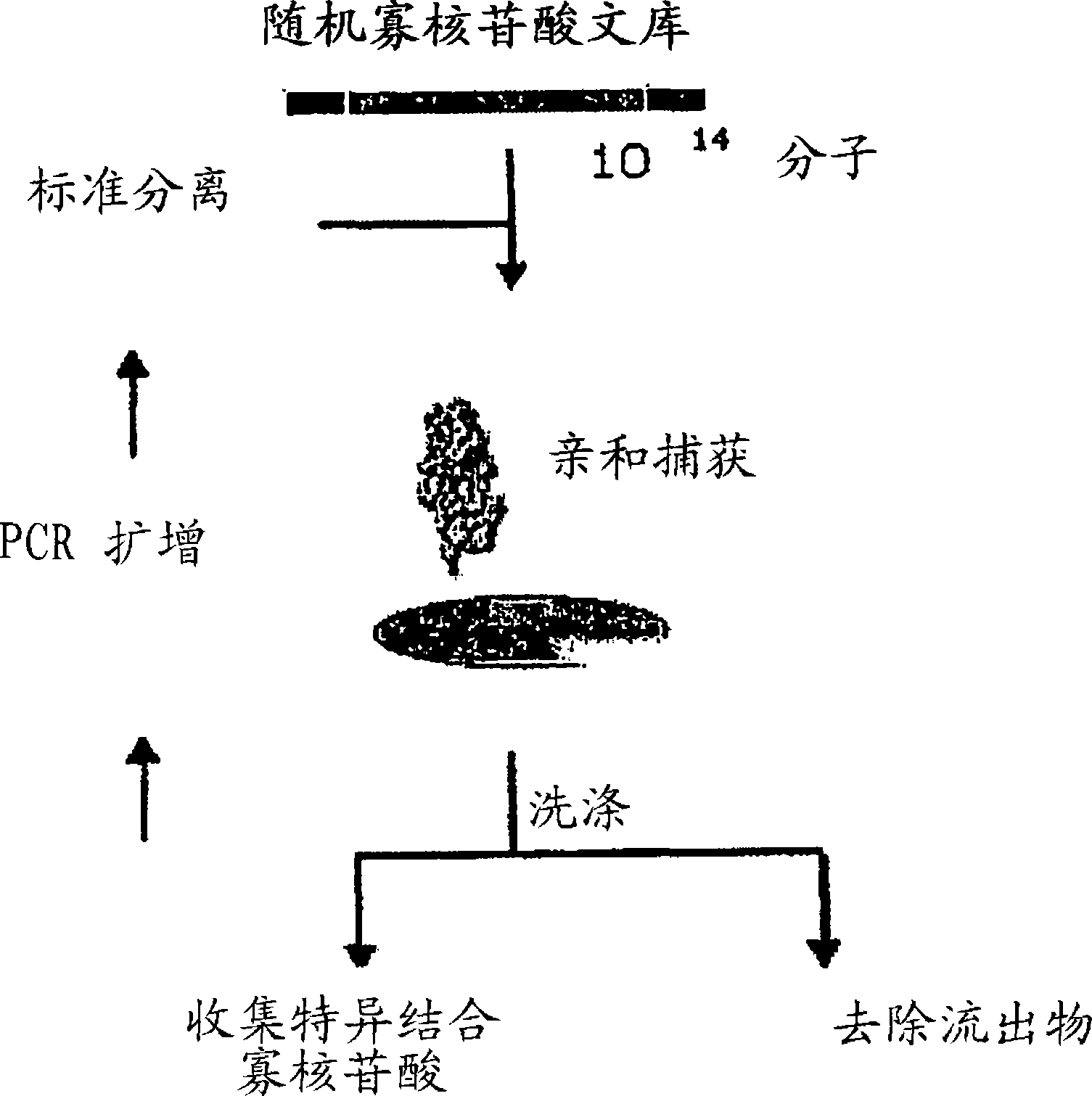
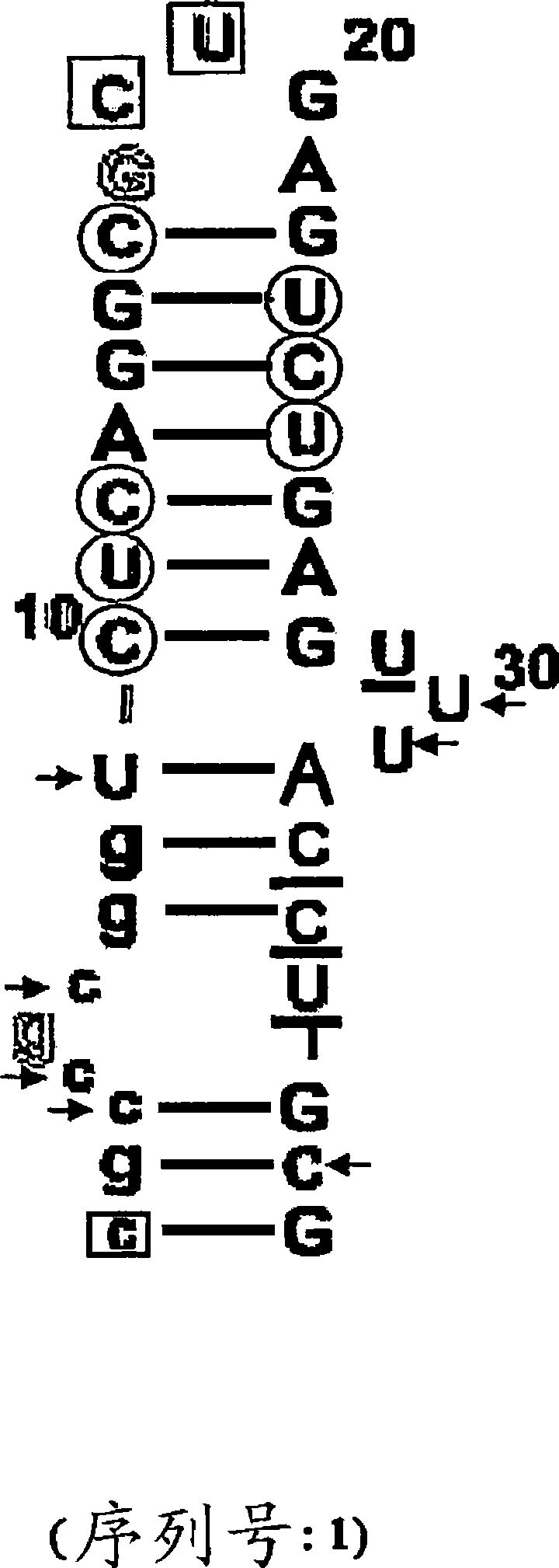Complement binding aptamers and anti-C5 agents useful in the treatment of ocular disorders
A complement and aptamer technology, applied in the field of nucleic acids, can solve problems such as toxicity and immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
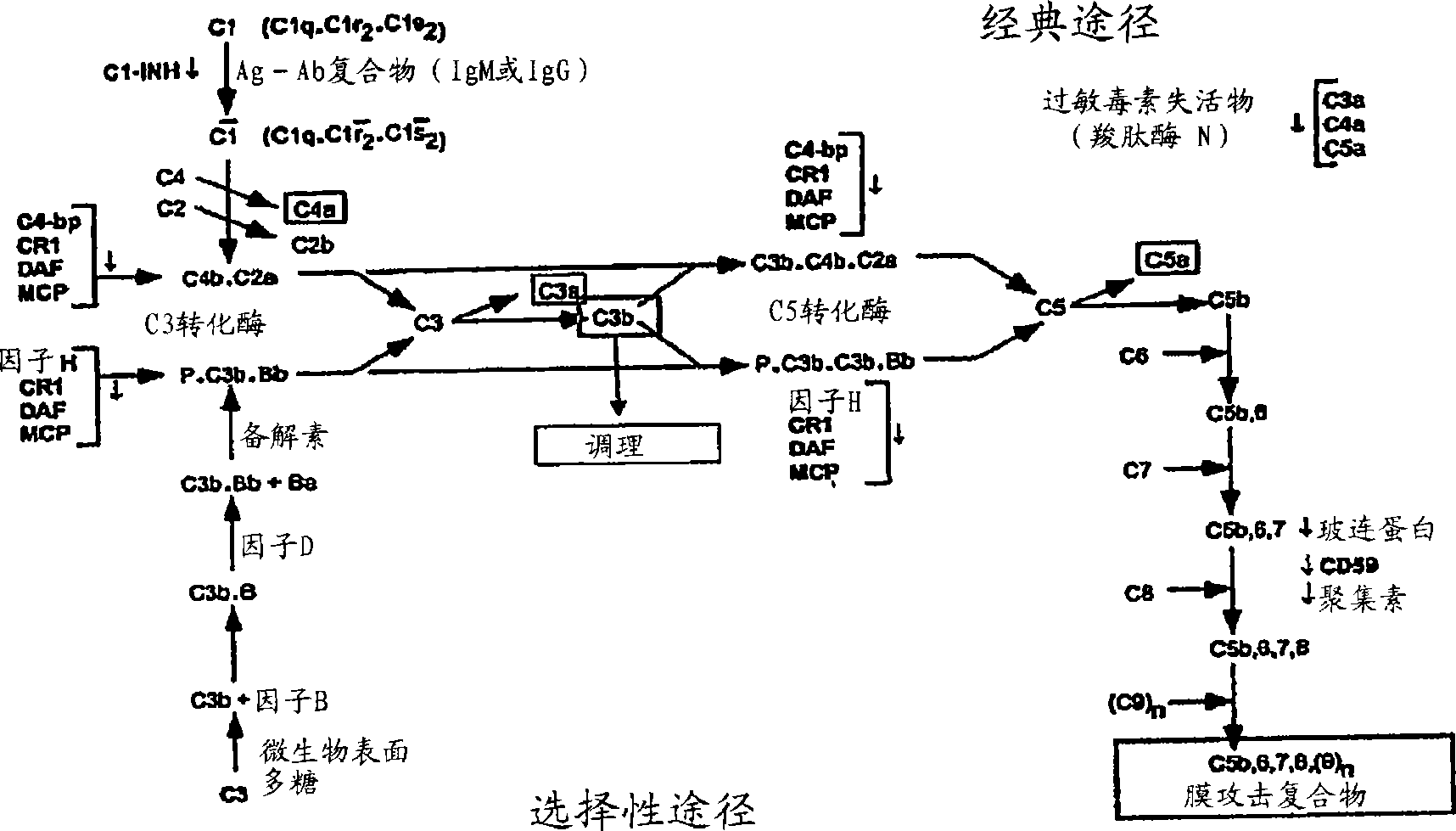
[0717] Anti-C5 aptamer activity in the classical and alternative complement pathways
Embodiment 1A
[0718] Example 1A: Hemolysis Test
[0719] The CH50 assay tests the ability of the complement system to solubilize 50% of a standard suspension of antibody-coated sheep red blood cells in a serum test sample. 0.2% human serum was mixed with antibody-coated sheep erythrocytes (DiamedixEZ Complement CH50 Kit, Diamedix Corp, Miami, FL) in the presence or absence of various anti-C5 aptamers. Operated according to the kit protocol, in barbiturate-buffered saline solution containing calcium, magnesium and 1% gel (GVB ++ complement buffer) at 37°C for 30 minutes. After incubation, samples were centrifuged to pellet intact red blood cells. The absorbance (OD) of the suspension was measured at 412nm 412 ) to quantify soluble hemoglobin, which is proportional to the degree of hemolysis (Green et al. (1995) Chem. Biol. 2:683-95). To verify that the aptamer inhibited C5 activity, some hemolyzed supernatants were analyzed for the presence of C5a and C5b-9 by ELISA according to the pr...
Embodiment 1B
[0737] Example 1B: Whole blood assay.
[0738] The following whole blood assay was used to analyze the effect of anti-C5 aptamers on the selective pathways of the complement system. Blood was isolated from normal human volunteers in the absence of anticoagulants. Aliquots of blood (without anti-anticoagulant) were reacted with increasing concentrations of ARC186 (sequence number: 4) at room temperature or 37°C for 5 hours. The serum was separated from the samples by centrifugation, and the presence of C5b in the serum was determined by sC5b-9 ELISA (C5b-9 ELISA kit, Quidel, San Diego, CA). Such as Figure 15 As shown, the anti-complement activity represented by C5b-9 has a deviation of 3 μM between samples incubated at different temperatures. The room temperature data showed that the aptamer concentrations required for quantitative inhibition ranged from 3–6 μM, while the reported C5 concentration was approximately 400 nM. These results show that more than a 10-fold mola...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com