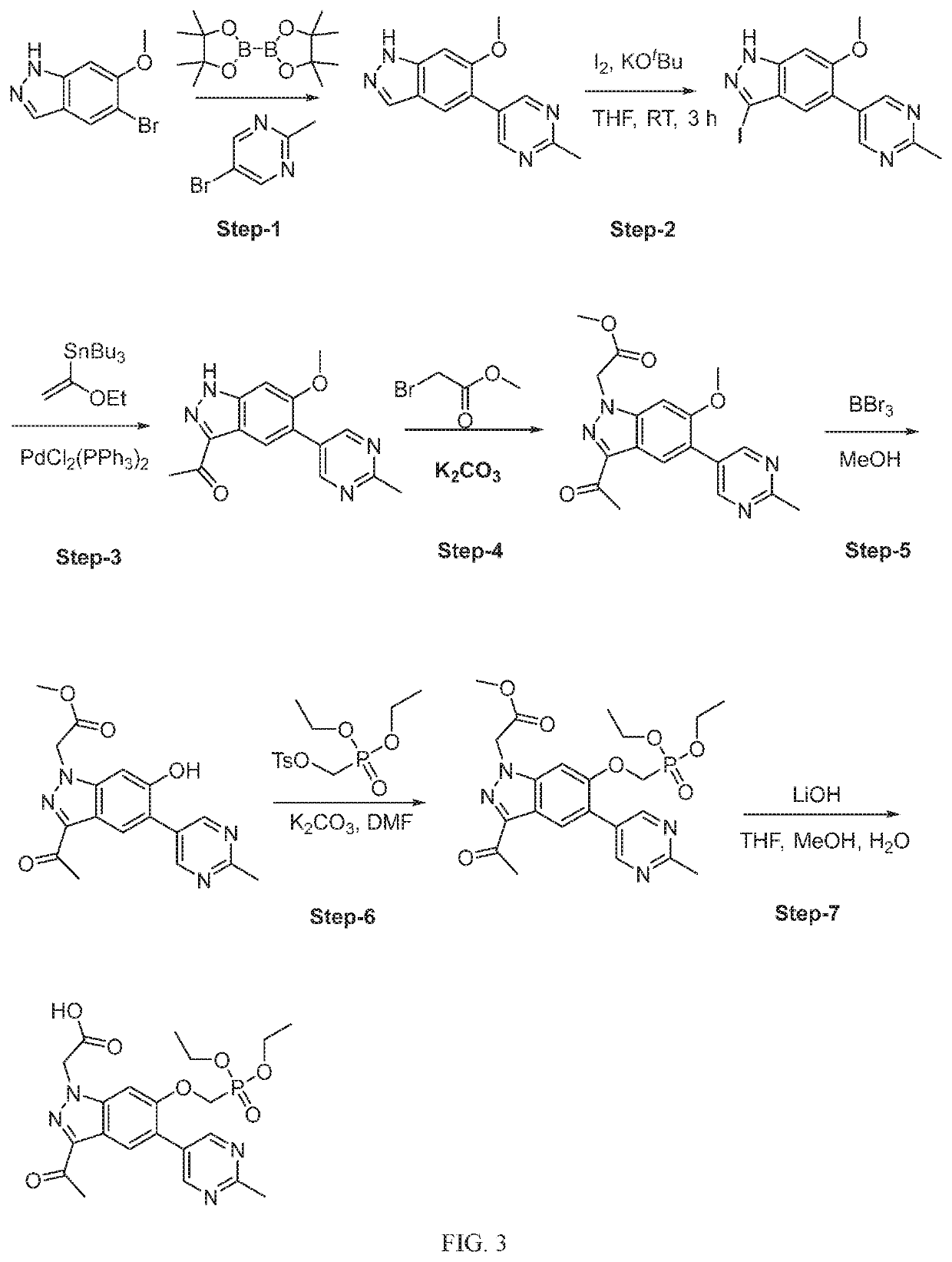
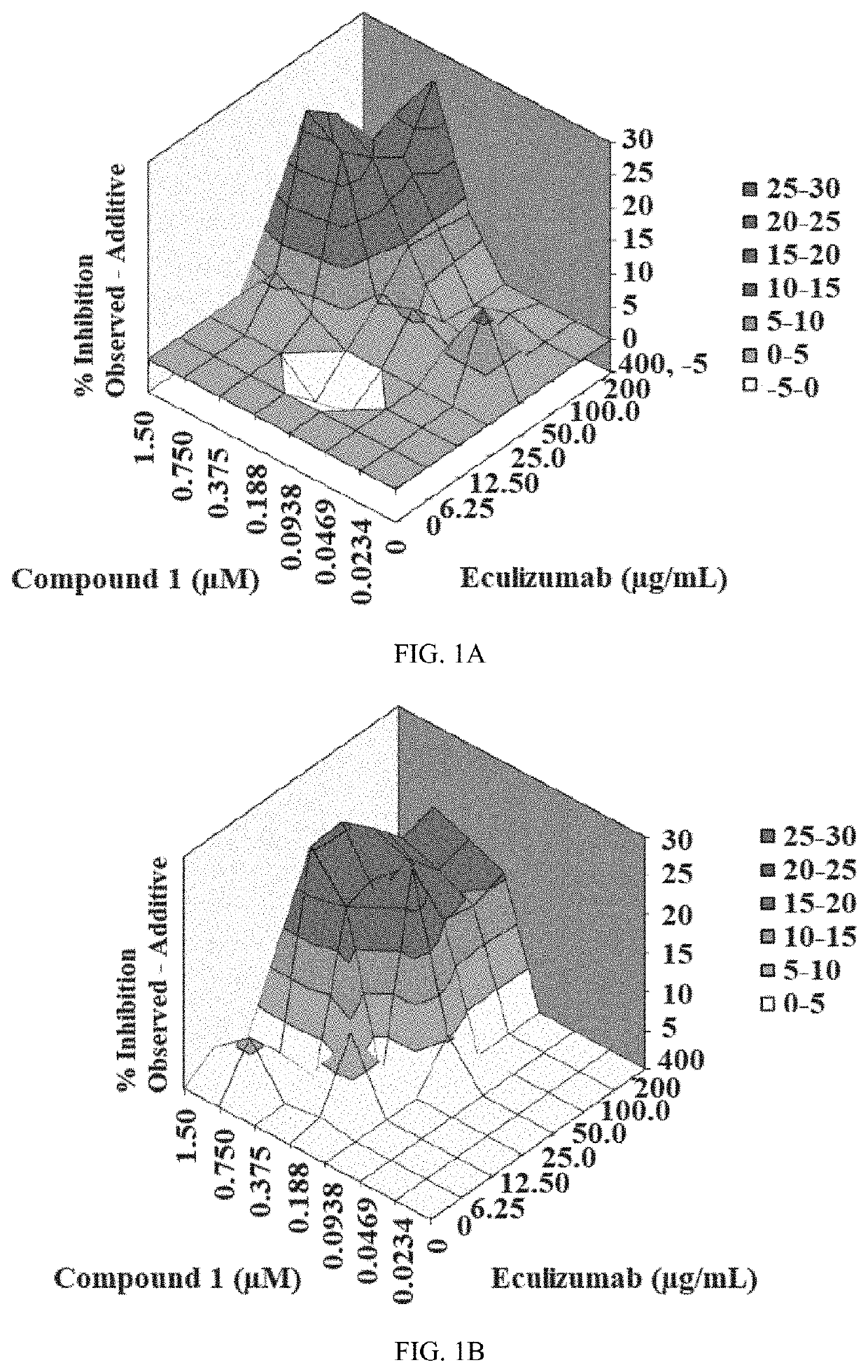
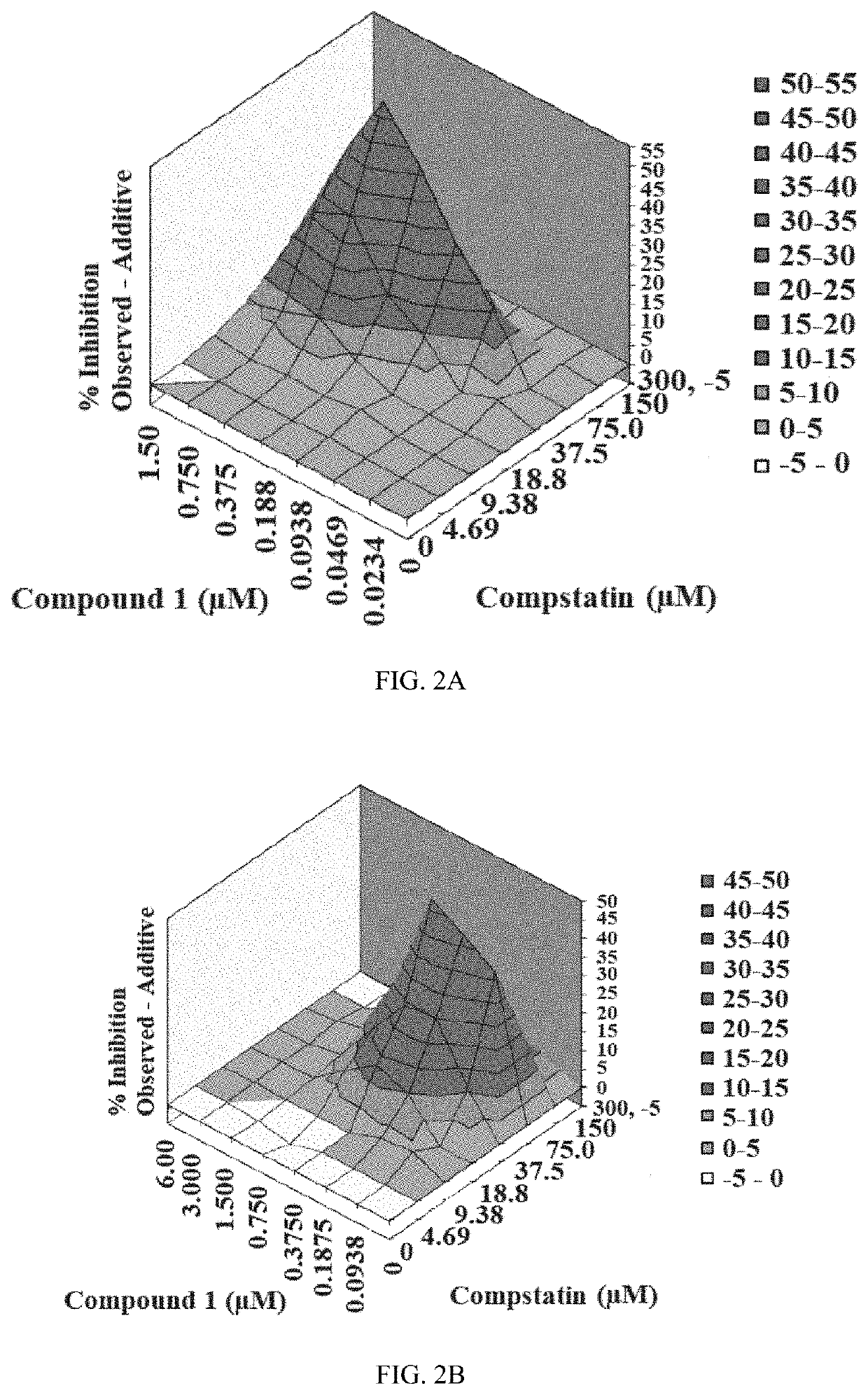
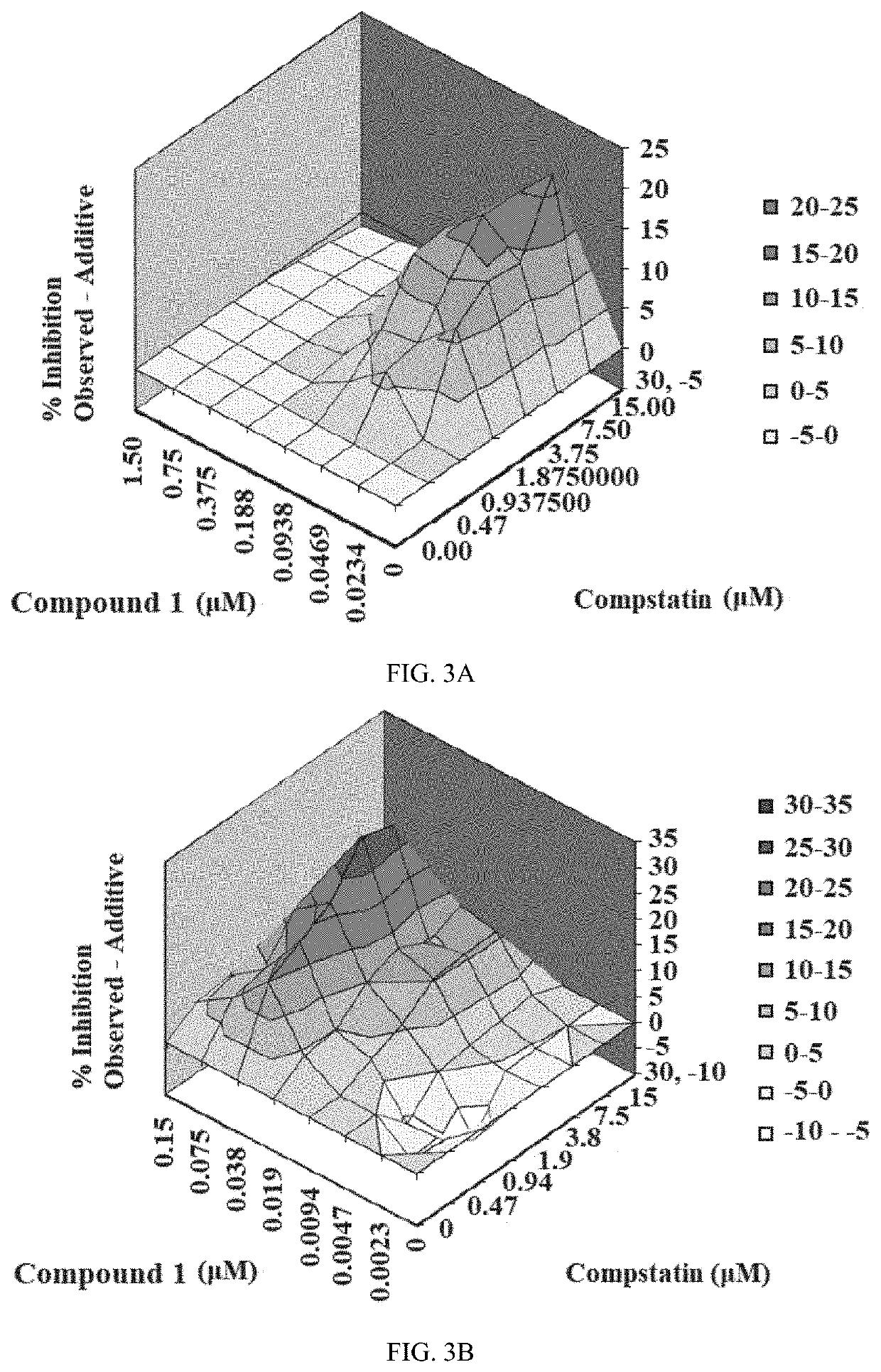
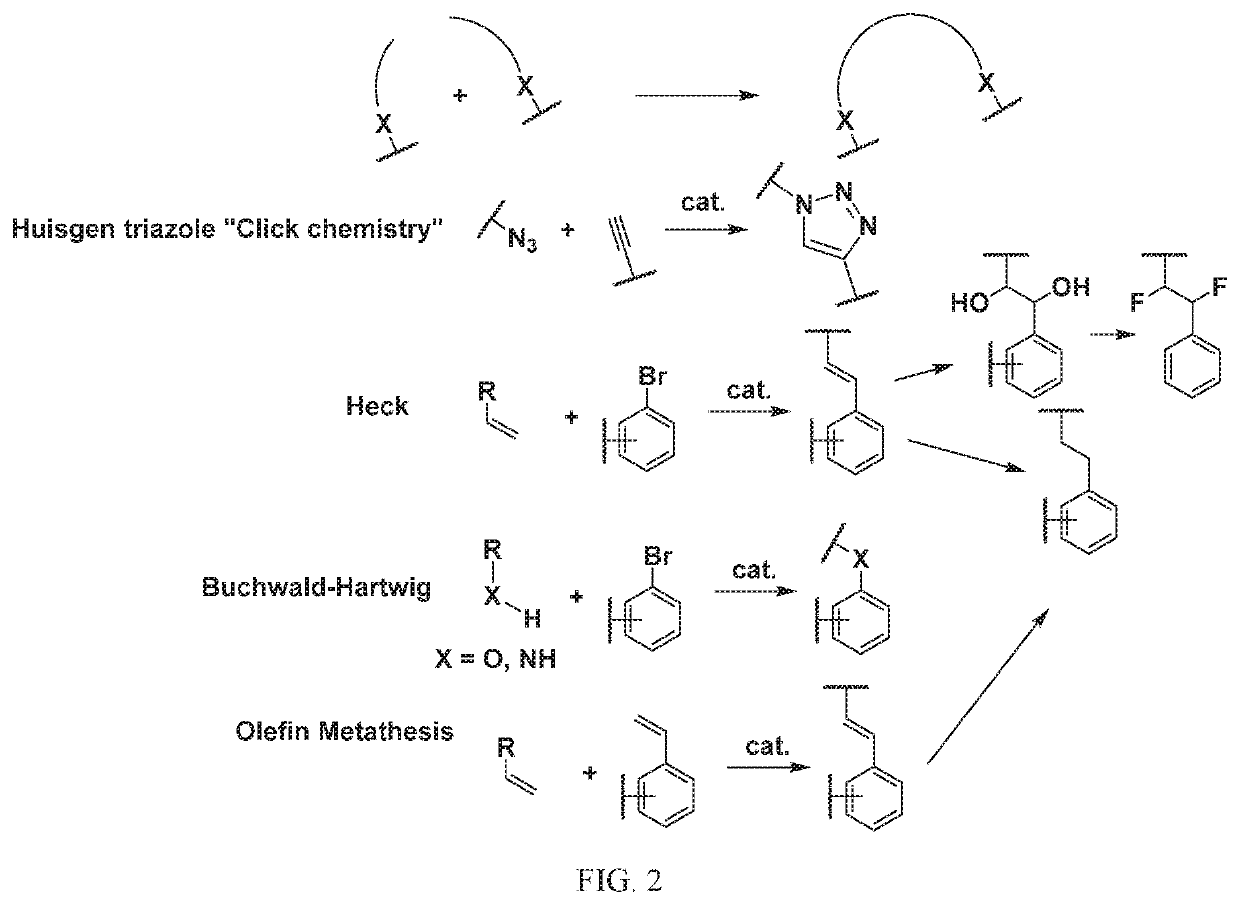
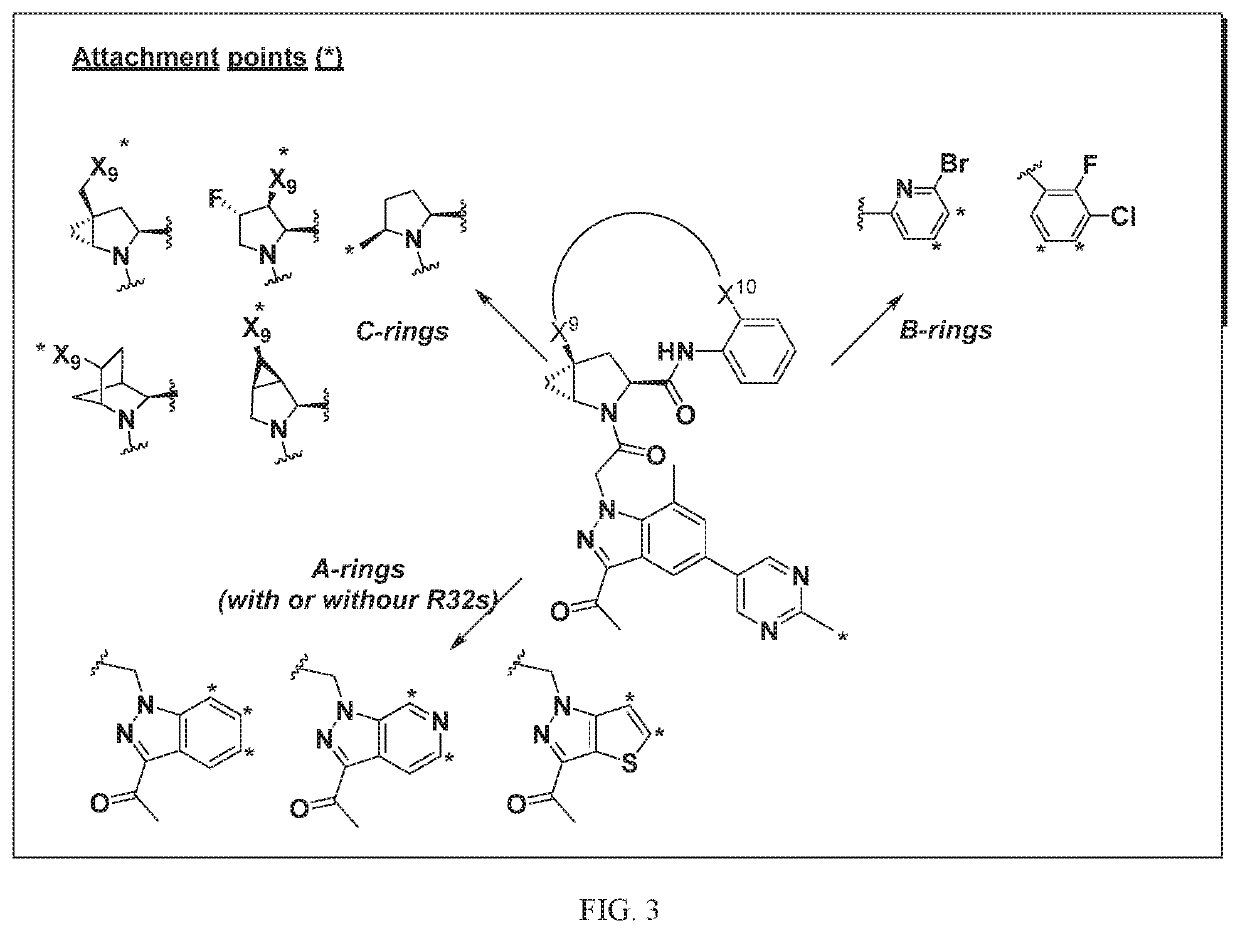
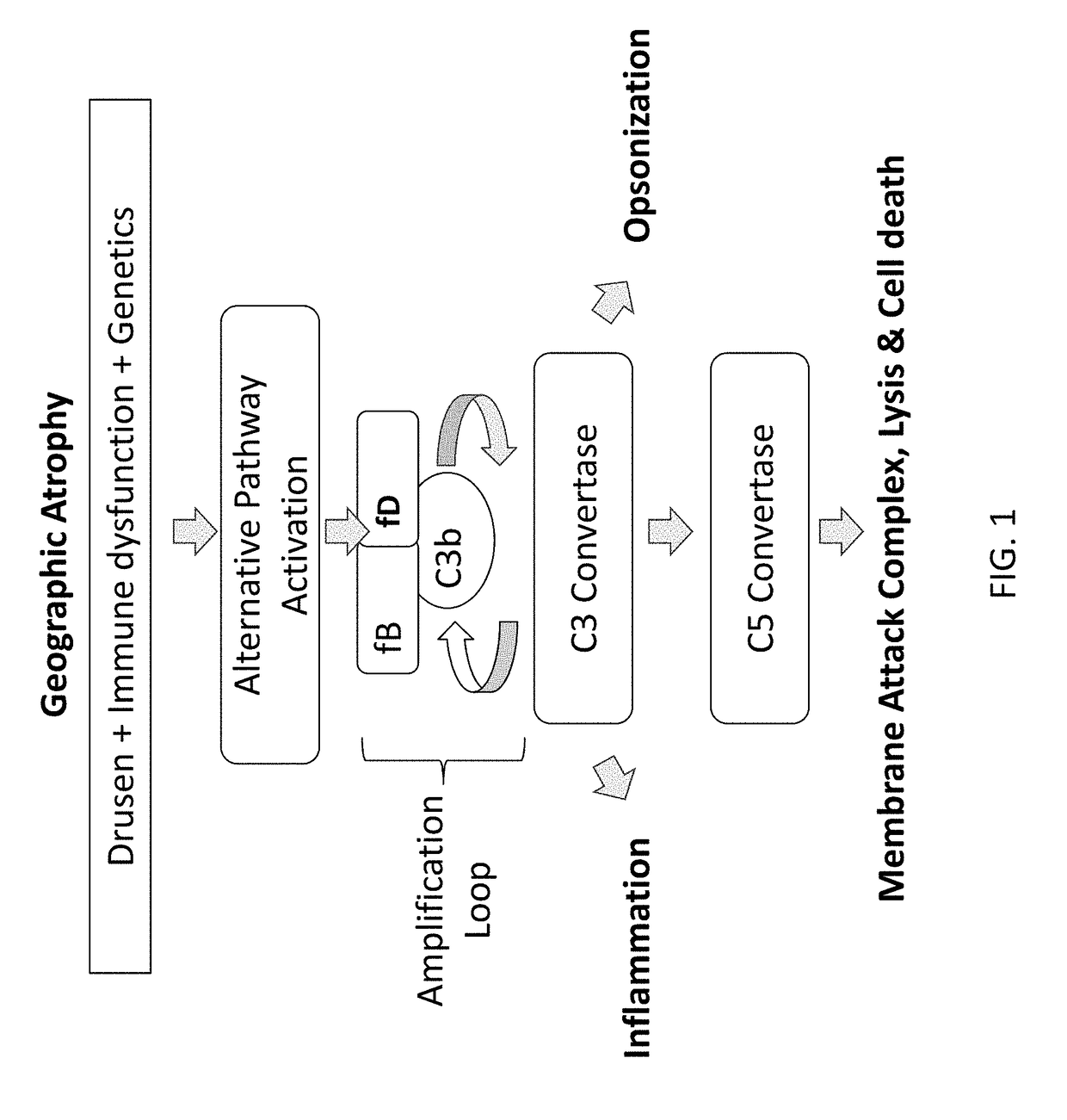
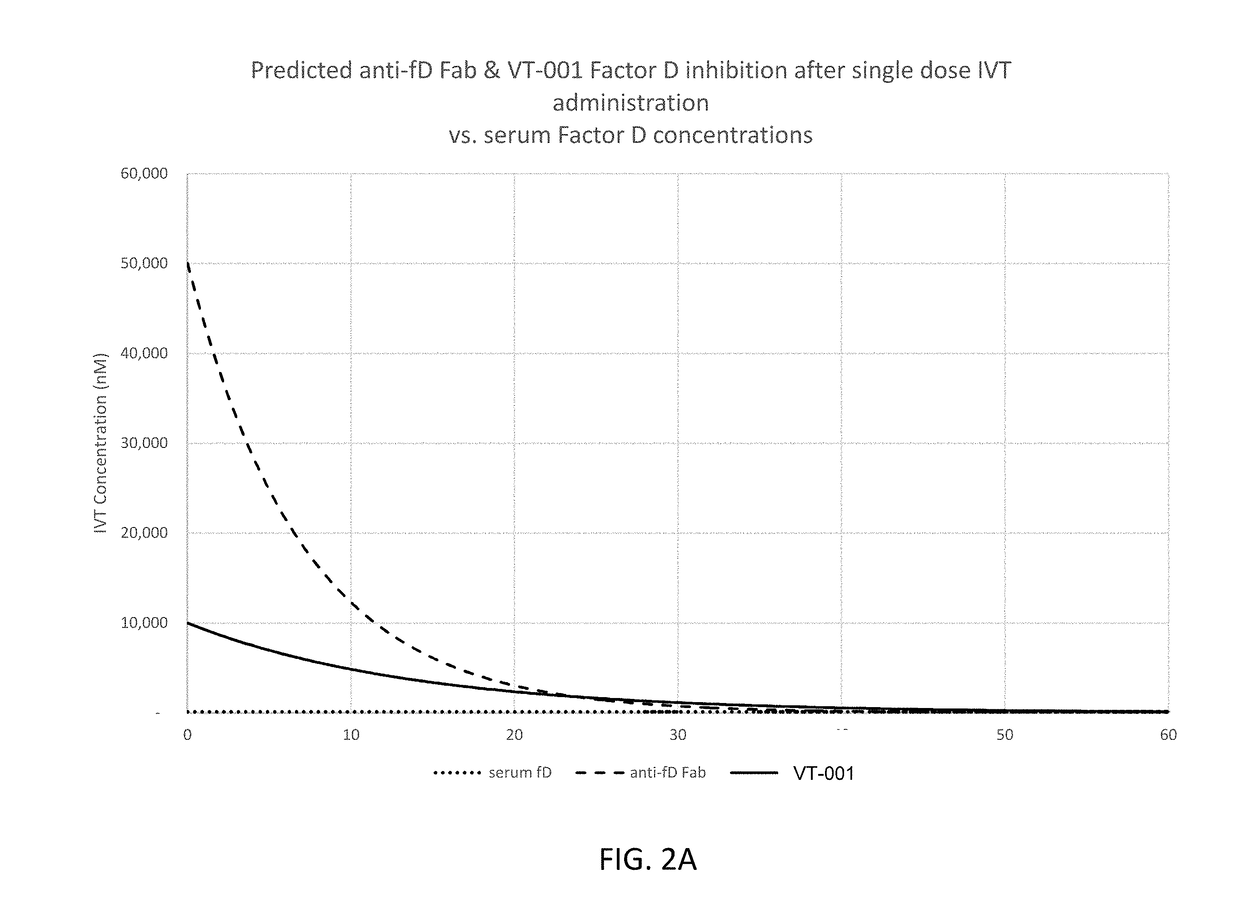
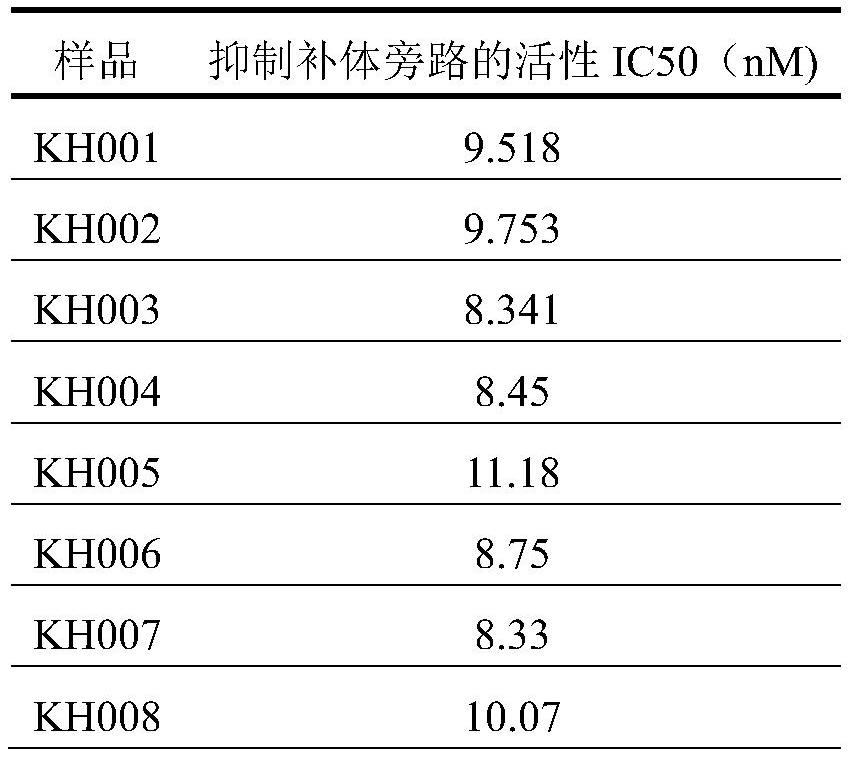
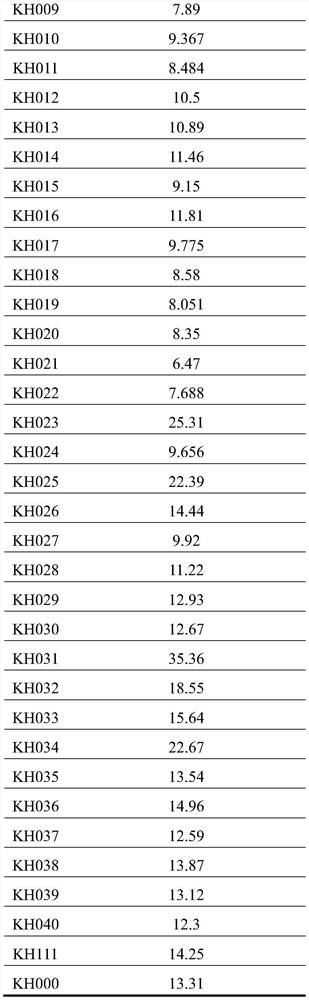
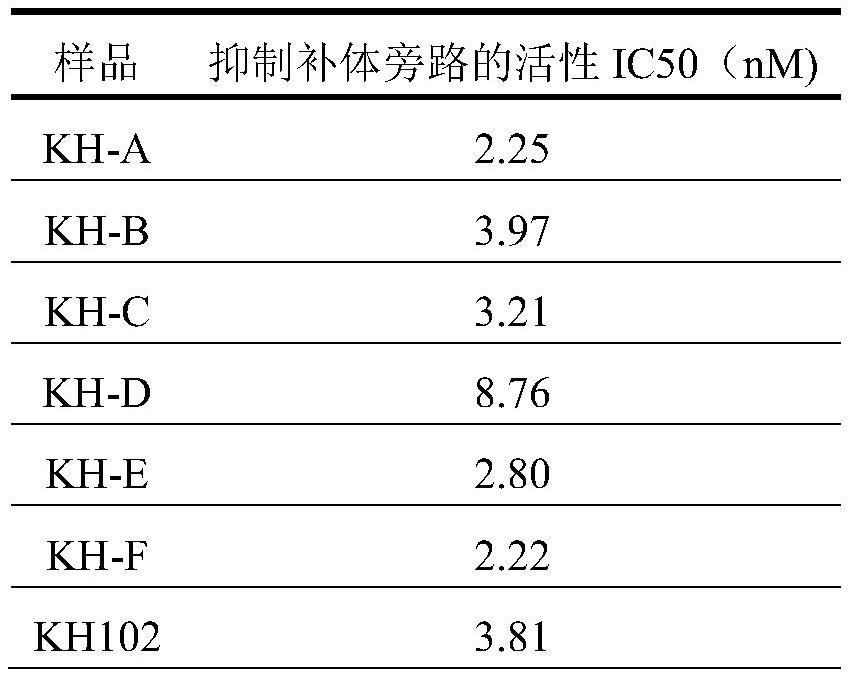
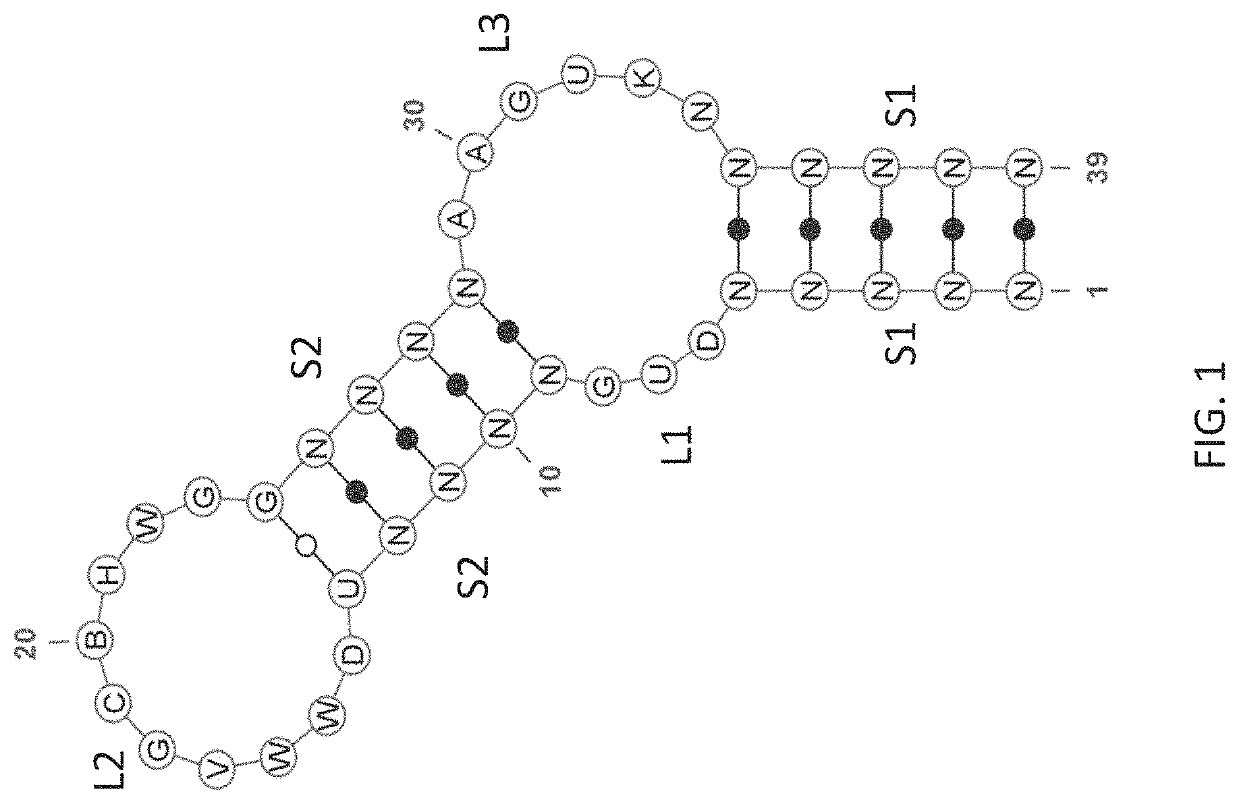
Patents
Literature
31 results about "Complement Factor D" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
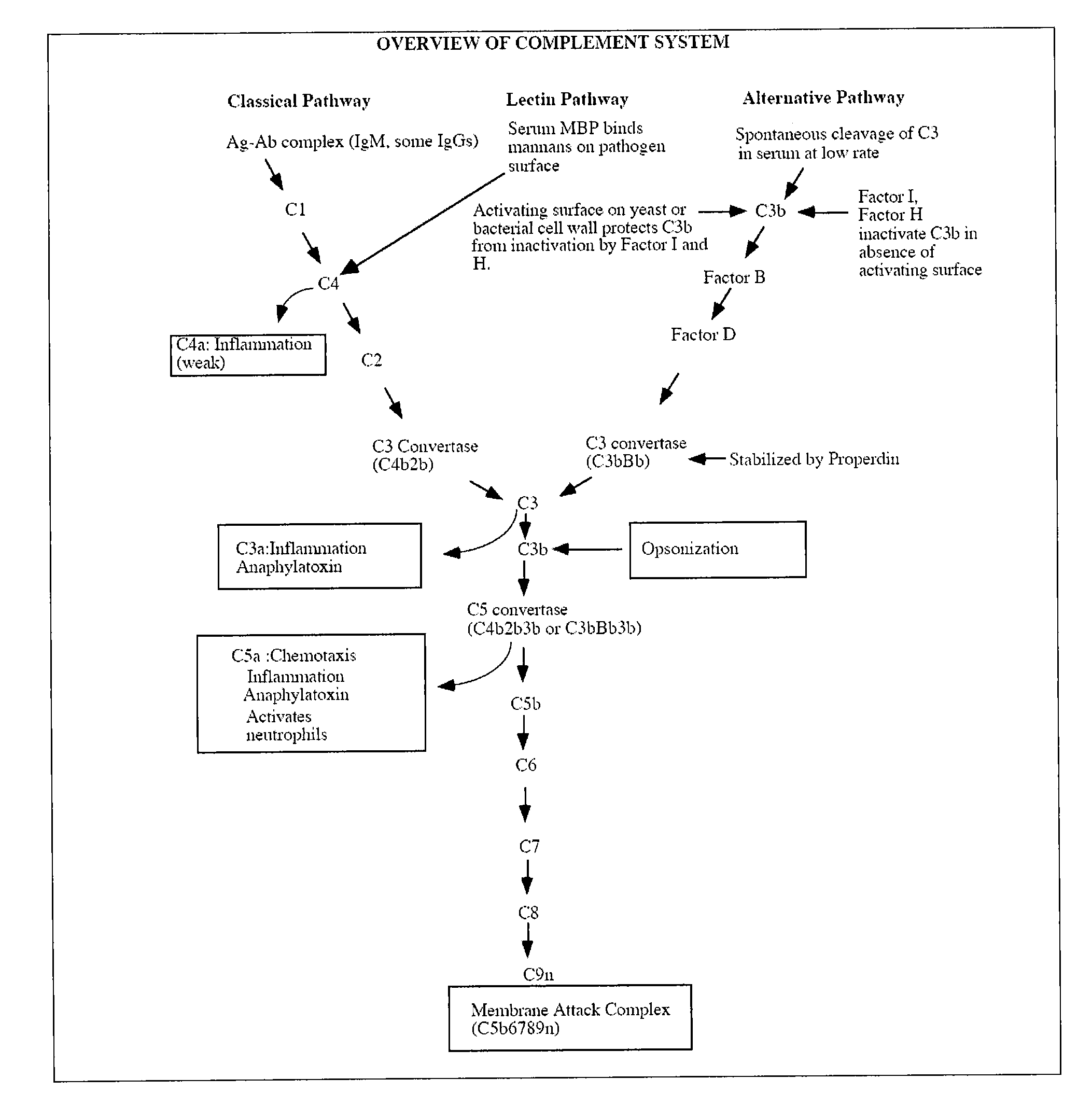
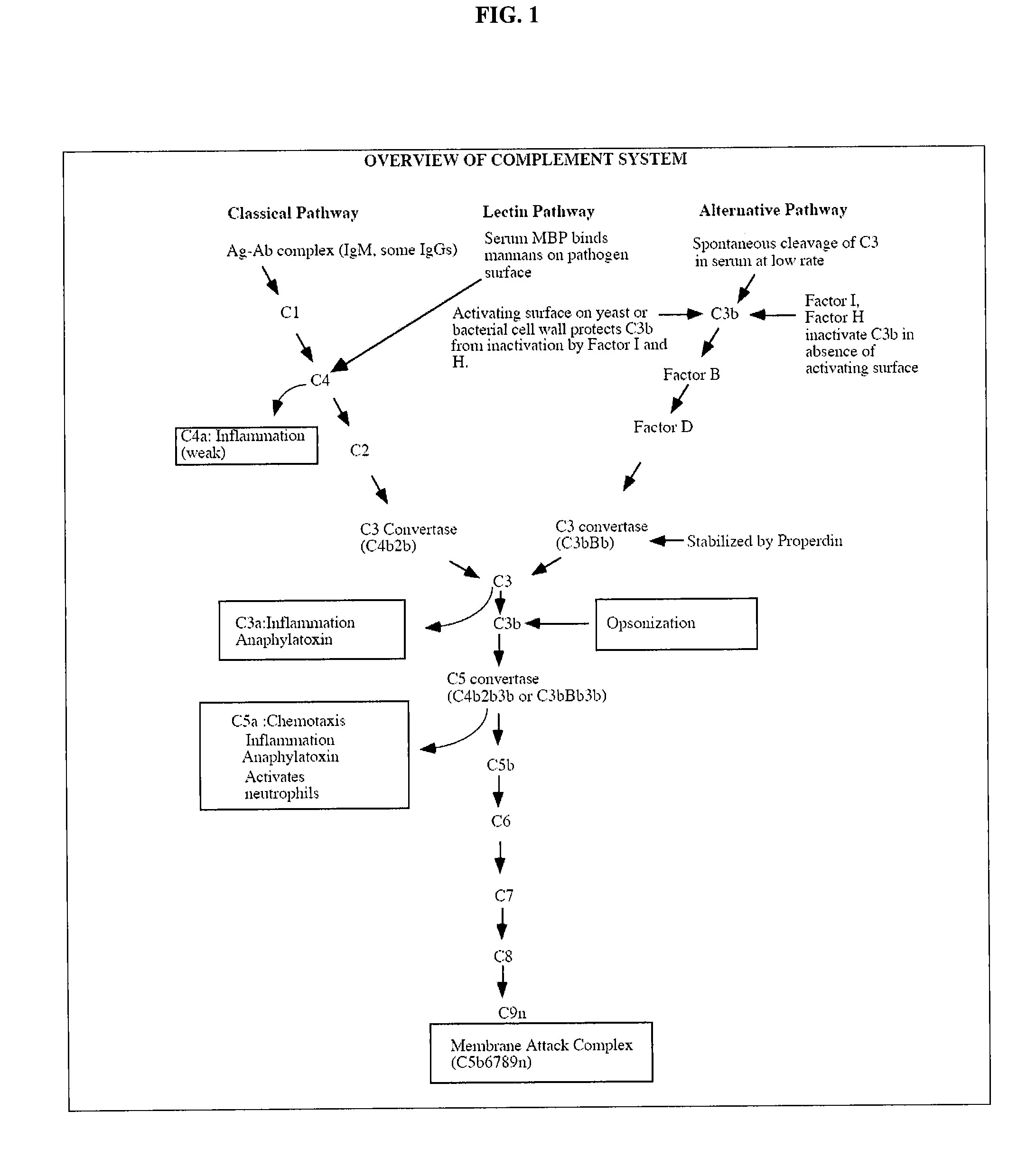
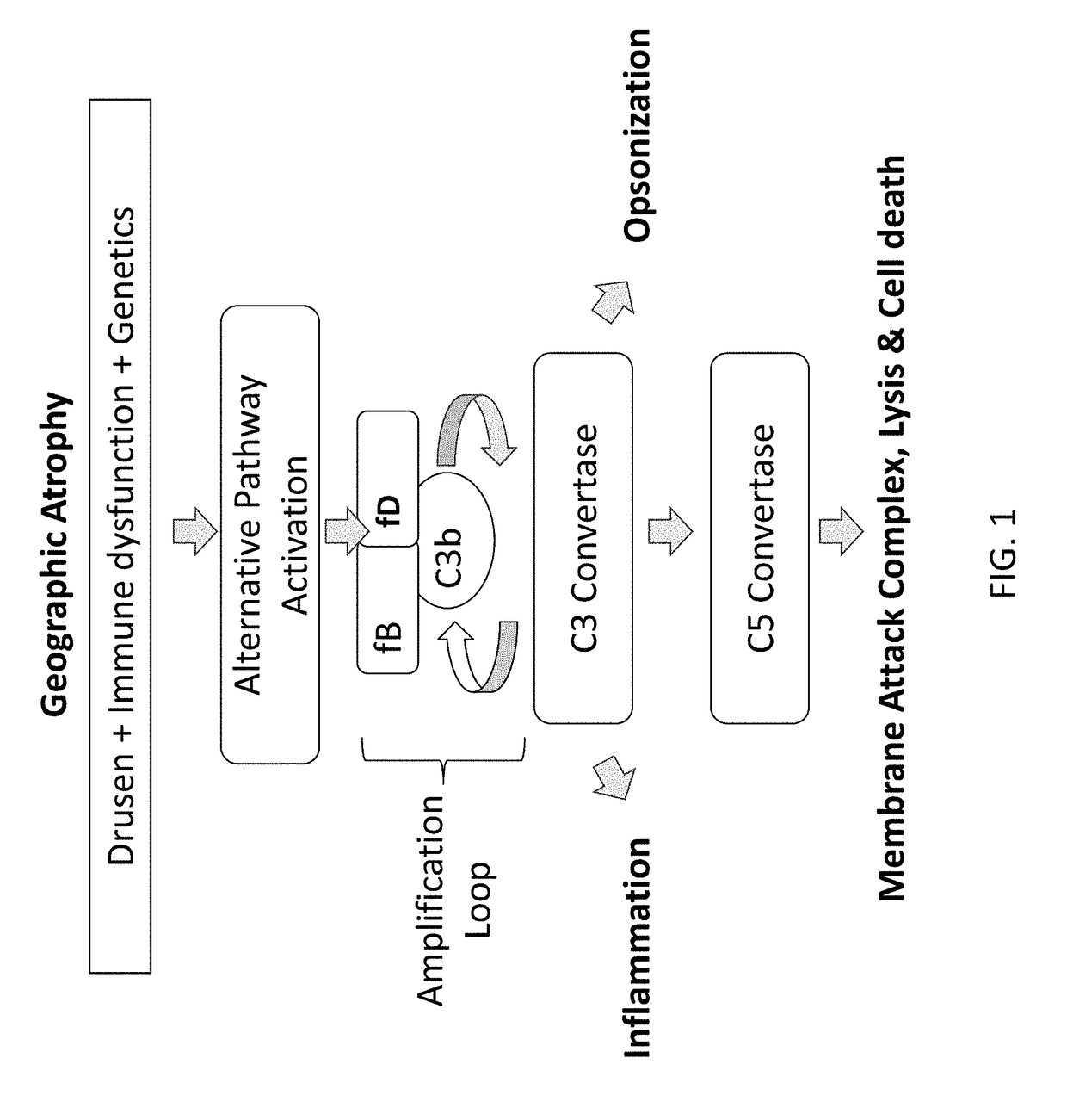
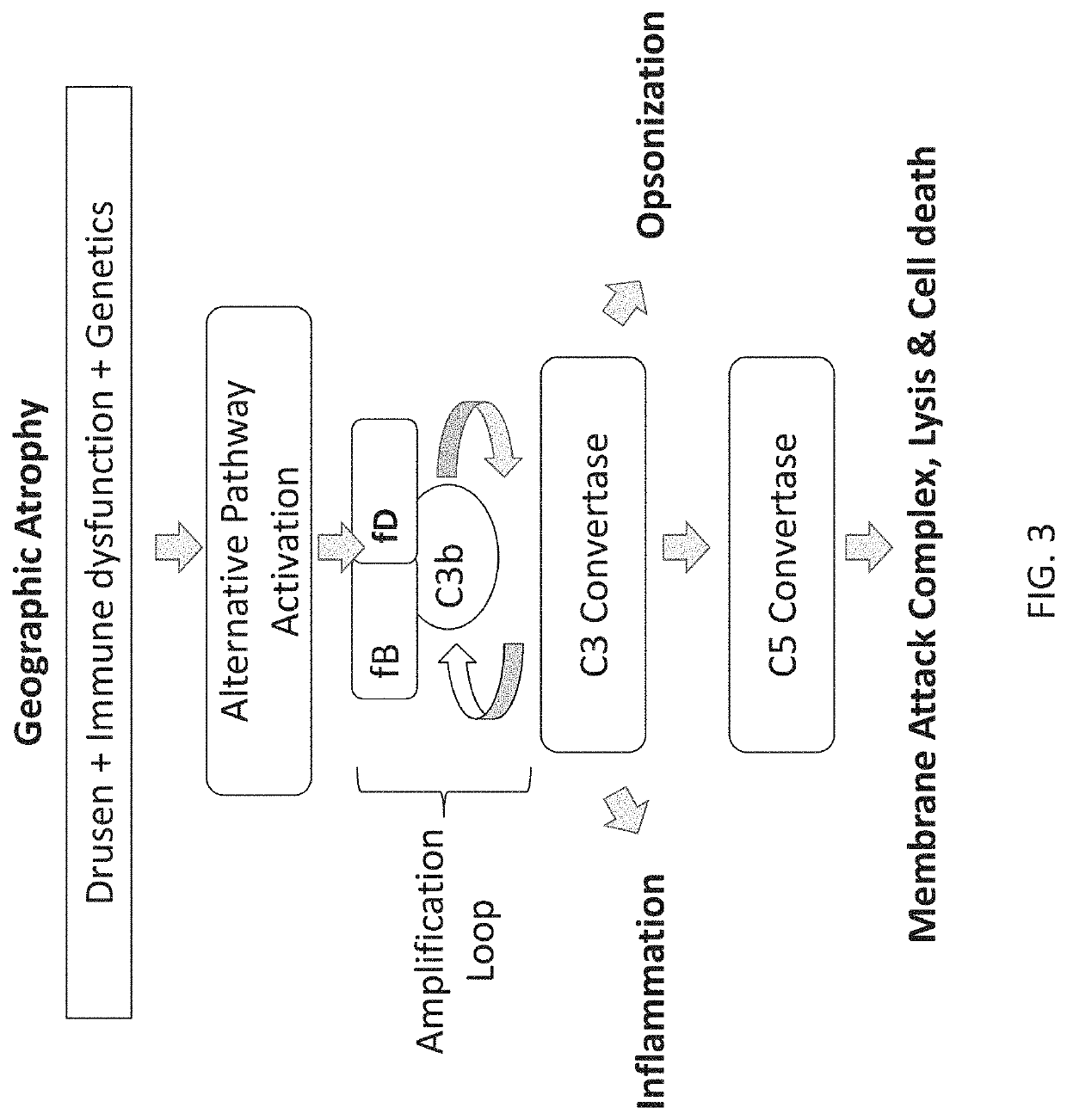
A serum protein which is important in the ALTERNATIVE COMPLEMENT ACTIVATION PATHWAY. This enzyme cleaves the COMPLEMENT C3B-bound COMPLEMENT FACTOR B to form C3bBb which is ALTERNATIVE PATHWAY C3 CONVERTASE.
Treatment of age-related macular degeneration using inhibitors of complement factor d
InactiveUS20080269318A1Avoid developmentInhibit the loss of visual acuityBiocideSenses disorderComplement Factor H GeneFactor ii
The present invention provides methods for identifying a patient at risk for developing AMD by identifying the presence of the Y402H polymorphism or other at risk variants in the complement factor H gene. The present invention further provides methods for treating persons having AMD or at risk for developing AMD as a result of having the Y402H polymorphism or other at risk variants in the complement factor H gene.
Owner:ALCON RES LTD
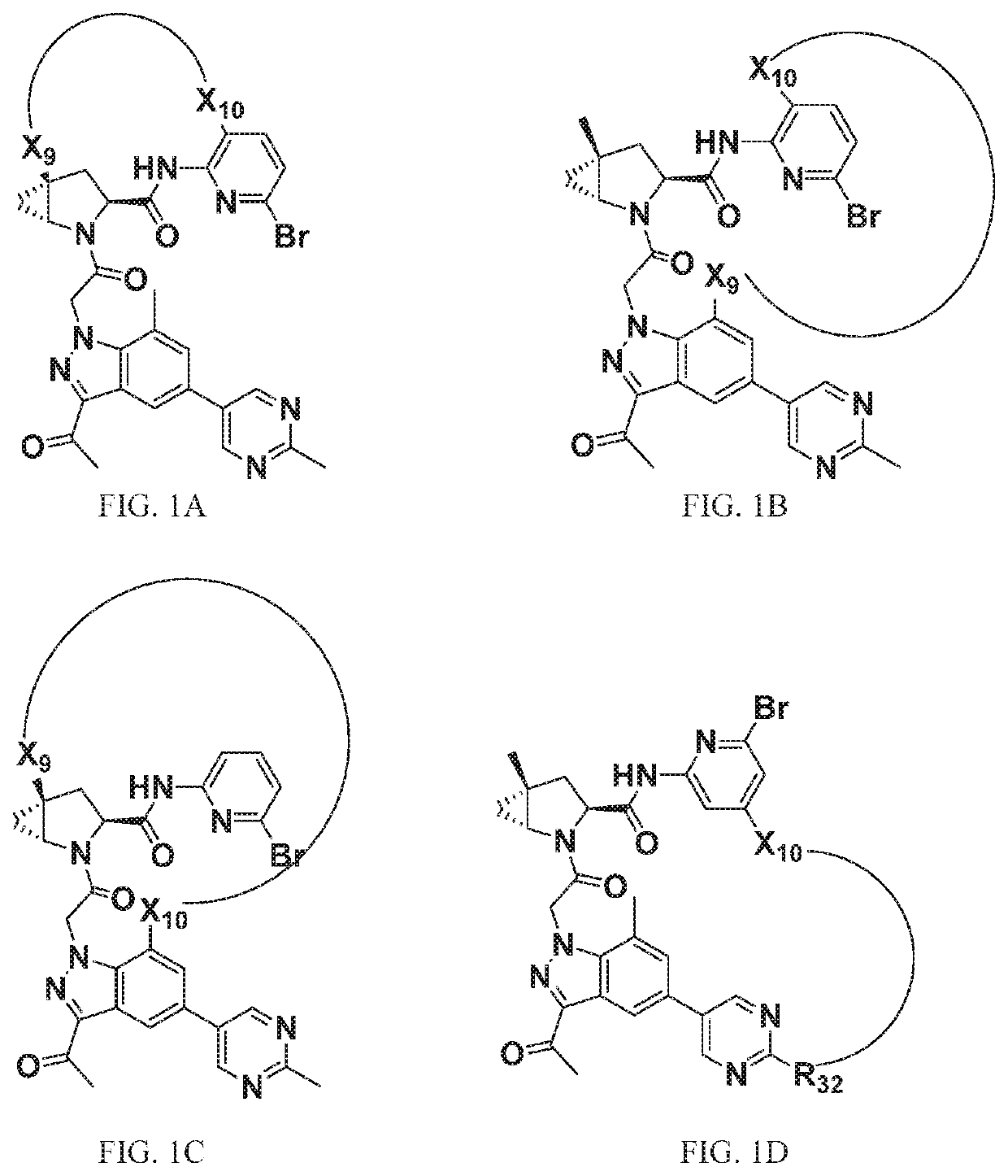
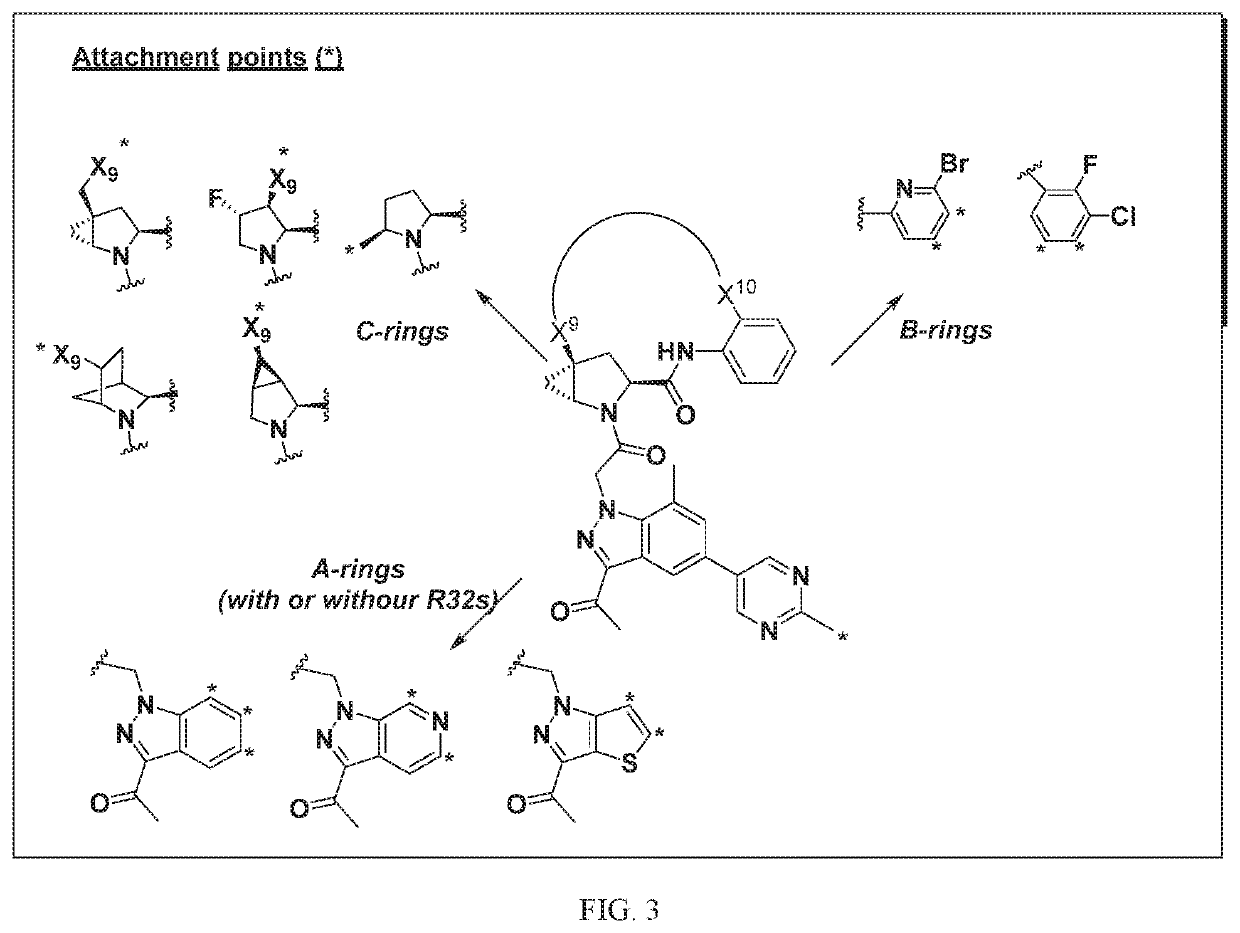
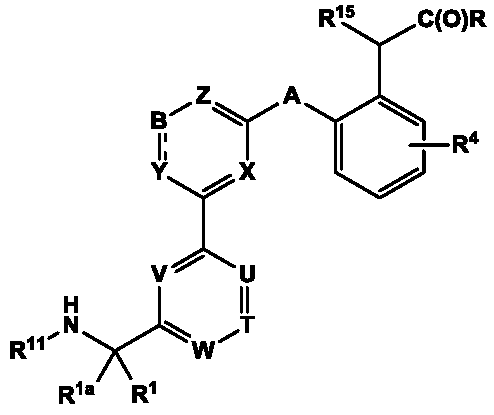
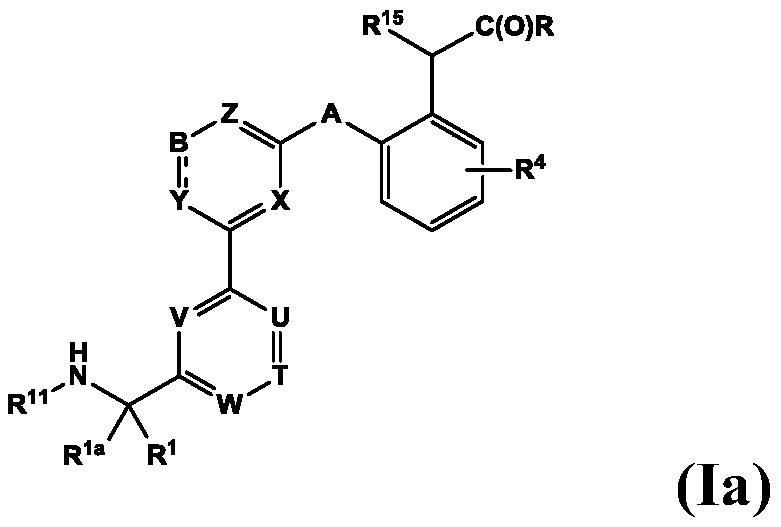
Compounds for Treatment of Complement Mediated Disorders
ActiveUS20180022767A1Dampen and inhibit detrimental complement activitySenses disorderNervous disorderDiseaseReperfusion injury
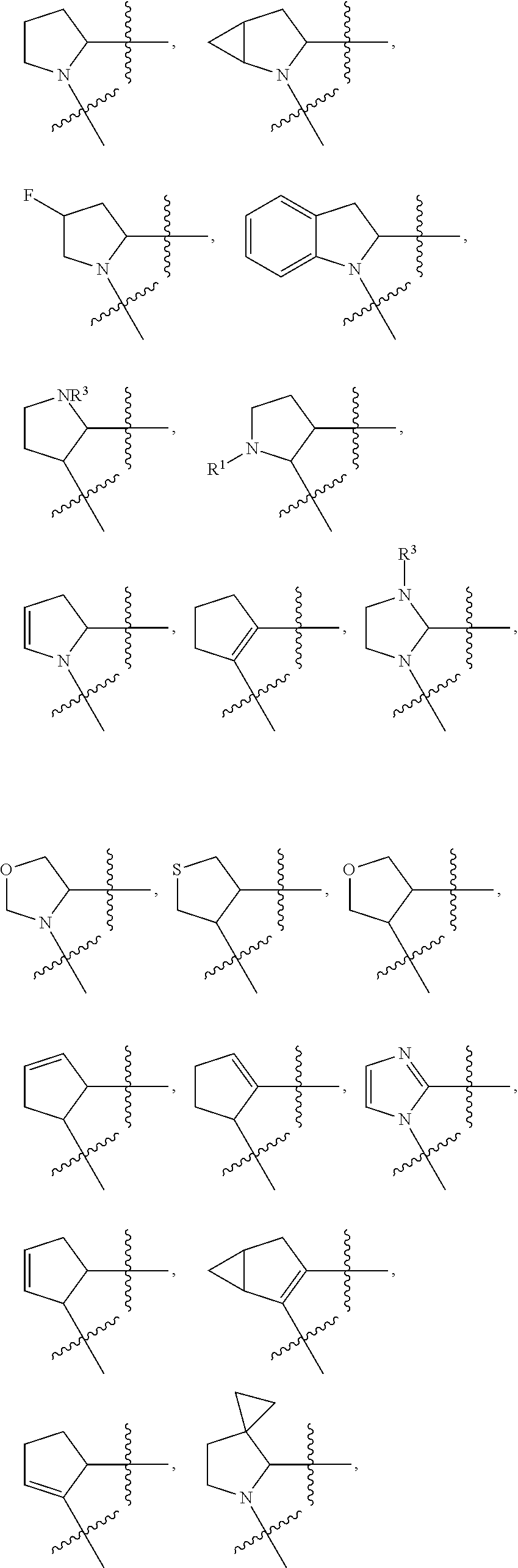
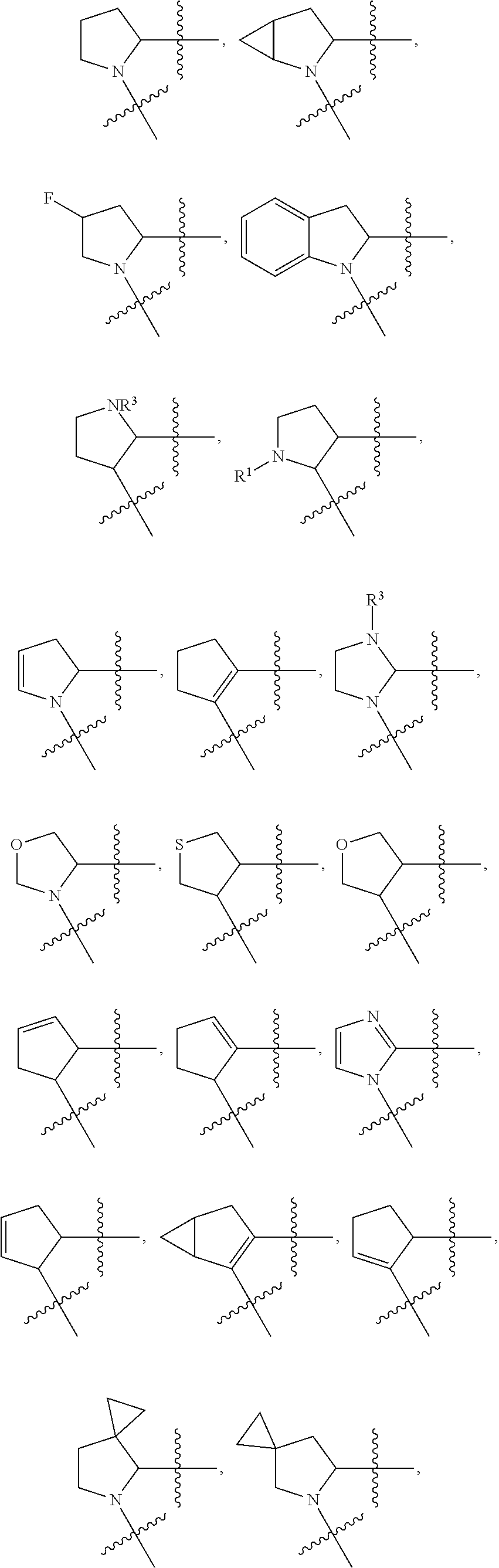
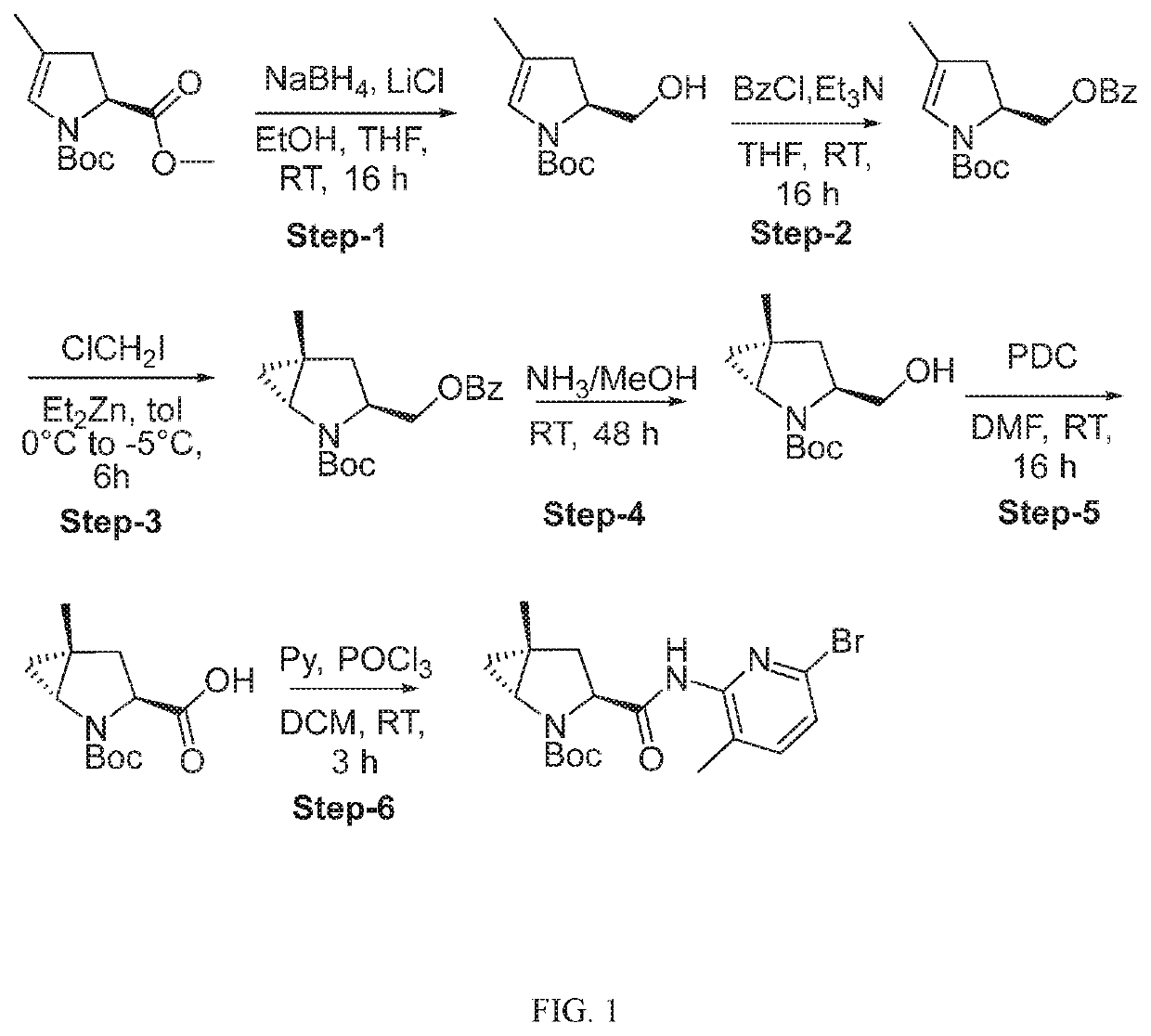
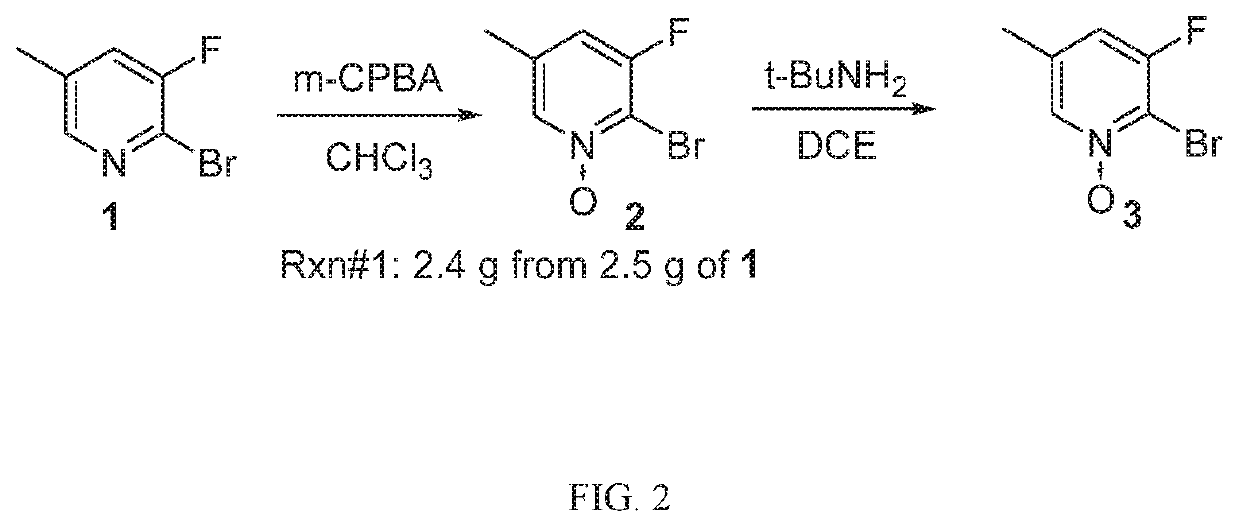
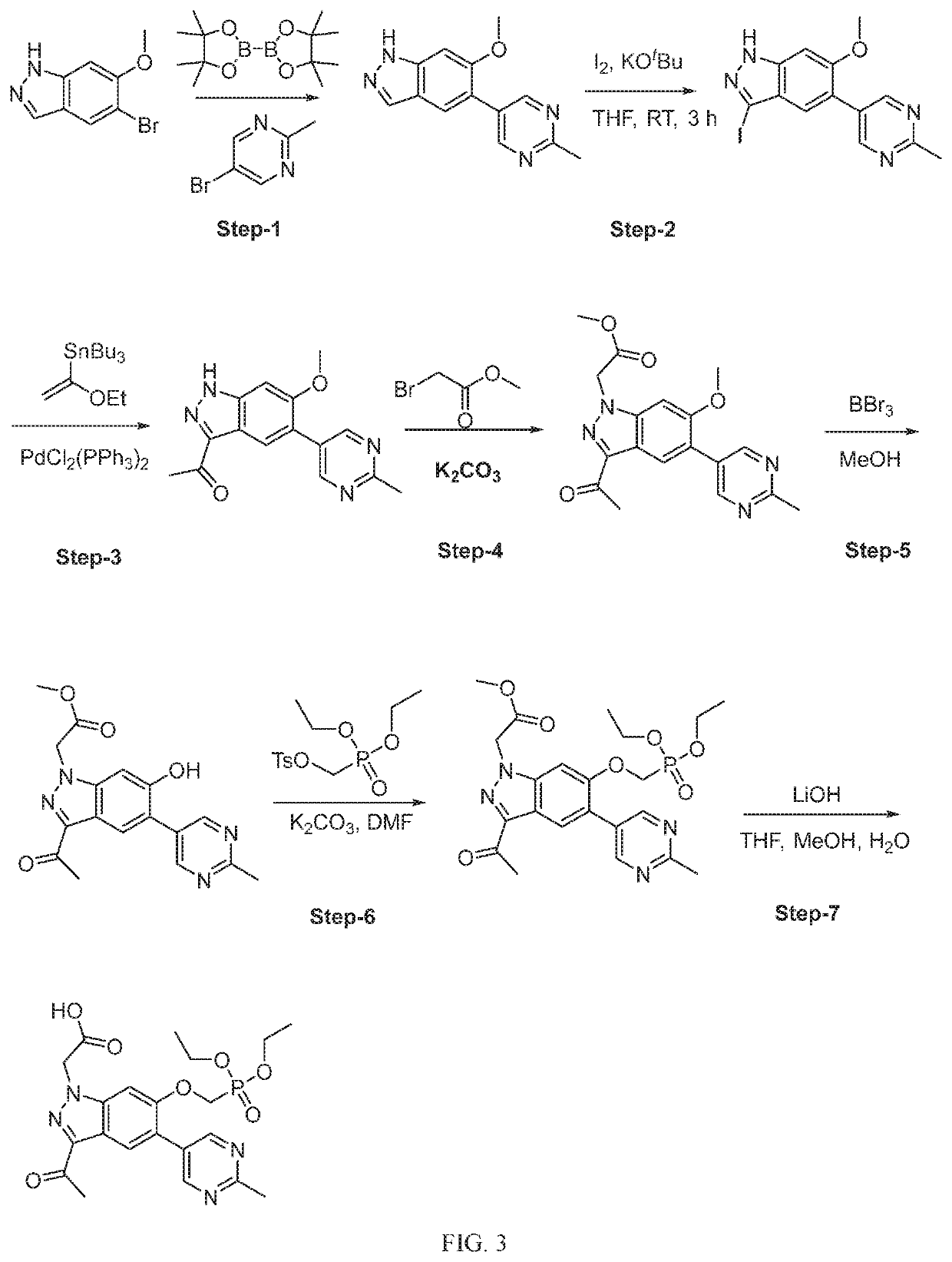
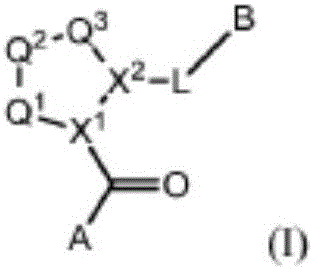
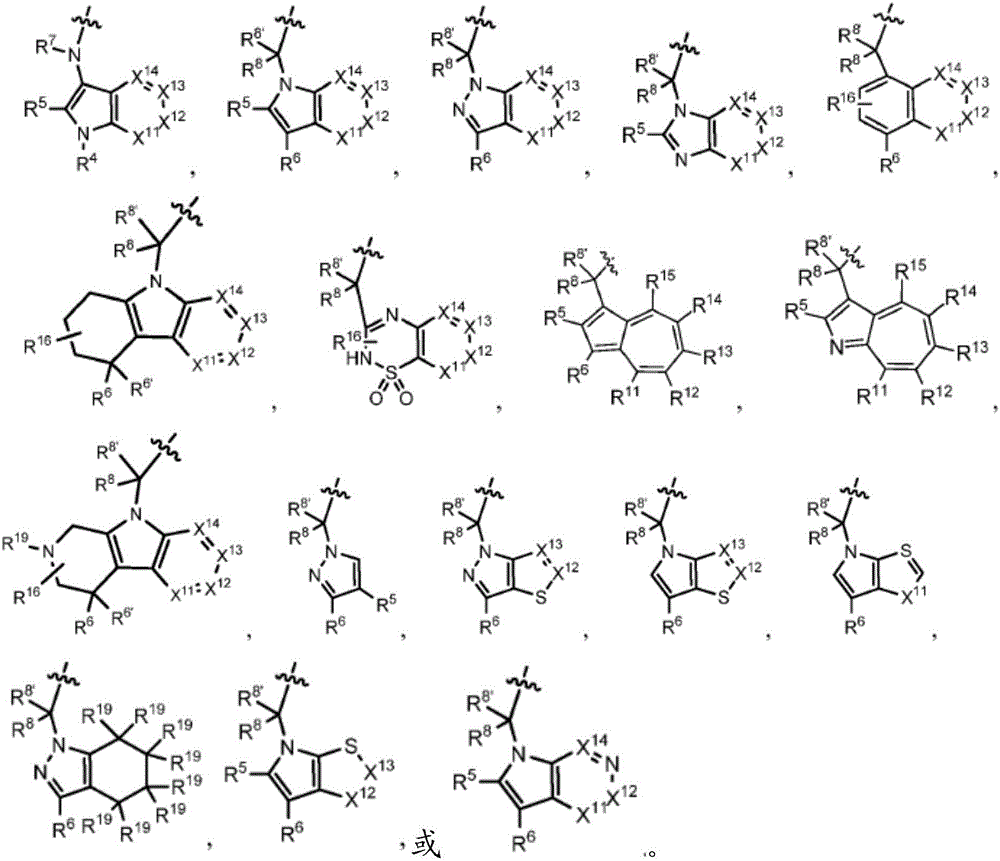
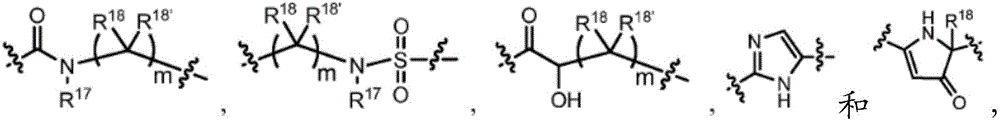
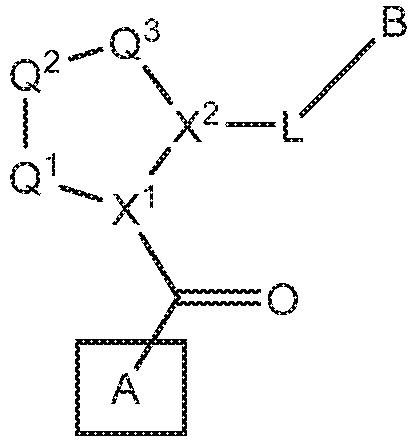
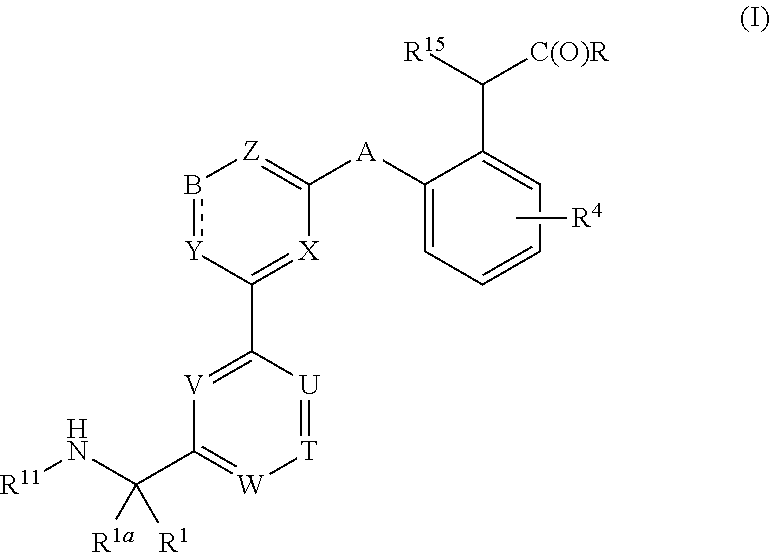
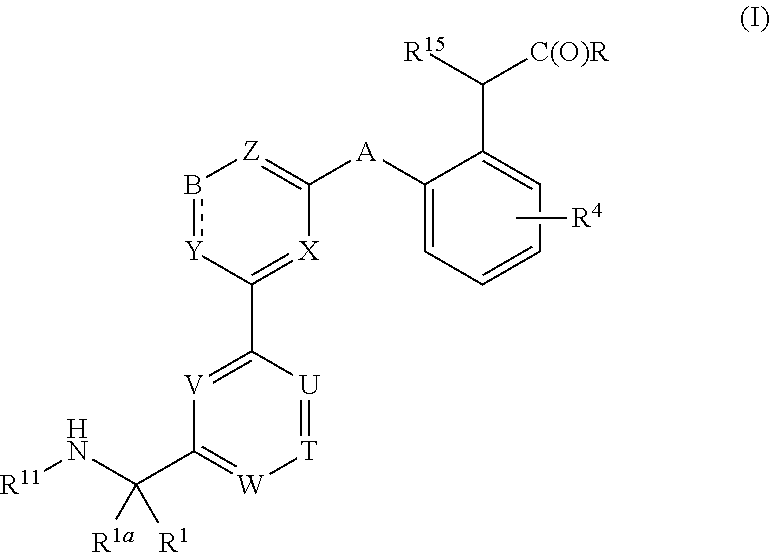
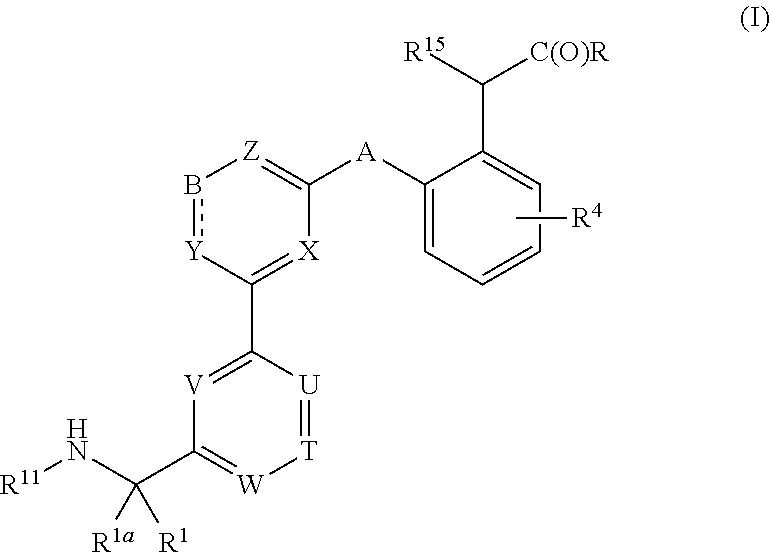
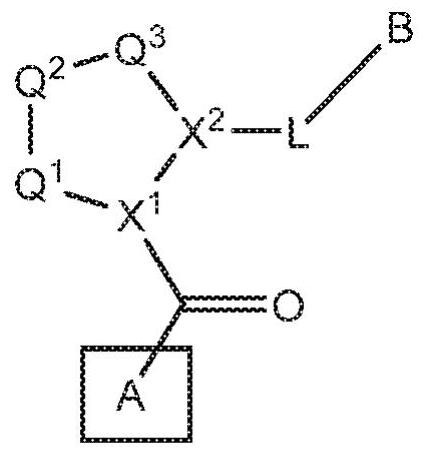
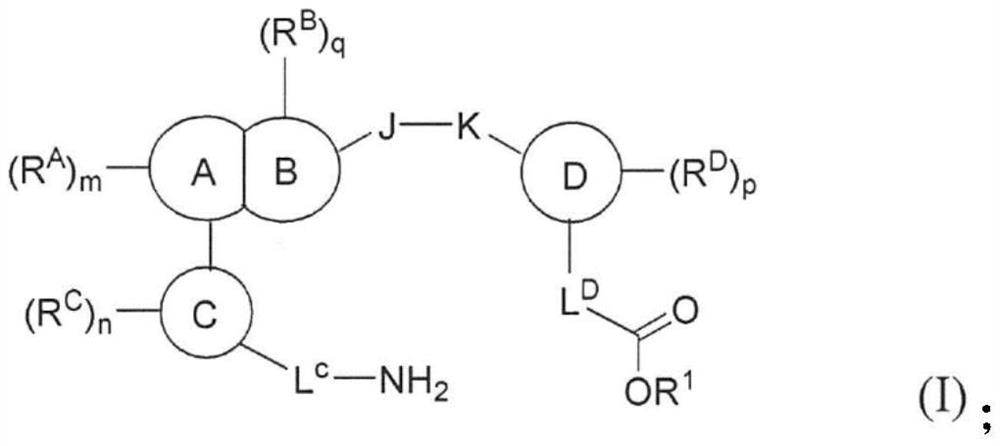
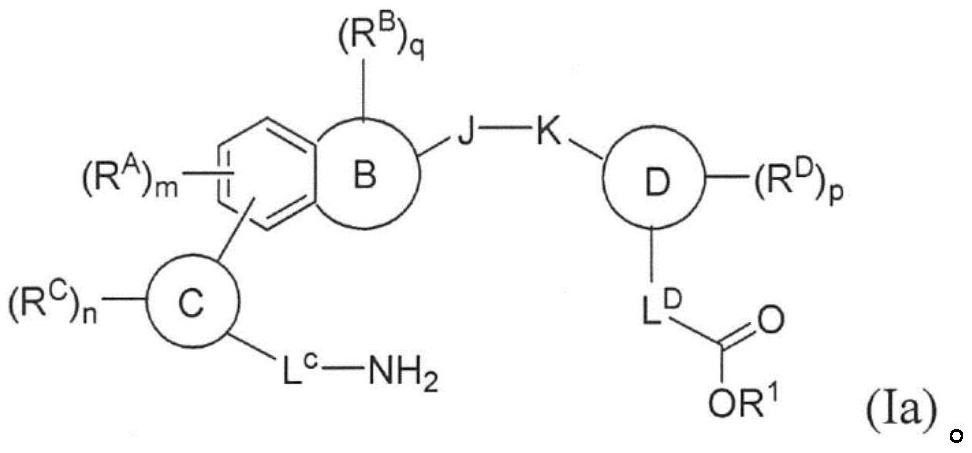
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade at an early and essential point in the alternative complement pathway, and reduce factor D's ability to modulate the classical and lectin complement pathways. The inhibitors of factor D described herein are capable of reducing the excessive activation of complement, which has been linked to certain autoimmune, inflammatory, and neurodegenerative diseases, as well as ischemia-reperfusion injury and cancer.
Owner:ACHILLION PHARMA INC
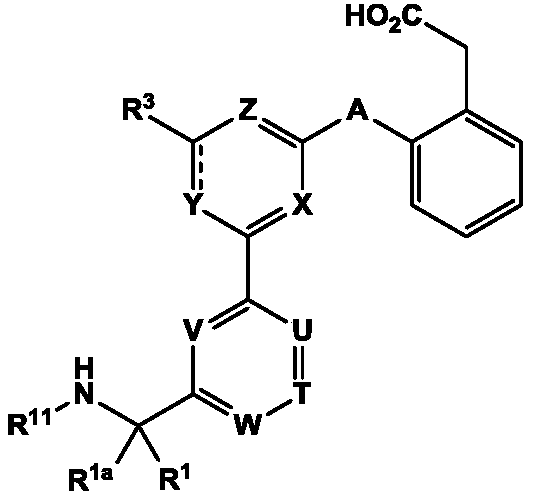
Amide compounds for treatment of complement mediated disorders
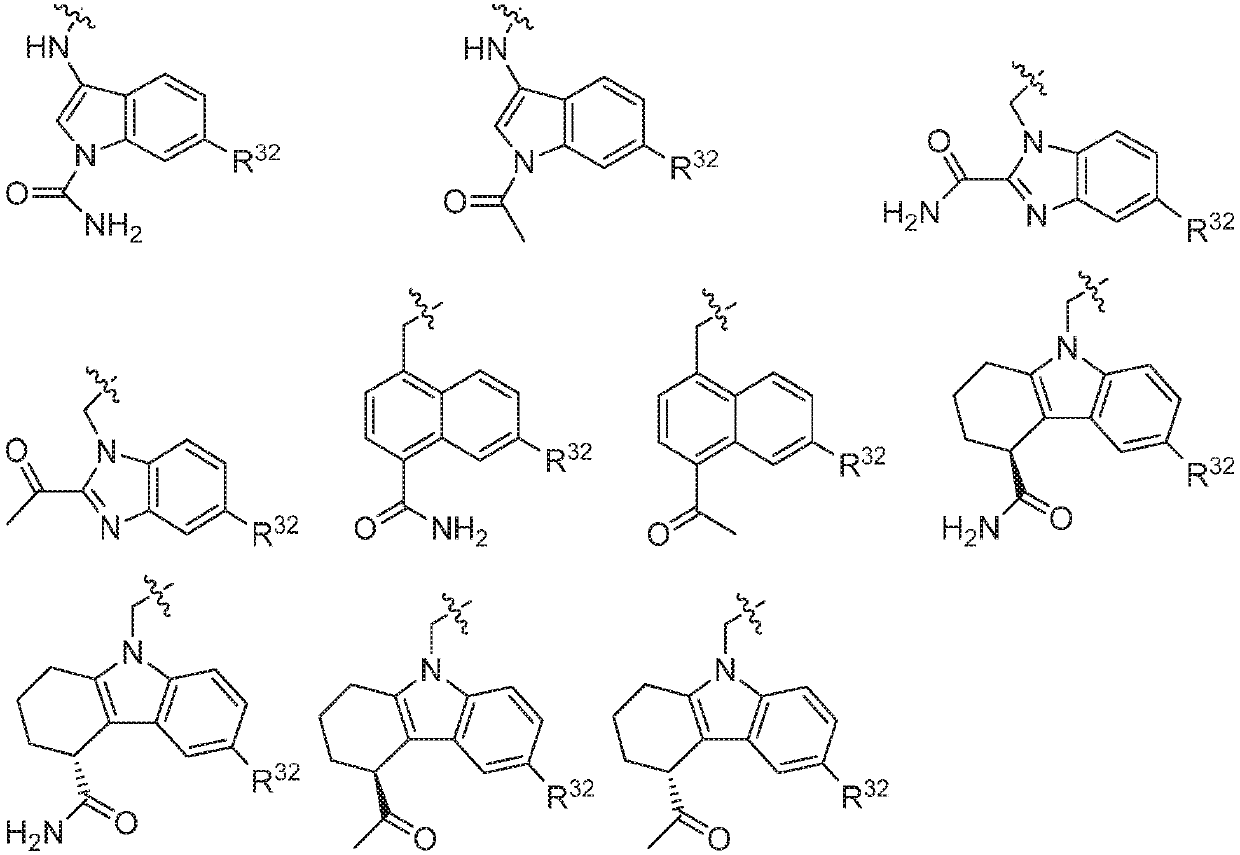
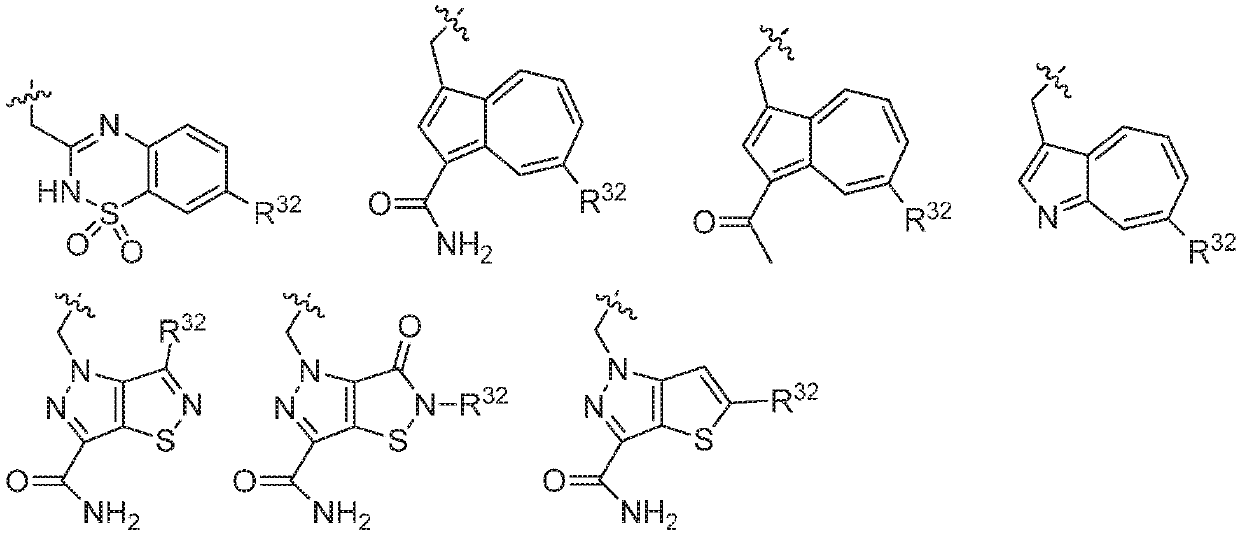
ActiveUS20160362398A1Dampen and inhibit detrimental complement activitySenses disorderNervous disorderDiseaseFactor D
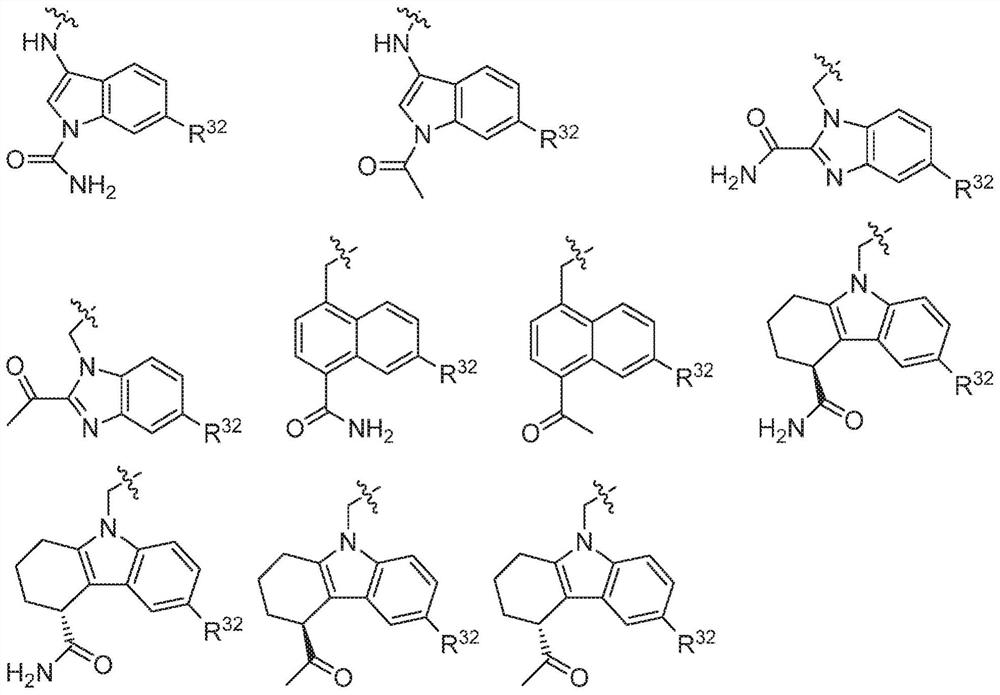
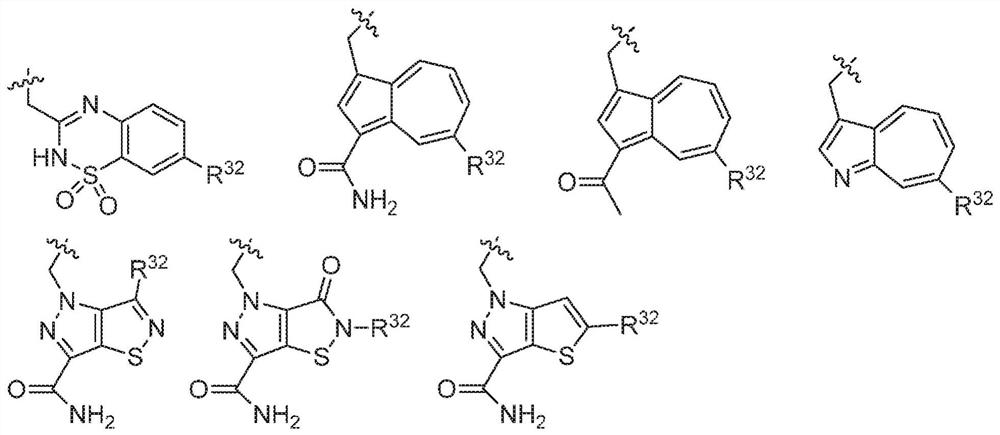
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof, wherein R12 or R13 on the A group is an amide substituent (R32) are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade in the alternative complement pathway. The inhibitors of factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Macrocyclic compounds for treatment of medical disorders
ActiveUS20200002347A1Minimal hydrolysisDecreases potential for formationOrganic chemistryAntiviralsDiseaseMedical disorder
Macrocyclic Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, Formula IV, Formula V, Formula VI, Formula VII, and Formula VIII or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
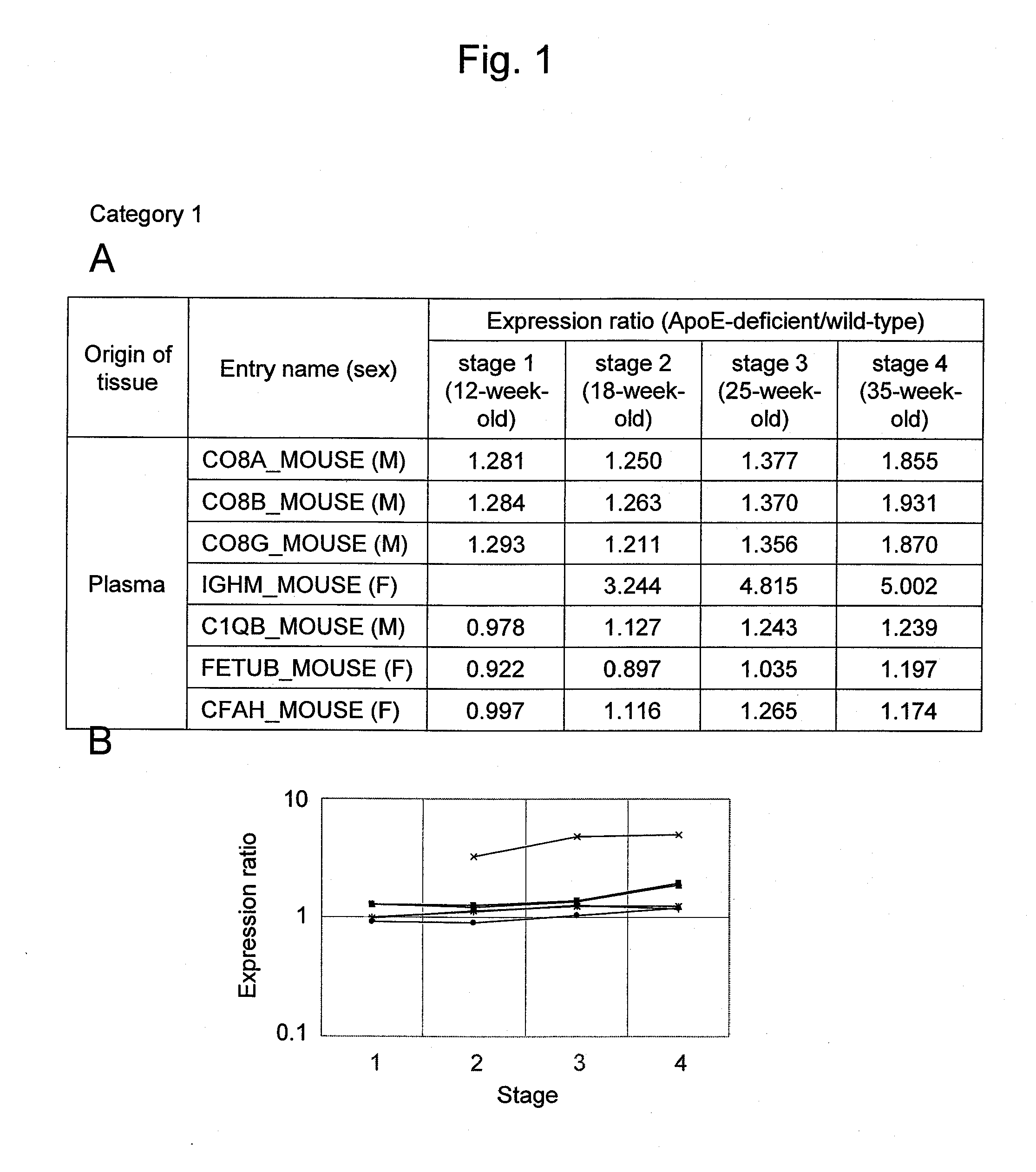
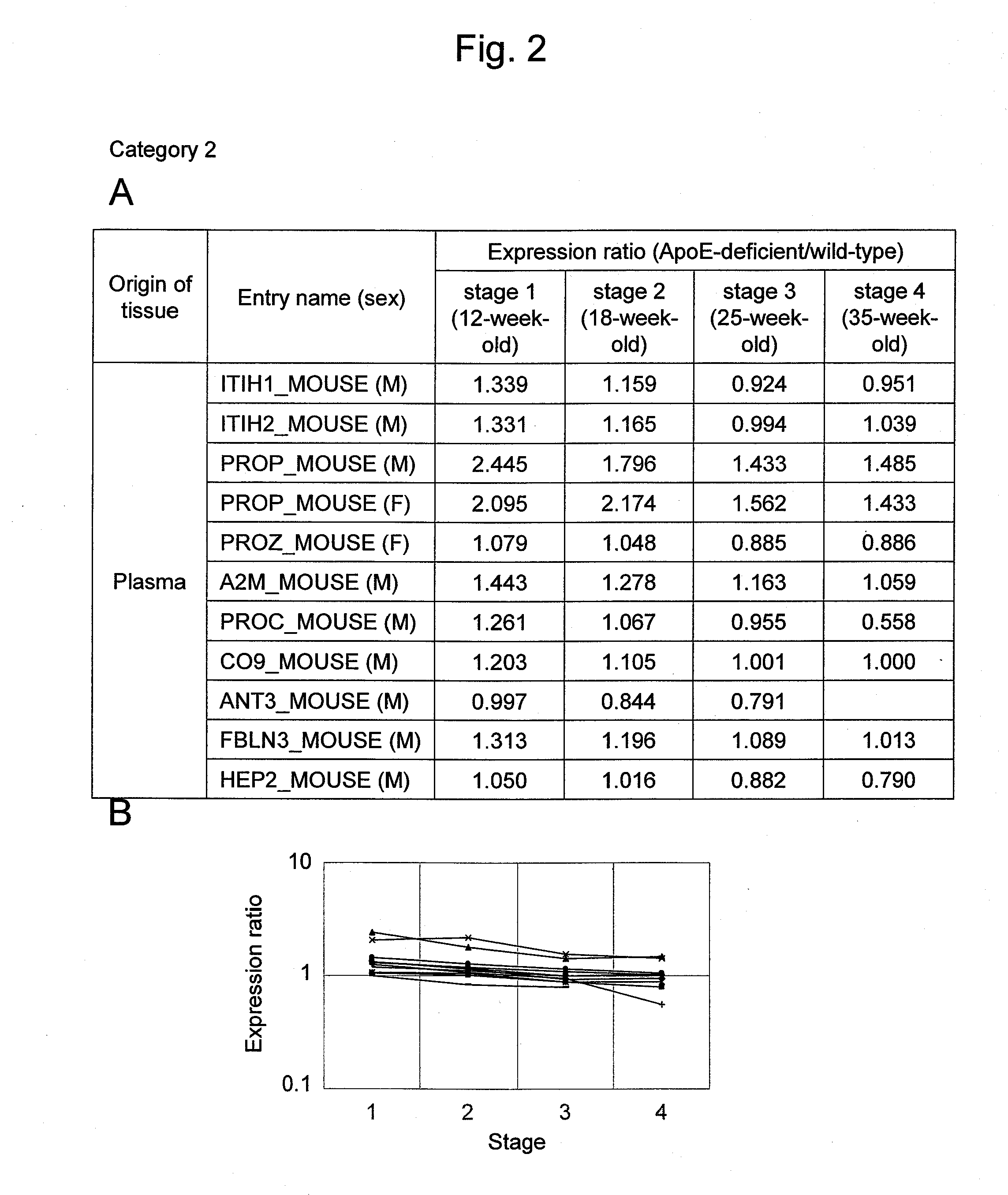
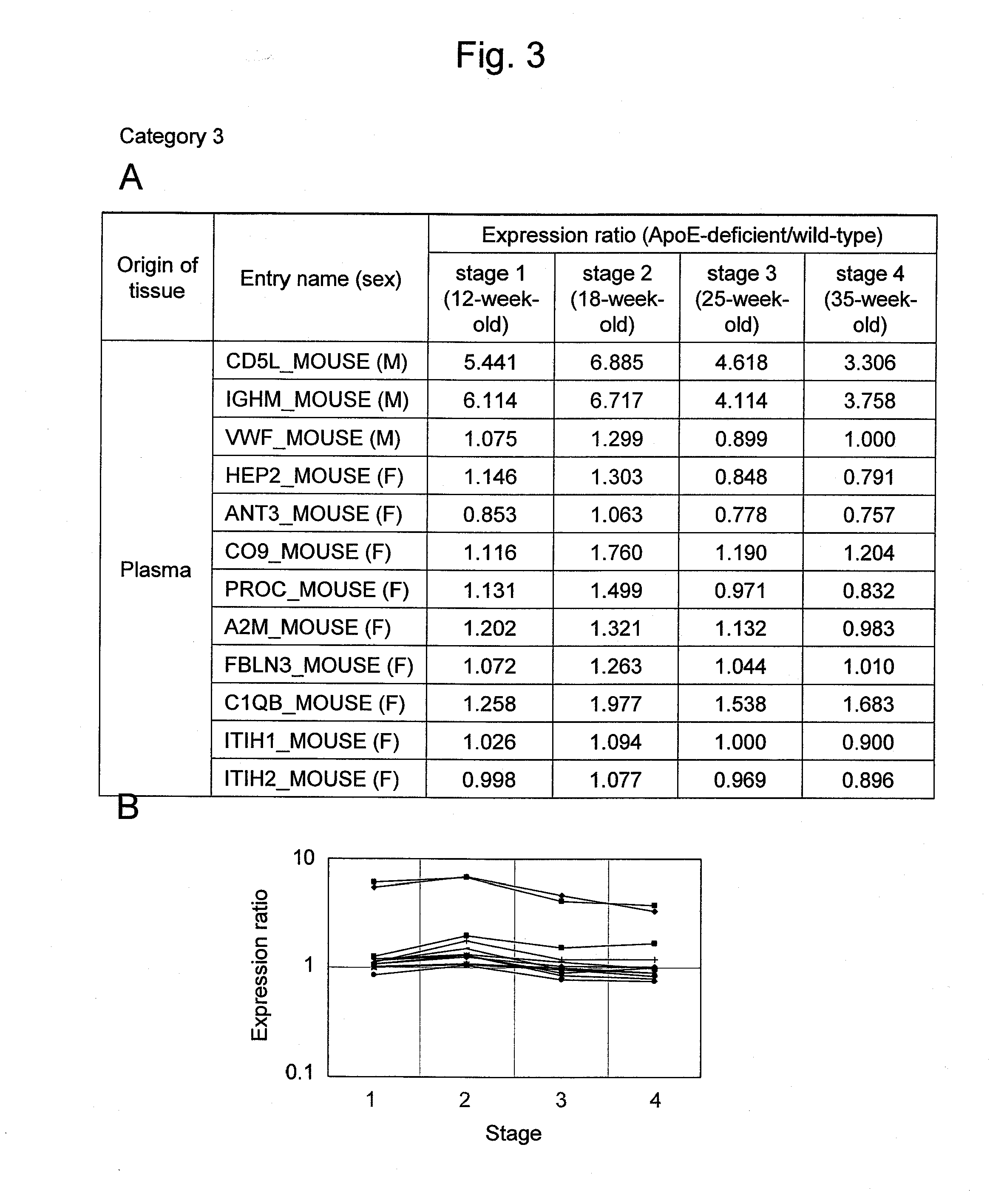
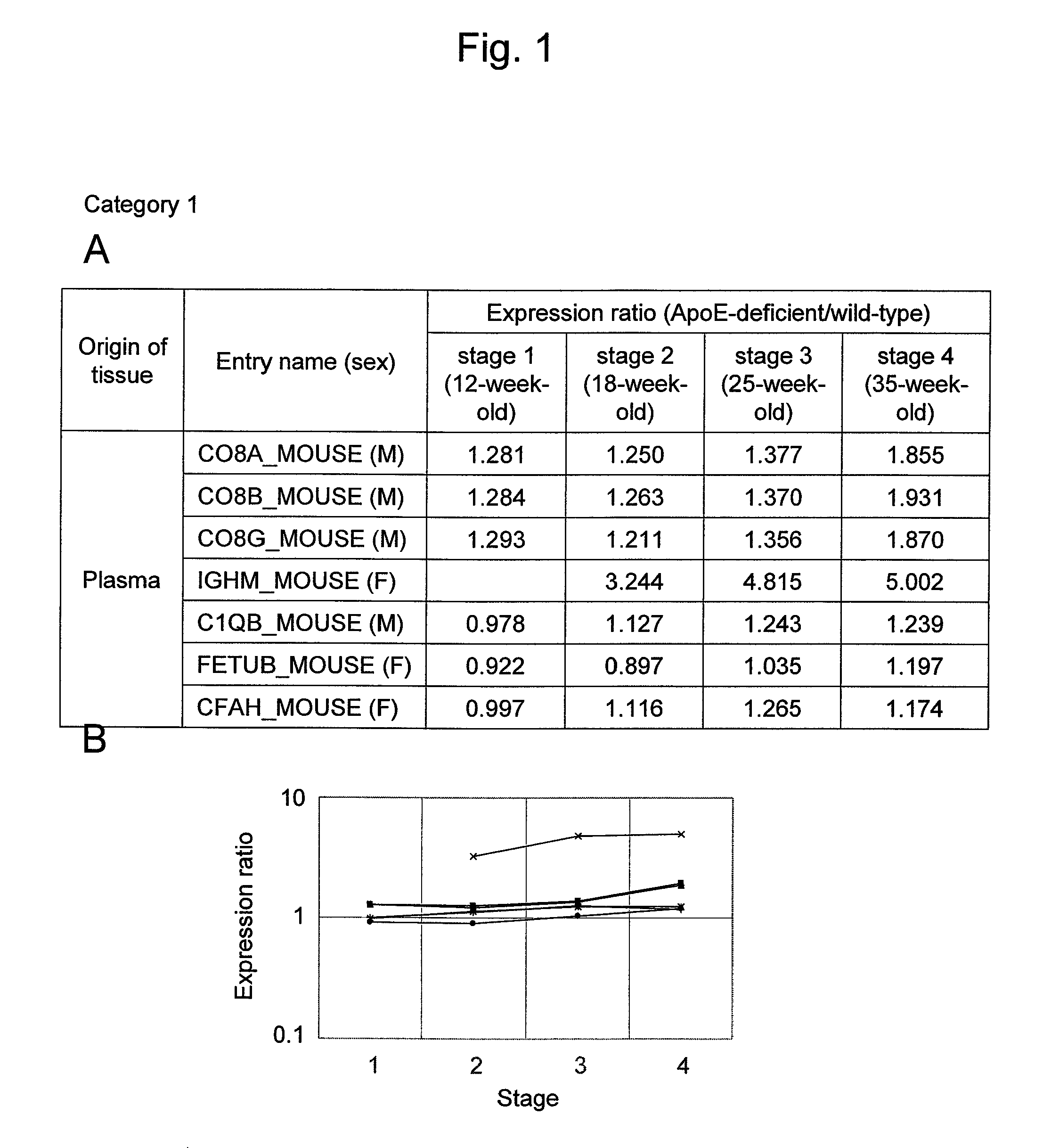
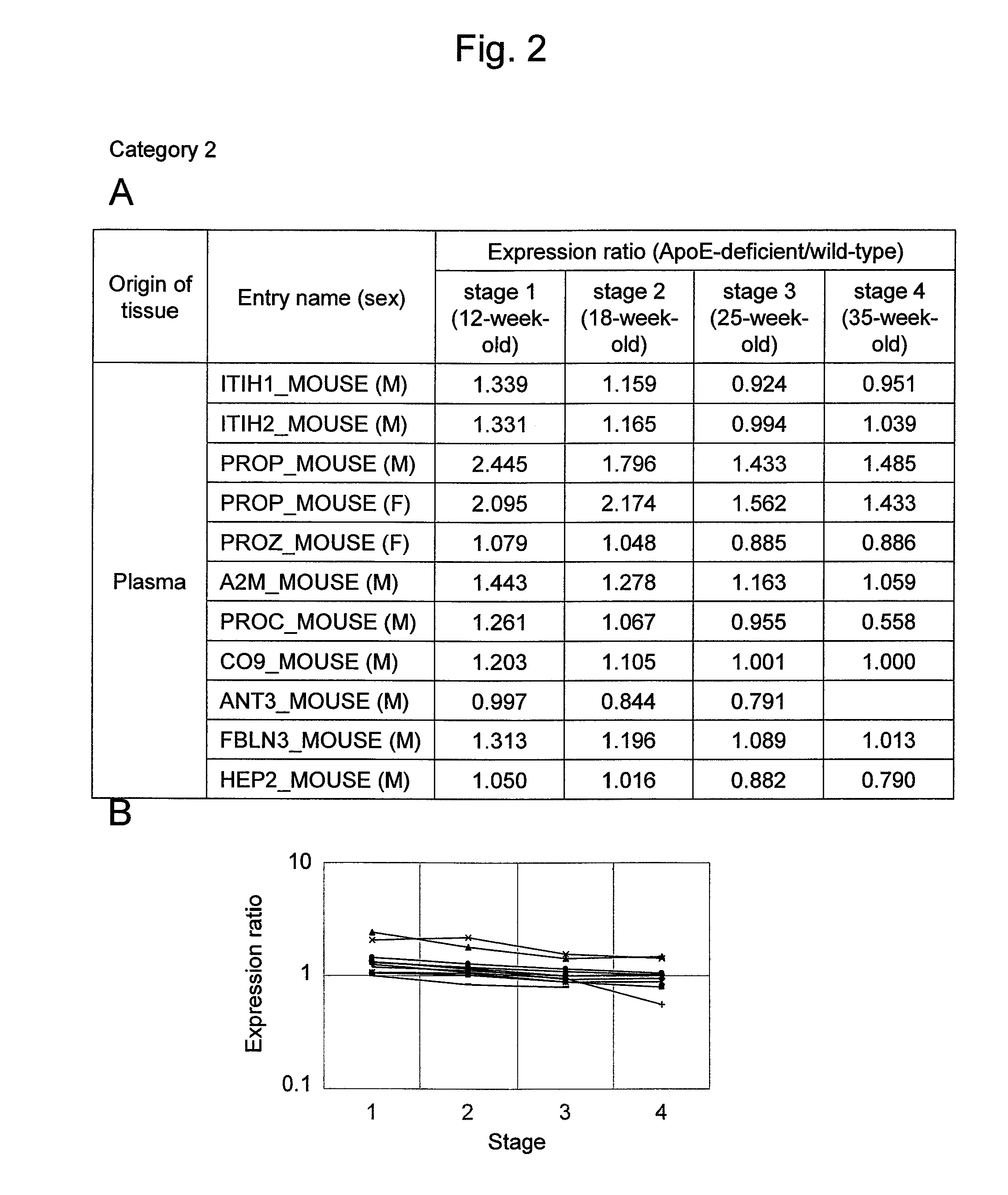
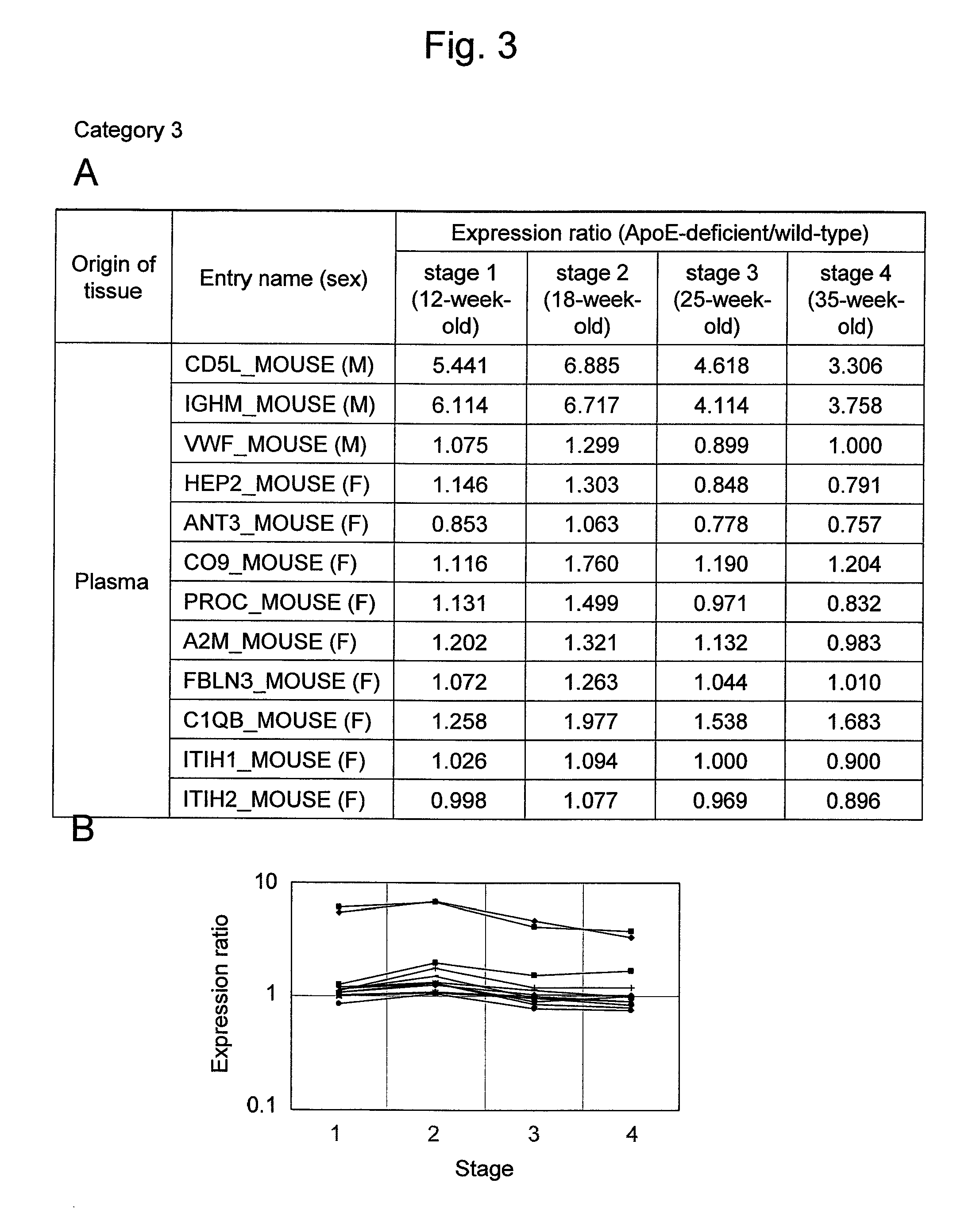
Evaluation Method for Arteriosclerosis
ActiveUS20130040851A1Microbiological testing/measurementLibrary screeningProtein markersVon Willebrand factor
Arteriosclerosis induces cerebral infarction and myocardial infarction. A multi-marker (a group of protein markers) that assesses the accurate pathogenesis of arteriosclerosis and enables the selection of an adequate treatment method for arteriosclerosis and prediction of the progression of arteriosclerosis, and an evaluation method for the diagnosis, prevention, and treatment of arteriosclerosis that uses said marker group as an indicator have been sought. The present invention relates to A method for evaluation of arteriosclerosis comprising the steps of (a) measuring the expression of von Willebrand factor and / or complement factor D in a sample derived from a subject, (b) measuring the expression of complement component C8 and / or vitamin K-dependent protein Z in the sample derived from the subject, and (c) evaluating arteriosclerosis in the subject on the basis of the results from (a) and (b).
Owner:HITACHI LTD
Pharmaceutical compounds for treatment of medical disorders
ActiveUS20190382376A1Reduce the possibilityMinimal hydrolysisGroup 5/15 element organic compoundsMedical disorderMedicine
Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, and Formula IV or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
Therapeutic regimens for treatment of paroxysmal nocturnal hemoglobinuria
PendingUS20200101071A1Growth inhibitionIncrease depositionOrganic active ingredientsImmunoglobulins against animals/humansParoxysmal AFRegimen
Provided herein are methods for treating a subject with PNH comprising administering to a subject a therapeutically effective amount of complement component C5 (C5) inhibitor, complement component C3 (C3) inhibitor, or complement factor B (CFB) inhibitor in combination with a therapeutically effective amount of small molecule complement factor D (CFD) inhibitor of Formula I or Formula II, or a pharmaceutically acceptable salt thereof.
Owner:ACHILLION PHARMA INC
Macrocyclic compounds for treatment of medical disorders
ActiveUS11053253B2Decreases potential for formationReduce the possibilityOrganic chemistryAntiviralsDiseaseMedical disorder
Macrocyclic Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, Formula IV, Formula V, Formula VI, Formula VII, and Formula VIII or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
Compositions and methods for inhibiting factor d
ActiveUS20180051287A1Inhibit functioningOrganic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
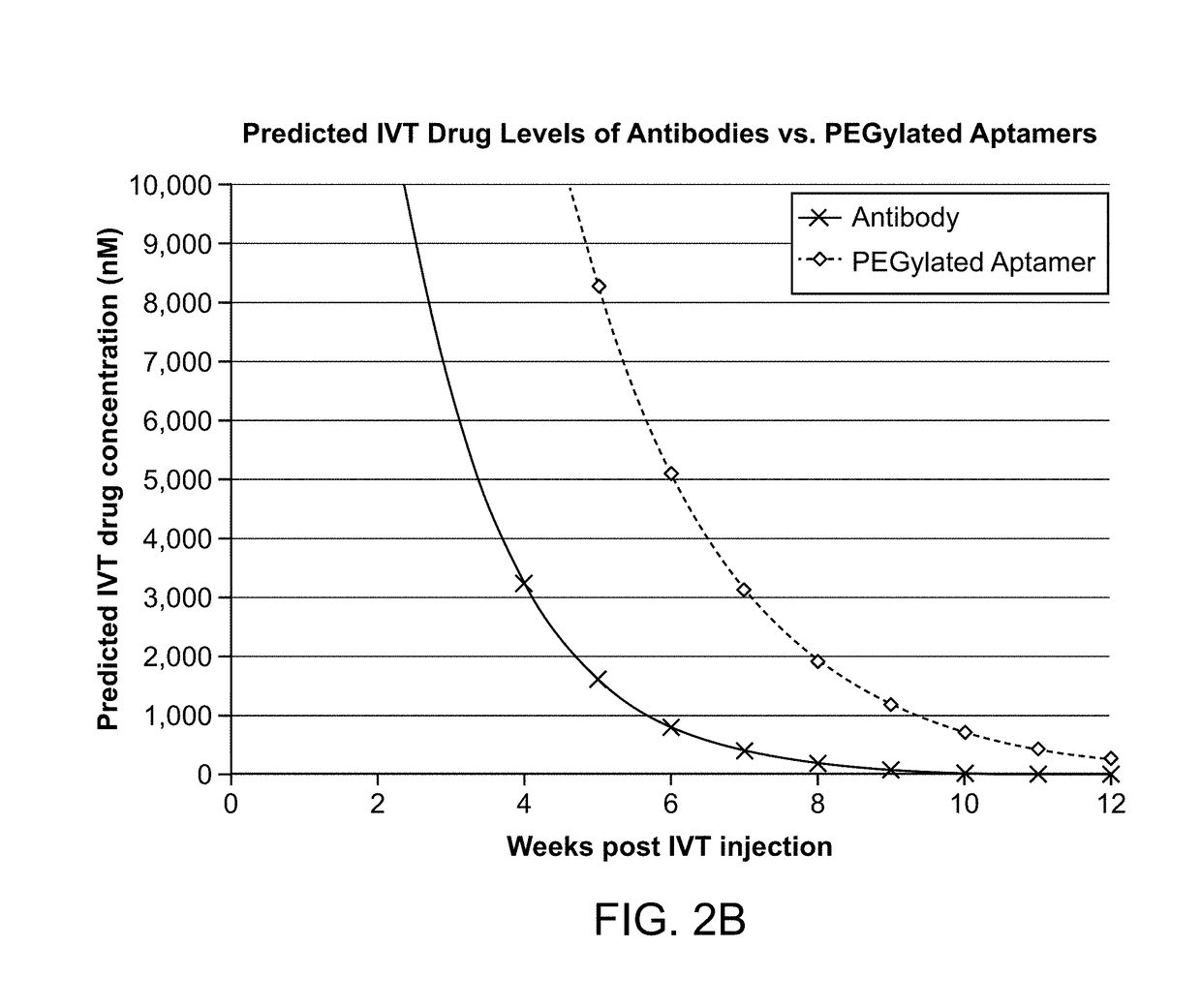
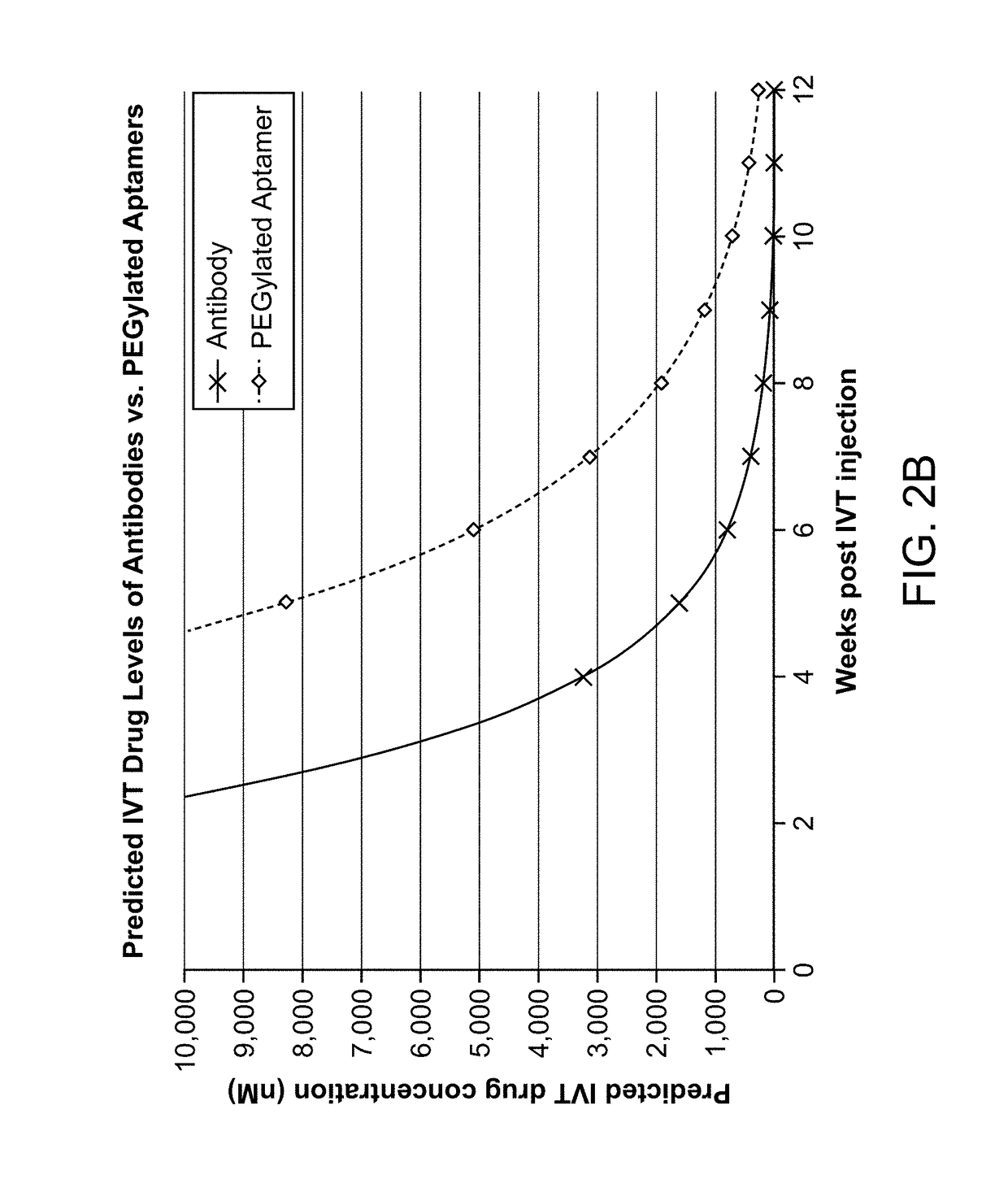
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Pharmaceutical compounds for treatment of medical disorders
ActiveUS11447465B2Decreases potential for formationReduce the possibilityGroup 5/15 element organic compoundsDiseaseMedical disorder
Complement Factor D inhibitors, pharmaceutical compositions, and uses thereof, as well as processes for their manufacture are provided. The compounds provided include Formula I, Formula II, Formula III, and Formula IV or a pharmaceutically acceptable salt, prodrug, isotopic analog, N-oxide, or isolated isomer thereof, optionally in a pharmaceutically acceptable composition. The inhibitors described herein target Factor D and inhibit or regulate the complement cascade.
Owner:ACHILLION PHARMA INC
Amide compounds for treatment of complement mediated disorders
InactiveCN106413707ALess quantityIncreased detectable levelsSenses disorderNervous disorderDepressantPharmaceutical medicine
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof, wherein R12 or R13 on the A group is an amide substituent (R32) are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade at an early and essential point in the alternative complement pathway, and reduce factor D's ability to modulate the classical and lectin complement pathways. The inhibitors of factor D described herein are capable of reducing the excessive activation of complement, which has been linked to certain autoimmune, inflammatory, and neurodegenerative diseases, as well as ischemia-reperfusion injury and cancer.
Owner:ACHILLION PHARMA INC
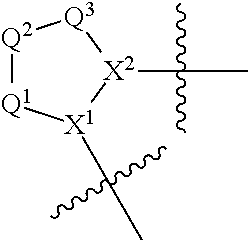
Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
Compounds, methods of use, and processes for making inhibitors of complement Factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof wherein R12 or R13 on the A group is an aryl, heteroaryl or heterocycle (R32) are provided. The inhibitors of Factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Compositions and methods for inhibiting Factor D
ActiveUS10174325B2Organic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Aminomethyl-Biaryl Derivatives Complement Factor D inhibitors and uses thereof
InactiveUS20160145247A1High affinityInhibition of catalytic activityAntibacterial agentsSenses disorderActive agentPharmaceutical drug
Owner:NOVARTIS AG
Evaluation method for arteriosclerosis
ActiveUS9175346B2Microbiological testing/measurementLibrary screeningProtein markersComplement factor I
Arteriosclerosis induces cerebral infarction and myocardial infarction. A multi-marker (a group of protein markers) that assesses the accurate pathogenesis of arteriosclerosis and enables the selection of an adequate treatment method for arteriosclerosis and prediction of the progression of arteriosclerosis, and an evaluation method for the diagnosis, prevention, and treatment of arteriosclerosis that uses said marker group as an indicator have been sought. The present invention relates to A method for evaluation of arteriosclerosis comprising the steps of (a) measuring the expression of von Willebrand factor and / or complement factor D in a sample derived from a subject, (b) measuring the expression of complement component C8 and / or vitamin K-dependent protein Z in the sample derived from the subject, and (c) evaluating arteriosclerosis in the subject on the basis of the results from (a) and (b).
Owner:HITACHI LTD
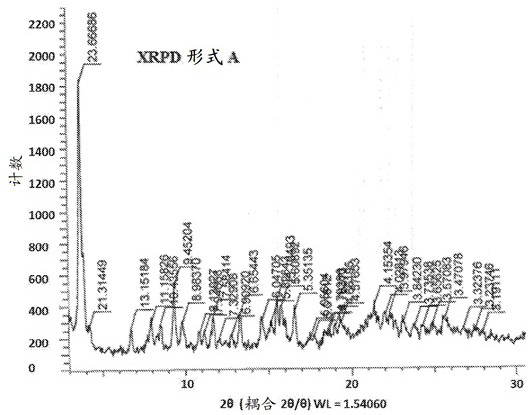
Morphic forms of complement factor d inhibitors
This invention provides stable, highly crystalline forms of Complement factor D inhibitors Compound 1 and Compound 2 for advantageous therapeutic pharmaceutical efficacy and dosage form stability.
Owner:ACHILLION PHARMA INC
Allelic Variants Associated with Advanced Age-Related Macular Degeneration
InactiveUS20120115925A1Increased riskOrganic active ingredientsMicrobiological testing/measurementComplement factor IMacular degeneration
Owner:MASSACHUSETTS EYE & EAR INFARY
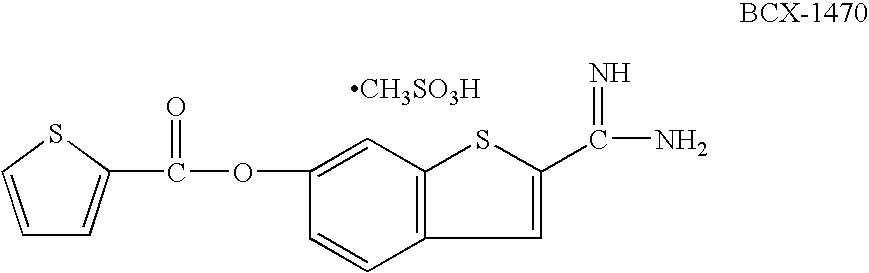
Oral complement factor D inhibitors
Disclosed are compounds of Formula (I)-(IV) and pharmaceutically acceptable salts thereof, which are inhibitors of the complement system. Also provided are pharmaceutical compositions comprising such compounds, and methods of using the compounds and compositions to treat or prevent diseases or conditions characterized by aberrant complement system activity.
Owner:BIOCRYST PHARM INC
Dosing regimens for oral complement factor D inhibitors
Compounds and pharmaceutically acceptable salts and prodrugs thereof are disclosed, which are inhibitors of the complement system. Also provided are oral dosage forms comprising such compounds, salts, or prodrugs. Also disclosed are methods of using the compounds, salts and prodrugs and oral dosage forms thereof to treat or prevent diseases or conditions characterized by aberrant complement system activity (e.g., paroxysmal sleep hemoglobinuria).
Owner:BIOCRYST PHARM INC
Urinary Protein Markers of Renal Injury in Malignant Hypertension and Its Diagnostic Application
ActiveCN109839505BAvoiding the Risks of a Kidney BiopsyComponent separationMaterial analysis by electric/magnetic meansProtein markersRNA - Ribonucleic acid
Owner:BEIJING NORMAL UNIVERSITY
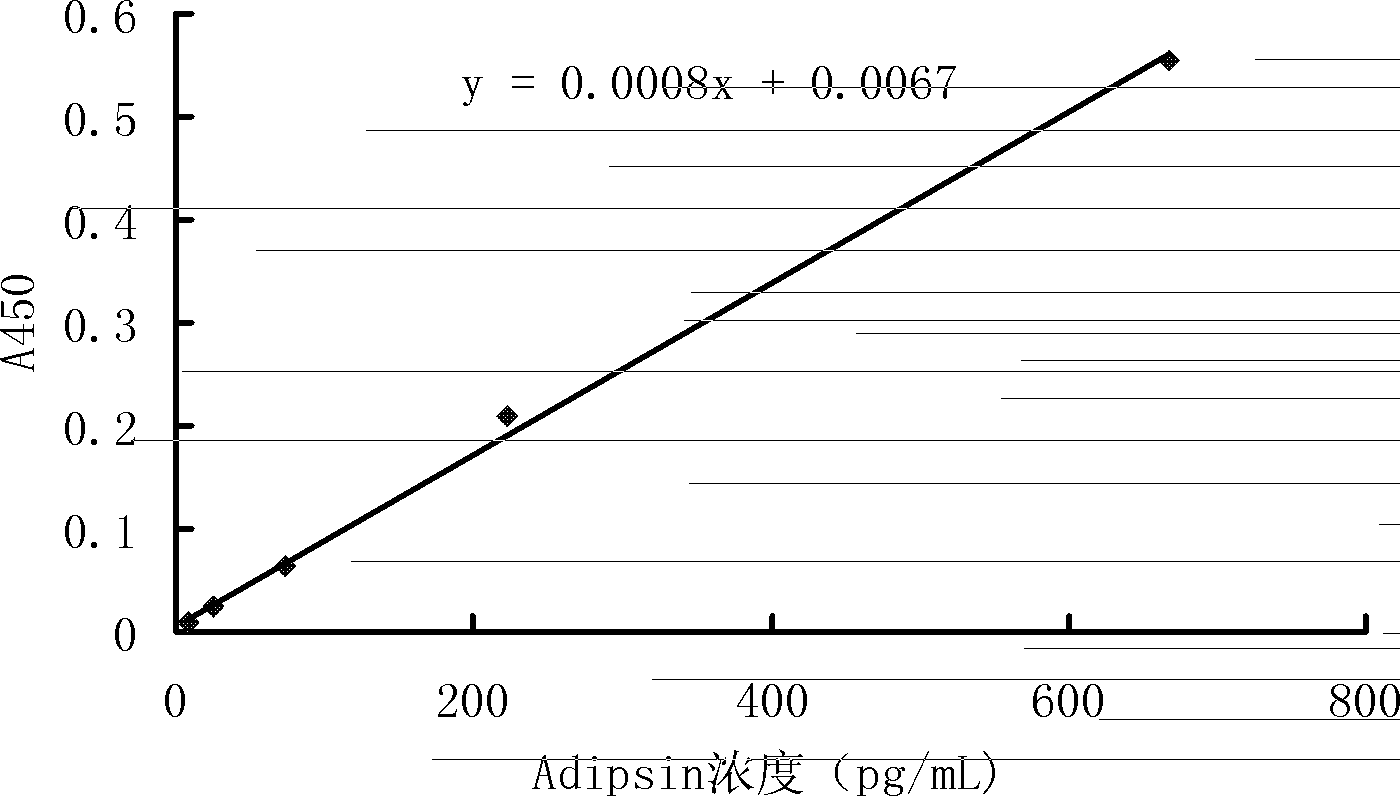
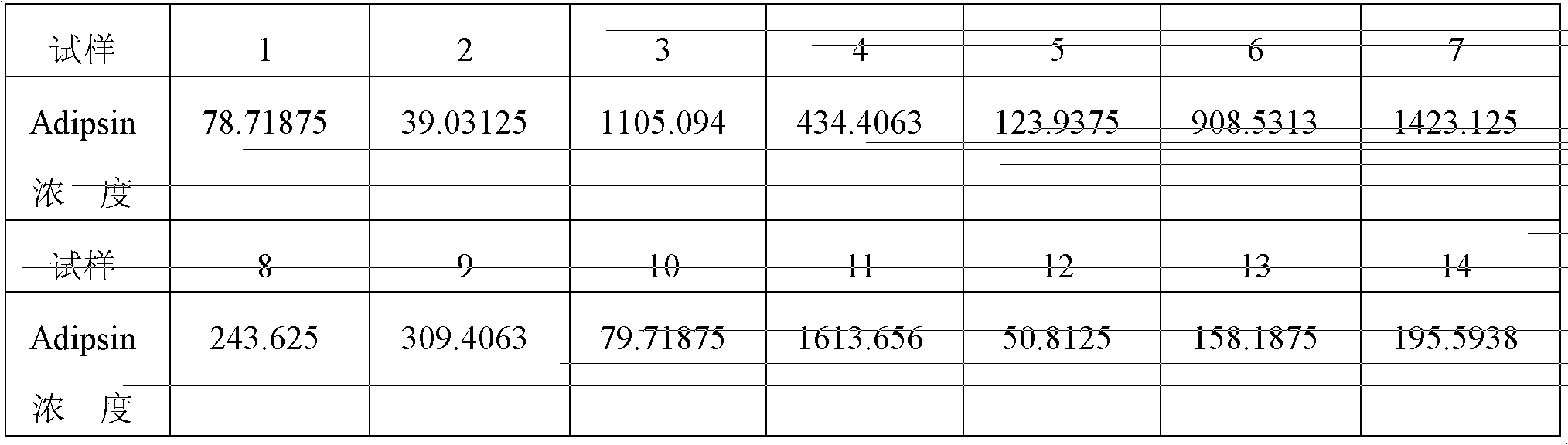
Preparation method of preeclampsia detection kit adopting Adipsin as detection index
ActiveCN102426243BQuantitative detection of accurate contentImprove accuracyDisease diagnosisChromogenic SubstratesMonoclonal antibody
A preeclampsia detecting kit using Adipsin as indicator, comprises porous plate coated with anti-human Adipsin monoclonal antibody, anti-human Adipsin monoclonal antibody detecting solution marked by biotin, avidin horseradish peroxidase combined with anti-human Adipsin monoclonal antibody marked by biotin, chromogenic substrate 3',3',5,5'-tetramethylbenzidine and Adipsin protein standard substance. The preparing method of the detecting kit comprises:(1)preparing the porous plate coated with anti-human Adipsin monoclonal antibody; (2) preparing the anti-human Adipsin monoclonal antibody detecting solution marked by biotin;(3) preparing the avidin horseradish peroxidase combined with anti-human Adipsin monoclonal antibody marked by biotin;(4) preparing the chromogenic substrate 3',3',5,5'-tetramethylbenzidine and Adipsin protein standard substance.
Owner:ORIGISSAY BIOLOGICS TECH
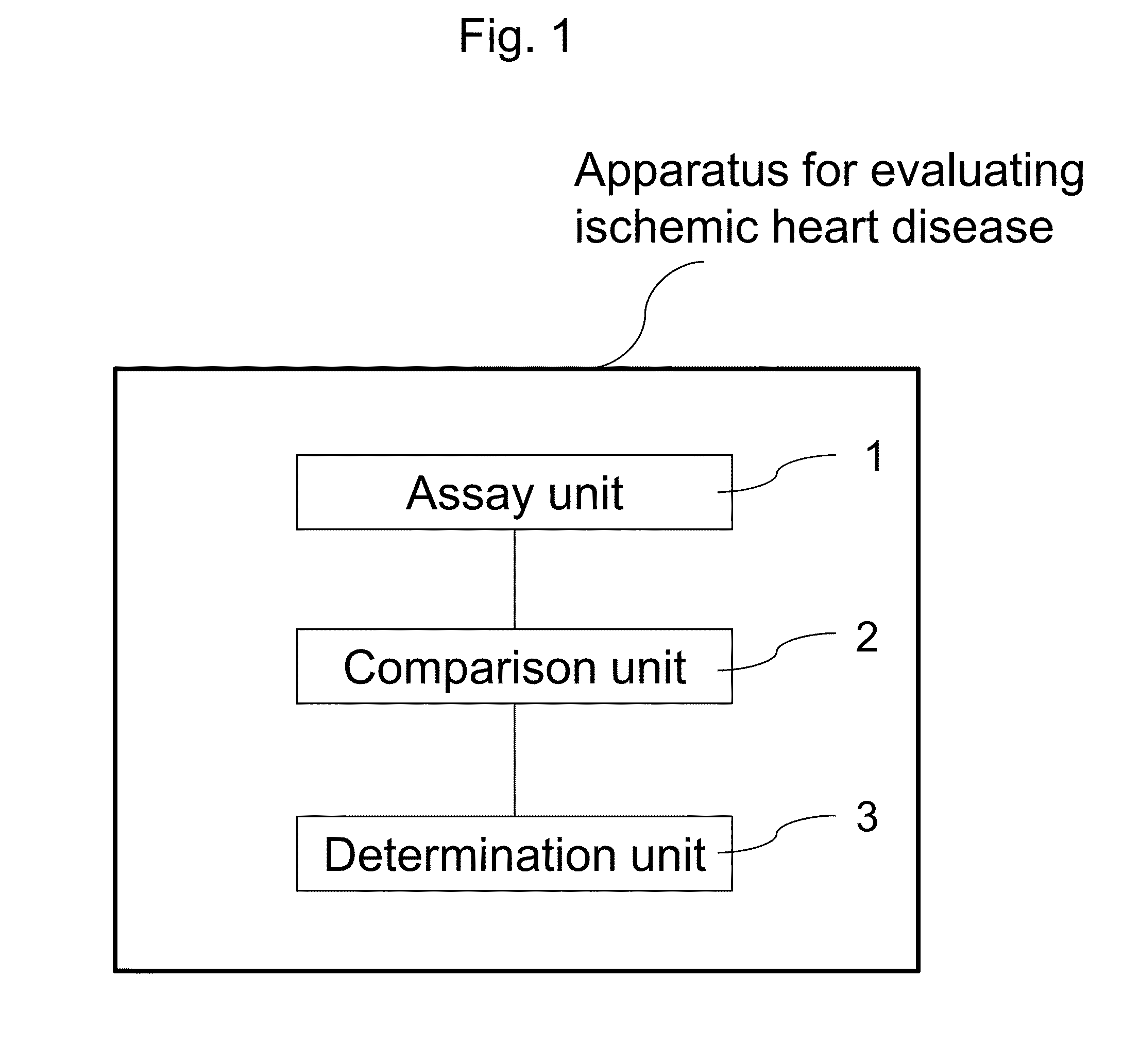
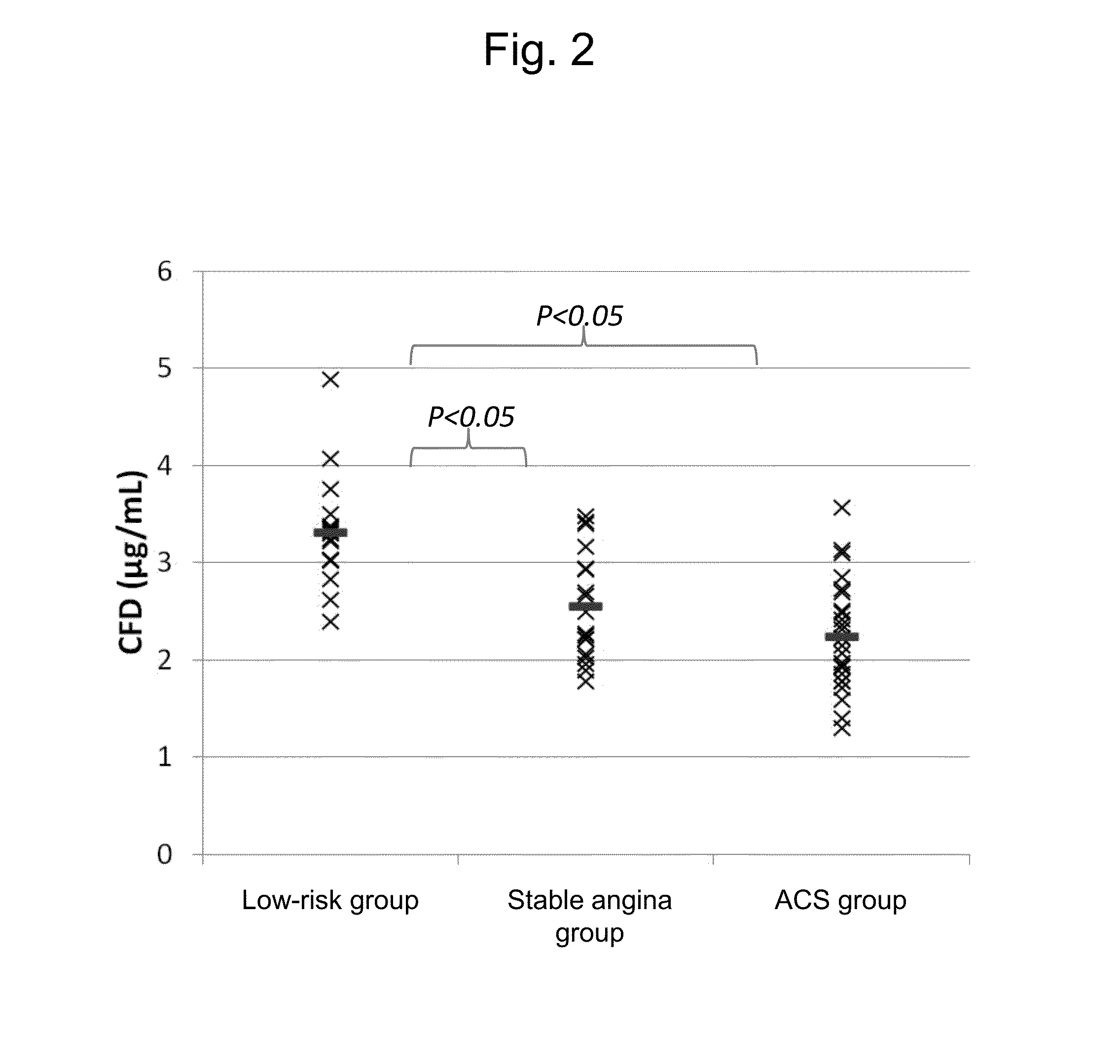
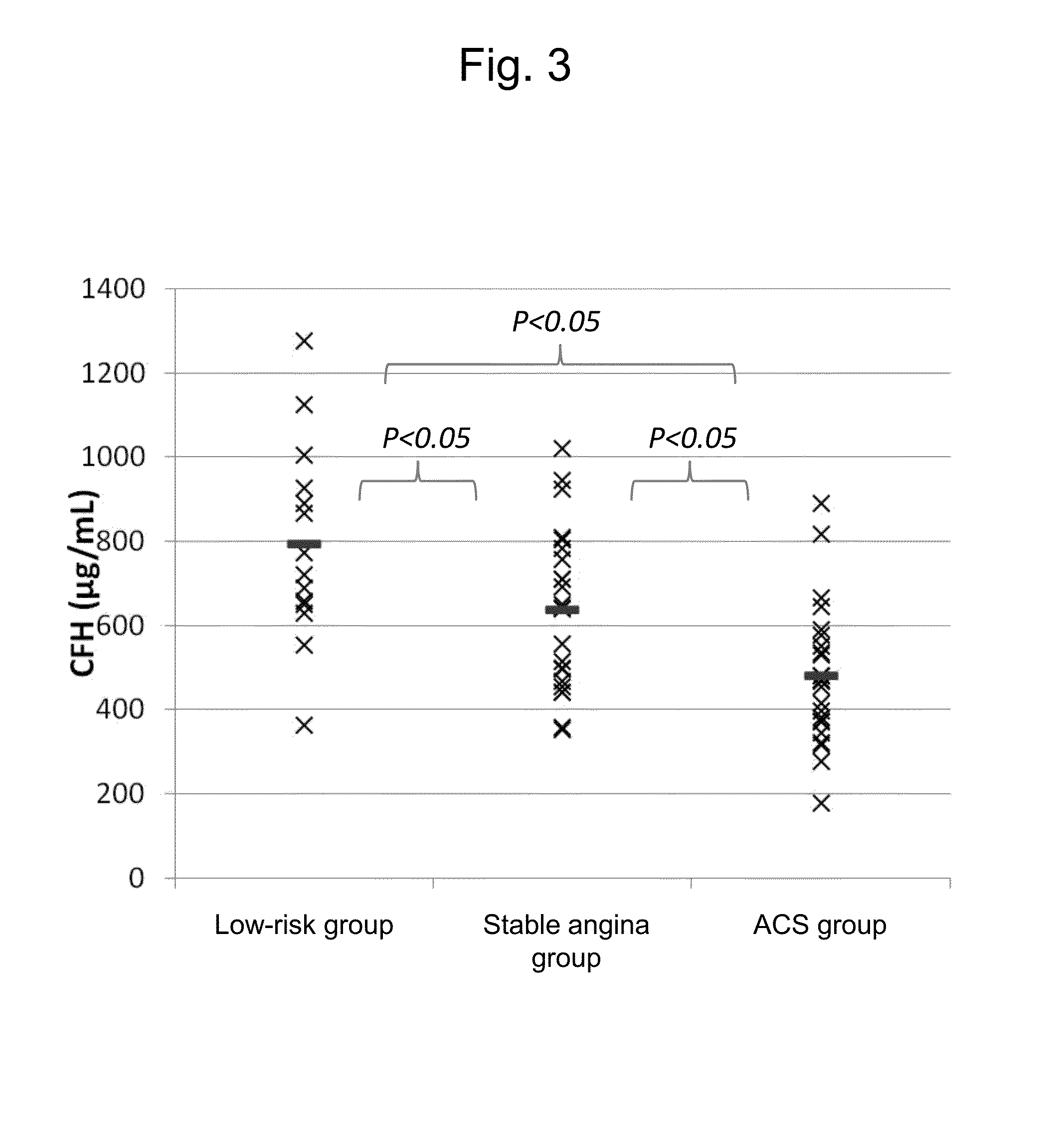
Method, kit, and apparatus for evaluating ischemic heart disease
ActiveUS20150105291A1Easy to operateAccurate and convenient examinationImmunoglobulins against blood coagulation factorsBioreactor/fermenter combinationsHeart diseaseIschemic Heart Diseases
It is intended to evaluate an ischemic heart disease with high accuracy by convenient operation. The method for evaluating an ischemic heart disease according to the present invention comprises the steps of: assaying complement factor H and / or complement factor D in a sample derived from the blood of a test subject; and comparing the concentration of the complement factor H and / or the concentration of the complement factor D assayed in the preceding step with a reference value(s), wherein it is determined that the seriousness of the ischemic heart disease is high when the concentration falls below the reference value.
Owner:HITACHI LTD
Humanized fabs and humanized antibodies against complement factor d and their uses
The present invention discloses an anti-complement factor D humanized Fab and humanized antibody and application thereof, the humanized Fab and humanized antibody contain light chain variable region and heavy chain variable region, the use Human complement factor D-related diseases are diseases caused by elevated human complement factor D.
Owner:CHENGDU KANGHONG BIOTECH
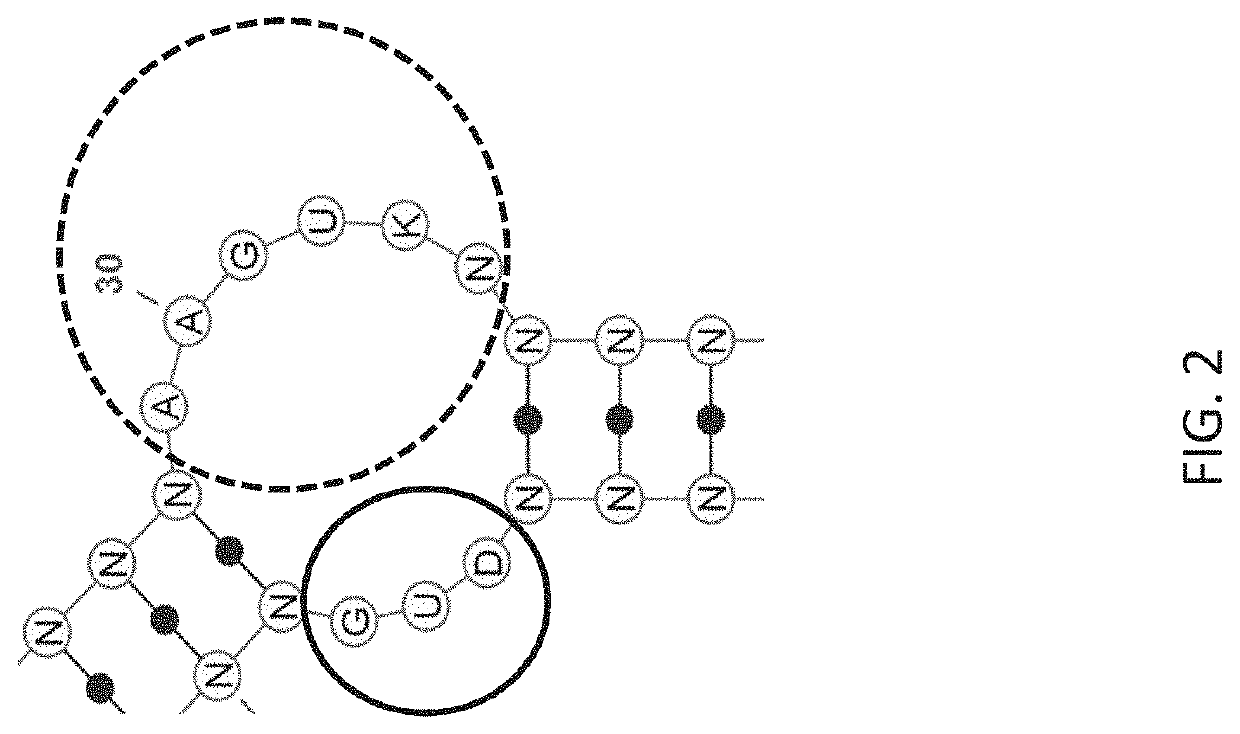
Stem-loop compositions and methods for inhibiting factor D
ActiveUS11466276B2Organic active ingredientsPharmaceutical non-active ingredientsAptamerGeographic atrophy
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease. In some cases, stem-loop aptamers are provided for the inhibition of Factor D.
Owner:396419 B C LTD +1
Morphic forms of complement factor d inhibitors
This invention provides stable, highly crystalline forms of Complement Factor D inhibitor Compound 3 for therapeutic applications.
Owner:ACHILLION PHARMA INC
Tool and box for quickly detecting early-stage puerperal convulsion by taking Adipsin as detection index and manufacturing method thereof
A means for rapidly detecting Adipsin for diagnosing preeclampsia comprises a water-adsorbing pad(2), a nitrocellulose membrane(5), a gold labeling pad (6) and a sampling pad (7), all of which are successively joined from the top down and fixed in the base plate(1). The gold-labeling pad (6) is partly overlapped by the sampling pad (7). A detecting line (4) coated by rabbit anti-human Adipsin polyclonal antibody, or goat anti-human Adipsin polyclonal antibody or mouse anti-human Adipsin polyclonal antibody and a controlling line (3) coated by goat anti-mouse IgG polyclonal antibody are provided on the nitrocellulose membrane (5). The detecting line (4) is located downstream and spaced from the controlling line (3).The gold-labeling pad (6) is made of water adsorbing material, and coated by gold labeled mouse anti-human Adispin monoclonal antibody. A tesk kit for rapidly detecting Adipsin for diagnosing preeclampsia comprises an outer house (8) and the said means therein. The method for preparing the said detecting means comprise: 1) coating of the detecting line (4) and the controlling line (3); 2) preparing the gold labeling pad (6); 3)combination.
Owner:ORIGISSAY BIOLOGICS TECH
Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
Compounds, methods of use, and processes for making inhibitors of complement Factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof wherein R12 or R13 on the A group is an aryl, heteroaryl or heterocycle (R32) are provided. The inhibitors of Factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Marker for predicting curative effect of harmful ventricular remodeling and immunotherapy of myocardial infarction patient and application of marker
PendingCN114859061AMeet specificityMeet the accuracyDisease diagnosisBiological testingLeft Ventricle RemodelingComplement factor I
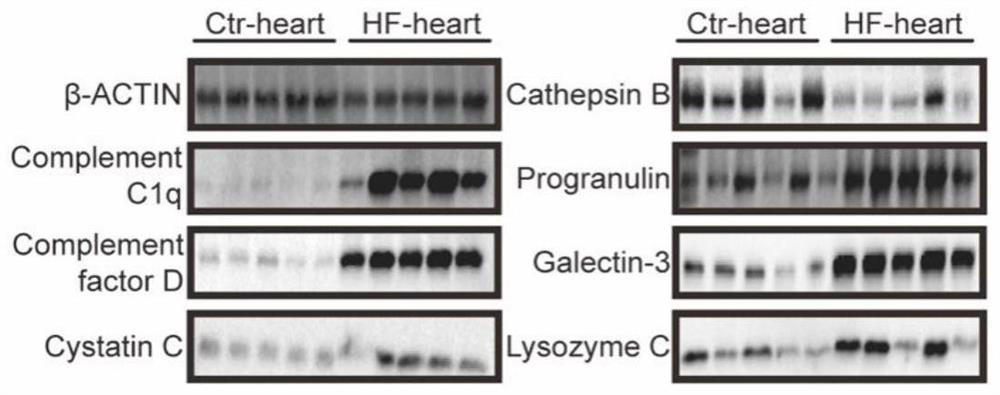
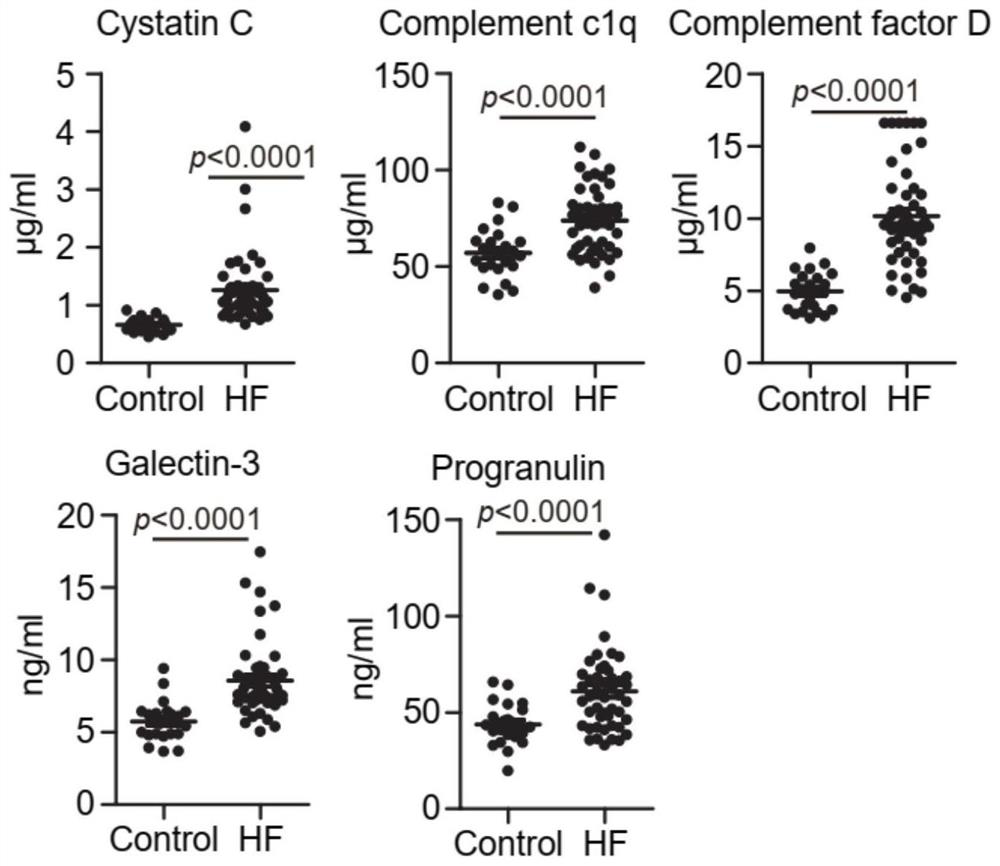
The invention provides a marker for predicting the curative effect of harmful ventricular remodeling and immunotherapy of myocardial infarction patients and application of the marker. The marker comprises granular protein, galectin 3, a complement factor D, a cysteine protease inhibitor 3 and C1q, the five markers are combined to be used as serum indexes for predicting harmful ventricular remodeling and immunotherapy curative effects of myocardial infarction patients, so that the requirements on specificity, accuracy, precision (including repeatability, day difference and personnel operation error) and the like in a verification process can be met; the method has important significance in predicting harmful ventricular remodeling and immunotherapy effects of myocardial infarction patients.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Oral complement factor D inhibitors
Compounds of Formula (I) and pharmaceutically acceptable salts thereof are disclosed, which are inhibitors of the complement system. Also provided are pharmaceutical compositions comprising such compounds, and methods of using the compounds and compositions to treat or prevent diseases or conditions characterized by aberrant complement system activity.
Owner:BIOCRYST PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com