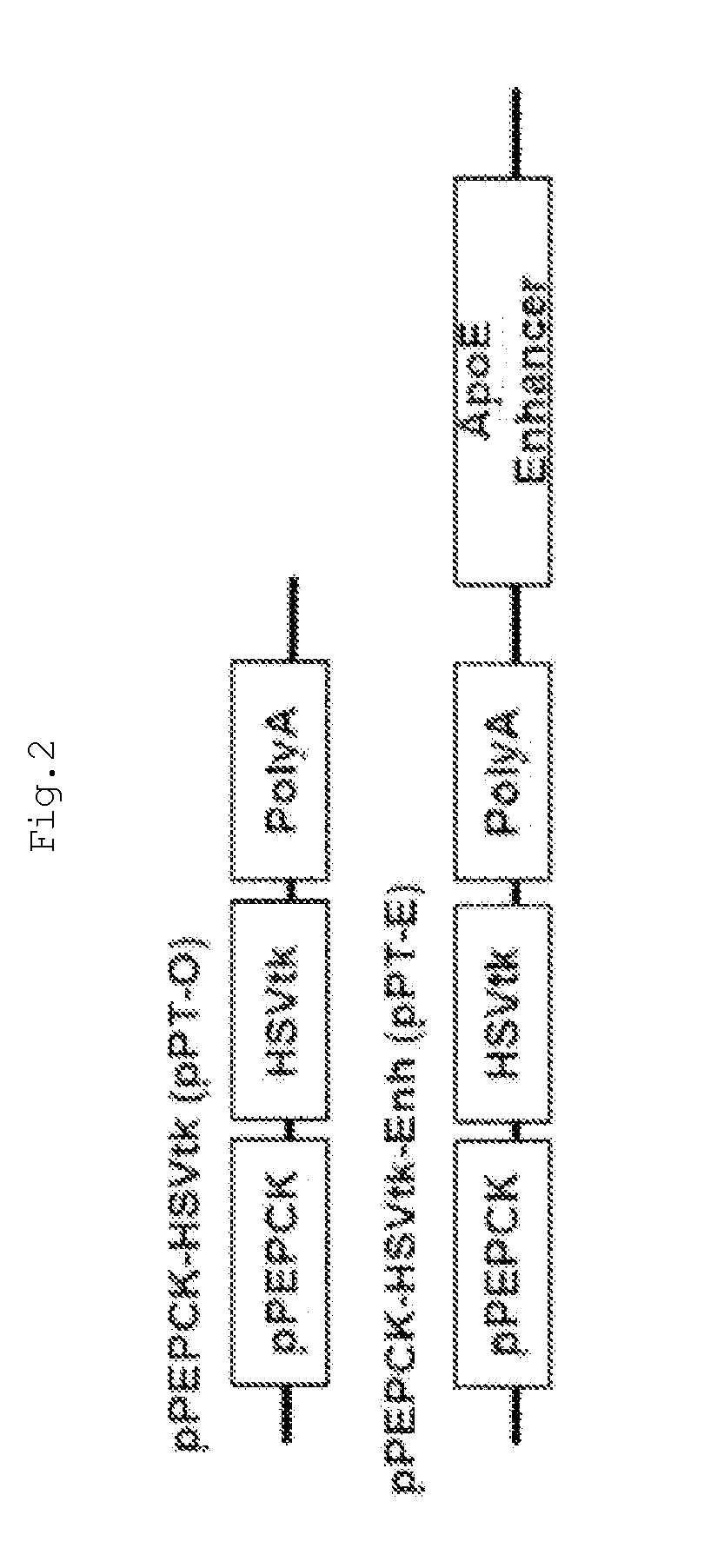
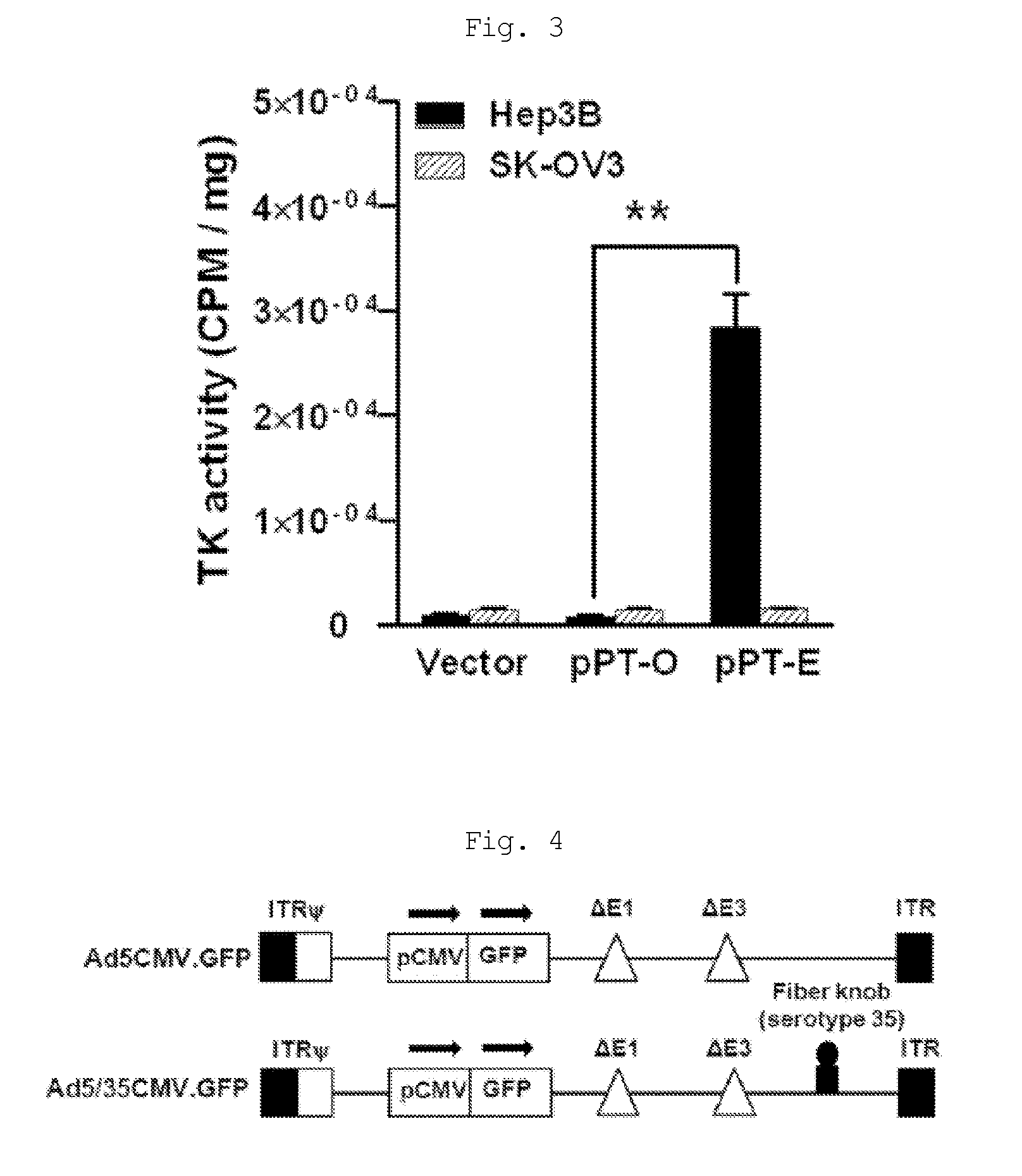
The present invention relates to a recombinant adenovirus with increased in-vivo safety, tissue specificity, and anticancer activities, and a use thereof. Specifically, the recombinant adenovirus comprising: a promoter of the liver tissue-specific phosphoenolpyruvate carboxykinase (PEPCK) gene; a trans-splicing ribozyme which is operably linked to the promoter and acts on a cancer-specific gene; a therapeutic gene or a reporter gene which is linked to the 3′ exon of the ribozyme; and a serotype 35 fiber knob and a serotype 5 shaft, in which the orf4 gene is deleted from adenovirus E1, E3 and E4 orf1, shows remarkable in-vivo safety, high specificity for a target tissue, and remarkable anticancer effects, and thus can be useful for an anticancer drug or a cancer diagnostic agent as a gene delivery vector.
Owner:NAT CANCER CENT
Real-time fluorescent quantitative RT-PCR detection primers for serotype of Palyam serogroup virus (PALV), probes and detection kit
ActiveCN110669870AStrong specificityShorten the timeMicrobiological testing/measurementMicroorganism based processesAnimal virusSingle strand
The invention relates to real-time fluorescent quantitative RT-PCR detection primers for a serotype of a Palyam serogroup virus (PALV), probes and a detection kit and belongs to the technical field ofmolecular biological detection on animal viruses. The kit comprises 3 pairs of primers and further comprises three probes, a negative control template, positive control templates, standard substancetemplates and a PCR amplification reagent. RNase-free water serves as the negative control template; the positive control templates are CHUV, BCV and DAV inactivated viruses separately; and the standard substance templates are gene segment 2 single-stranded RNAs of CHUV, BCV and DAV. The kit disclosed by the invention can be used for detecting three kinds of serotype PALVs popular in China and hasthe advantages of specificity, sensitivity, rapidness, high efficiency and the like; and a standard curve established by utilizing the standard substance templates in a matched manner can be used forcarrying out quantitative detection on RNAs of all PALV serotype strains in clinical samples, and thus, the working efficiency is increased greatly.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Methods and microarrays for detecting enteric viruses
InactiveUS20090136916A1Better diagnose gastrointestinal illnessRapid and reliable assaySugar derivativesMicrobiological testing/measurementAssayNucleic acid sequencing
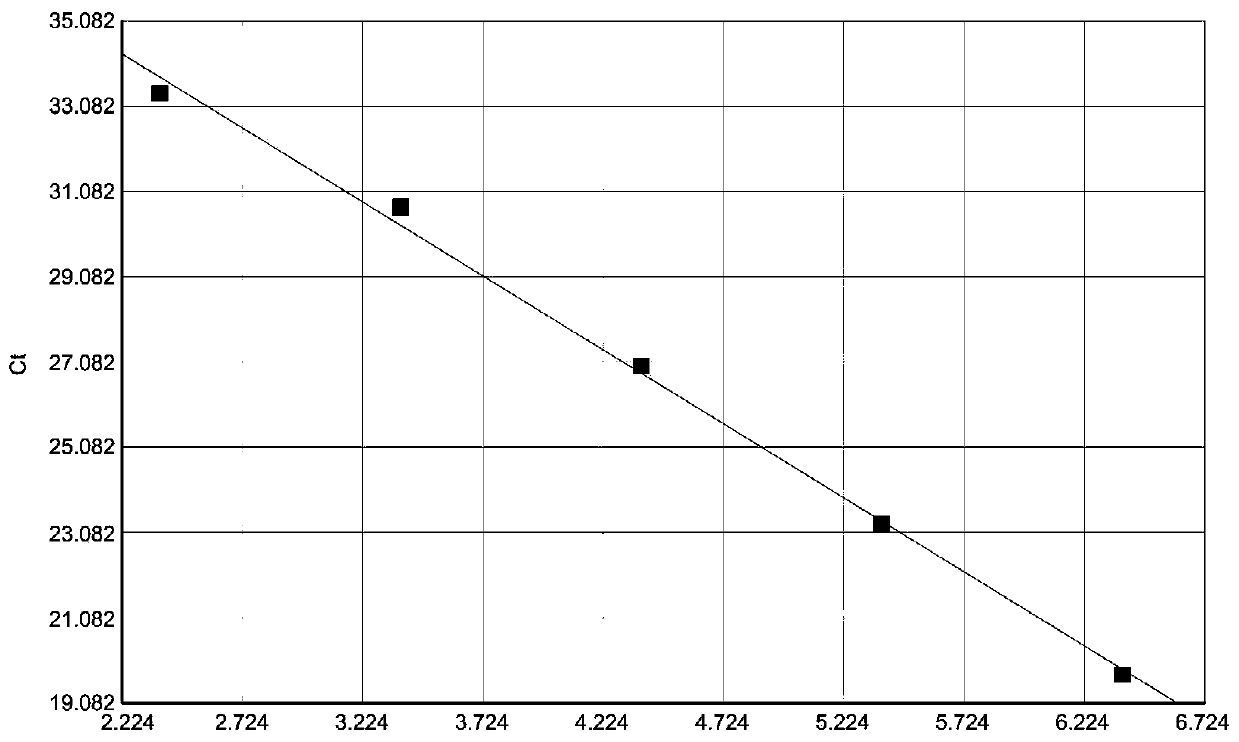
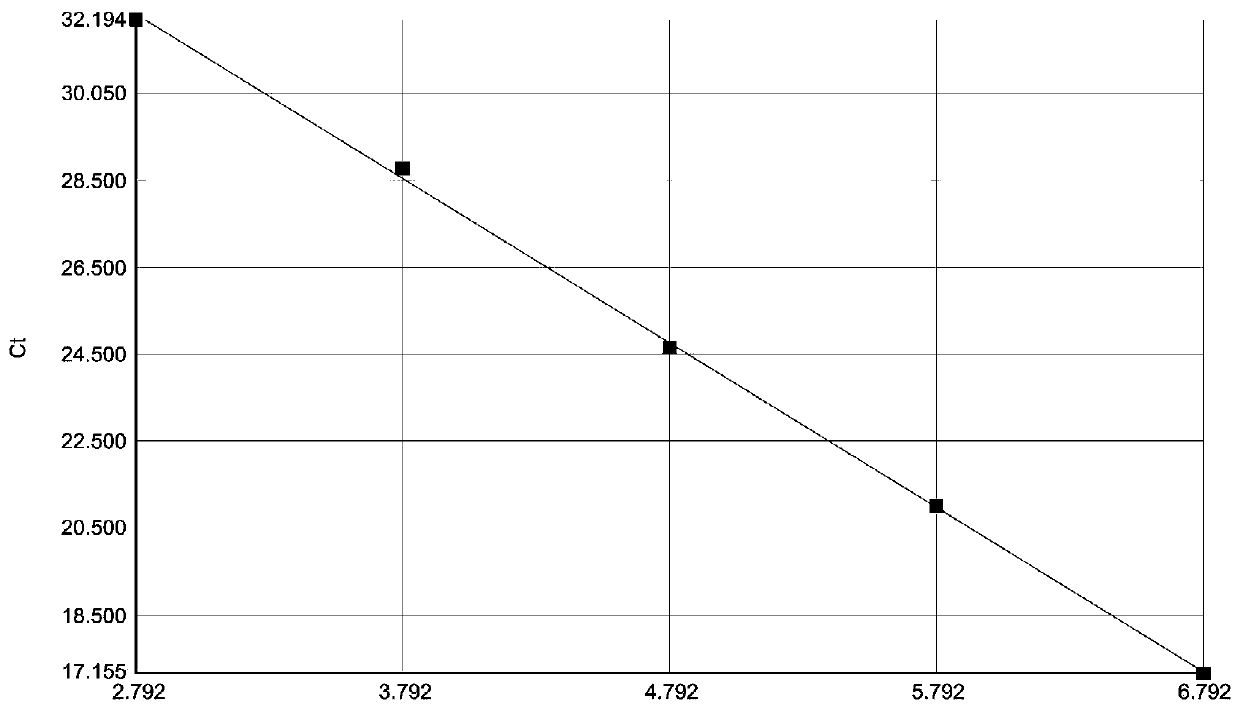
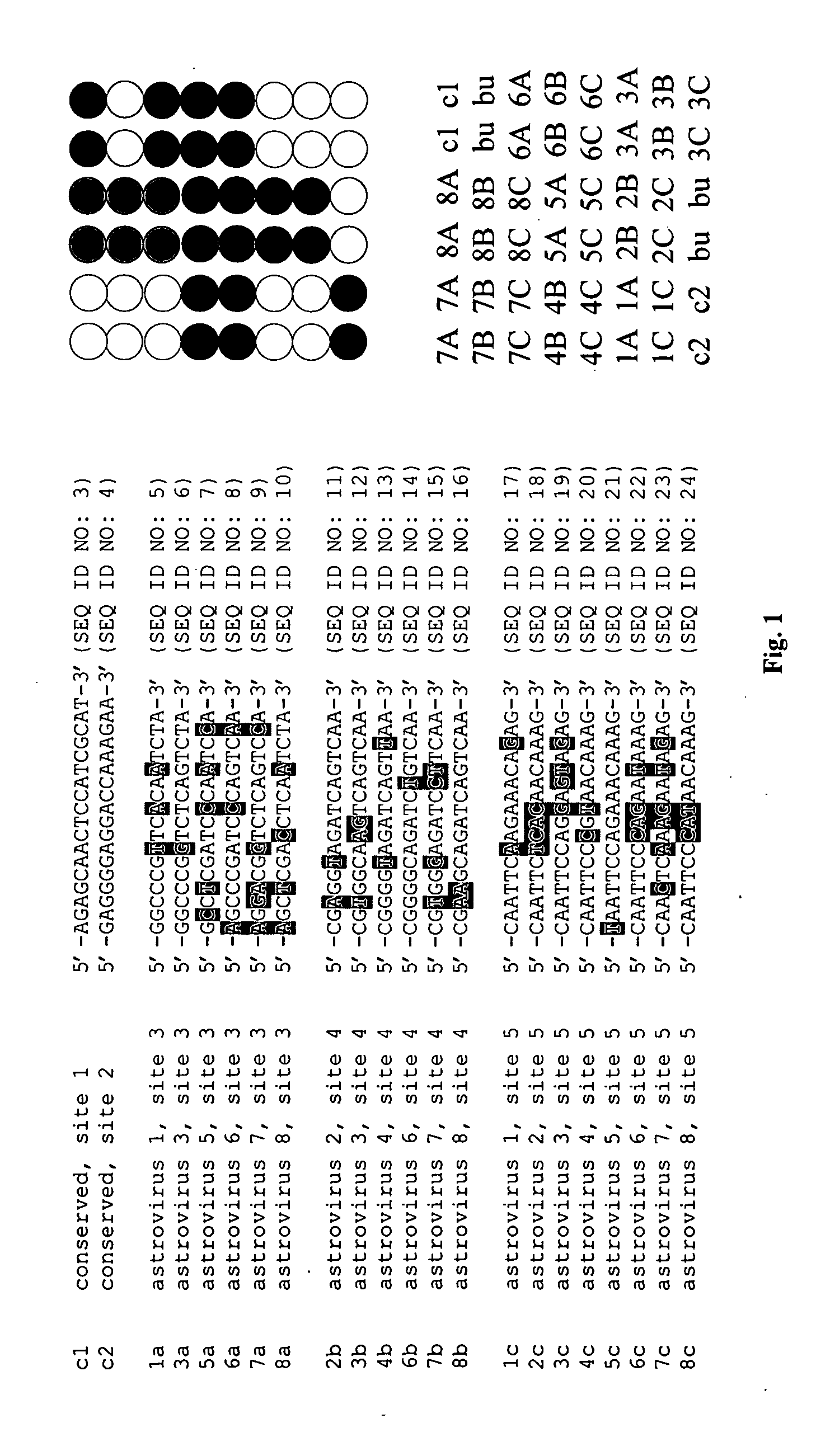
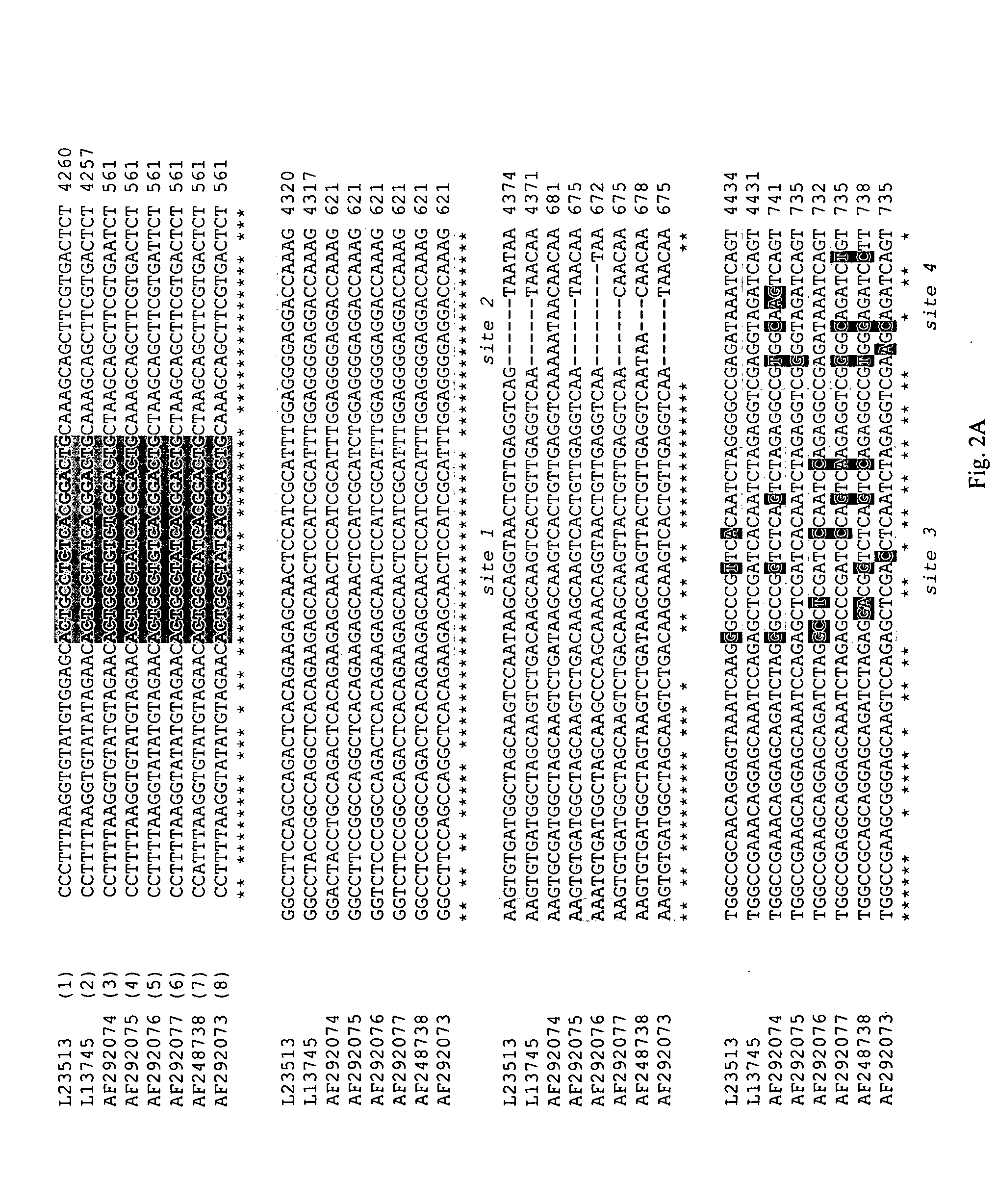
The present invention relates to methods, microarrays and kits for detecting one or more human astrovirus serotypes in a sample (e.g., a fecal sample) from an individual. The method includes amplifying nucleic acid molecules of the sample with one or more primers, to thereby obtain an amplified nucleic acid product; contacting the amplified nucleic acid product with one or more serotype specific probes having a nucleic acid sequence that is specific for only one astrovirus serotype in the group of astroviruses being assessed, wherein the nucleic acid sequence includes between about 9 and 25 nucleic acid bases (e.g., SEQ ID NO: 5-24); and detecting the hybridization complex. The presence of hybridization complexes with a serotype specific probe indicates the presence of one or more specific astrovirus serotypes, and the absence of hybridization complexes with a serotype specific probe indicates the absence of the specific astrovirus serotype. Identification of the astrovirus serotypes allows for one to diagnose an individual infected with the serotype. The present invention further includes microarrays having any one of the astrovirus specific probe, or kits having microarrays and reagents for carrying out the assay.
Owner:TRUSTEES OF TUFTS COLLEGE
Vaccine in the form of a recombinant sero type 9 avian adenovirus vector
ActiveUS20170232096A1Adequate responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsAntigenMaternal antibody
A recombinant vaccine comprising a serotype 9 fowl adenovirus vector (FAdV-9) having at least one exogenous nucleotide sequence inserted encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region, and a pharmaceutically acceptable vehicle, adjuvant and / or excipient, wherein the at least one exogenous nucleotide sequence encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region is located between the 491 and 2782 nucleotides.The vector of this vaccine is stable for industrial scale production. Likewise, even when administering this vaccine in combination with a vaccine against Marek's disease, both vaccines produce an adequate immune response which is not affected by interference between each other. In the same way, effectiveness of the recombinant vaccine is not affected by maternal antibodies, and is capable of inducing both an early and lasting protective response, even with only one application.
Owner:GRUPO IND PECUARIO S A DE CV
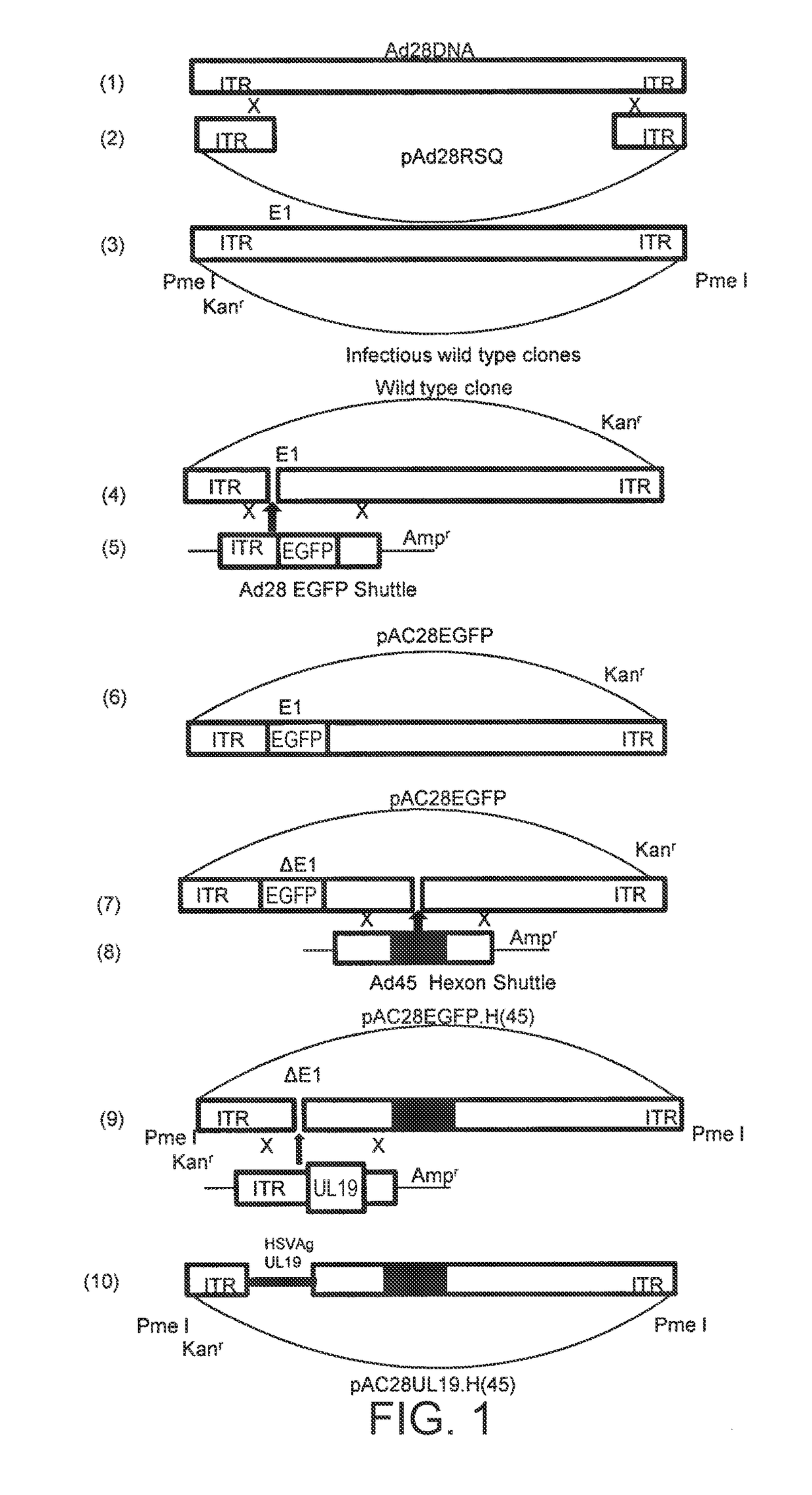
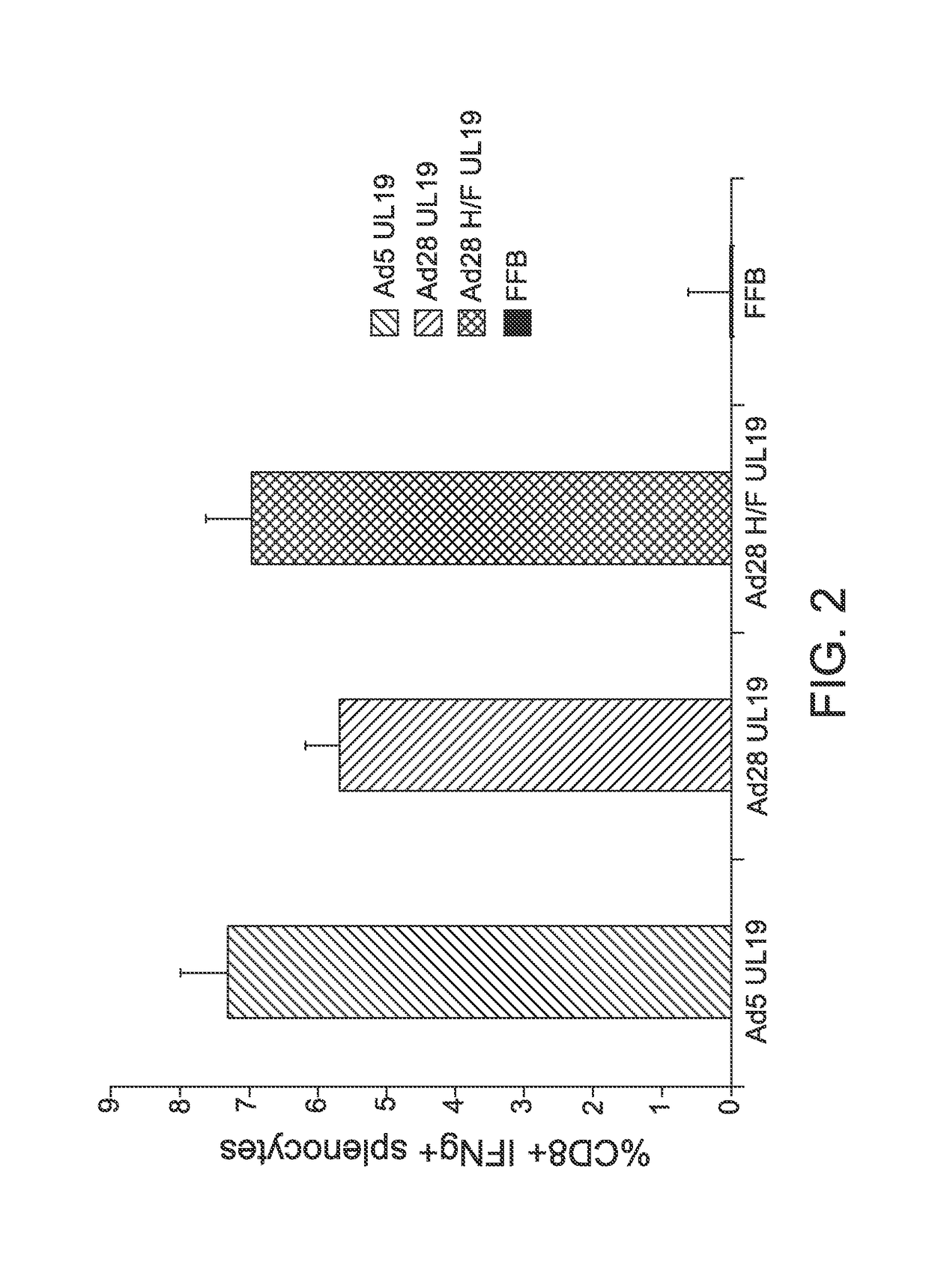
Modified serotype 28 adenoviral vectors
The invention provides a replication-deficient serotype 28 adenoviral vector characterized by comprising a portion of a serotype 45 adenoviral hexon protein and / or a portion of a serotype 45 fiber protein in place of the endogenous serotype 28 hexon and / or fiber protein.
Owner:GENVEC INC
Hybridoma cell line capable of secreting foot and mouth disease virus sharable monoclonal antibody 3D9 and application of hybridoma cell line
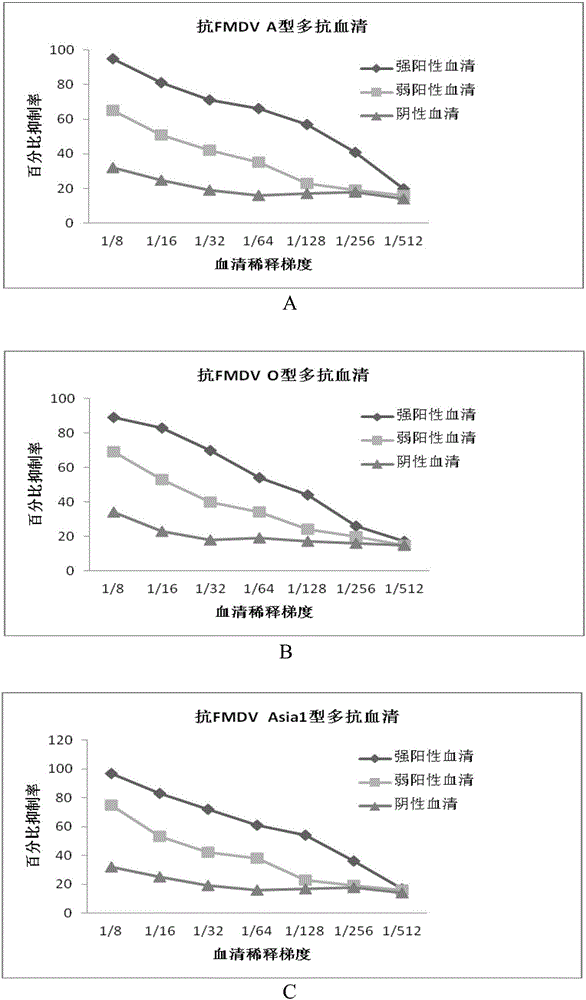
The invention discloses a hybridoma cell line capable of secreting foot and mouth disease virus sharable monoclonal antibody 3D9 and application of the hybridoma cell line. Firstly, the invention discloses a strain of hybridoma cell line capable of secreting foot and mouth disease virus sharable monoclonal antibody 3D9, the microbial preservation No. is CGMCC No.13293. The invention further discloses the foot and mouth disease virus sharable monoclonal antibody 3D9 secreted by the hybridoma cell line. IFA specificity identification shows that the monoclonal antibody 3D9 is a strain of a serotype sharable monoclonal antibody which can react with O-type, A-type and Asia1-type foot and mouth disease virus antigens. In the invention, the monoclonal antibody 3D9 is used as a detection antibody and can be used for specific detection of O-type, A-type and Asia1-type foot and mouth disease virus antigens or antibodies. The hybridoma cell line and the foot and mouth disease virus sharable monoclonal antibody 3D9 secreted by the hybridoma cell line have an application prospect in preparation of reagents or drugs for diagnosing, preventing or treating foot-and-mouth diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Riemerella anatipestifer tervalent inactivated vaccine and preparation method thereof
ActiveCN102908616AAvoid infectionGood immune protectionAntibacterial agentsBacterial antigen ingredientsAdjuvantRiemerella anatipestifer
The invention belongs to the field of biotechnology, and discloses a riemerella anatipestifer tervalent inactivated vaccine. The inactivated vaccine comprises a riemerella anatipestifer serotype I inactivated whole bacterium, a serotype II inactivated whole bacterium, a serotype X inactivated whole bacterium and an adjuvant. The immune protection ratio of the inactivated vaccine against the toxicity attack from the serotype I, II and X type riemerella anatipestifer virulent strains reaches up to 100%. The inactivated vaccine provided by the invention has a good immune protection effect on all serotype strain infections.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI +1
Subgroup B recombinant human adenovirus vector, and methods for constructing and for using the same
ActiveUS9315827B2Improve survivalReduce efficacyUnknown materialsAntineoplastic agentsWilms' tumorBiology
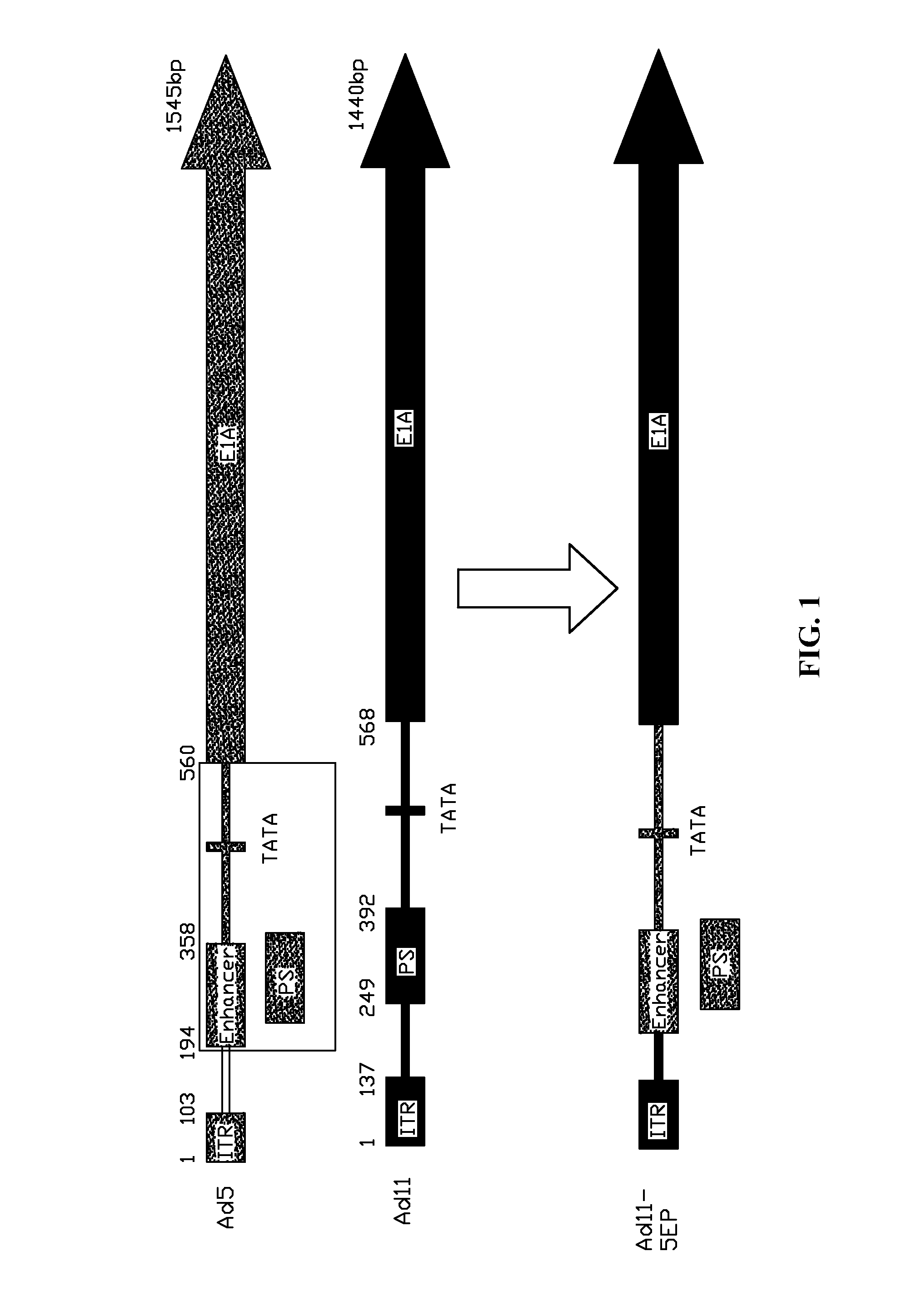
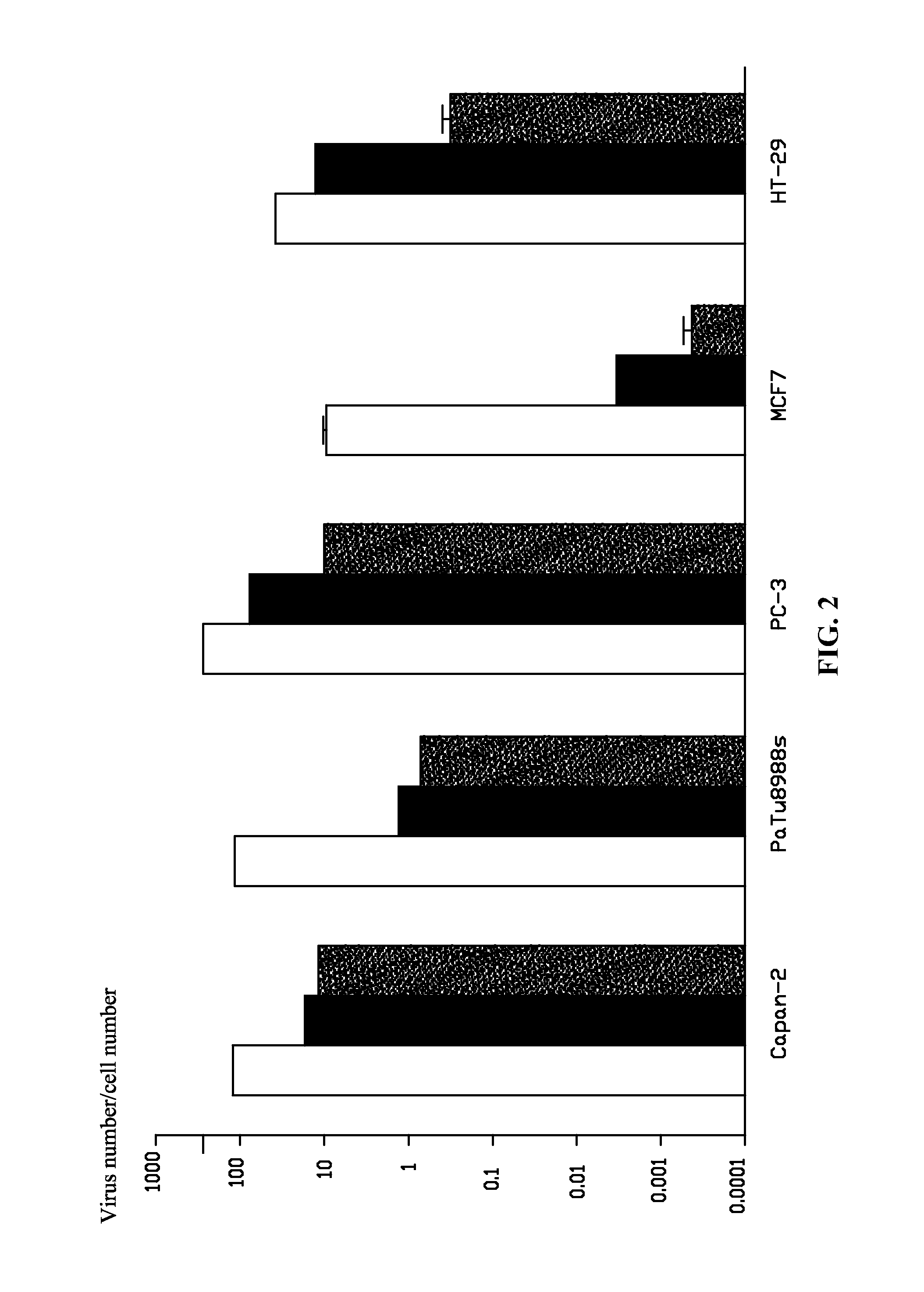
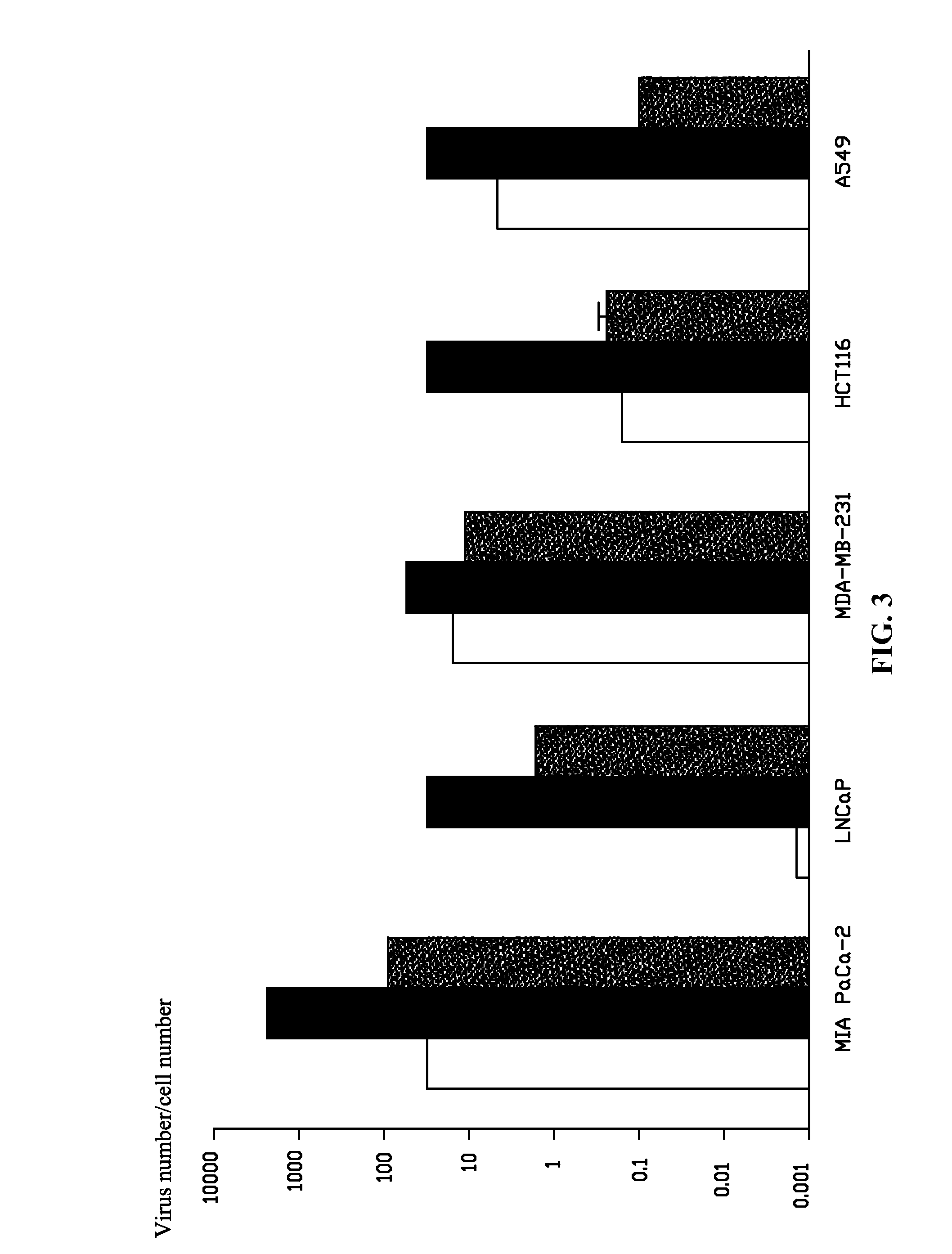
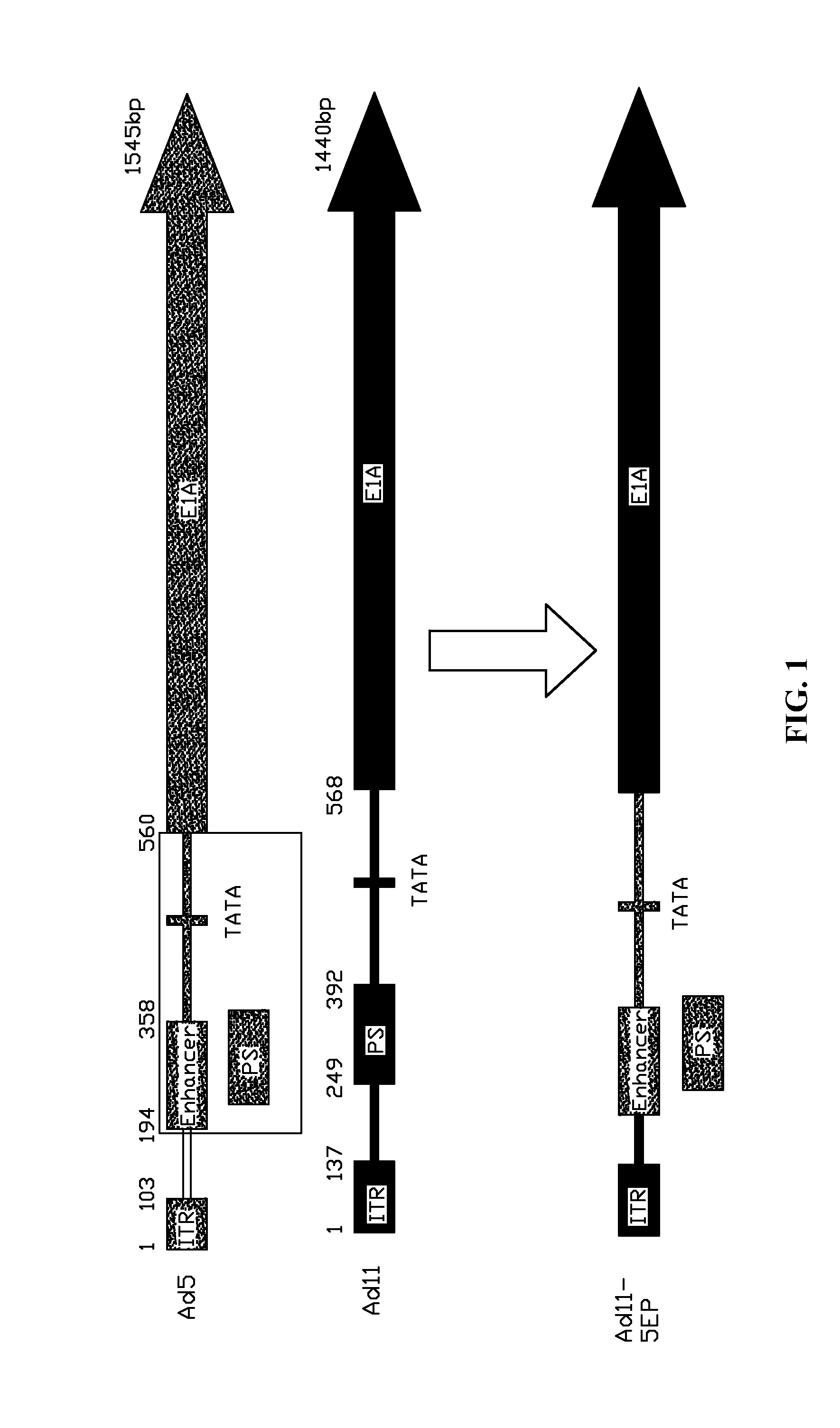
A method for constructing a subgroup B recombinant human adenovirus vector Ad11-5EP. The method includes substituting a 365 bp fragment including an enhancer and a promoter of an upstream coding sequence of Ad5 E1A for a corresponding region of a serotype Ad11 of the subgroup B human adenovirus vector by homologous recombination to construct the subgroup B recombinant human adenovirus vector Ad11-5EP. A subgroup B recombinant human adenovirus vector Ad11-5EP constructed by the method and the use thereof for treatment of tumors are also provided.
Owner:BEIJING BIO TARGETING THERAPEUTICS TECH
Detection method for human erythrocyte rare blood type genotype and detection kit
InactiveCN110079590AAccurate Blood Type ResultsComprehensive blood group resultsMicrobiological testing/measurementRed blood cellGenotype
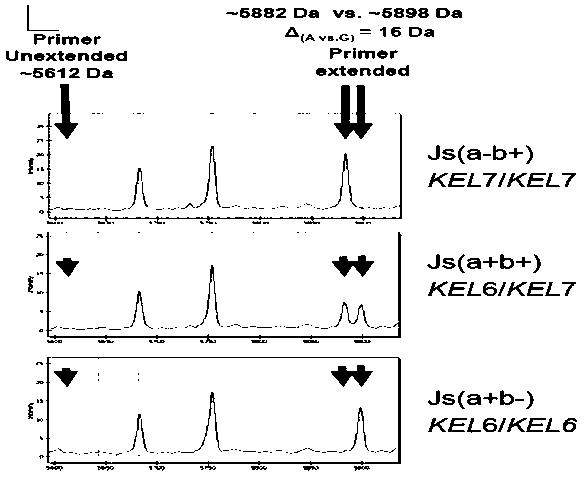
The invention provides a detection method for human erythrocyte rare blood type genotype and a detection kit. The detection method comprises the following steps: designing a specific primer and then utilizing single-tube multiple PCR amplified reaction, thereby acquiring a target segment; designing a single-base extension primer and then performing single-base extension; adopting mass spectrometryfor accurately measuring a target DNA sequence. The gene detection method is capable of simplifying a blood compatibility test and identifying rare blood type and is an alternative of a serotype identification method. The new method is capable of helping doctors to acquire a more accurate and comprehensive blood type result and helping patients to save cost.
Owner:为康(苏州)基因科技有限公司
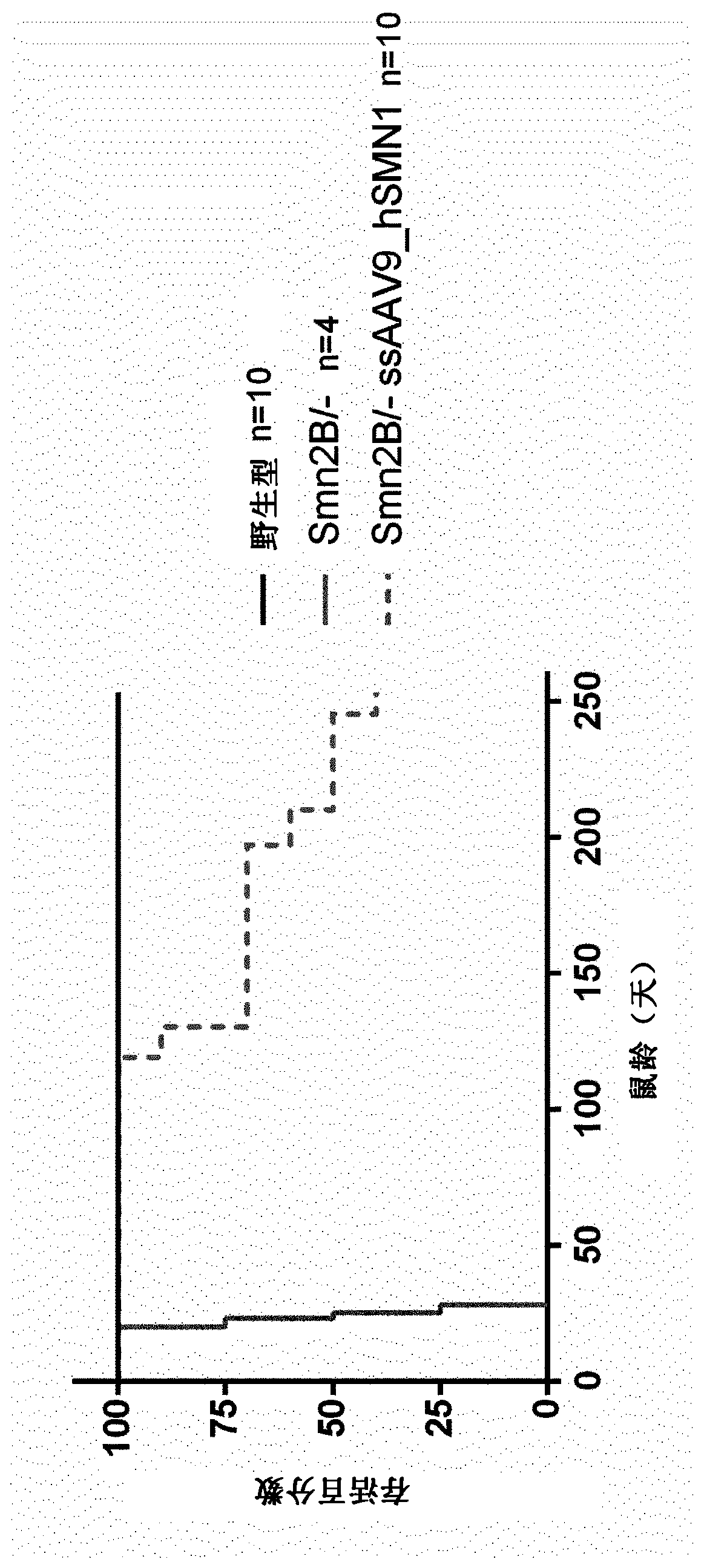
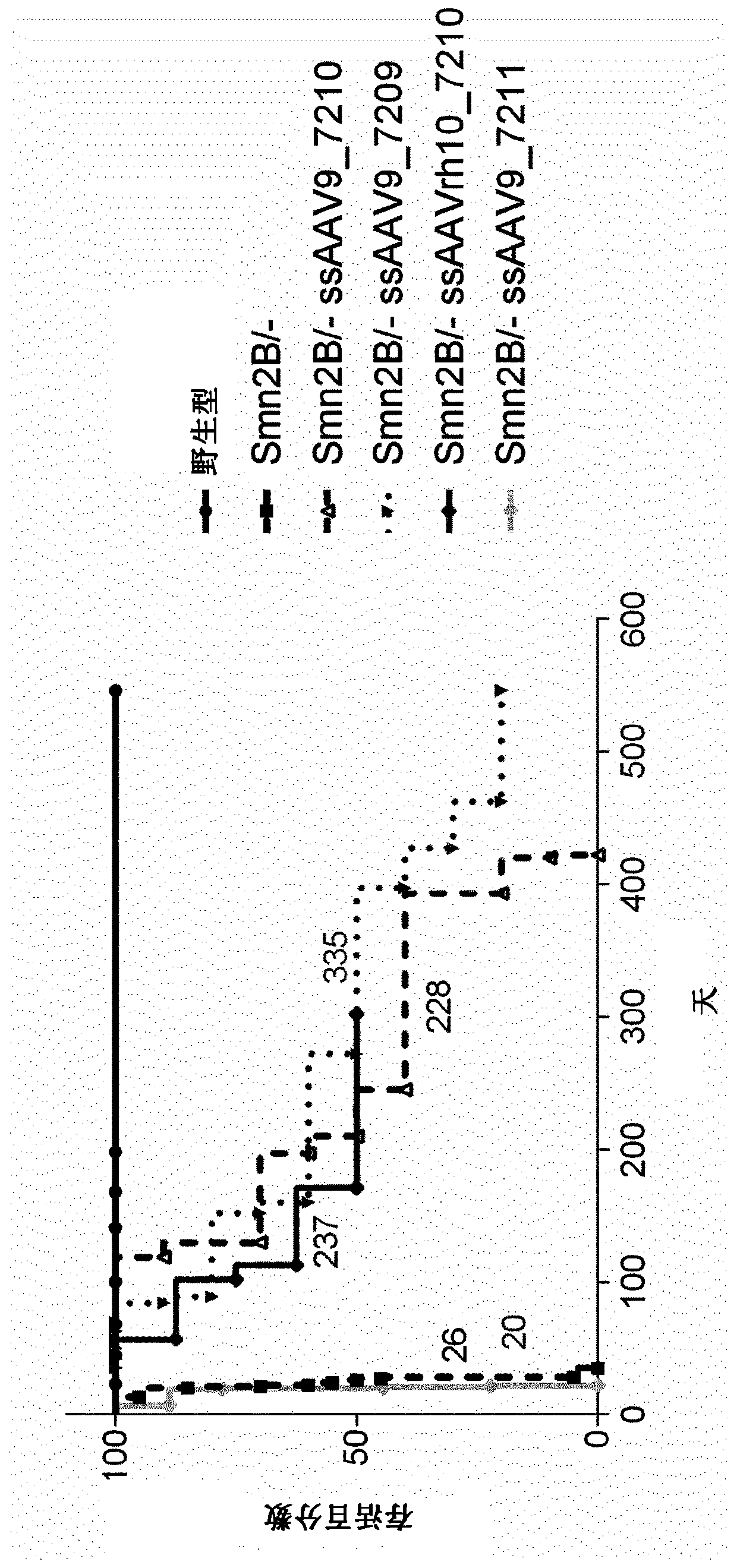
Treatment of spinal muscular atrophy
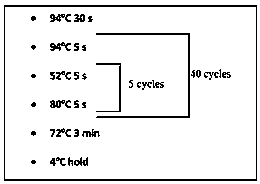
The present invention relates to a recombinant adeno-associated virus (rAAV) vector comprising a serotype 9 or rh10 AAV capsid, for use in a method for the treatment of spinal muscular atrophy (SMA).
Owner:GENETHON +2
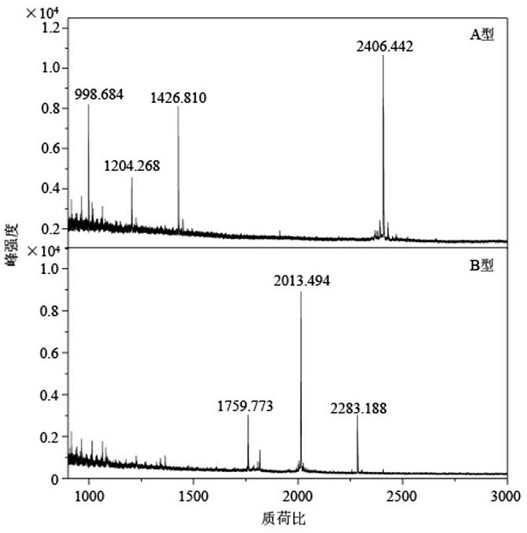
Botulinum toxin specific substrate peptide, detection kit and detection method
ActiveCN114539362ASpecific botulinum toxinSpecific serotype identificationMaterial analysis by electric/magnetic meansDepsipeptidesTarget peptideToxin
The invention relates to the technical field of analysis and detection, in particular to a botulinum toxin specific substrate peptide, a detection kit and a detection method. The invention provides a botulinum toxin specific substrate peptide. The botulinum toxin specific substrate peptide is one or more of polypeptides with amino acid sequences as shown in SEQ ID NO.1-4. The specific substrate peptide is combined with a serotype specific target peptide fragment generated by cutting the specific substrate peptide through the botulinum toxin, so that relatively specific, sensitive and accurate identification of the botulinum toxin and the serotype of the botulinum toxin can be realized. The invention also provides a detection kit and a detection method based on the specific substrate peptide, by using the kit and the method, the botulinum toxin and the serotype thereof can be rapidly, sensitively and accurately identified with high throughput, and a technical support is provided for botulinum toxin detection.
Owner:ICDC CHINA CDC
Recombinant adenovirus with increased safety and anticancer activities, and use thereof
InactiveUS20150337270A1High activityImprove securityBiocideOrganic active ingredientsFiberGene delivery
The present invention relates to a recombinant adenovirus with increased in-vivo safety, tissue specificity, and anticancer activities, and a use thereof. Specifically, the recombinant adenovirus comprising: a promoter of the liver tissue-specific phosphoenolpyruvate carboxykinase (PEPCK) gene; a trans-splicing ribozyme which is operably linked to the promoter and acts on a cancer-specific gene; a therapeutic gene or a reporter gene which is linked to the 3′ exon of the ribozyme; and a serotype 35 fiber knob and a serotype 5 shaft, in which the orf4 gene is deleted from adenovirus E1, E3 and E4 orf1, shows remarkable in-vivo safety, high specificity for a target tissue, and remarkable anticancer effects, and thus can be useful for an anticancer drug or a cancer diagnostic agent as a gene delivery vector.
Owner:NAT CANCER CENT
Bivalent DNA vaccine connecting peptide and applications thereof
ActiveCN103864902AStrong ability to neutralize virusesHigh titerGenetic material ingredientsAntiviralsDiseaseAdjuvant
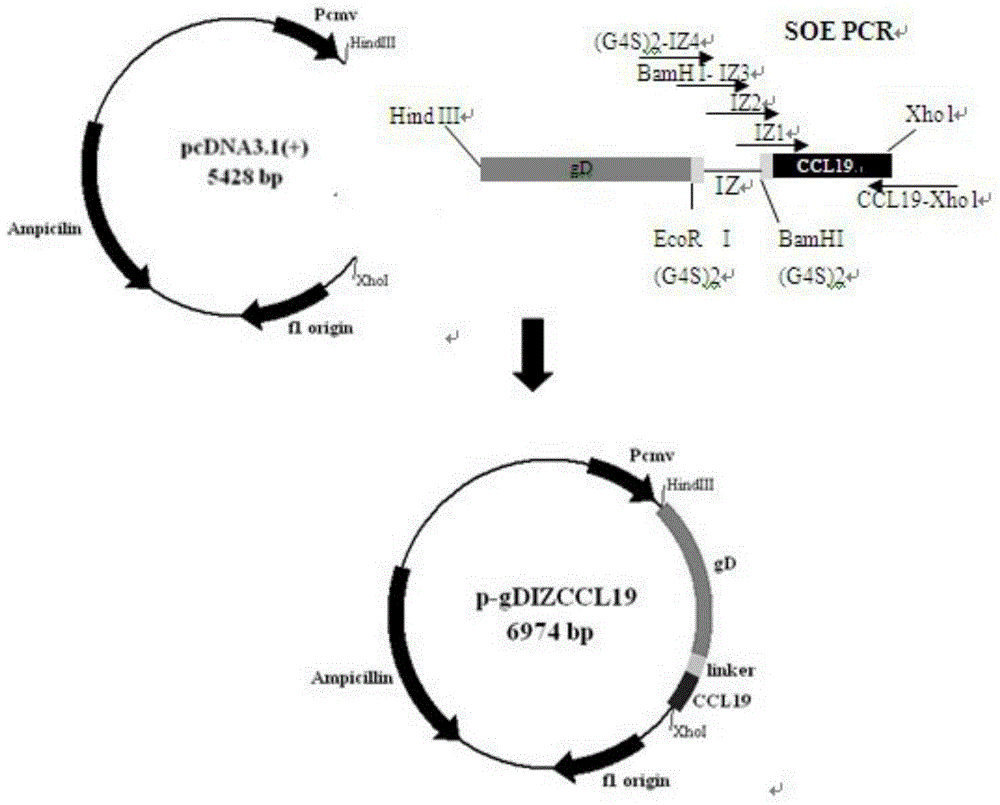
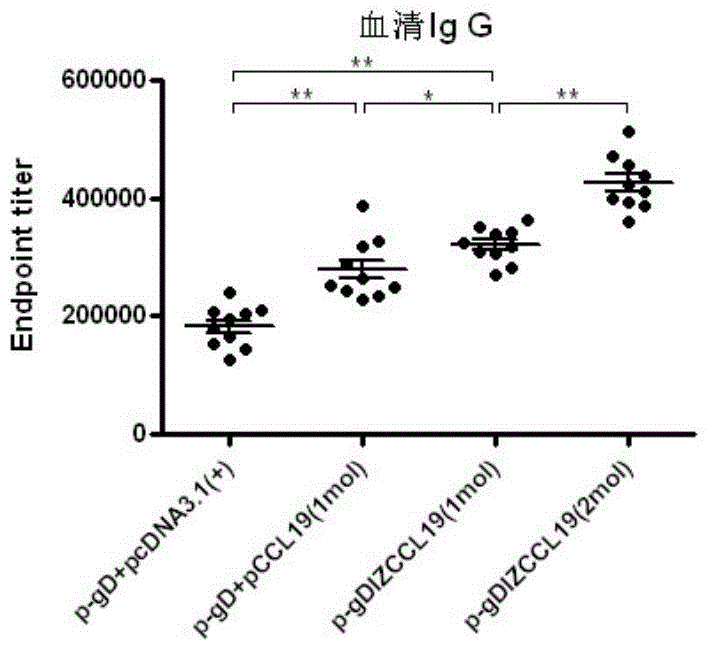
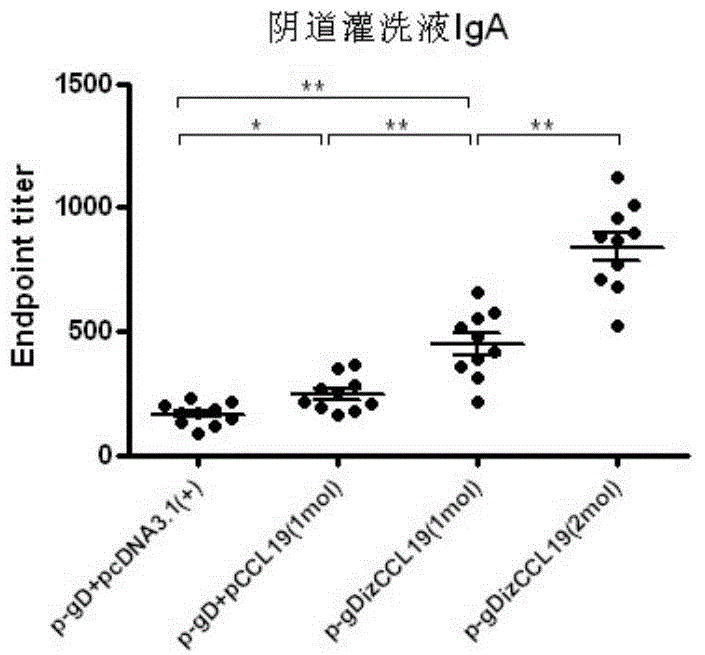
The invention discloses a bivalent DNA vaccine connecting peptide and applications thereof. A bivalent DNA vaccine is obtained through the following steps: performing PCR amplification on an HSV-2 glycoprotein gene and an adjuvant gene, introducing enzyme cutting sites, and introducing a connecting peptide (G4S)2IZ(G4S)2 via overlapping PCR. Humoral immunity, humoral immunity and mucosal immunity response reactions triggered by the bivalent DNA vaccine are detected by prime-boost principle immunized mice; a serotype antibody is detected and the severity of illness of each group within 15 days is evaluated by vaginal infection of150LD50 HSV-2. Compared with a univalent DNA vaccine, the recombinant bivalent DNA vaccine generates more intense humoral immunity, humoral immunity and mucosal immunity response reactions and a powerful capability of neutralizing the virus is detected out; high-titer antibodies can be rapidly generated by the immunity reactions and the anamnestic immunity reactions of the recombinant bivalent vaccine, so that a good protection effect on the mice is achieved.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
A kind of bivalent dna vaccine connection peptide and its application
ActiveCN103864902BStrong ability to neutralize virusesHigh titerGenetic material ingredientsAntiviralsDiseaseAdjuvant
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Vaccine in the form of a recombinant sero type 9 avian adenovirus vector
ActiveUS10758608B2Adequate responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsMaternal antibodyAntigen
A recombinant vaccine comprising a serotype 9 fowl adenovirus vector (FAdV-9) having at least one exogenous nucleotide sequence inserted encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region, and a pharmaceutically acceptable vehicle, adjuvant and / or excipient, wherein the at least one exogenous nucleotide sequence encoding at least one antigen of a disease of interest and replacing the adenovirus genome non-essential region is located between the 491 and 2782 nucleotides. The vector of this vaccine is stable for industrial scale production. When administering this vaccine in combination with a vaccine against Marek's disease, both vaccines produce an adequate immune response which is not affected by interference between each other. In the same way, effectiveness of the recombinant vaccine is not affected by maternal antibodies, and is capable of inducing both an early and lasting protective response, even with only one application.
Owner:GRUPO IND PECUARIO S A DE CV
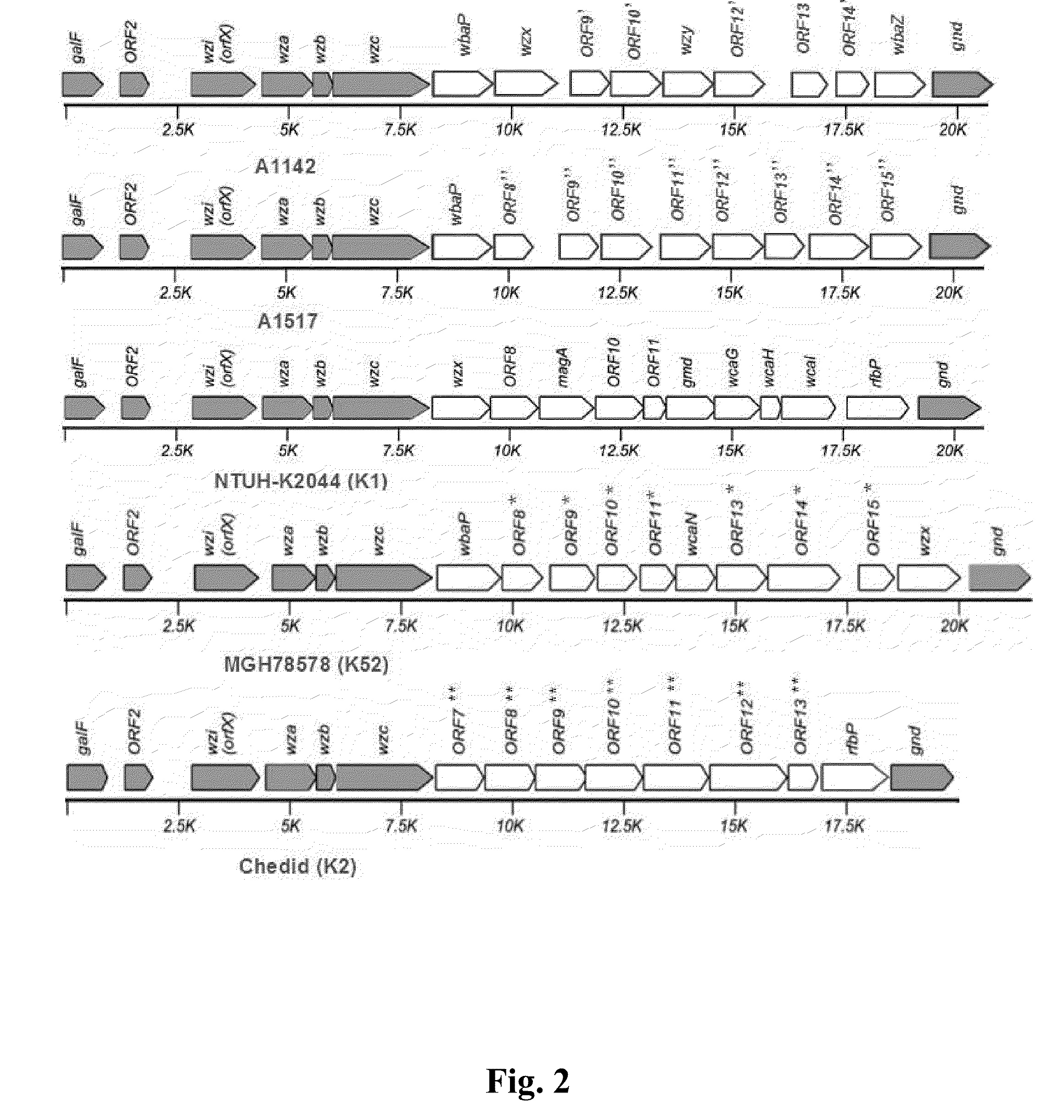
Method of identifiying a serotype of klebsiella pneumoniae and application thereof
ActiveUS20100136534A1Reduce mortalityRapid diagnosisSugar derivativesMicrobiological testing/measurementPolysaccharide synthesisClinical diagnosis
The present invention relates to a method of identifying a serotype of Klebsiella pneumoniae, in particular to a method using specific polymerase chain reaction (PCR) primer sets designed according to a fragment of a capsular polysaccharide synthesis (cps) region to identify a K57 or a NTUH-N1 serotype and its application. NTUH-N1 is a novel serotype which differs from the previously reported 77 serotypes. This PCR-based cps genotyping method not only solves the problems of insufficient specificity and sensitivity caused by conventional immune method, but can be applied in clinical diagnosis with the advantages of rapidity and low cost. In addition, the rate of unidentifiable strains can also be reduced by this method.
Owner:NAT TAIWAN UNIV
Porcine contagious pleuropneumonia trivalent inactivated vaccine and its preparation method
ActiveCN103212067AEasy to preparePrevention of contagious pleuropneumoniaAntibacterial agentsBacterial antigen ingredientsSerum igeAntigen
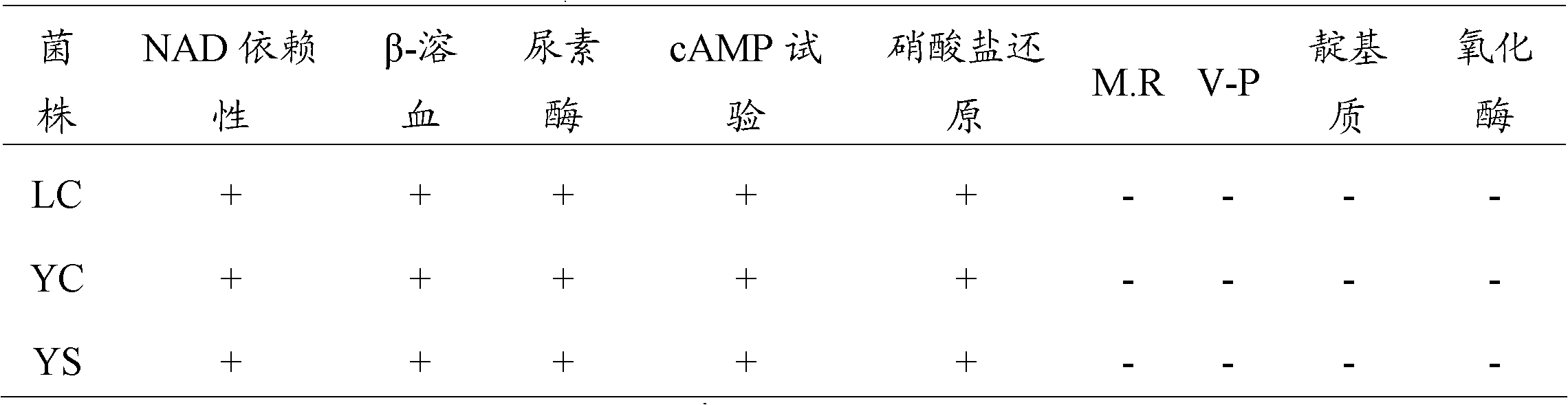
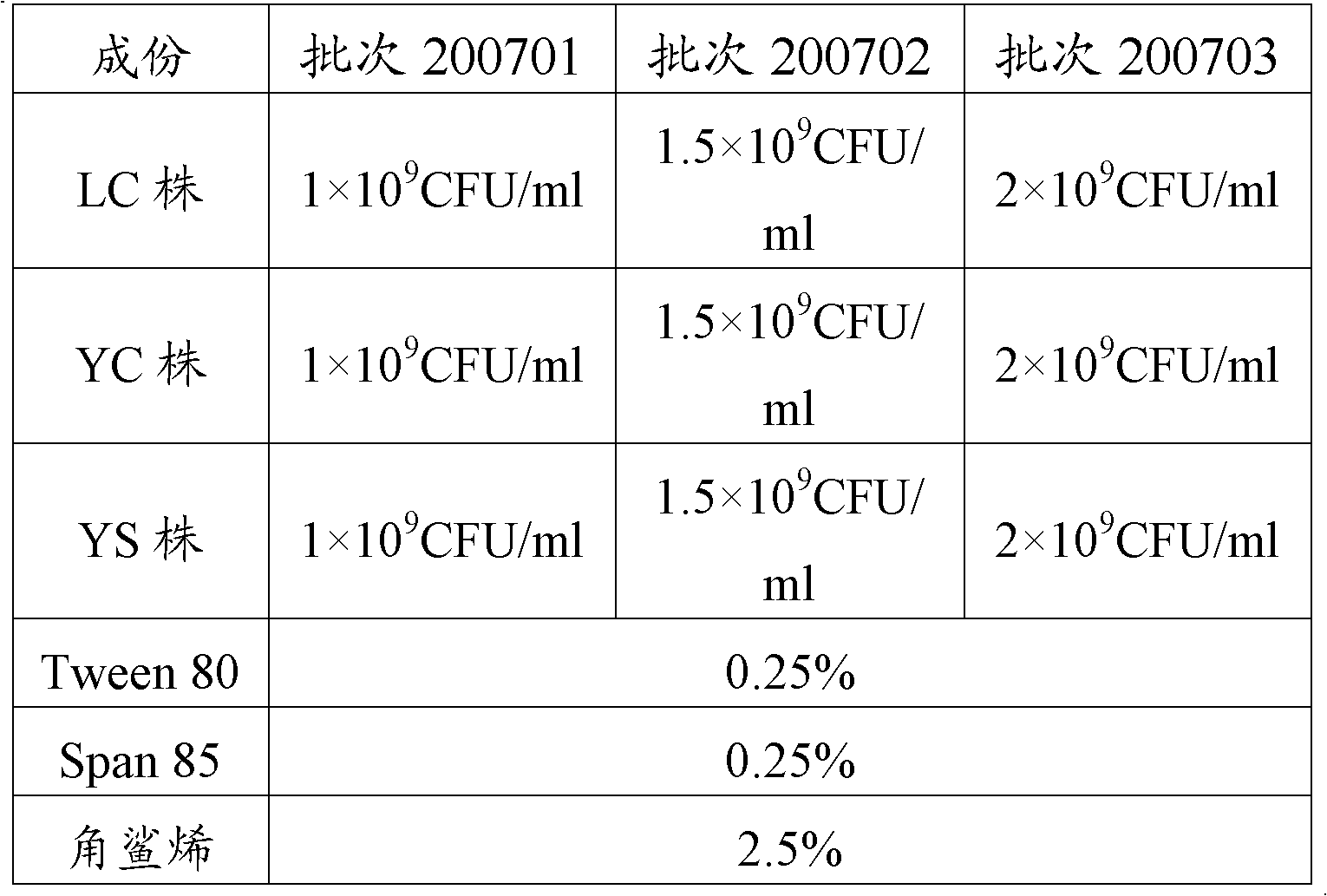
Three porcine contagious Actinobacillus pleuropneumoniae comprising a serotype 1 LC strain, a serotype 5 YC strain and a serotype 7 YS strain are obtained in the invention; and a porcine contagious pleuropneumonia trivalent inactivated vaccine and its preparation method are obtained through the reasonable preparation of inactivated antigens of the above three strains to effectively prevent and control the propagation and the burst of porcine contagious pleuropneumonia in a culturing farm. The invention also provides a porcine contagious pleuropneumonia trivalent inactivated vaccine using an aqueous adjuvant. The porcine contagious pleuropneumonia trivalent inactivated vaccine furthest reduces the stress response during injection, and continuously improves the immune protection effect, so the porcine contagious pleuropneumonia trivalent inactivated vaccine is especially suitable for the large-scale application in Chinese culturing farms.
Owner:PU LIKE BIO ENG
Subgroup b recombinant human adenovirus vector, and methods for constructing and for using the same
ActiveUS20140088180A1Improve survivalReduce efficacyGenetic material ingredientsUnknown materialsNeoplasmA serotype
A method for constructing a subgroup B recombinant human adenovirus vector Ad11-5EP. The method includes substituting a 365 bp fragment including an enhancer and a promoter of an upstream coding sequence of Ad5 E1A for a corresponding region of a serotype Ad11 of the subgroup B human adenovirus vector by homologous recombination to construct the subgroup B recombinant human adenovirus vector Ad11-5EP. A subgroup B recombinant human adenovirus vector Ad11-5EP constructed by the method and the use thereof for treatment of tumors are also provided.
Owner:BEIJING BIO TARGETING THERAPEUTICS TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com