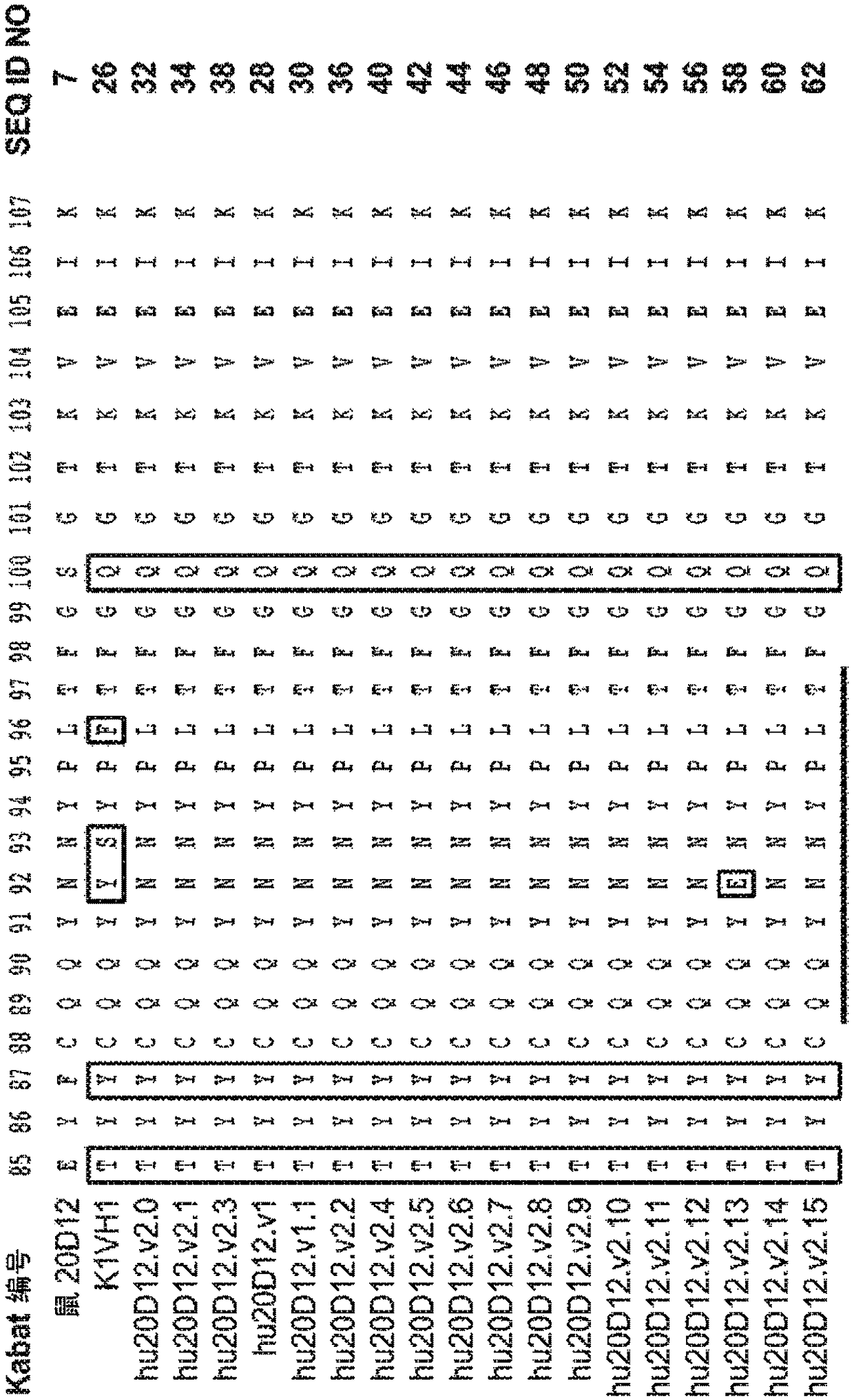
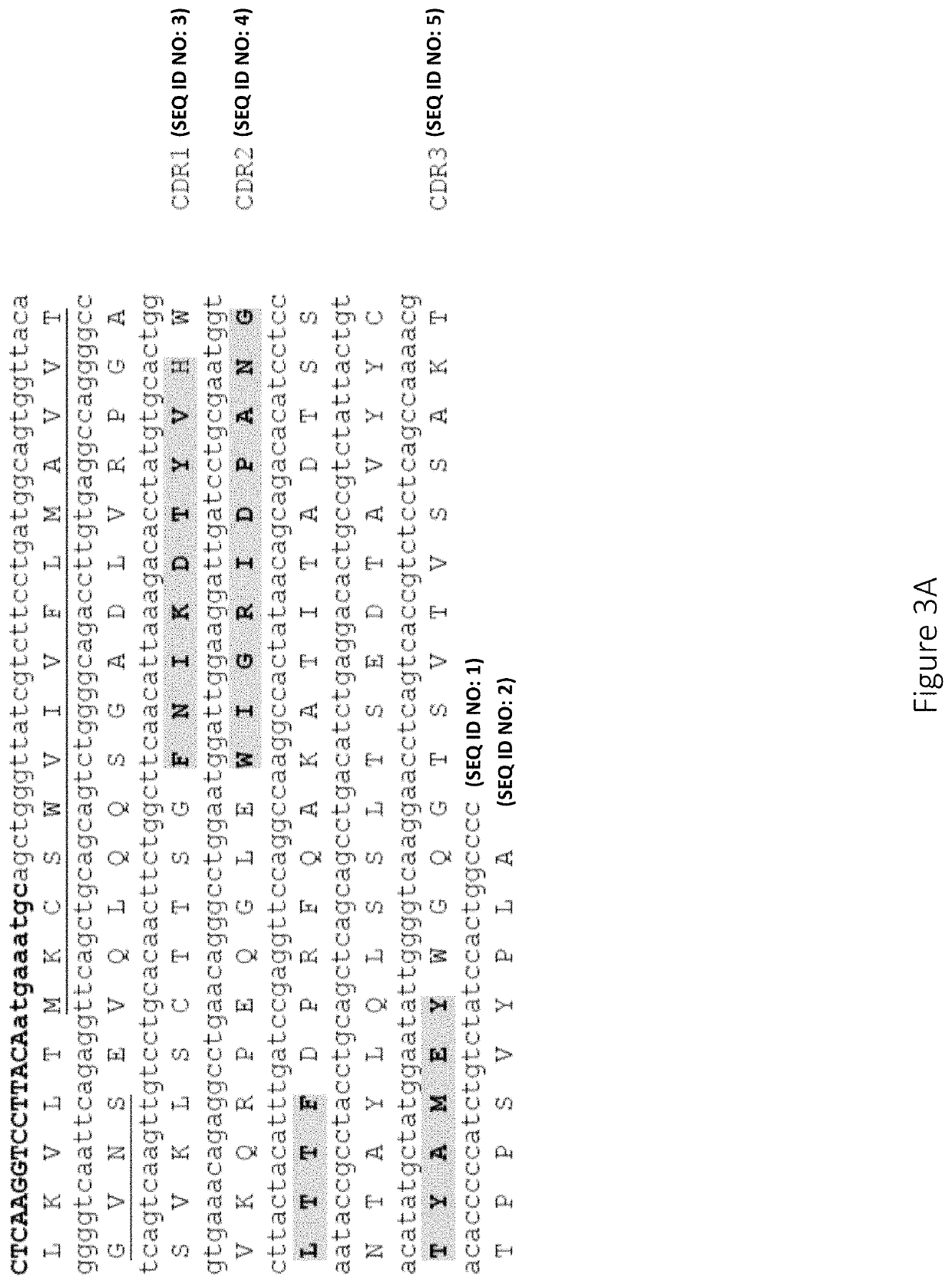
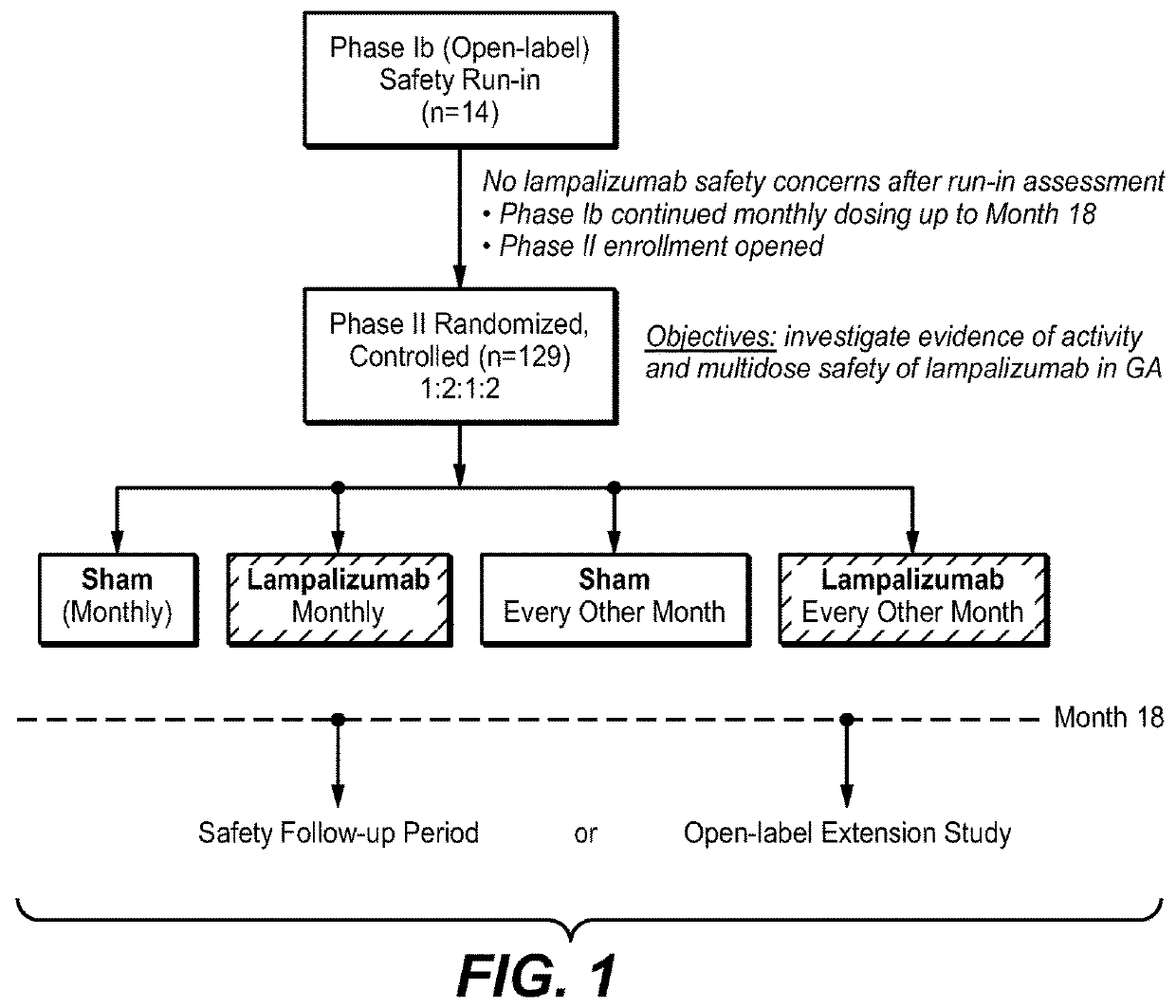
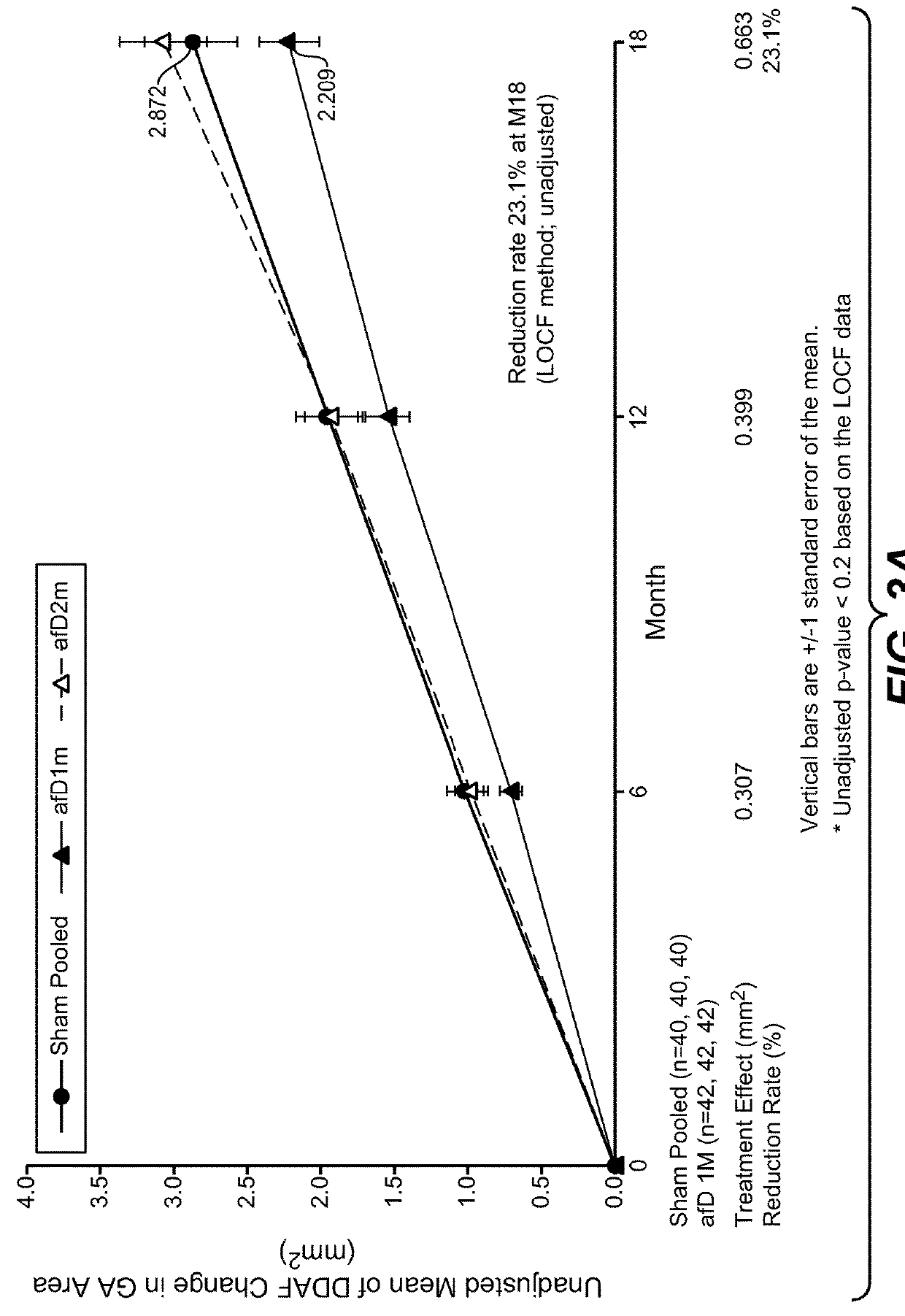
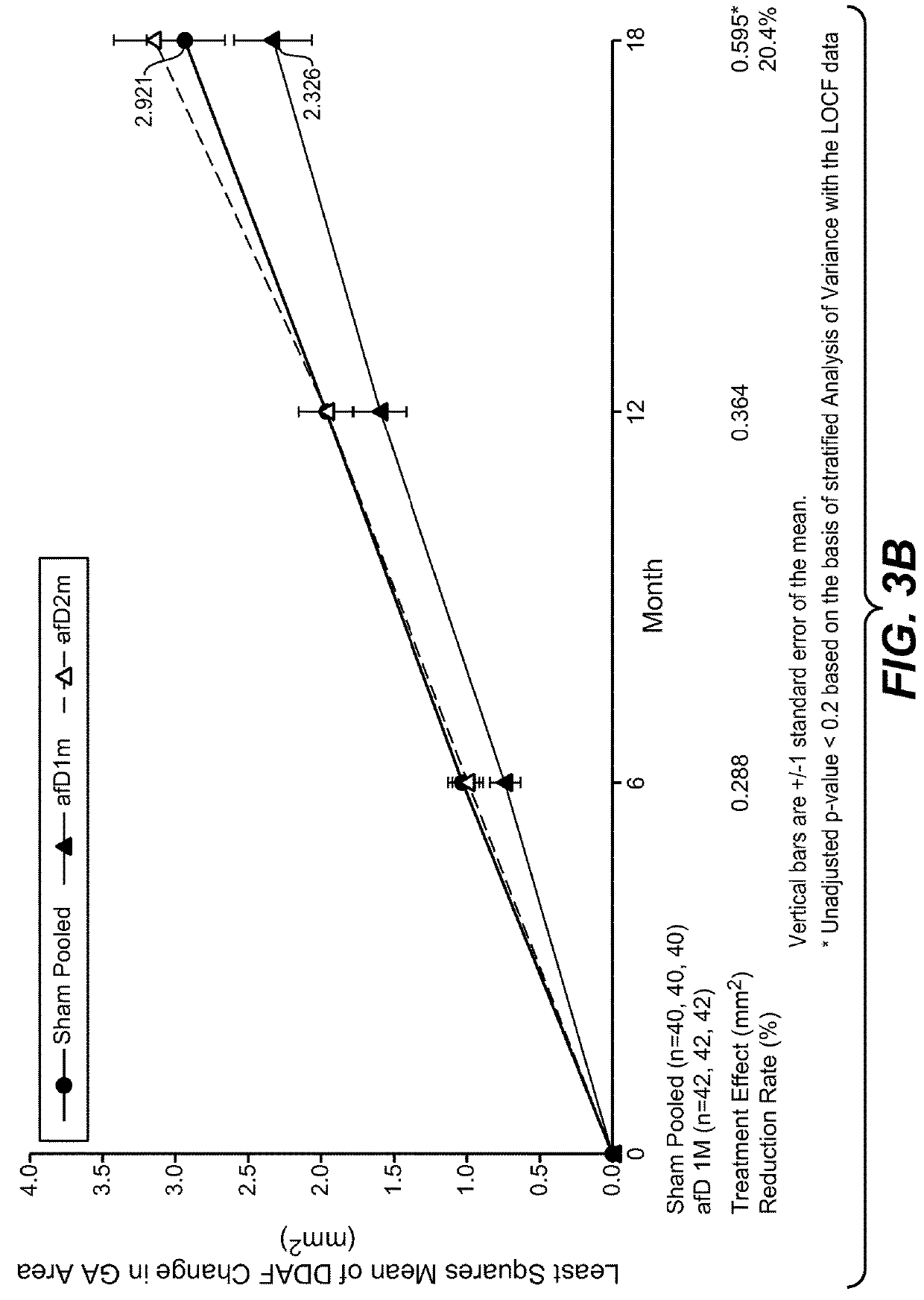
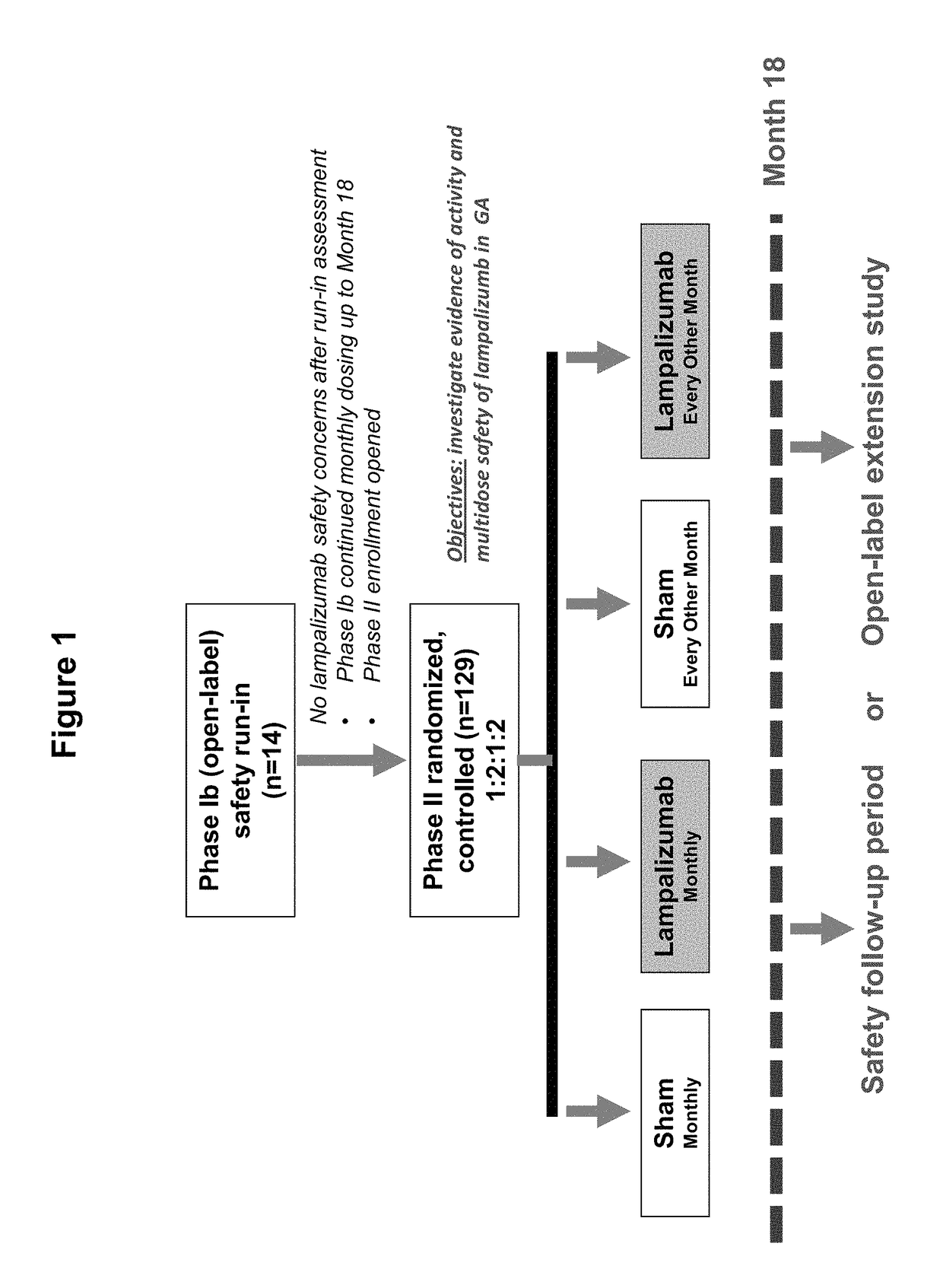
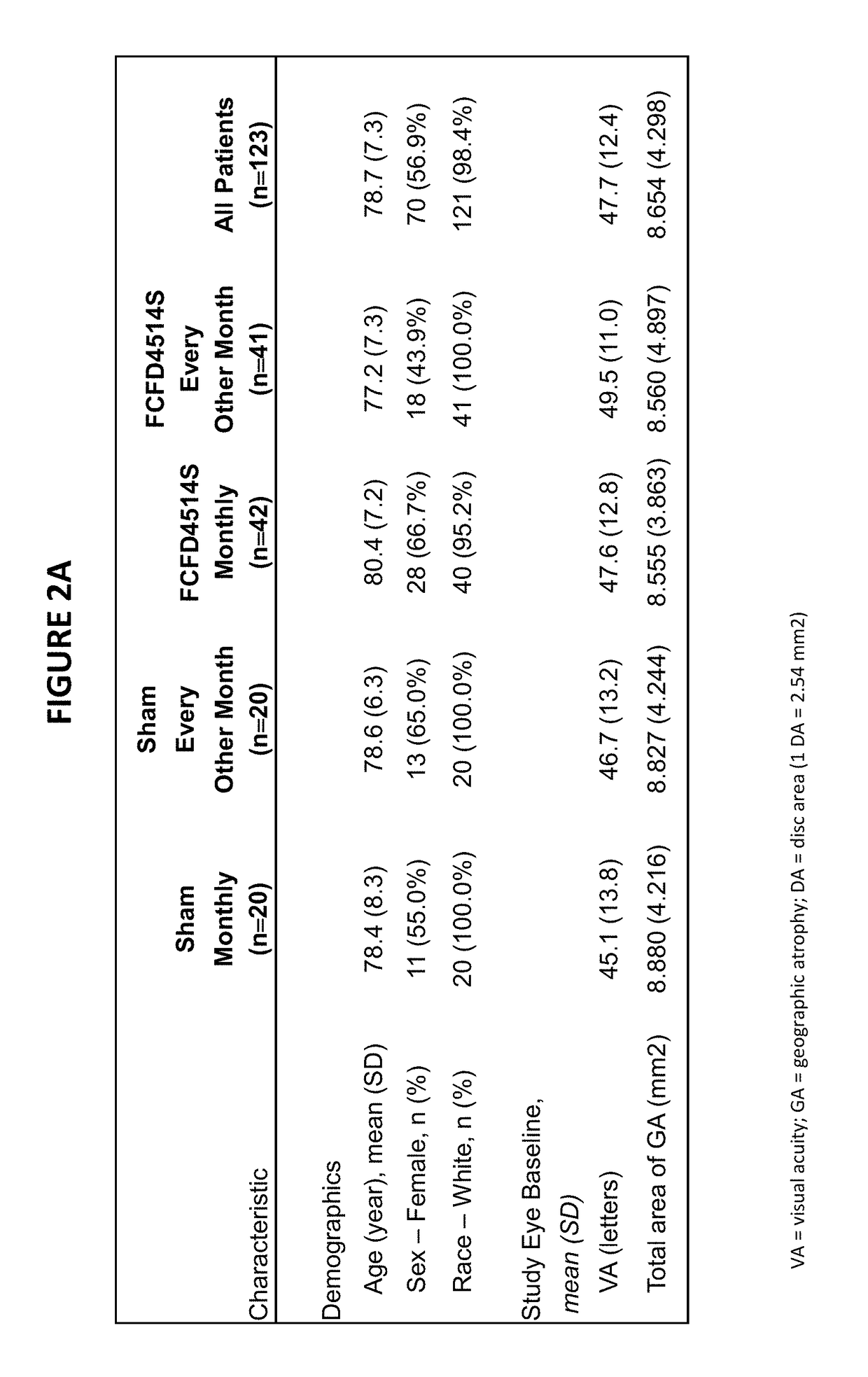
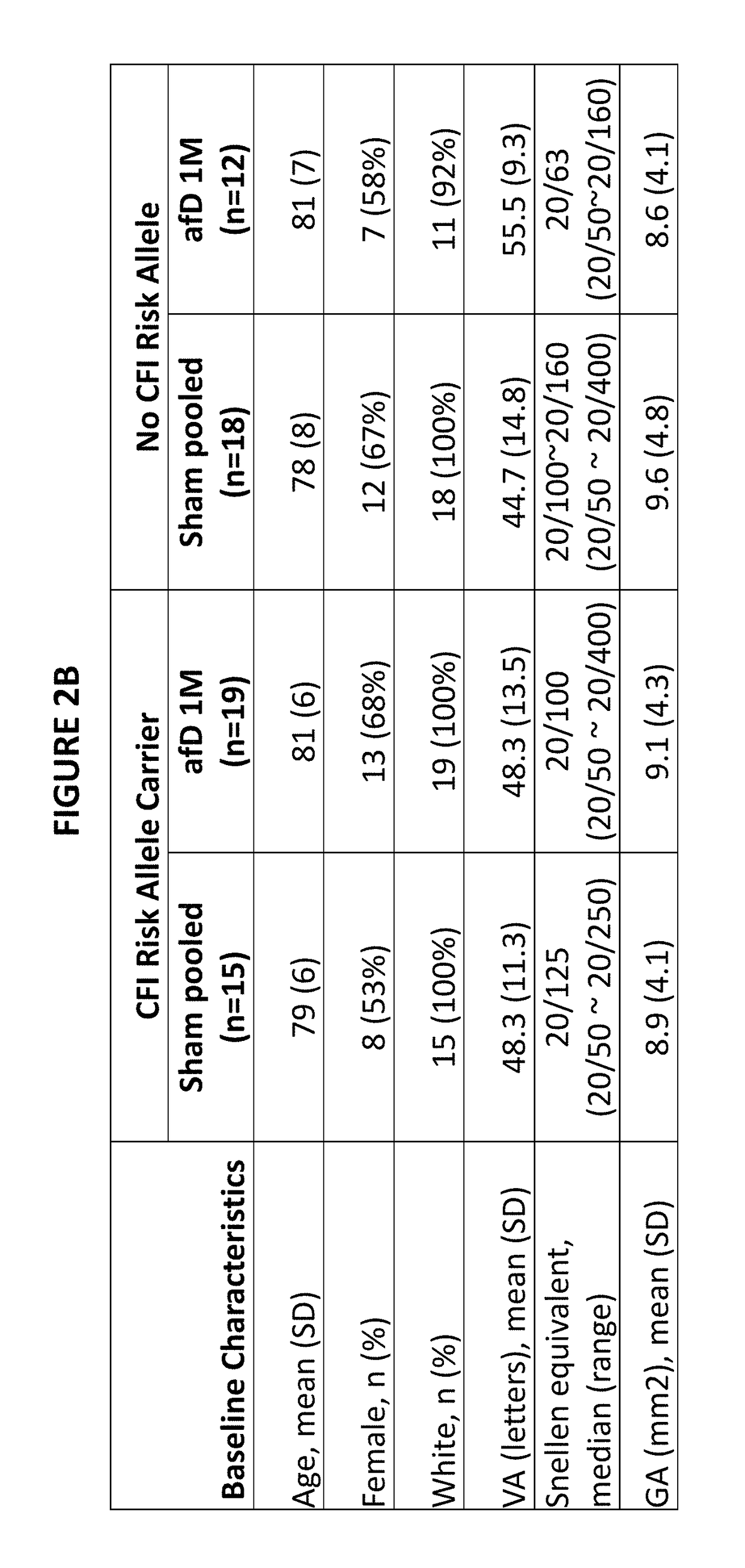
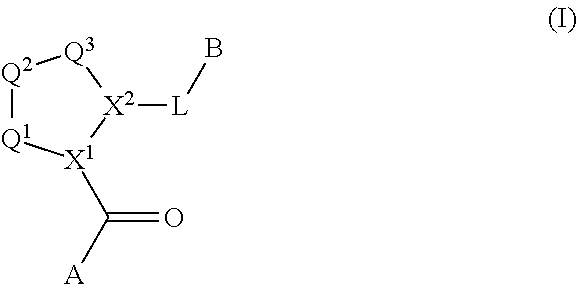Patents
Literature
30 results about "Factor D" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
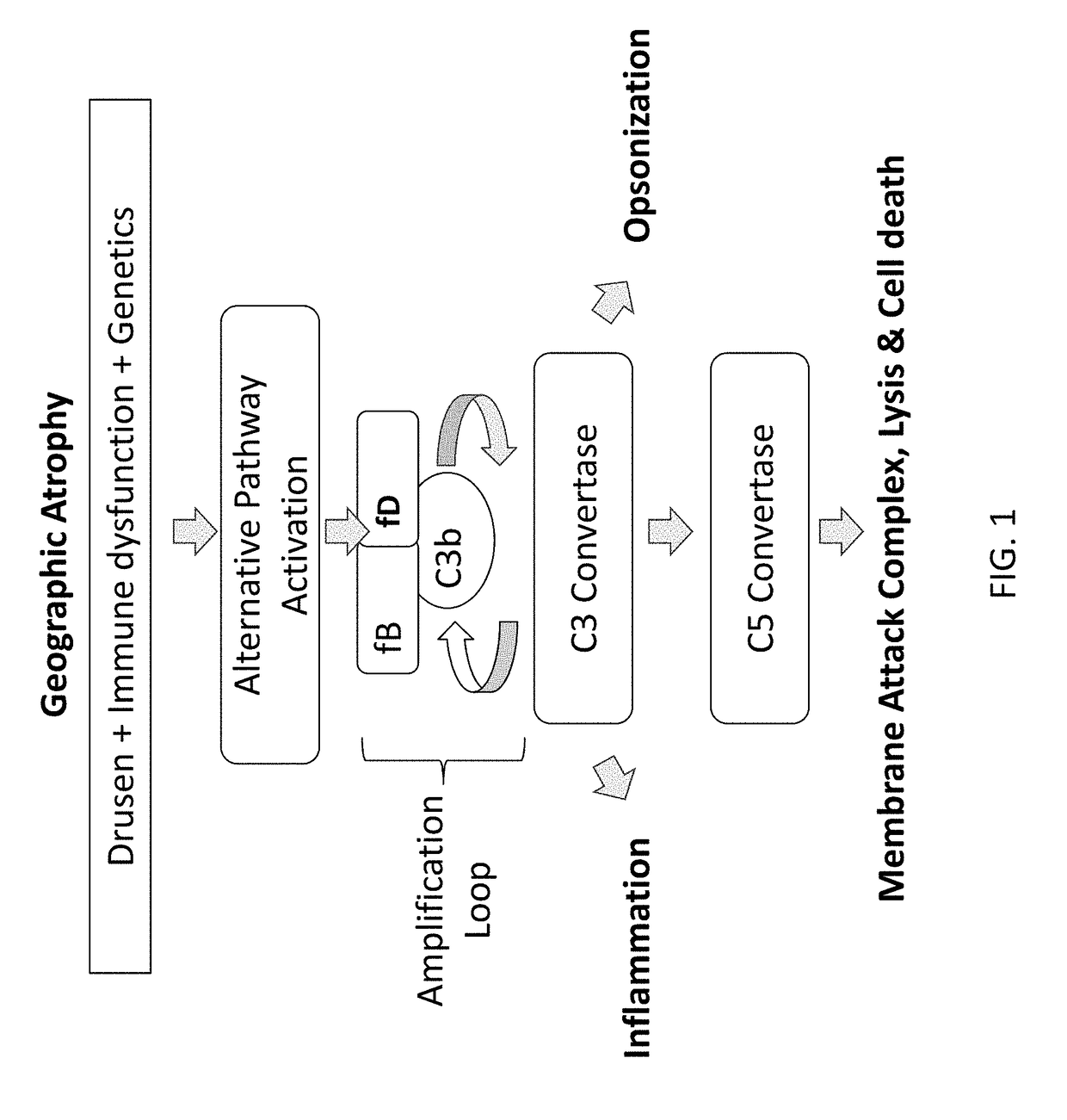
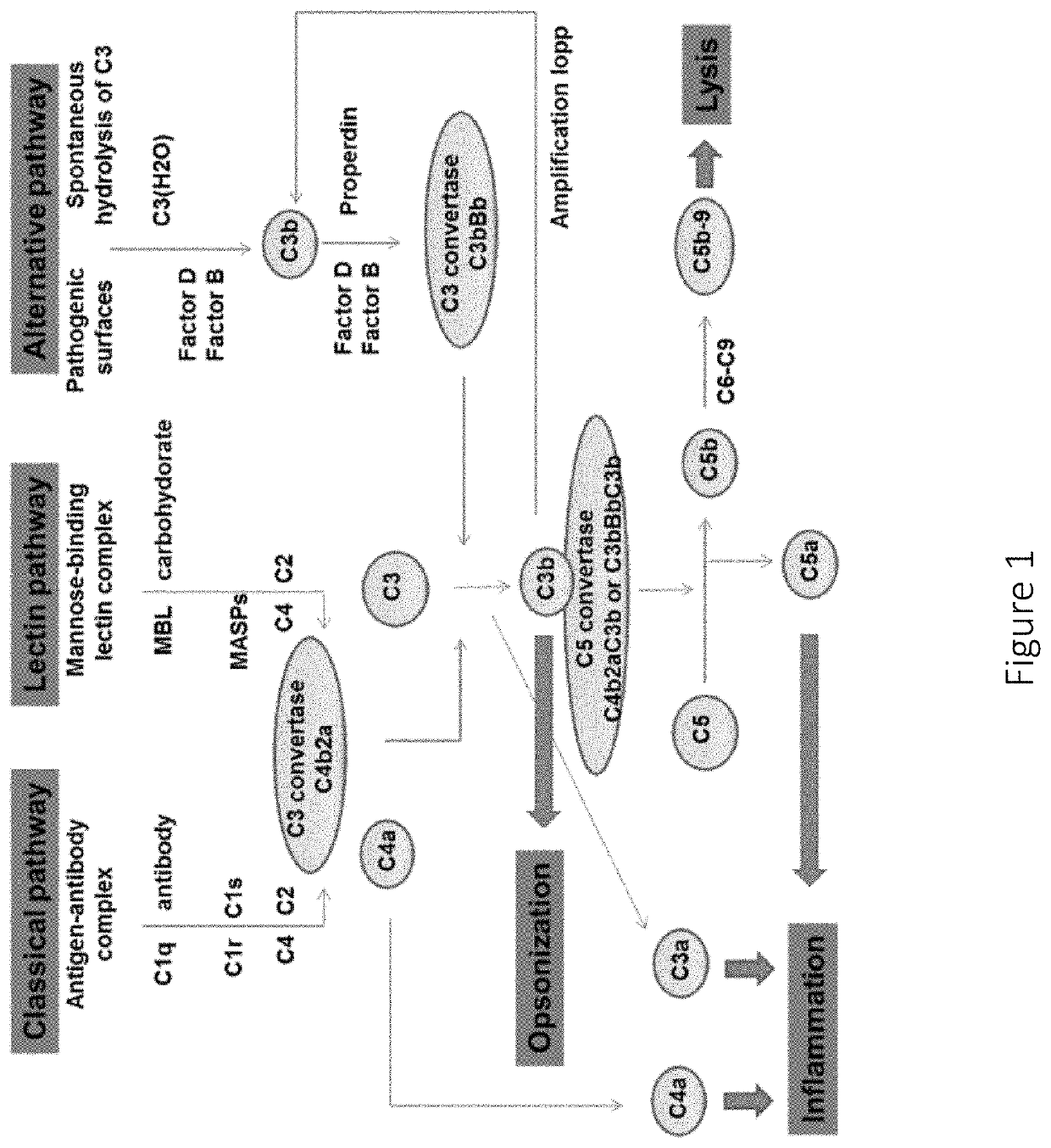
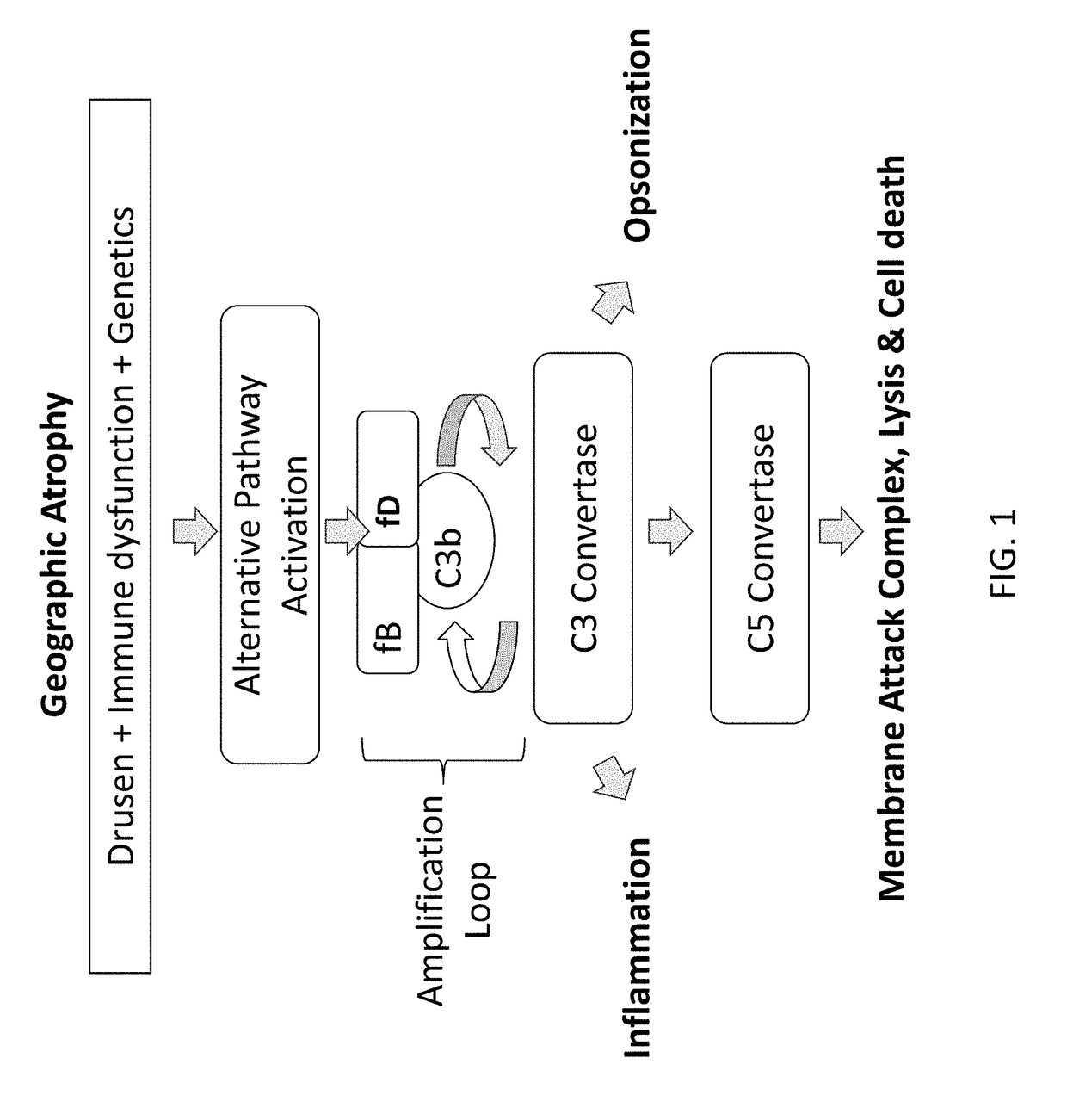
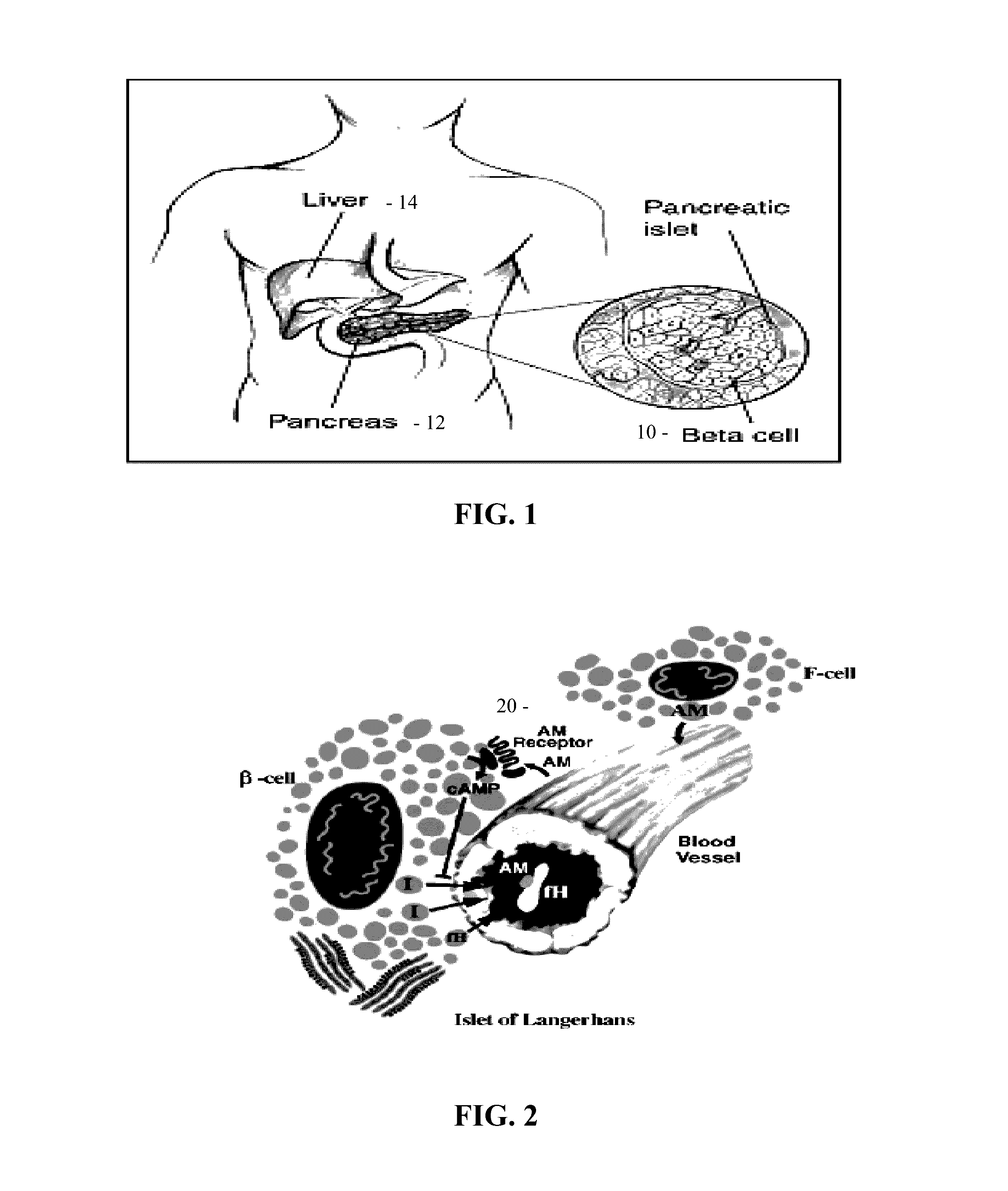
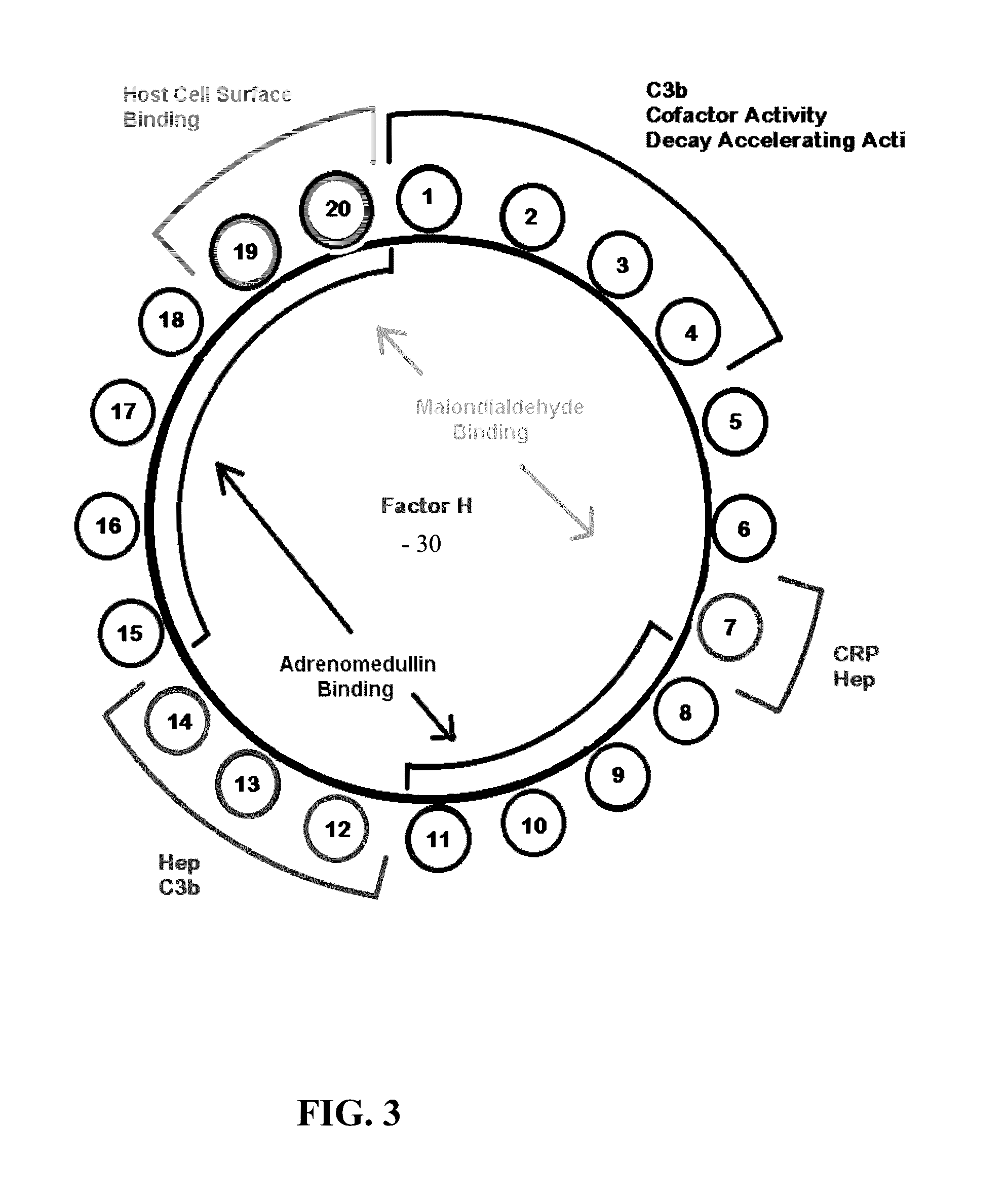
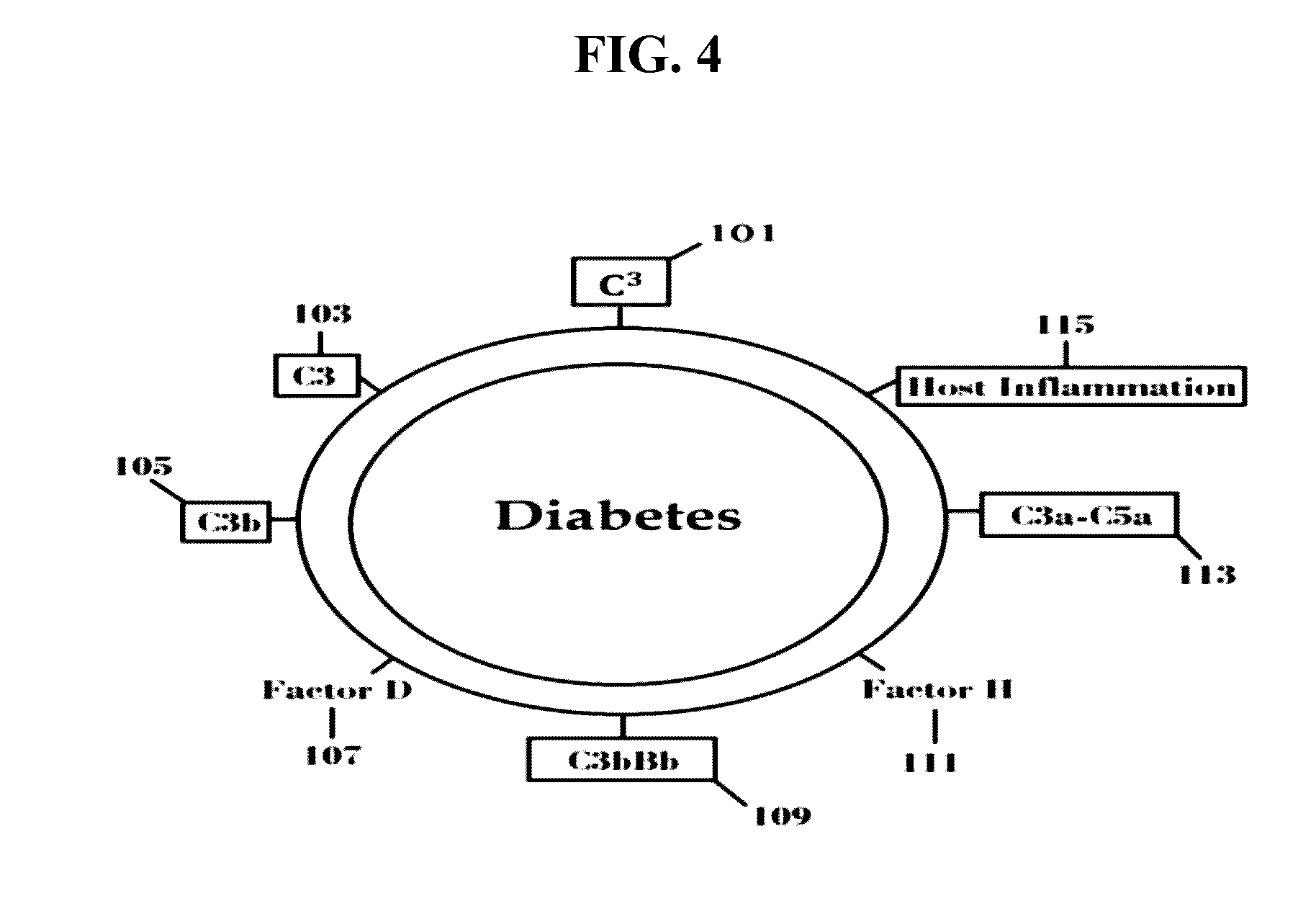
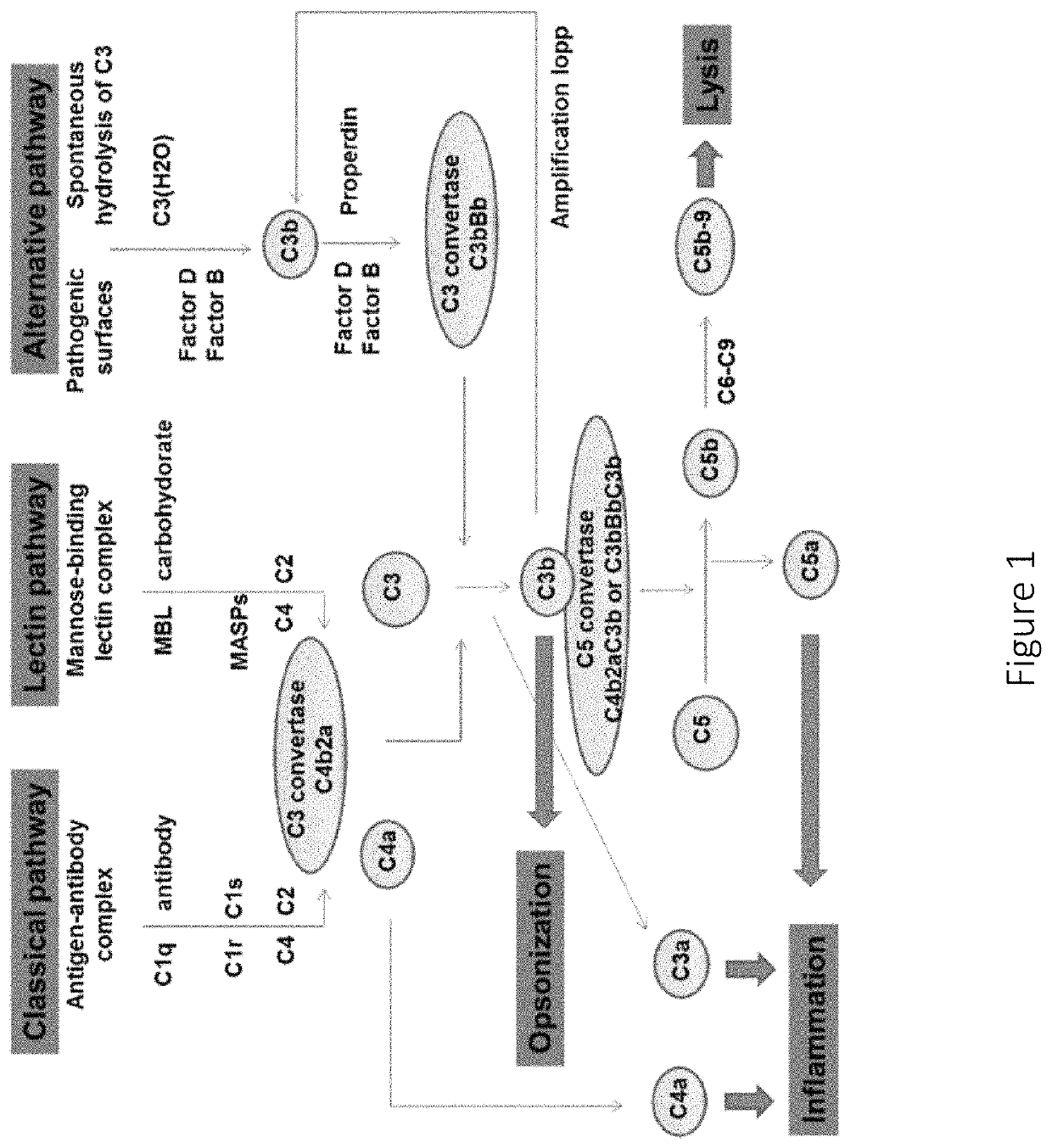
Factor D (EC 3.4.21.46, C3 proactivator convertase, properdin factor D esterase, factor D (complement), complement factor D, CFD, adipsin) a protein which in humans is encoded by the CFD gene. Factor D is involved in the alternative complement pathway of the complement system where it cleaves factor B.
Compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation
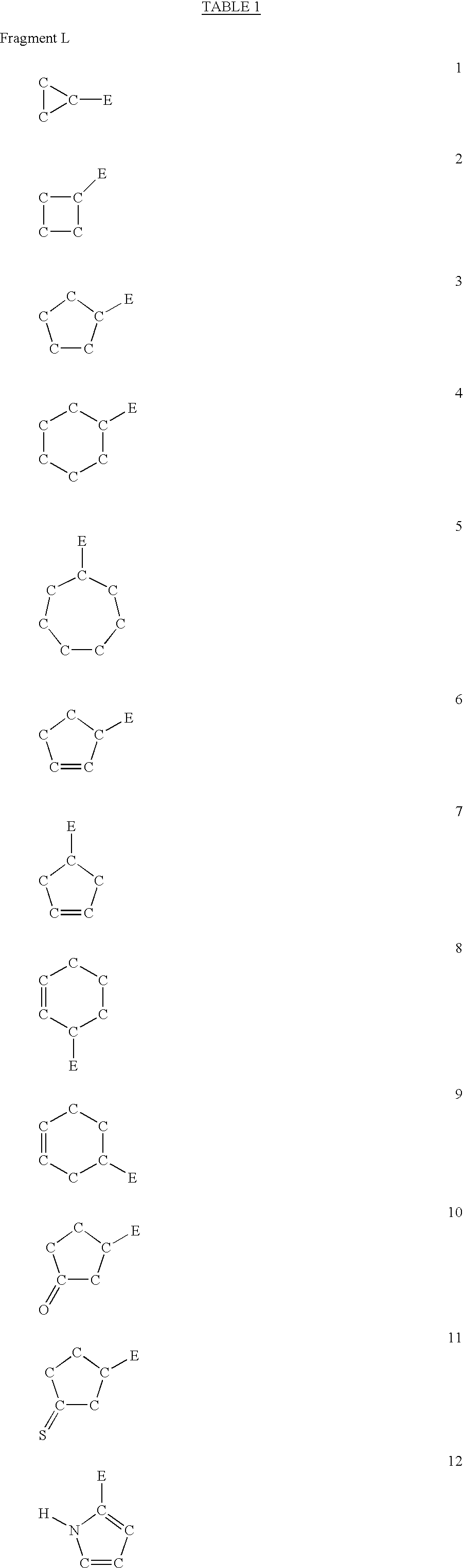
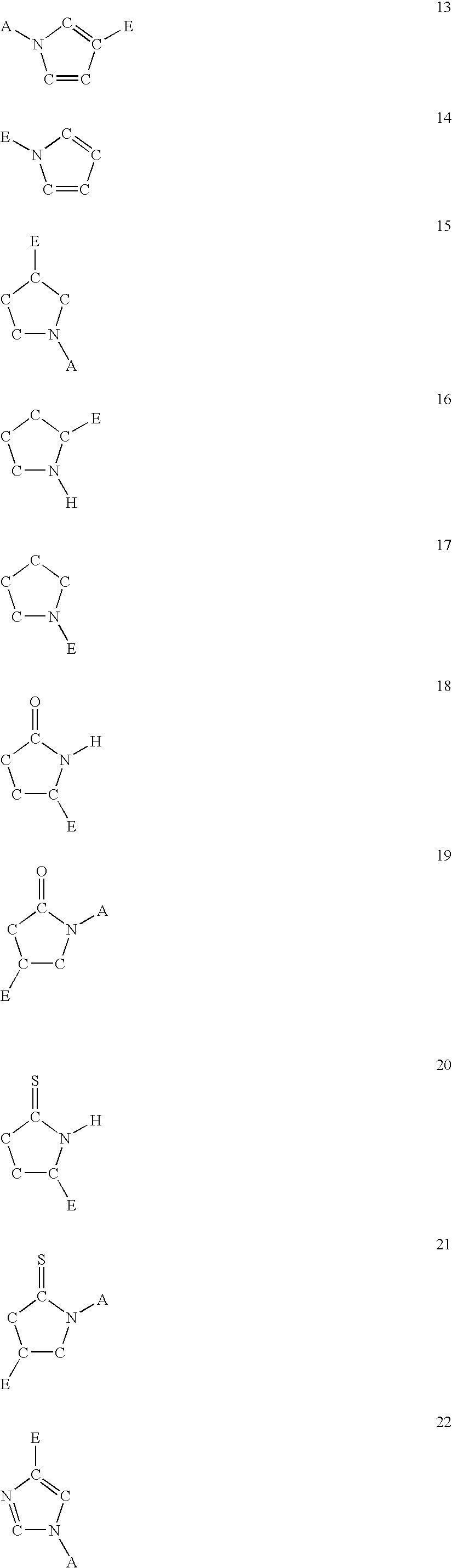
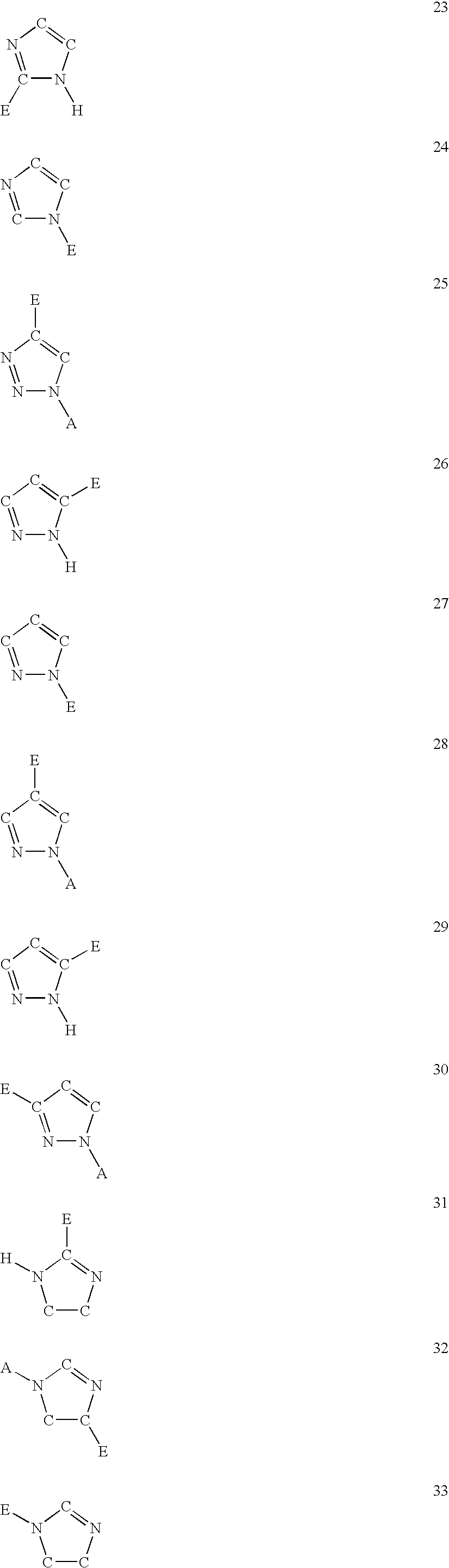
The present invention is concerned with new compounds, and particularly those having a fused bicyclic ring substituted with an amidine moiety. These compounds are each potent inhibitors of Factor D of the alternate pathway of complement, C1s of the classical pathway of complement, Factors Xa, XIIa, VIIa and thrombin of the coagulation pathway, plasmin in the fibrinolytic pathway, and kallikrein and high molecular weight kininogen in the inflammatory pathways. These proteases, which have serine in their active site, are called serine proteases and they are pivotal to most of the processes of inflammation and coagulation. In fact, these various systems are interactive with one another and it is difficult to activate one pathway without it influencing the others.
Owner:BIOCRYST PHARM INC
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Methods and compositions for the treatment of meconium aspiration syndrome
InactiveUS20070065433A1Reduce morbidityReduce mortalityImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntiendomysial antibodiesComplement S-Protein
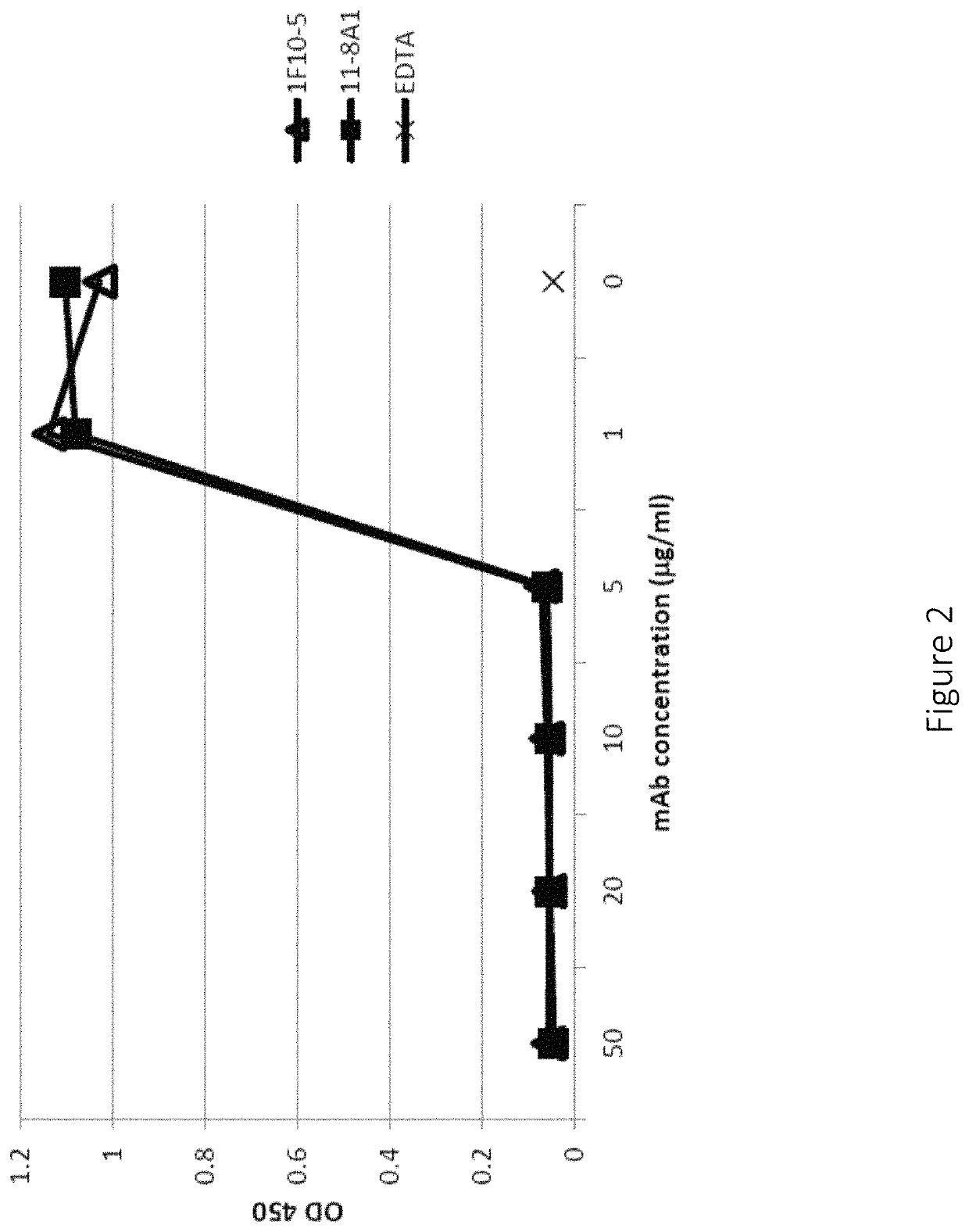
A method for preventing or treating meconium aspiration syndrome (“MAS”) by administering a meconium aspiration syndrome preventing or treating amount of one or more complement inhibitors to a patient likely to develop or suffering from MAS. The complement inhibitors are preferably antibodies that bind to and inhibit complement proteins involved in the formation of the membrane attach complex, preferably anti-Factor D or anti-C5 antibodies. The complement inhibitors can be used alone or in combination with other MAS therapies to decrease the morbidity and mortality caused by MAS.
Owner:MOLLNES TOM EIRIK +1
Prevention and treatment of complement-associated eye conditions
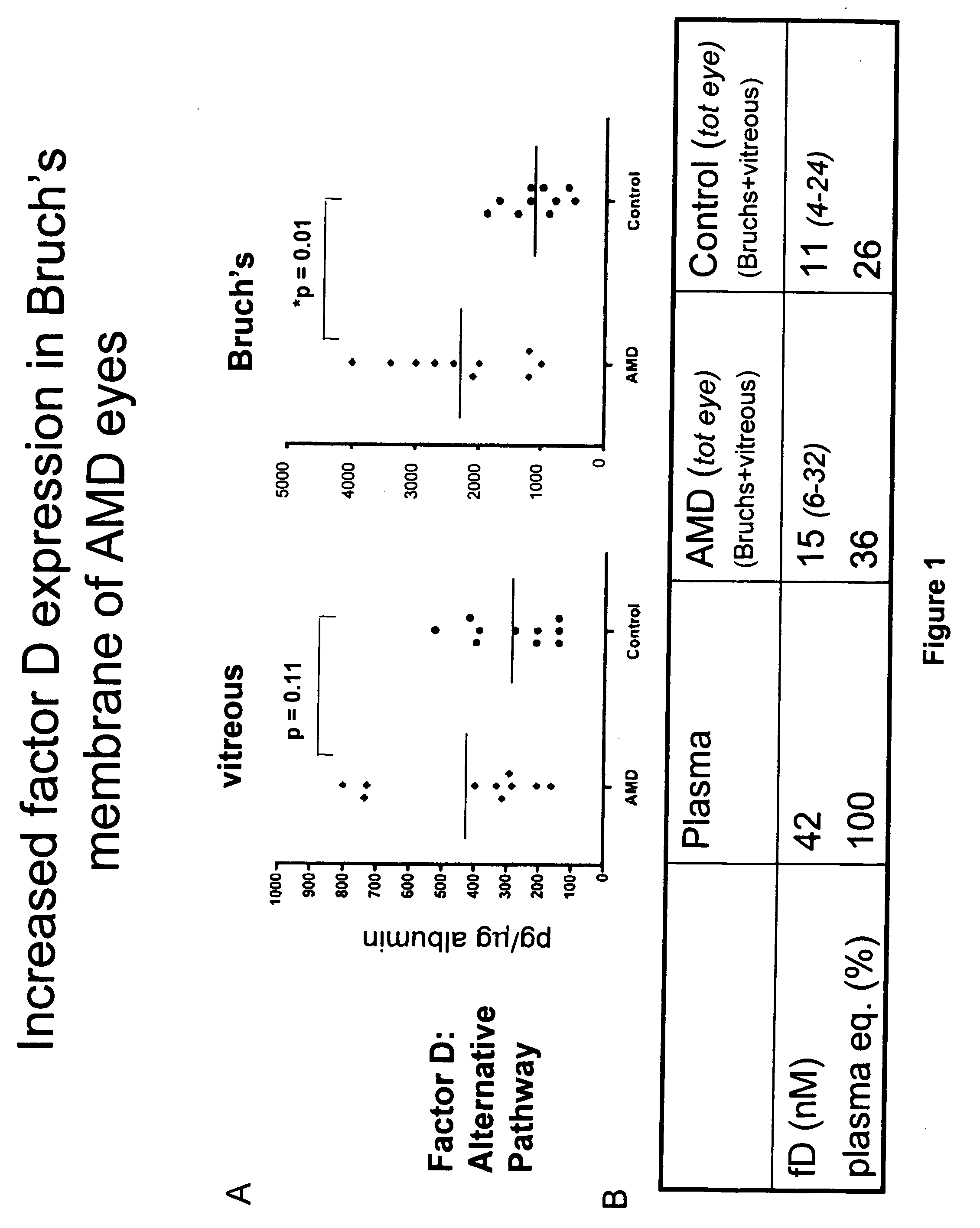
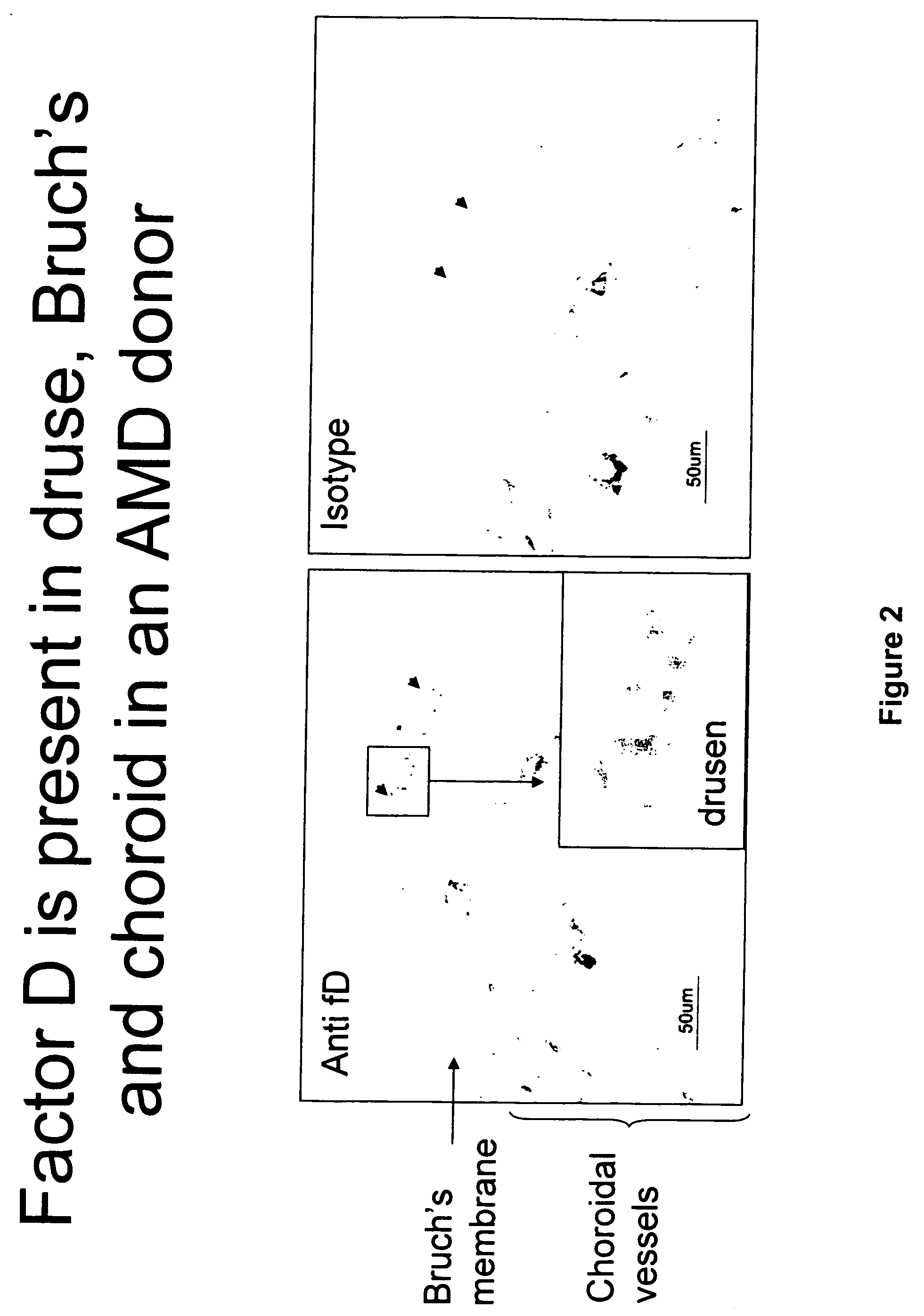
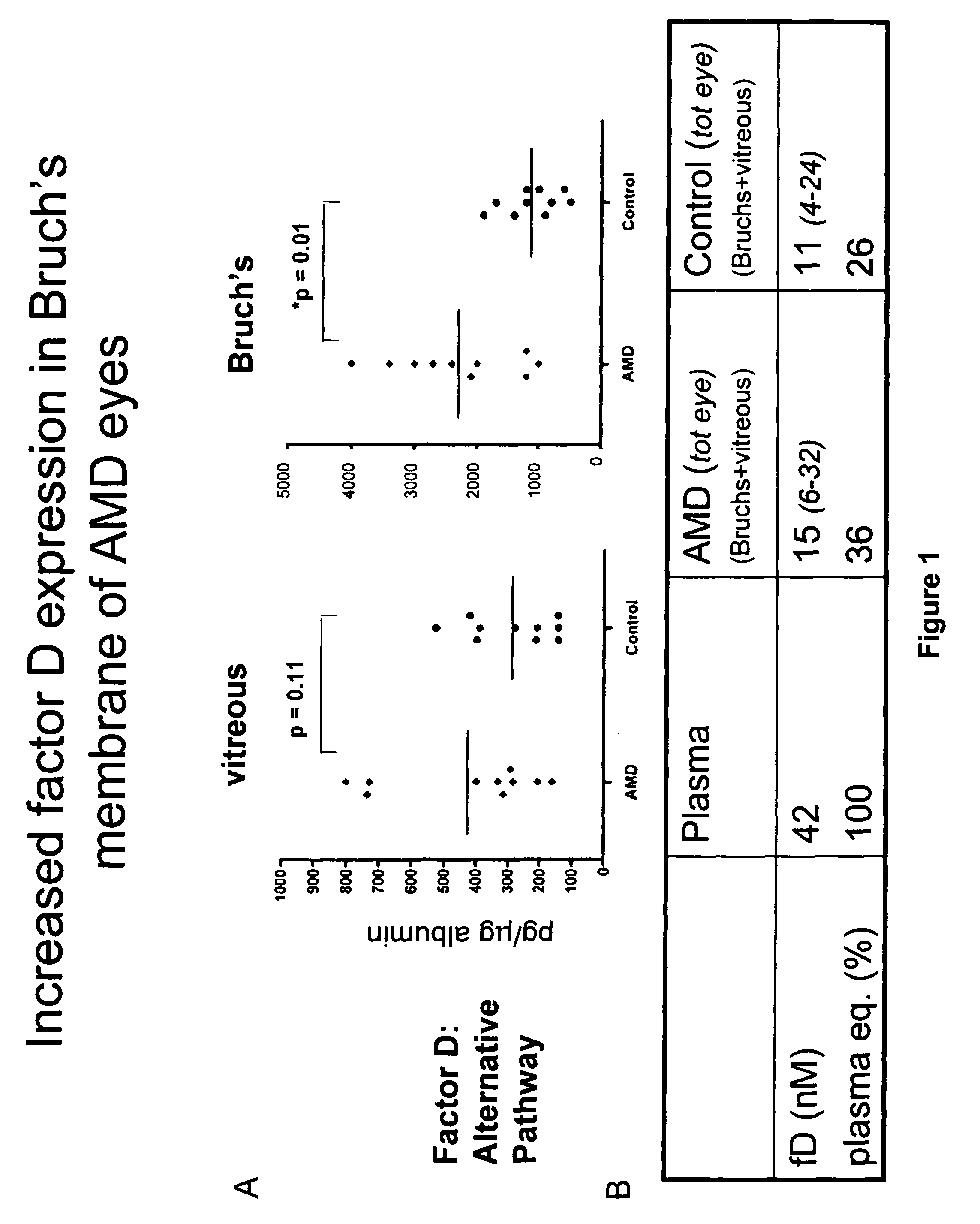
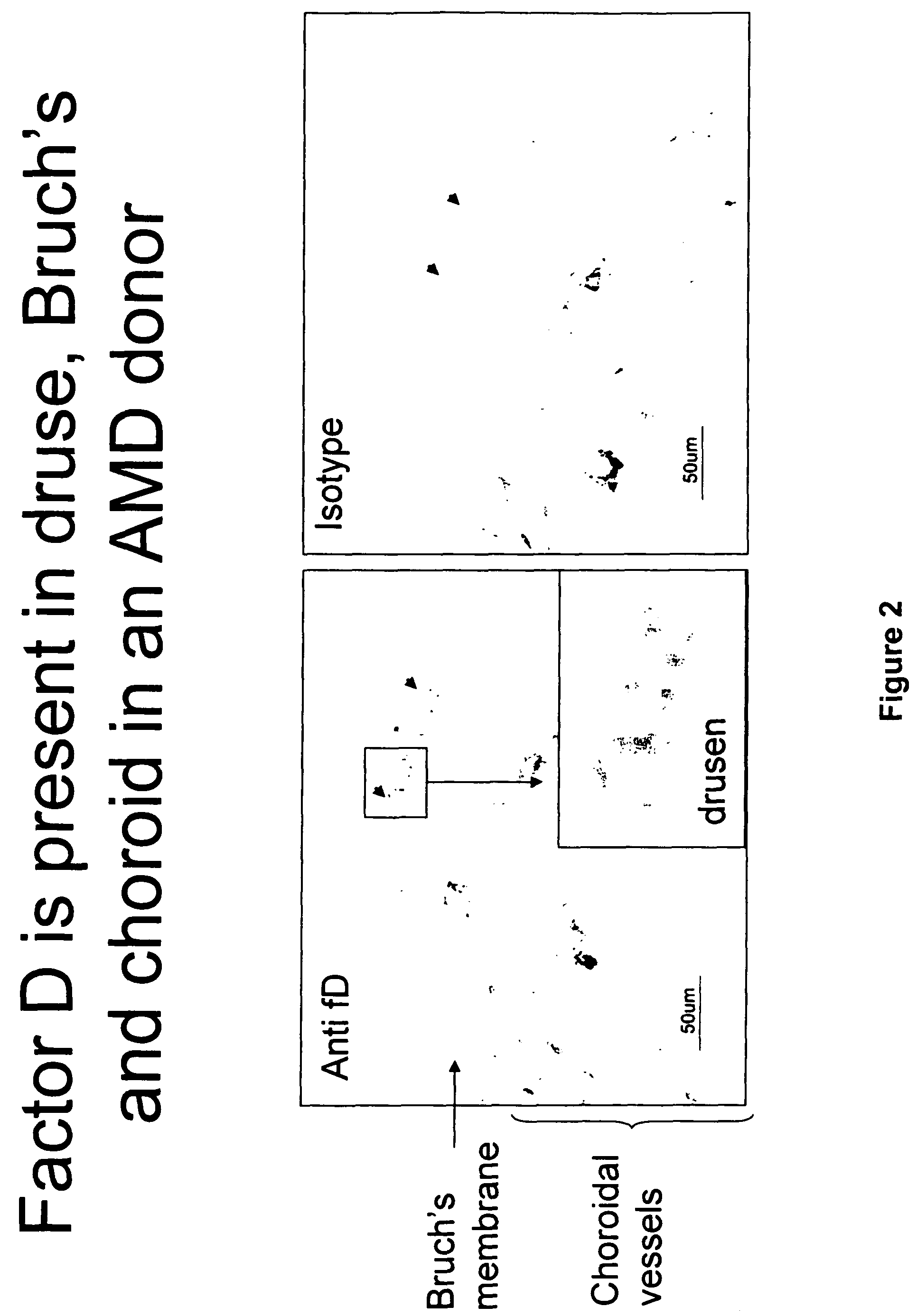
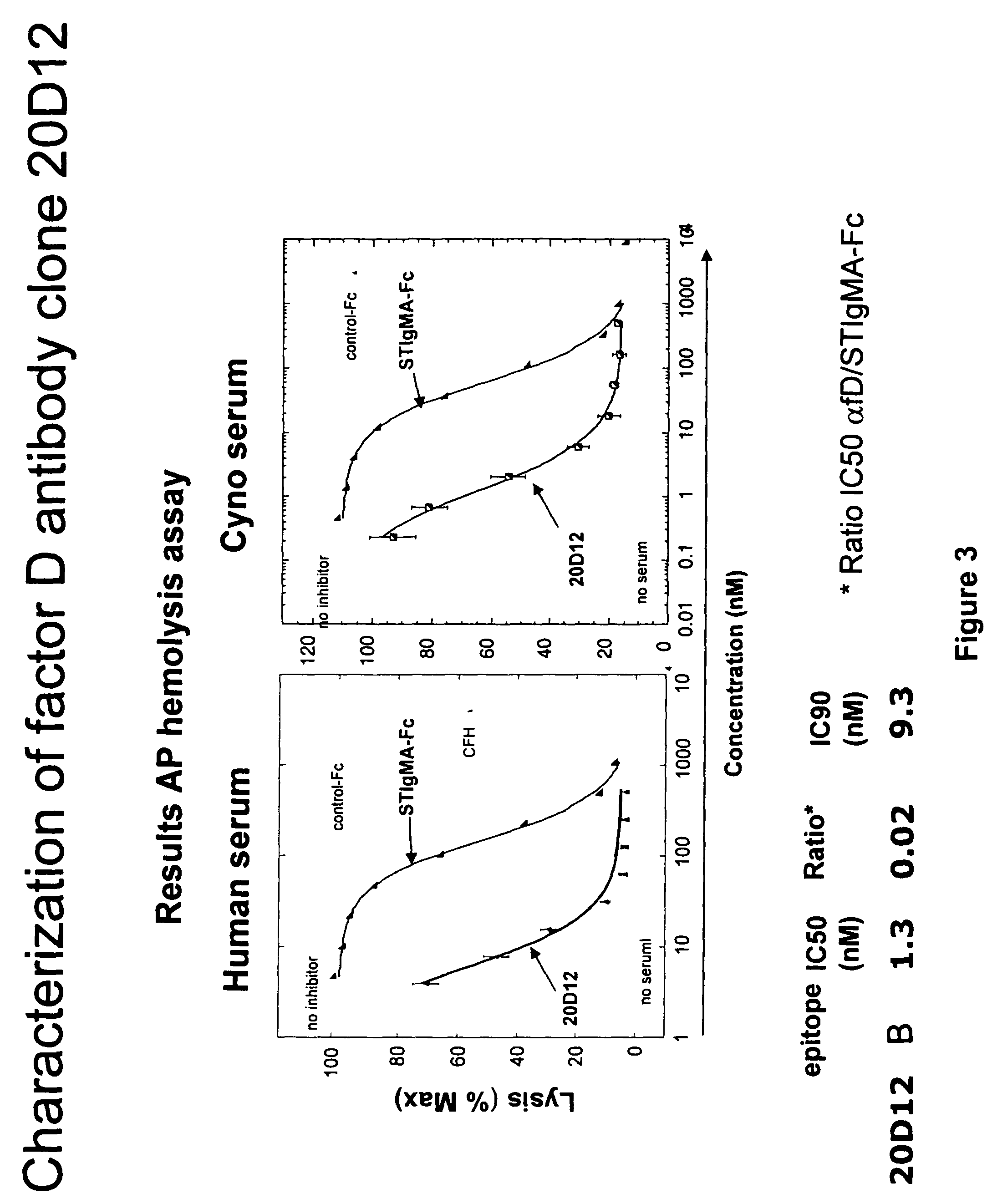
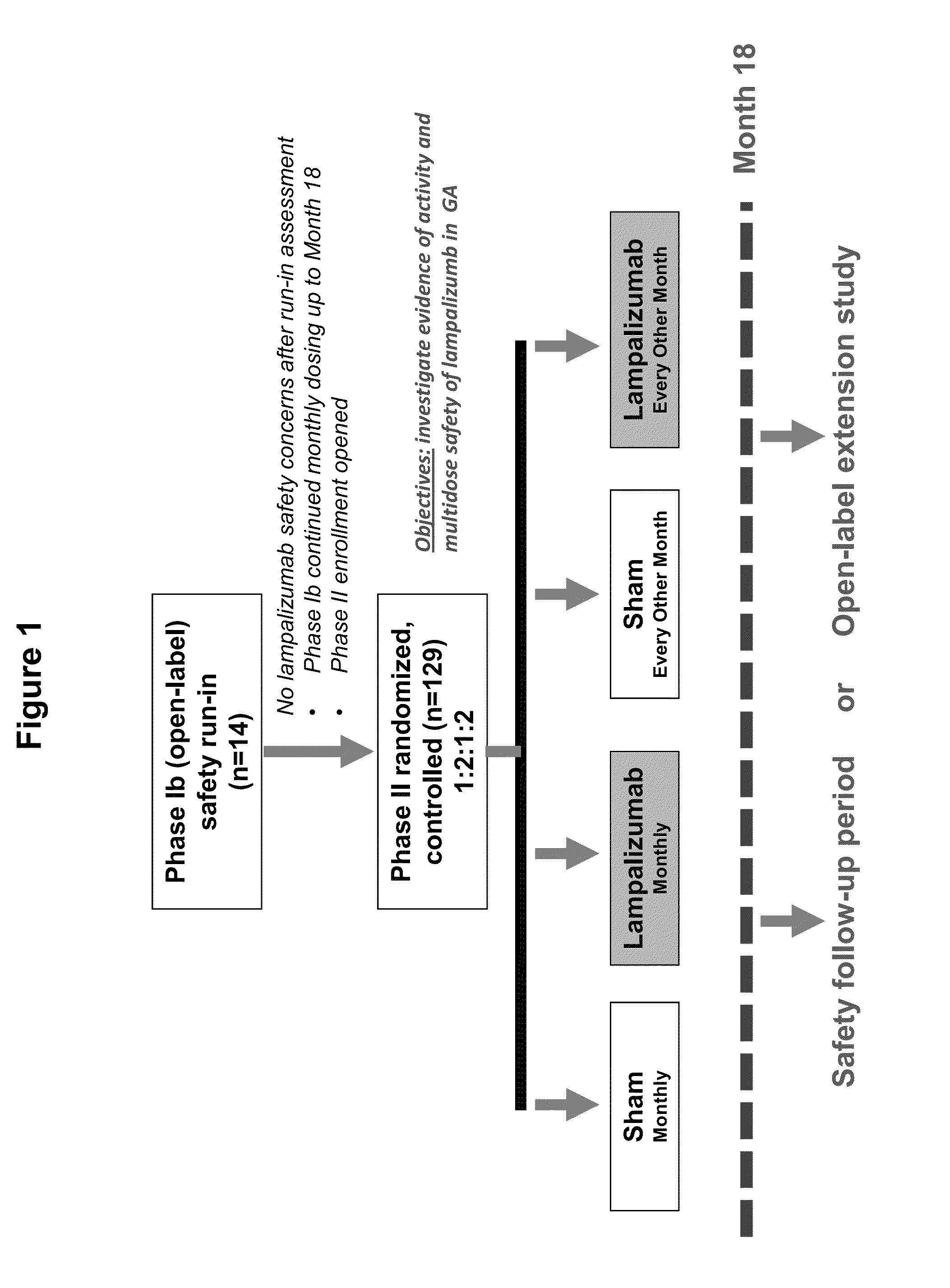
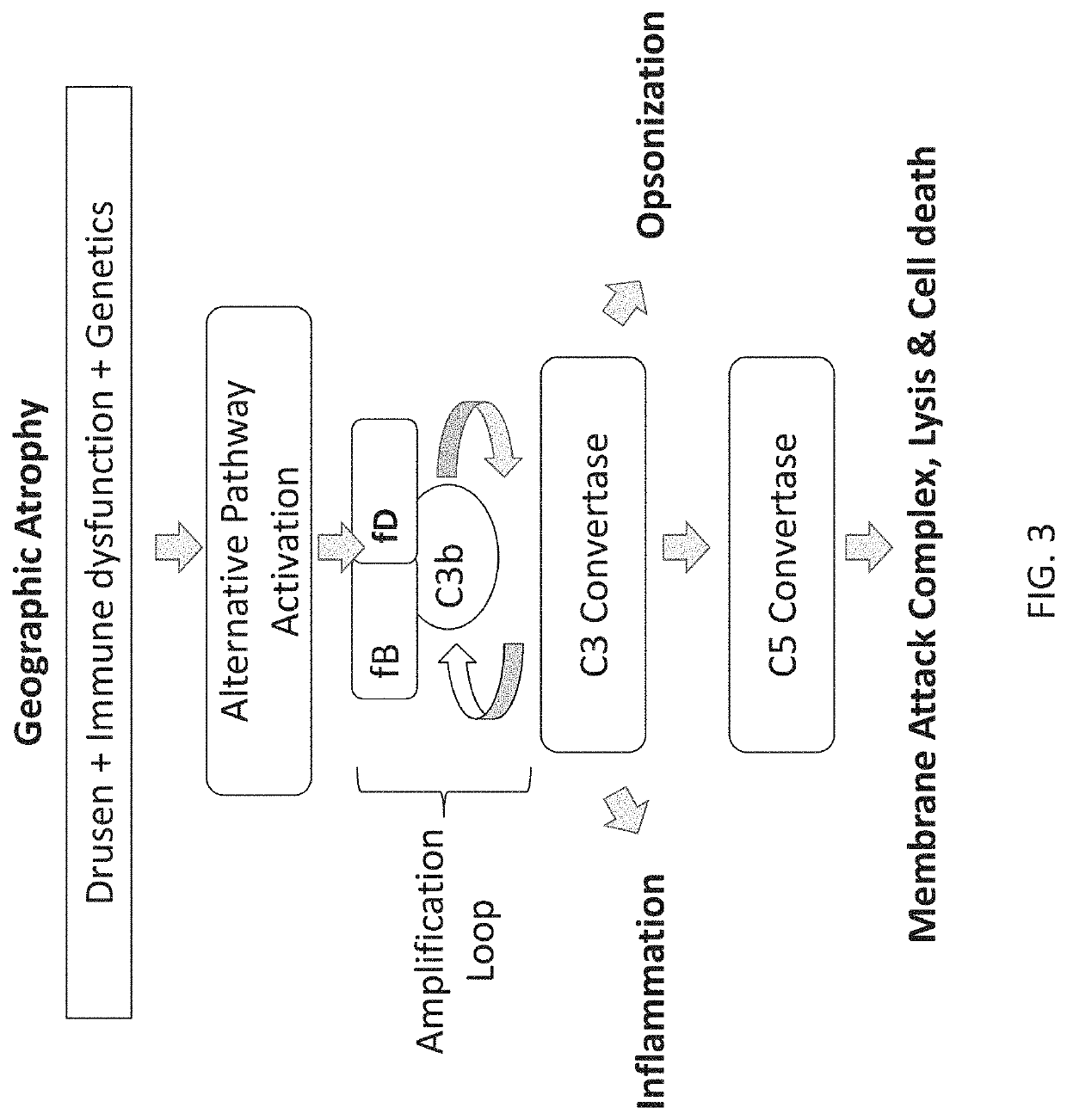
The invention concerns the prevention and treatment of complement-associated eye conditions, such as choroidal neovascularization (CNV) and age-related macular degeneration (AMD), by administration of Factor D antagonists.
Owner:GENENTECH INC
Prevention and treatment of complement-associated eye conditions
The invention concerns the prevention and treatment of complement-associated eye conditions, such as choroidal neovascularization (CNV) and age-related macular degeneration (AMD), by administration of Factor D antagonists.
Owner:GENENTECH INC
Amide compounds for treatment of complement mediated disorders
ActiveUS20160362398A1Dampen and inhibit detrimental complement activitySenses disorderNervous disorderDiseaseFactor D
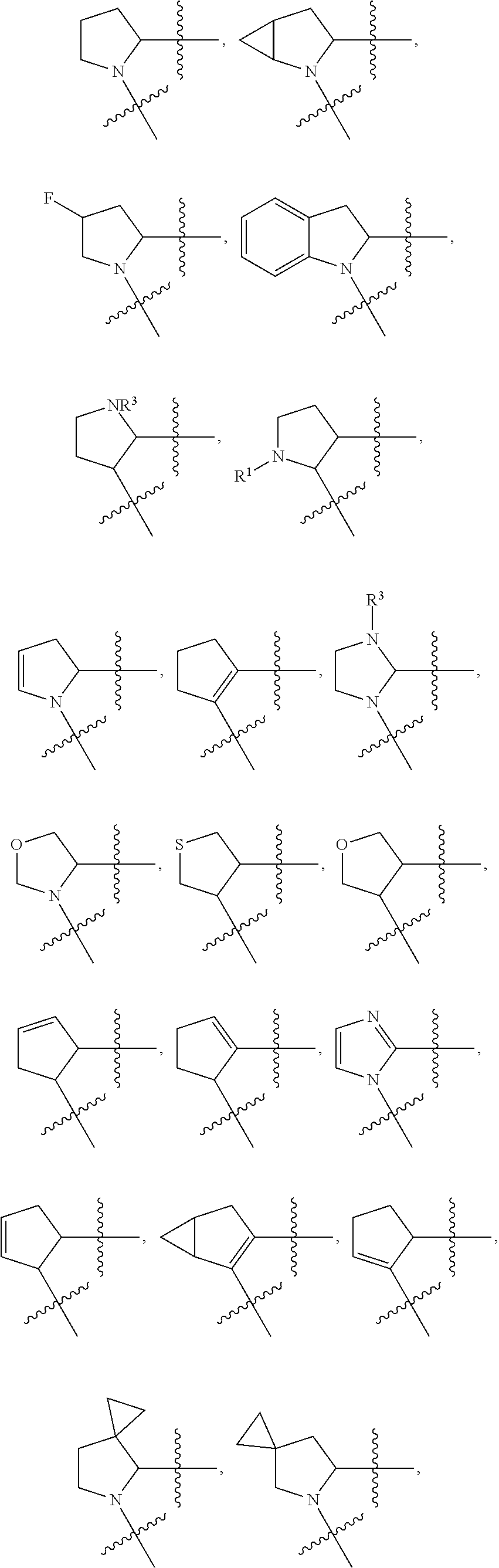
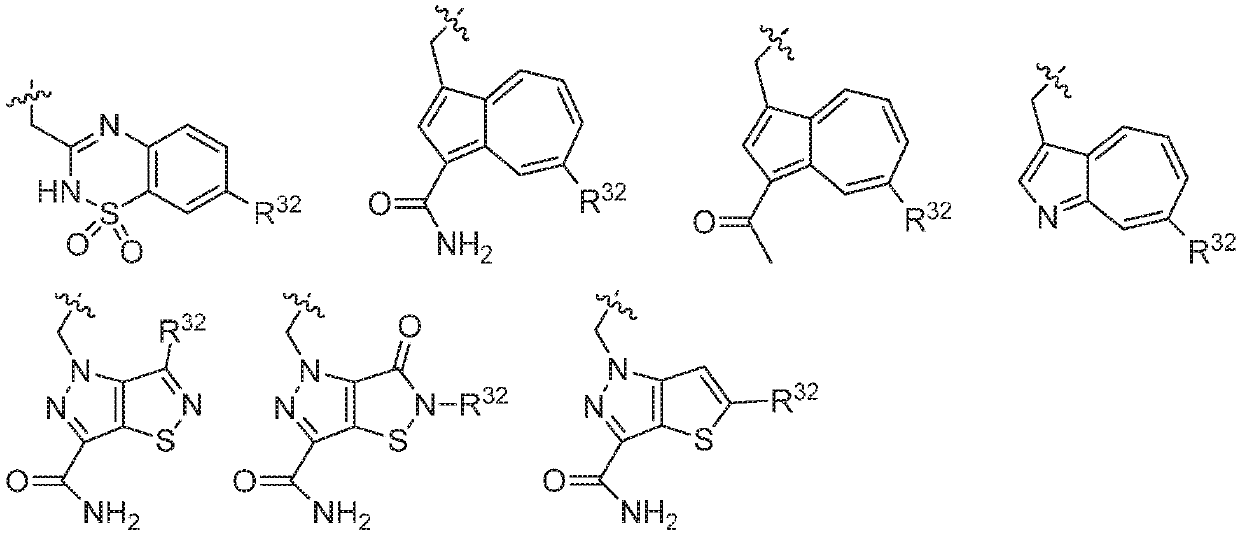
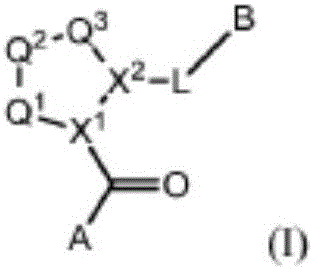
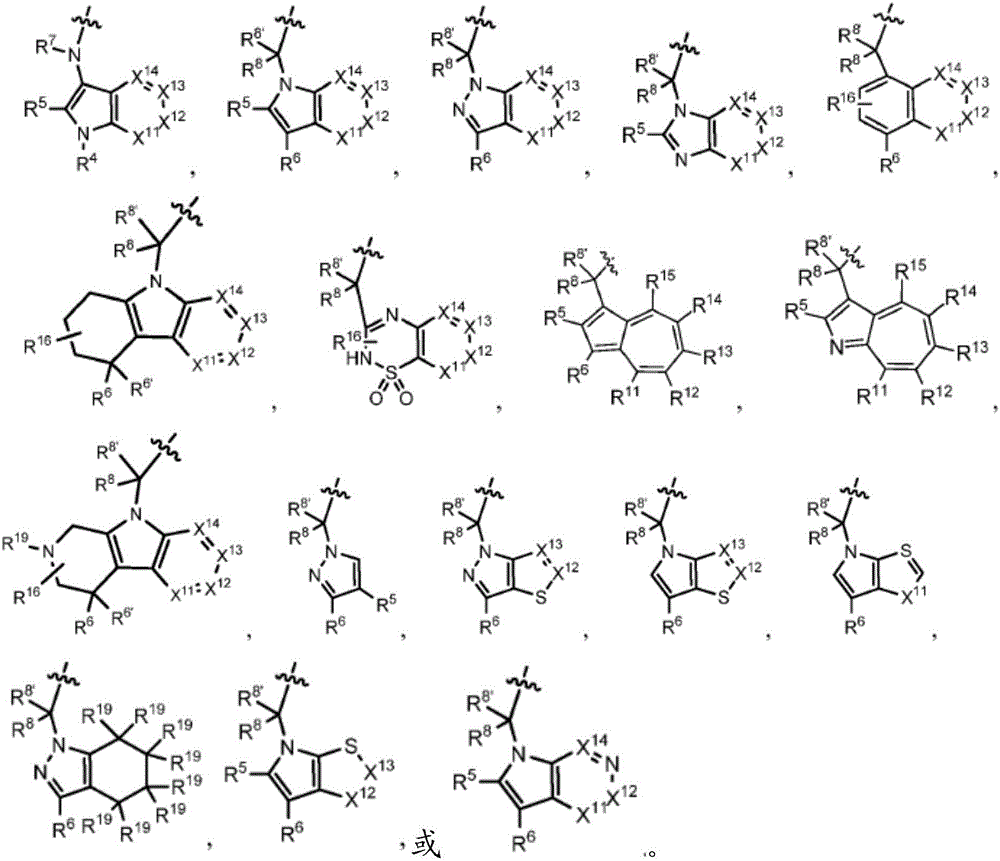
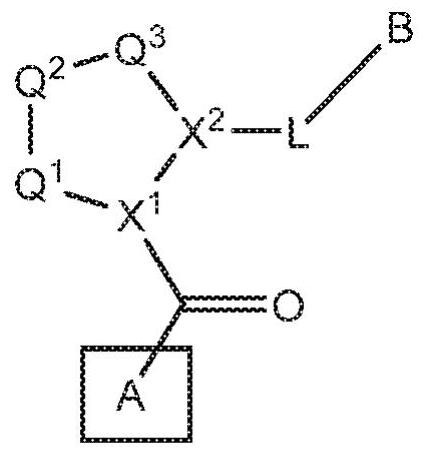
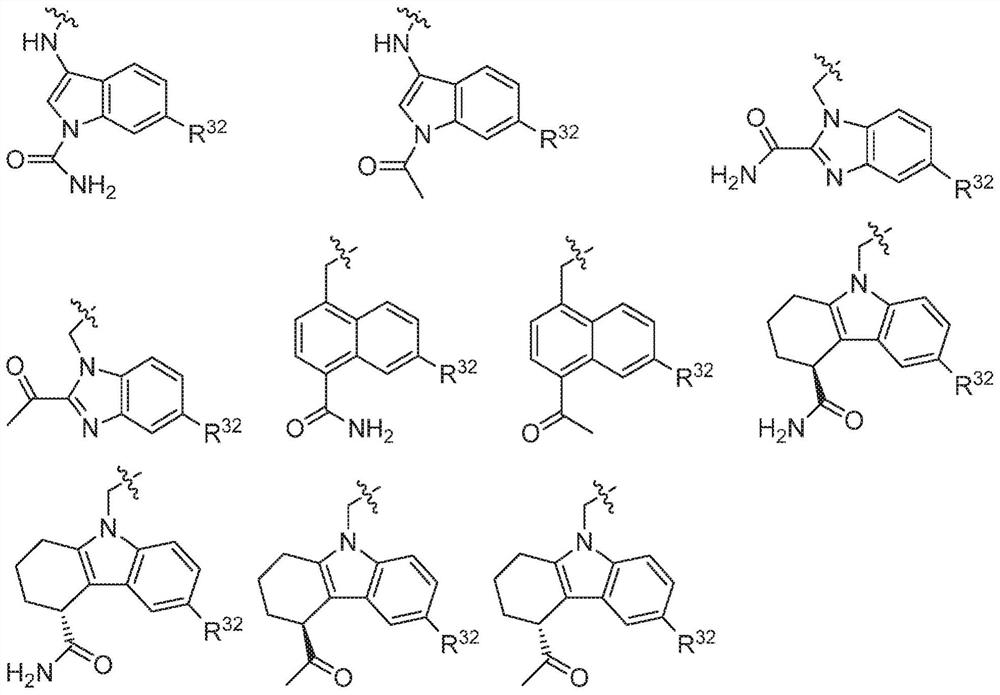
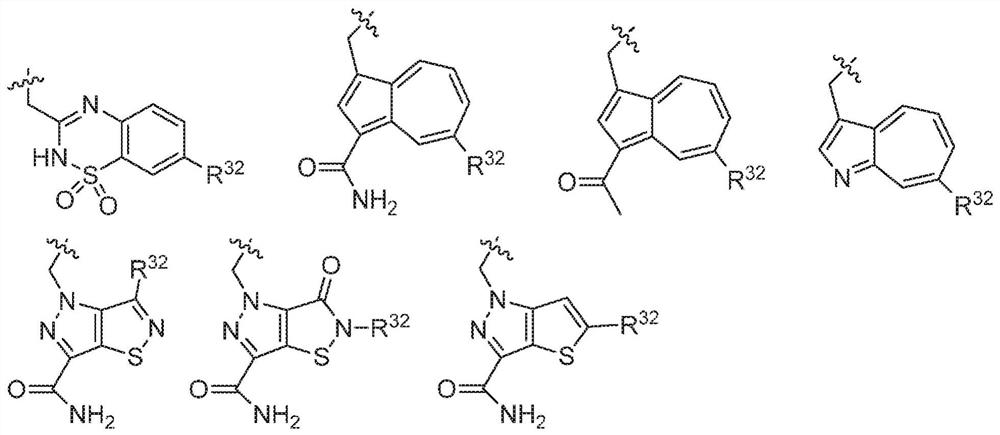
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof, wherein R12 or R13 on the A group is an amide substituent (R32) are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade in the alternative complement pathway. The inhibitors of factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Method of treating recurrent miscarriages
InactiveUS20070123466A1Prevent and reduce riskCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsRecurrent miscarriageScreening method
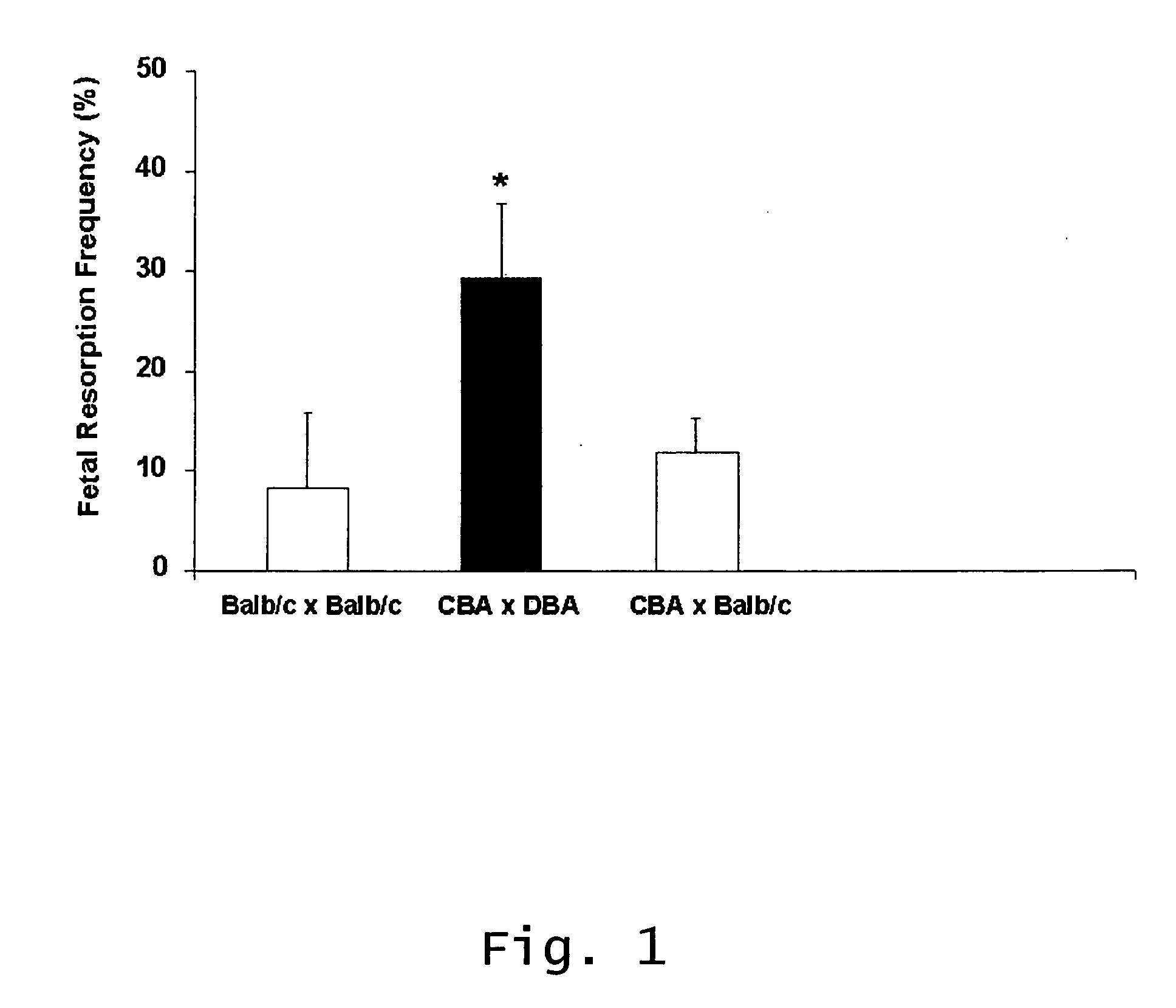
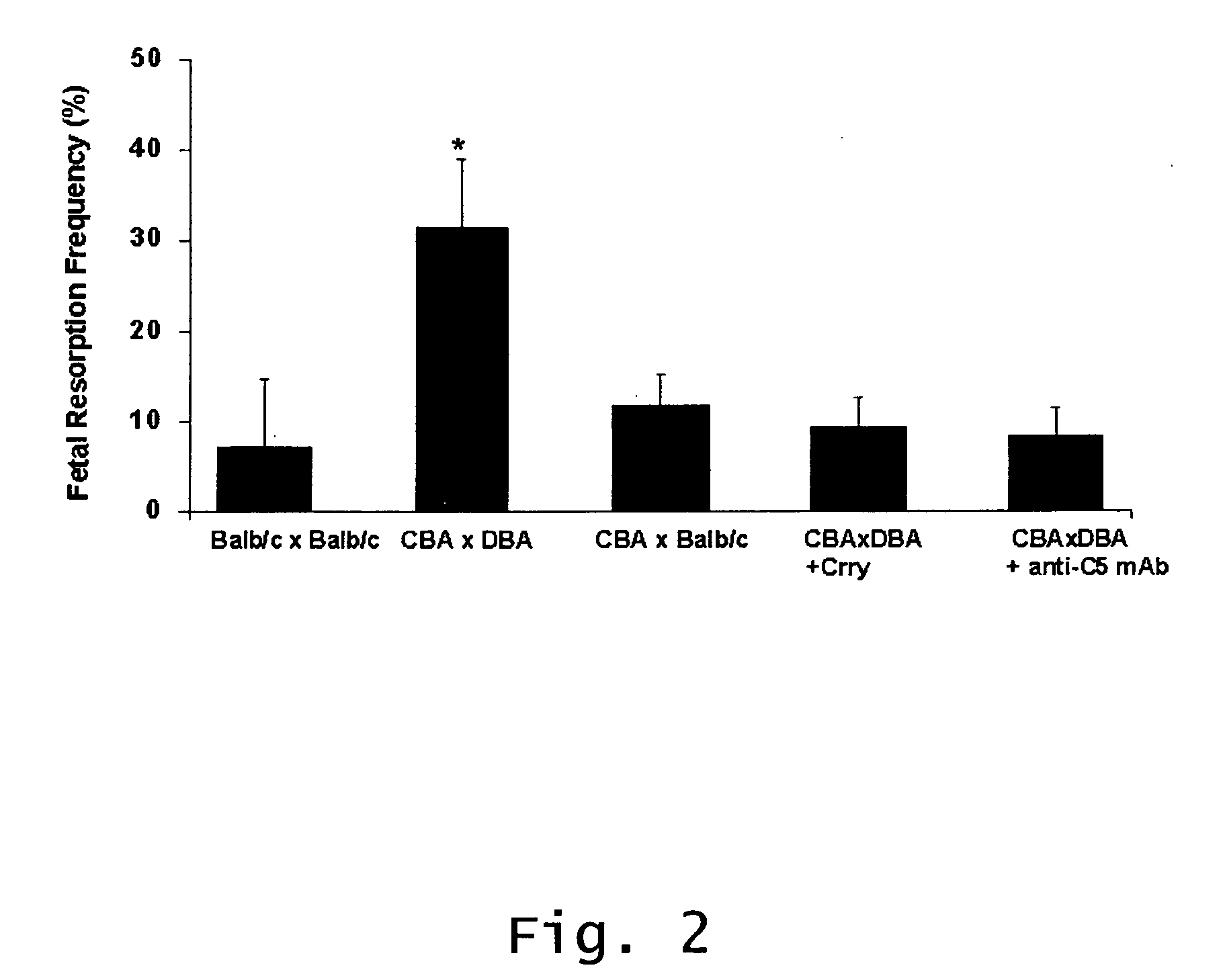
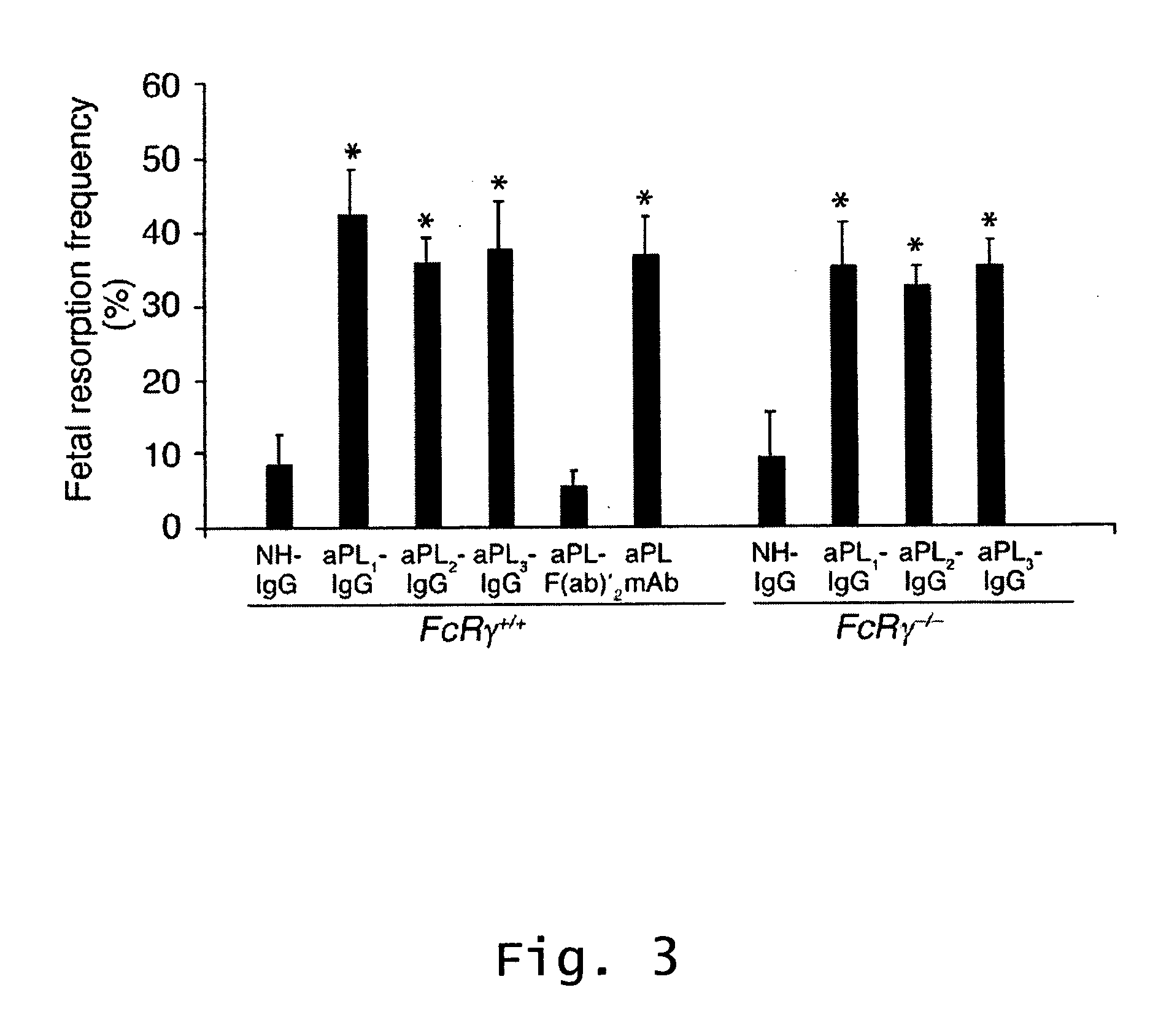
Described are methods for treating, preventing, or reducing the risk of, miscarriages, especially recurrent miscarriages. The methods comprise administering to a female subject a therapeutic agent that modulates the activity or binding of components of the complement system, together with a pharmaceutically acceptable carrier. For example, the therapeutic agent can be a C3-convertase inhibitor, an antibody against C5, an antagonist of the C5a receptor, or an antibody against factor B or factor D. Screening methods for agents that can prevent or reduce the risk of miscarriages, especially recurrent miscarriages, are also described.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY +1
Compositions and method for treating compliment-associated conditions
InactiveUS20150044205A1Increased riskReduce riskSenses disorderSugar derivativesAntigenAntigen Binding Fragment
The invention provides methods and compositions for treating various degenerative diseases (e.g., AMD) with a factor D inhibitor (e.g., anti-factor D antibody or antigen-binding fragment thereof). Also provided are methods of selecting or identifying patients for treatment with a factor D inhibitor. Methods include the use of prognostic and / or predictive biomarkers.
Owner:GENENTECH INC
Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
Compounds, methods of use, and processes for making inhibitors of complement Factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof wherein R12 or R13 on the A group is an aryl, heteroaryl or heterocycle (R32) are provided. The inhibitors of Factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Compositions and methods for inhibiting factor d
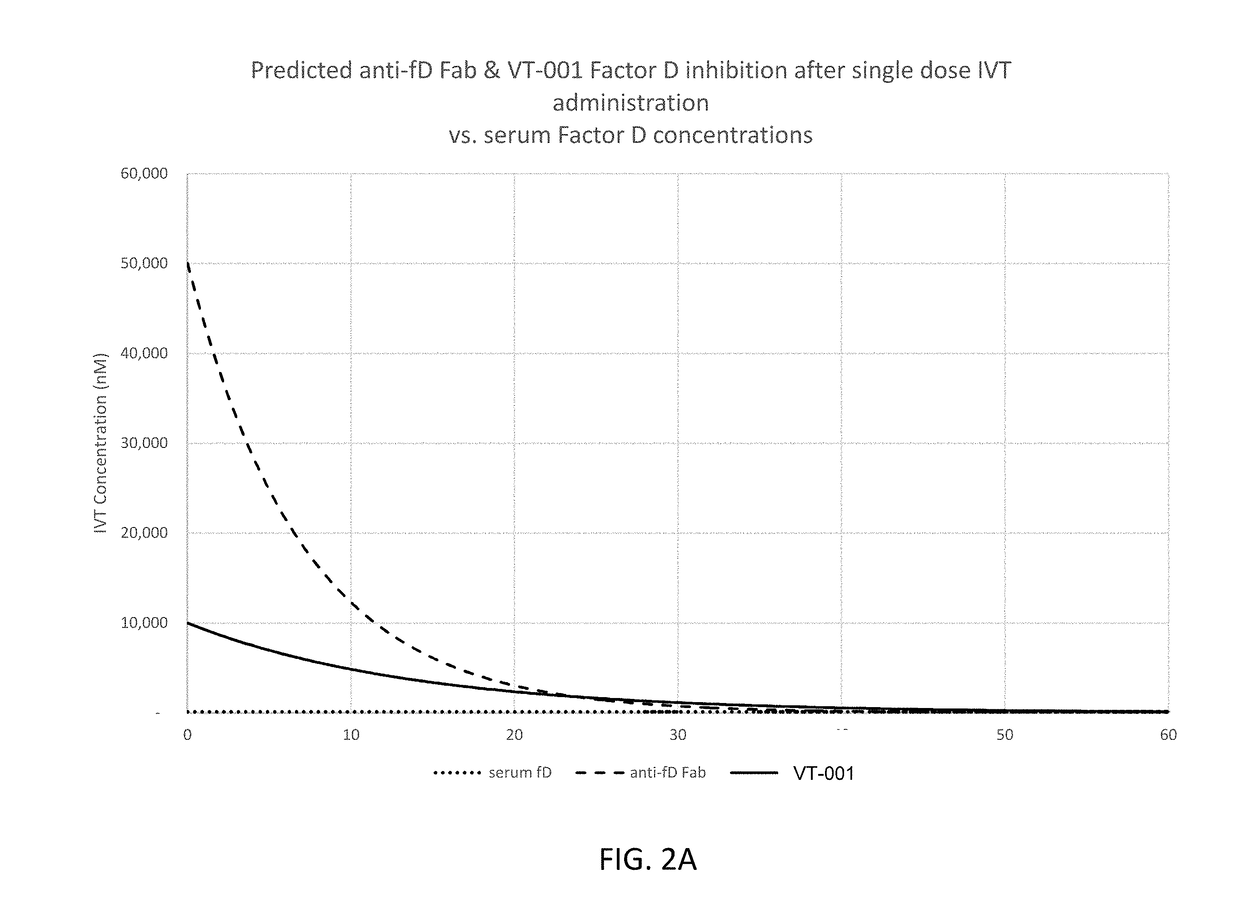
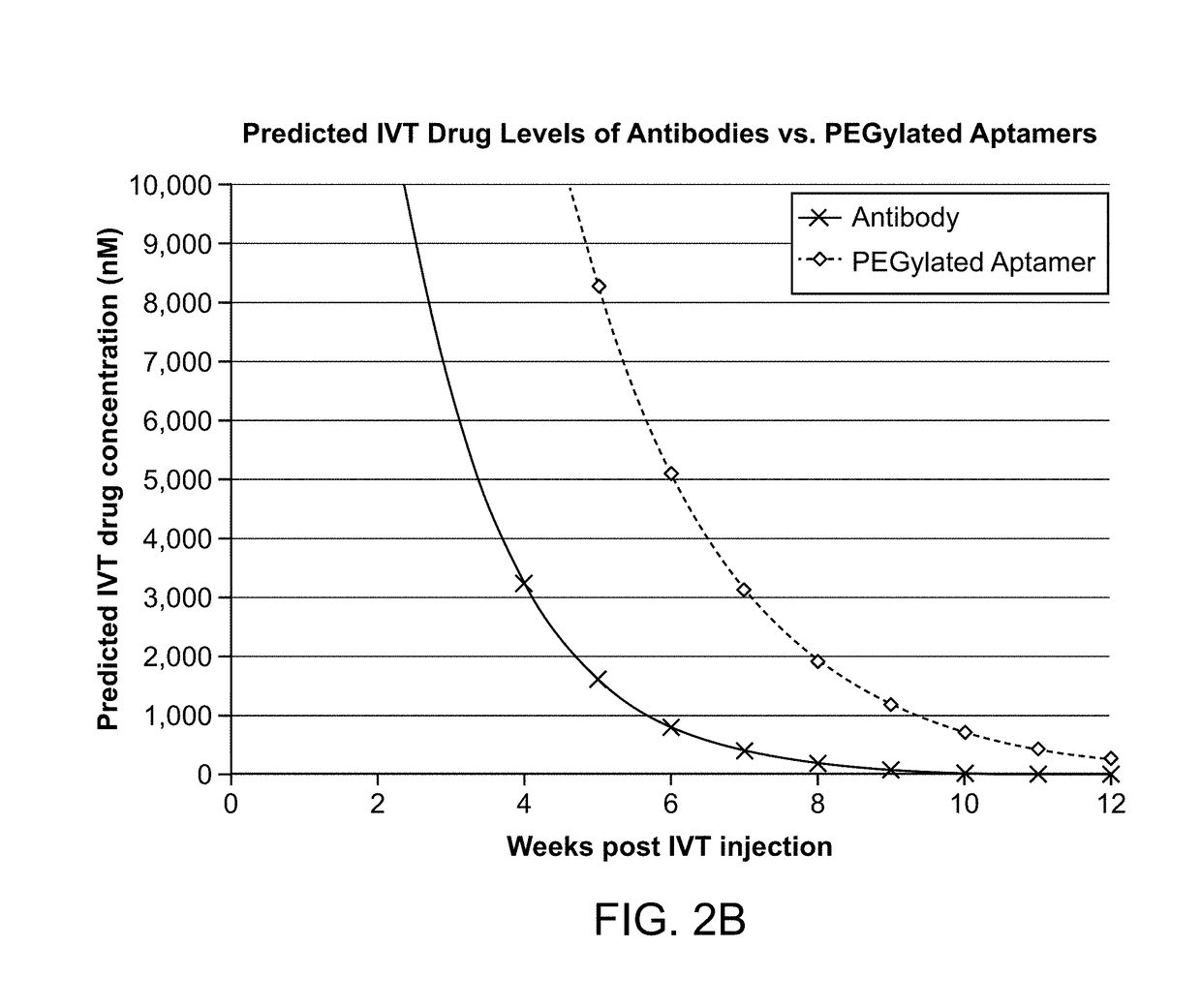
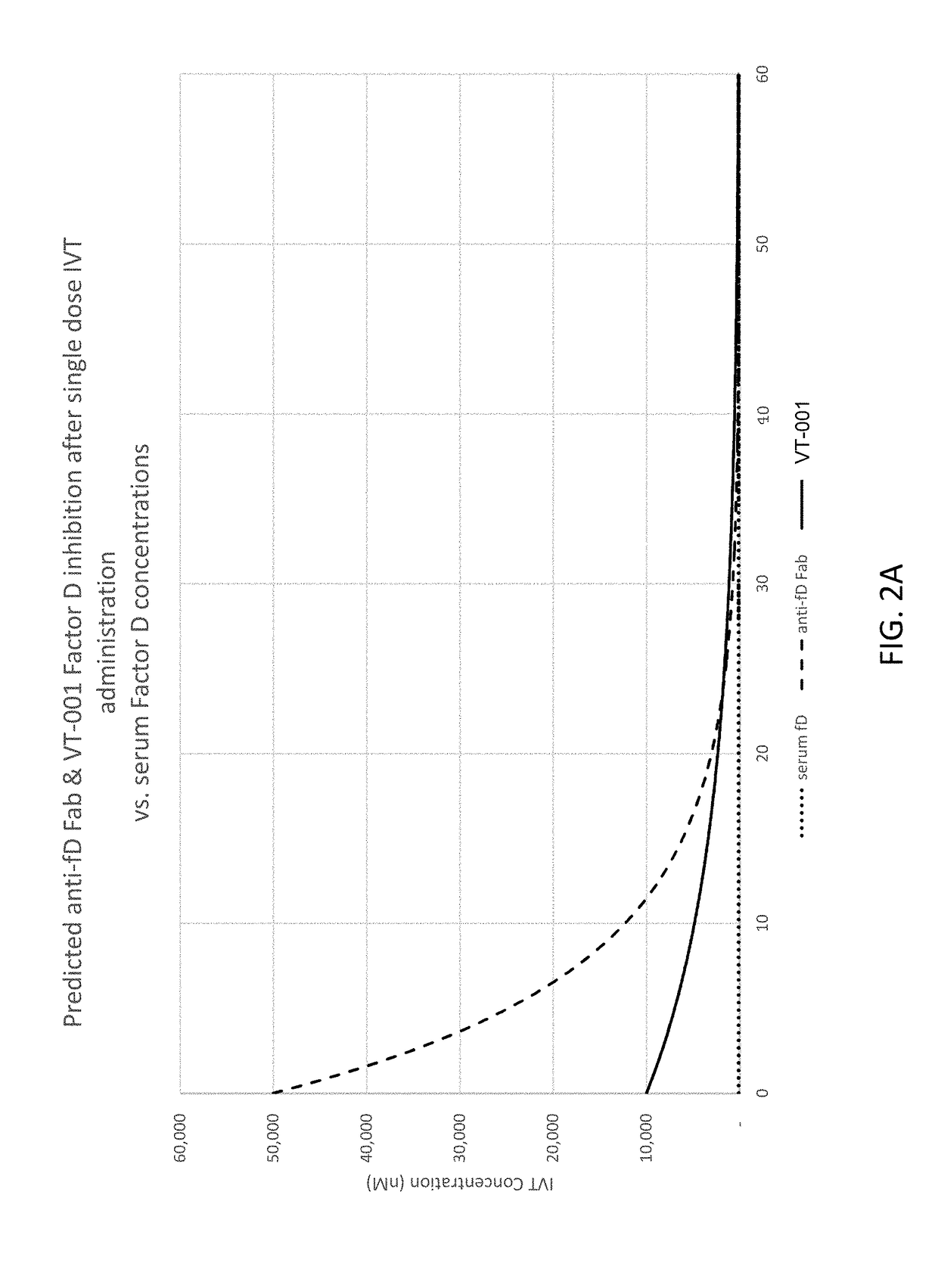
ActiveUS20180051287A1Inhibit functioningOrganic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
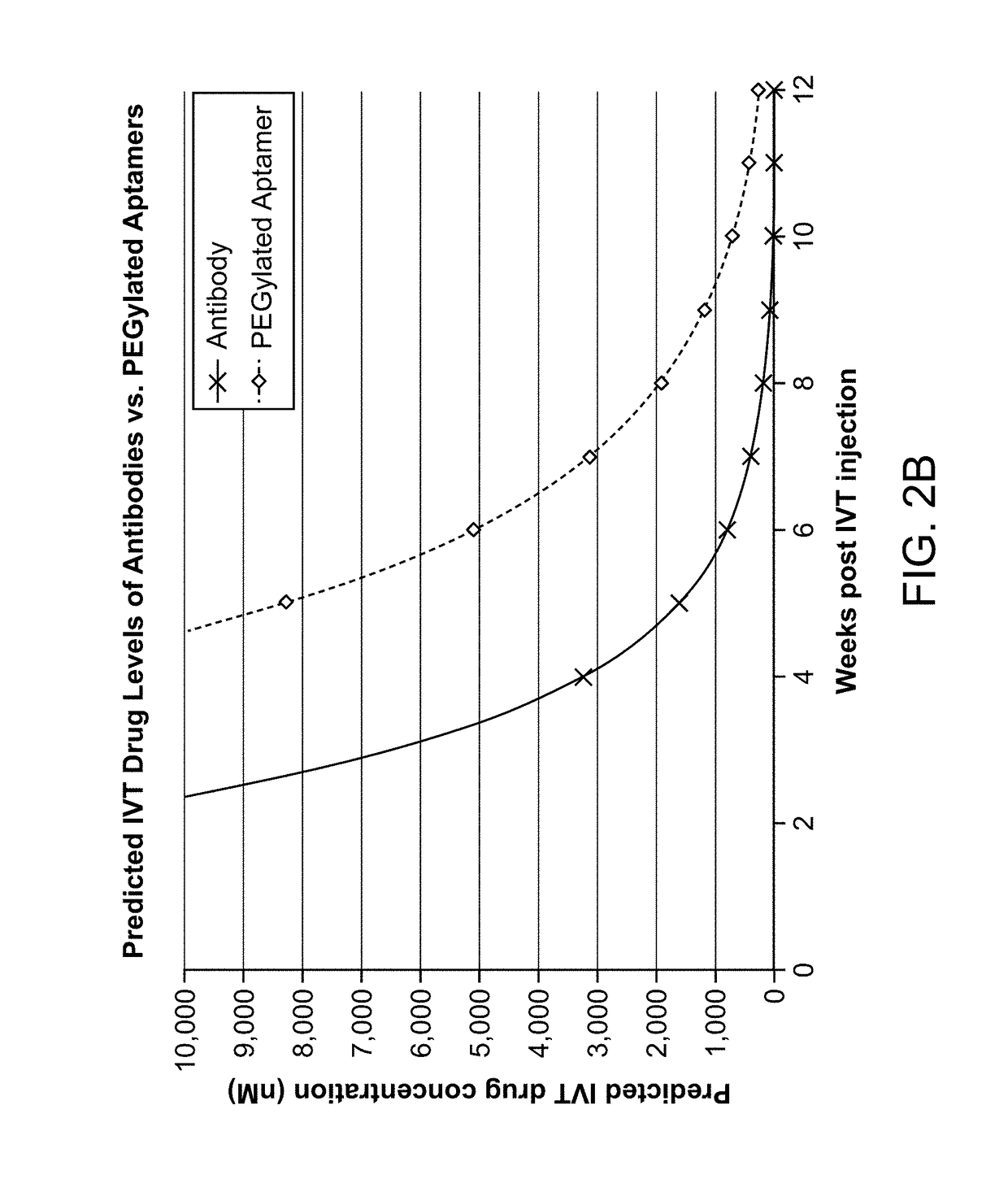
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Amide compounds for treatment of complement mediated disorders
InactiveCN106413707ALess quantityIncreased detectable levelsSenses disorderNervous disorderDepressantPharmaceutical medicine
Compounds, methods of use, and processes for making inhibitors of complement factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof, wherein R12 or R13 on the A group is an amide substituent (R32) are provided. The inhibitors described herein target factor D and inhibit or regulate the complement cascade at an early and essential point in the alternative complement pathway, and reduce factor D's ability to modulate the classical and lectin complement pathways. The inhibitors of factor D described herein are capable of reducing the excessive activation of complement, which has been linked to certain autoimmune, inflammatory, and neurodegenerative diseases, as well as ischemia-reperfusion injury and cancer.
Owner:ACHILLION PHARMA INC
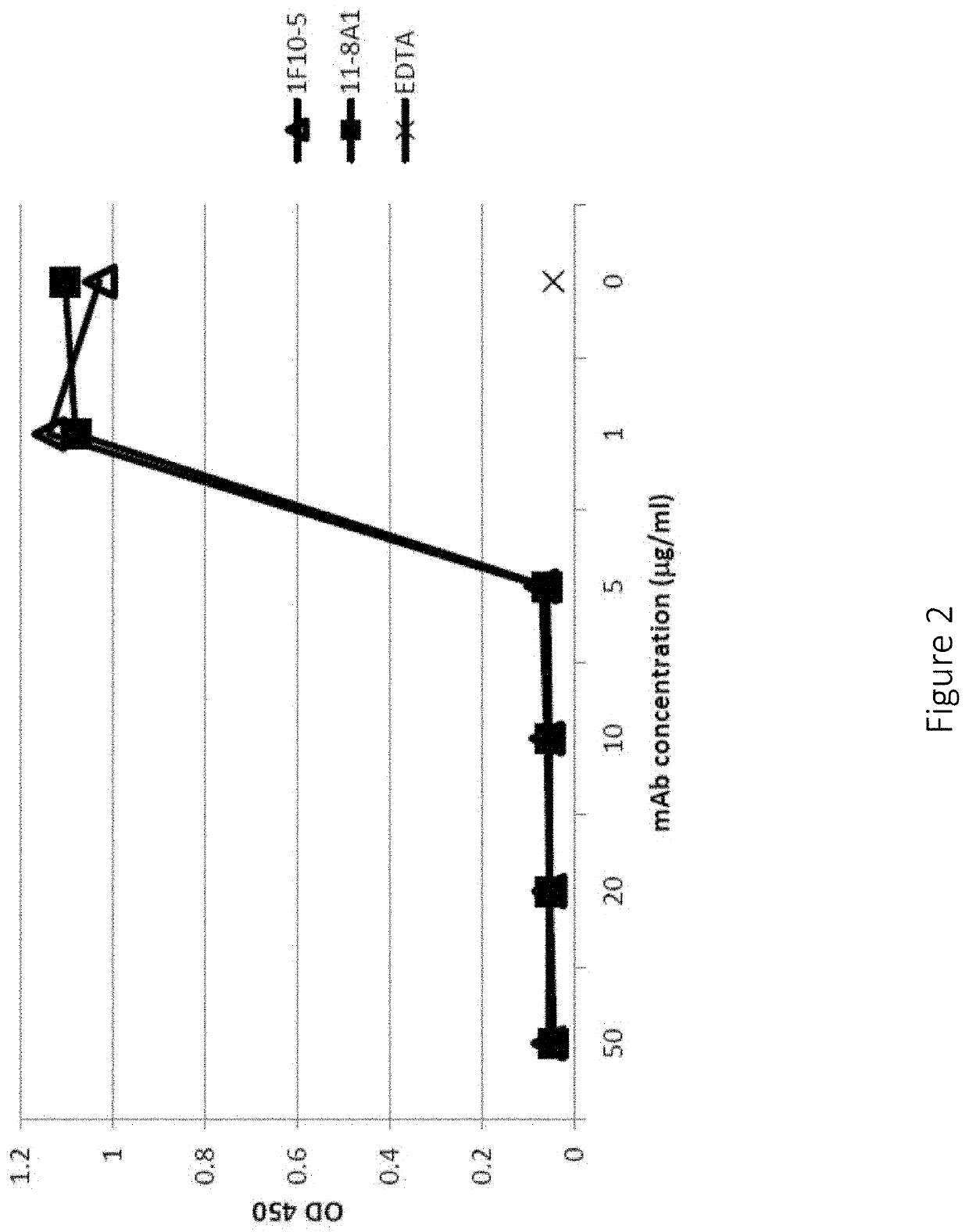
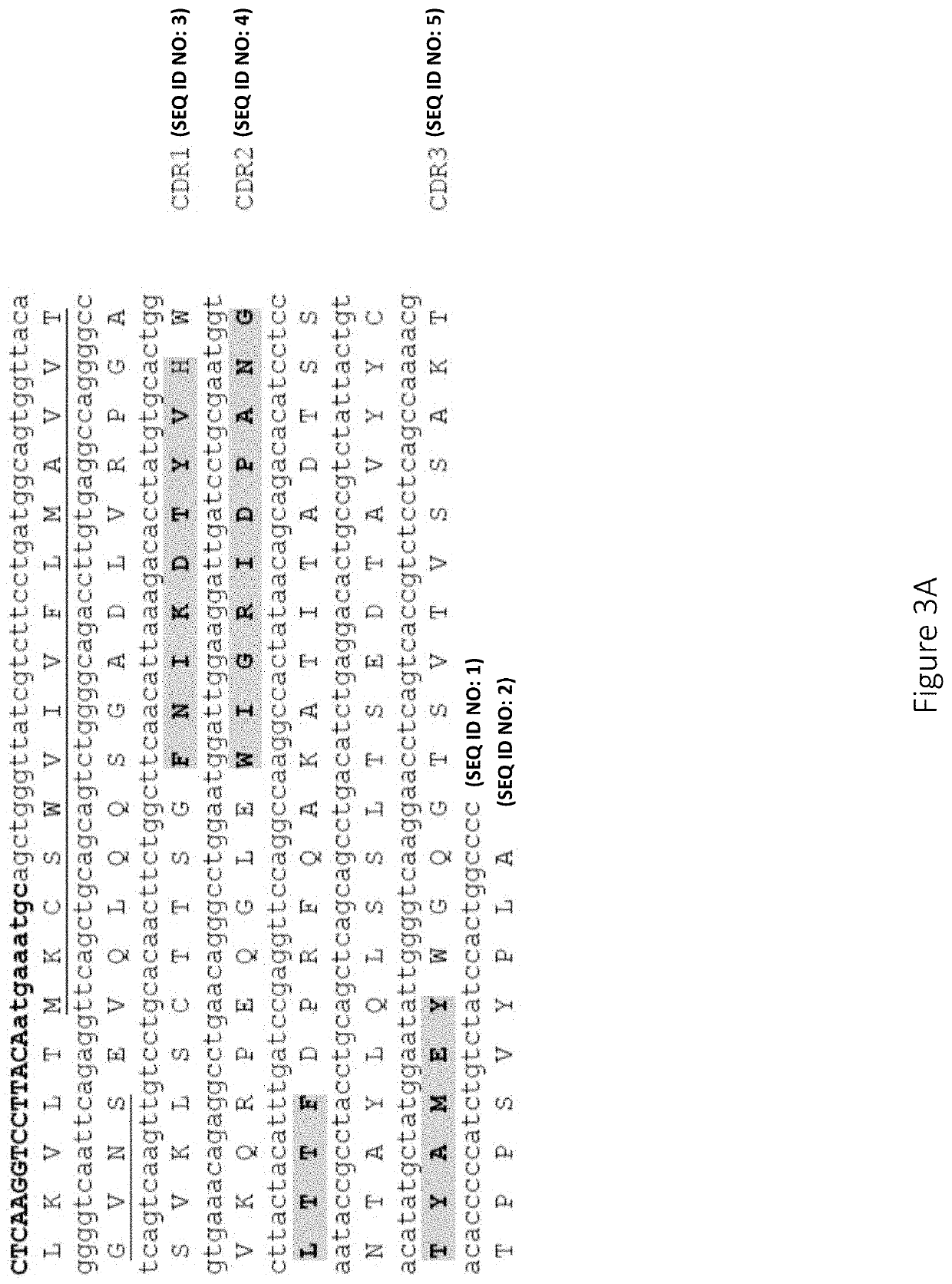
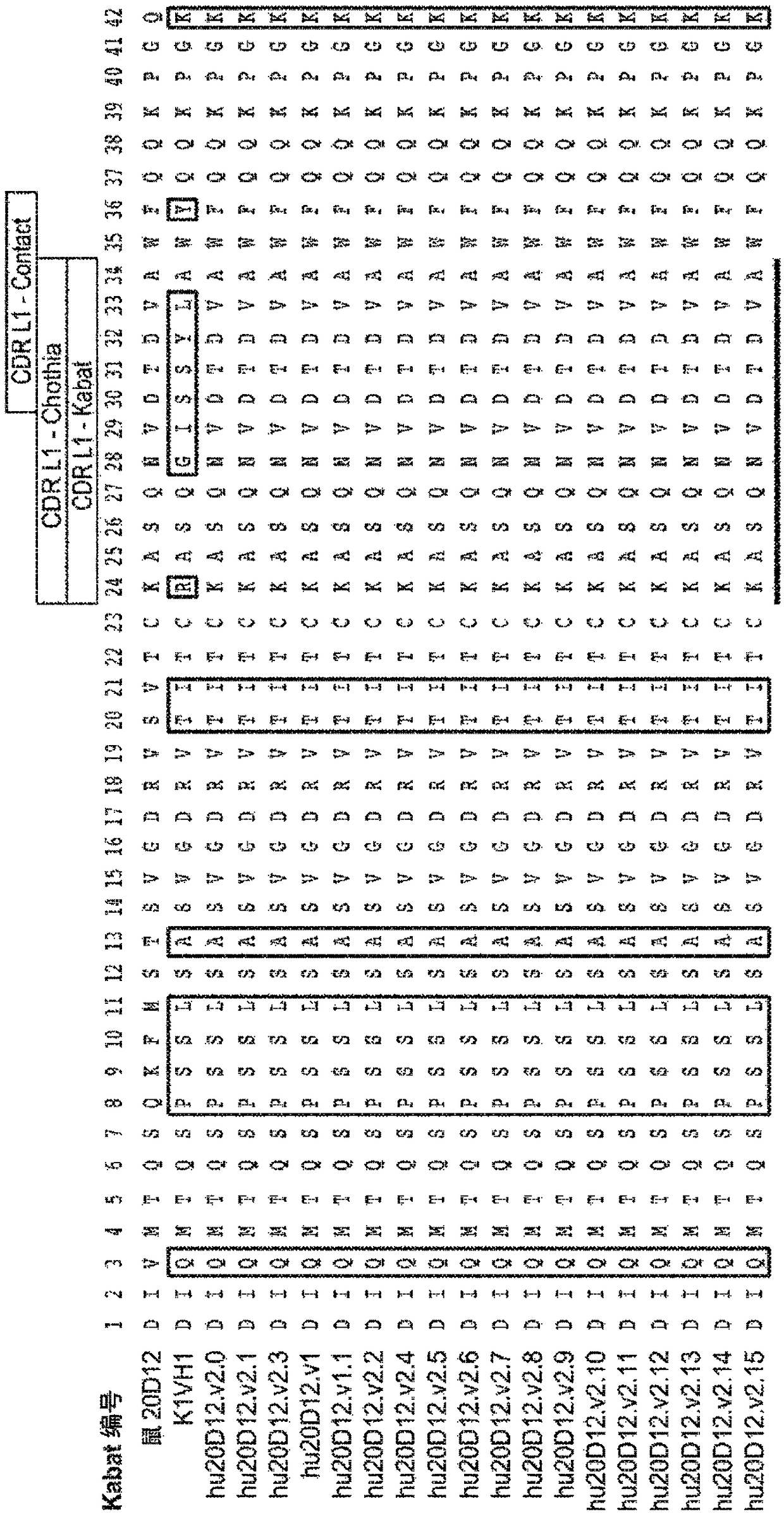
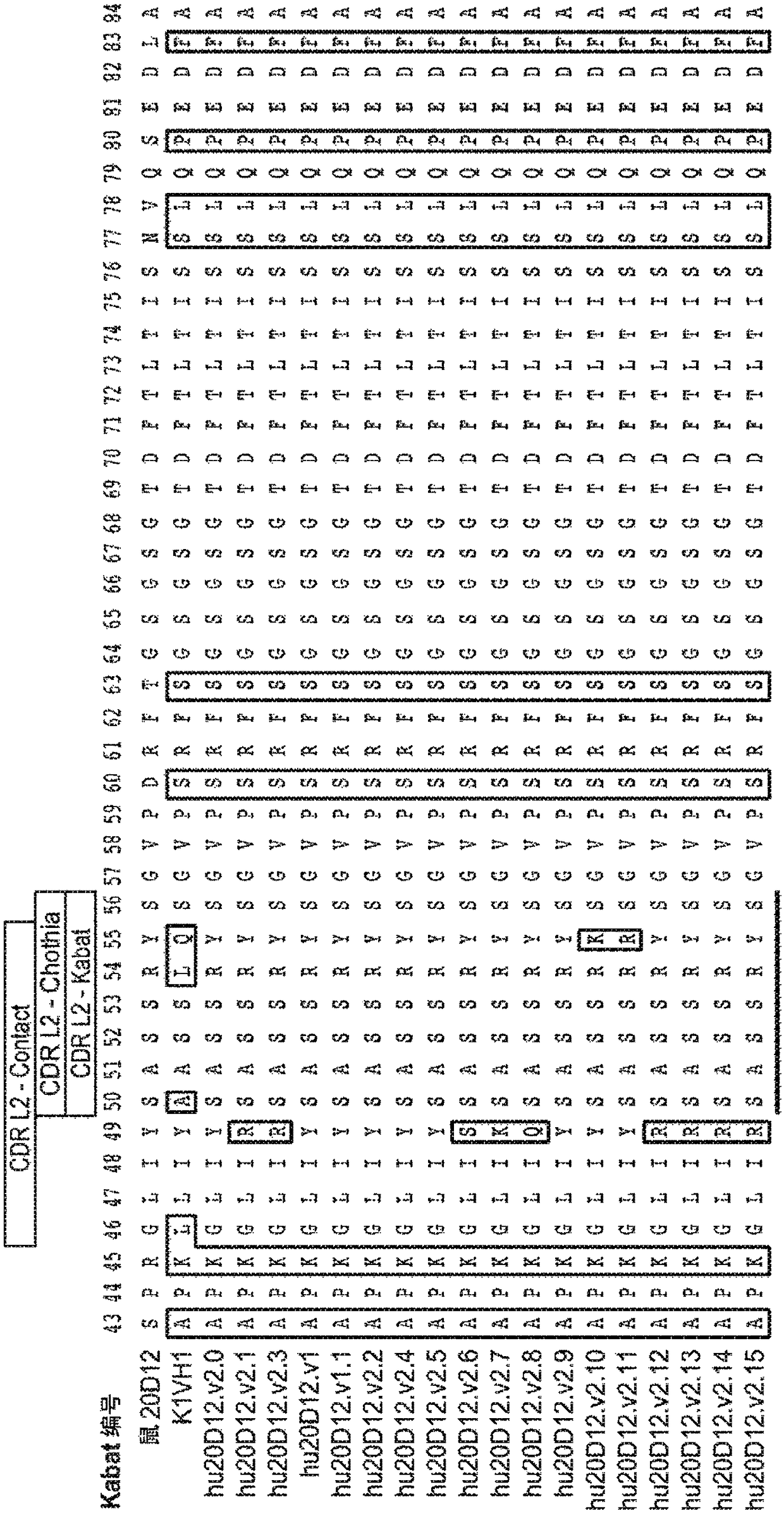
Anti-factor d antibodies and uses thereof
ActiveUS20190359699A1Immunoglobulins against animals/humansAntibody ingredientsComplement systemFactor ii
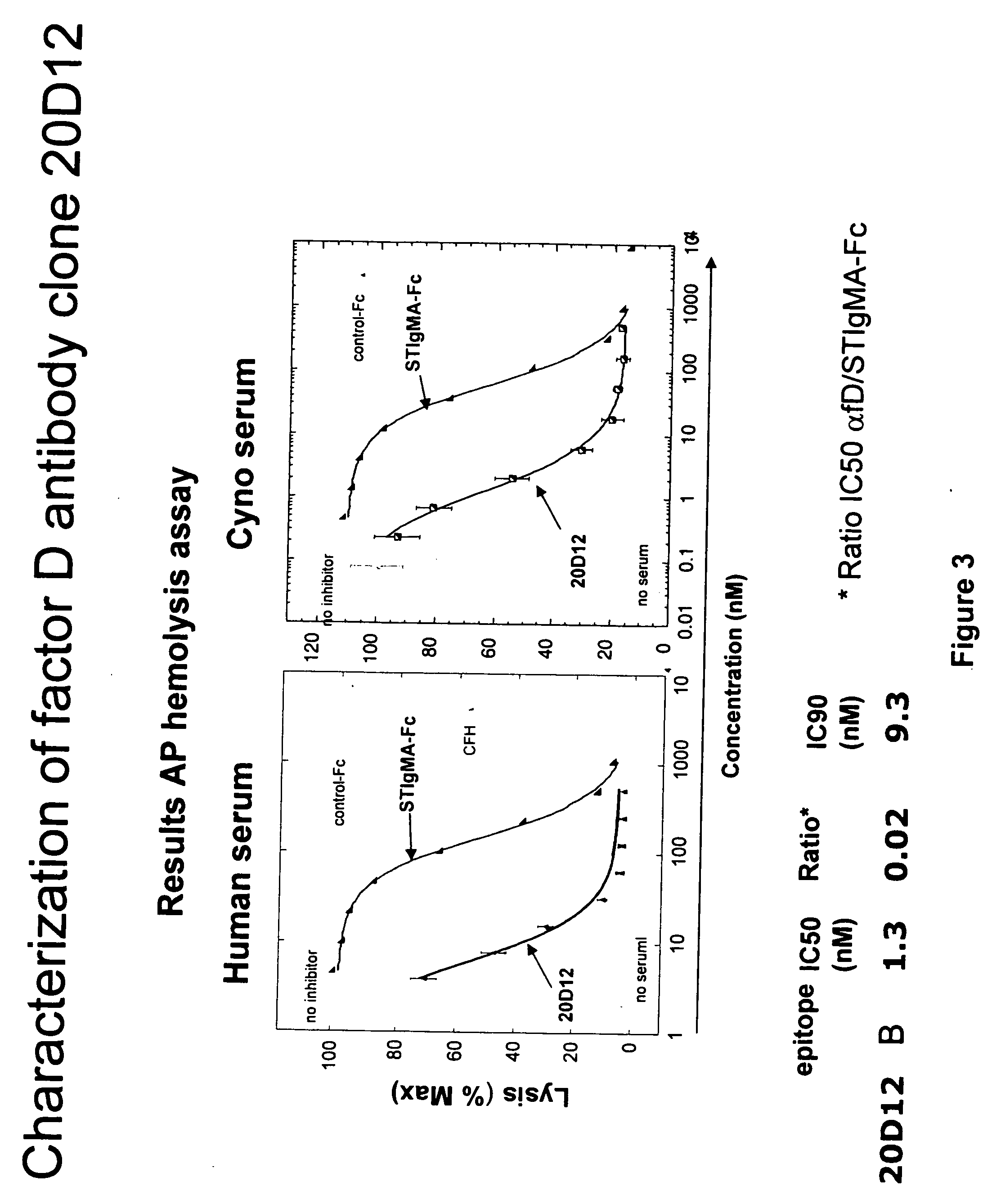
This invention relates to selective inhibition of the alternative pathway (AP) of the complement system using an anti-factor D antibody. Specifically, the invention relates to methods of treating an AP-mediated disease or AP-mediated disorder in an individual by contacting the individual with an anti-factor D antibody.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for inhibiting Factor D
ActiveUS10174325B2Organic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Anti-factor d antibody variant conjugates and uses thereof
ActiveUS20170143843A1Improve stabilityExtended half-lifeSenses disorderPharmaceutical delivery mechanismDiseaseBiological activation
Owner:GENENTECH INC
Anti-factor D antibody variants and uses thereof
InactiveCN106536561ASenses disorderPharmaceutical delivery mechanismDiseaseAntiendomysial antibodies
The invention relates to anti-Factor D antibody variants, their production and their use in the preparation of compositions and medicaments for treatment of diseases and disorders associated with excessive or uncontrolled complement activation.
Owner:F HOFFMANN LA ROCHE & CO AG
Prophylaxis and therapy for age related illnesses
InactiveUS20160317573A1Reduce adverse effectsPowder deliveryOrganic active ingredientsAge related diseaseRegulating factors
A method for treating a subject suffering from age related illnesses such as Type 2 diabetes by administering to the subject a compound to down regulate Factor D or Factor H thereby providing prophylaxis and therapy to inhibit age related illnesses. The compound is a functionalized Nano Polymer of less than 100 nm, which primarily inhibits Factor D, Factor H, or both.
Owner:SHAH KUMARPAL A
Cell growth regulating factor D and its prepn process
InactiveCN1506065APromote regenerationPromote repairOrganic active ingredientsSkeletal disorderHistiocyteAmino acid
The present invention is one kind of natural cell growth regulating factor D and its preparation process. The preparation process includes screening Gram-positive coccus or bacillus and Gram-negative coccus or bacillus, freeze preserving coccus or bacillus strain, conventional culture of the coccus or bacillus strain at 4-38 deg.c, once filtering to eliminate harmful bacteria while leaving beneficial thallus segment, extracting natural gene, adding isoleucine into the natural gene, safety test, preparing into solution or powder, and sealing. The cell growth regulating factor D has the functions of stimulating and strengthening immunity, promoting the amplification of capillary vessel in fracture and pathological change part and repairing and reforming pathological change histiocyte.
Owner:常山汇永生物技术有限公司
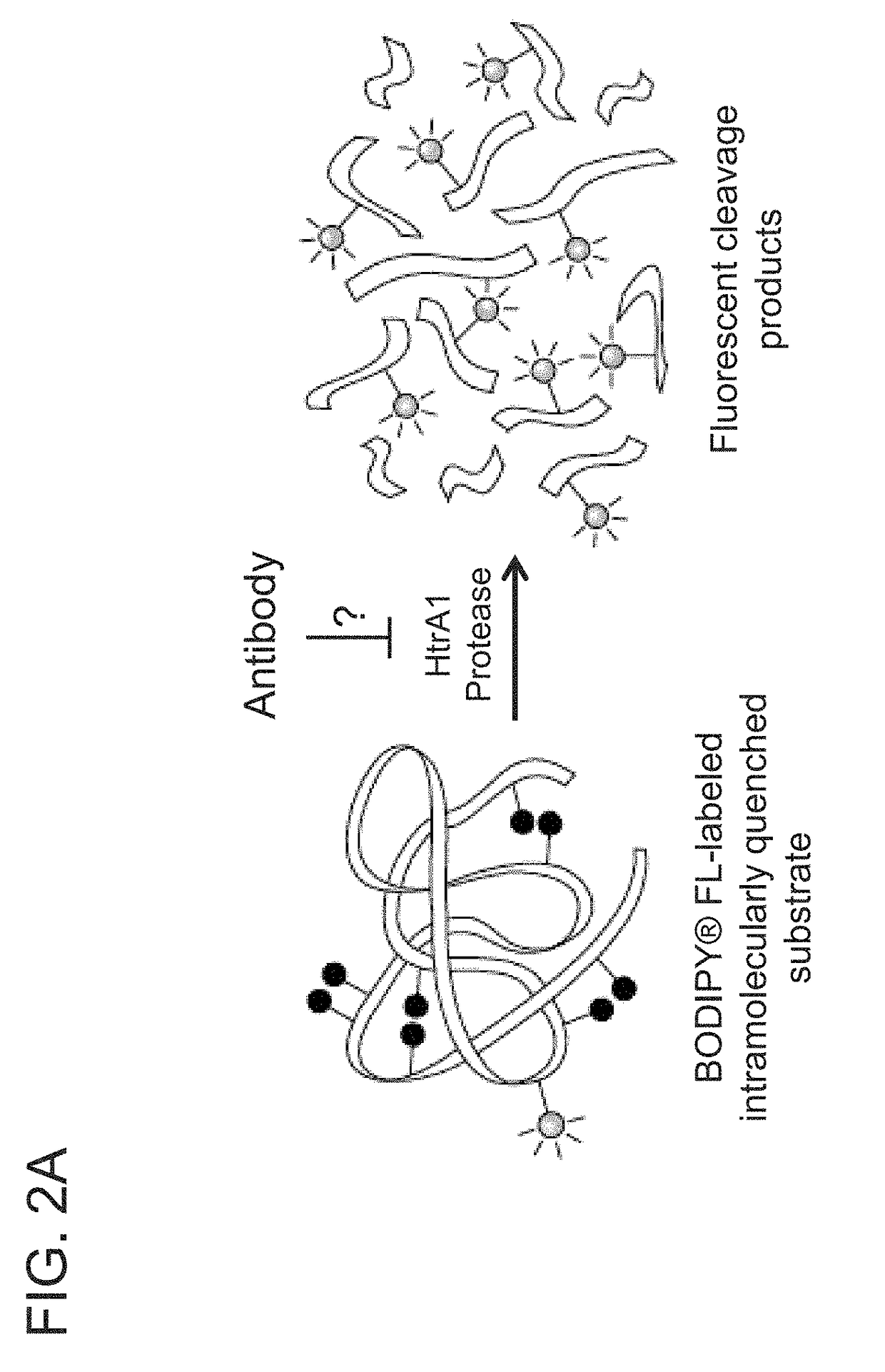
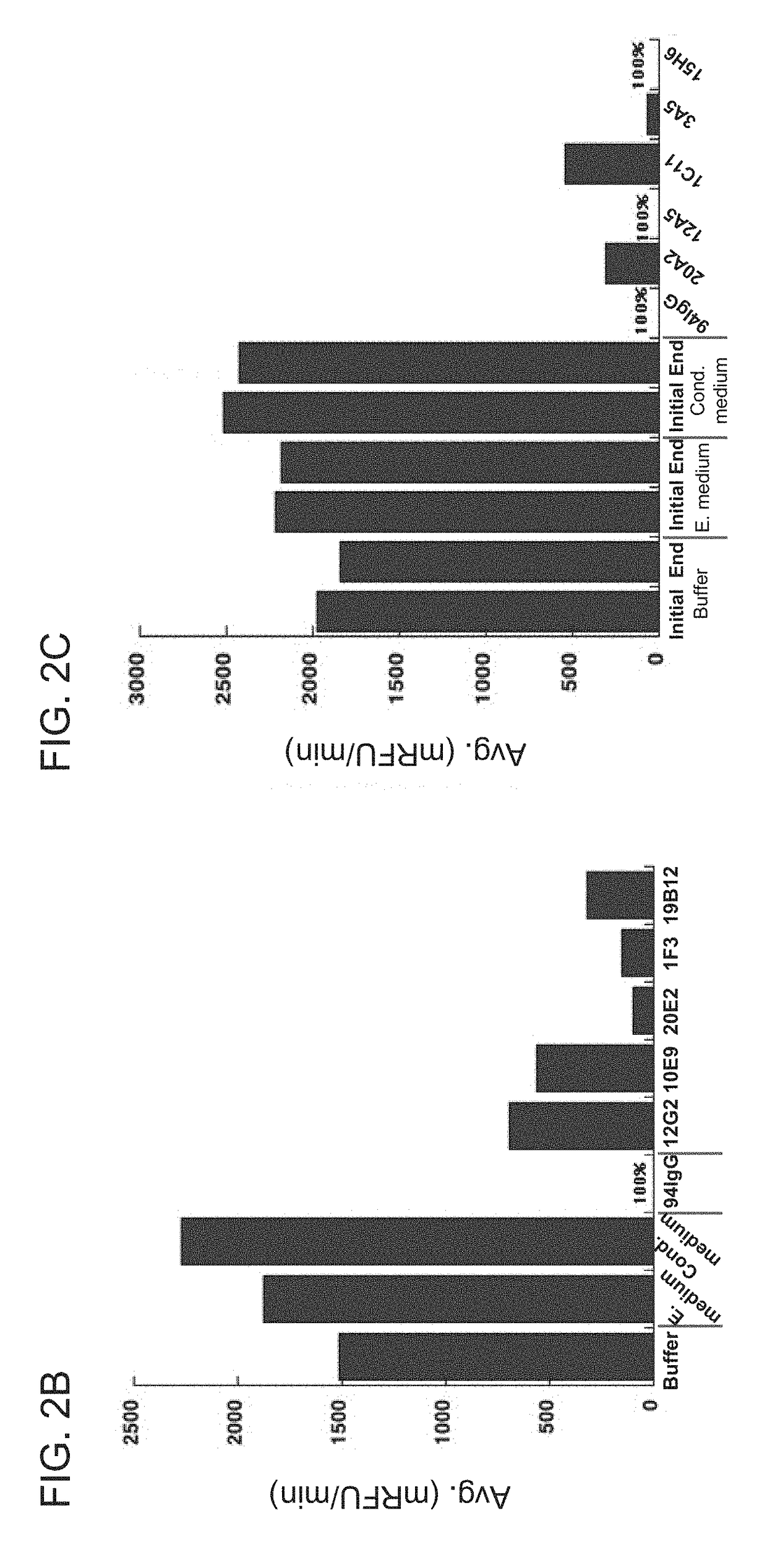
ANTI-HtrA1 ANTIBODIES AND METHODS OF USE THEREOF
ActiveUS20190048097A1Raise the potentialHigh binding affinitySenses disorderPharmaceutical delivery mechanismAntibodyFactor D
Owner:GENENTECH INC
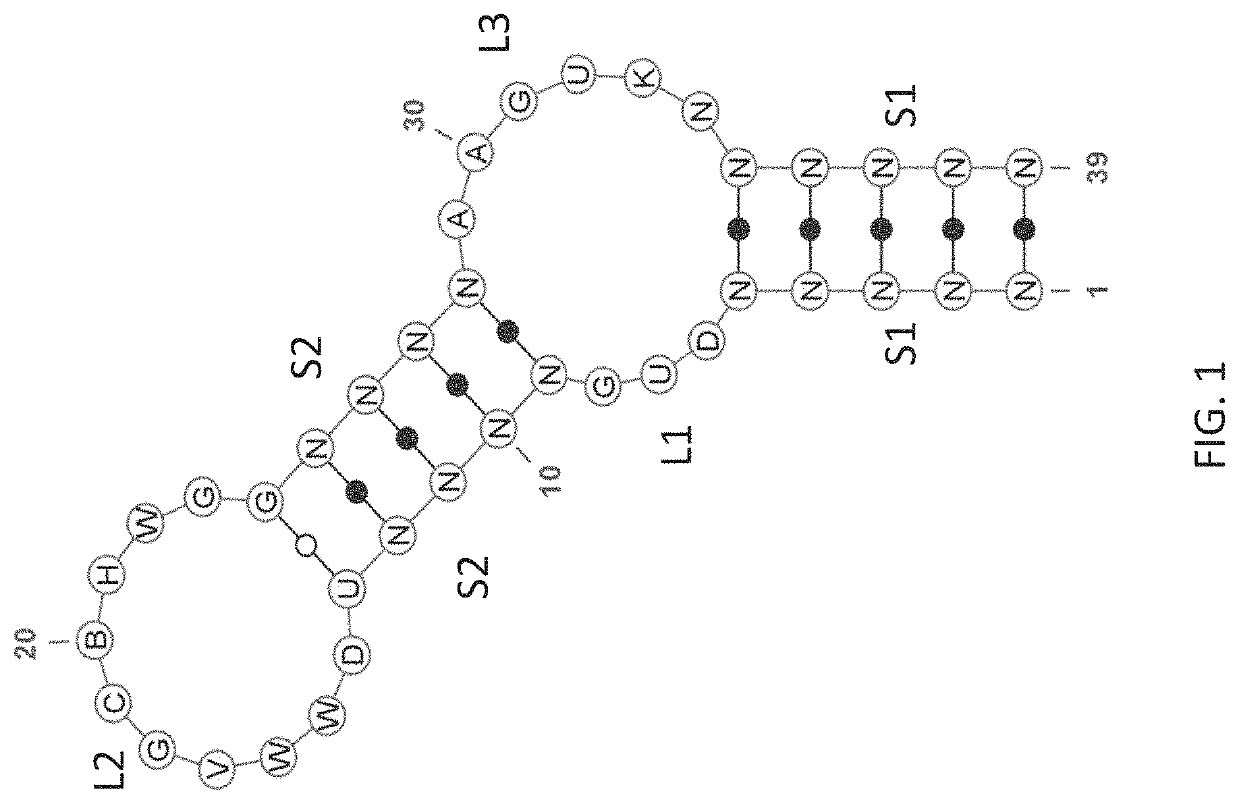
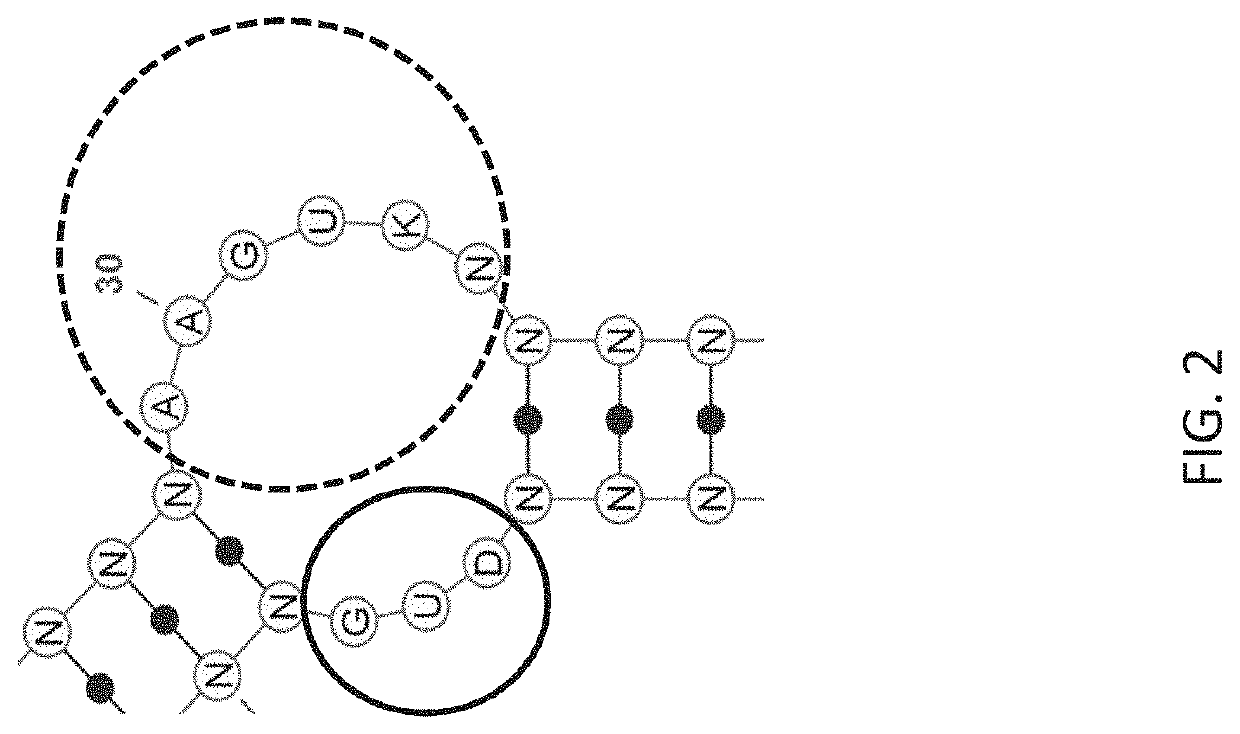
Stem-loop compositions and methods for inhibiting factor D
ActiveUS11466276B2Organic active ingredientsPharmaceutical non-active ingredientsAptamerGeographic atrophy
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease. In some cases, stem-loop aptamers are provided for the inhibition of Factor D.
Owner:396419 B C LTD +1
Nicandra physalodes stem total alkaloid extraction process
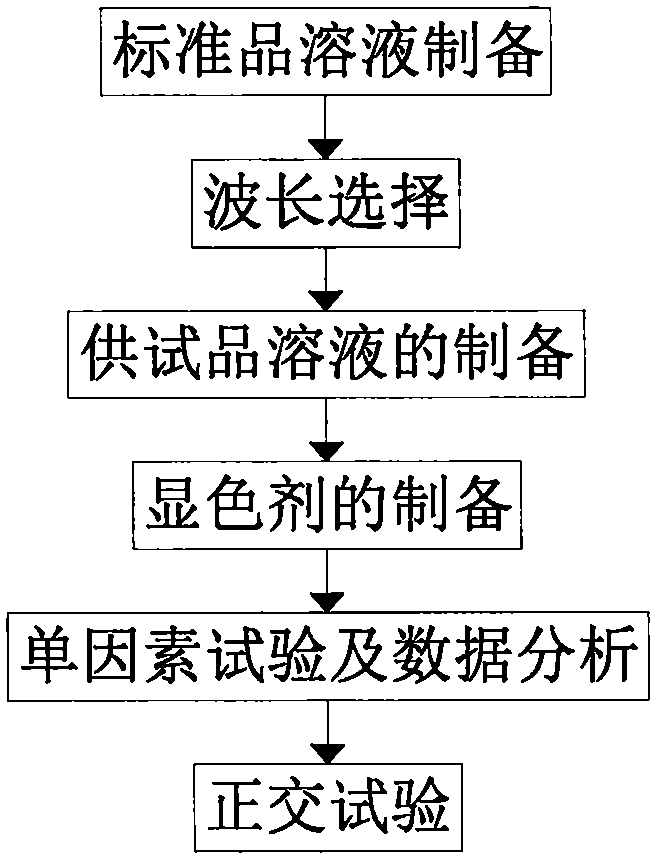
The invention discloses a nicandra physalodes stem total alkaloid extraction process. The extraction process includes the step A of standard solution preparation: accurately weighing 5.5mg of standardatropine sulfate with a 1 / 10000 scale, and metering the volume with ethanol into a 50 ml volumetric flask to prepare 0.11mg.mL-1 standard solution. First a single factor test is carried out to determine the optimal extraction conditions, and then an orthogonal test is designed based on the optimal extraction conditions obtained by the single factor test. By calculating the alkaloid yield, the nicandra physalodes stem total alkaloid ultrasonic extraction optimal process conditions are obtained: ultrasonic 50min; ratio of material to liquid 1:25; ethanol concentration 60%; ultrasonic power 400w; temperature 50 DEG C; extraction rate 2.58%. The influence of a factor A, a factor B, a factor C, a factor D and a factor E can be analyzed according to analysis of variance, the sequence is that B>A>D>E>C, that is, time>material ratio>concentration>power>temperature.
Owner:JILIN AGRI SCI & TECH COLLEGE
Anti-factor d antibodies and conjugates
InactiveCN108289951ASenses disorderImmunoglobulins against growth factorsAntiendomysial antibodiesBiochemistry
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-factor D antibodies and uses thereof
ActiveUS11434279B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
This invention relates to selective inhibition of the alternative pathway (AP) of the complement system using an anti-factor D antibody. Specifically, the invention relates to methods of treating an AP-mediated disease or AP-mediated disorder in an individual by contacting the individual with an anti-factor D antibody.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and method for treating complement-associated conditions
ActiveUS20190185935A1Reduce areaReduced vision lossSenses disorderMicrobiological testing/measurementAntigenAntiendomysial antibodies
The invention provides methods and compositions for treating various degenerative diseases (e.g., AMD) with a factor D inhibitor (e.g., anti-factor D antibody or antigen-binding fragment thereof). Also provided are methods of selecting or identifying patients for treatment with a factor D inhibitor. Methods include the use of prognostic and / or predictive biomarkers.
Owner:GENENTECH INC
Compositions for detecting complement factor H (CFH) and complement factor I (CFI) polymorphisms
InactiveUS10093978B2Reduce areaReduced vision lossSenses disorderMicrobiological testing/measurementAntigenAntigen Binding Fragment
The invention provides methods and compositions for treating various degenerative diseases (e.g., AMD) with a factor D inhibitor (e.g., anti-factor D antibody or antigen-binding fragment thereof). Also provided are methods of selecting or identifying patients for treatment with a factor D inhibitor. Methods include the use of prognostic and / or predictive biomarkers.
Owner:GENENTECH INC
Method for the diagnosis of c3nef-mediated membranoproliferative glomerulonephritis
Owner:红公里科学技术园股份公司
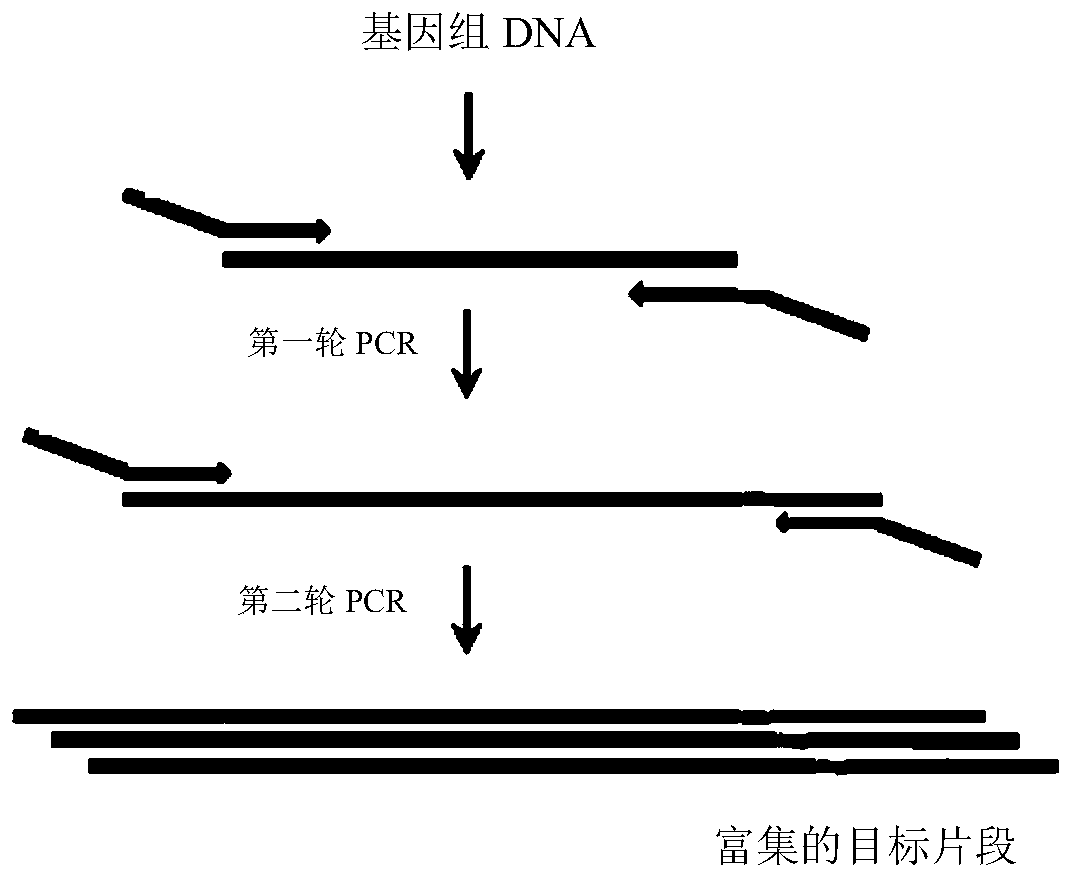
Method for enriching target DNA fragments by multiplex PCR
The invention discloses a method for enriching target DNA fragments by multiple PCR. The method for enriching target DNA fragments disclosed by the invention includes: carrying out multiplex PCR for enriching target DNA fragments by using a complete set of primers meeting the following conditions: factor J of each primer pair < 50% and at most one factor of nine factors is not in the standard range; the standard ranges of the nine factors are: 35% <= factor A <= 60%; 68 DEG C <= factor B <= 79 DEG C; 30% <= factor C <= 70%; 30% <= factor D <= 70%; 15 kcal / mol <= absolute value of factor E <= 70 kcal / mol; absolute value of factor F < 100 kcal / mol; absolute value of factor G < 100 kcal / mol; 4 kcal / mol <= absolute value of factor H <= 10 kcal / mol; and factor I < 100 DEG C. The method of the invention can be used to successfully enrich target DNA fragments in genome.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
Compounds, methods of use, and processes for making inhibitors of complement Factor D comprising Formula I, or a pharmaceutically acceptable salt or composition thereof wherein R12 or R13 on the A group is an aryl, heteroaryl or heterocycle (R32) are provided. The inhibitors of Factor D described herein reduce the excessive activation of complement.
Owner:ACHILLION PHARMA INC
Anti-factor d antibody variant conjugates and uses thereof
ActiveUS20200115462A1Improve stabilityExtended half-lifeSenses disorderPharmaceutical delivery mechanismDiseaseAntiendomysial antibodies
Owner:GENENTECH INC
Method for the diagnosis of c3nef-mediated membranoproliferative glomerulonephritis
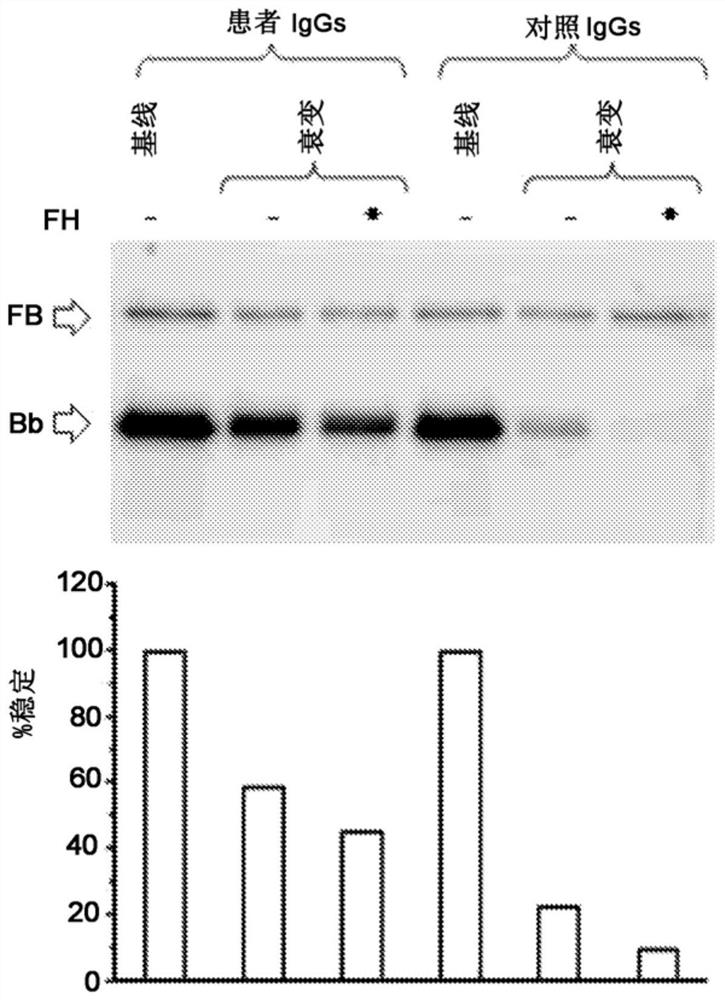
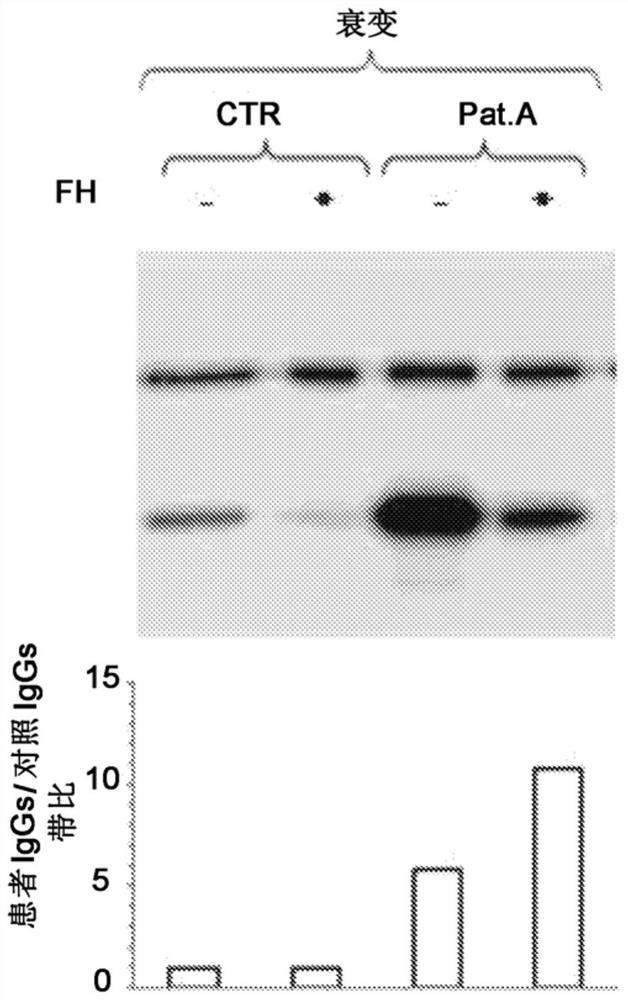
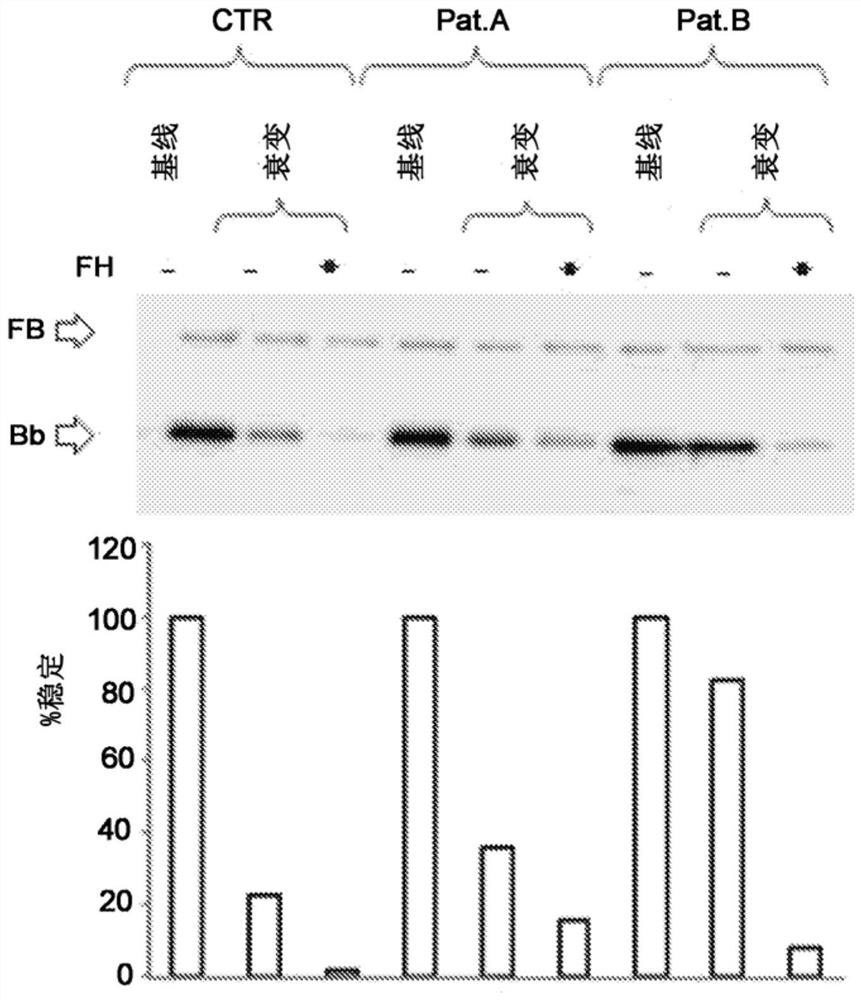
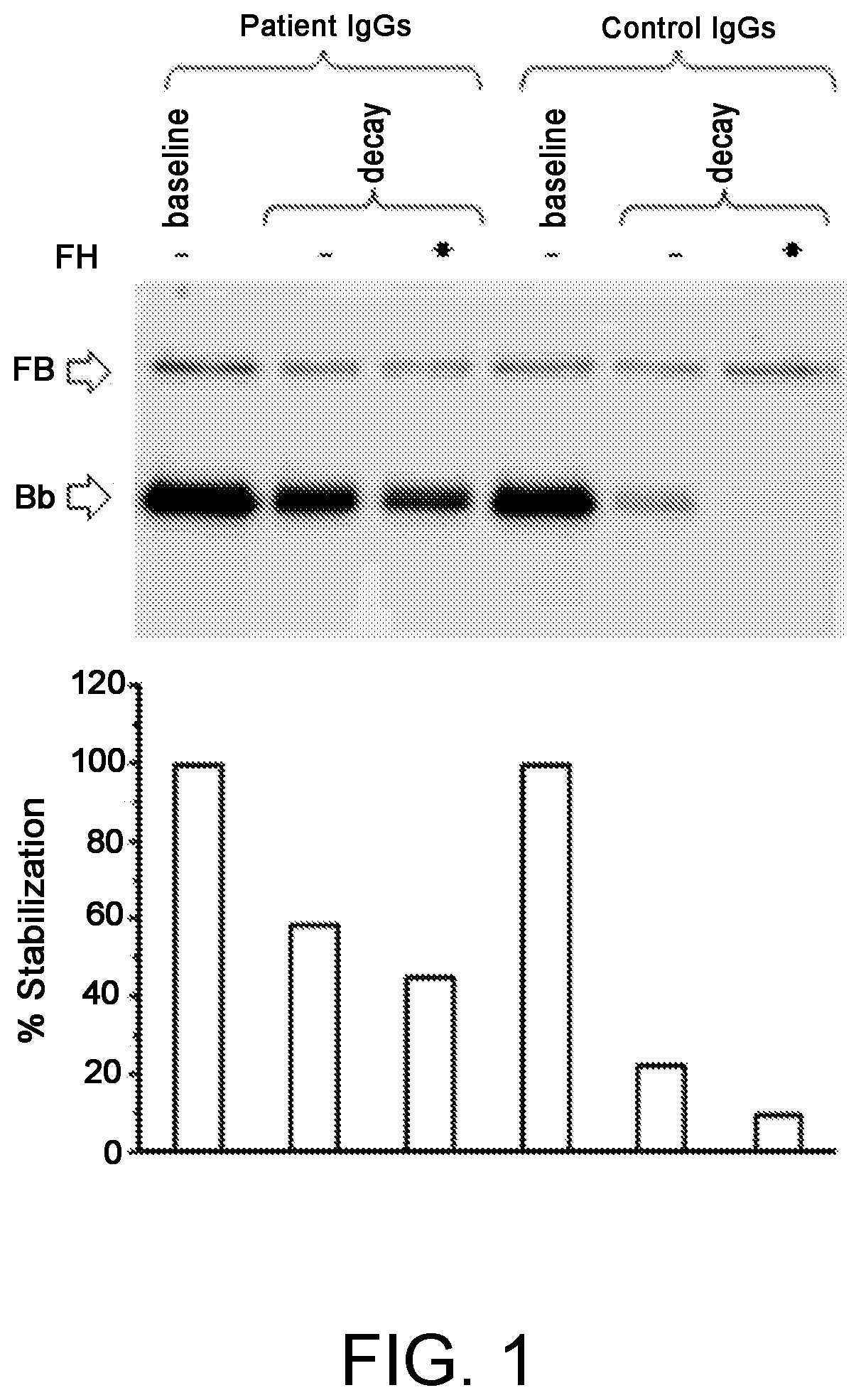
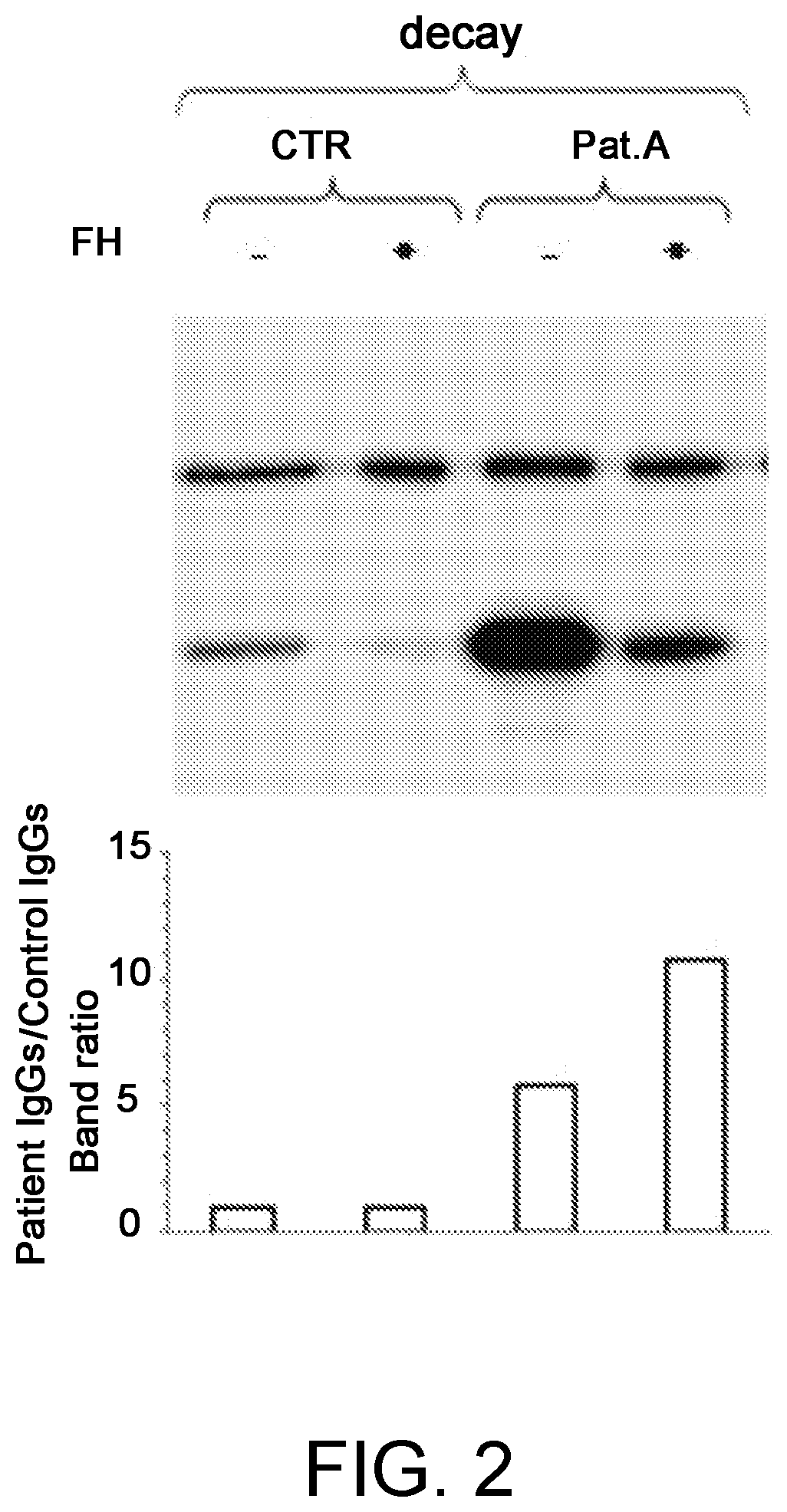
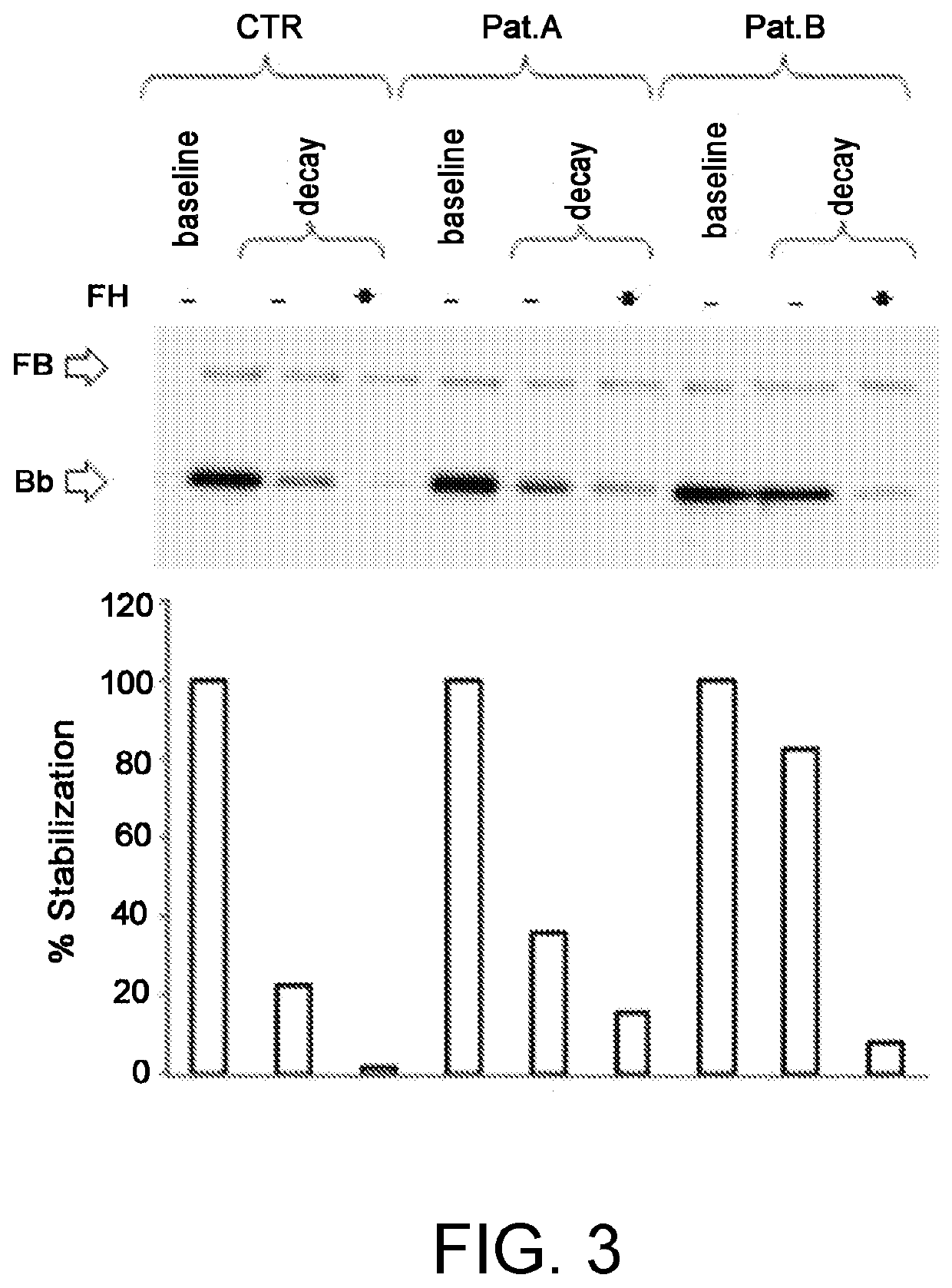
A method for the diagnosis of C3NeF-mediated membranoproliferative glomerulonephritis (MPGN) in a sample may include the step of immobilization of C3b in at least two wells of a plate. The method may further include formation in the wells of the C3bBb complex, where the formation of the complex is mediated by the addition of IgGs purified from the sample and / or control in the presence of Factor B (FB), Factor D (FD), bovine serum albumin (BSA), or magnesium ions. The method may also include detachment of the formed complexes and an electrophoretic run under denaturing conditions of the complexes and transfer on a membrane. Further, the method may include detection of the protein of interest, Bb. The ratio of the intensities measured for the Bb band in the samples collected by each of the wells is indicative of C3NeF activity in the sample.
Owner:PARCO SCIO TECHCO KILOMETRO ROSSO SPA
Cell growth regulating factor D and its preparation process
InactiveCN1255113CPromote regenerationPromote repairOrganic active ingredientsSkeletal disorderVascular proliferationFactor ii
A kind of natural cell growth regulating factor D and its preparation method, it is first to screen Gram-positive, negative cocci or bacilli and freeze the strains, culture the strains routinely at 4-38°C, and filter at least once Extract the natural gene after removing the harmful bacteria, quantitatively add isoleucine amino acid to the natural gene, and seal it under the GMP standard after safety testing. 1 μg of the natural gene in the natural cell growth regulator D can be configured There are more than 1 μg of isoleucine amino acid, which can better promote the activation of the body and enhance immune function, can promote the proliferation and proliferation of capillaries at fracture ends and diseased parts, and promote the repair and remodeling of diseased tissue cells.
Owner:常山汇永生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com