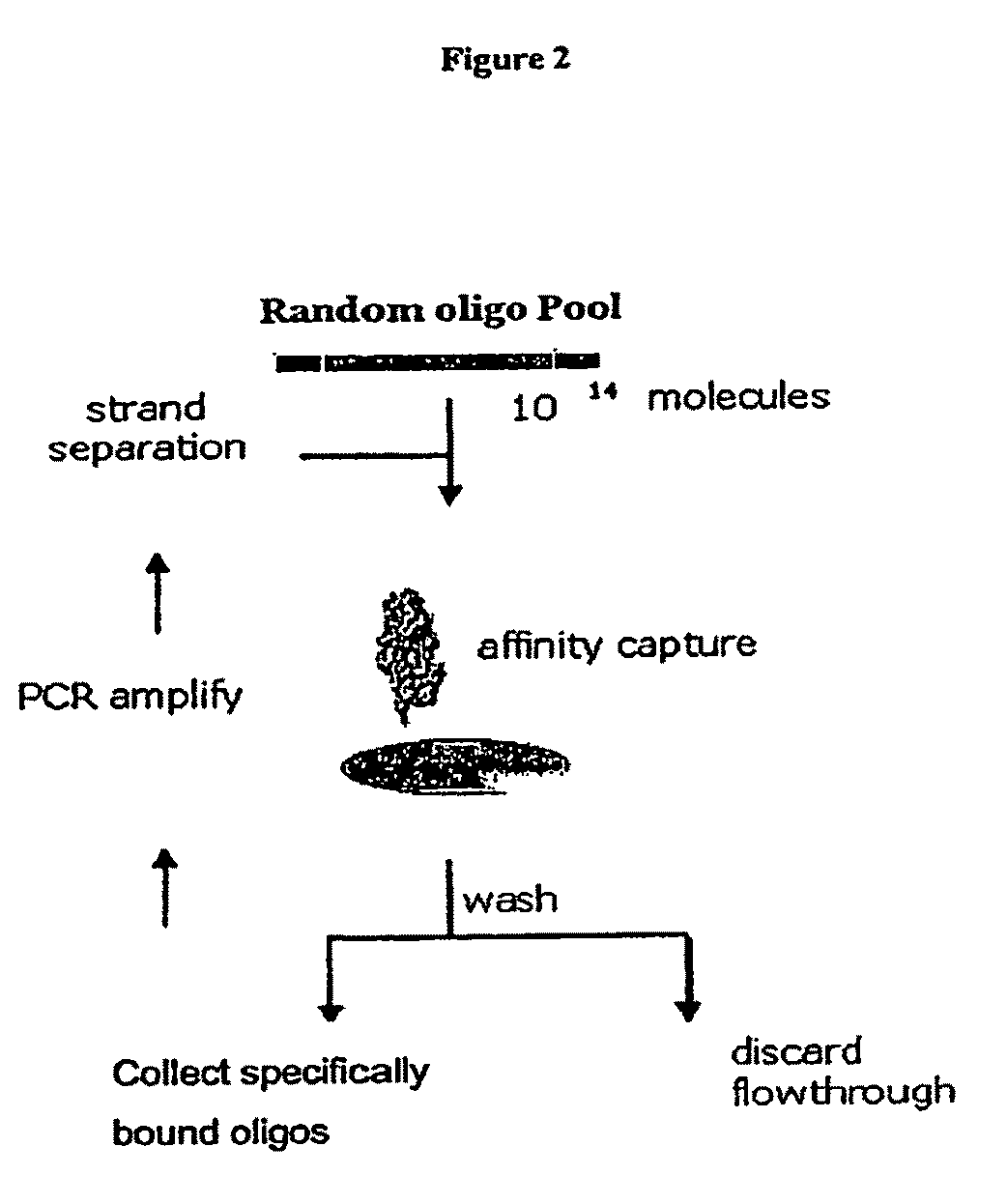
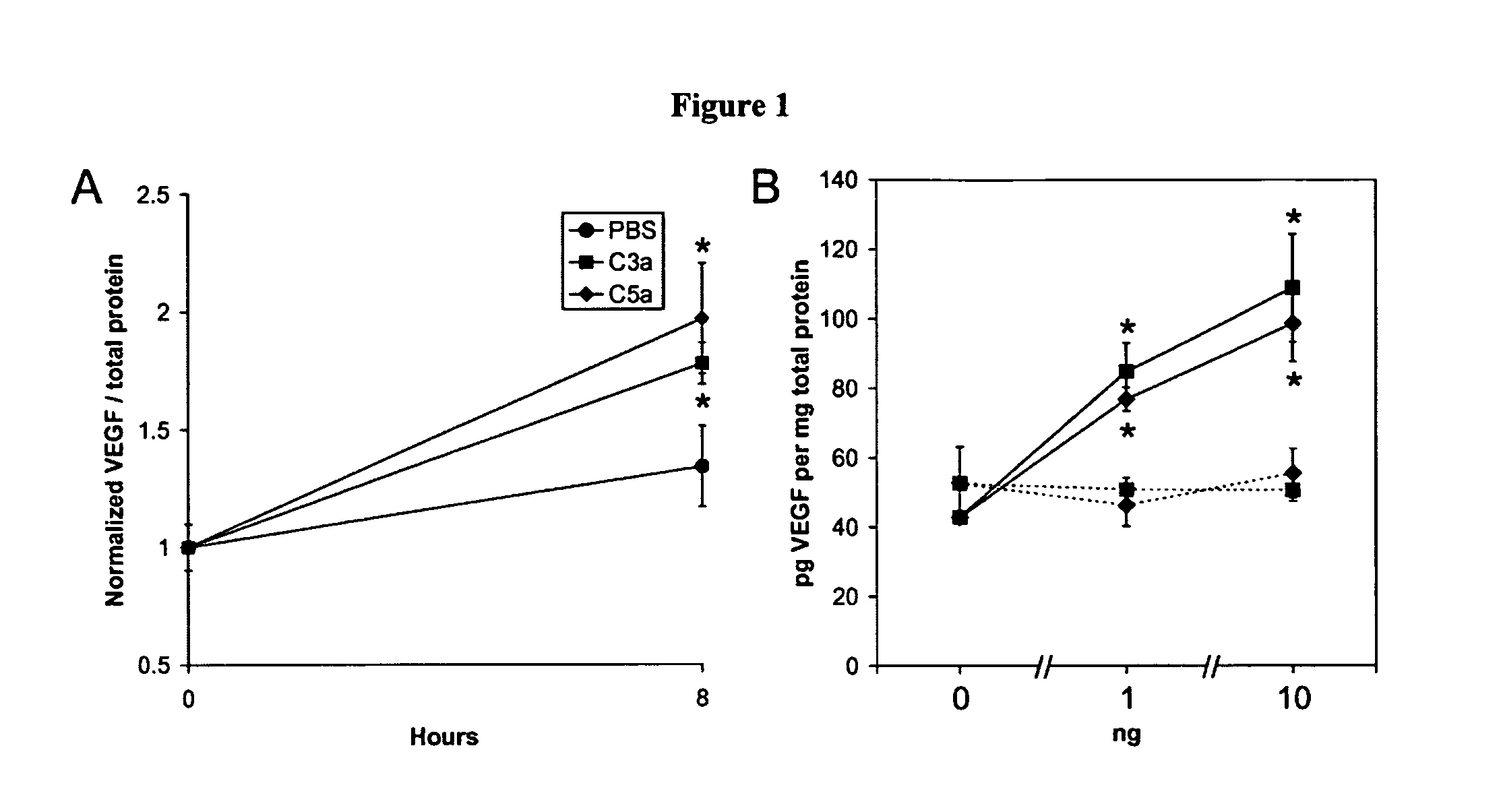
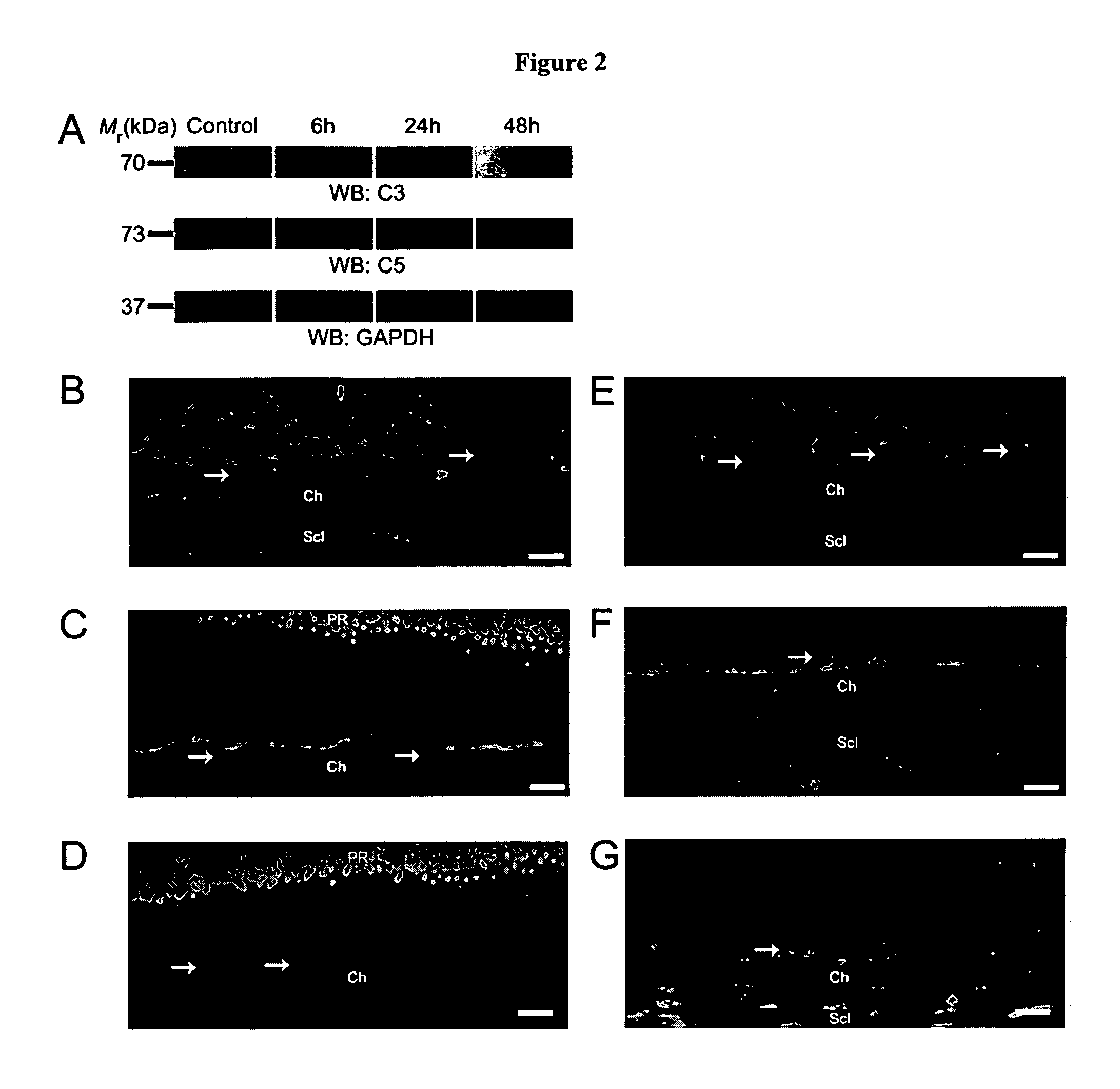
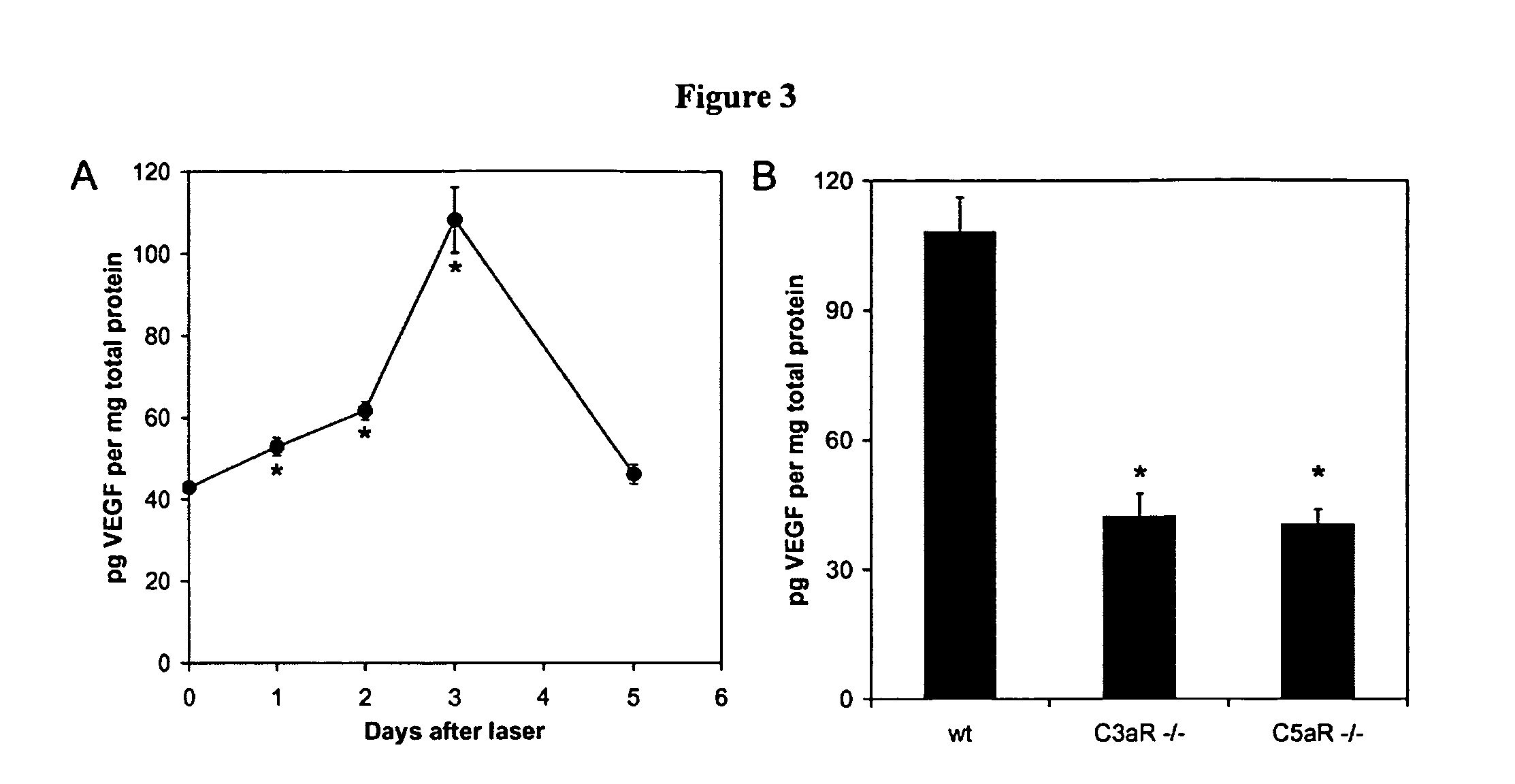
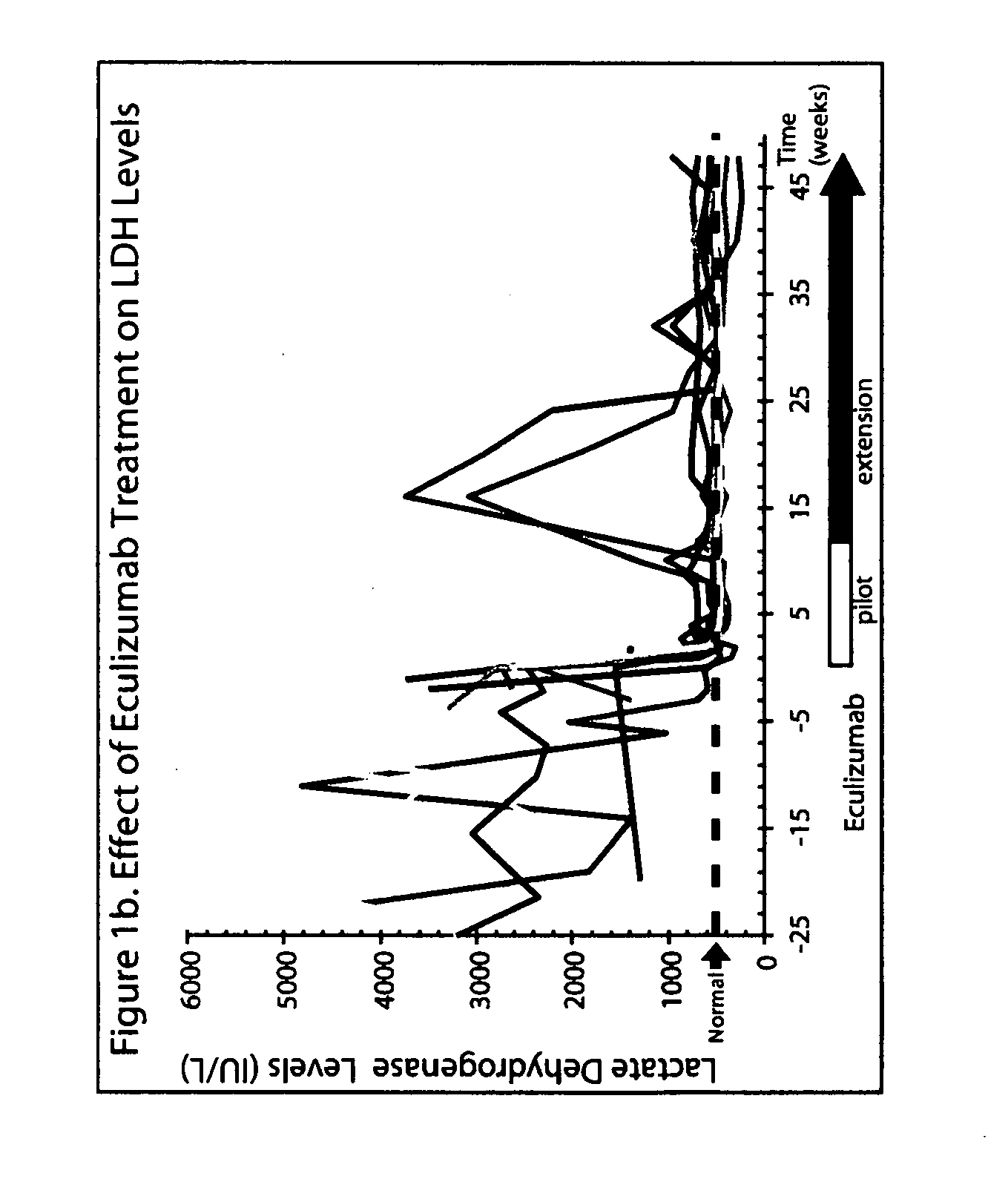
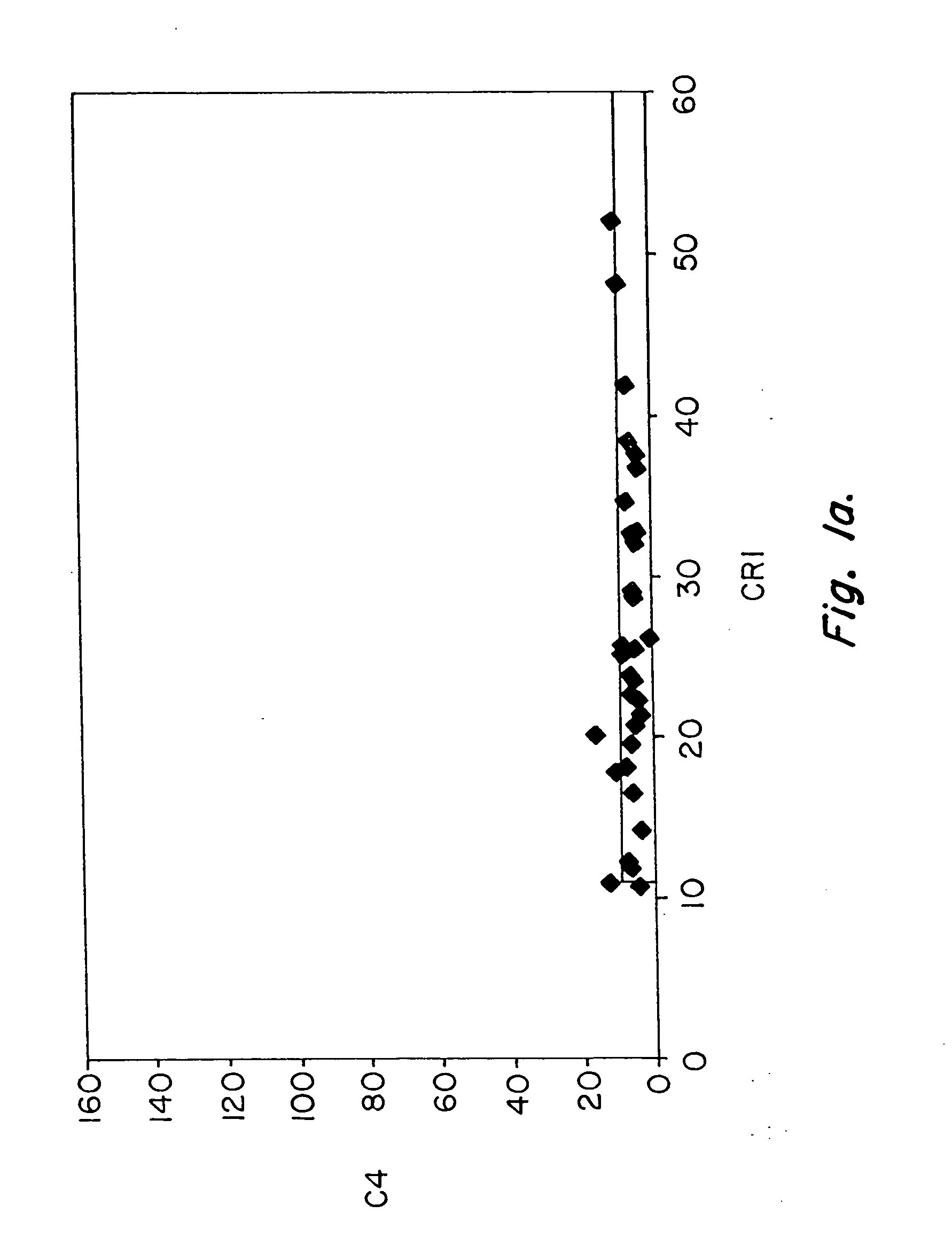
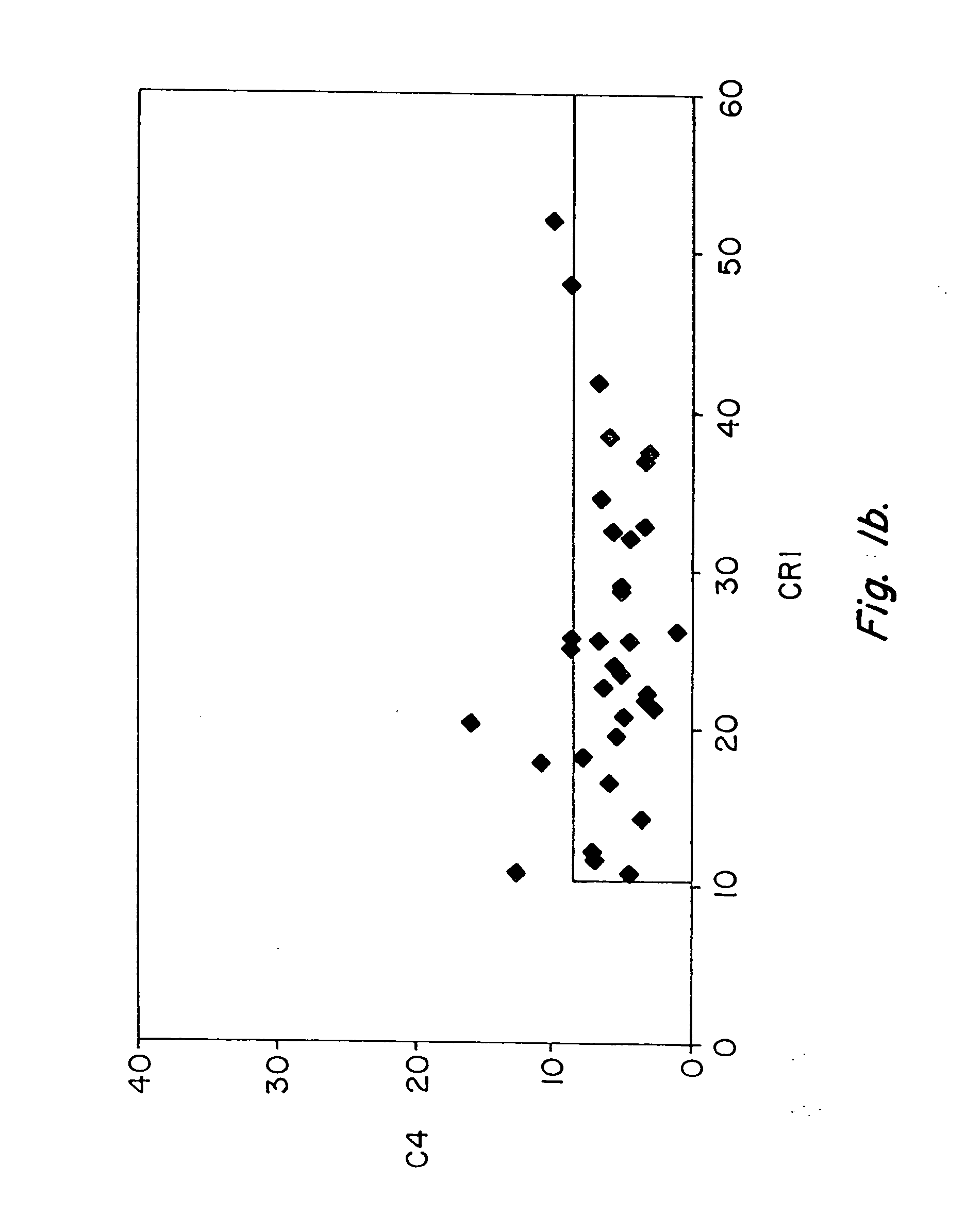
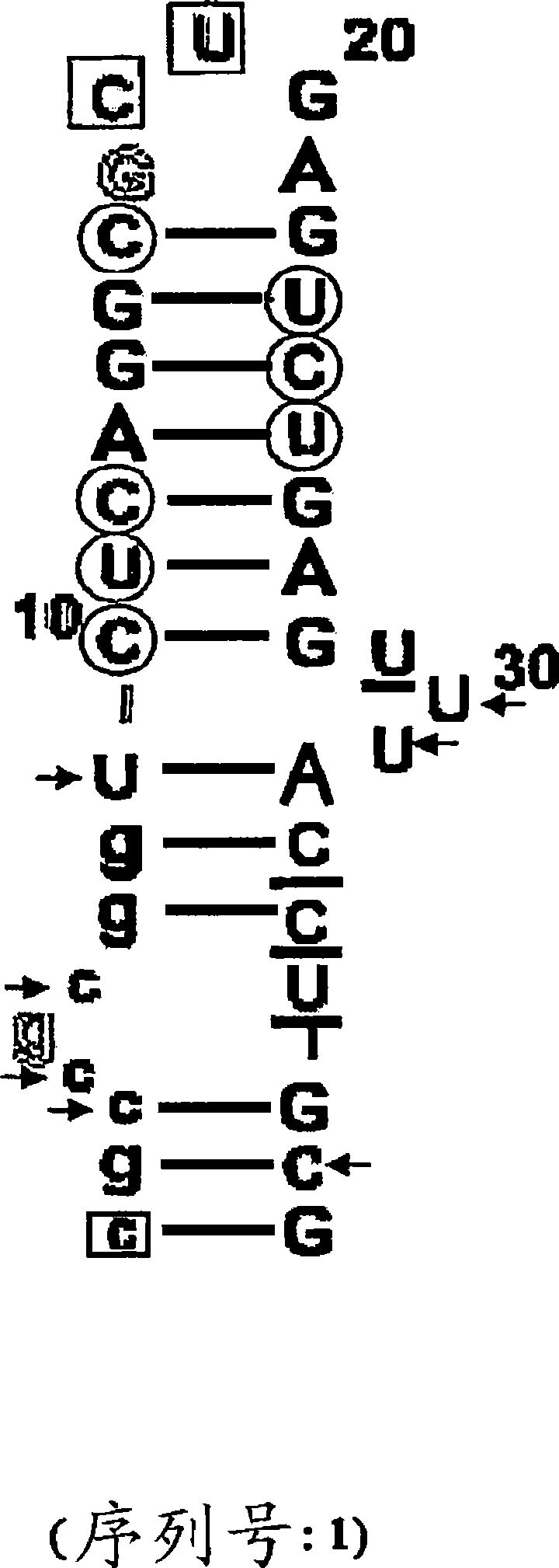Patents
Literature
89 results about "Complement components" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
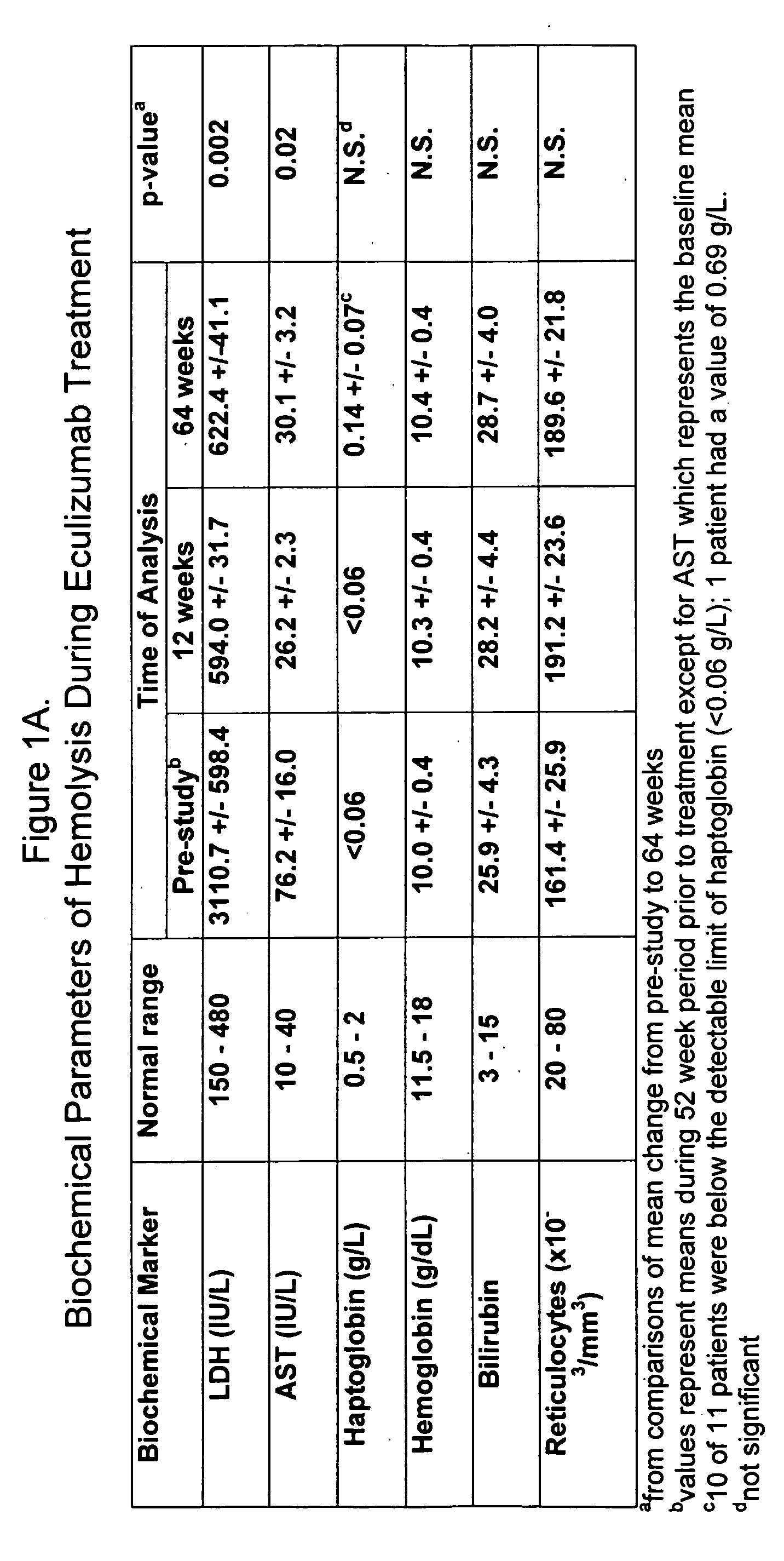
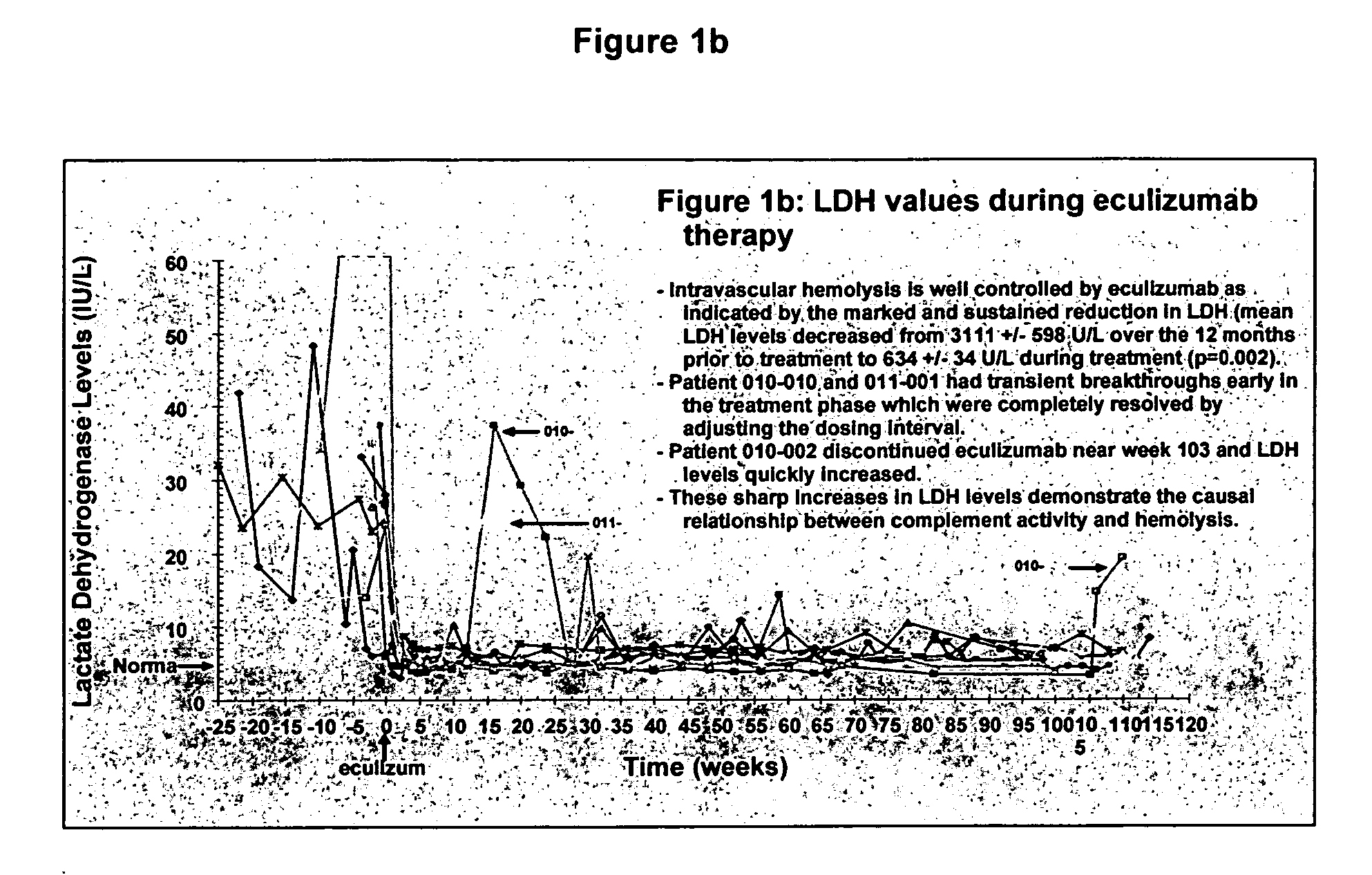
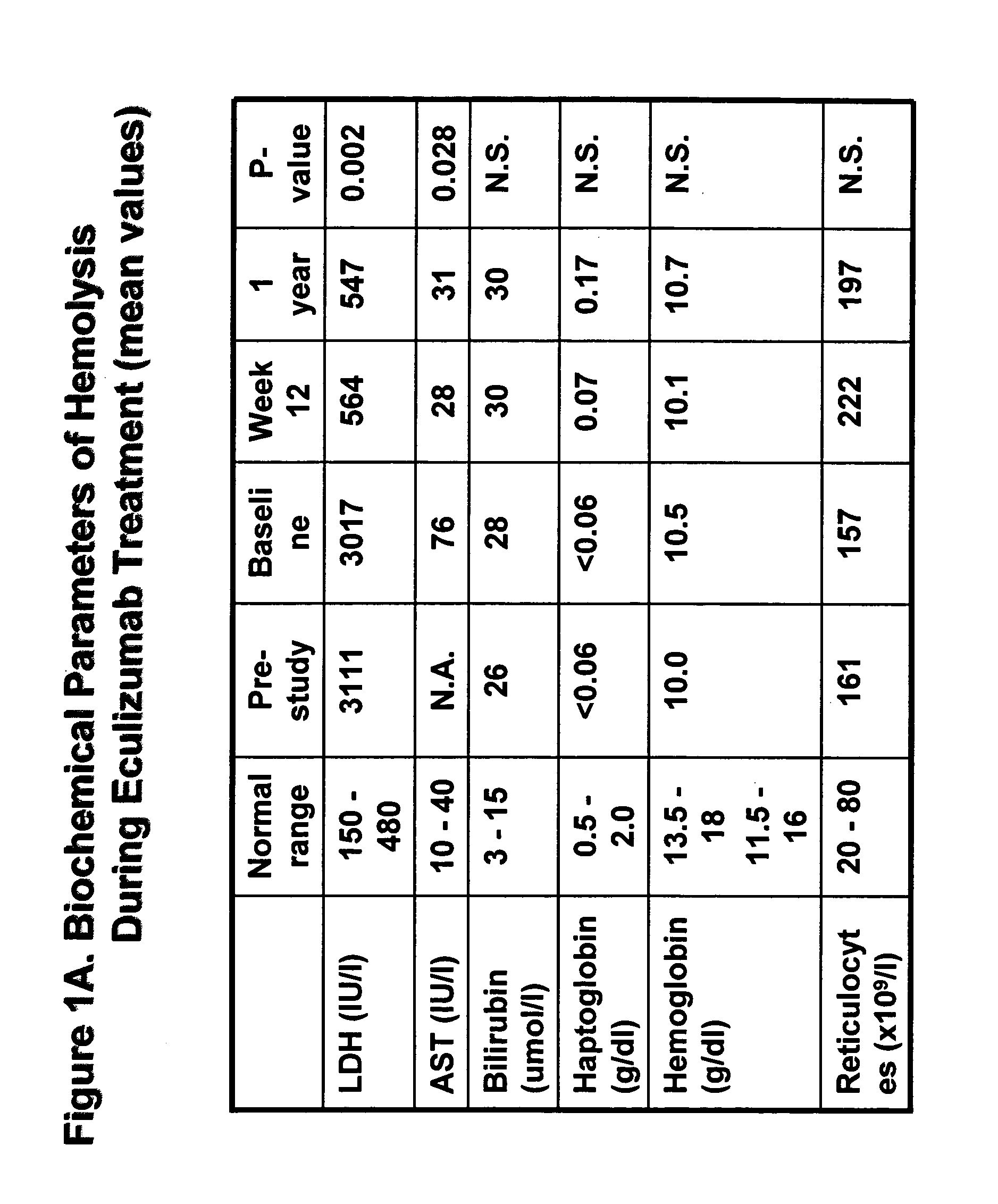
Method of treating hemolytic disease
InactiveUS20050191298A1Reduce crackingRaising serum levelPeptide/protein ingredientsGenetic material ingredientsCompound (substance)Paroxysmal nocturnal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria or other hemolytic diseases are treated using a compound which binds to or otherwise blocks the generation and / or the activity of one or more complement components, such as, for example, a complement-inhibiting antibody.
Owner:ALEXION PHARMA INC
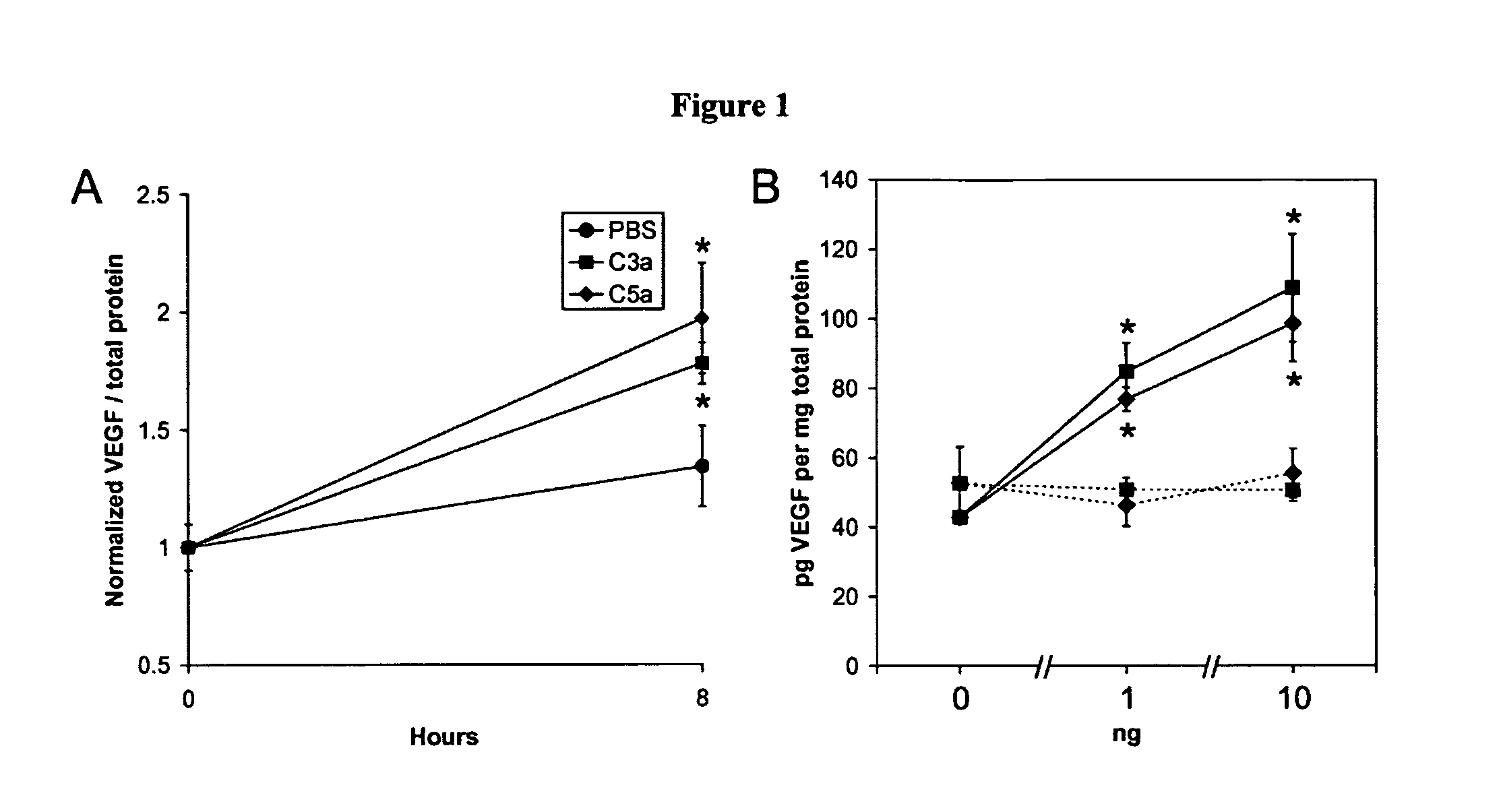
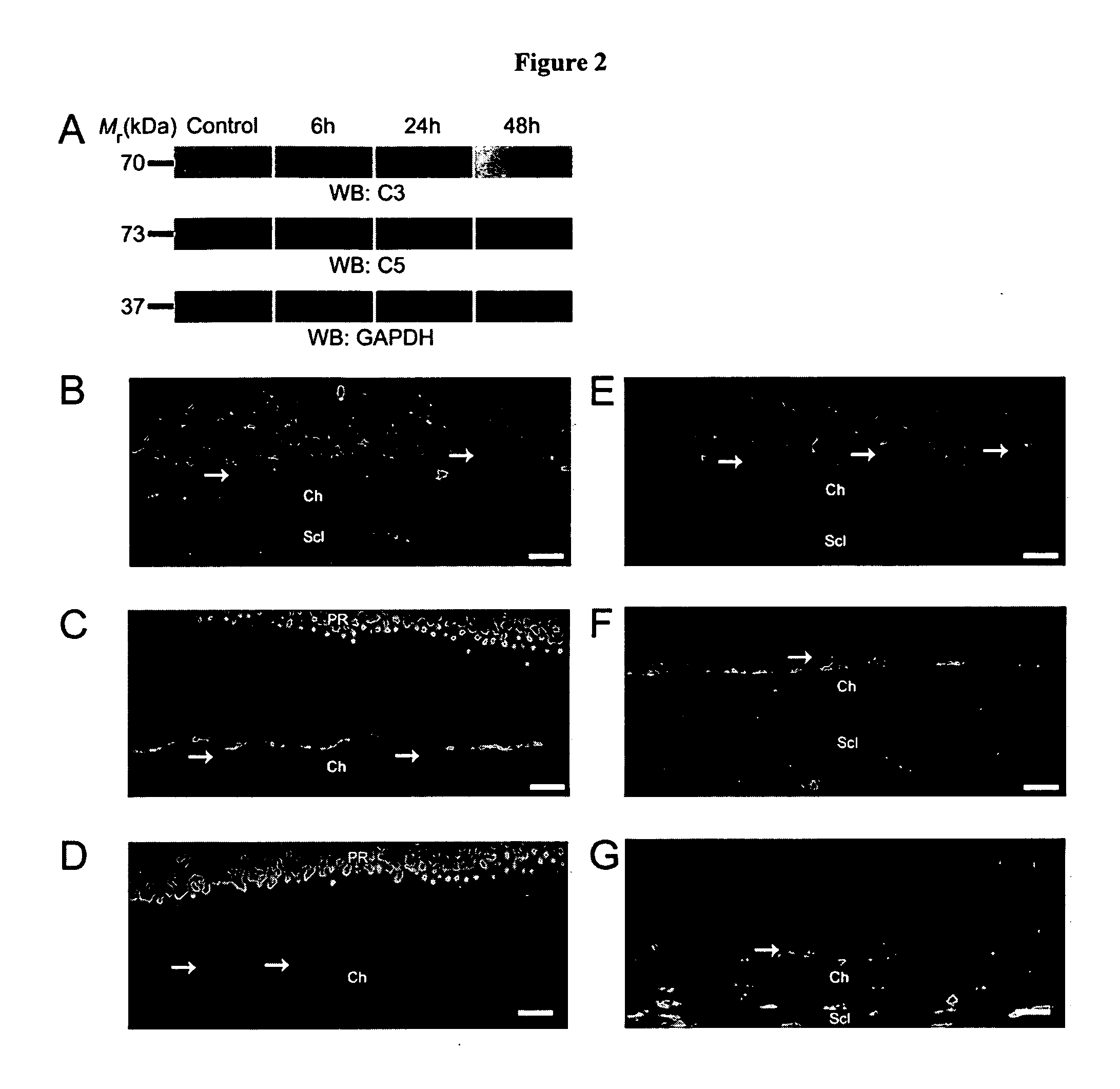
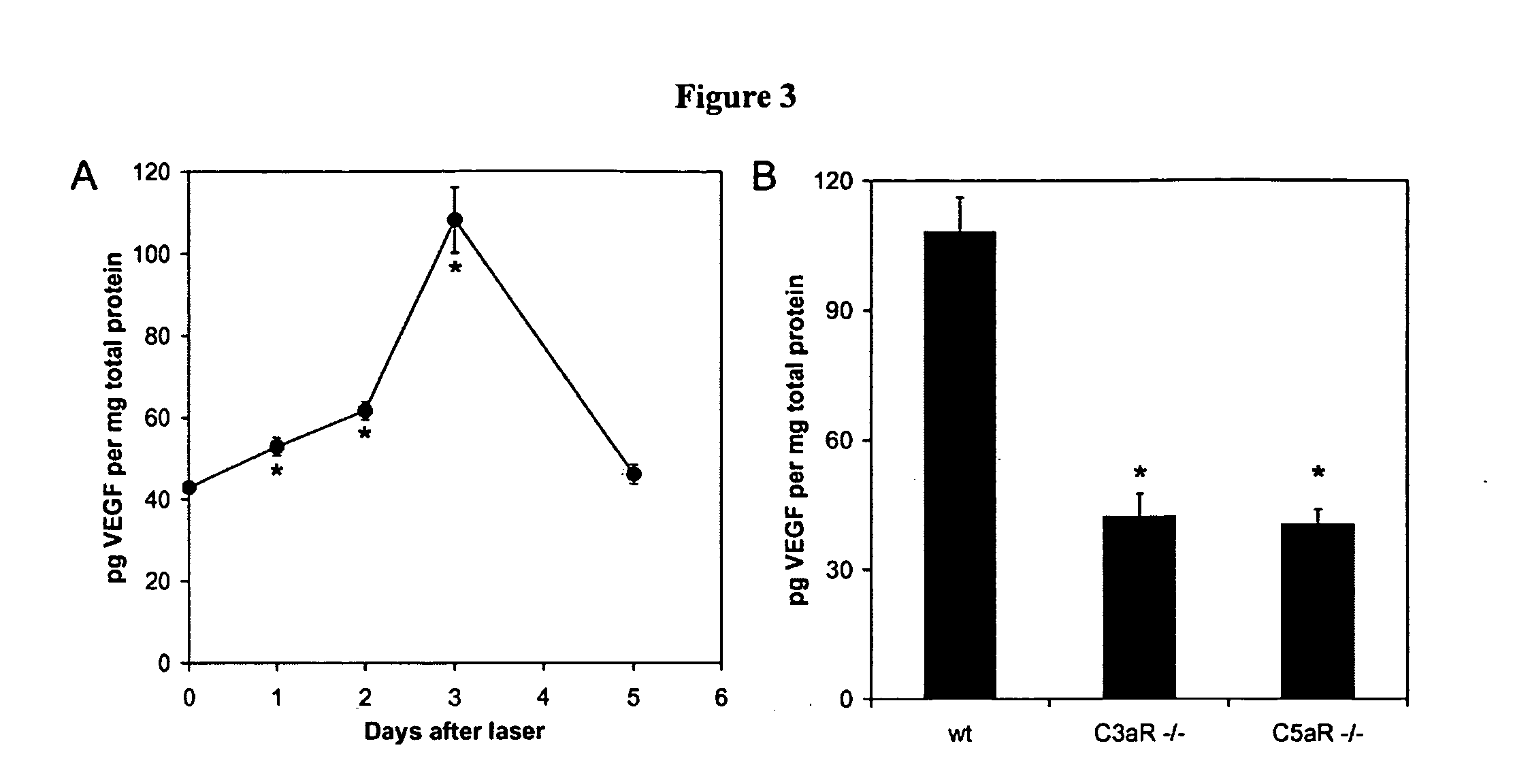
Compositions and methods for inhibiting drusen complement components C3a and C5a for the treatment of age-related macular degeneration
InactiveUS20060067935A1Reduces VEGF expressionReduce expressionGenetic material ingredientsAntibody ingredientsIn vivoBiology
Activated C3 (C3a) and its receptor (C3aR) and activated C5 (C5a) and its receptor (C5aR) have been shown to induce vascular endothelial growth factor (VEGF) expression in vitro and in vivo. Compositions and methods for inhibiting C3a, C3aR, C5a and C5aR for the treatment and / or prevention of neovascular disease are provided. Also provided are Novel therapeutic targets and diagnostic markers for choroidal neovascularization.
Owner:KENTUCKY UNIVERISTY OF
Complement Binding Aptamers and Anti-C5 Agents Useful in the Treatment of Ocular Disorders
InactiveUS20090269356A1Lower Level RequirementsImprove acuityBiocideSenses disorderDiseaseEye disease
Methods of treating complement-mediated ocular disorders by administering agents that inhibit a subject's complement component in an amount sufficient to treat the ocular disorder wherein, in a selected embodiment, said agent is an anti-complement aptamer that, in a preferred embodiment, is an anti-C5 aptamer.
Owner:ARCHEMIX CORP
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Compositions and methods for inhibiting drusen complement components C3a and C5a for the treatment of age-related macular degeneration
Activated C3 (C3a) and its receptor (C3aR) and activated C5 (C5a) and its receptor (C5aR) have been shown to induce vascular endothelial growth factor (VEGF) expression in vitro and in vivo. Compositions and methods for inhibiting C3a, C3aR, C5a and C5aR for the treatment and / or prevention of neovascular disease are provided. Also provided are Novel therapeutic targets and diagnostic markers for choroidal neovascularization.
Owner:KENTUCKY UNIVERISTY OF
Methods for reducing morality associated with acute myocardial infarction
InactiveUS7361339B2Reduce mortalityImmunoglobulins against blood coagulation factorsOrganic active ingredientsMortality rateAntibody
Methods of reducing mortality in myocardial infarction patients receiving a stent in connection with percutaneous transluminal coronary angioplasty include administering an anti-inflammatory compound to the patient. In one embodiment, the anti-inflammatory compound is an antibody to a complement component.
Owner:ALEXION PHARMA INC
Diagnosis and monitoring of systemic lupus erythematosus and of scleroderma
ActiveUS7390631B2Bioreactor/fermenter combinationsBiological substance pretreatmentsRed CellSclerosis skin
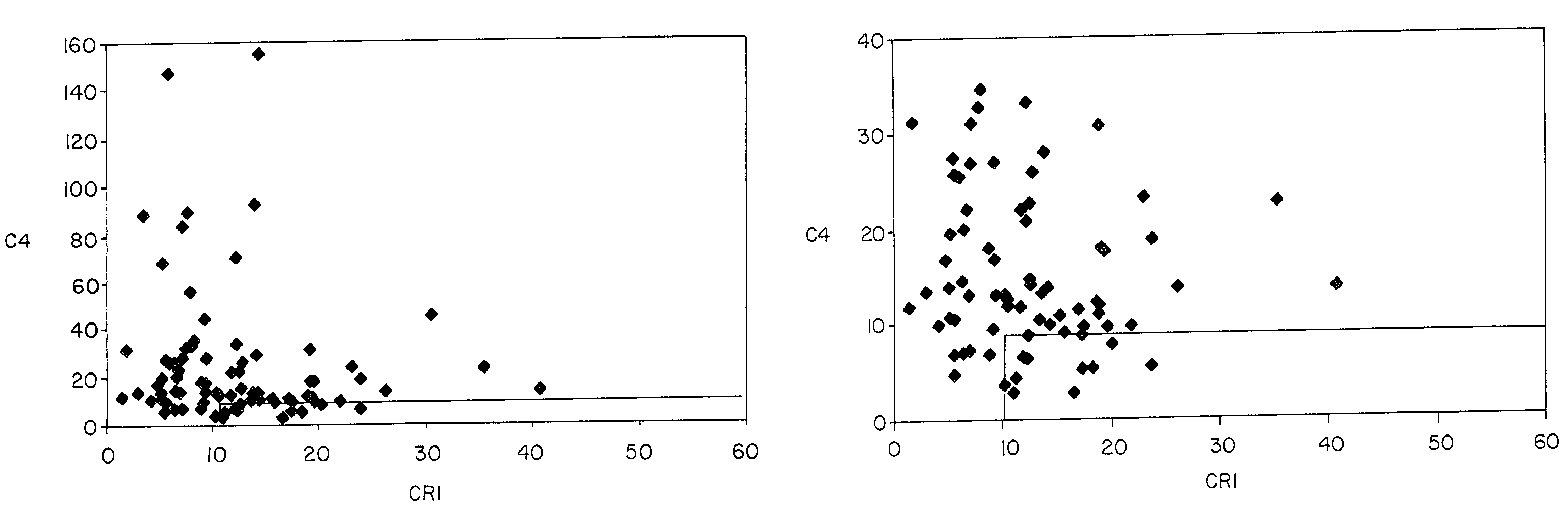
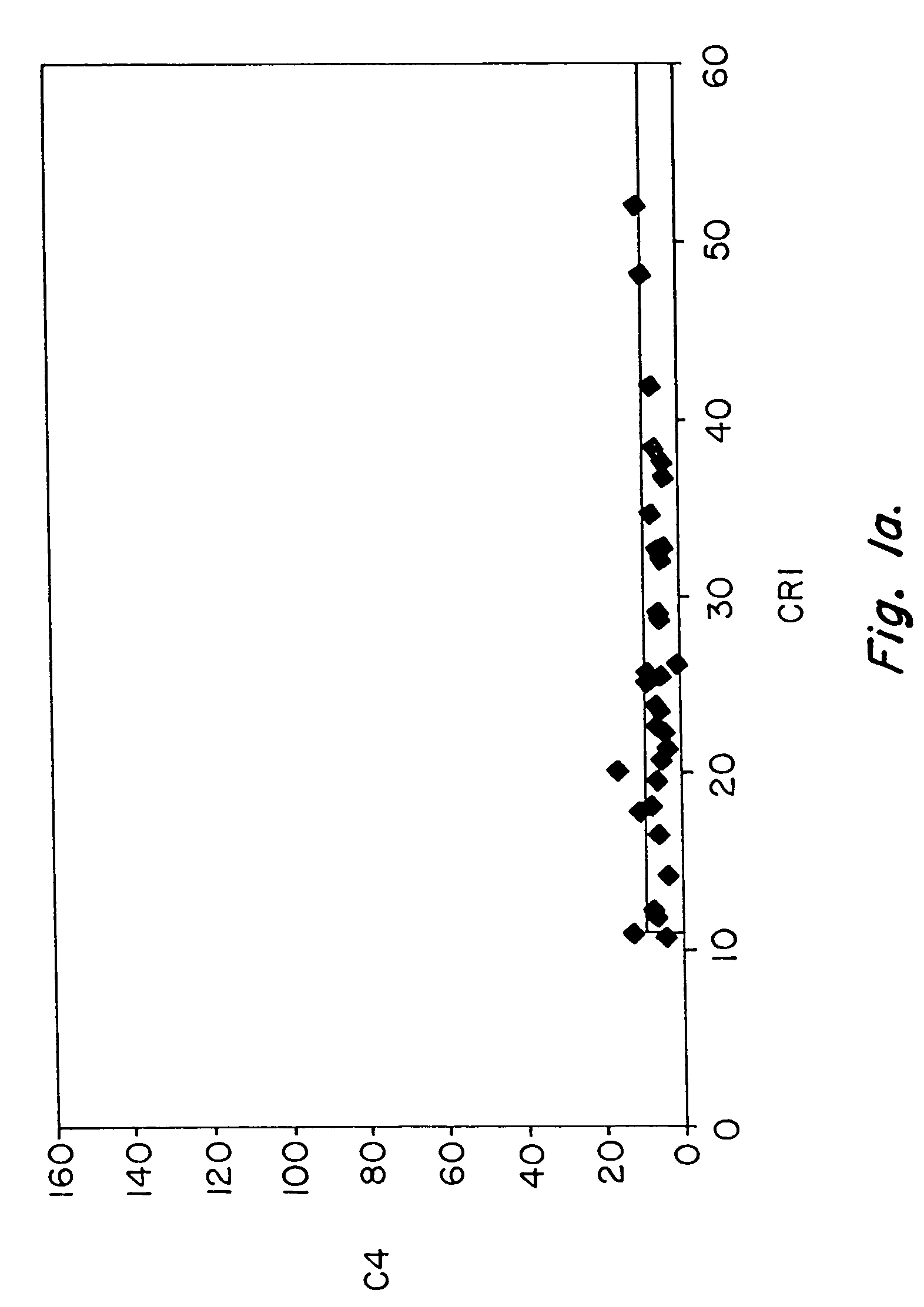
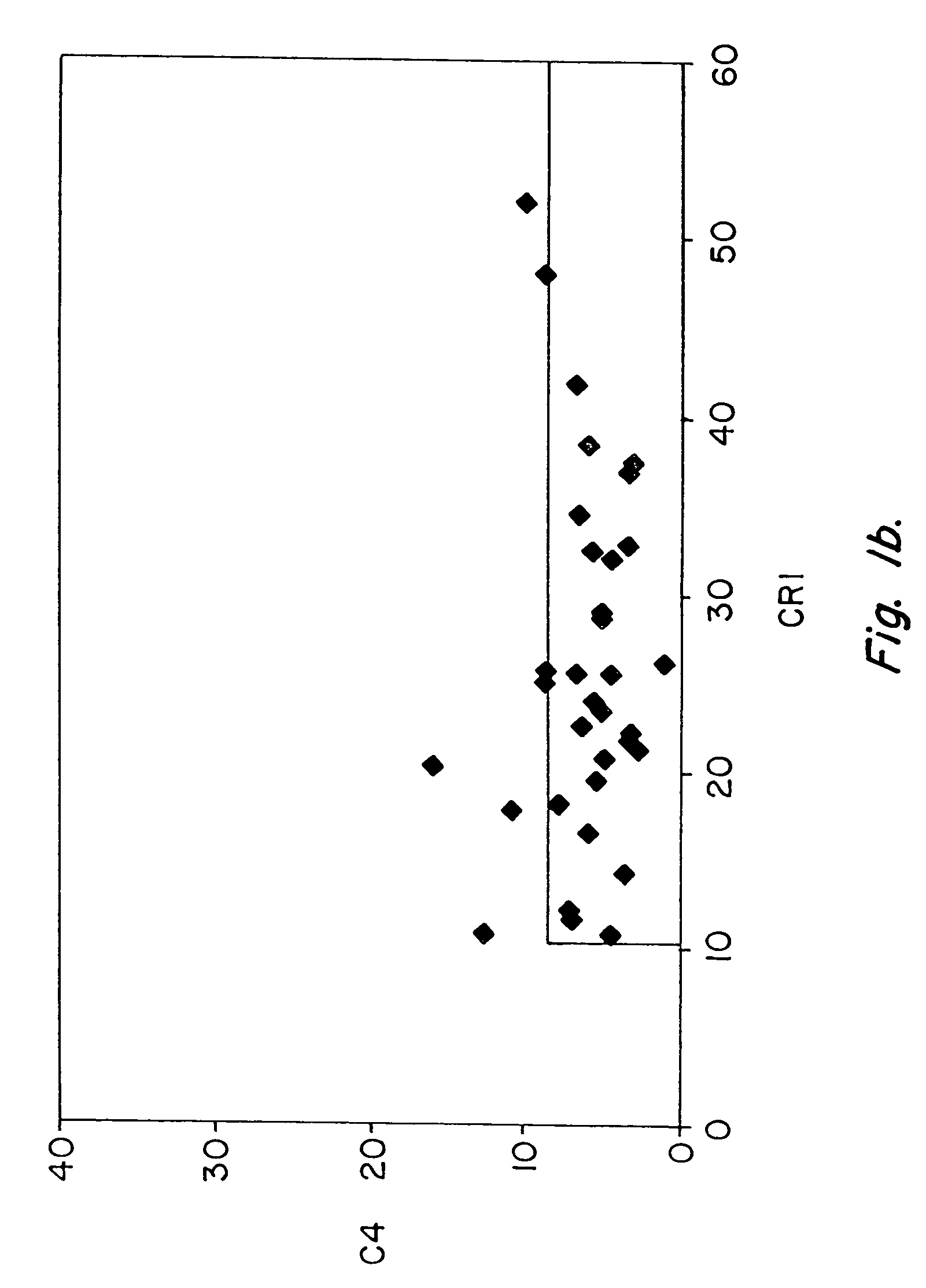
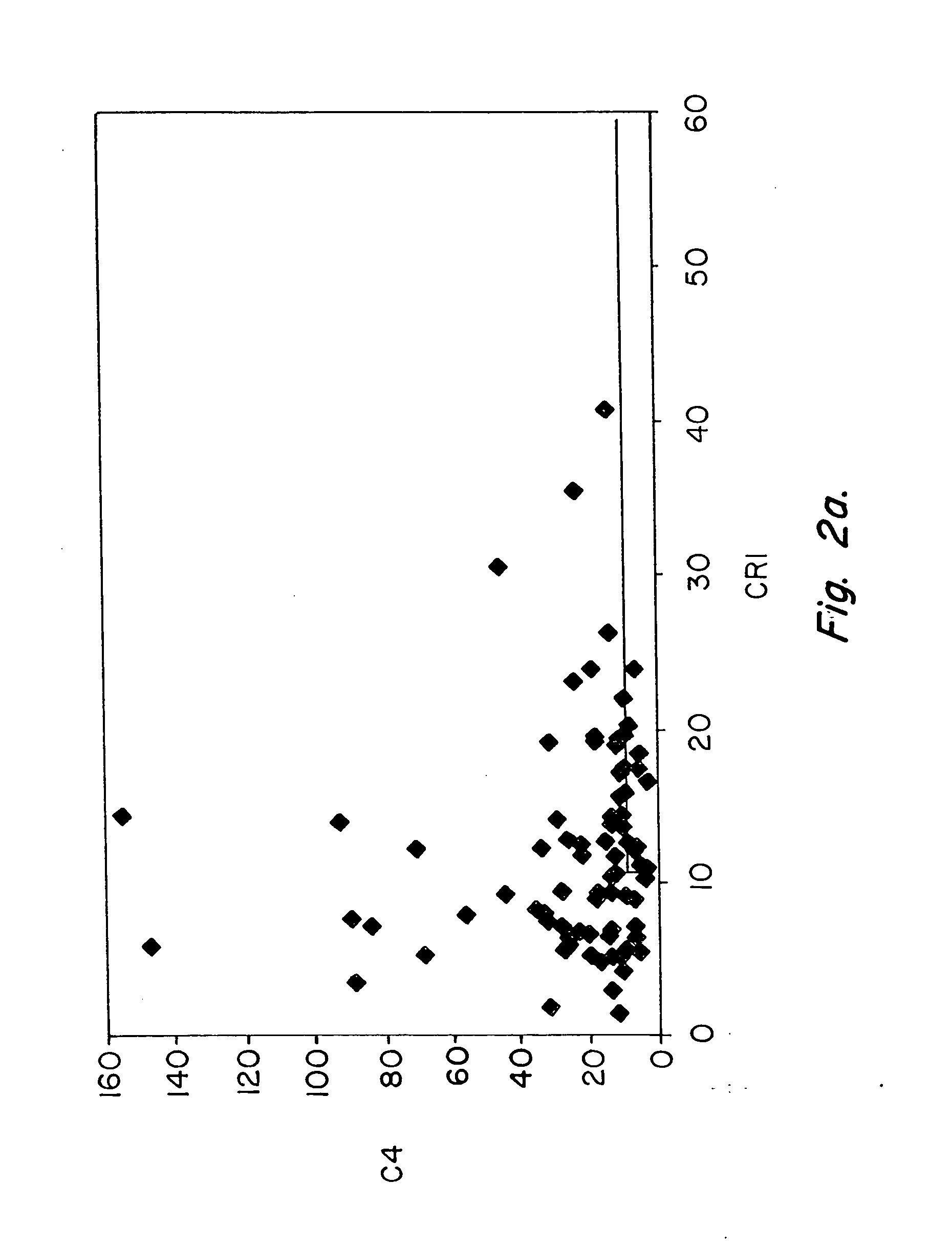
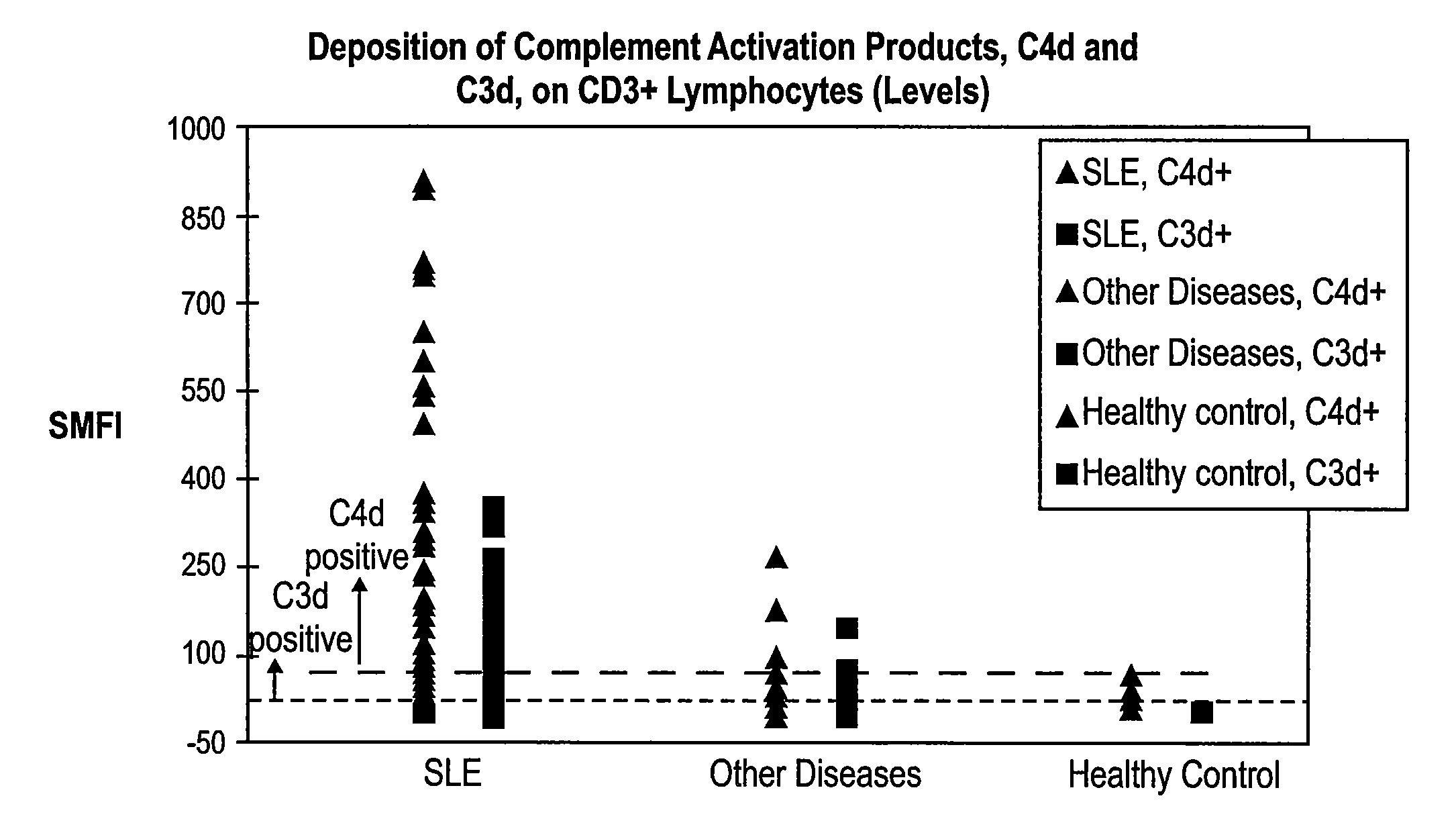
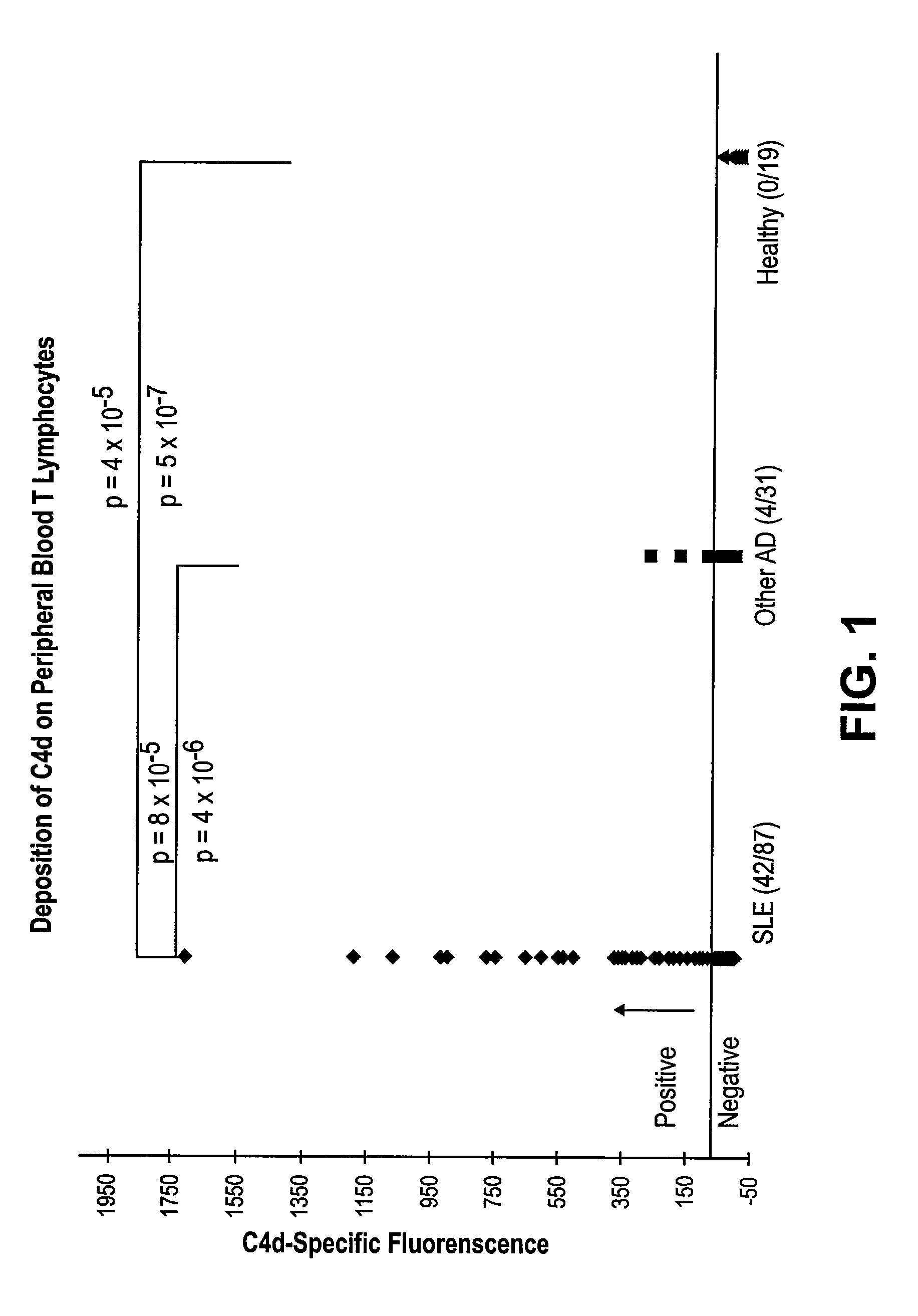
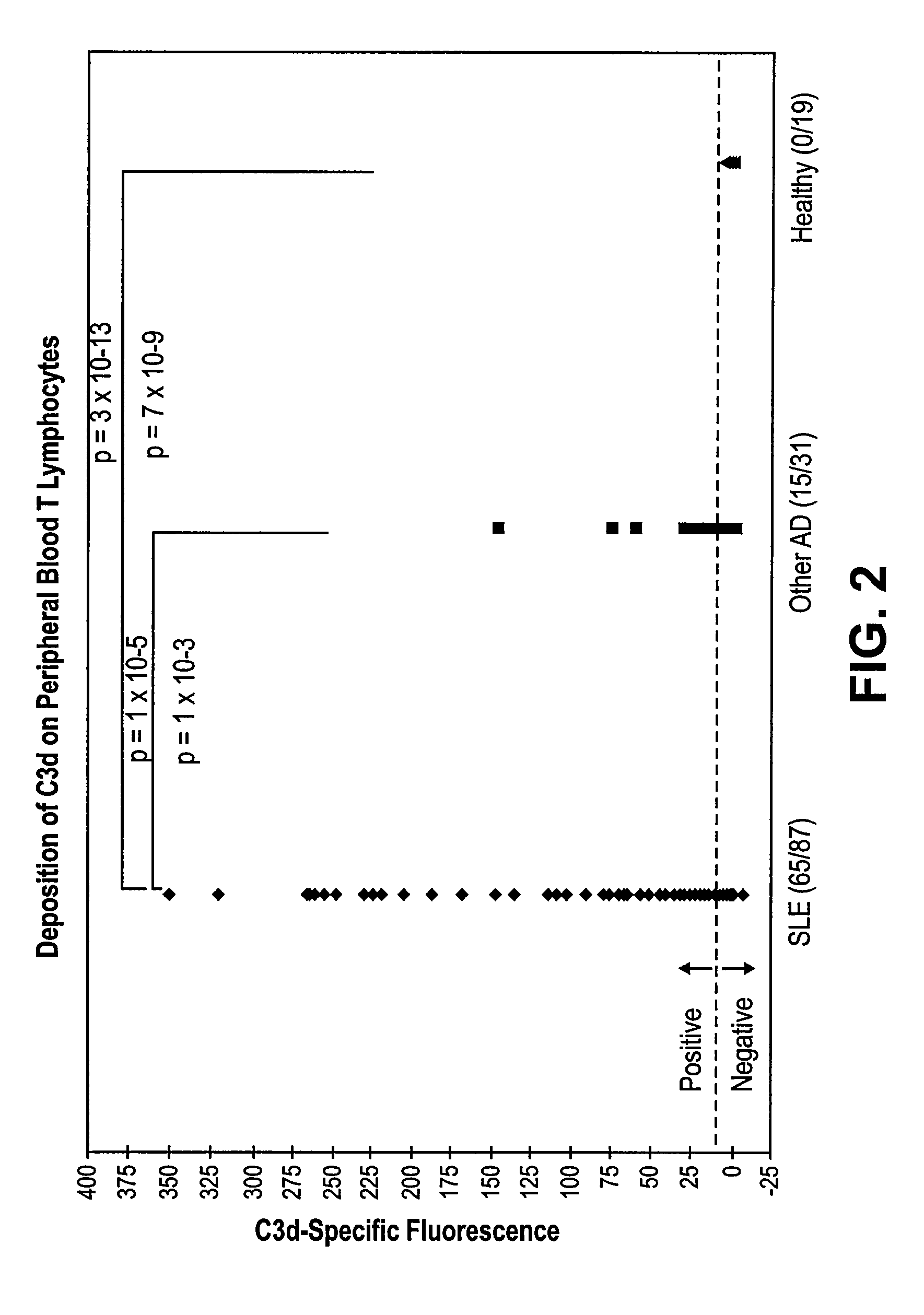
Methods for diagnosing and monitoring systemic lupus erythematosus (SLE) or scleroderma by determining, in a blood sample from the individual being diagnosed or monitored, complement component C4d deposited on surfaces of red blood cells in the sample, and optionally also determining complement receptor CR1 deposited on the red blood cell surfaces. For diagnosis this is compared with the quantity of C4d (and optionally CR1) present on red blood cells of normal individuals. For monitoring it is compared with a value in a sample or samples previously obtained from the individual patient. The comparison may be made with individual values for C4d and CR1 and / or with a ratio of the two found in normal individuals.
Owner:UNIVERSITY OF PITTSBURGH
Method of treating hemolytic disease
InactiveUS20050169921A1Reduce crackingRaising serum levelGenetic material ingredientsDigestive systemCompound (substance)Paroxysmal nocturnal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria or other hemolytic diseases are treated using a compound which binds to or otherwise blocks the generation and / or the activity of one or more complement components, such as, for example, a complement-inhibiting antibody.
Owner:ALEXION PHARMA INC
Diagnosis and monitoring of systemic lupus erythematosus and of scleroderma
ActiveUS20050037441A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRed CellSclerosis skin
Methods for diagnosing and monitoring systemic lupus erythematosus (SLE) or scleroderma by determining, in a blood sample from the individual being diagnosed or monitored, complement component C4d deposited on surfaces of red blood cells in the sample, and optionally also determining complement receptor CR1 deposited on the red blood cell surfaces. For diagnosis this is compared with the quantity of C4d (and optionally CR1) present on red blood cells of normal individuals. For monitoring it is compared with a value in a sample or samples previously obtained from the individual patient.The comparison may be made with individual values for C4d and CR1 and / or with a ratio of the two found in normal individuals.
Owner:UNIVERSITY OF PITTSBURGH
Diagnosing and monitoring inflammatory diseases by measuring complement components on white blood cells
ActiveUS7585640B2Bioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellDisease cause
Owner:UNIVERSITY OF PITTSBURGH
Serum protein marker group for diagnosing MODY (maturity-onset-diabetes of the young) and application thereof
InactiveCN106645757AAccurate diagnosisReliable resultsApolipeptidesHydrolasesFactor iiMass spectrometry
The invention relates to a serum protein marker group for diagnosing MODY (maturity-onset-diabetes of the young) and application thereof. The serum protein marker group comprises five proteins: apolipoprotein C-IV (APOC4), apolipoprotein a (LPA), a complement component C6 (C6), a blood coagulation factor v (F5) and thyroid binding globulin (SERPINA7). ITRAQ (isobaric tags for relative and absolute quantitation) are used to combine with an MALDI-TOF / MS technology to detect; mass spectrum detection shows that expression levels of the serum apolipoprotein C-IV, the apolipoprotein a, the complement component C6 and the blood coagulation factor v protein in serum of a MODY patient are obviously improved, and an expression level of the thyroid binding globulin in the serum of the MODY patient is obviously reduced. Mass spectrum MRM (multiple-reaction-monitoring) also verifies that the five proteins are abnormal in expression in serums of the MODY patient, type-1 and type-2 diabetics and a healthy control group, and are specific proteins.
Owner:XINJIANG MEDICAL UNIV
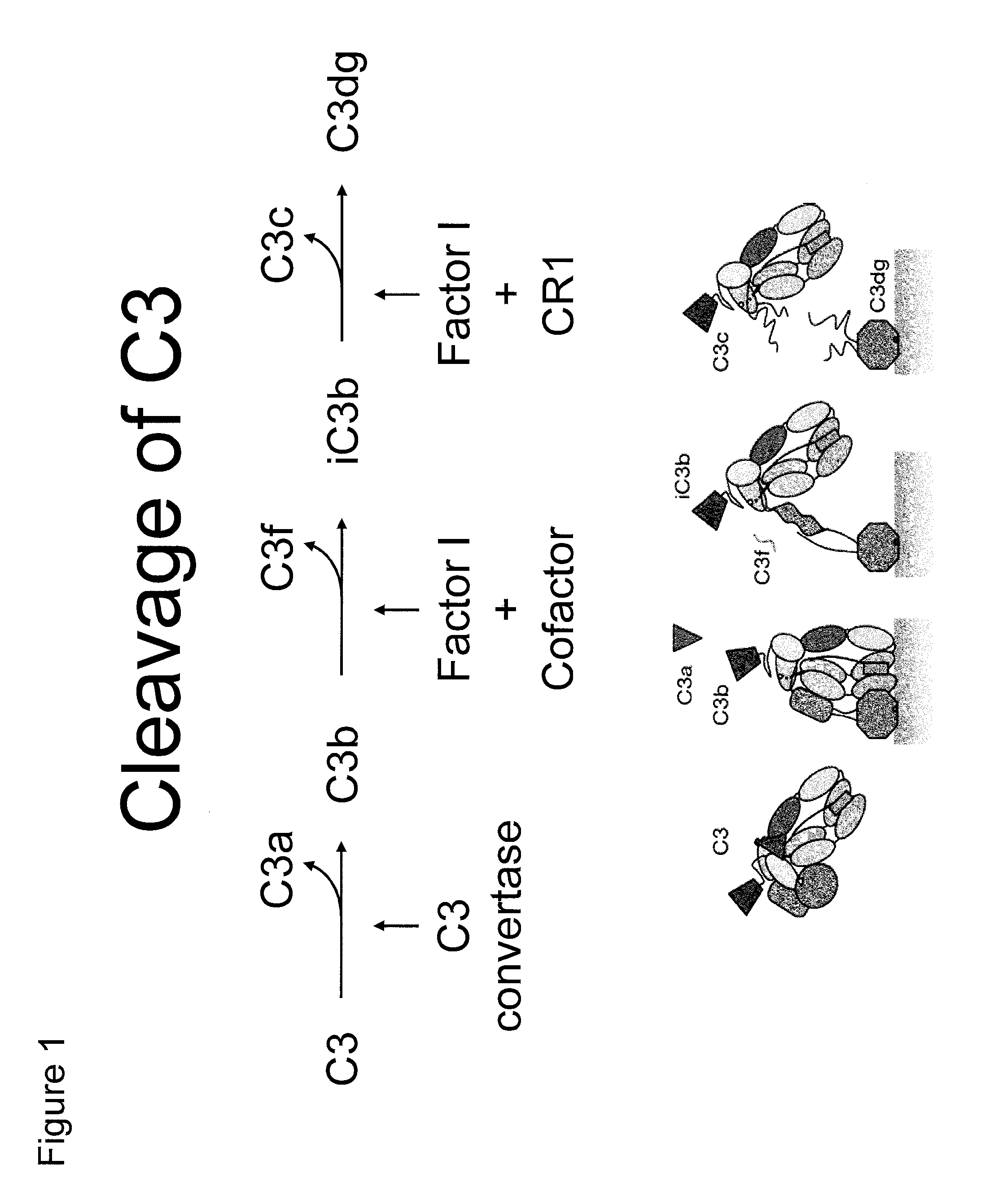
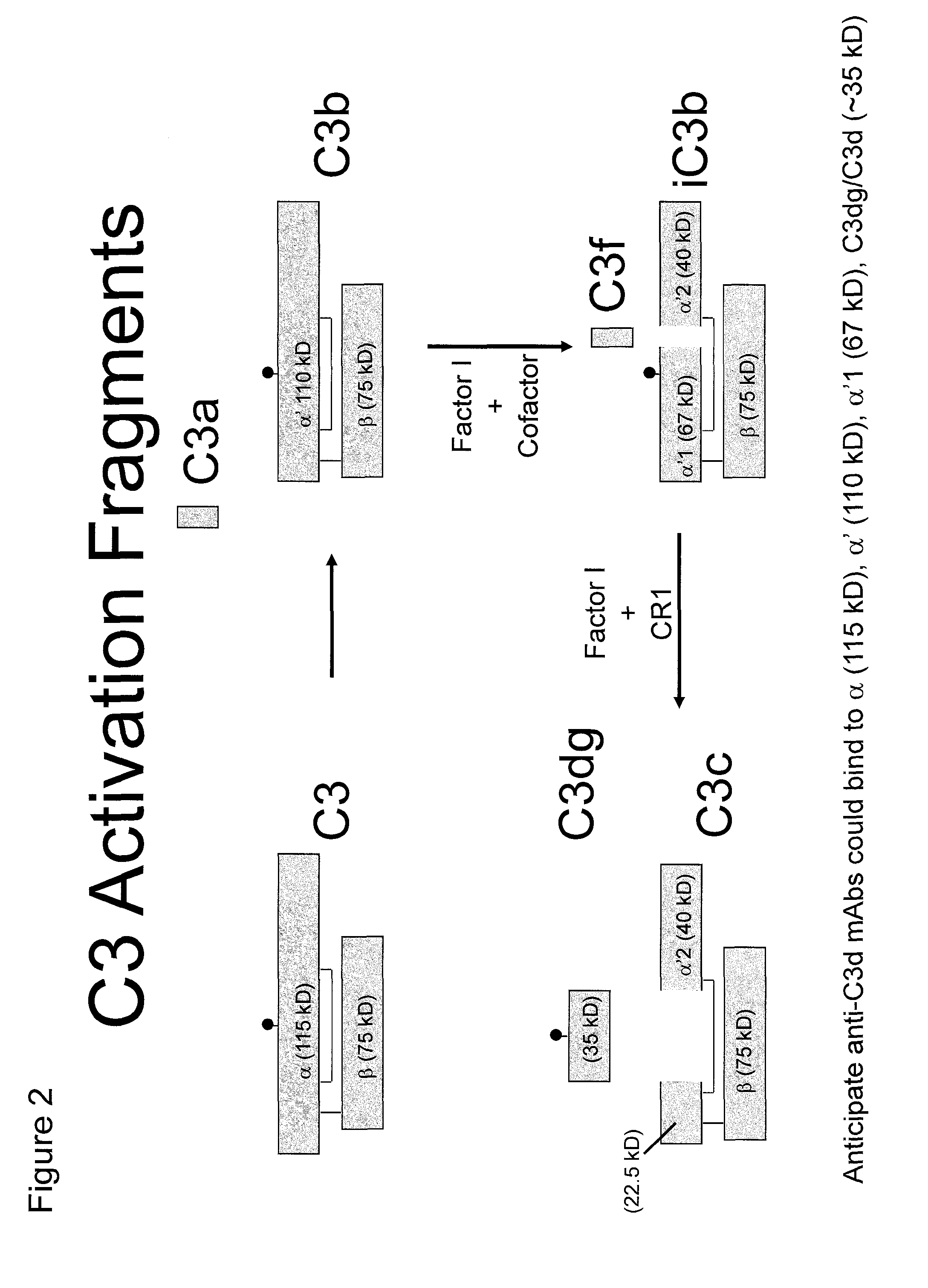
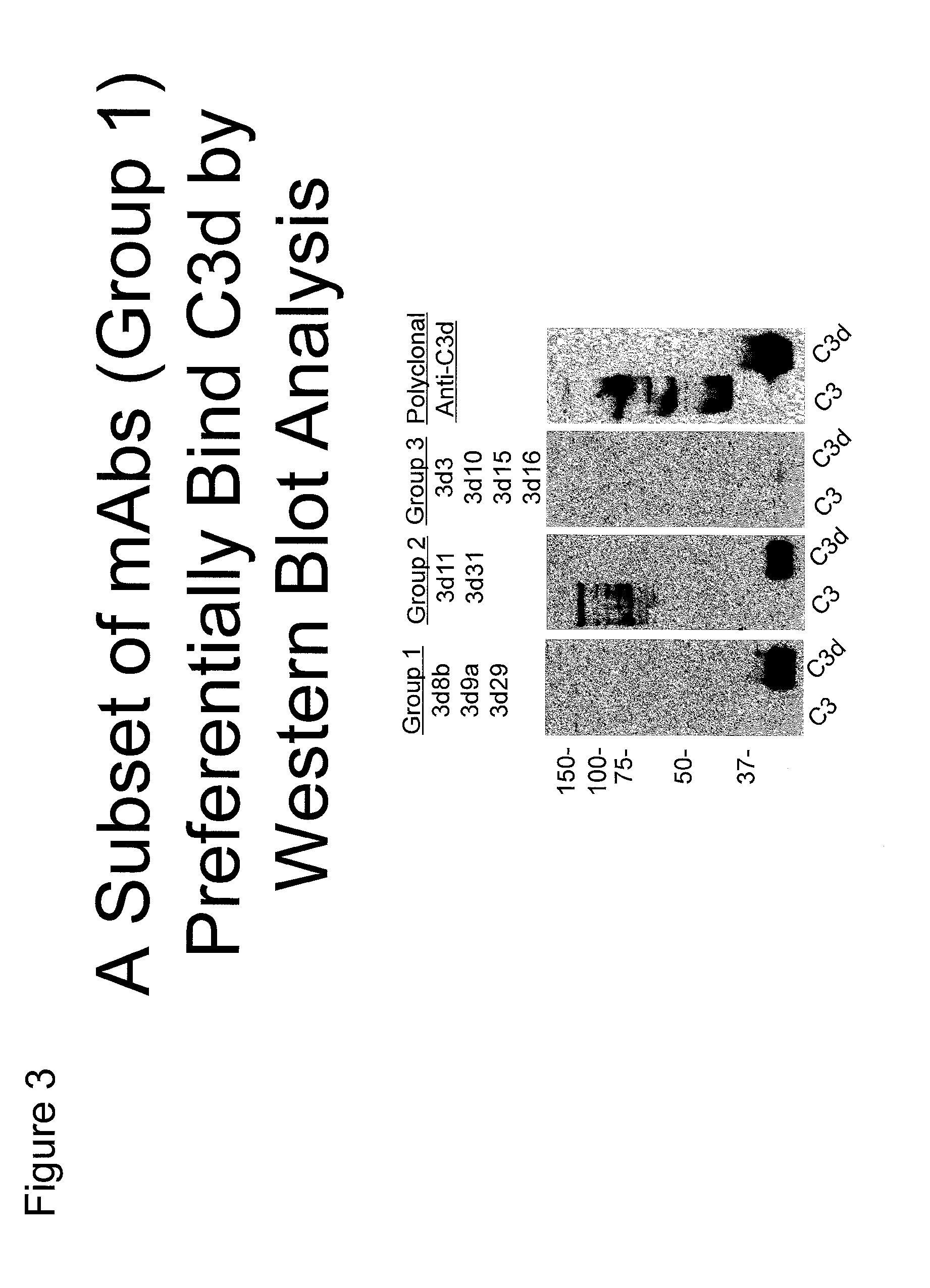
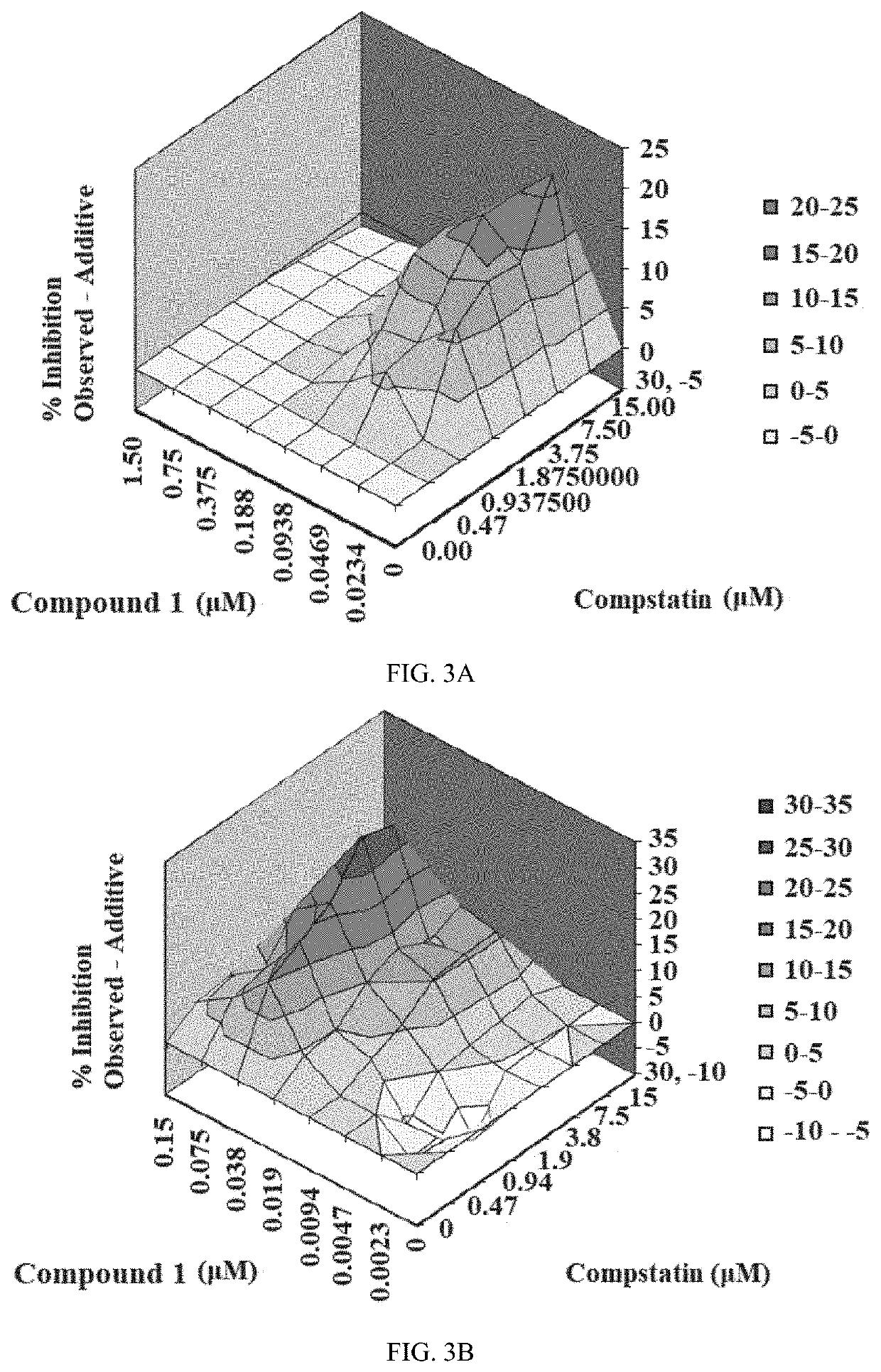
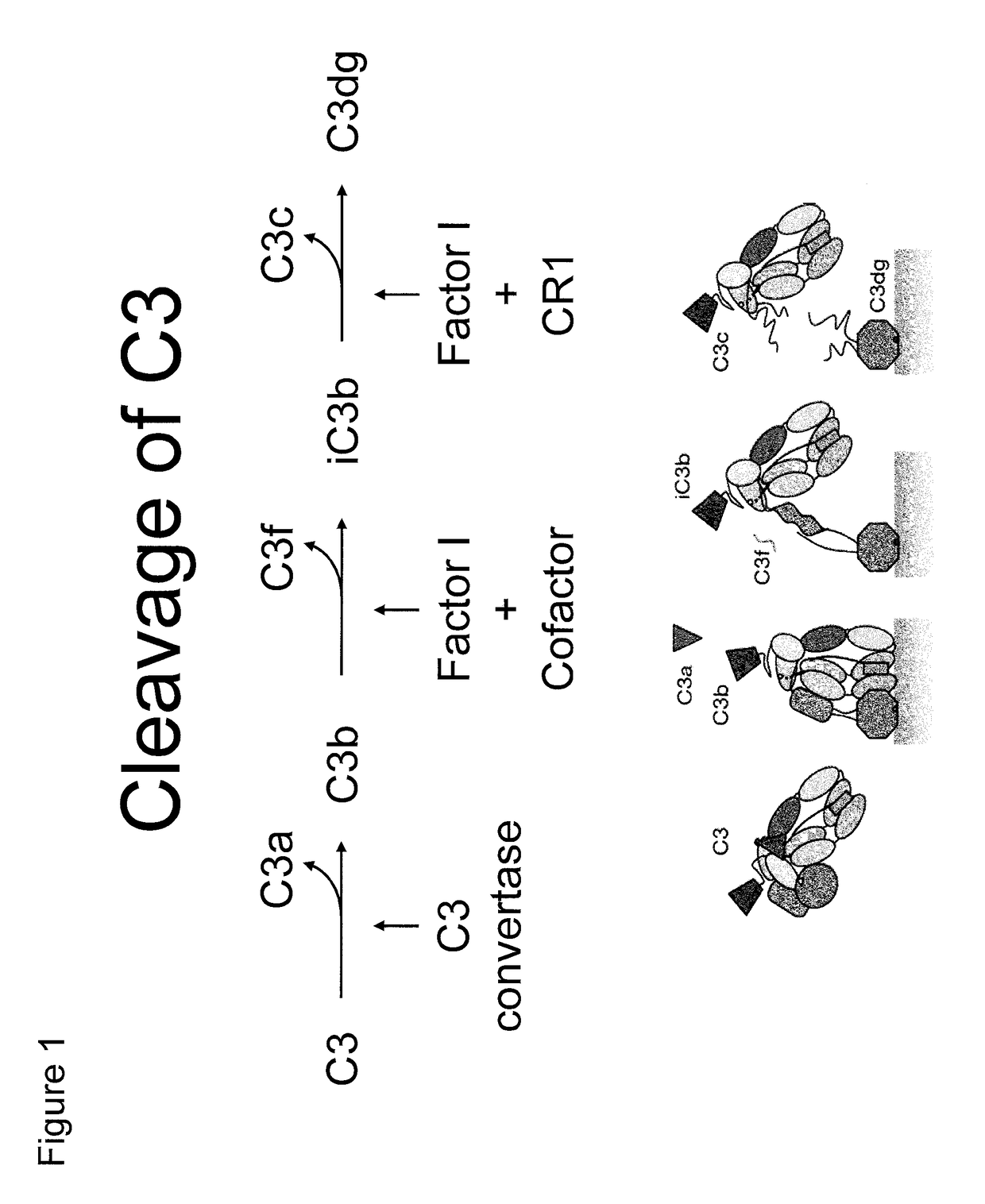
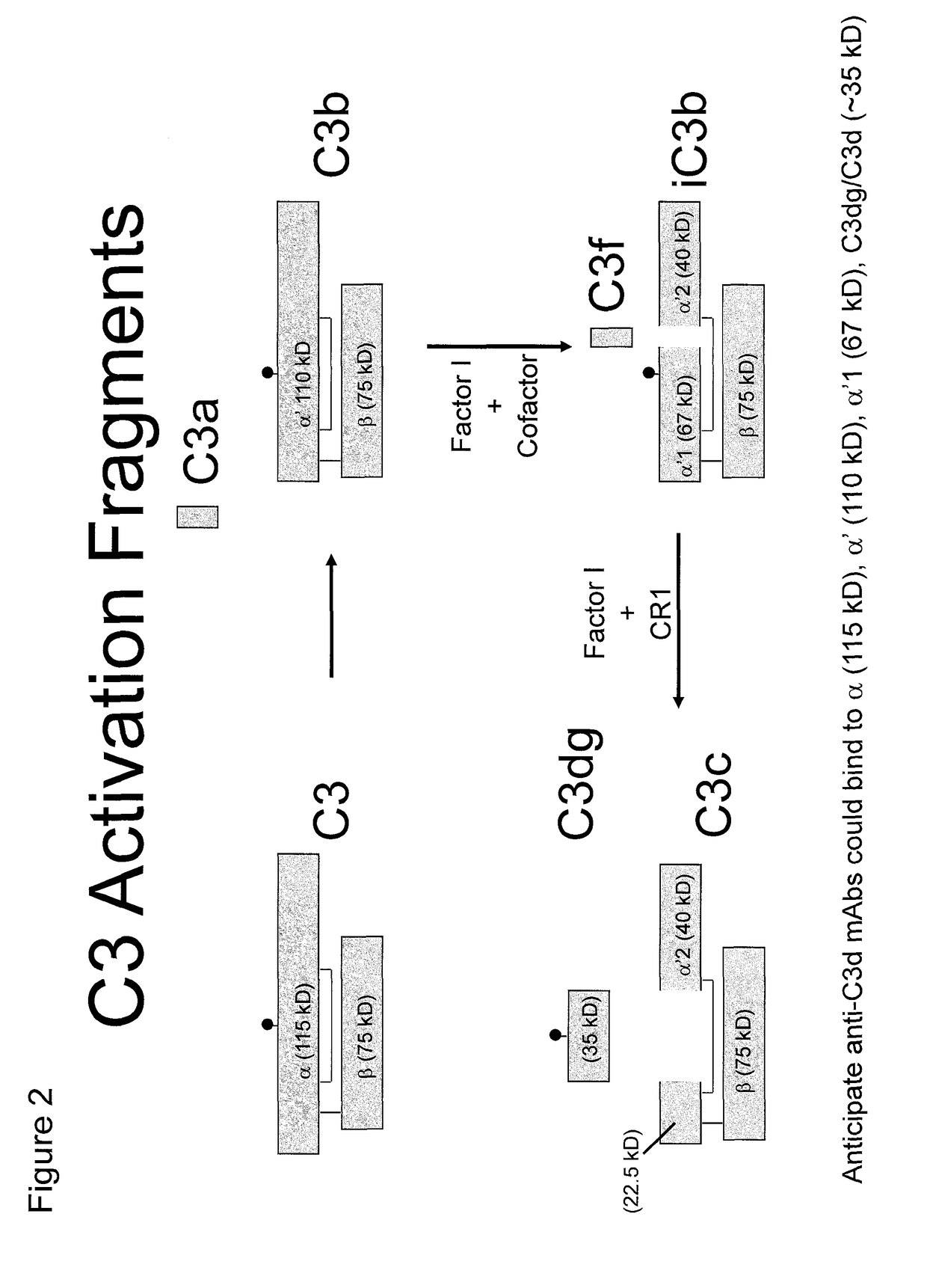
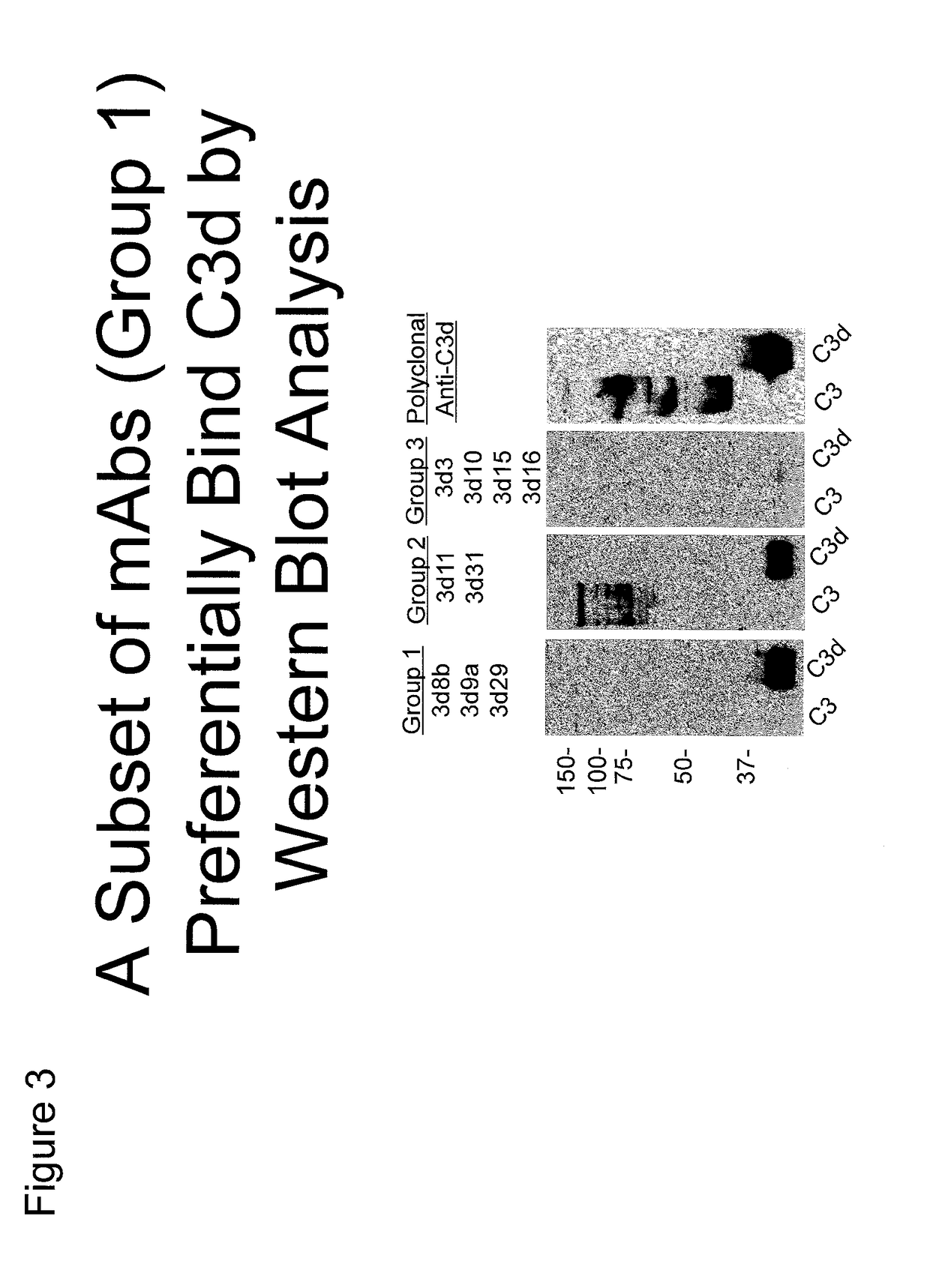
Antibodies to the c3d fragment of complement component 3
ActiveUS20130129728A1Effective treatment and preventionReduce systemic side effectsAntibacterial agentsSenses disorderWhole bodyComplement component 3
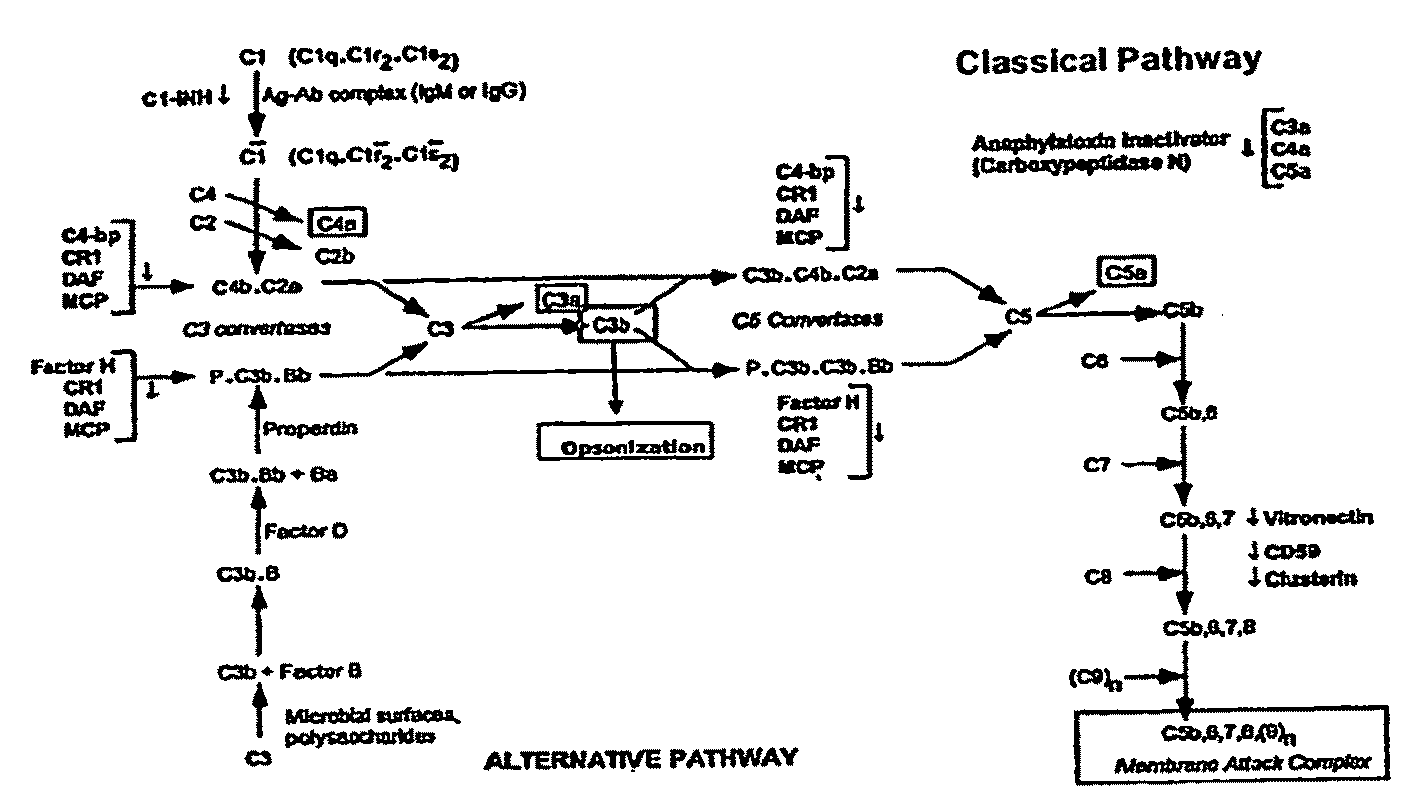
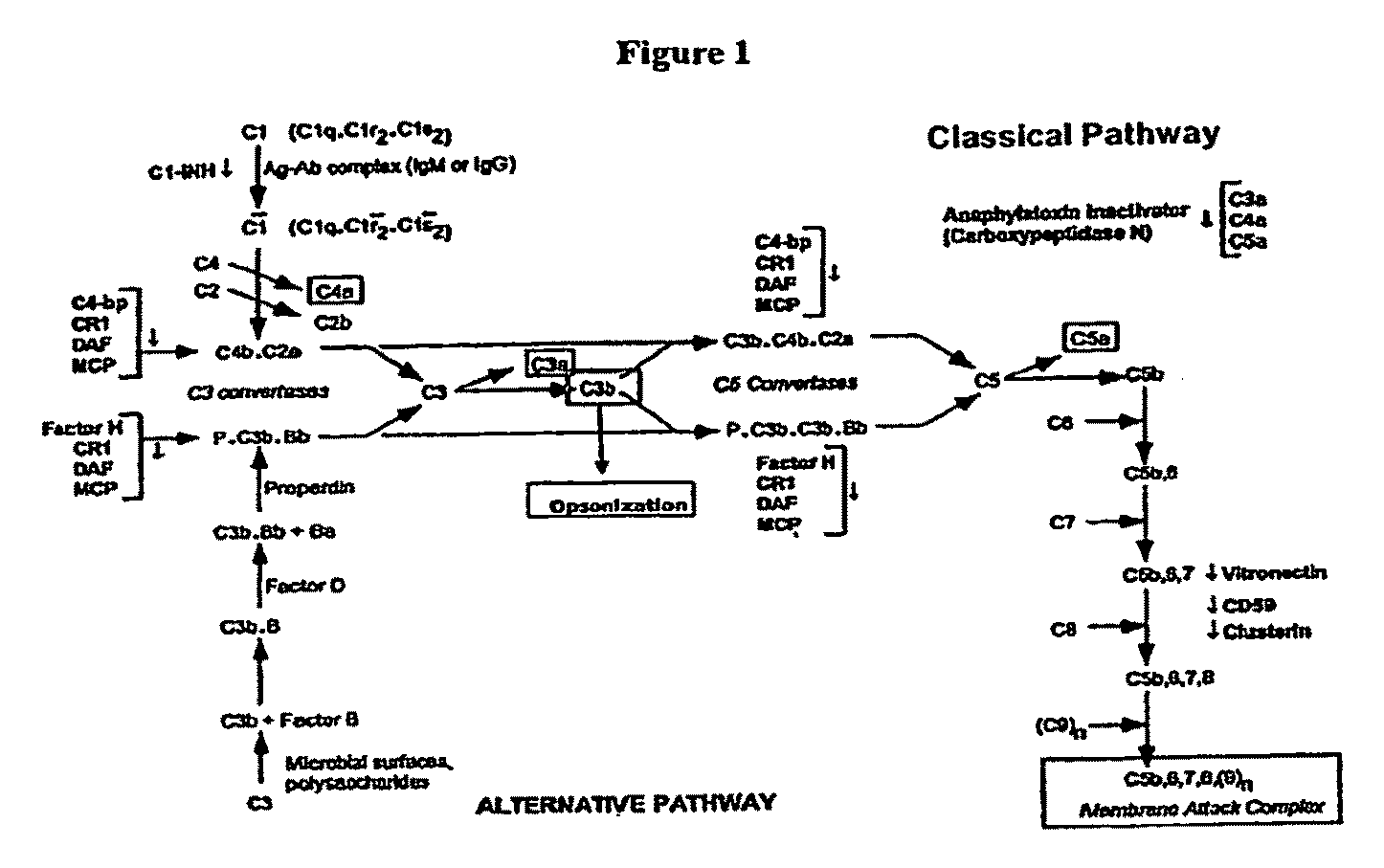
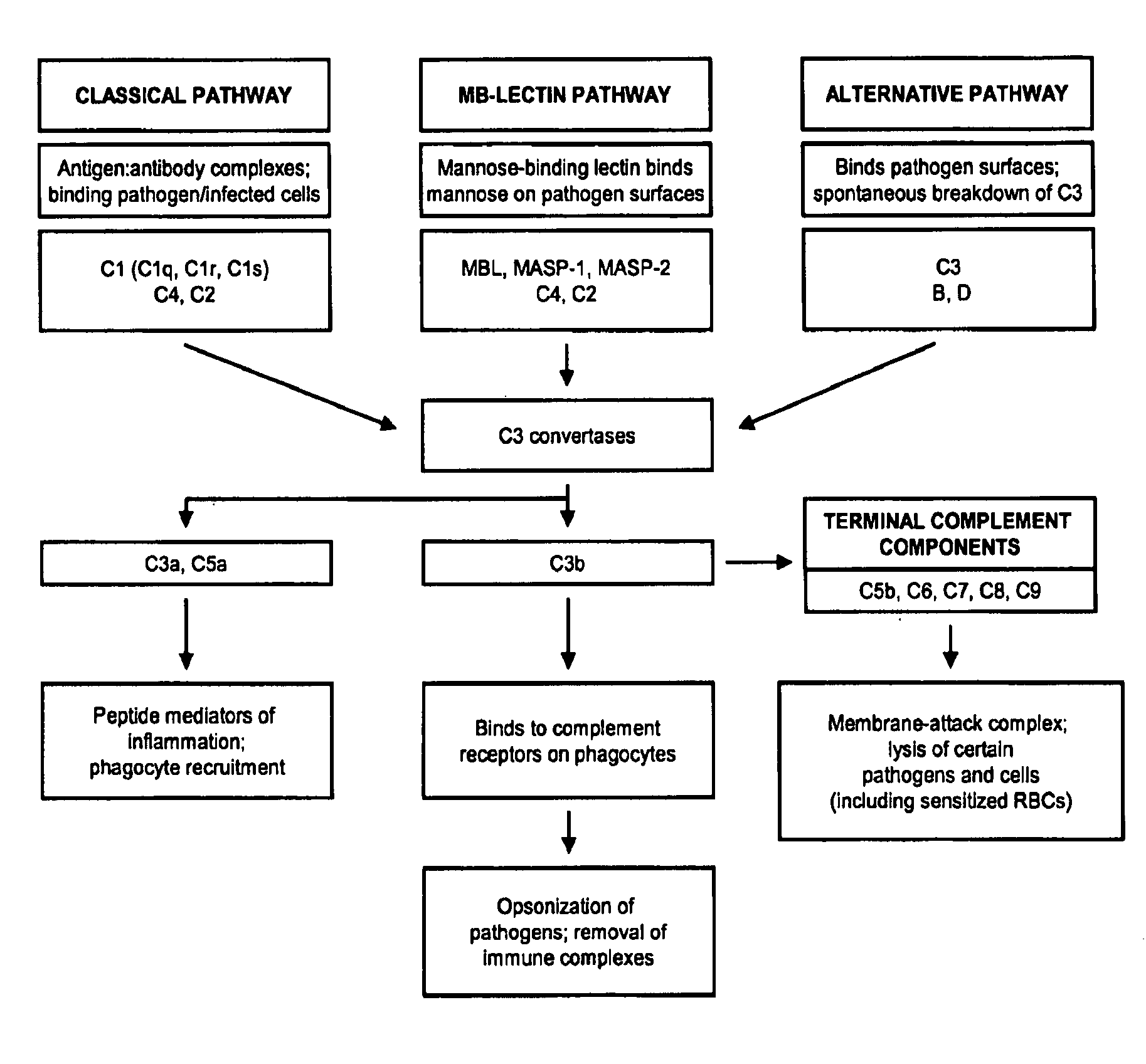
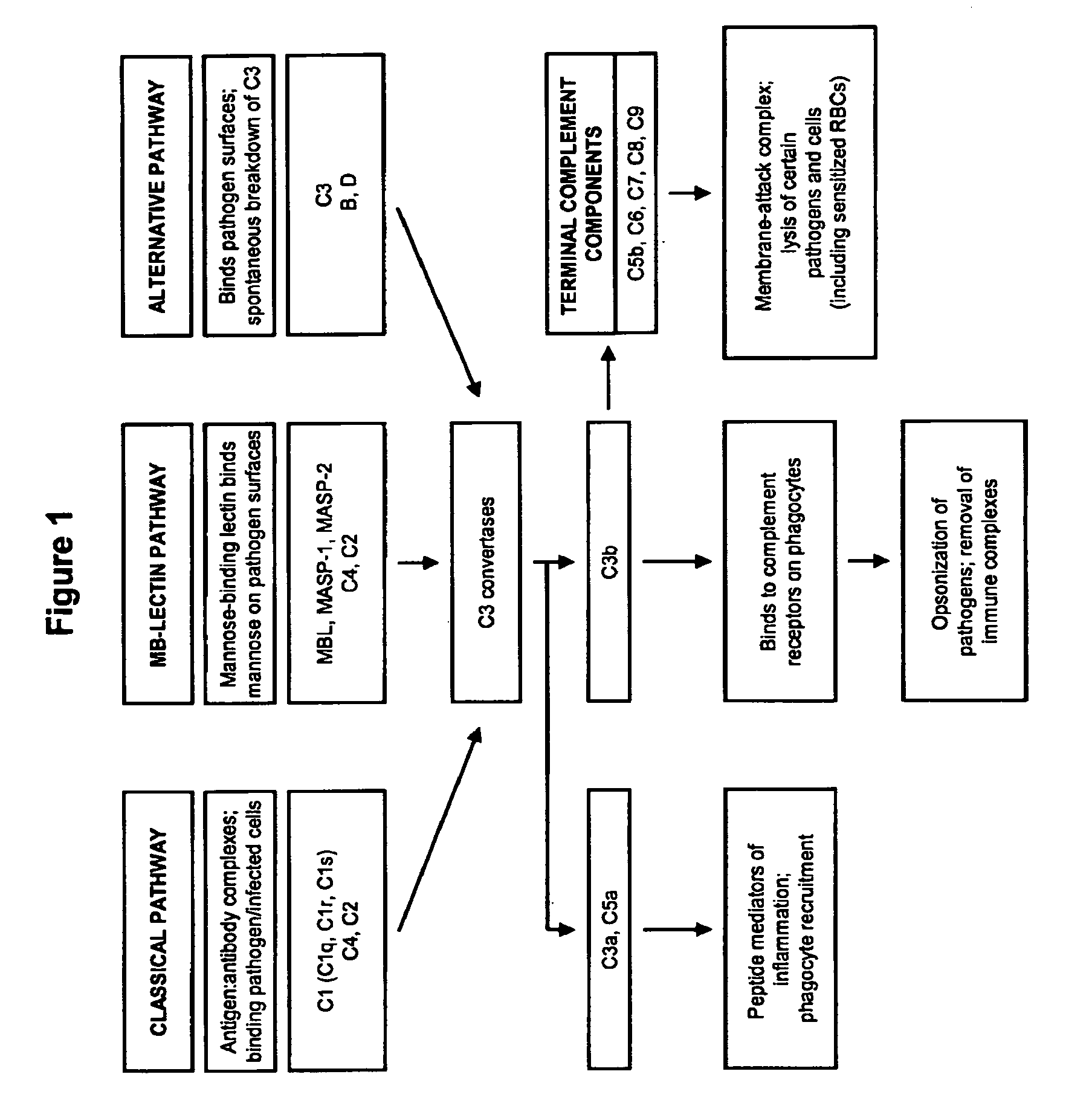
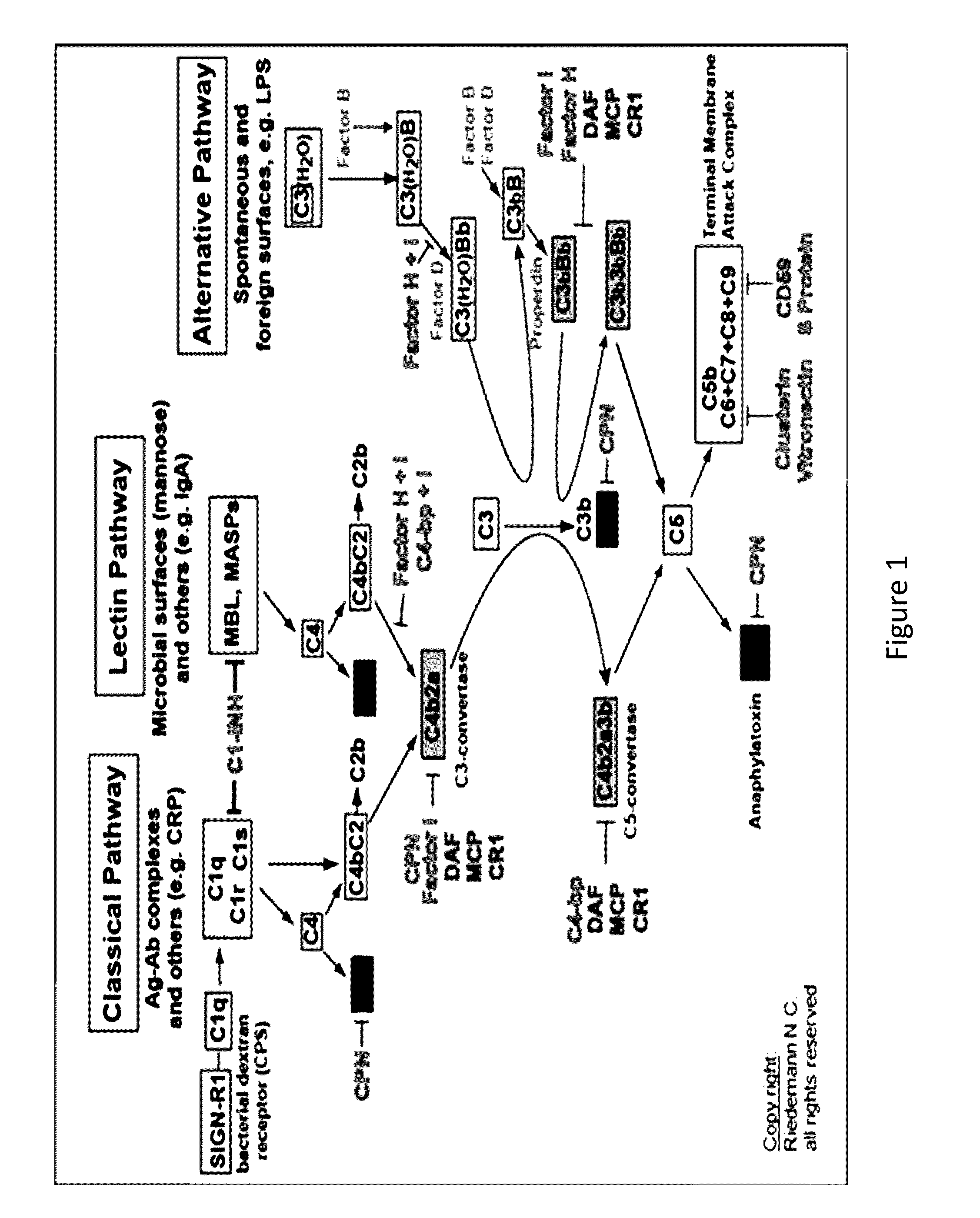
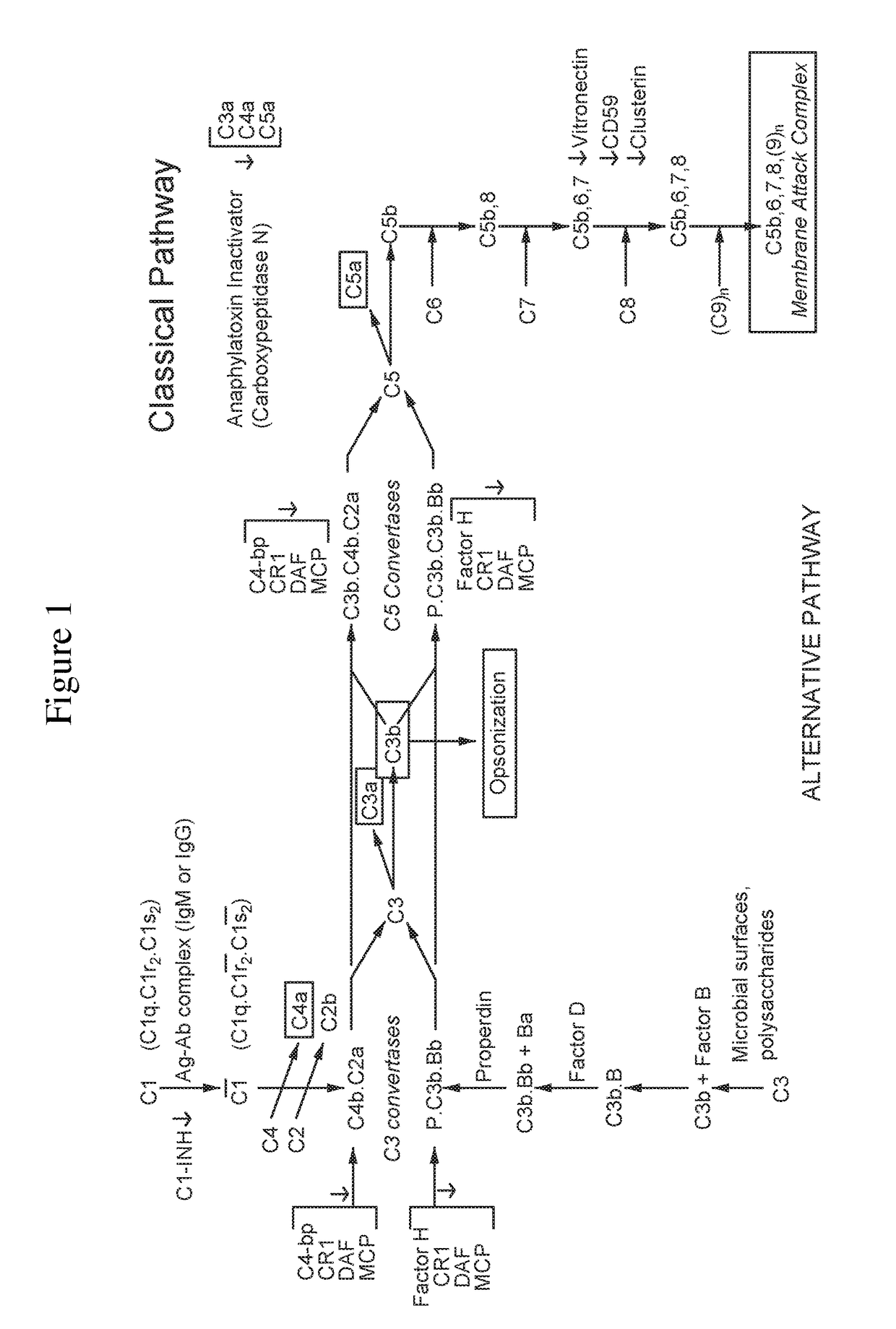
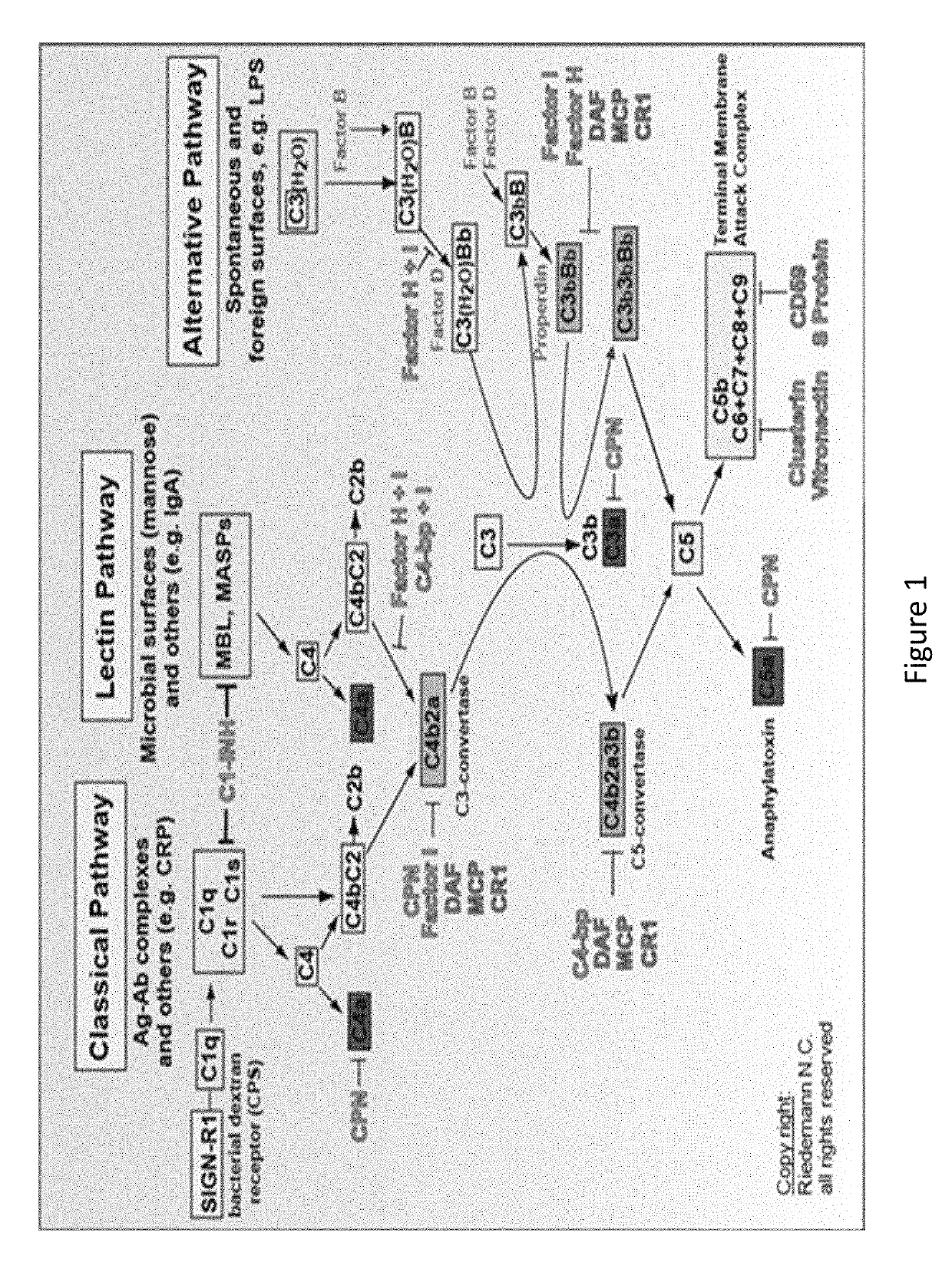
The present invention relates to methods and materials for modulating the complement alternative pathway (CAP), the complement classical pathway (CCP), the complement lectin / mannose pathway (CMP), or combinations thereof, as well as methods and materials for targeting diagnostic, prophylactic and therapeutic agents to localized areas of tissue within the body where they may more directly exert their effects upon the intended target cells or tissue, with reduced, associated systemic effects compared with administration of the same or similar agents in an untargeted, systemic manner. The methods and materials of the present invention may therefore allow for increased efficacy, lower threshold effective dosages and / or lower effective maintenance doses, and / or reduced associated undesired or adverse effects in terms of frequency or severity of occurrence, or both. The present invention also relates to methods and materials for modulating a host humoral immune response, especially reducing, inhibiting, or preventing a host humoral immune response.
Owner:MUSC FOUND FOR RES DEV +1
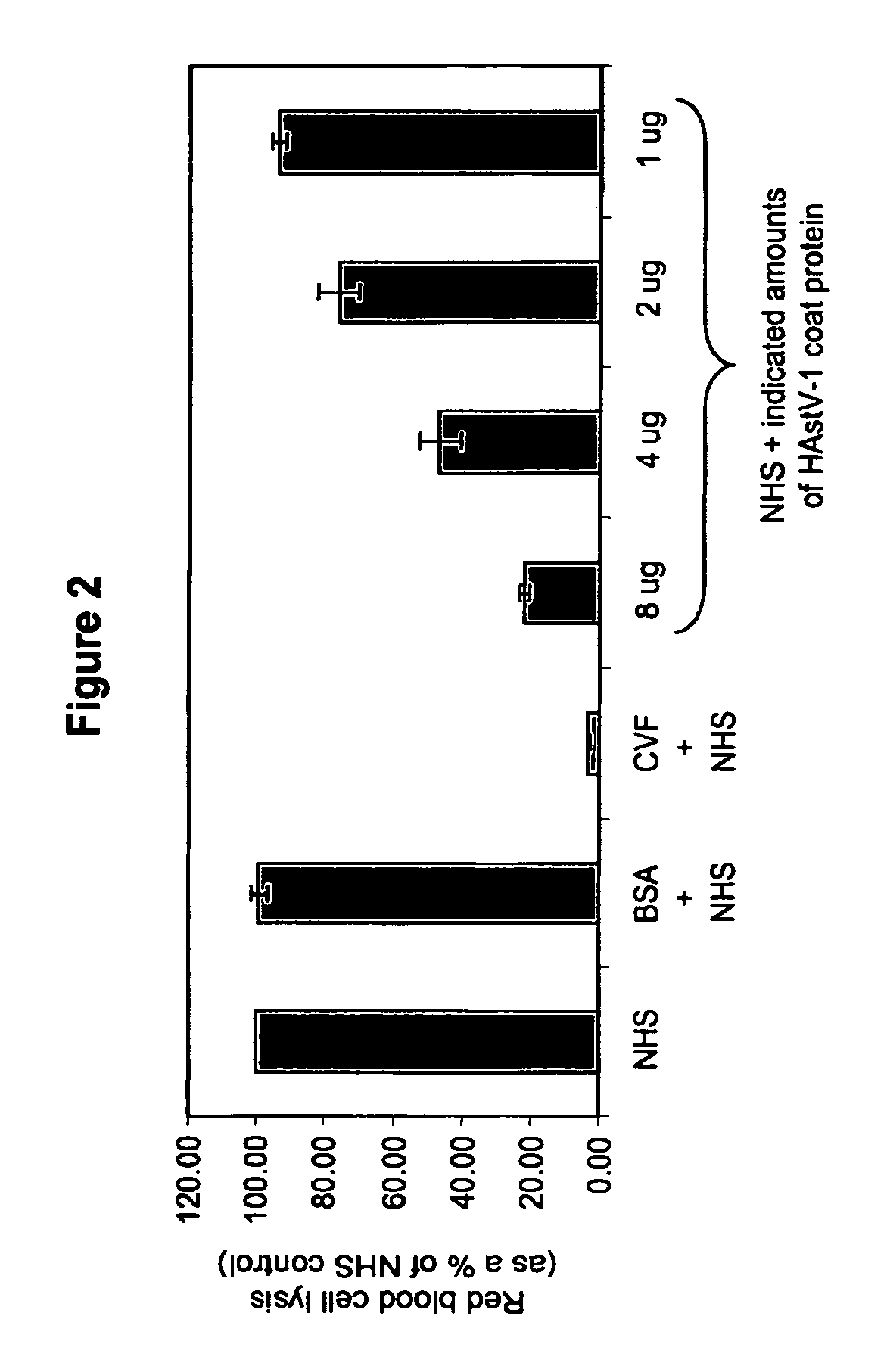
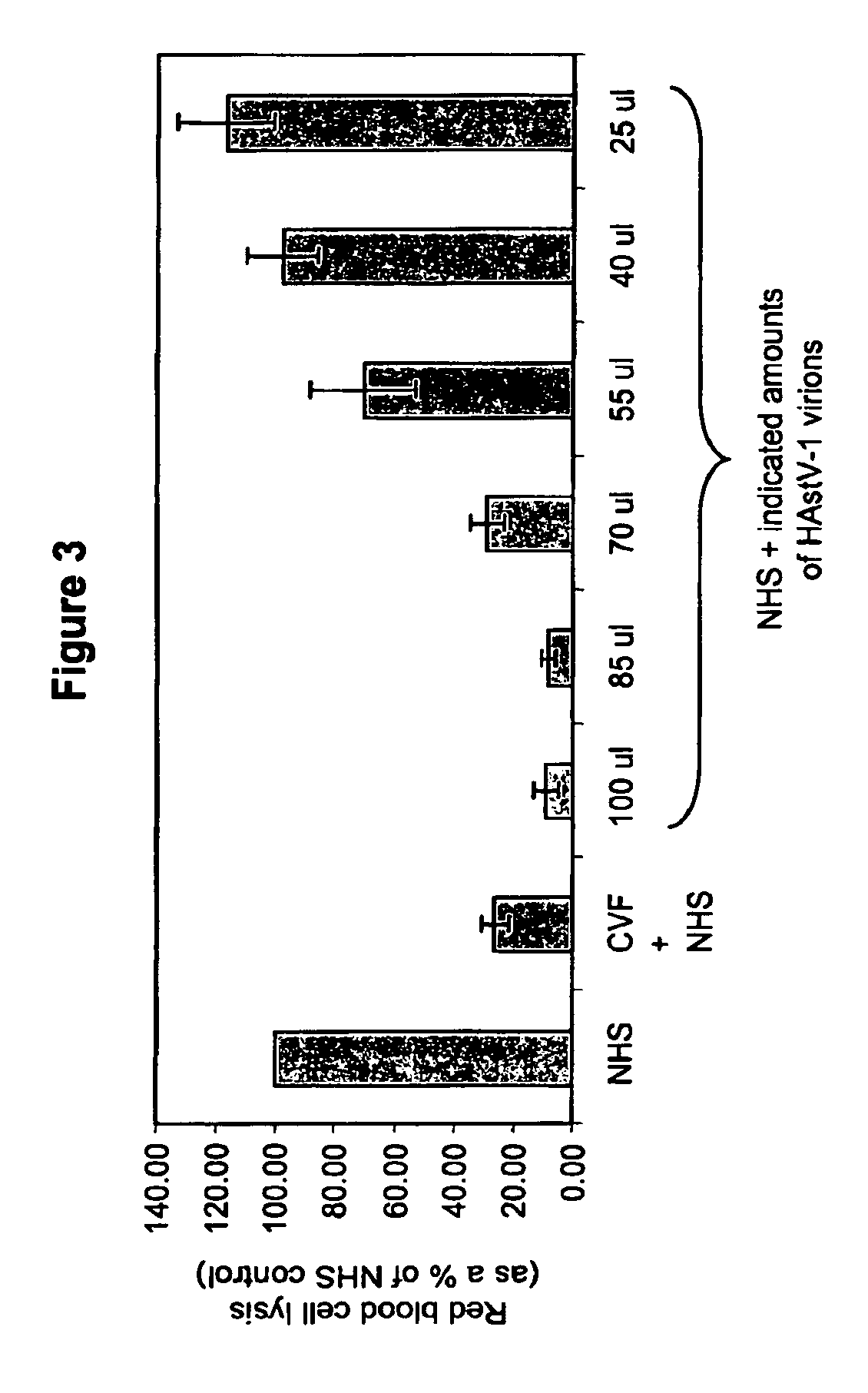
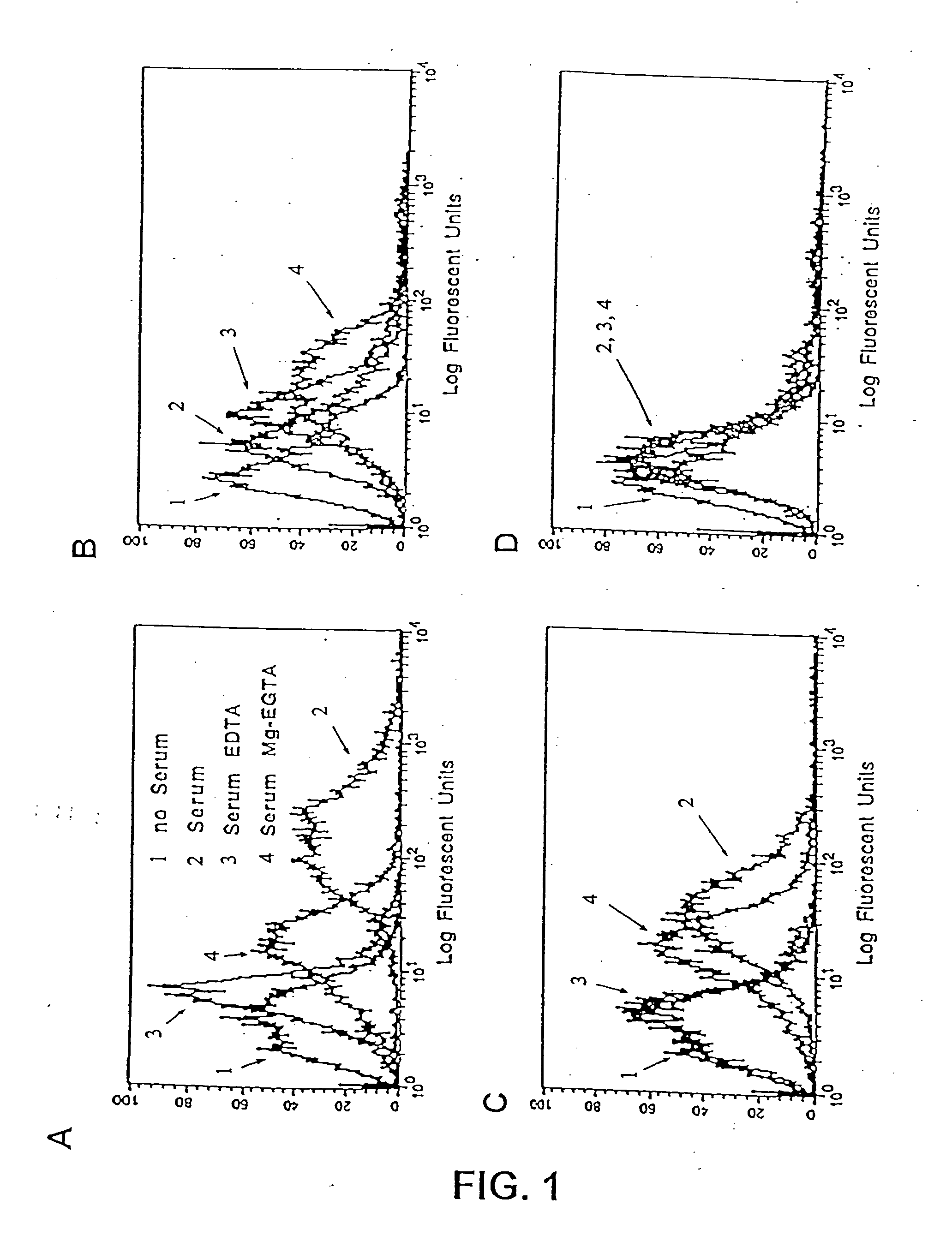
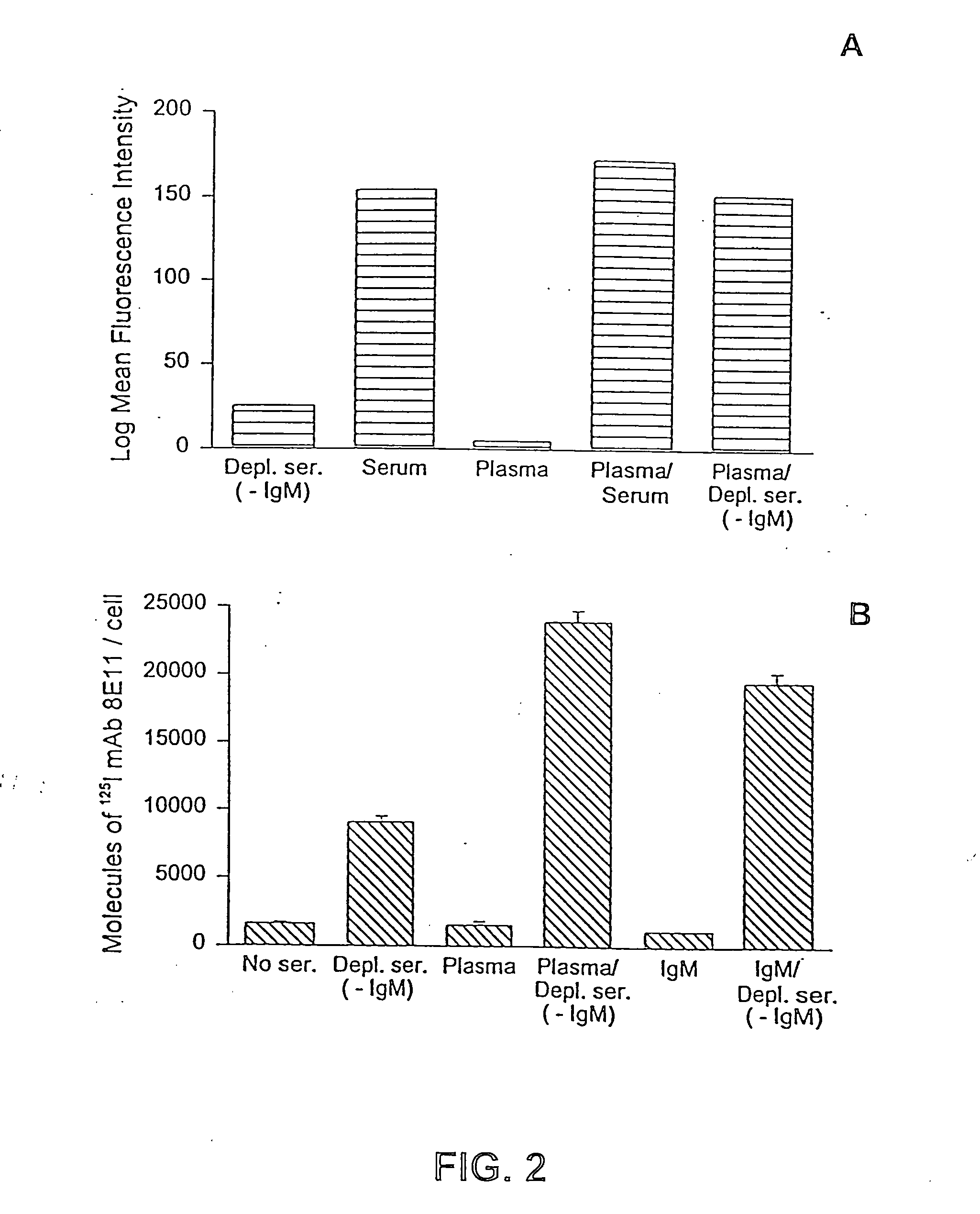
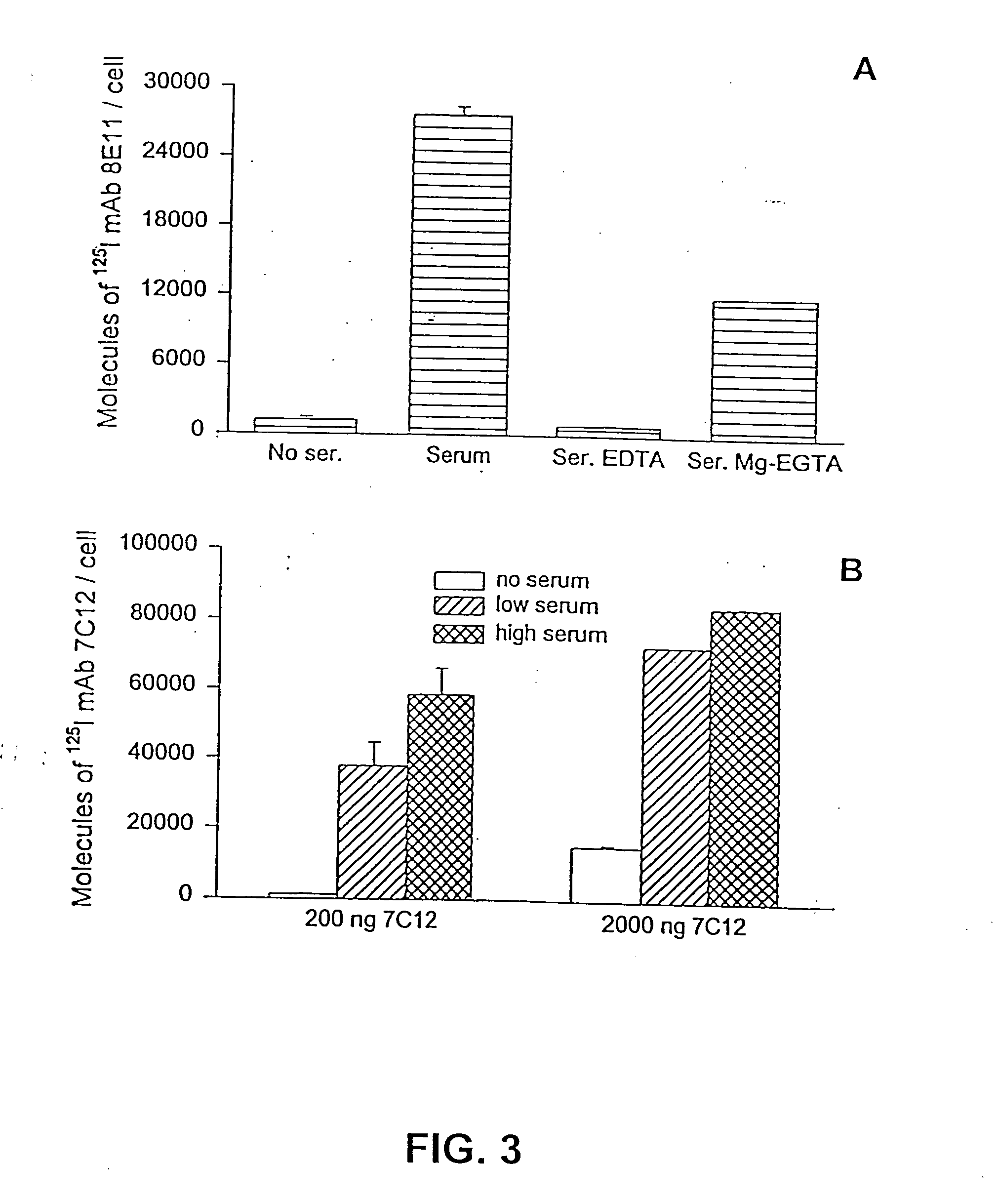
Methods for regulating complement cascade proteins using astrovirus coat protein and derivatives thereof
ActiveUS20100055106A1Decrease complement-related tissue damageReduce tissue damageNervous disorderSsRNA viruses positive-senseDiseaseFunctional activity
The present invention provides a method for modulating the complement cascade by depleting the plasma of the functional activity of complement proteins and thereby reducing or eliminating complement-mediated cell lysis. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated cell lysis and peptide mediators of inflammation. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated diseases. Methods are described herein where complement cascade, triggered by either the classical or alternative complement pathways, is prevented from effecting cell lysis and inflammation due to inhibition or depletion of one or more complement components in the serum following administration of astrovirus coat proteins or derivatives.
Owner:CHILDRENS RES HLDG LLC +1
Adsorption conduction device of residual harmful ingredient after removing intracranial hematoma
InactiveCN101288783AEffective drainageSimple structureWound drainsSuction devicesGynecologyAdditive ingredient
The invention relates to an adsorption drainage device for residual harmful composition after the clearance of intracranial hematoma, which includes a tee body, a drainage catheter and a plurality of adsorbent tubes of different materials. The main body of the tee body is hollow tube shape, the back end of the tee body is provided with internal threads, and a drainage port is arranged on the lateral part of the back end. The drainage catheter is connected with the drainage port of the tee body. The adsorbent tube is arranged in the hollow tube of the main body of the tee body, and the exposing part at the front end of the adsorbent tube is a semicircular sacculus which is loaded with RPPGF polypeptide, complement component monoclonal antibody and ZnPP in a way of mixing. The back end of the absorbent tube is matched with the internal threads at the back end of the tee body by external threads. Therefore, the absorbent tube is fixed in the tee body. The outer diameter of the adsorbent tube is smaller than the inner diameter of the tee body, and a space for liquid to flow to the drainage catheter is arranged between the adsorbent tube and the tee body. The back end of the adsorbent tube is sealed with a cap without a hole. The adsorption drainage device can be used for being matched with a hematoma grinding puncture needle and be used after a minimally invasive hematoma removing operation and provides an instrument which effectively drains the residual harmful compositions for the hemorrhage hematoma clearance operation. The adsorption drainage device is applied to neurology, neurosurgery, emergency department, etc. for the therapy of intracranial hematoma.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Methods for regulating complement cascade proteins using astrovirus coat protein and derivatives thereof
ActiveUS8241843B2Reduce tissue damageSsRNA viruses positive-senseNervous disorderDiseaseFunctional activity
The present invention provides a method for modulating the complement cascade by depleting the plasma of the functional activity of complement proteins and thereby reducing or eliminating complement-mediated cell lysis. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated cell lysis and peptide mediators of inflammation. The invention provides a method for the therapeutic use of coat proteins and derivatives thereof from the Astroviradae family of viruses in the treatment of complement-mediated diseases. Methods are described herein where complement cascade, triggered by either the classical or alternative complement pathways, is prevented from effecting cell lysis and inflammation due to inhibition or depletion of one or more complement components in the serum following administration of astrovirus coat proteins or derivatives.
Owner:CHILDRENS RES HLDG LLC +1
Antibodies to a tumor-associated surface antigen for delivery of diagnostic and therapeutic agents
InactiveUS20040228860A1Prevent and inhibitInhibition is effectiveOrganic active ingredientsPeptide/protein ingredientsCancer preventionIgm antibody
The present invention relates to the treatment, inhibition and prevention of cancer by the administration of anti-C3b(i) antibodies. The invention also relates to the treatment, inhibition and prevention of cancer by the administration of IgM antibodies and / or complement components prior to the administration of anti-C3b(i) antibodies. The present invention further relates to the detection, imaging, diagnosis and monitoring of cancer utilizing C3b(i) specific antibodies.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Diagnostic markers of liver dysfunction
InactiveUS20030087316A1Rapid and reproducibleLess expensiveMicrobiological testing/measurementBiological material analysisDiseaseIn vivo
The present invention provides methods and kits for detecting and monitoring liver damage in a subject. The methods and kits rely on the correlation between the presence or increase in a kallikrein-like peptidase in a sample from the subject and liver disease. Additionally, the present invention provides in vivo and in vitro methods for detecting toxicity of a therapeutic agent. Methods for detecting, diagnosing, or monitoring liver damage by measuring a panel of components, including kallikrein-like peptidase, along with other blood enzymes and / or complement components are also provided.
Owner:THE SCRIPPS RES INST +1
Controlled activation of complement components for use as endogenous adjuvant
InactiveUS20130337045A1Effective stimulationEnhance immune responsePowder deliverySnake antigen ingredientsAntigenAdjuvant
The invention relates to a pharmaceutical composition made of one or more preparation and comprising a therapeutically effective dose of at least one recombinant human C3-derivative and at least one antigen für vaccination.
Owner:PLS DESIGN +1
Identification and monitoring of systemic lupus erythematosus
ActiveUS7588905B2Bioreactor/fermenter combinationsBiological substance pretreatmentsPlateletSystemic lupus
A method for identifying or monitoring SLE in an individual is provided. The method includes quantitating complement component C4d on the surfaces of platelets and comparing the amounts of C4d to reference levels of C4d on platelets of individuals without SLE and / or on platelets of the individual obtained at a different time. Kits for use in the above-described methods are provided along with computer readable media tangibly embodying executable instructions to perform the methods.
Owner:UNIVERSITY OF PITTSBURGH
Evaluation Method for Arteriosclerosis
ActiveUS20130040851A1Microbiological testing/measurementLibrary screeningProtein markersVon Willebrand factor
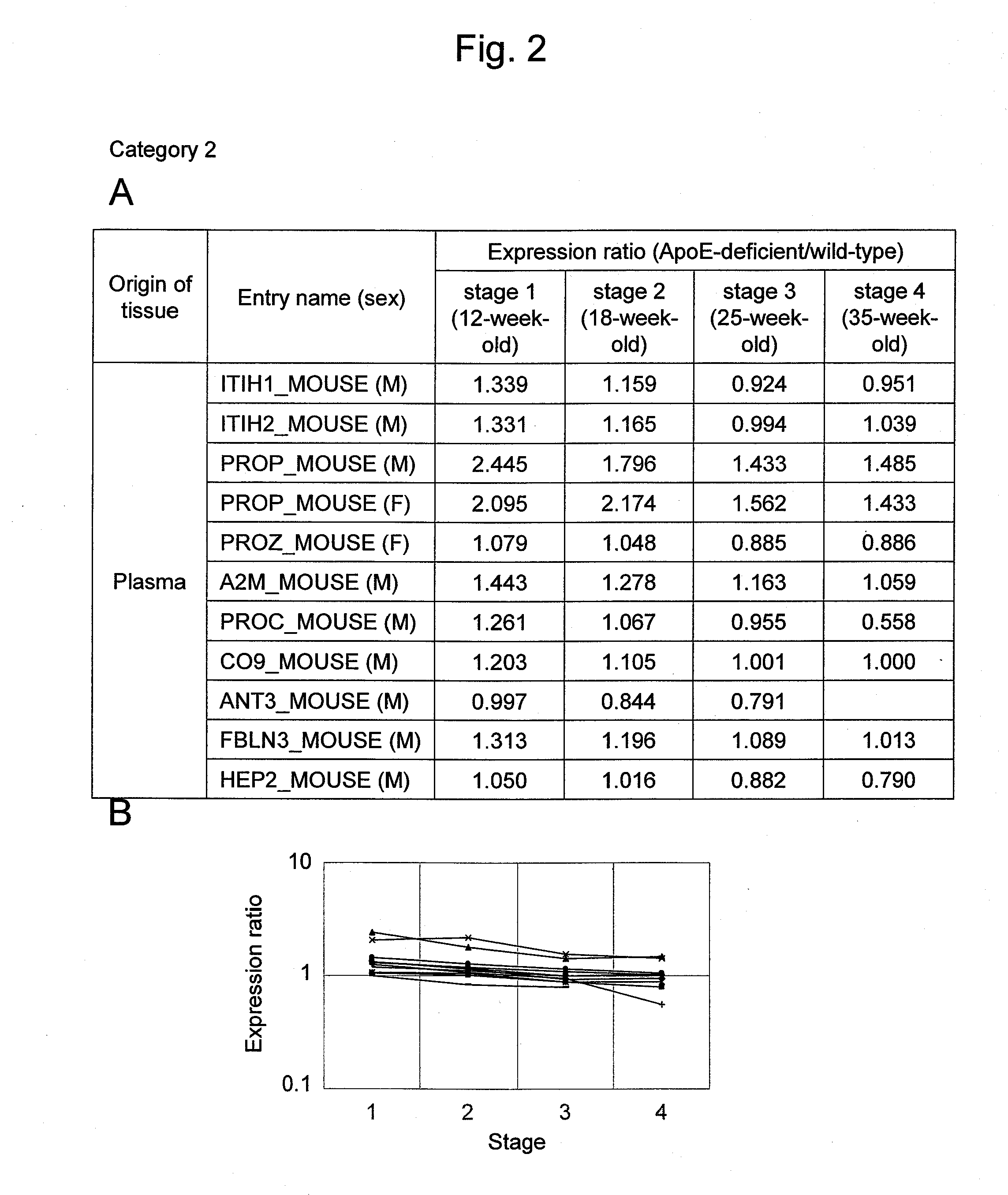
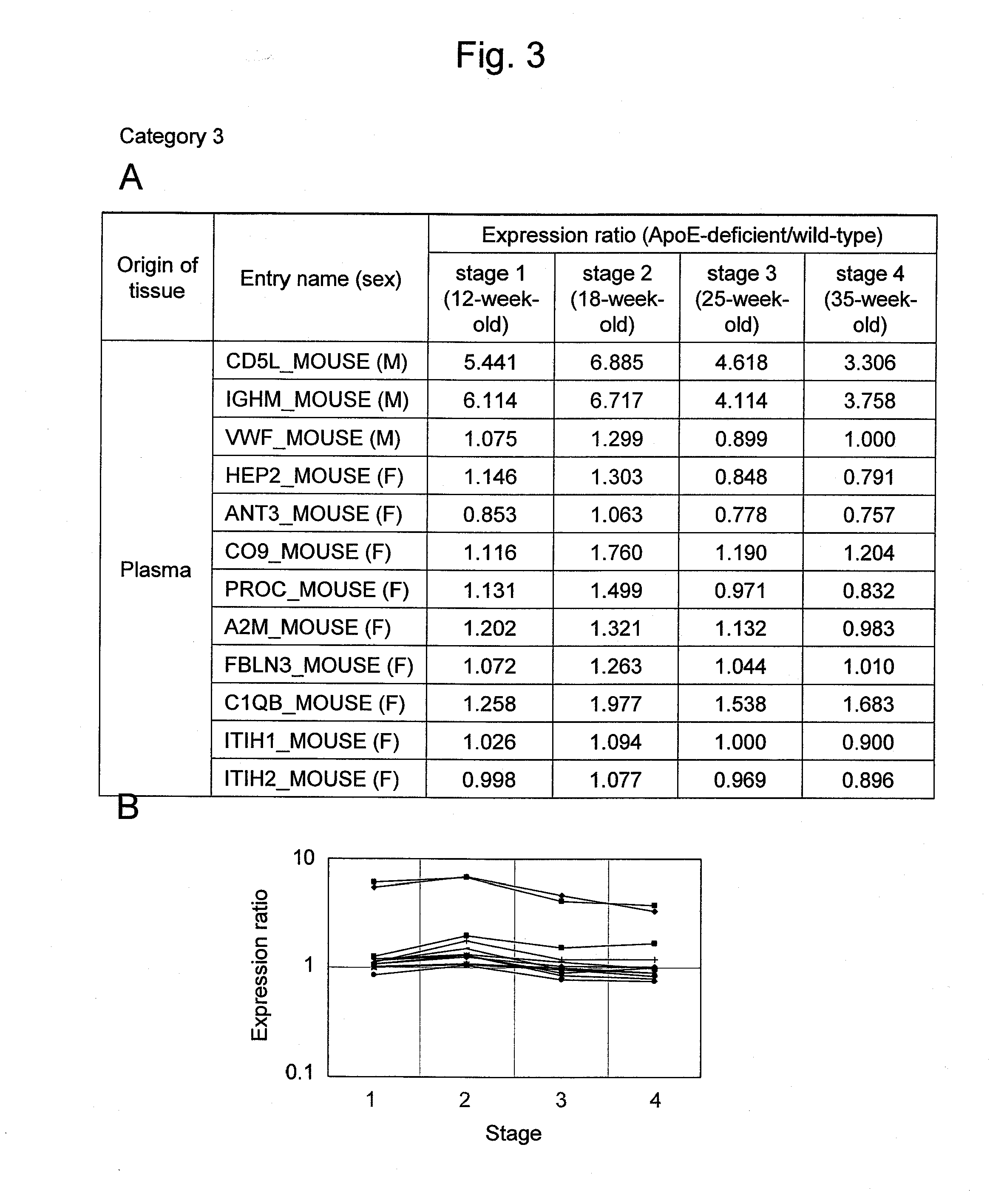
Arteriosclerosis induces cerebral infarction and myocardial infarction. A multi-marker (a group of protein markers) that assesses the accurate pathogenesis of arteriosclerosis and enables the selection of an adequate treatment method for arteriosclerosis and prediction of the progression of arteriosclerosis, and an evaluation method for the diagnosis, prevention, and treatment of arteriosclerosis that uses said marker group as an indicator have been sought. The present invention relates to A method for evaluation of arteriosclerosis comprising the steps of (a) measuring the expression of von Willebrand factor and / or complement factor D in a sample derived from a subject, (b) measuring the expression of complement component C8 and / or vitamin K-dependent protein Z in the sample derived from the subject, and (c) evaluating arteriosclerosis in the subject on the basis of the results from (a) and (b).
Owner:HITACHI LTD
Complement binding aptamers and anti-C5 agents useful in the treatment of ocular disorders
Methods of treating complement-mediated ocular disorders by administering agents that inhibit a subject's complement component in an amount sufficient to treat the ocular disorder wherein, in a selected embodiment, said agent is an anti-complement aptamer that, in a preferred embodiment, is an anti-C5 aptamer.
Owner:ARCHEMIX CORP
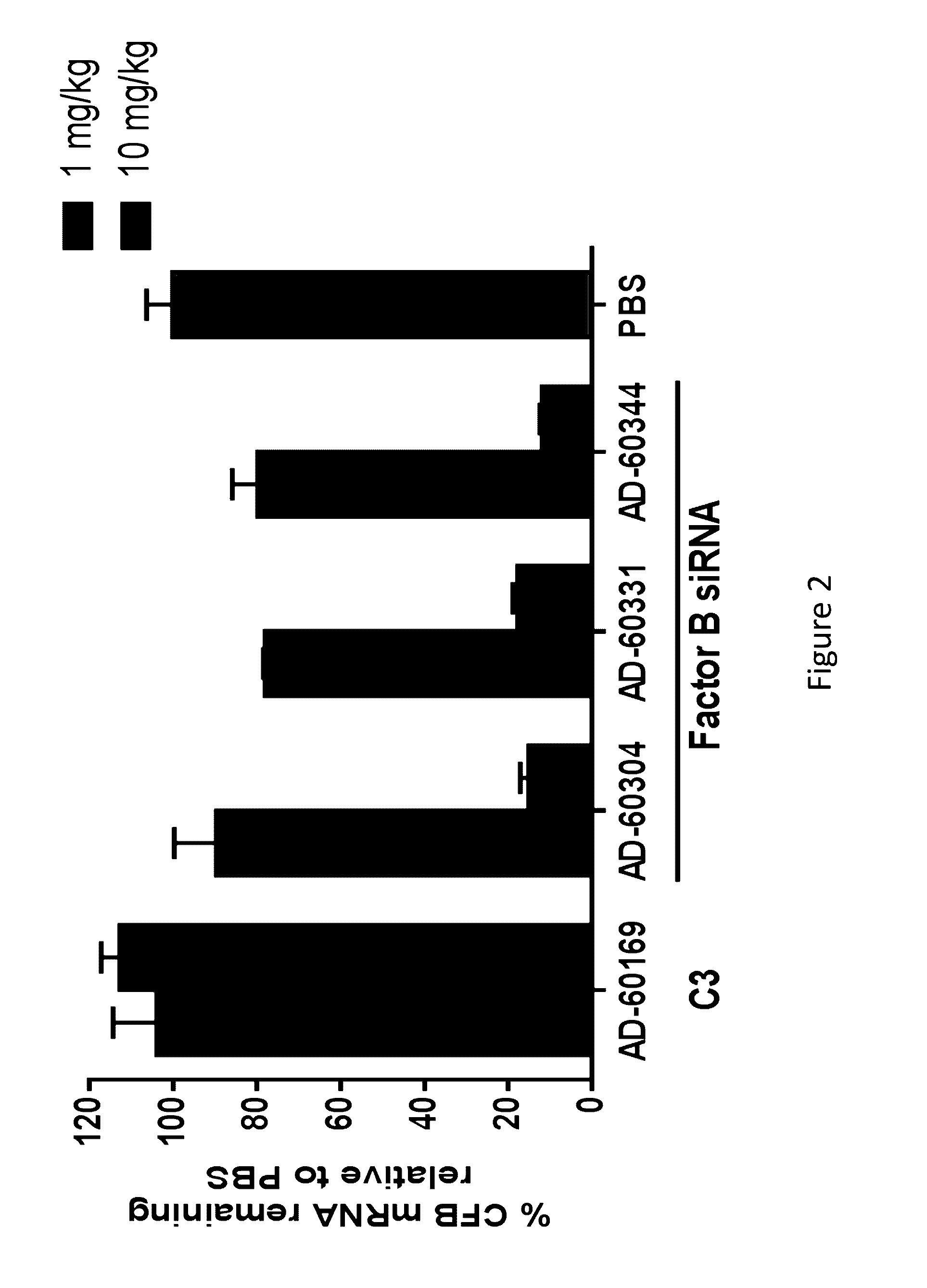
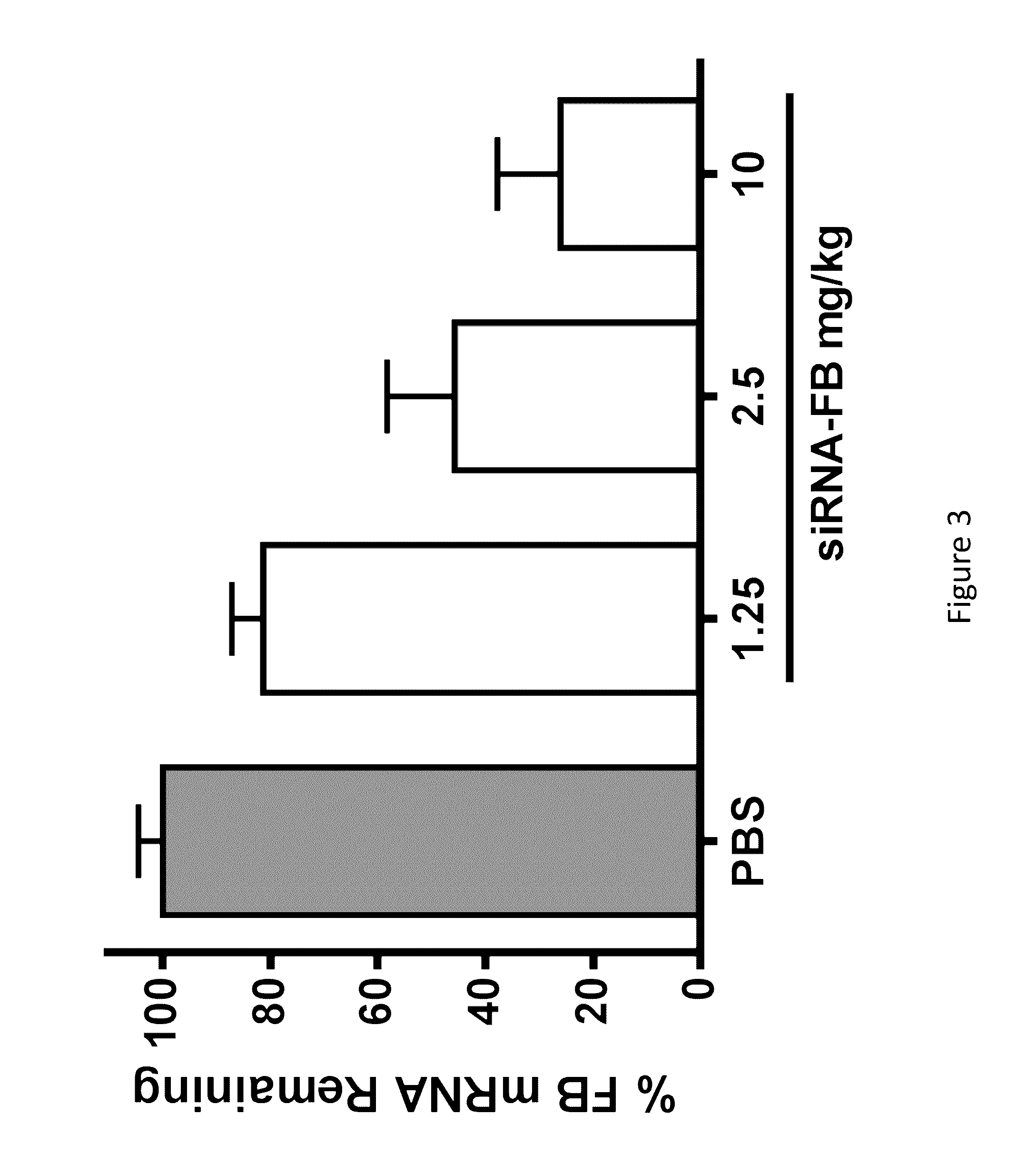
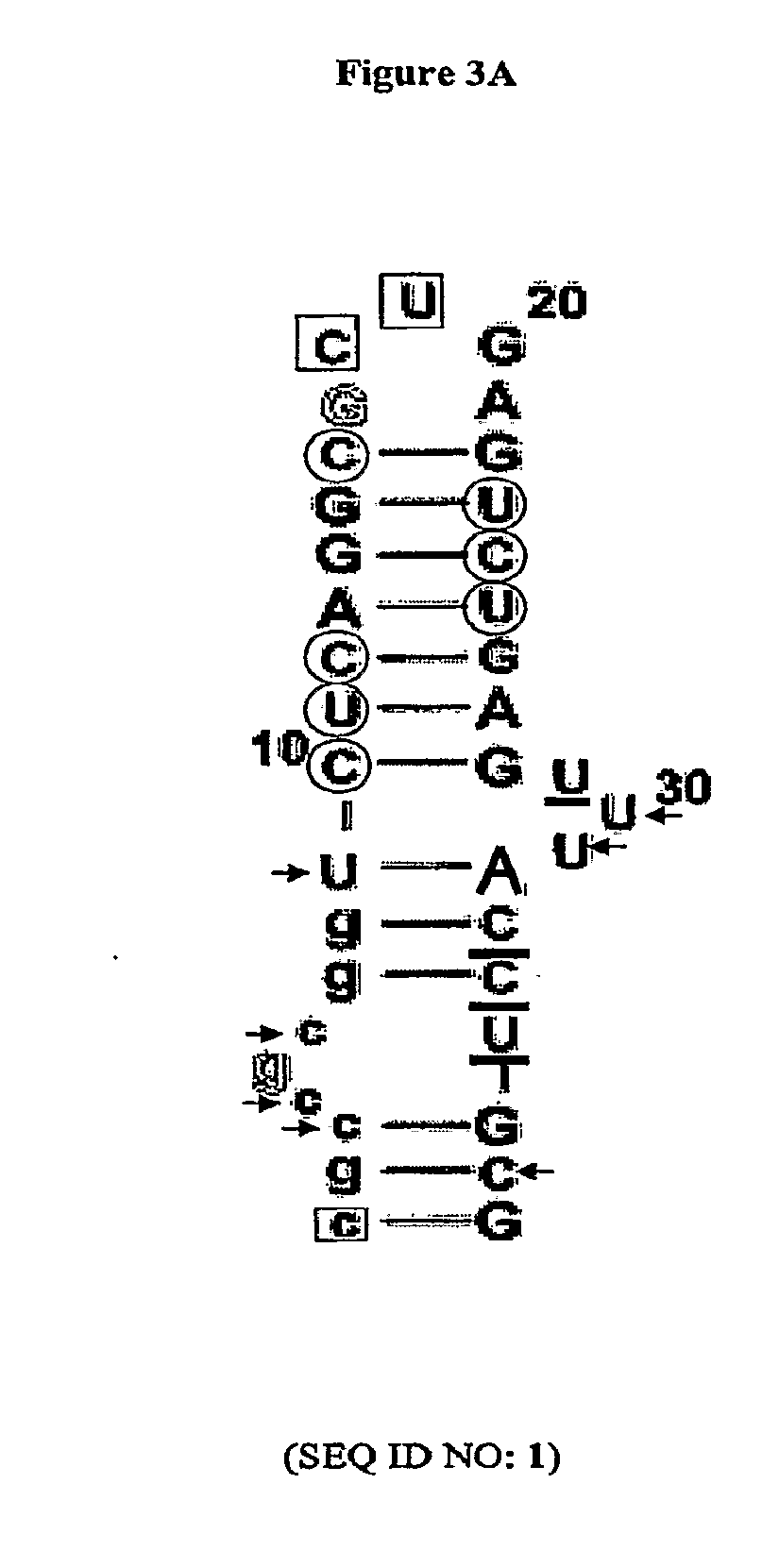
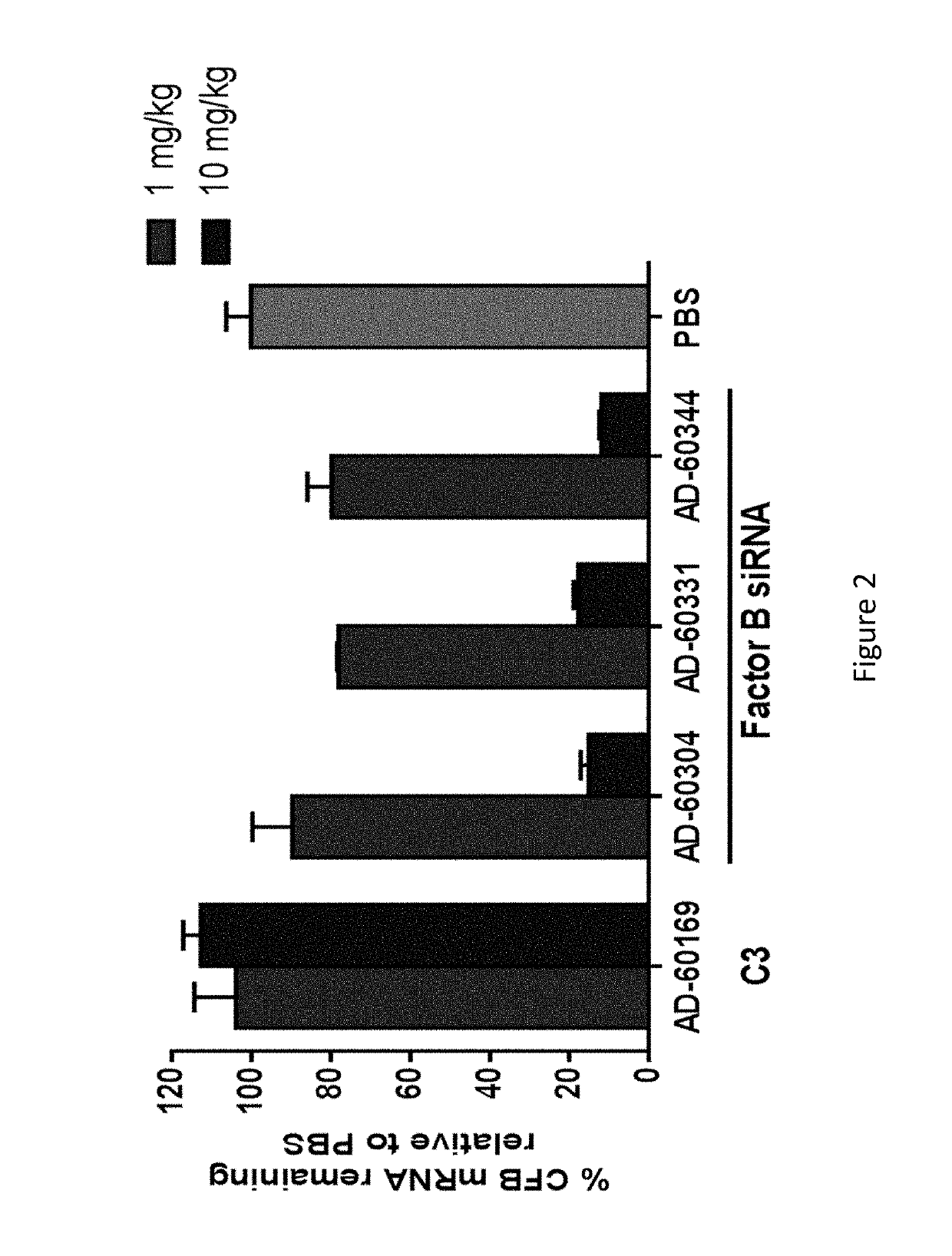
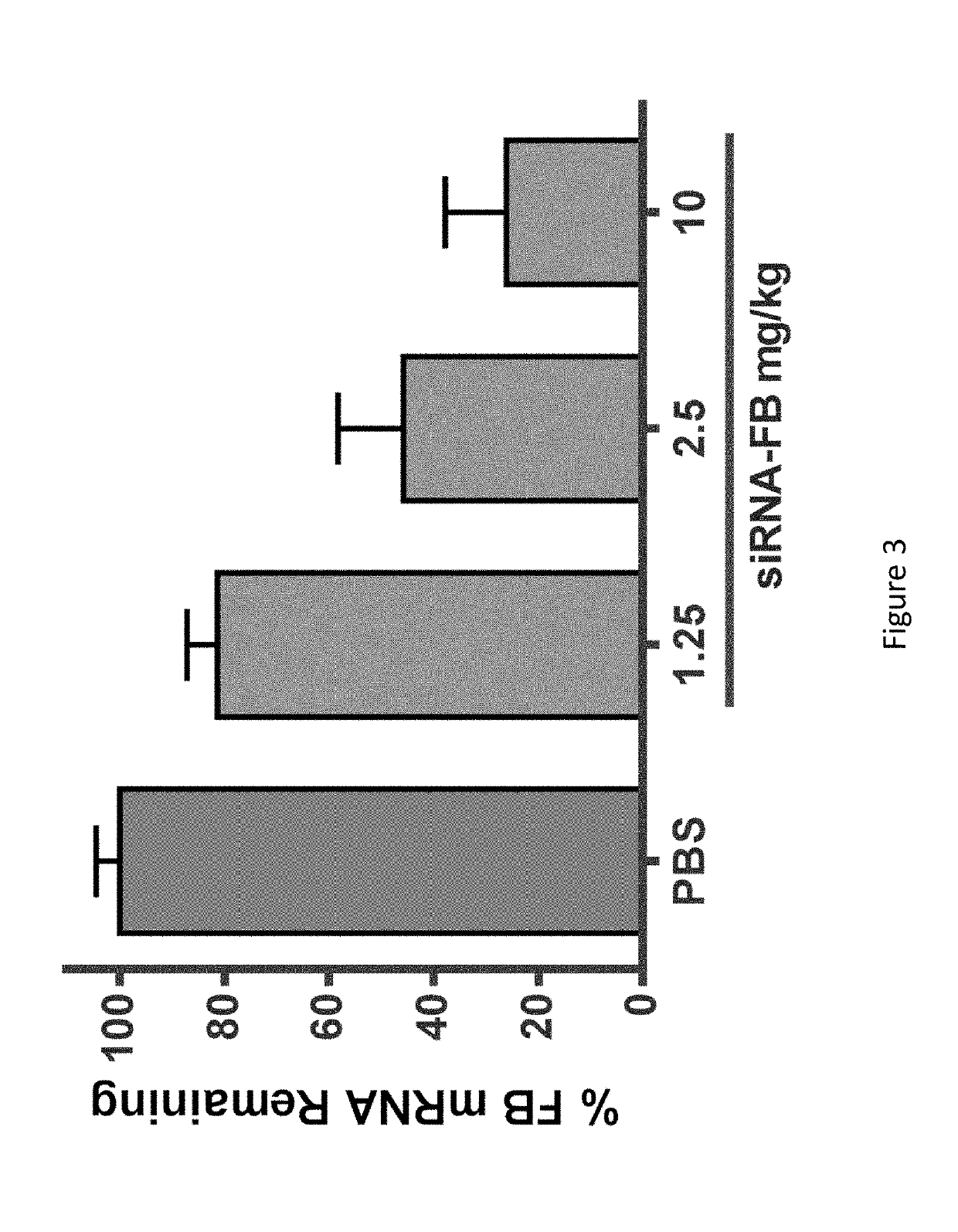
COMPLEMENT COMPONENT iRNA COMPOSITIONS AND METHODS OF USE THEREOF
ActiveUS20160298124A1Avoid symptomsReduce expressionAntibacterial agentsOrganic active ingredientsParoxysmal AFDisease
The invention relates to iRNA, e.g., double-stranded ribonucleic acid (dsRNA), compositions targeting the complement factor B (CFB) gene, the complement component C3 gene, and the complement component C9 gene and methods of using such iRNA, e.g., dsRNA, compositions to inhibit expression of CFB, C9 and / or C3 and to treat subjects having a complement component-associated disease, e.g., paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Owner:ALNYLAM PHARM INC
Apparatus and method for detecting multiple hits in CAM arrays
An apparatus and method are disclosed for detecting multiple hits in CAM arrays. A binary address value is stored for each entry of the CAM array and is output to identify the matching entry for a single hit. However, to facilitate multiple hit detection, both the true and complement components of this address are stored and output to determine whether or not a multiple hit occurred. If a multiple hit occurs (e.g., more than one address location has been matched), all the bits that make up the binary address and the complement will not be complements of each other and a multiple hit condition can be detected by XORing each bit of an address location value with the complement of that address location value. If the XORed bits are equal to “1”, then a single hit has occurred. Otherwise, a multiple hit has occurred.
Owner:INT BUSINESS MASCH CORP
Complement Binding Aptamers and Anti-C5 Agents Useful in the Treatment of Ocular Disorders
Methods of treating complement-mediated ocular disorders by administering agents that inhibit a subject's complement component in an amount sufficient to treat the ocular disorder wherein, in a selected embodiment, said agent is an anti-complement aptamer that, in a preferred embodiment, is an anti-C5 aptamer.
Owner:ARCHEMIX CORP
Kits comprising satiety-inducing formulations
InactiveUS20190110514A1Efficient deliveryImprove integrityFood coatingFood shapingMeal replacementPowder mixture
The invention provides a kit comprising a satiety inducing component A, useful for the treatment and / or prevention of obesity; and a component B complementing component A. Component A comprises a first ingestible solid formulation of a lipid material and a water-swellable or water-soluble polymer, said first ingestible solid formulation being provided in form of ingestible particles that may be used as a partial or full meal replacement. Component B complements component A in that it provides e.g. specific nutrients such as unsaturated fatty acids, vitamins, proteins, amino acids, micro-nutrients, dietary elements, dietary fibres or combinations thereof to a human subject in order to reduce the risk of any deficiencies thereof during a dieting schedule. These nutrients in component B may be provided in an oil, a granulate and / or a powder or powder blend. Alternatively, component B may comprise a second ingestible solid formulation similar to the first ingestible solid formulation, which may be provided in form of ingestible particles, but also in other forms than component A, e.g. as snack-bars.
Owner:PERORA
Complement component iRNA compositions and methods of use thereof
ActiveUS10465194B2Avoid symptomsReduce expressionAntibacterial agentsOrganic active ingredientsParoxysmal AFDisease
The invention relates to iRNA, e.g., double-stranded ribonucleic acid (dsRNA), compositions targeting the complement factor B (CFB) gene, the complement component C3 gene, and the complement component C9 gene and methods of using such iRNA, e.g., dsRNA, compositions to inhibit expression of CFB, C9 and / or C3 and to treat subjects having a complement component-associated disease, e.g., paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Owner:ALNYLAM PHARMA INC
Targeting specificity complement system inhibitor and preparation method and application thereof
ActiveCN104231085AComplement inhibition is achievedNervous disorderPeptide/protein ingredientsPharmacyMedicine
The invention belongs to the field of biological pharmacy, and particularly relates to a targeting specificity complement system inhibitor and the design, preparation and clinical application of the targeting specificity complement system inhibitor. CRIg which can be combined with fragments C3b and / or iC3b which are formed after a complement component C3 is activated to generate targeting effect is directly connected with another component with a complement inhibiting effect, for example factor H or is indirectly connected by virtue of a flexible peptide fragment (Gly4Ser)3 and the like to prepare a fusion protein by methods such as genetic engineering, thus achieving the effect of inhibiting complement activation in a targeting manner. The medicine can be applied to treatment and prevention of a plurality of human diseases which are related to abnormal complement activation.
Owner:SHANGHAI COMGEN BIO PHARMA CO LTD
Therapeutic regimens for treatment of paroxysmal nocturnal hemoglobinuria
PendingUS20200101071A1Growth inhibitionIncrease depositionOrganic active ingredientsImmunoglobulins against animals/humansParoxysmal AFRegimen
Provided herein are methods for treating a subject with PNH comprising administering to a subject a therapeutically effective amount of complement component C5 (C5) inhibitor, complement component C3 (C3) inhibitor, or complement factor B (CFB) inhibitor in combination with a therapeutically effective amount of small molecule complement factor D (CFD) inhibitor of Formula I or Formula II, or a pharmaceutically acceptable salt thereof.
Owner:ACHILLION PHARMA INC
Antibodies to the C3d fragment of complement component 3
ActiveUS9815890B2Effective treatment and preventionReduce systemic side effectsAntibacterial agentsSenses disorderEfficacyComplement component 3
The present invention relates to methods and materials for modulating the complement alternative pathway (CAP), the complement classical pathway (CCP), the complement lectin / mannose pathway (CMP), or combinations thereof, as well as methods and materials for targeting diagnostic, prophylactic and therapeutic agents to localized areas of tissue within the body where they may more directly exert their effects upon the intended target cells or tissue, with reduced, associated systemic effects compared with administration of the same or similar agents in an untargeted, systemic manner. The methods and materials of the present invention may therefore allow for increased efficacy, lower threshold effective dosages and / or lower effective maintenance doses, and / or reduced associated undesired or adverse effects in terms of frequency or severity of occurrence, or both. The present invention also relates to methods and materials for modulating a host humoral immune response, especially reducing, inhibiting, or preventing a host humoral immune response.
Owner:MUSC FOUND FOR RES DEV +1
Stable polypeptides binding to human complement c5
ActiveUS20160311870A1Improve stabilityRetain biological activitySenses disorderNervous disorderComplement component 5Biology
The invention relates to a polypeptide capable of binding human complement component 5 (C5), said polypeptide comprising the amino acid sequence(SEQ ID NO: 296)[BM]-[L2]-QSX42X43LLX46EAKKLX52X53X54Qwherein [BM] is a C5 binding motif; [L2] is an interconnecting loop; X42 is selected from A and S; X43 is selected from N and E; X46 is selected from A, S and C; X52 is selected from E, N and S; X53 is selected from D, E and S, provided that X53 is not D when X52 is N; and X54 is selected from A and S.
Owner:IPC RES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com