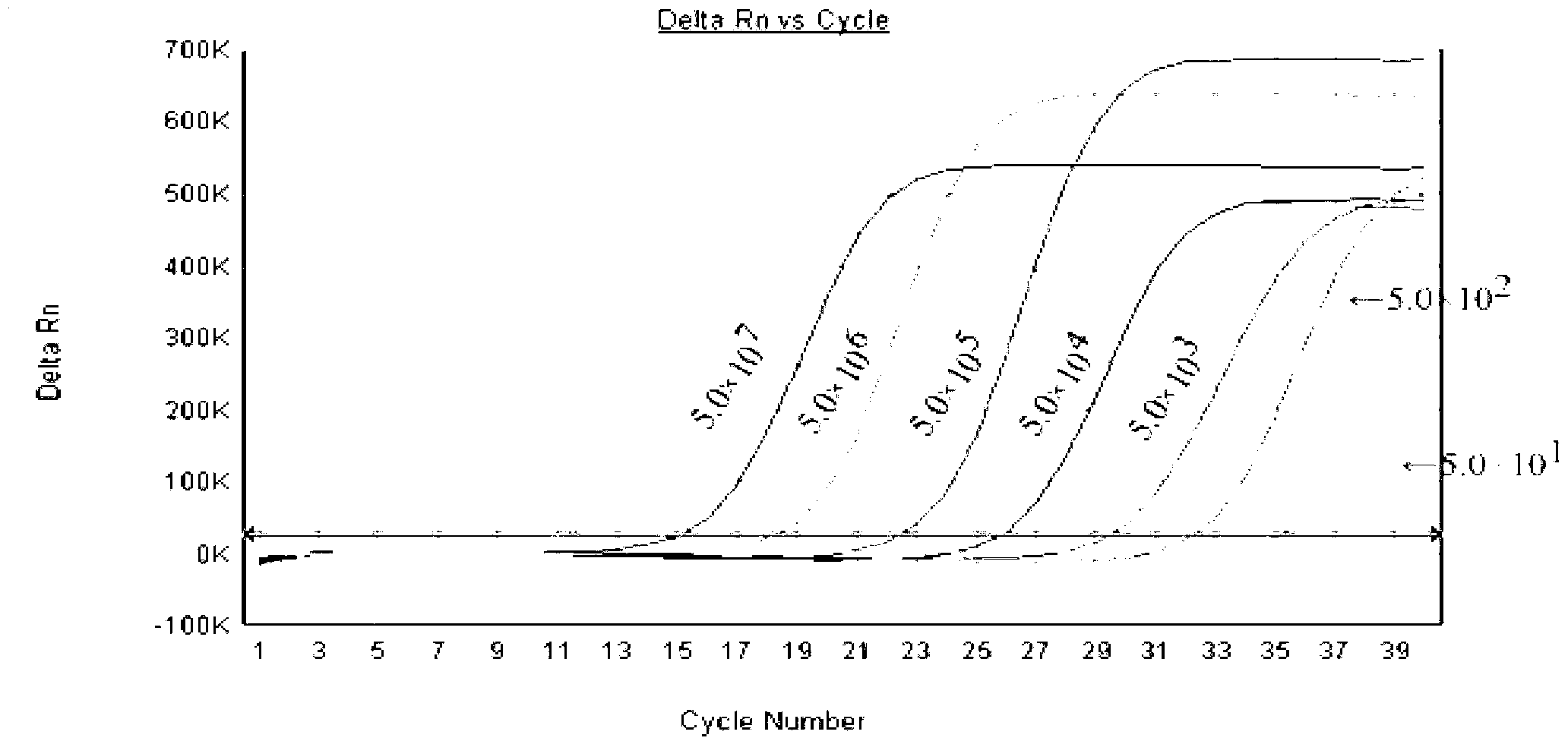
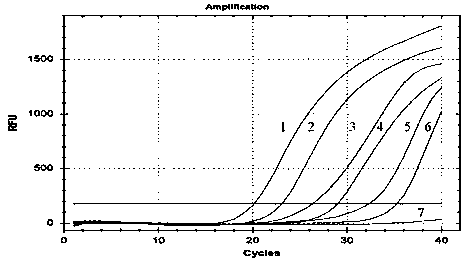
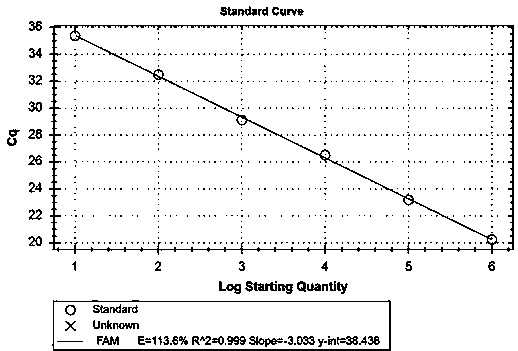
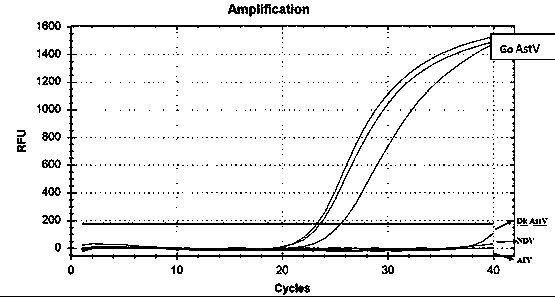
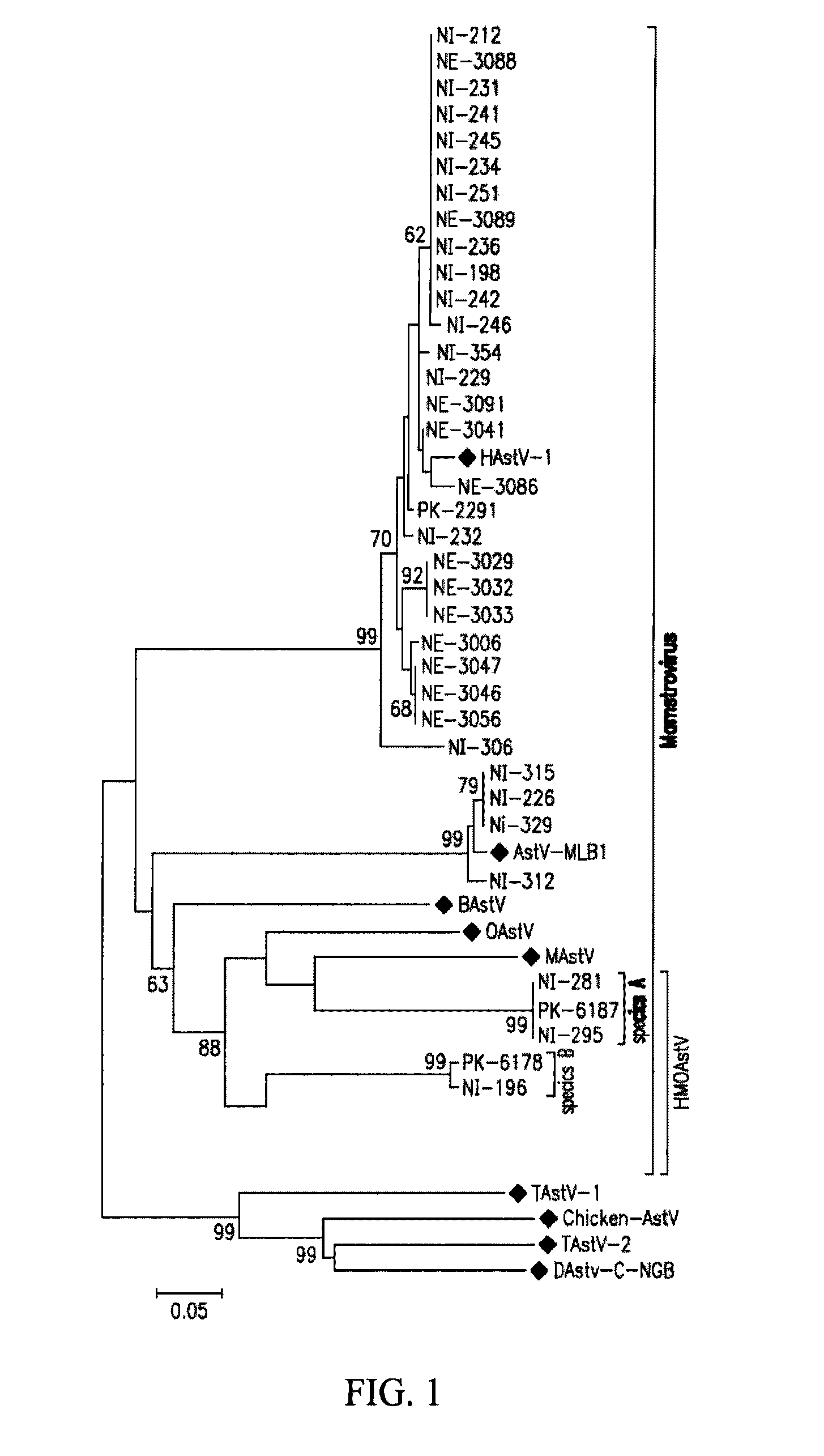
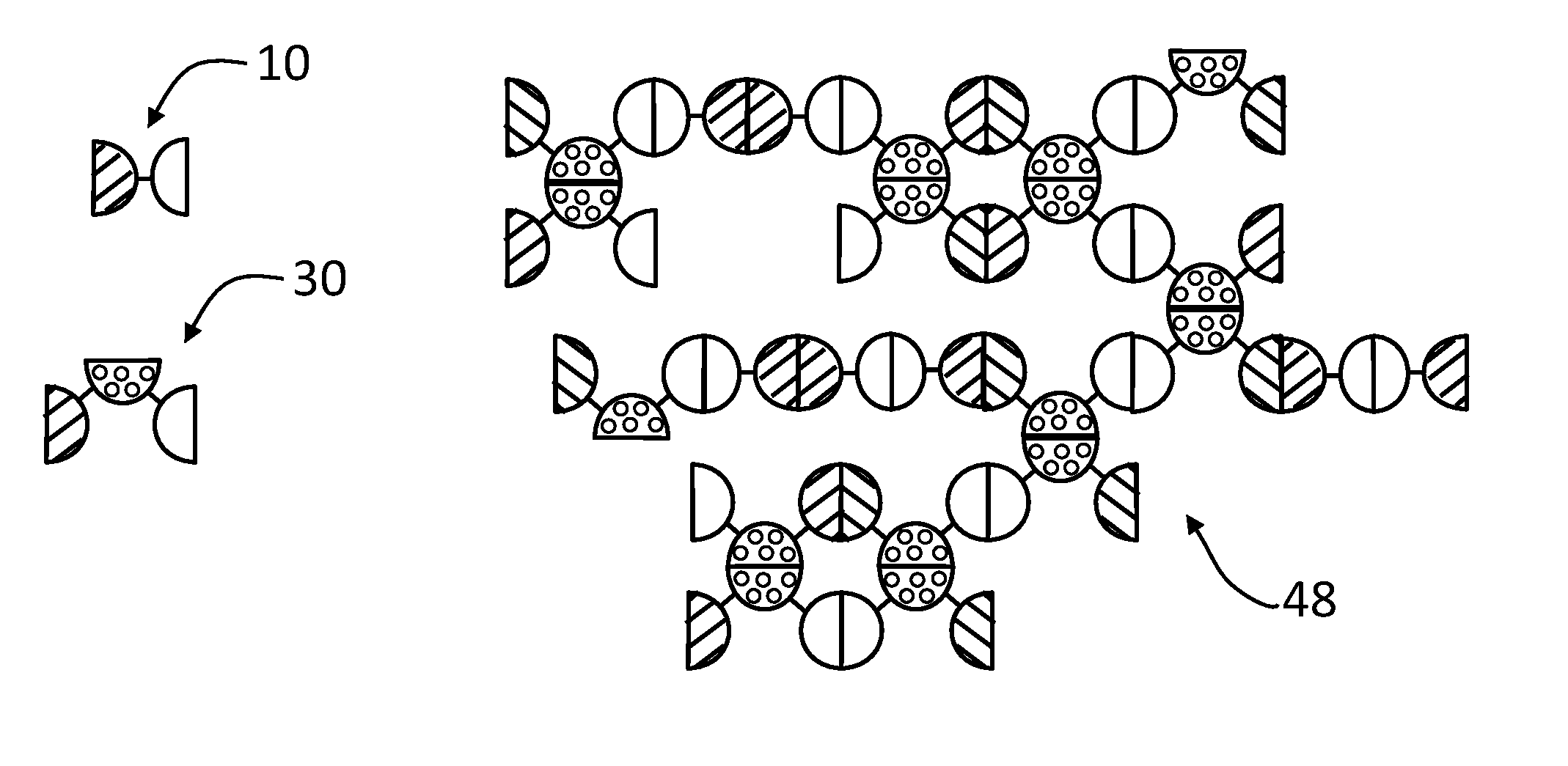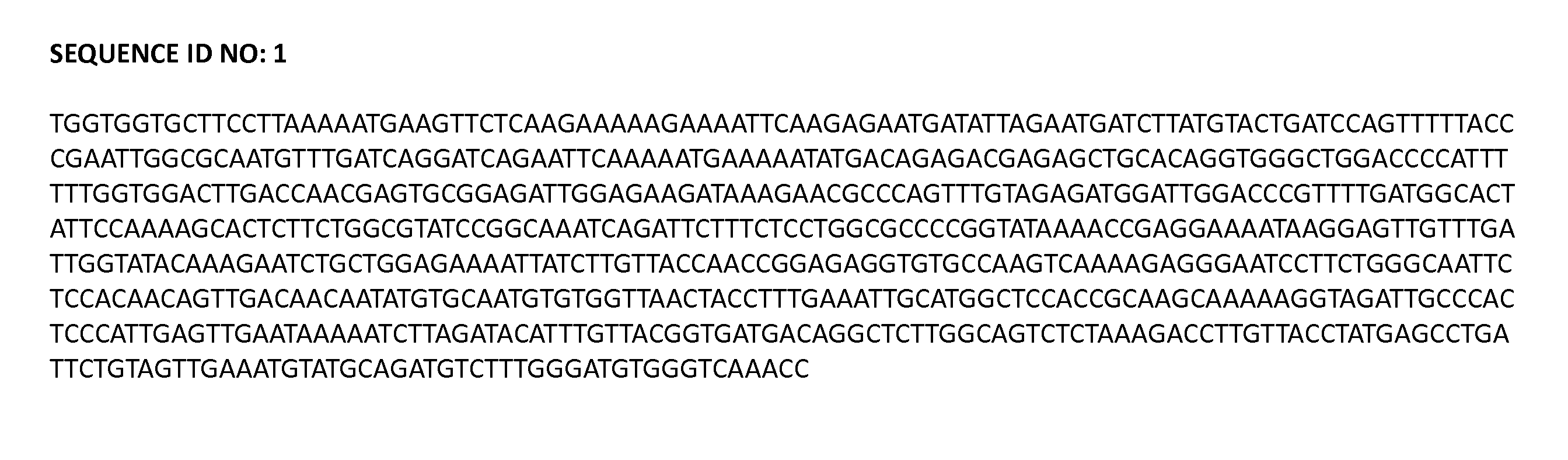Patents
Literature
43 results about "Human astrovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
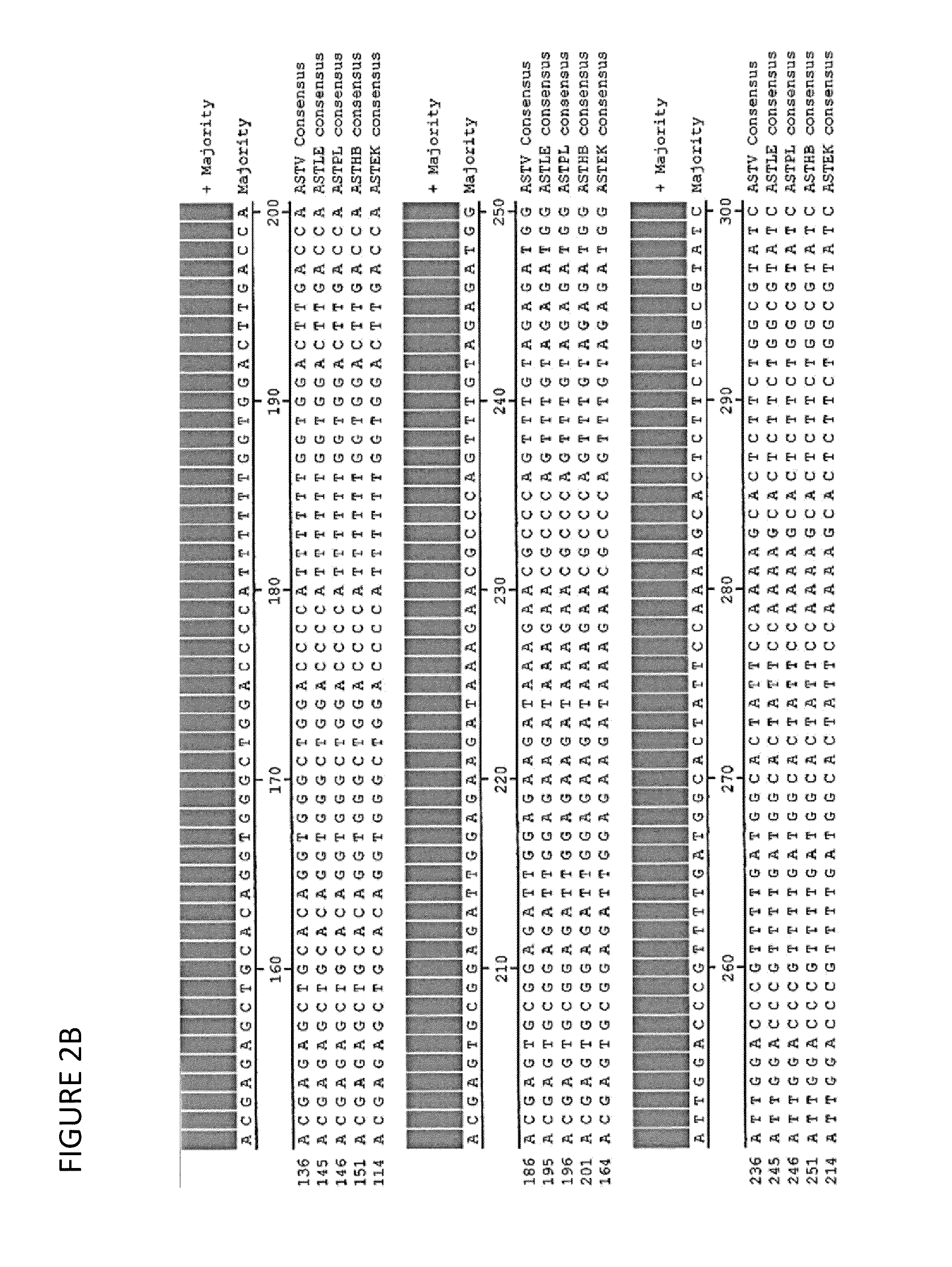
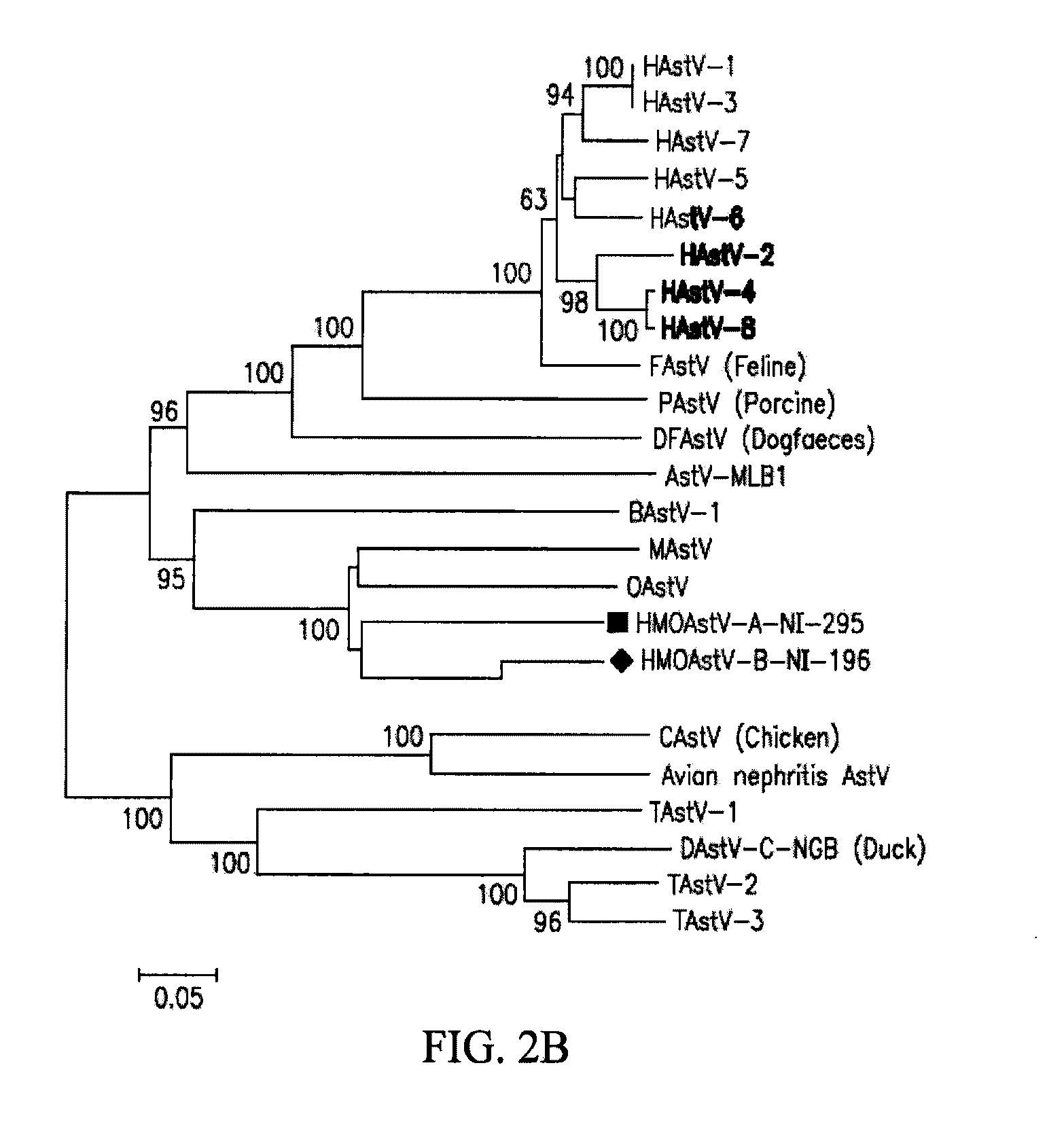
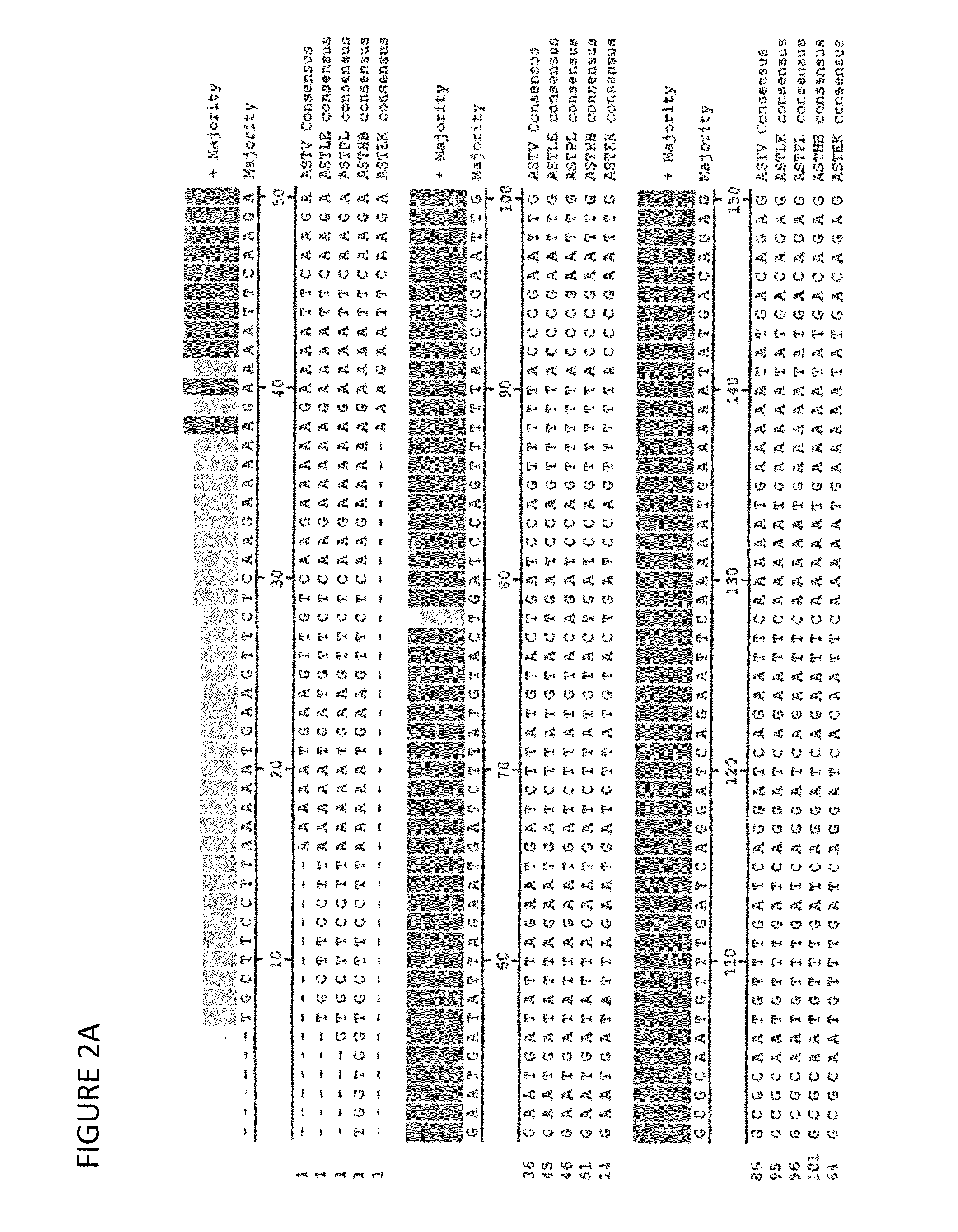
Astrovirus is a type of virus that was first discovered in 1975 using electron microscopes following an outbreak of diarrhea in humans.
Methods for regulating complement cascade proteins using astrovirus coat protein and derivatives thereof
ActiveUS20100055106A1Decrease complement-related tissue damageReduce tissue damageNervous disorderSsRNA viruses positive-senseDiseaseFunctional activity
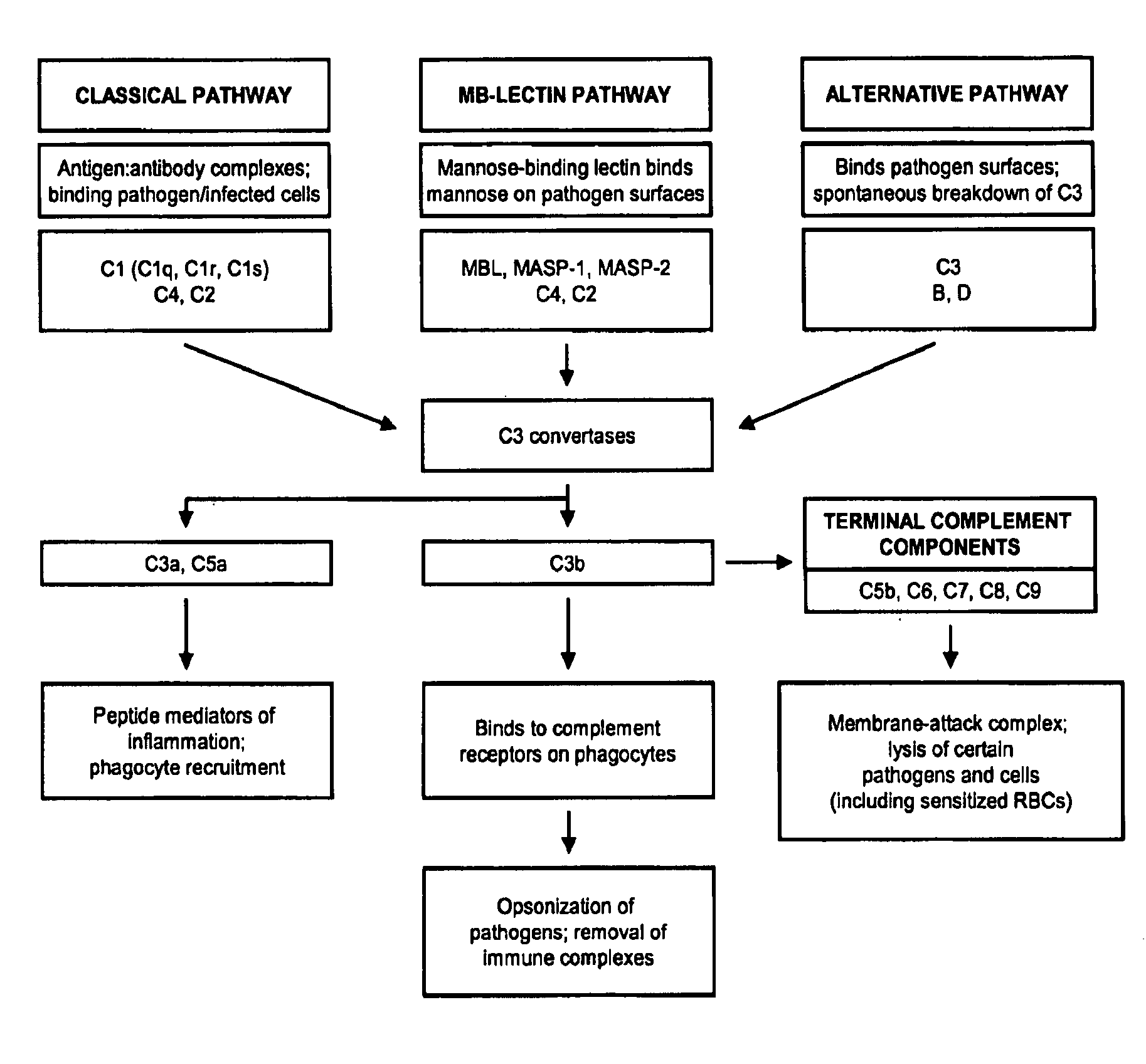
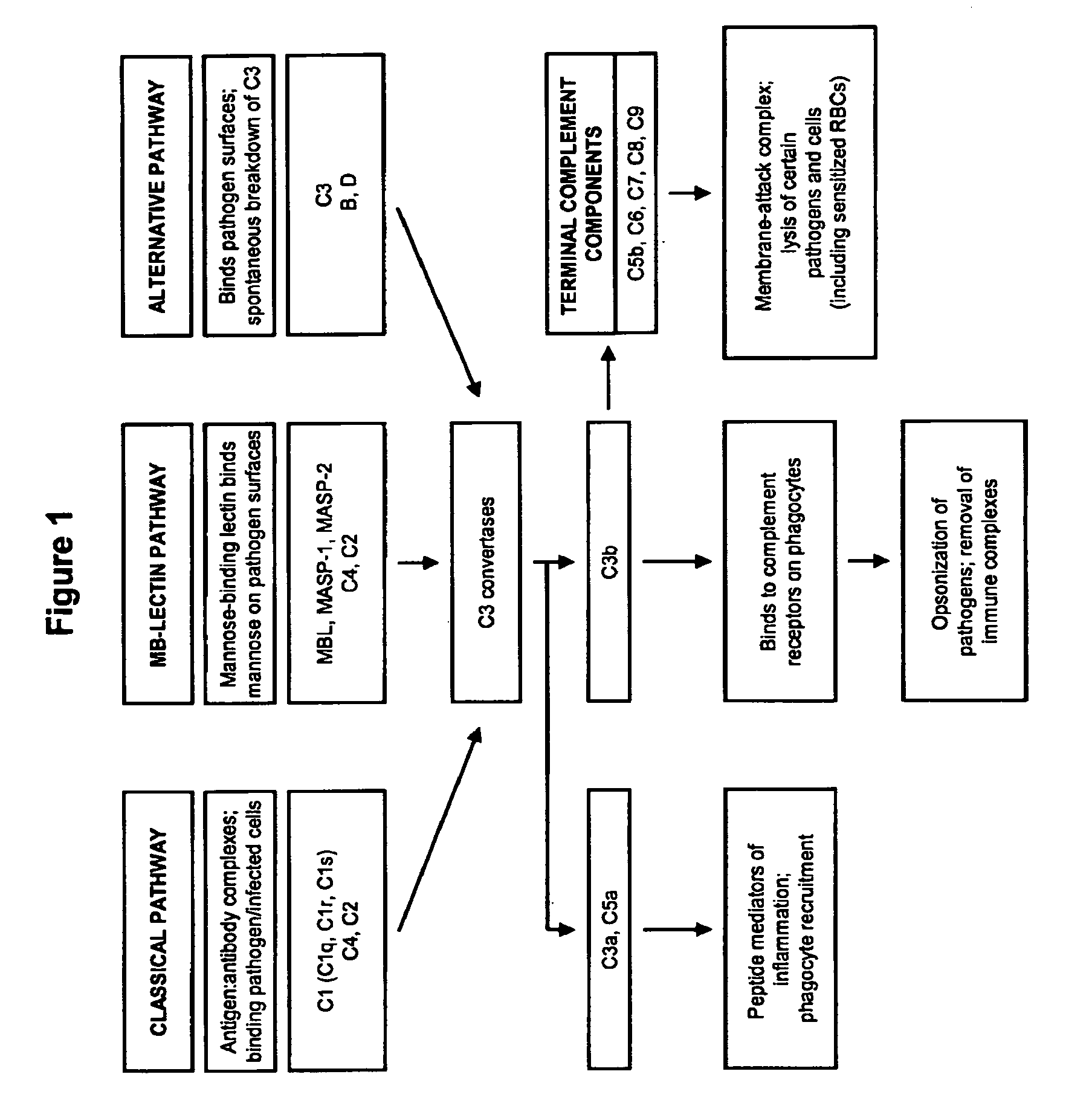
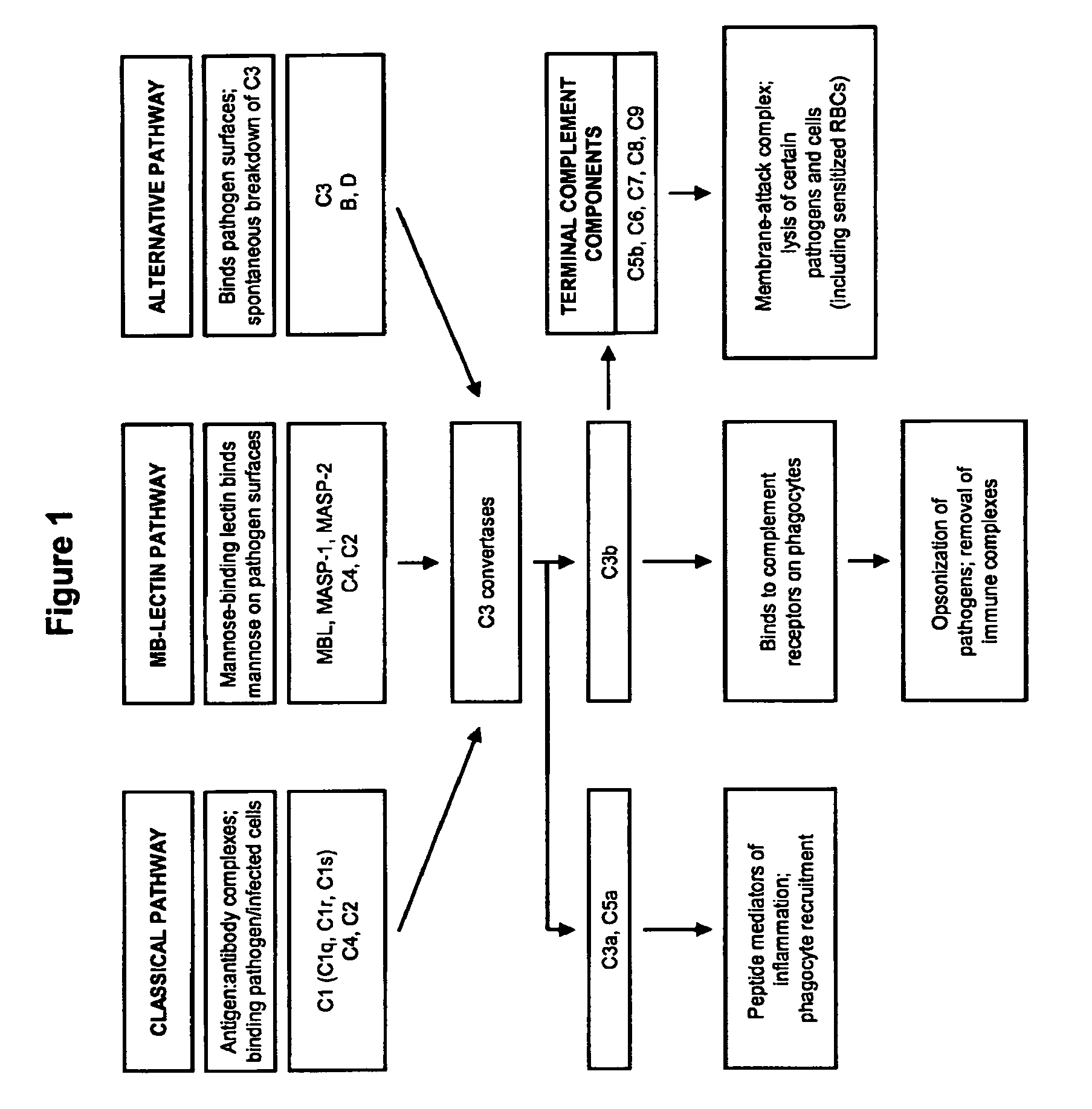
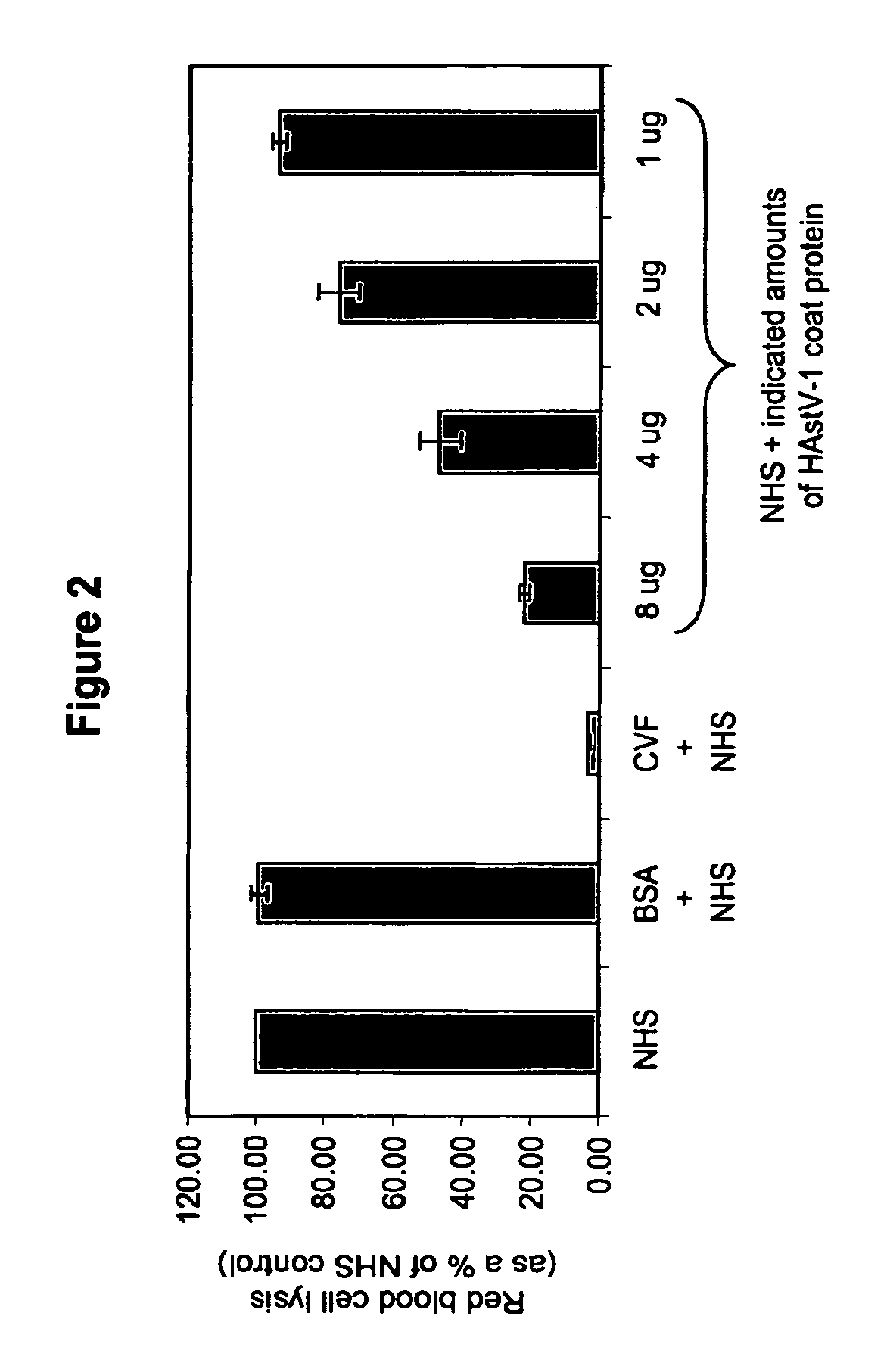
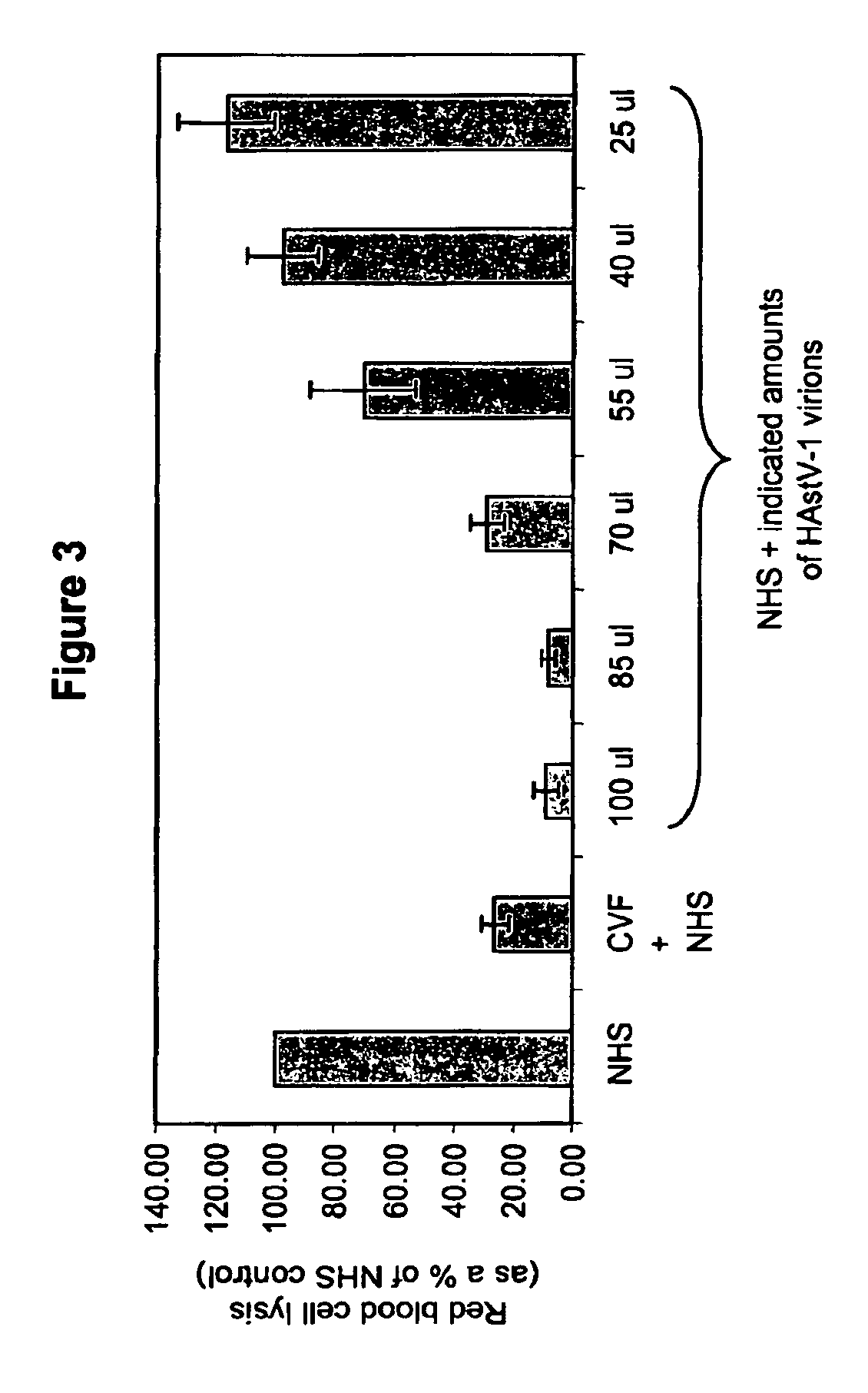
The present invention provides a method for modulating the complement cascade by depleting the plasma of the functional activity of complement proteins and thereby reducing or eliminating complement-mediated cell lysis. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated cell lysis and peptide mediators of inflammation. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated diseases. Methods are described herein where complement cascade, triggered by either the classical or alternative complement pathways, is prevented from effecting cell lysis and inflammation due to inhibition or depletion of one or more complement components in the serum following administration of astrovirus coat proteins or derivatives.
Owner:CHILDRENS RES HLDG LLC +1
Methods for regulating complement cascade proteins using astrovirus coat protein and derivatives thereof
ActiveUS8241843B2Reduce tissue damageSsRNA viruses positive-senseNervous disorderDiseaseFunctional activity
The present invention provides a method for modulating the complement cascade by depleting the plasma of the functional activity of complement proteins and thereby reducing or eliminating complement-mediated cell lysis. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated cell lysis and peptide mediators of inflammation. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated diseases. Methods are described herein where complement cascade, triggered by either the classical or alternative complement pathways, is prevented from effecting cell lysis and inflammation due to inhibition or depletion of one or more complement components in the serum following administration of astrovirus coat proteins or derivatives.
Owner:CHILDRENS RES HLDG LLC +1
Diarrhea virus detection kit and method
ActiveCN103215379AImprove detection efficiencySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceRotavirus RNAFluorophore
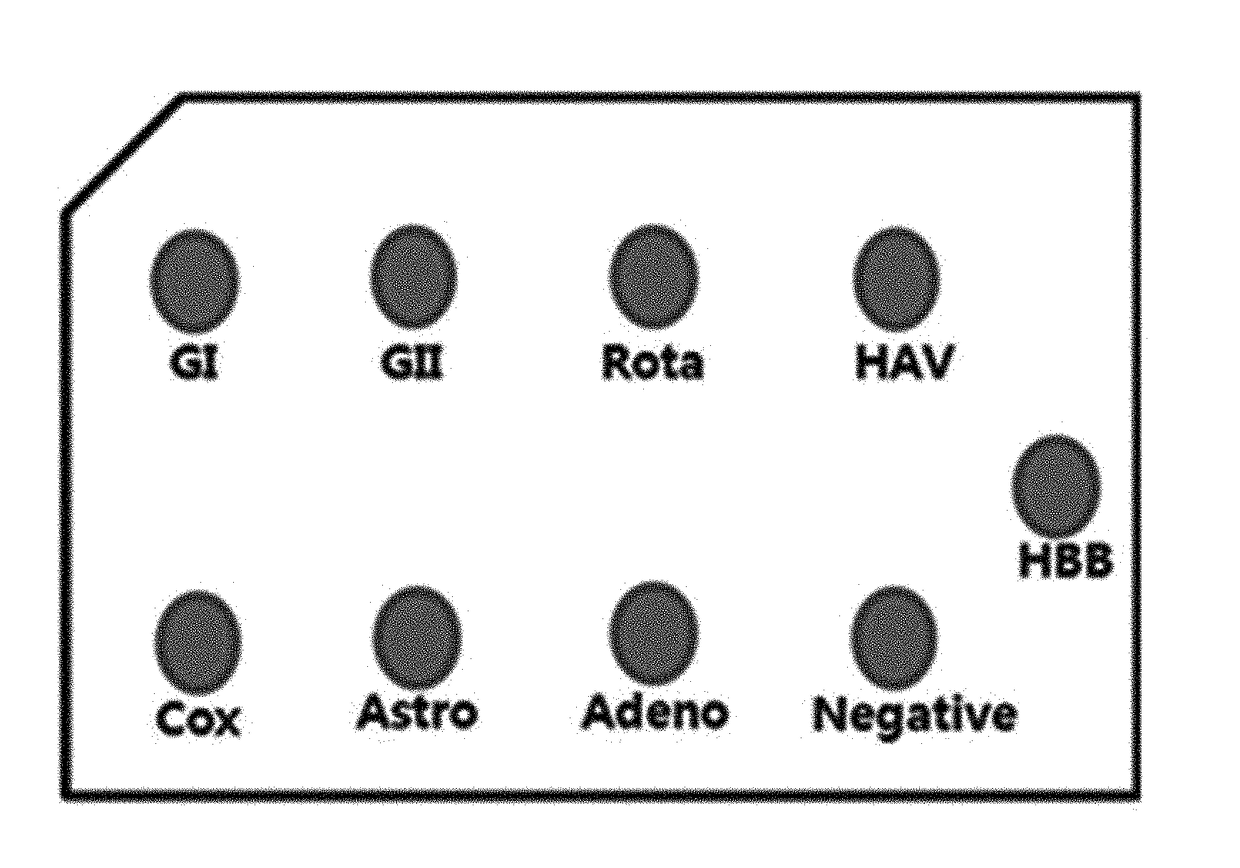
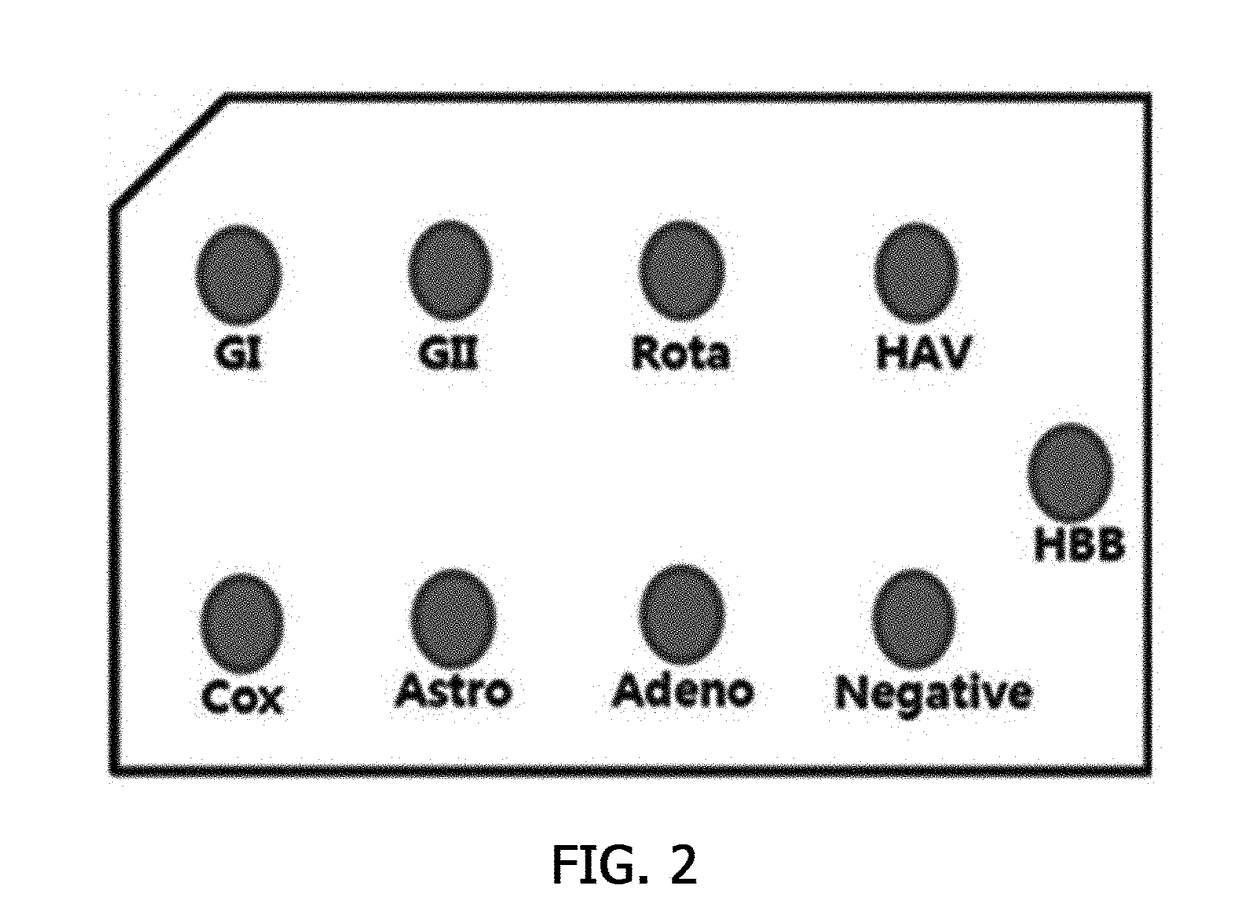
The invention is applicable to the technical field of biotechnology and medical detection and provides a diarrhea virus detection kit and method. The detection kit comprises PCR (polymerase chain reaction) liquid which comprises a PCR buffer solution, MgCl2, dNTP, DNA polymerase, primer pairs, probe sequences and Homo-tag, wherein the primer pairs and the probe sequences respectively aim at type I, type II and type IV of sapovirus, type I and type II of norovirus, rotavirus, adenovirus and astrovirus, the 5' ends of the probe sequences are connected with fluorophore, and the 3' ends of the probe sequences are connected with quencher. The detection kit provided by the invention can detect seven viruses (subtype) related to diarrhea at one time, and compared with a detection method in the prior art, the detection method provided by the invention has the advantages that operation is simple and convenient, a result is quick to read, and false positive is avoided.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
Kit and oligonucleotide sequences for detecting astrovirus
InactiveCN101671745AHigh detection sensitivityShort reaction timeMicrobiological testing/measurementMicroorganism based processesMagnesium pyrophosphateTurbidity
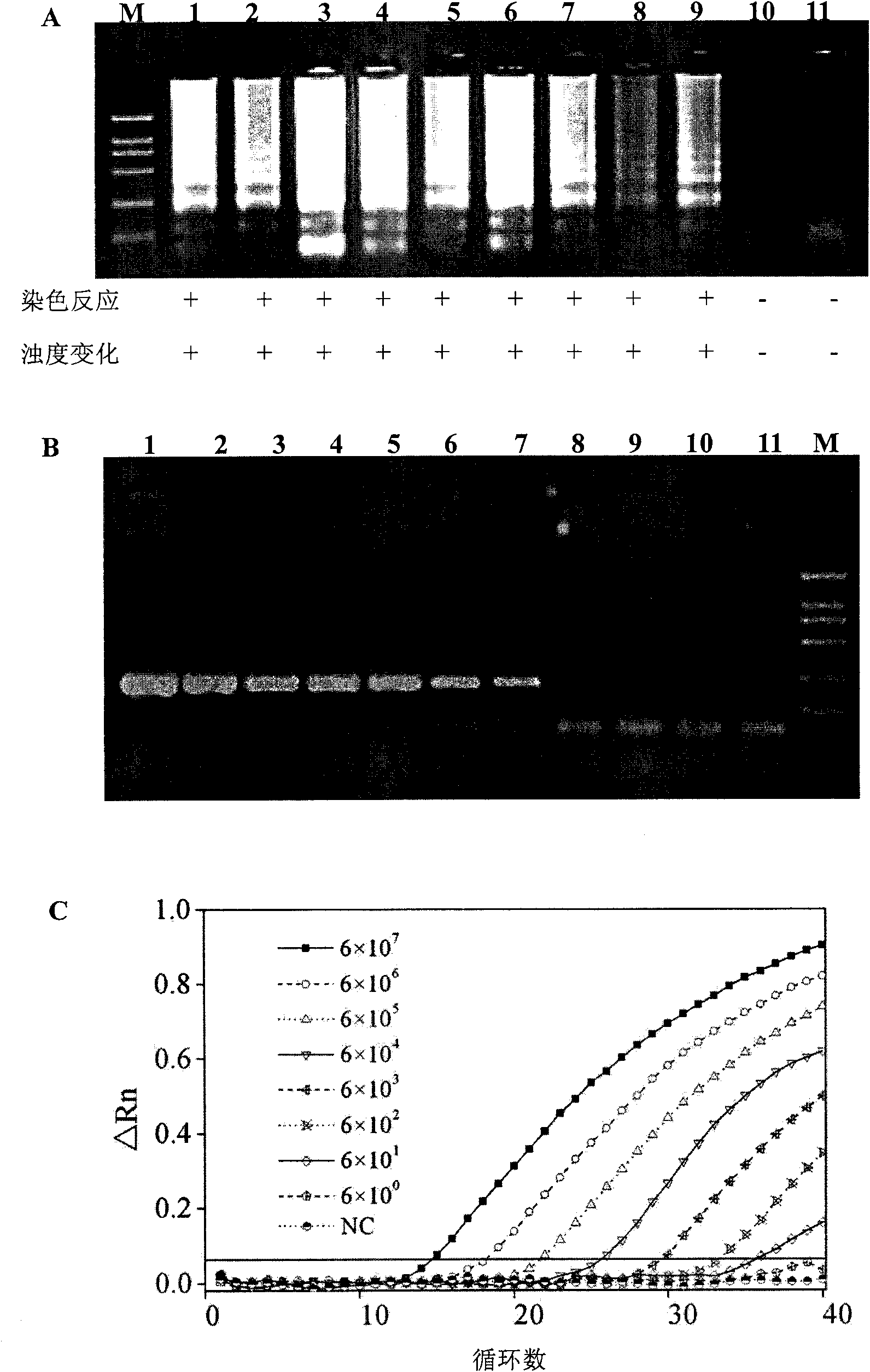
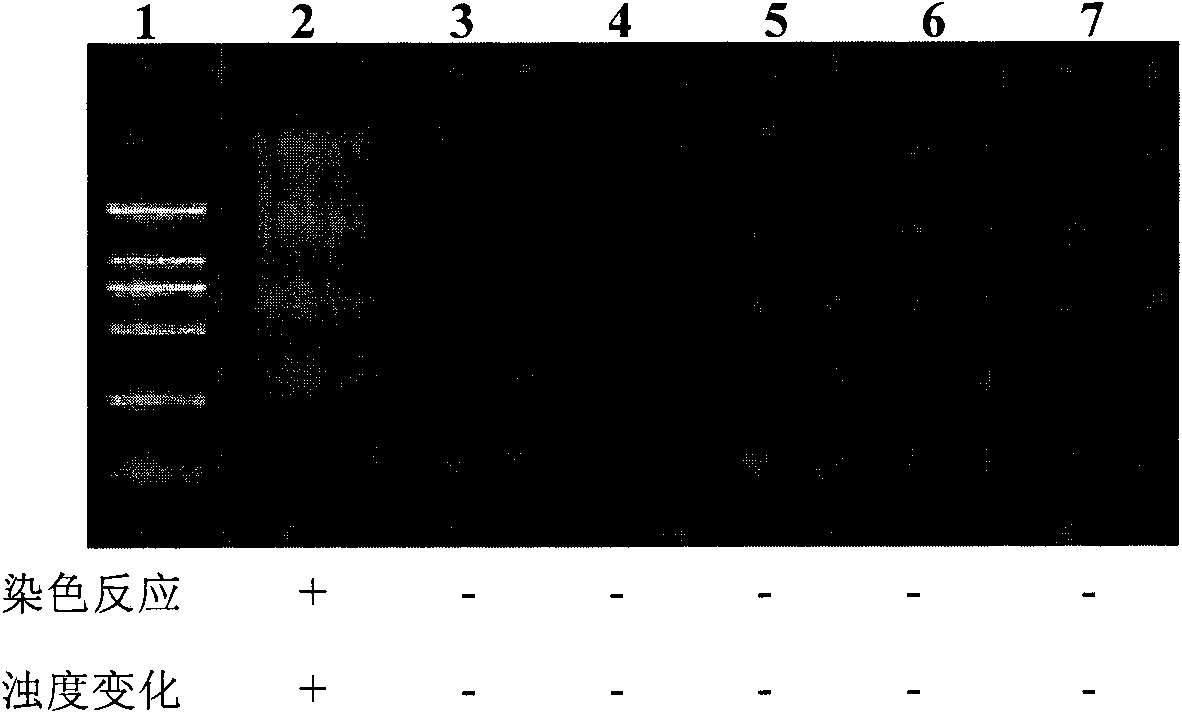
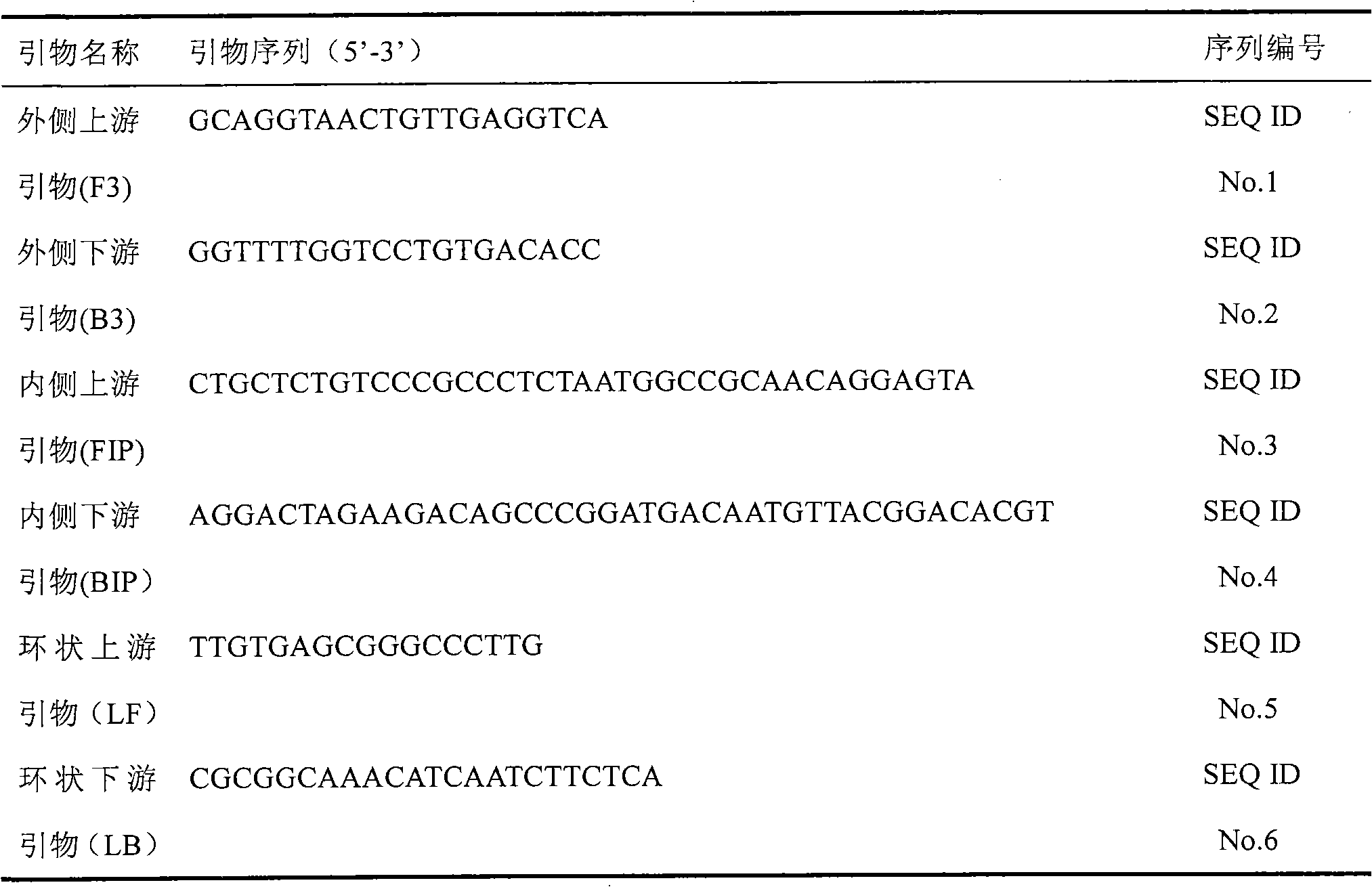
The invention discloses a kit and oligonucleotide sequences for detecting astrovirus, in particular to a group of oligonucleotides which are used for detecting astrovirus and have the oligonucleotidesequences shown from the sequence table SEQ ID No.1 to the sequence table SEQ ID No.6, the kit containing the oligonucleotides and a detection method thereof. The kit of the invention has high sensitivity, strong specificity, low cost and simple and convenient operation. During detection, when lots of nucleic acid is synthesized, a by-product magnesium pyrophosphate precipitate is generated, whichhas high specificity. Whether the products are amplified or not can be judged only by observing the turbidity of the products with naked eyes.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Nucleic acid composition and kit for detecting diarrhea viruses as well as use method of kit
InactiveCN109593890AMicrobiological testing/measurementMicroorganism based processesRotavirus RNASapovirus
The invention relates to nucleic acid composition and a kit for detecting diarrhea viruses as well as a use method of the kit. The nucleic acid composition for detecting diarrhea viruses comprises anamplification primer pair and detection probes corresponding to amplification primers. The nucleic acid composition for detecting diarrhea viruses can at least detect three of group A rotavirus, groupB rotavirus, group C rotavirus, norovirus, norovirus I type, norovirus II type, sapovirus, astrovirus and adenovirus simultaneously and has high detection sensitivity and good specificity.
Owner:深圳市刚竹医疗科技有限公司
Gene chip and reagent box for detecting food-borne virus
InactiveCN101328505AGuaranteed reproducibilityGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesFood borneRotavirus RNA
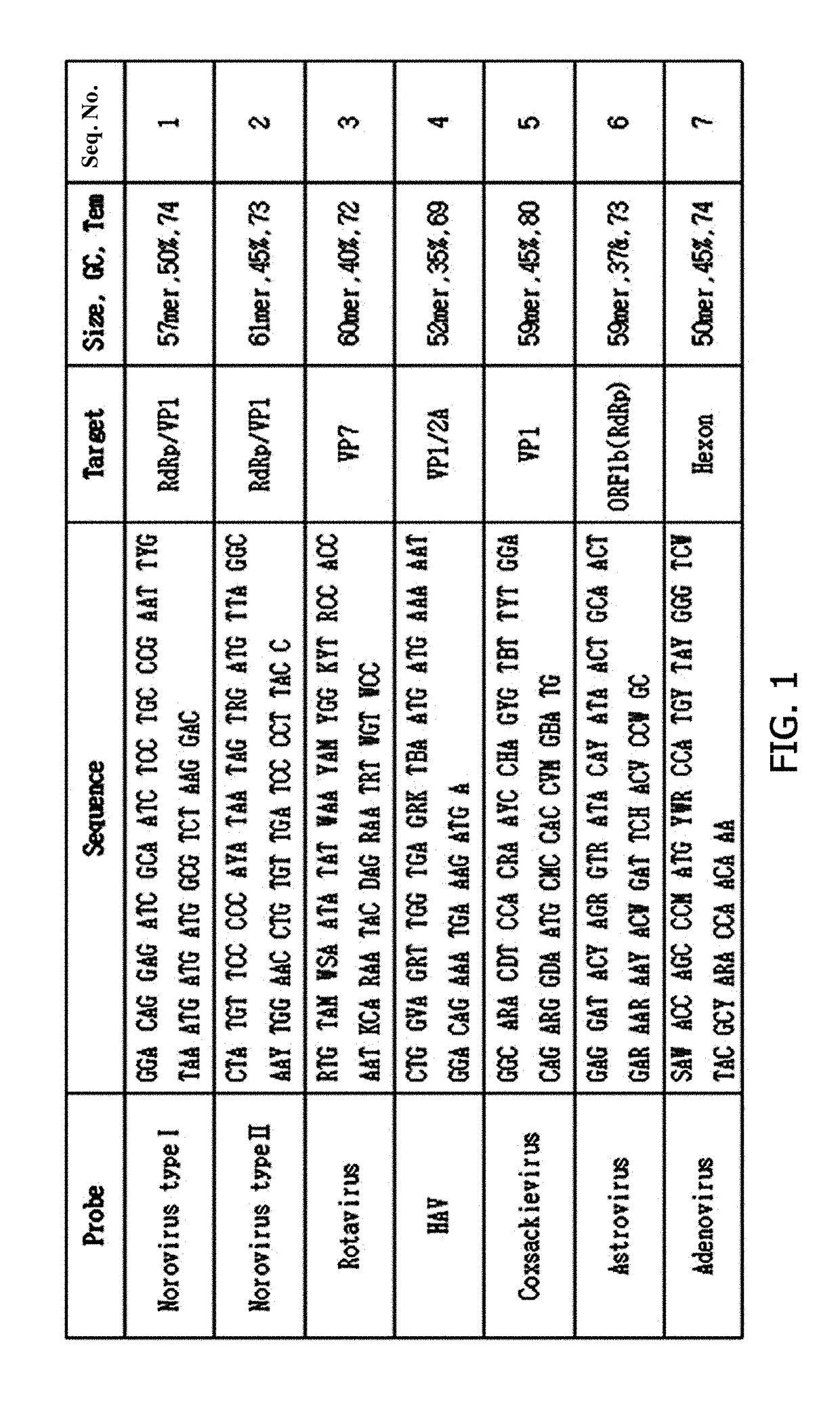
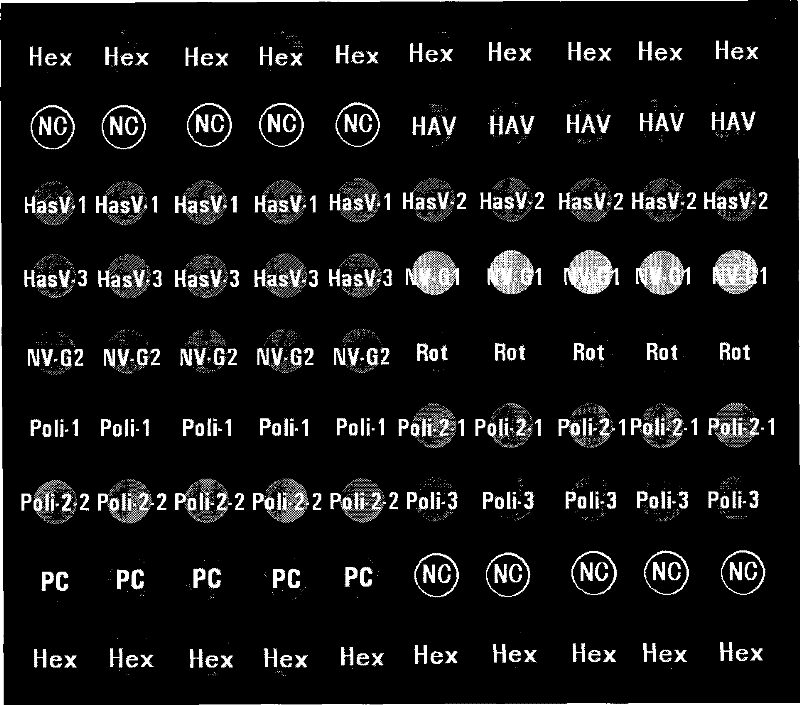
The invention relates to a gene chip for detecting food-home virus and a kit, belonging to the inspection field. The surface of a solid phase carrier is fixed with a plurality of detection probes and quality control contrast probes, wherein, the detection probes consist of the probes for detecting hepatitis A virus, human astrovirus, norwalk virus G1, norwalk virus G2, rotavirus, polio virus 1, polio virus 2 and polio virus 3; and the quality control contrast probes consist of a spotting positive quality control probe, a chip hybridization positive quality control probe and a chip negative quality control probe. The gene chip and the kit have the advantages of: (1) high throughout: five common viruses are integrated and detected simultaneously, and the practicability is strong; (2) rapidness: the detection time is only 4 hours; (3) specificity: the false positive caused by the cross reaction is avoided; (4) flexibility: the detection flexibility of the chip is 10<8> virus particles per gram of the tissue sample, which is higher than that of RT-PCR; and (5) good repetitiveness and stable results. The gene chip and the kit can be widely applied to the food safety inspection of the inspections system.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
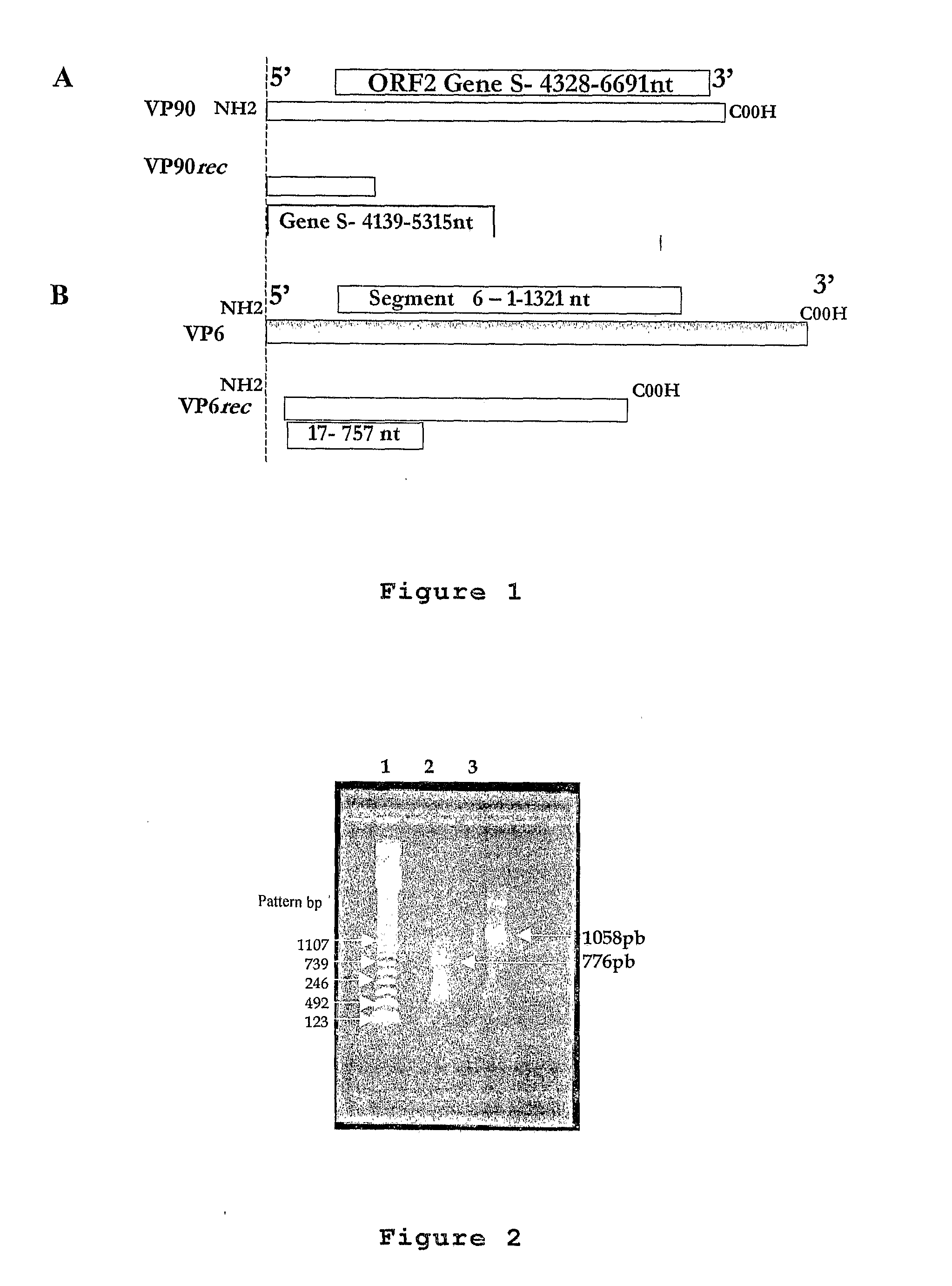
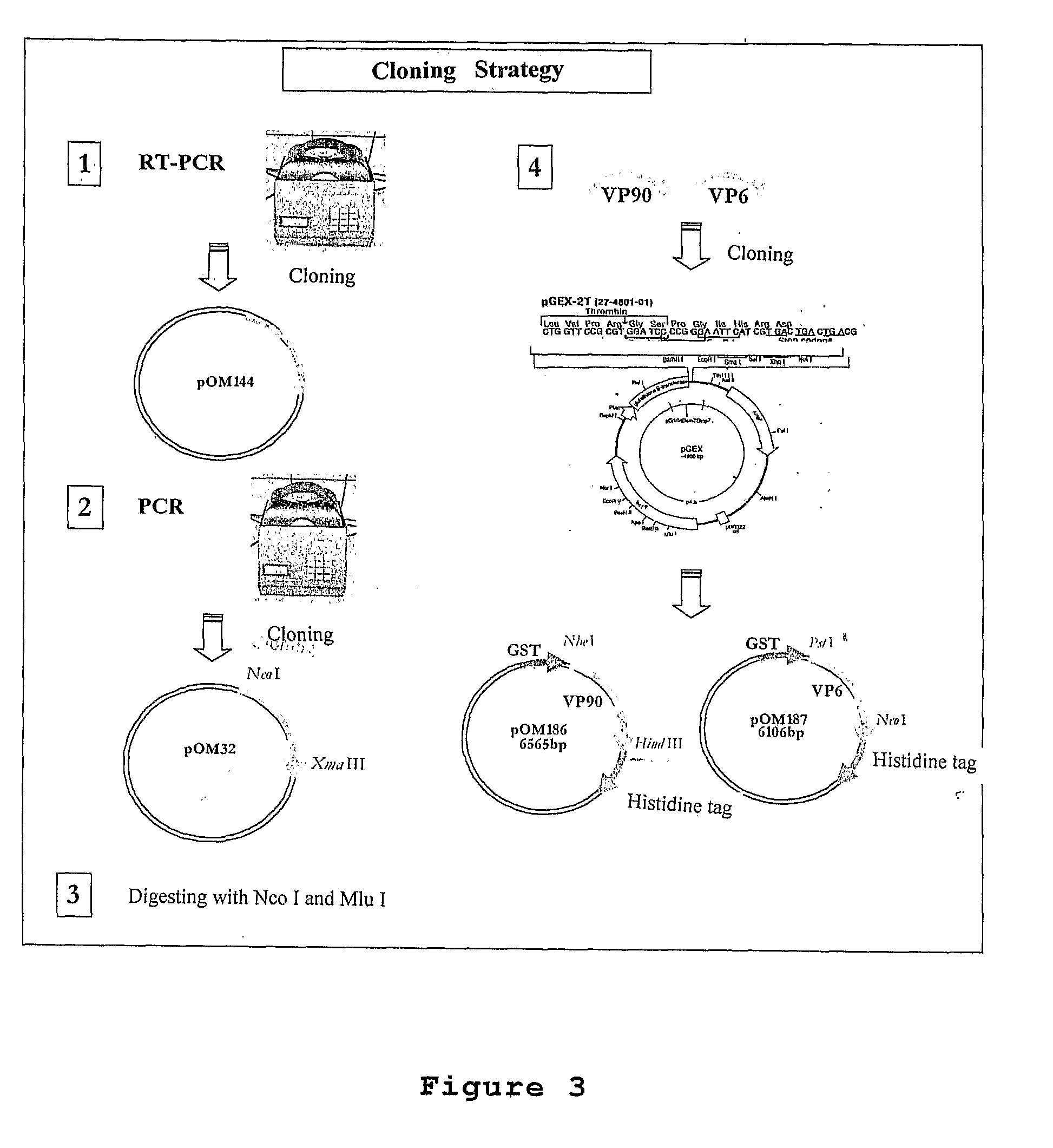
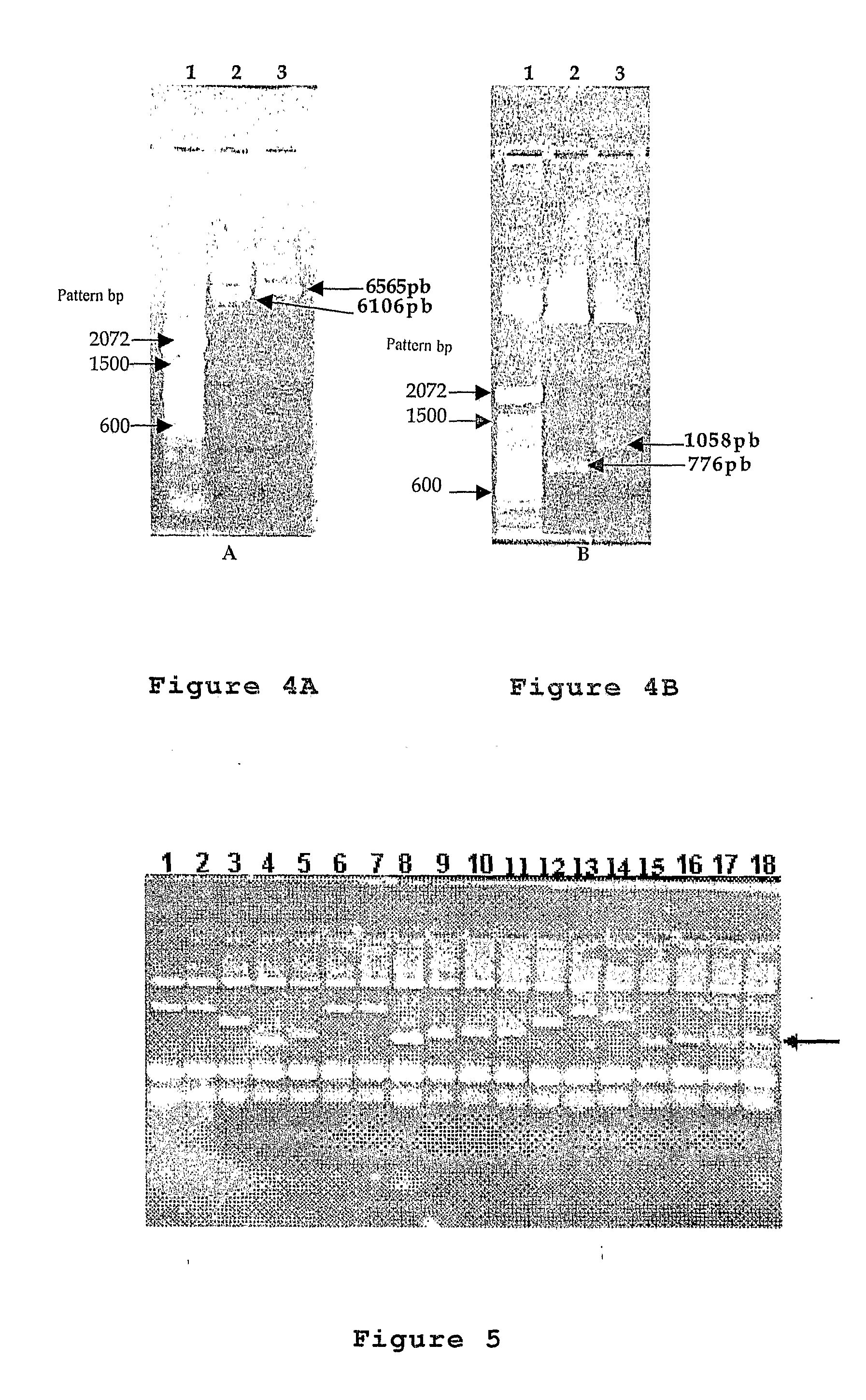
Plasmid Expression Vectors for Expression of Recombinant Rotavirus and Astrovirus Proteins or Epitopes
The present invention refers to the production of specific recombinant viral proteins intended for use in the construction of a diagnostic kit for the simultaneous detection of the two most important gastroenteric viruses, namely rotavirus and astrovirus.
Owner:GOES ANA CAROLINA MAGALHA +5
Fluorescent quantization PCR detection method for swine astrovirus
InactiveCN101705310AAvoid contamination riskWide detection rangeMicrobiological testing/measurementPlasmidBioinformatics
The invention relates to a fluorescent quantitation PCR detection method for swine astrovirus, which belongs to the technical field of inspection, which comprises the following steps: step one, using a conservative segment SEQ ID NO:1 of a swine astrovirus gene as a target, designing a primer and a probe, and amplifying a gene segment; step two, constructing recombined plasmid by using the gene segment obtained in the step one; step three, extracting RNA of a sample and reversely transcribing to obtain cDNA; and step four, detecting the content of the swine astrovirus in the sample according to a conventional fluorescent quantization PCR detection method. The method has the characteristics of high sensitivity, high stability, quantifiability, good specificity, low false positive and the like.
Owner:SHANGHAI JIAO TONG UNIV
Novel astrovirus
This invention relates to the isolation and uses of novel avian astroviruses. The present invention also relates to vaccines, kits and methods for detection of a novel astrovirus. The present invention further relates to vaccination of avians, prevention and / or treatment of avian infections associated with astrovirus. Infections of poultry by this novel astrovirus are associated with runt stunting syndromes.
Owner:BIOMUNE
Single standard product-based four-color fluorogenic quantitative PCR (Polymerase Chain Reaction) method and kit
InactiveCN103146846AEasy to operateQuick checkMicrobiological testing/measurementFluorescence/phosphorescencePcr methodDiagnosis laboratory
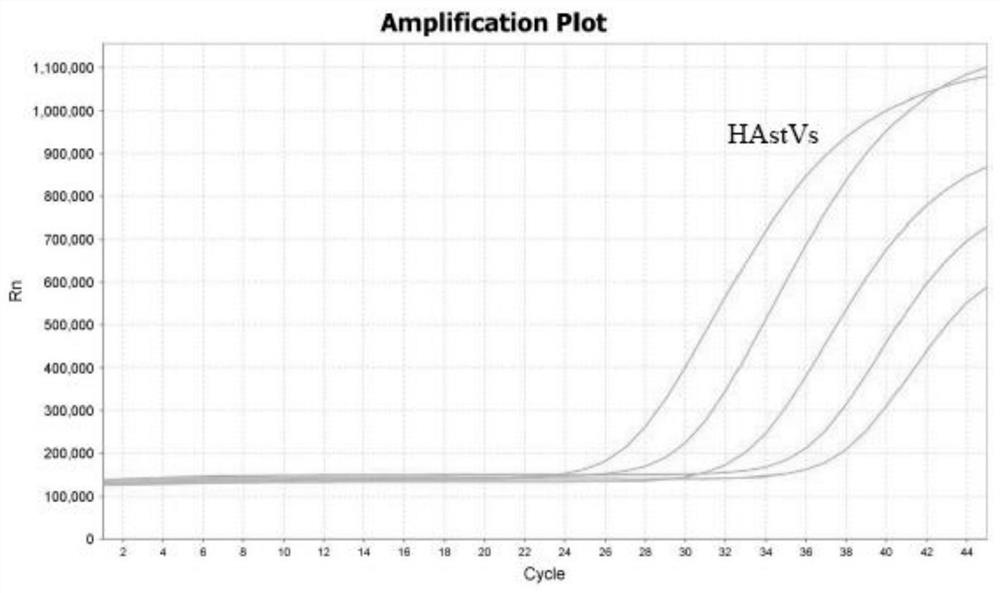
The invention discloses a single standard product-based quardruple four-color fluorogenic quantitative PCR (Polymerase Chain Reaction) technology. The technology is capable of detecting group A rotavirus (HRVA), norovirus group I (NVGI), norovirus group II (NVG II) and human astrovirus (HAstV) RNA in various treated specimen through one-step quardruple PCR amplification and quantifying virus load. The technology is characterized in that single standard product is adopted as positive control and quantitative standard in the quardruple four-color fluorogenic quantitative PCR detection for four viruses, thereby breaking through the deficiency that the traditional multiple fluorogenic quantitative PCR needs a plurality of standard products, improving the experiment detection efficiency, reducing the pollution probability and improving the preparation and production efficiency of the kit. The method and kit can be used for fast high-throughput test and epidemic monitoring in laboratories of HRVA, NVG I, NVG II and HAstV which can cause diarrhea syndromes. The single standard product-based four-color florogenic quantitative PCR method and the kit belong to the biotechnology field.
Owner:GUANGZHOU VIPOTION BIOTECH
Protein complex system for increased immunogenicity and functionality, and methods making and use
ActiveUS20140017269A1Improving immunogenicityImprove functionalitySsRNA viruses negative-senseSsRNA viruses positive-senseEscherichia coliRotavirus RNA
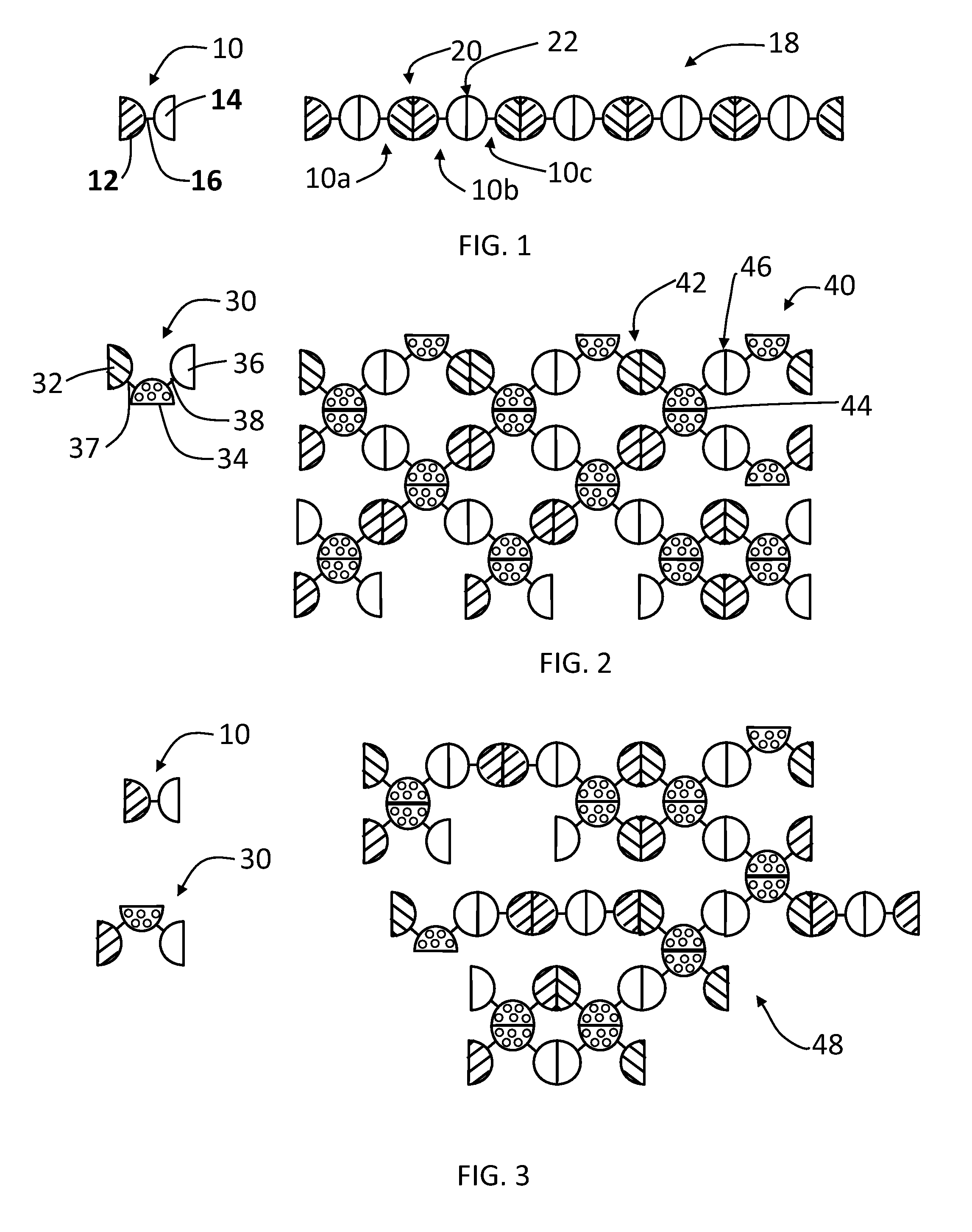
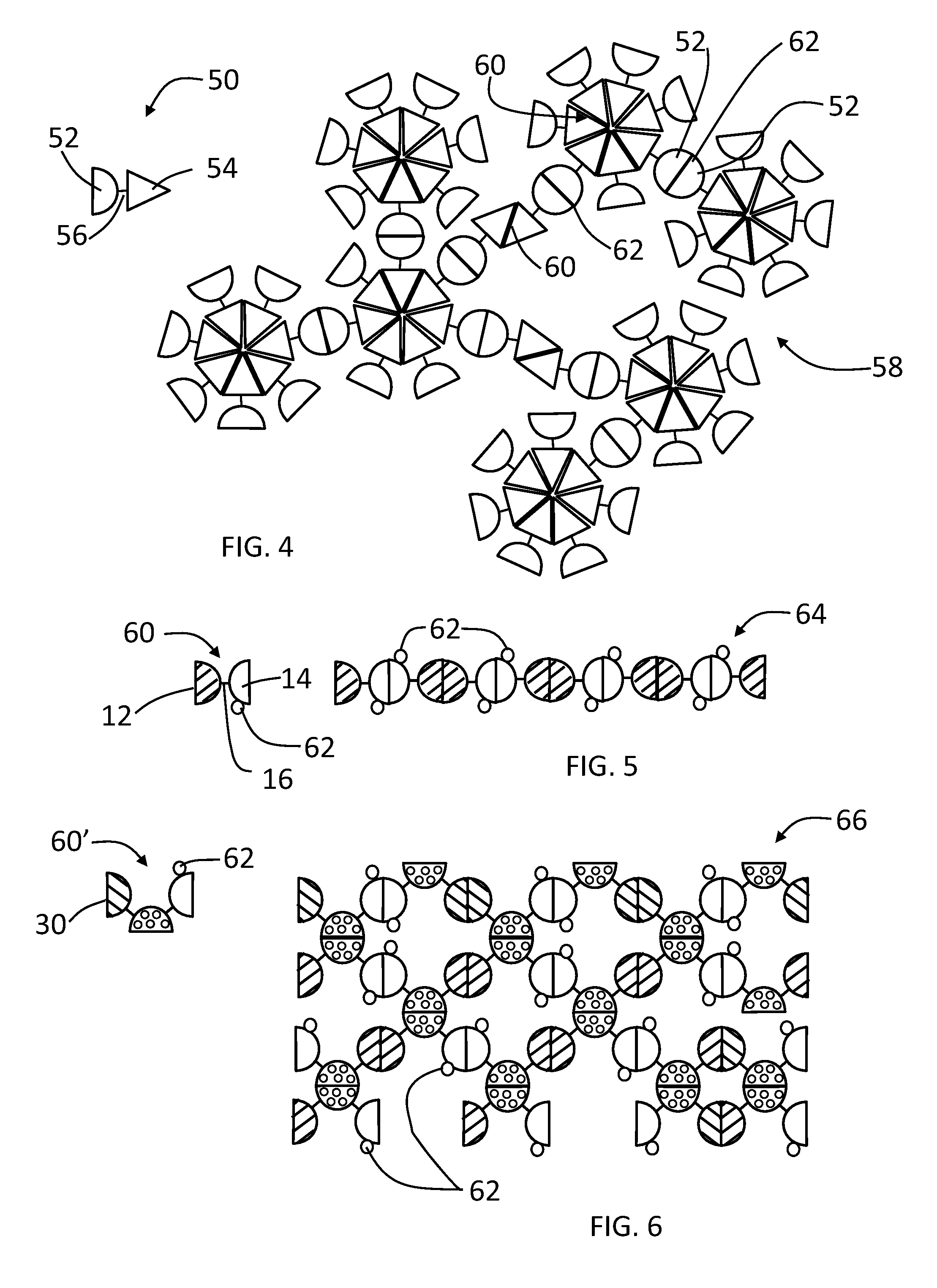
Genes for proteins which spontaneously form dimers and / or oligomers can be recombinantly linked together, which upon expression in E. coli produces stable dimeric fusion proteins that spontaneously self-assemble into enormous, polyvalent complexes having increased immunogenicity and functionality. Linear, network and agglomerate complexes with enormous sizes and polyvalences are constructed using glutathione S-transferase, Norovirus P domains (NoV P− and NoV+), the protruding (P) domain of hepatitis E virus (HEV P), the astrovirus P domain (AstV), a monomeric peptide epitope (M2e of influenza virus), and / or a protein antigen (VP8* of rotavirus) fused in different combinations. The resulting complexes can contain hundreds to thousands NoV P-protein, HEV, AstV, M2e and / or VP8* copies and exhibit higher immunogenicity than the individual proteins alone. The large size and multivalent nature of the complexes are candidates as a bivalent or multivalent vaccines against Norovirus and other pathogens, and for generation of antibodies for diagnosis and research purposes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Preparation method and product of goose astrovirus egg yolk antibody
PendingCN109336971AHigh recovery rateHigh purityEgg immunoglobulinsViral antigen ingredientsCarrageenanEggshell
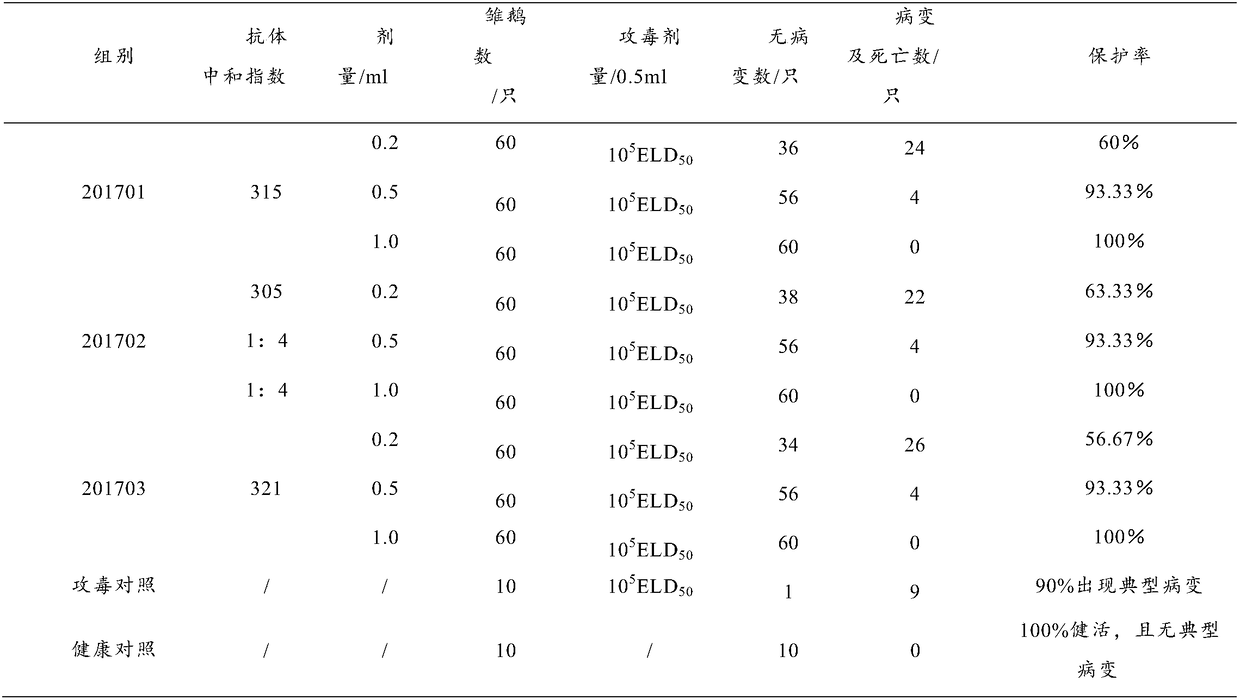
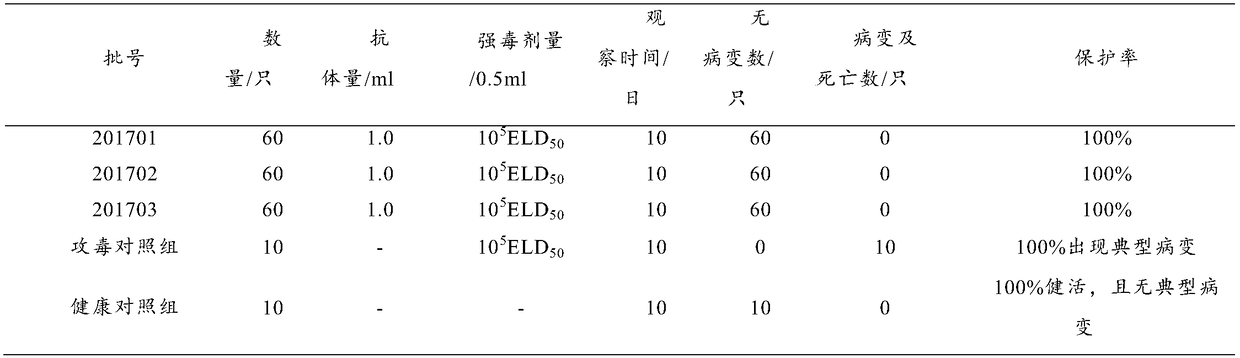
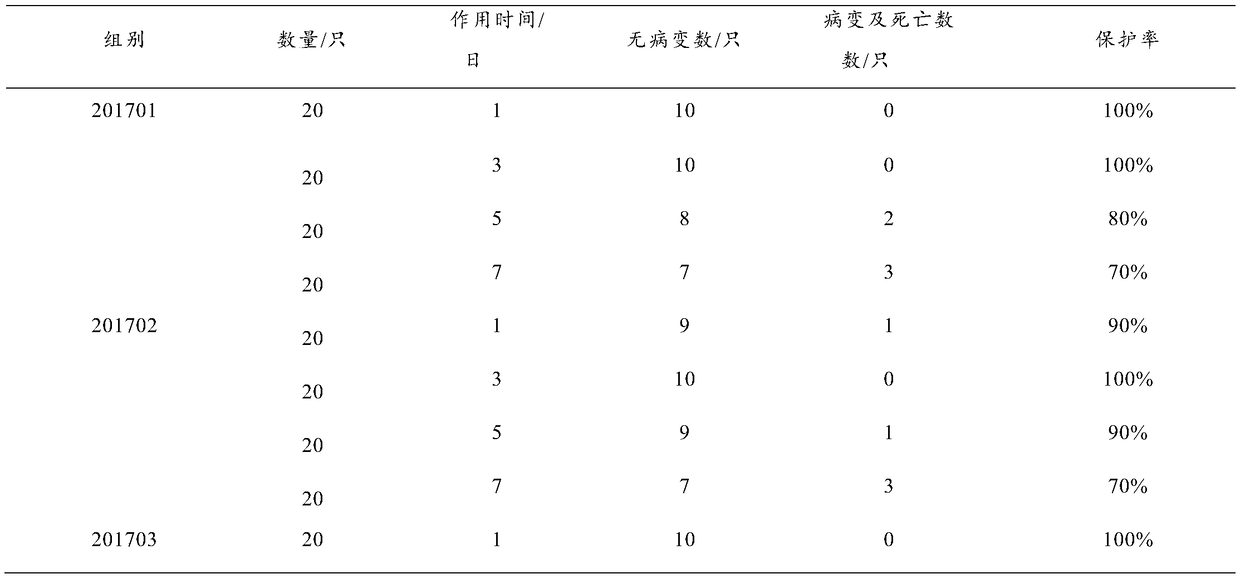
The invention discloses a preparation method and a product of a goose astrovirus egg yolk antibody. The preparation method comprises the following steps: (1) preparing an astrovirus liquid and an adjuvant, using the astrovirus liquid and the adjuvant as immunogens to immunize a healthy laying goose to prepare a highly immunized egg, collecting the highly immunized egg; (2) disinfecting the highlyimmunized egg, collecting an egg yolk; and extracting the egg yolk antibody from the egg yolk by using an acid water-carrageenan method. The recovery rate of the goose astrovirus egg yolk antibody extracted through the preparation method is more than 93%, the purity is more than 95%, and the indexes like the recovery rate, the extracting quantity and the purity of the goose astrovirus egg yolk antibody are obviously better than those of the goose astrovirus egg yolk antibody prepared by using the existing method. The goose astrovirus egg yolk antibody prepared by using the preparation method achieves the better protecting effect only by 0.5 ml of a preventive dose, and the rate of protection against the virulent strain reaches 100%. The infection and the outbreak of the goose astrovirus isprevented specifically, meanwhile, the production process is simplified, and the preparation method can implement large-scale production and is applied clinically.
Owner:哈药集团生物疫苗有限公司
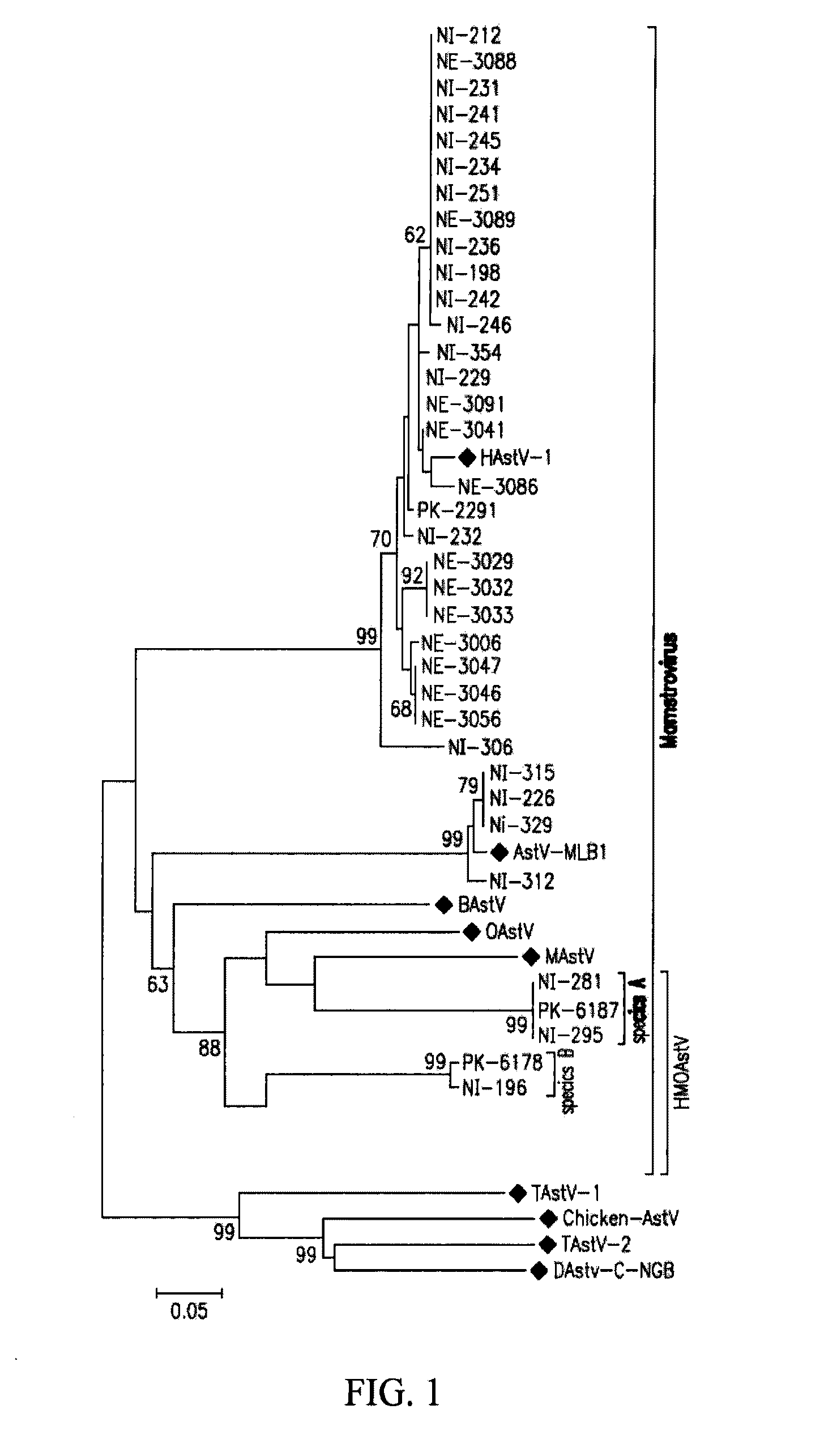
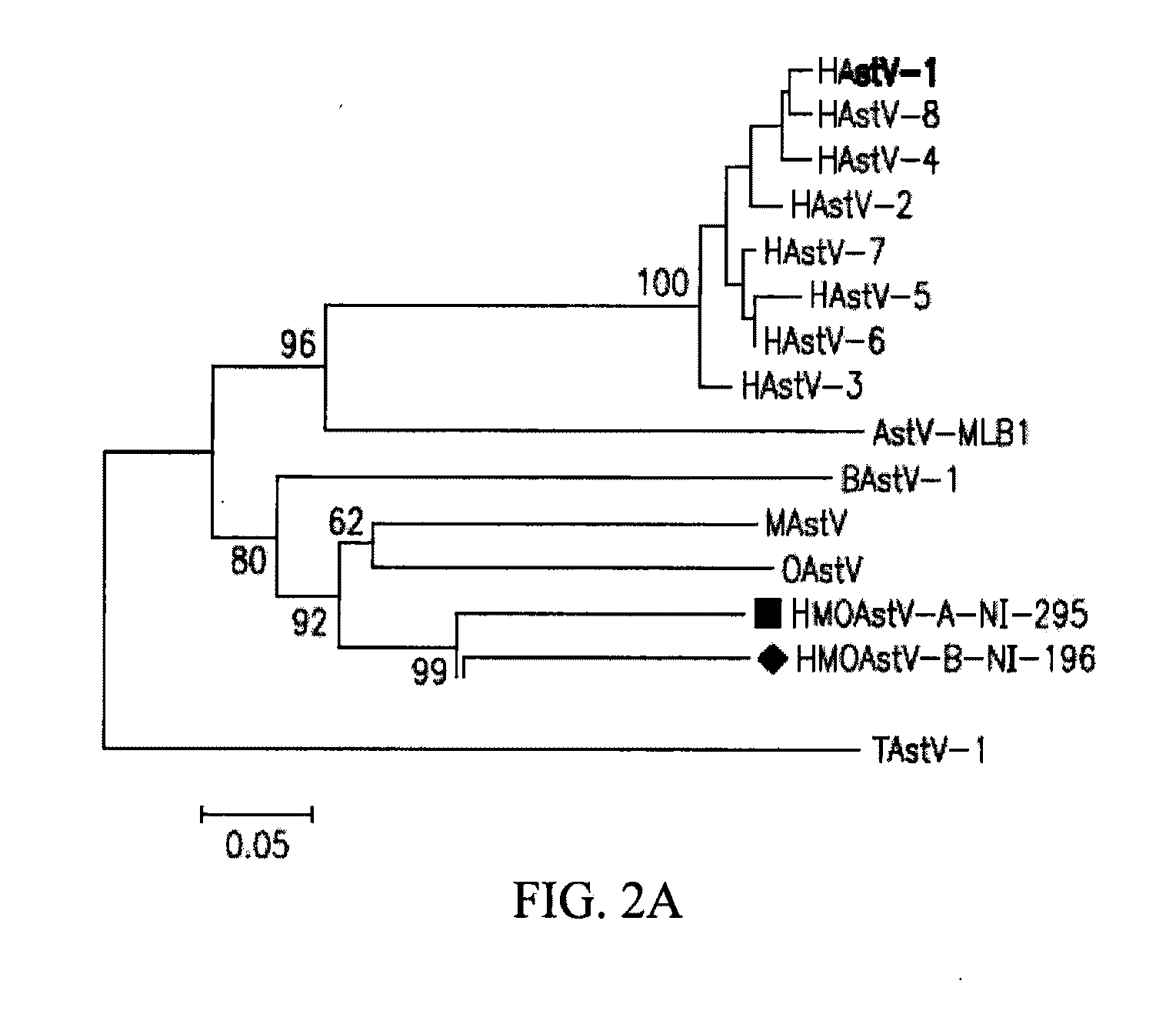
Astrovirus species
Owner:VITALANT
Primer group and kit for simultaneously detecting four diarrhea viruses
InactiveCN104651538AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesRotavirus RNAConserved sequence
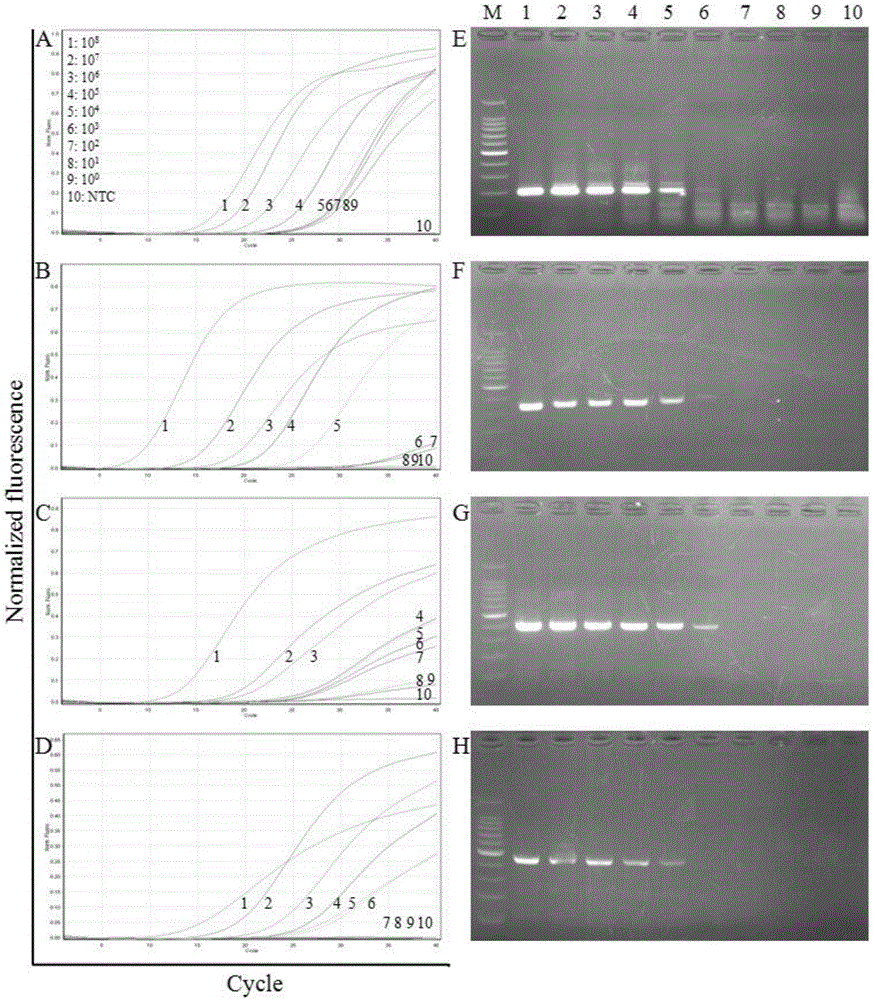
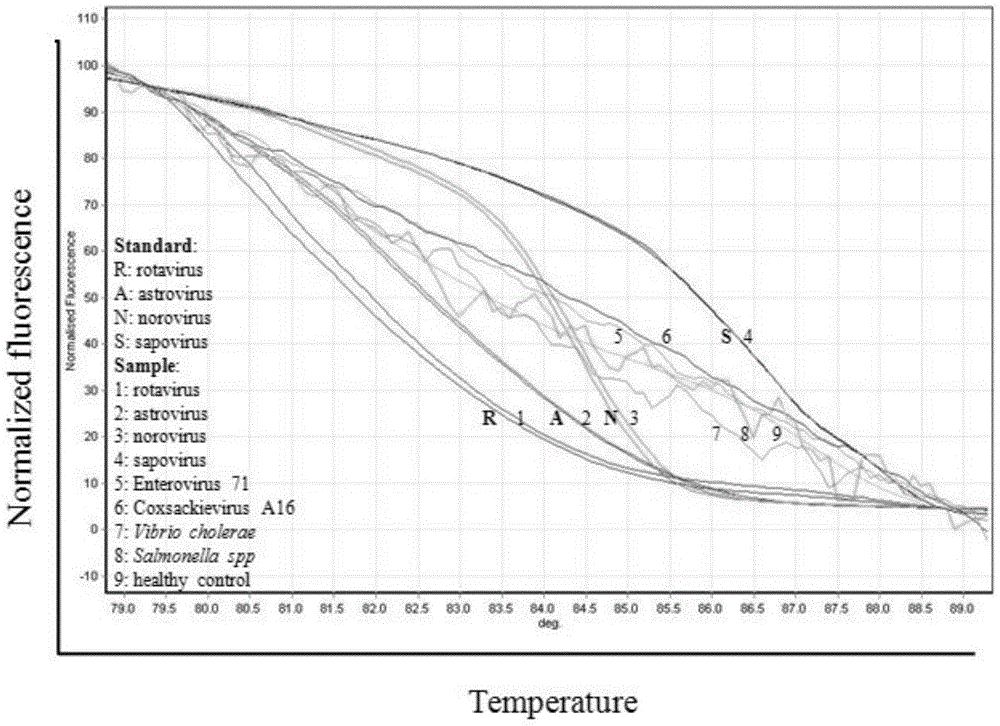
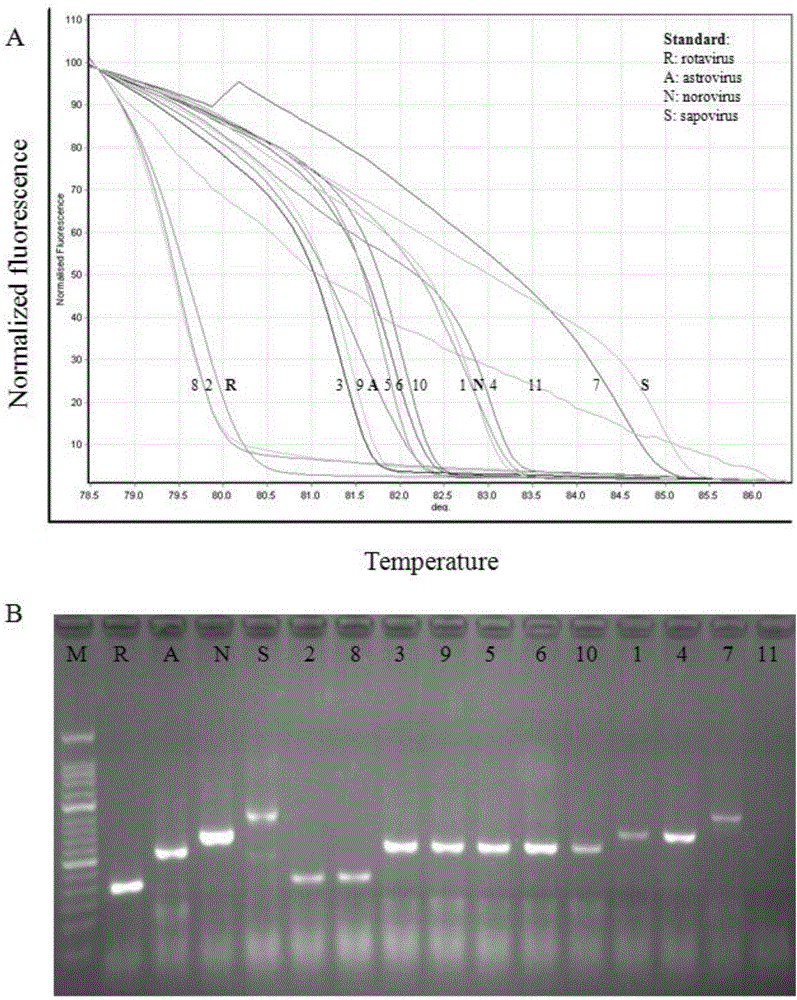
The invention discloses a primer group which is based on a high-resolution melting curve analysis technique and used for simultaneously detecting four diarrhea viruses, and a quadruple detection kit containing primers. The kit is capable of specifically detecting four diarrhea viruses, that is, rotavirus, astrovirus, norovirus and sapovirus. Through comparative analysis on respective homologies of the four viral genomes, respective conserved sequence design primers with high homologies can be found, the four diarrhea viruses can be simultaneously detected by using the high-resolution melting curve analysis technique, the primer group has the characteristics of being simple, convenient, rapid, cheap, safe, high in sensitivity and high in specificity, and powerful technical support can be provided for prevention and control and clinical diagnosis of dysentery.
Owner:珠海国际旅行卫生保健中心
ELISA kit used for detecting goose astrovirus antibodies, and preparation method thereof
The invention relates to an ELISA kit used for detecting goose astrovirus antibodies, and a preparation method thereof. The ELISA kit used for detecting goose astrovirus antibodies comprises an enzymelabeled plate, an enzyme labeled second antibody, goose astrovirus positive reference serum, goose astrovirus negative reference serum, a diluents, 10* washing liquid, TMB substrate solution, and a stopping solution. The antigen used for coating the enzyme labeled plate is goose astrovirus Cap protein; the goose astrovirus is Avastrovirus III type. According to the ELISA kit, expression purifiedgoose astrovirus (Avastrovirus III type) Cap protein is taken as the coating antigen; indirect ELISA method is adopted in detection of goose serum samples to be detected; and the kit used for detecting goose astrovirus antibodies in serum is assembled. The ELISA kit can be used for goose astrovirus infection serology diagnosis and antibody level monitoring, and epidemiological investigation, and asimple and rapid method is provided for goose astrovirus prevention and treatment.
Owner:YANGZHOU UNIV
Chicken Virus Vaccine and Diagnostic
ActiveUS20090092634A1Good curative effectEasy to useBiocideSsRNA viruses positive-senseNucleotideNucleotide sequencing
A novel astrovirus designated chicken astrovirus type 3 has been isolated and characterised. Nucleotide sequences and polypeptide sequences of this astrovirus are provided with uses of the same and the isolated astrovirus in assay kits and vaccines.
Owner:QUEENS UNIV OF BELFAST
Methods and microarrays for detecting enteric viruses
InactiveUS20090136916A1Better diagnose gastrointestinal illnessRapid and reliable assaySugar derivativesMicrobiological testing/measurementAssayNucleic acid sequencing
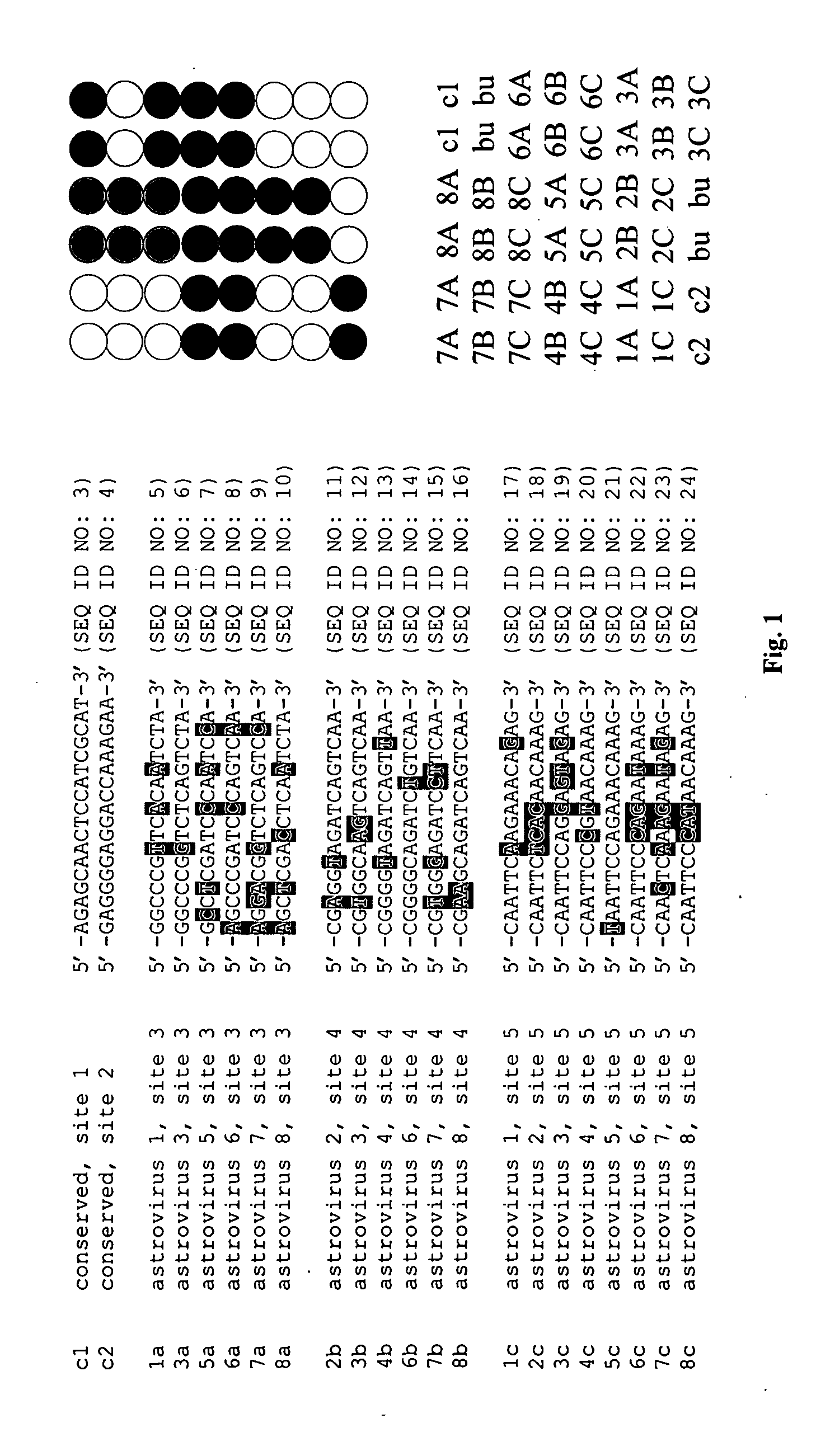
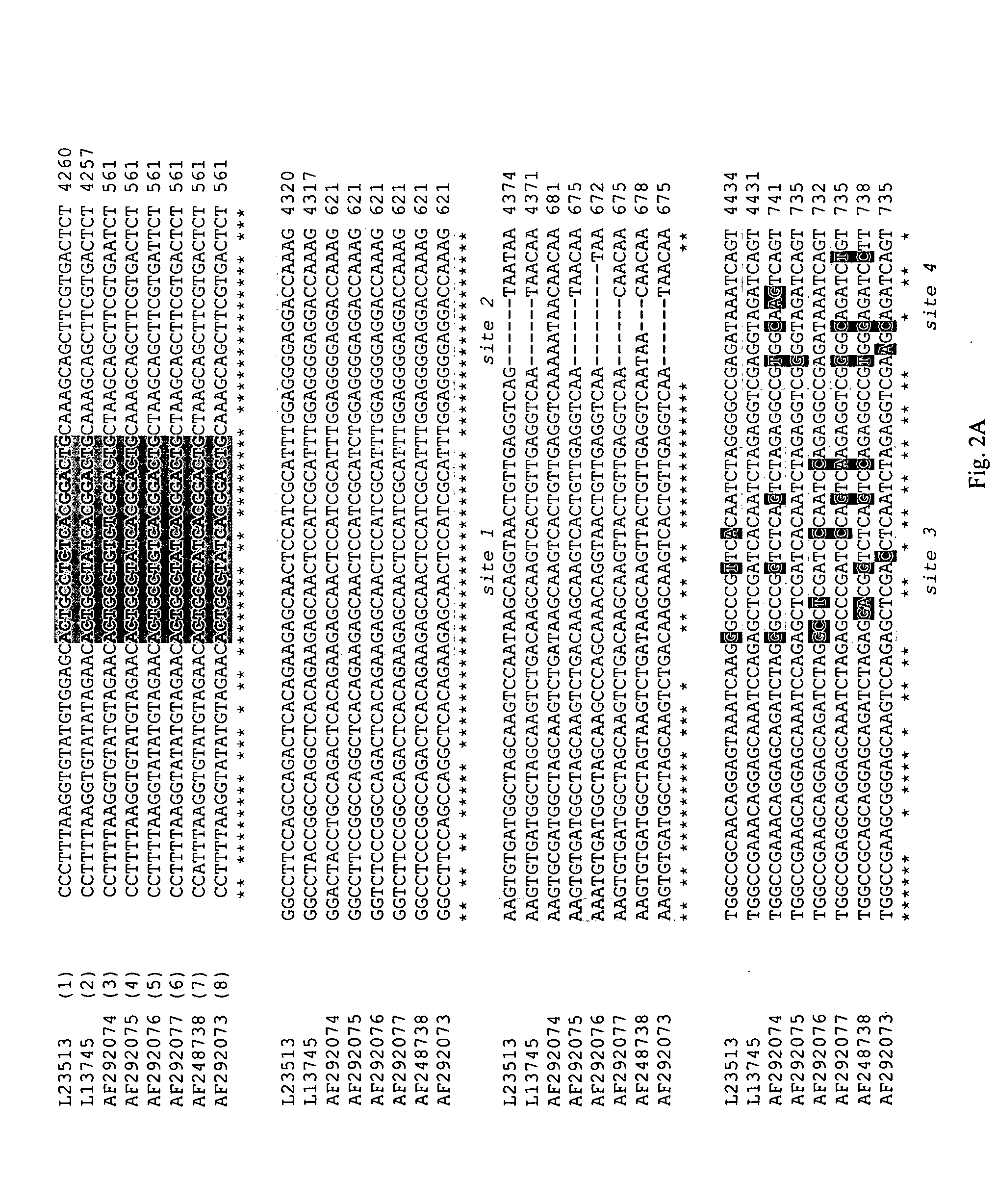
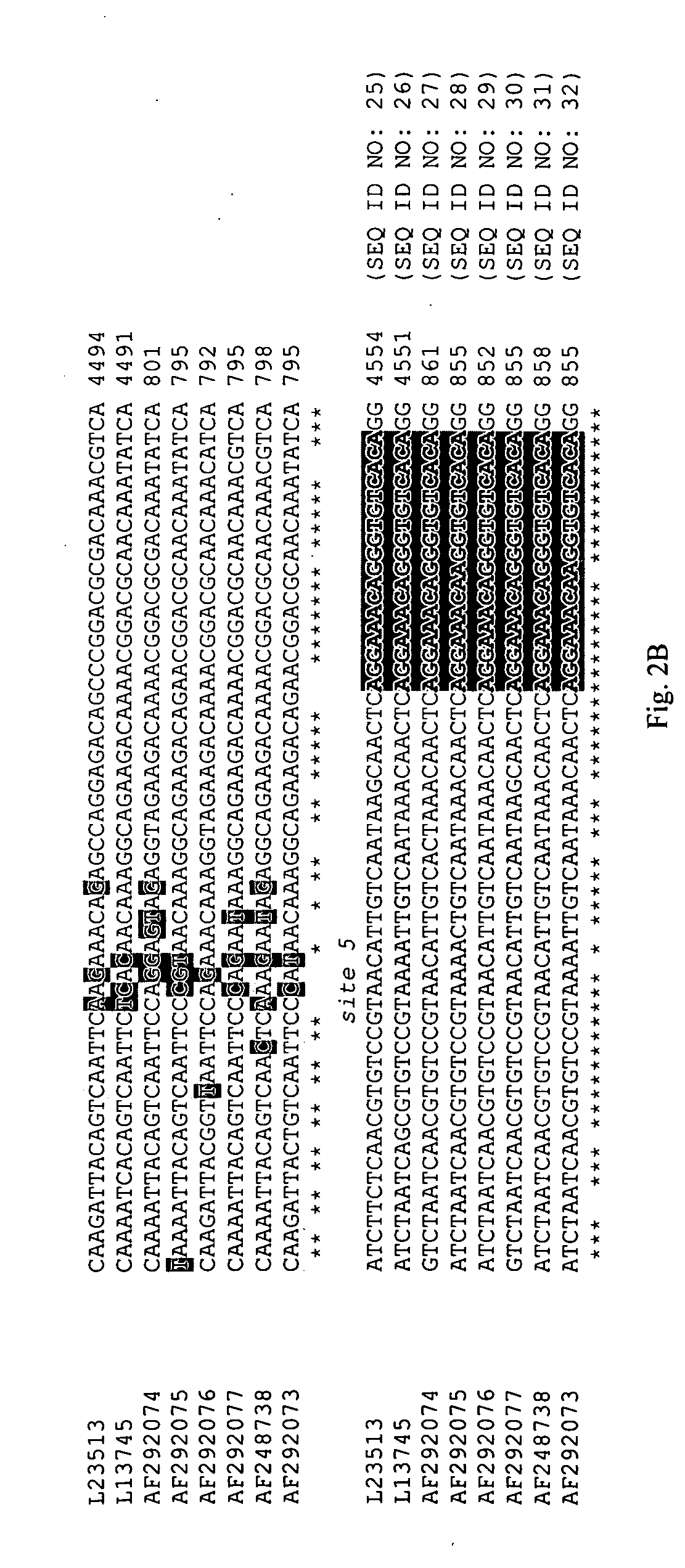
The present invention relates to methods, microarrays and kits for detecting one or more human astrovirus serotypes in a sample (e.g., a fecal sample) from an individual. The method includes amplifying nucleic acid molecules of the sample with one or more primers, to thereby obtain an amplified nucleic acid product; contacting the amplified nucleic acid product with one or more serotype specific probes having a nucleic acid sequence that is specific for only one astrovirus serotype in the group of astroviruses being assessed, wherein the nucleic acid sequence includes between about 9 and 25 nucleic acid bases (e.g., SEQ ID NO: 5-24); and detecting the hybridization complex. The presence of hybridization complexes with a serotype specific probe indicates the presence of one or more specific astrovirus serotypes, and the absence of hybridization complexes with a serotype specific probe indicates the absence of the specific astrovirus serotype. Identification of the astrovirus serotypes allows for one to diagnose an individual infected with the serotype. The present invention further includes microarrays having any one of the astrovirus specific probe, or kits having microarrays and reagents for carrying out the assay.
Owner:TRUSTEES OF TUFTS COLLEGE
Detection primer, probe and detection method of human astrovirus nucleotide
InactiveCN103014180AGood primer specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention discloses a detection primer, a probe and a detection method of human astrovirus nucleotide, belonging to the technical field of biological detection. The detection primer of the human astrovirus nucleotide comprises a forward primer and a reverse primer, the nucleotide sequences of which are shown as SEQ NO.1 and SEQ NO.2. A nucleotide sequence of the probe which is matched with the detection primer is shown as SEQ NO.3; and one end of the probe is signed with a report fluorescent dye, and the other end of the probe is signed with a quenching fluorescent dye. The detection method of the human astrovirus nucleotide comprises the following steps of: carrying out real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification by taking a sample RNA (Ribonucleic Acid) to be detected as a template and utilizing the forward primer, the reverse primer and the probe, collecting data after every one circulation is finished, and judging a result according to an amplification curve after the reaction is finished. The primer designed according to a human astrovirus genomic sequence is good in specificity and is high in sensitivity when being used for real-time fluorescent RT-PCR detection.
Owner:SOUTH CHINA UNIV OF TECH
Goose-origin kidney-type astrovirus GoAstV rapid diagnosis primer probe group, detecting method and application
InactiveCN108823333AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceBasic research
The invention belongs to the technical field of the gene detection, and particularly relates to a goose-origin kidney-type astrovirus GoAstV rapid diagnosis primer probe group, a detecting method andapplication. The goose-origin kidney-type astrovirus GoAstV rapid diagnosis primer probe group comprises probe primer groups in the following sequences: GoAstV-F: 5' TGGTGGTGGTGCGGTTTT 3'; GoAstV-R: 5' GGGCAACGTACCATCATAACG 3'; and a TaqMan MGB probe: 5' FAM TGTAGAGACGGACTGGAC MGB 3'. The 5' end is marked as a fluorescence group by the probe, and the 3' end is marked as a quenching fluorescence group. The provided primer probe group has the advantages of higher sensibility, specificity, good repeatability, rapidness, high flux, less pollution and the like, and is used as a useful and powerfultechnology in the fields of a fundamental research, an application research and the like.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI SHANDONG SPECIFIC PATHOGEN FREEN CHICKS RES CENT
Protein complex system for increased immunogenicity and functionality, and methods making and use
ActiveUS9562077B2Improving immunogenicity functionalityEasy to produceSsRNA viruses negative-senseSsRNA viruses positive-senseEscherichia coliRotavirus RNA
Genes for proteins which spontaneously form dimers and / or oligomers can be recombinantly linked together, which upon expression in E. coli produces stable dimeric fusion proteins that spontaneously self-assemble into enormous, polyvalent complexes having increased immunogenicity and functionality. Linear, network and agglomerate complexes with enormous sizes and polyvalences are constructed using glutathione S-transferase, Norovirus P domains (NoV P− and NoV+), the protruding (P) domain of hepatitis E virus (HEV P), the astrovirus P domain (AstV), a monomeric peptide epitope (M2e of influenza virus), and / or a protein antigen (VP8* of rotavirus) fused in different combinations. The resulting complexes can contain hundreds to thousands NoV P-protein, HEV, AstV, M2e and / or VP8* copies and exhibit higher immunogenicity than the individual proteins alone. The large size and multivalent nature of the complexes are candidates as a bivalent or multivalent vaccines against Norovirus and other pathogens, and for generation of antibodies for diagnosis and research purposes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Astrovirus
This invention relates to the isolation and uses of novel avian astroviruses. The present invention also relates to vaccines, kits and methods for detection of a novel astrovirus. The present invention further relates to vaccination of avians, prevention and / or treatment of avian infections associated with astrovirus. Infections of poultry by this novel astrovirus are associated with runt stunting syndromes.
Owner:BIOMUNE
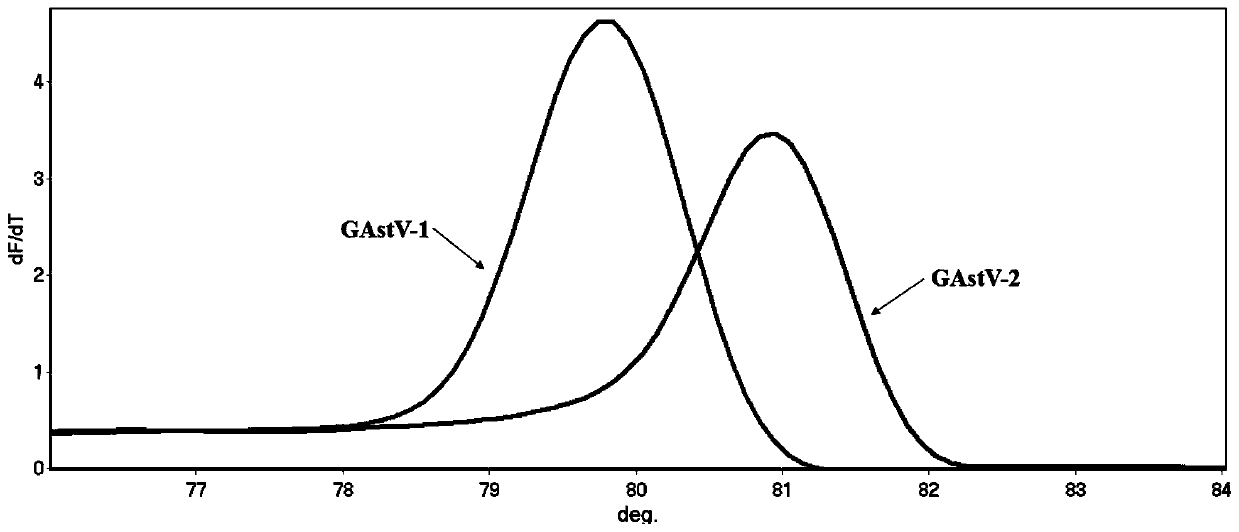
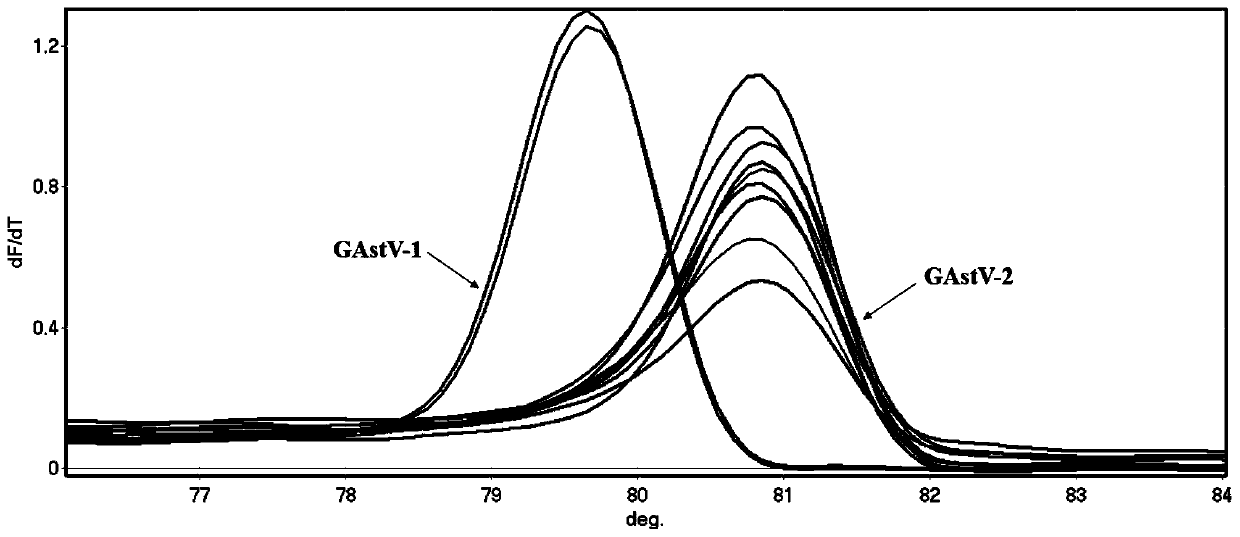
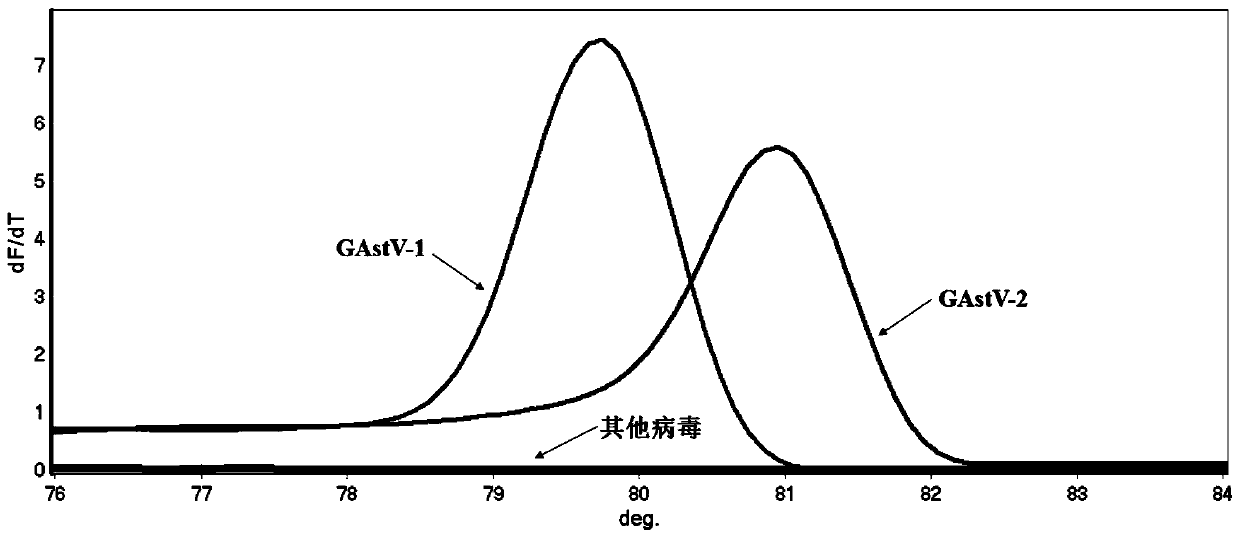
HRM detection method for quickly distinguishing goose type I astrovirus from goose type II astrovirus and primer of HRM detection method
ActiveCN110241259AQuick analysisEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesHigh fluxBiology
The invention discloses an HRM detection method for quickly distinguishing goose type I astrovirus from goose type II astrovirus and a primer of the HRM detection method. The HRM detection method disclosed by the invention is simple to operate, only a pair of universal primers is needed, and before a PCR reaction, fluorescence saturable dye is added. The detection speed is high and the flux is high, and time for subtyping is shortened; a specific fluorescence labelling probe is not needed; the specificity is high, the accuracy is high, samples can be analyzed in a quick, accurate and high-flux manner, popularization and application of the HRM detection method in clinical practice are facilitated, and the HRM detection method has higher application value.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) detection kit for human astrovirus and application thereof
ActiveCN103436638AEasy to judgeAvoid cross reactionMicrobiological testing/measurementFluorescence/phosphorescenceQualitative analysisVirus
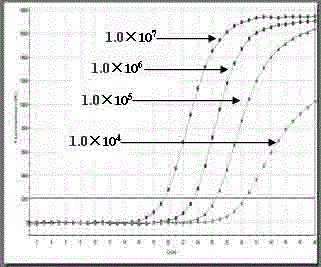
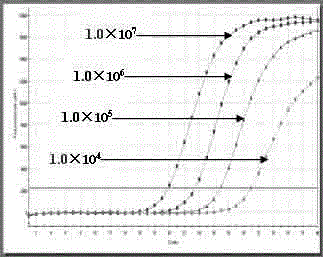
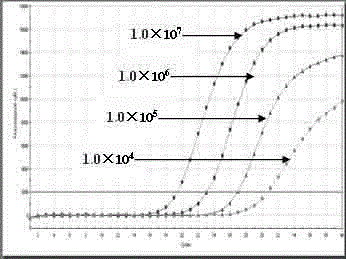
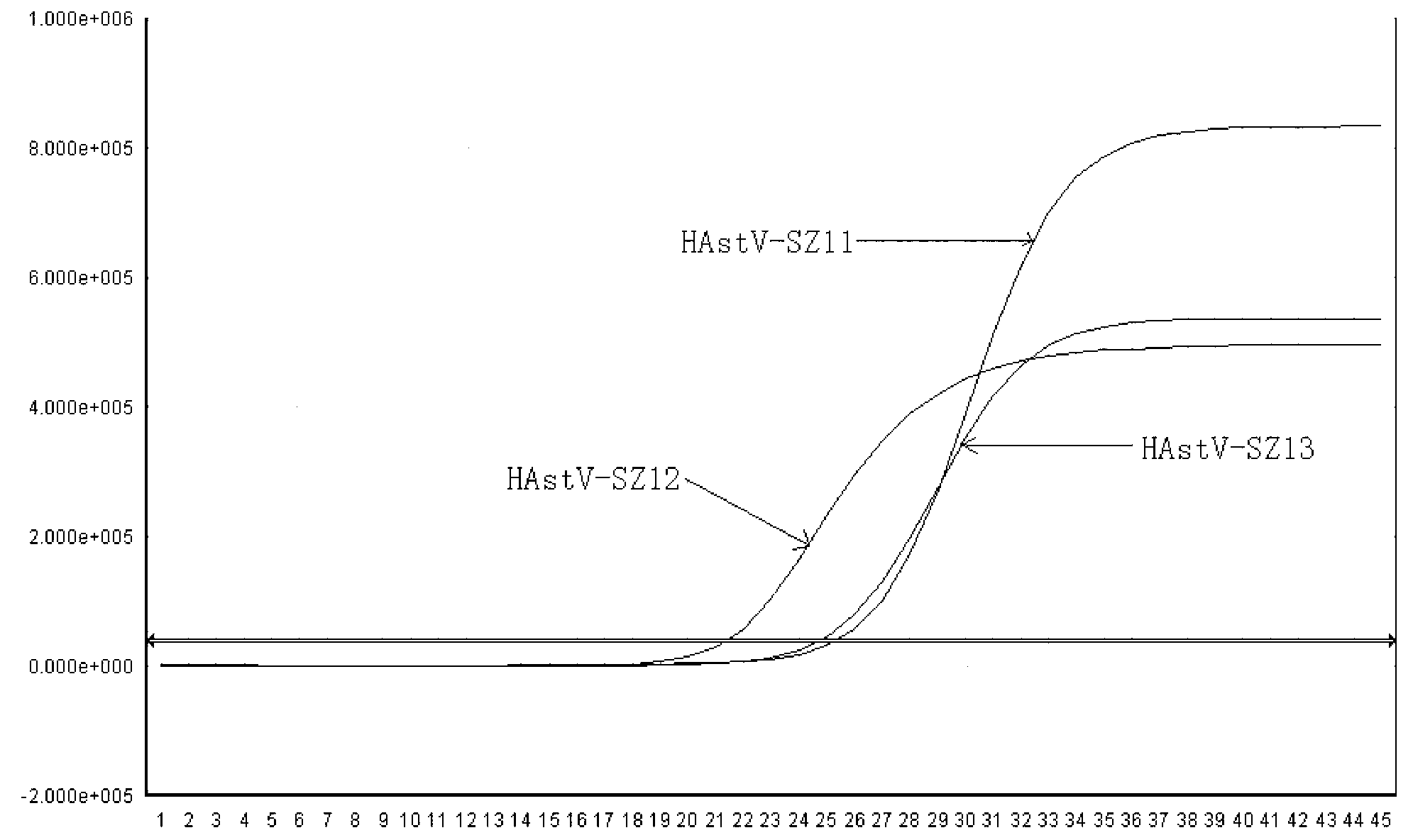
The invention belongs to the field of gene detection, and relates to a real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) detection kit for human astrovirus and application thereof. The kit provided by the invention has very high sensitivity and specificity. The kit provided by the invention implements quick early detection and quantitative analysis on human astrovirus in excrement, anus swab or any other sample. The invention has the advantages of short detection period, high efficiency, high specificity and accuracy for detecting virus, and favorable repetitiveness of experimental results, can simultaneously perform qualitative analysis and quantitative analysis on virus, and is simple to operate and easy to popularize; and the kit can detect the virus with the lowest concentration of 1.0*10<2> copies / mL, and thus, has higher sensitivity than a common PCR (polymerase chain reaction) and immunologic detection method.
Owner:湖北朗德医疗科技有限公司
Astrovirus species
Owner:VITALANT
Kit, primer probe composition and method for jointly detecting multiple enteroviruses
PendingCN114292956AImprove featuresIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesEnterovirusAvastrovirus
The invention discloses a kit, a primer probe composition and a method for jointly detecting various enteroviruses. The kit comprises a first nucleic acid reaction solution and a second nucleic acid reaction solution, and in the first nucleic acid reaction solution, nucleotide sequences of a primer pair and a probe of a norovirus GI group are sequentially shown as SEQ ID NO.1-3; the nucleotide sequences of the primer pair and the probe of the norovirus G2 group are as shown in SEQ ID NO.4-6 in sequence; the nucleotide sequences of the primer pair and the probe of the fiveleaf akebia virus are shown as SEQ ID NO.7-9 in sequence; in the second nucleic acid reaction liquid, the nucleotide sequences of the primer pair and the probe of the rotavirus group A are shown as SEQ ID NO.10-12 in sequence; the nucleotide sequences of the primer pair and the probe of the rotavirus B group are sequentially shown as SEQ ID NO.13 to SEQ ID NO.15; the nucleotide sequences of the primer pair and the probe of the astrovirus are as shown in SEQ ID NO. 16-18 in sequence. The kit can be used for jointly detecting norovirus, fiveleaf virus, rotavirus and human astrovirus, and has relatively high specificity and sensitivity.
Owner:HEMOSMART MEDICAL TECH LTD
Probe for detecting virus
ActiveUS20170356912A1Low costSsRNA viruses positive-senseMicrobiological testing/measurementDNA microarrayCoxsackievirus
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOPERATION FOUND
A kind of kit and detection method for accurate and quantitative detection of astrovirus
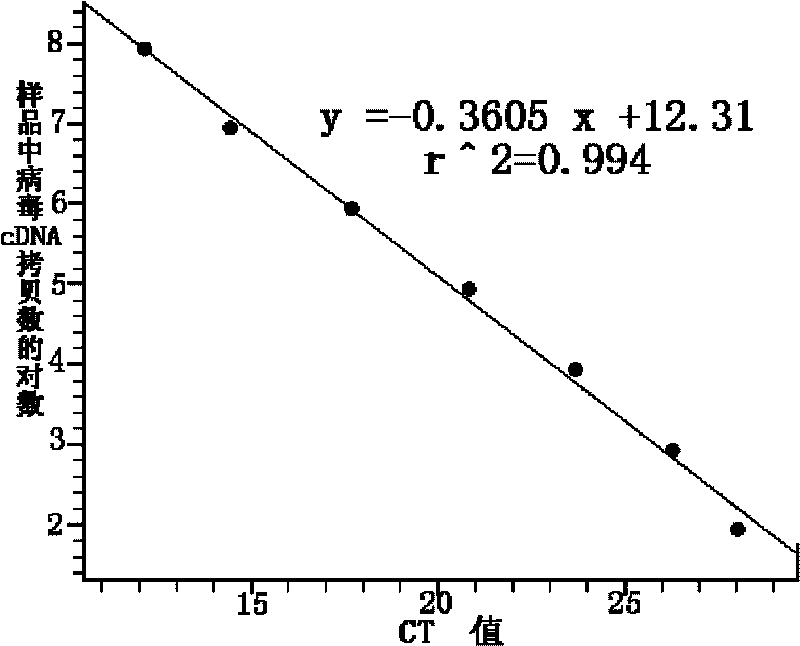
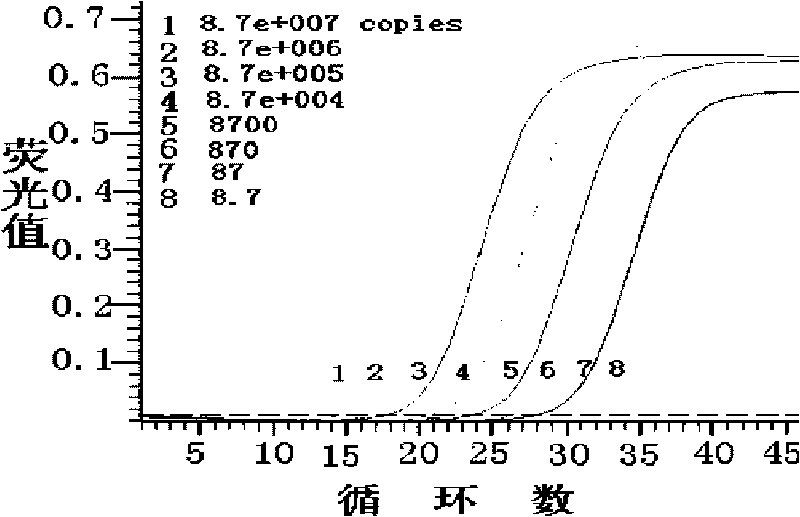
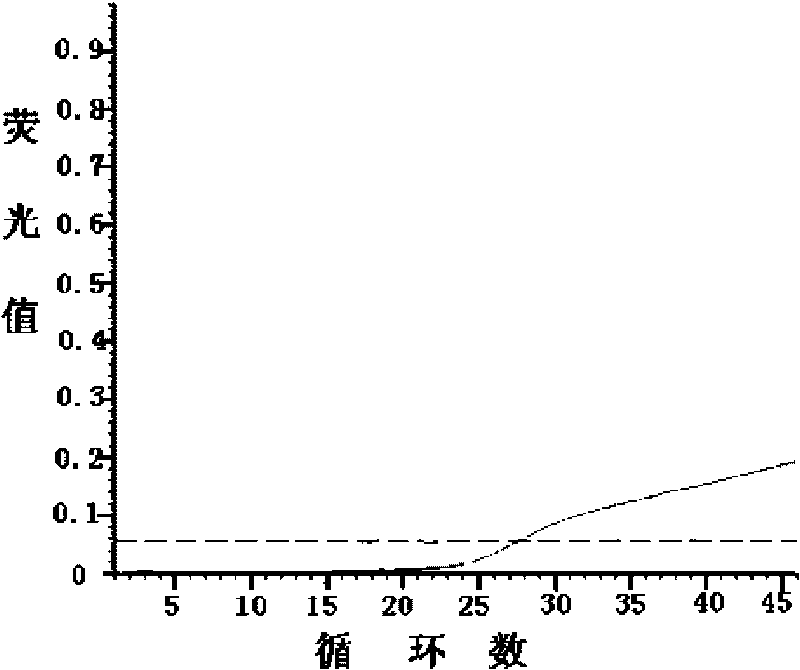
ActiveCN106119416BImprove tolerancePrevent deviationMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyPcr ctpp
The invention discloses a kit and a detection method for accurately and quantitatively detecting astrovirus and particularly relates to a group of primers and probes for detecting the astrovirus as well as the kit containing the primers and the probes and a detection method of the astrovirus, wherein the primers and the probes have nucleotide sequences shown in SEQ ID No. 1 to SEQ ID No. 3 in a sequence table. The invention provides the detection method for accurately and quantitatively detecting the astrovirus by using a droplet digital PCR (Polymerase Chain Reaction) technology; the method does not need to rely on certified reference materials or other standard substances, and has higher accuracy, sensitivity and repeatability, and is easy to standardize.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
Diarrhea virus detection kit and method
ActiveCN103215379BImprove detection efficiencySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceRotavirus RNAFluorophore
The invention is applicable to the technical field of biotechnology and medical detection and provides a diarrhea virus detection kit and method. The detection kit comprises PCR (polymerase chain reaction) liquid which comprises a PCR buffer solution, MgCl2, dNTP, DNA polymerase, primer pairs, probe sequences and Homo-tag, wherein the primer pairs and the probe sequences respectively aim at type I, type II and type IV of sapovirus, type I and type II of norovirus, rotavirus, adenovirus and astrovirus, the 5' ends of the probe sequences are connected with fluorophore, and the 3' ends of the probe sequences are connected with quencher. The detection kit provided by the invention can detect seven viruses (subtype) related to diarrhea at one time, and compared with a detection method in the prior art, the detection method provided by the invention has the advantages that operation is simple and convenient, a result is quick to read, and false positive is avoided.
Owner:SHENZHEN CENTER FOR DISEASE CONTROL AND PREVENTION (SHENZHEN HEALTH INSPECTION CENTER SHENZHEN INSTITUTE OF PREVENTIVE MEDICINE)
Gene chip and reagent box for detecting food-borne virus
InactiveCN101328505BGuaranteed reproducibilityGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesFood borneRotavirus RNA
The invention relates to a gene chip for detecting food-home virus and a kit, belonging to the inspection field. The surface of a solid phase carrier is fixed with a plurality of detection probes and quality control contrast probes, wherein, the detection probes consist of the probes for detecting hepatitis A virus, human astrovirus, norwalk virus G1, norwalk virus G2, rotavirus, polio virus 1, polio virus 2 and polio virus 3; and the quality control contrast probes consist of a spotting positive quality control probe, a chip hybridization positive quality control probe and a chip negative quality control probe. The gene chip and the kit have the advantages of: (1) high throughout: five common viruses are integrated and detected simultaneously, and the practicability is strong; (2) rapidness: the detection time is only 4 hours; (3) specificity: the false positive caused by the cross reaction is avoided; (4) flexibility: the detection flexibility of the chip is 10<8> virus particles per gram of the tissue sample, which is higher than that of RT-PCR; and (5) good repetitiveness and stable results. The gene chip and the kit can be widely applied to the food safety inspection of the inspections system.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Diarrhea-associated virus multiple gene detection system and its kit and application
ActiveCN107164562BOptimize the reaction systemGuaranteed accuracyMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingMultiplex
The invention relates to a diarrhea-associated virus multiple gene detection system, a kit and application thereof. The detection system has a total of 9 pairs of primers, including 6 pairs of diarrhea virus, 2 pairs of human genome internal reference and 1 pair of system quality control internal reference primers. Diarrhea pathogens are respectively targeting human astrovirus, norovirus II, human enteric adenovirus and rotavirus, etc. The diarrhea pathogen multiple detection system and its kit of the present invention do not need conventional detection, and can directly perform synchronous detection and analysis of various diarrhea-related viruses on stool samples in the same reaction system, making up for the low throughput and consumption of conventional detection methods. Time-consuming and other shortcomings, it provides a comprehensive, accurate, and low-cost etiological diagnosis for the clinic at the first time, and provides an important reference for individualized drug use and precision medicine.
Owner:HUADONG HOSPITAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com