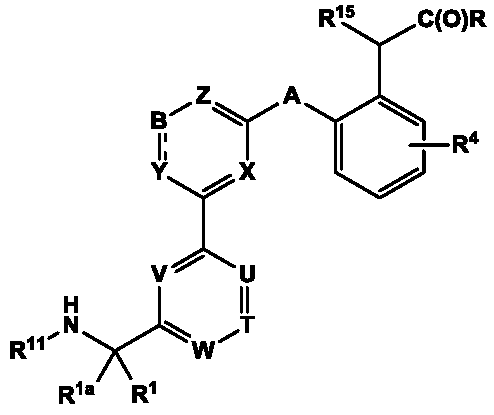
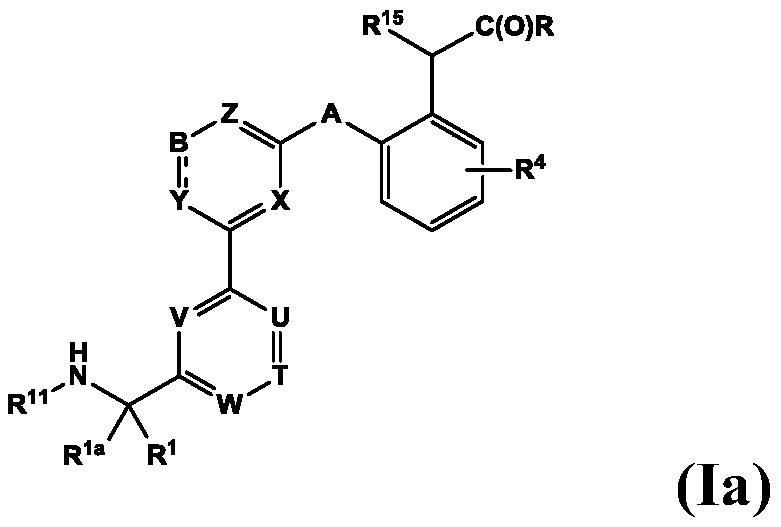
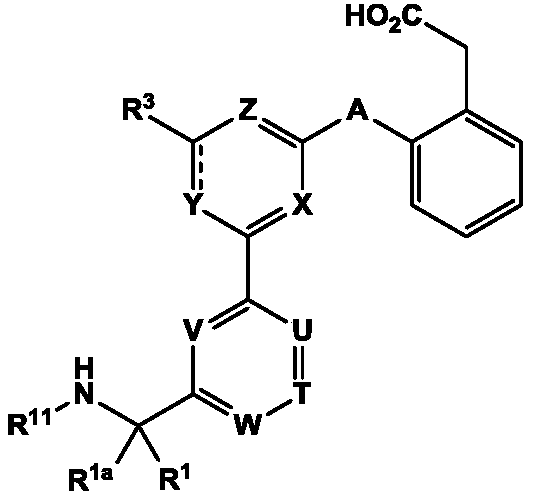
Aminomethyl-biaryl derivatives as complement factor D inhibitors and uses thereof
A technology of heteroaryl and alkyl, applied to aminomethyl-biaryl derivatives as complement factor D inhibitors and application fields thereof, can solve problems such as blindness in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1189] Biological Example 1: Human Complement Factor DELISA Assay
[1190] Recombinant human factor D (expressed in E. coli and purified using standard methods) was treated at a concentration of 10 nM together with various concentrations of test compounds in 7.5 mM MgCl 2Incubate with 0.075% (w / v) CHAPS in 0.1 MPBS pH 7.4 for 1 hour at room temperature. Cobra venom factor and human complement factor B substrate complex were added to a final concentration of 200 nM. After incubation at room temperature for 1 hour, the enzyme reaction was stopped by adding 0.1 M sodium carbonate buffer, pH 9.0, containing 0.15 M NaCl and 40 mM EDTA. The reaction product Ba was quantified by enzyme-linked immunosorbent assay. ICs were calculated as percent inhibition of Factor D-activity as a function of test compound concentration 50 value.
Embodiment 2
[1191] Biological Example 2: Human Complement Factor DTR-FRET Assay
Embodiment 21
[1192] Biological Example 2.1.2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-(tert-butoxycarbonyl)-5-((3- Chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-yl)methyl)amino)-6-oxohexyl)-3,3-dimethyl-5-sulfodihydro Indole-2-ylidene)pent-1,3-dien-1-yl)-1-ethyl-3,3-dimethyl-5-sulfo-3H-indole-1-
[1193]
[1194] To 2-((1E,3E,5E)-5-(1-(5-carboxypentyl)-3,3-dimethyl-5-sulfoindoline-2-ylidene)pentyl-1 ,3-dien-1-yl)-1-ethyl-3,3-dimethyl-5-sulfo-3H-indole-1- To a solution of potassium salt (Cy-5, CAS#449175-58-0) (162 mg, 0.233 mmol) in DMF (1 mL) was added HBTU (112 mg, 0.295 mmol) at room temperature. After stirring for 10 minutes, (2S,4S)-4-(aminomethyl)-2-((3-chloro-2-fluorobenzyl)carbamoyl)-4-fluoro-pyrrolidine-1-carboxylic acid tert Butyl ester (Intermediate B15 of WO2012093101, Step C) (129 mg, 0.32 mmol) and DIEA (86 μL, 0.491). The blue solution was stirred for 12 hours and then purified by preparative HPLC (WatersSunFire C18OBD, 5 μm, 30*100 mm, eluent A:H 2 O+0.1% TFA, B:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com