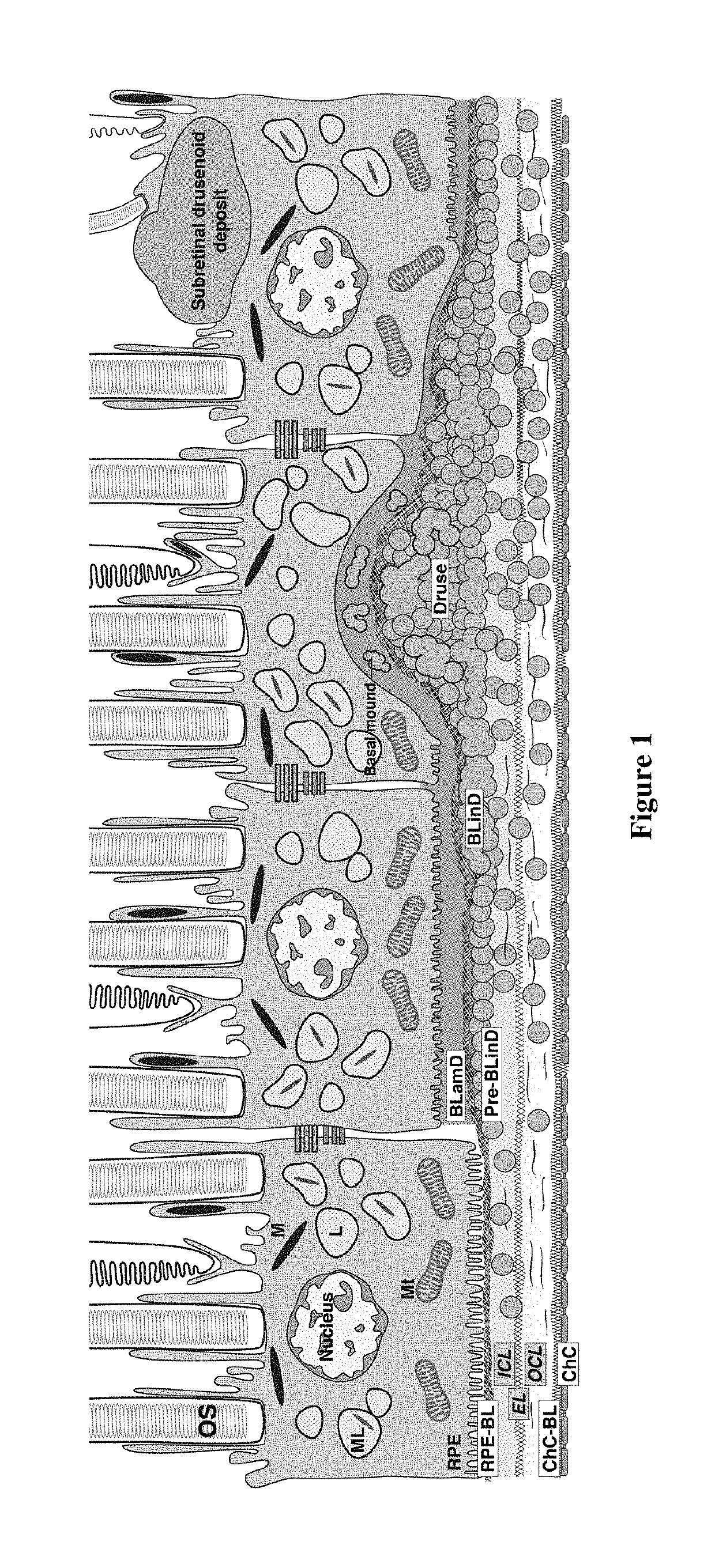
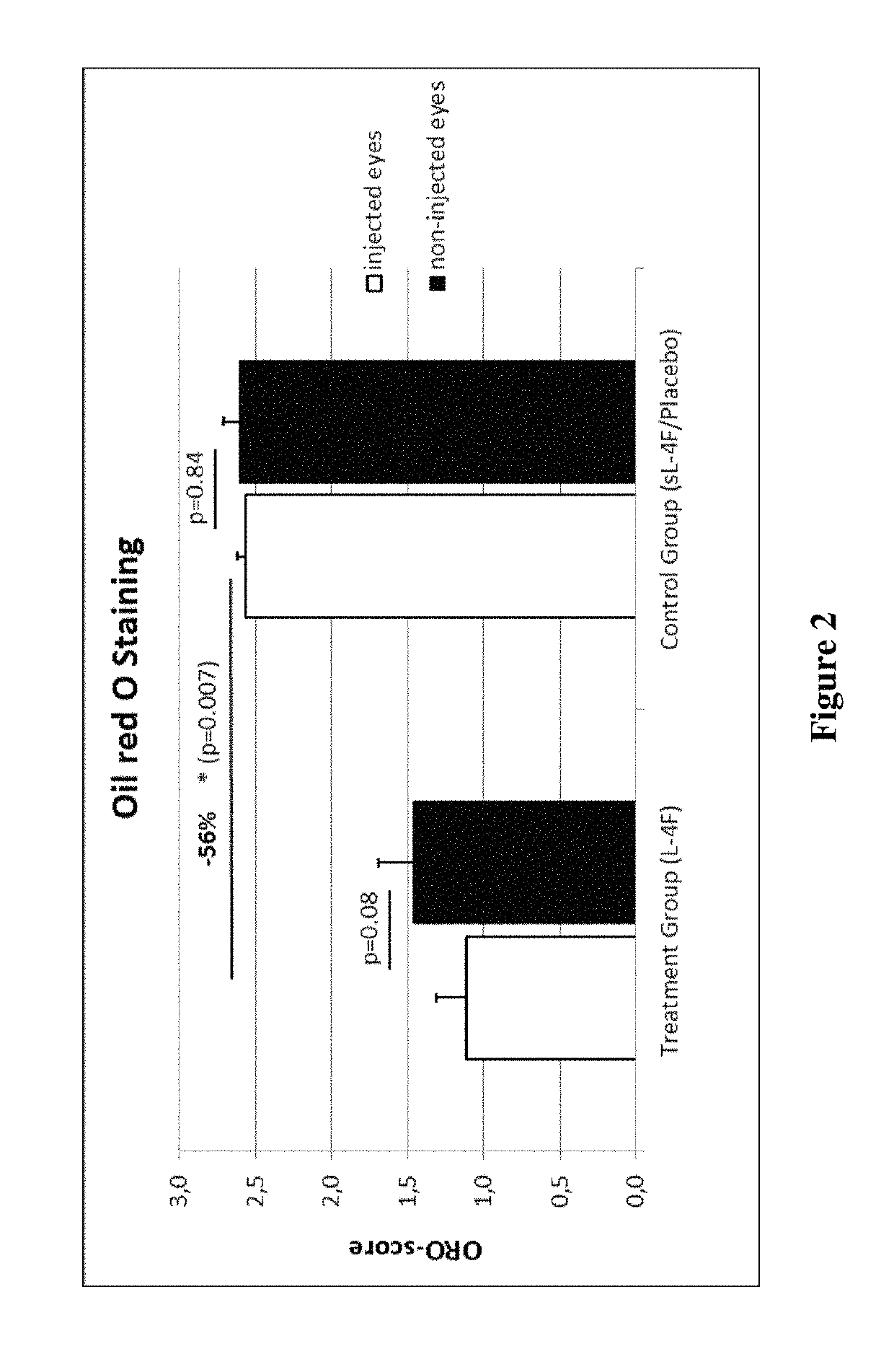
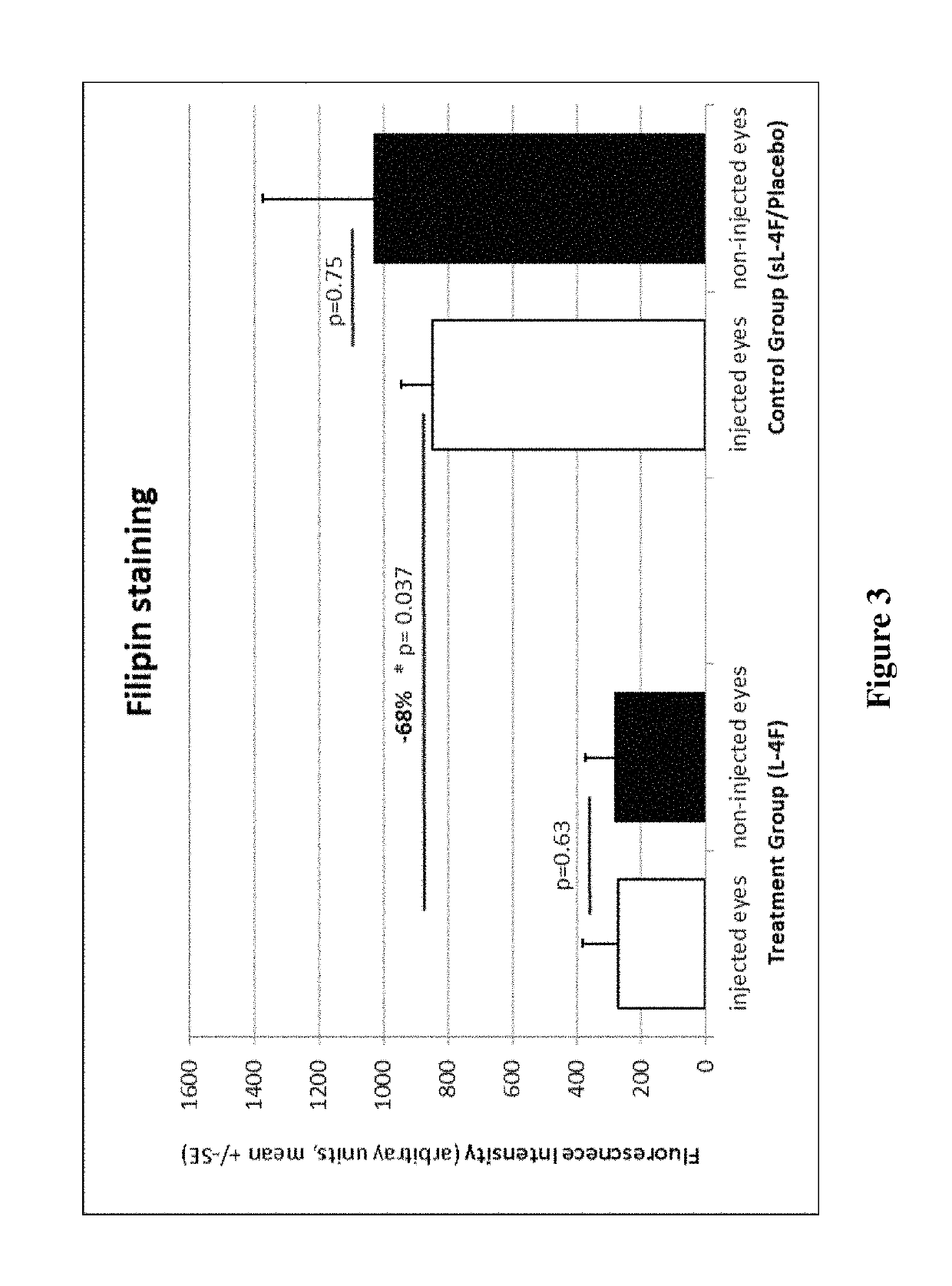
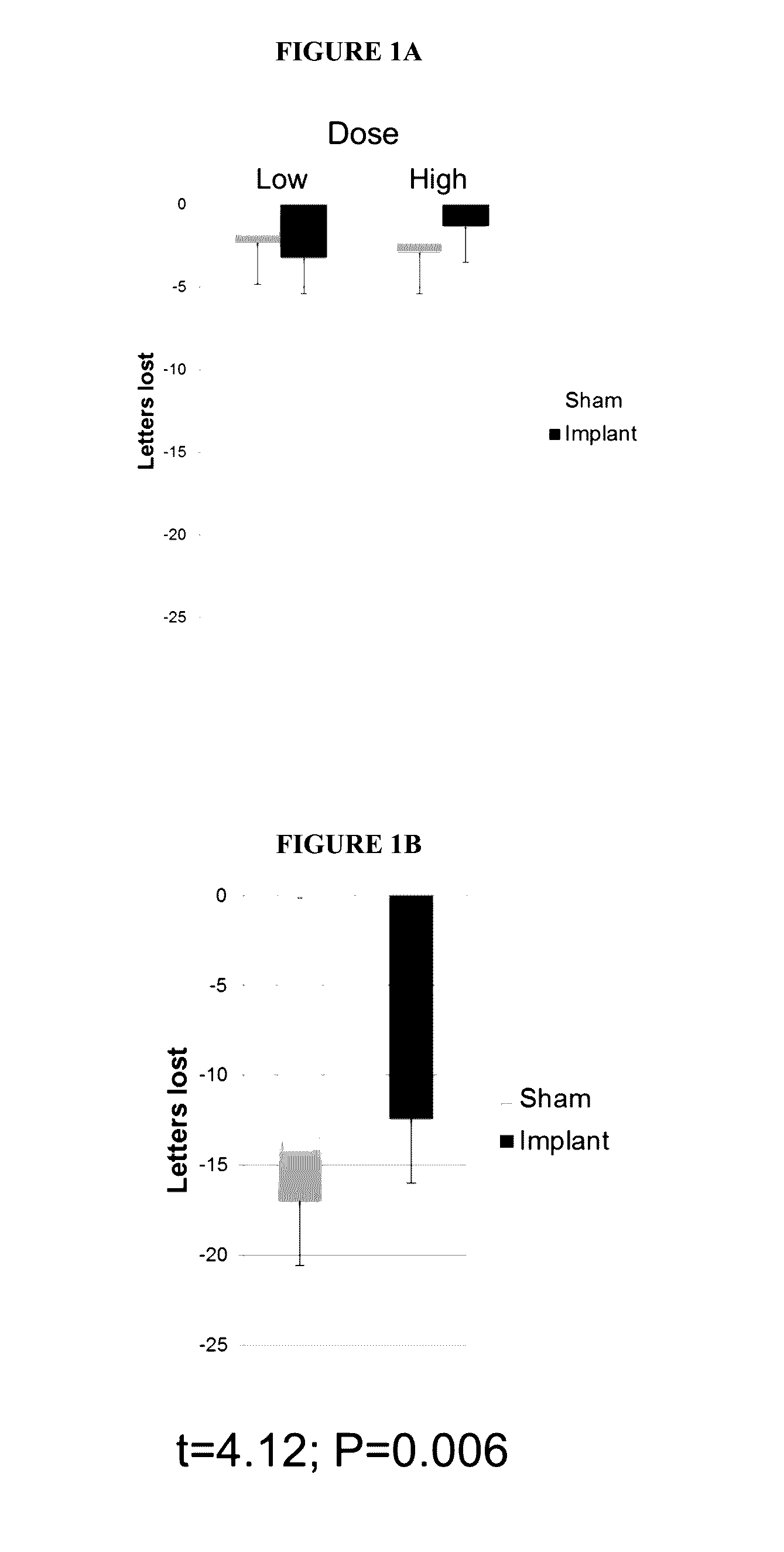
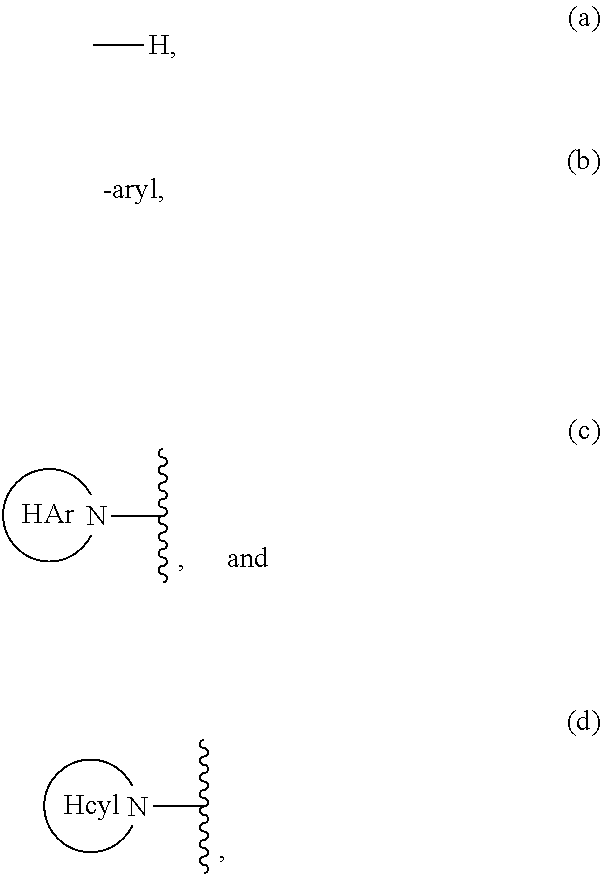
Patents
Literature
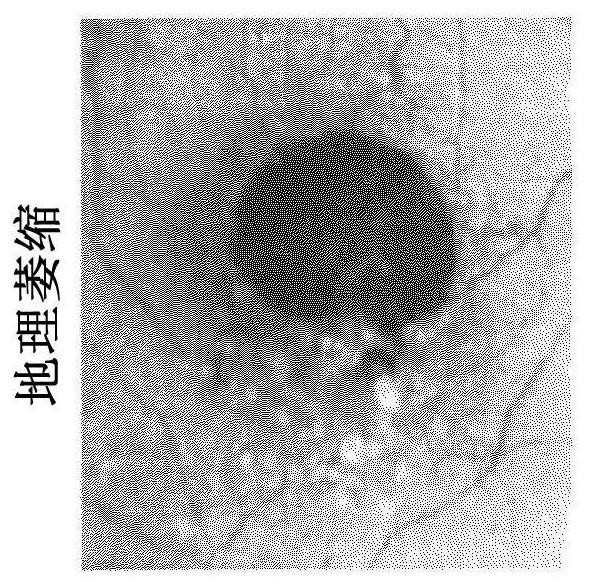
39 results about "Geographic atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
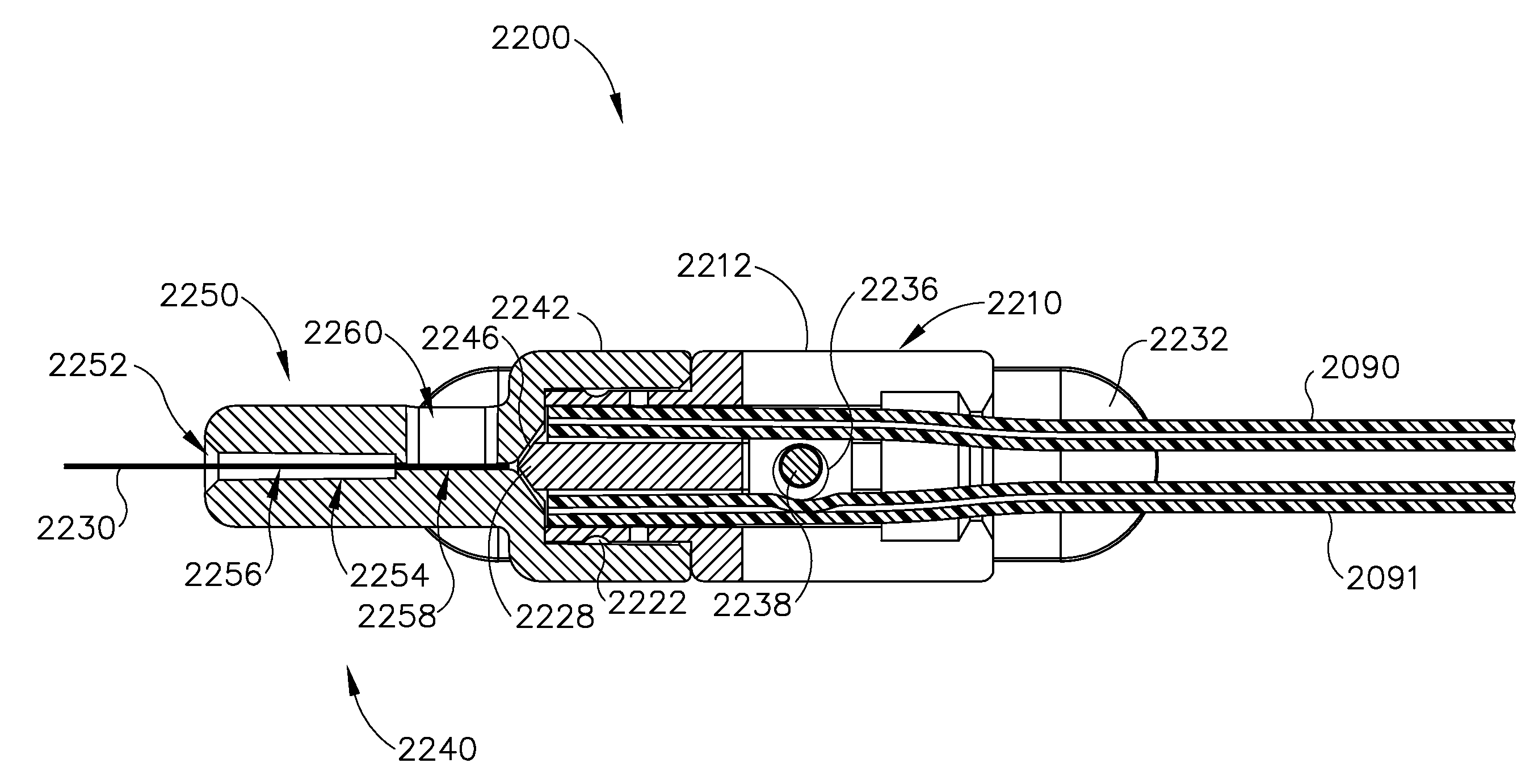
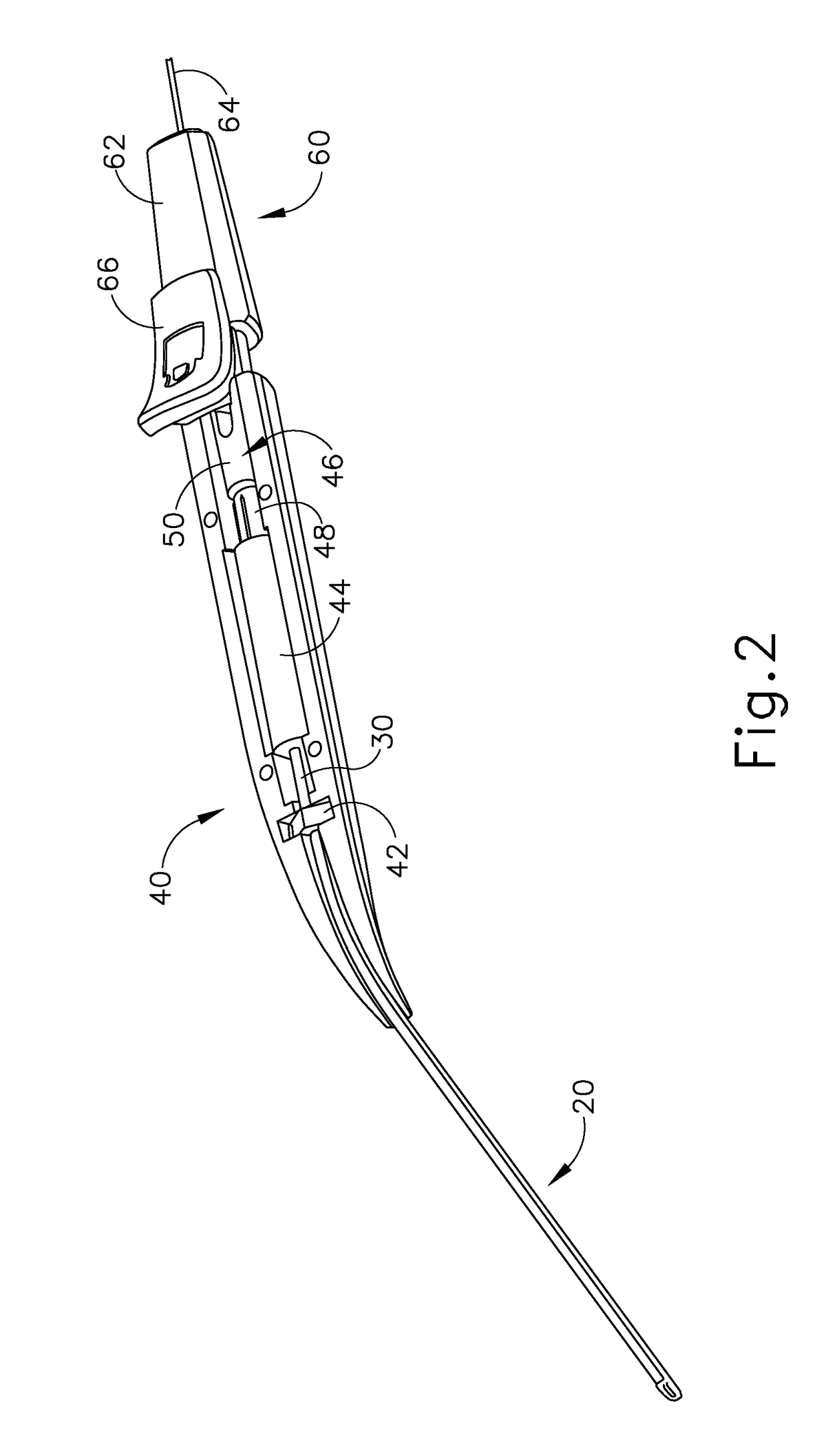
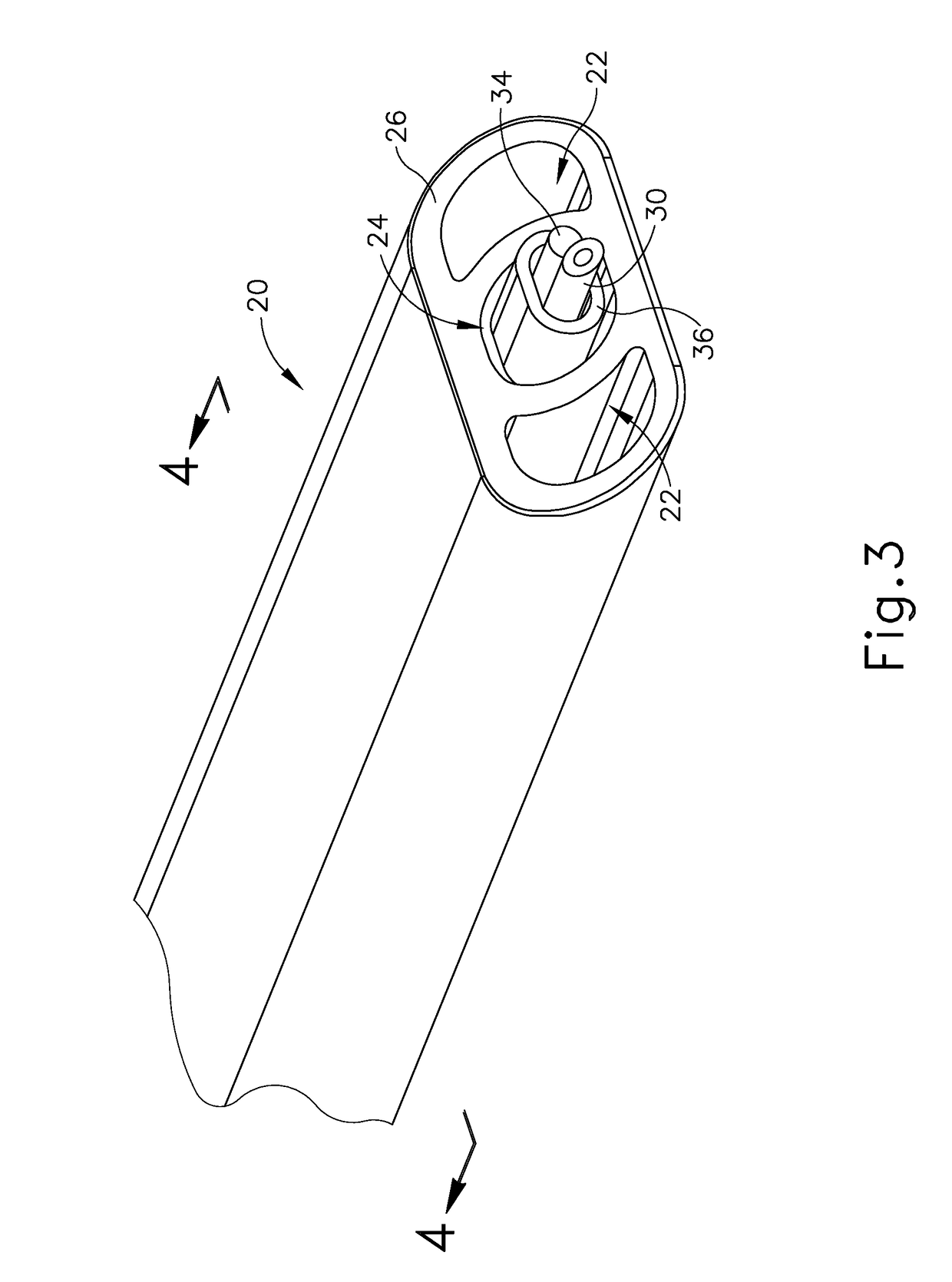
Method and apparatus for suprachoroidal administration of therapeutic agent
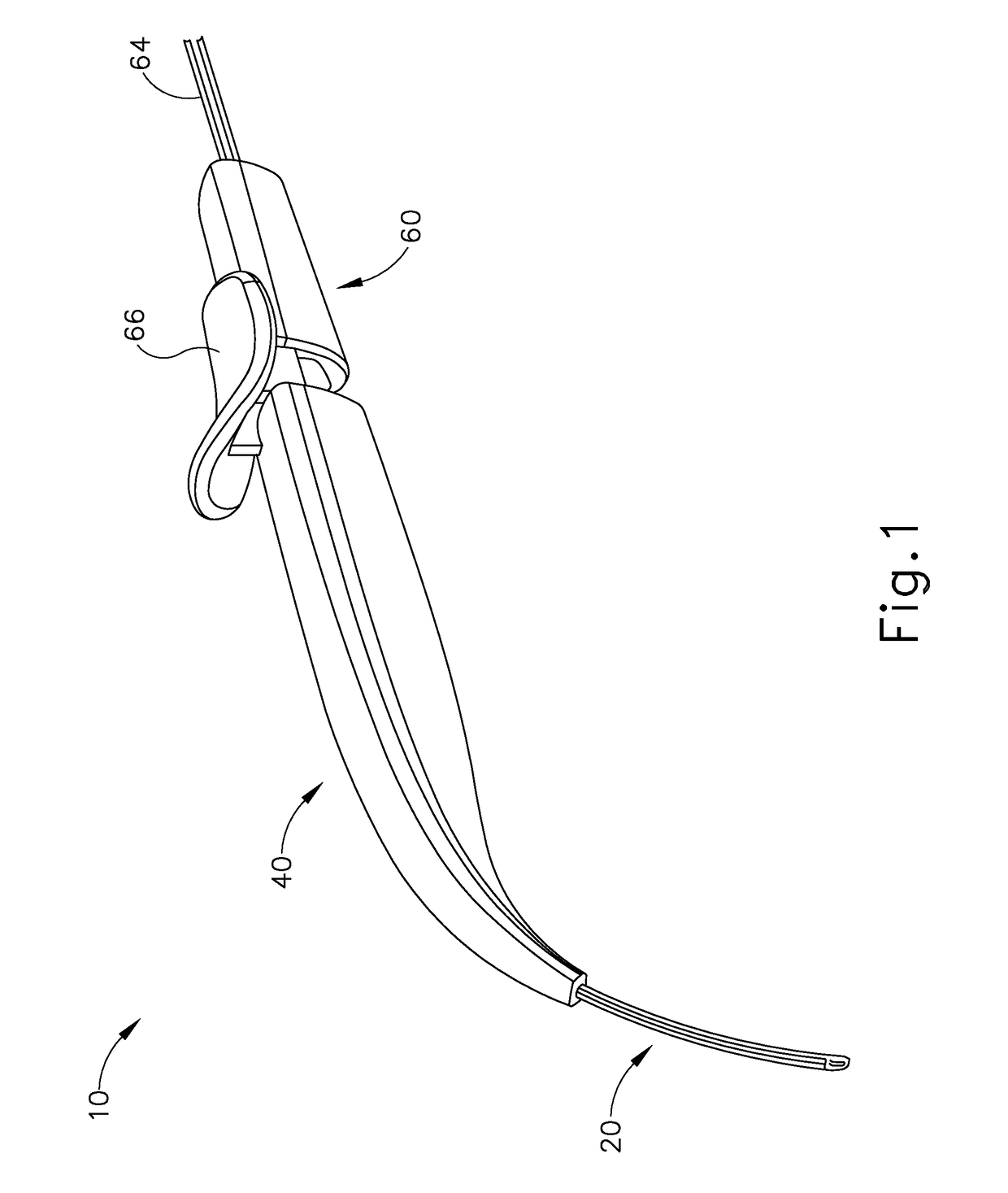
An apparatus for delivering therapeutic agent to an eye comprises a body, a cannula, a hollow needle, and an actuation assembly. The cannula extends distally from the body and is sized and configured to be insertable between a choroid and a sclera of a patient's eye. The actuation assembly is operable to actuate the needle relative to the cannula to thereby drive a distal portion of the needle along an exit axis that is obliquely oriented relative to the longitudinal axis of the cannula. The cannula may be inserted through a sclerotomy incision to position a distal end of the cannula at a posterior region of the eye, between the choroid and sclera. The needle may be advanced through the choroid to deliver the therapeutic agent adjacent to the potential space between the neurosensory retina and the retinal pigment epithelium layer, adjacent to the area of geographic atrophy.
Owner:GYROSCOPE THERAPEUTICS LTD +1
Topical delivery of therapeutic agents using cell-penetrating peptides for the treatment of age-related macular degeneration and other eye diseases
ActiveUS20190015521A1Hydroxy compound active ingredientsPeptide/protein ingredientsAntioxidantApolipoproteins E
The present disclosure provides therapeutic agents for the treatment of age-related macular degeneration (AMD) and other eye disorders. One or more therapeutic agents can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (including non-central GA and central GA) and neovascularization (including types 1, 2 and 3 NV). In some embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent, an antioxidant, an anti-inflammatory agent, a complement inhibitor, a neuroprotector or an anti-angiogenic agent, or any combination thereof. In certain embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent (e.g., an apolipoprotein mimetic or / and a statin). In some embodiments, the one or more therapeutic agents are mixed with, non-covalently associated with or covalently bonded to a cell-penetrating peptide (CPP), encapsulated in CPP-conjugated nanoparticles, micelles or liposomes, or modified (e.g., stapled, prenylated, lipidated or coupled to a small-molecule α-helix mimic) to acquire membrane-translocating ability. In certain embodiments, the one or more therapeutic agents are administered by eye drop.
Owner:MACREGEN INC
Use of cyclodextrins as an active ingredient for treating dry AMD and solubilizing drusen
The present invention is directed to the treatment of disorders involving the accumulation of drusen, such as dry age-related macular degeneration and geographic atrophy via administration of therapeutically effective amounts of at least one monomeric or polymeric cyclodextrin.
Owner:NOVARTIS AG
Geographic atrophy identification and measurement
Owner:KK TOPCON
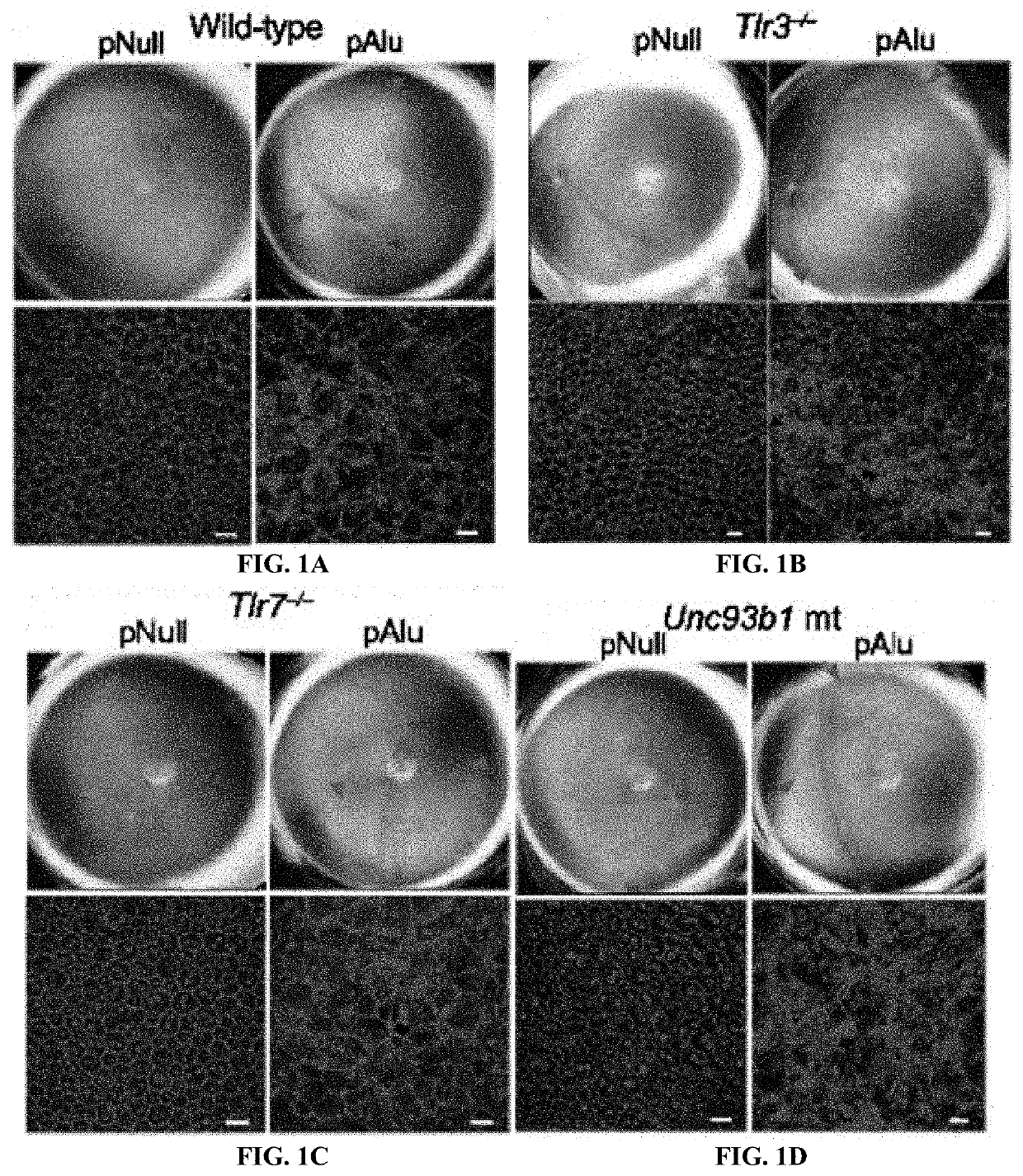
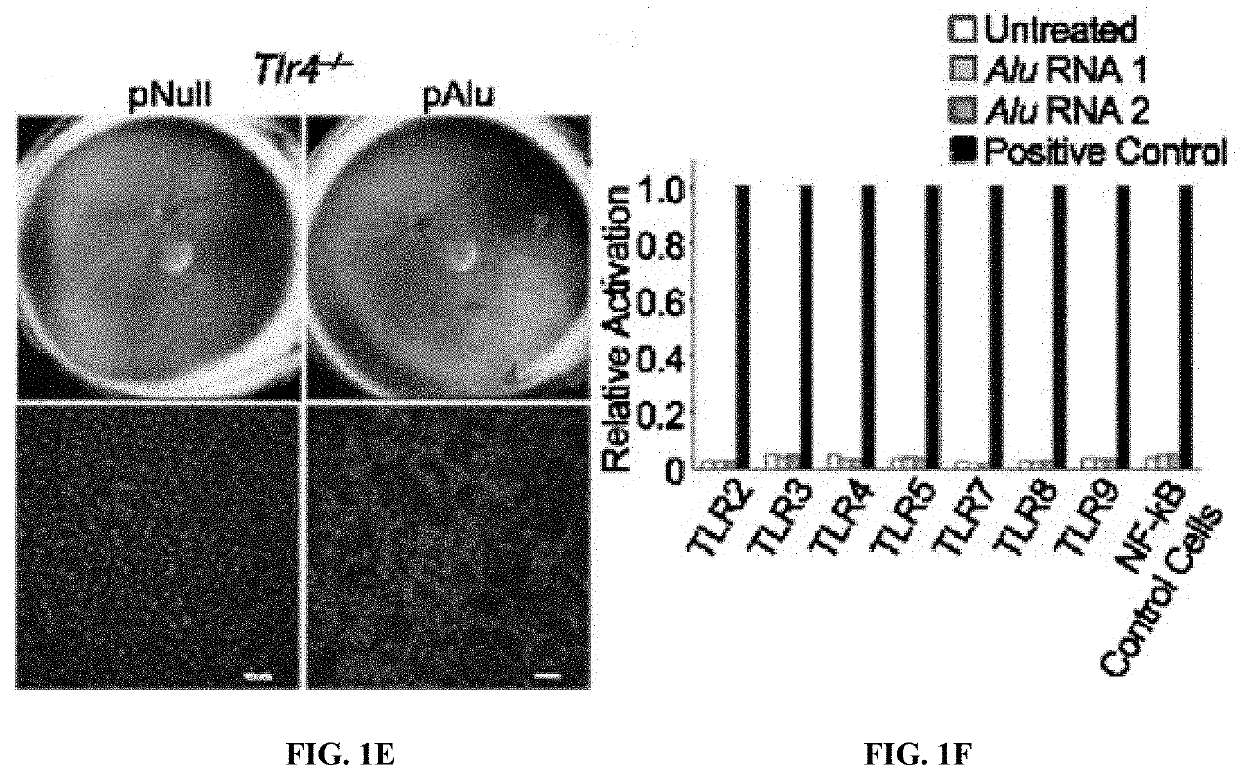
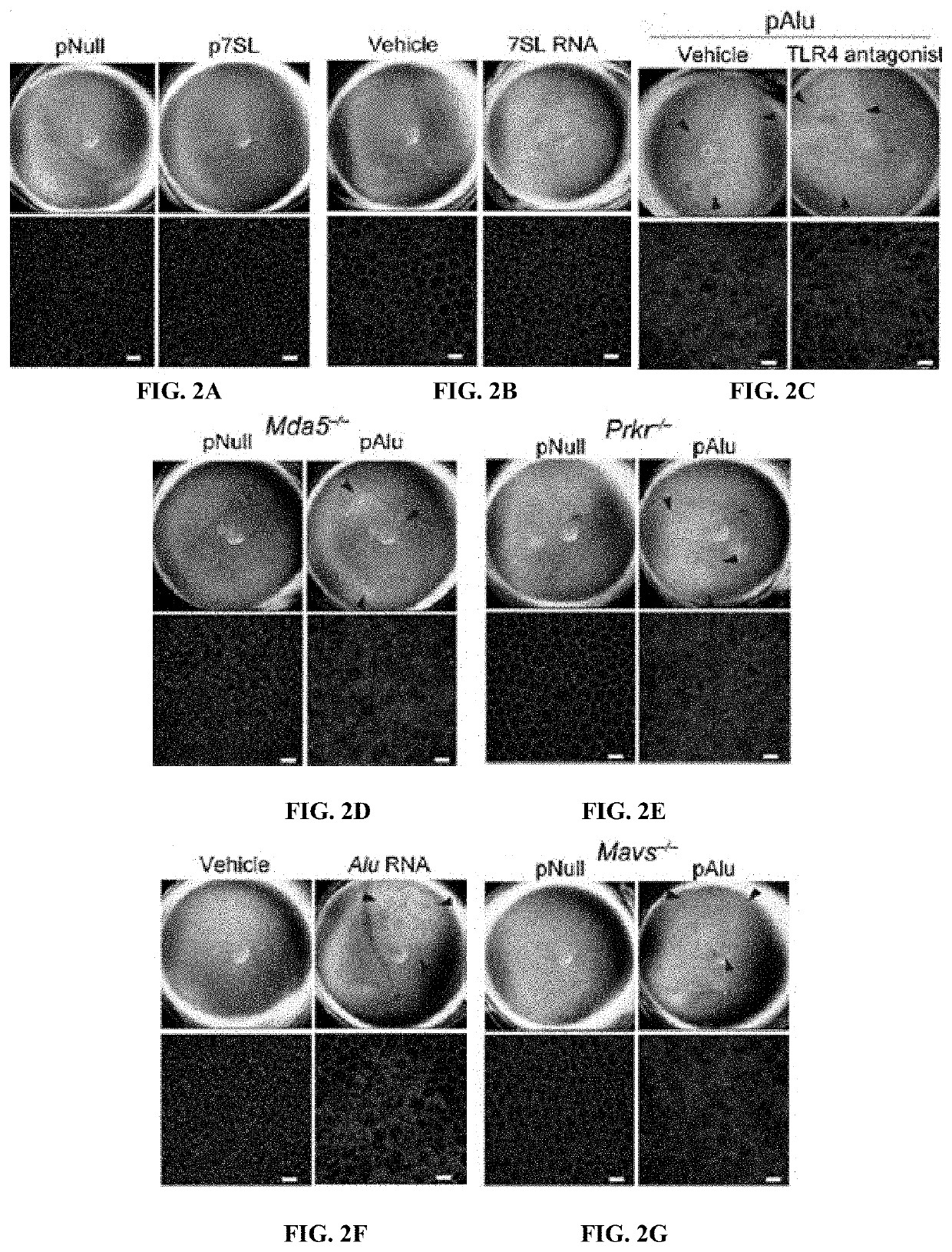
Method of inhibiting Alu RNA and therapeutic uses thereof
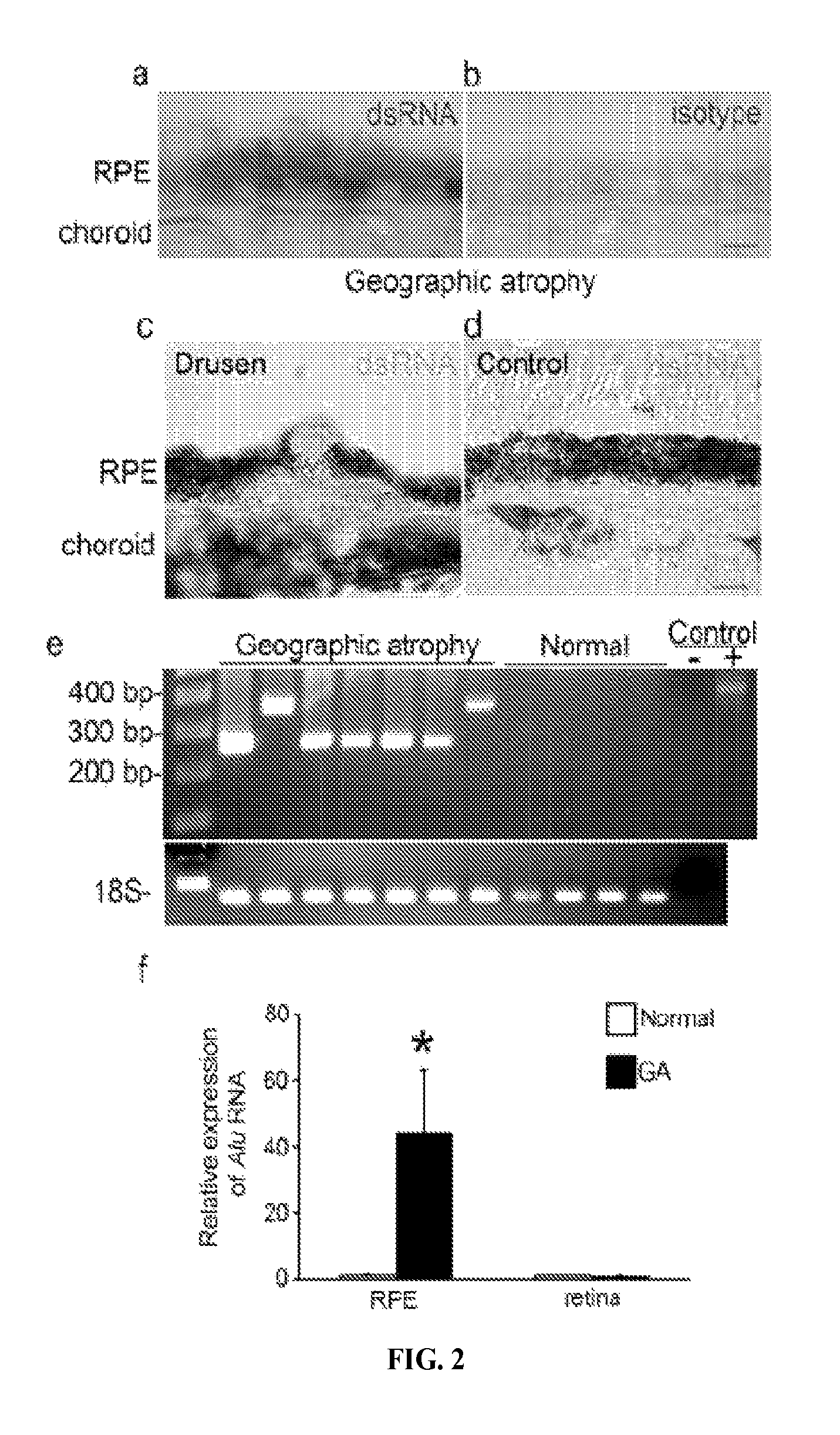
The presently-disclosed subject matter includes methods of identifying an Alu RNA inhibitor, and methods and compositions for inhibiting Alu RNA. Methods and compositions can be used for the treatment of geographic atrophy and other conditions of interest.
Owner:UNIV OF KENTUCKY RES FOUND
Treatment of age-related macular degeneration and other eye diseases with one or more therapeutic agents
InactiveUS20180296525A1Prevent and slow progressionSlow onsetSenses disorderPeptide/protein ingredientsDyslipidemiaAntioxidant
The present disclosure provides therapeutic agents for the treatment of age-related macular degeneration (AMD) and other eye disorders. One or more therapeutic agents can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (including non-central GA and central GA) and neovascularization (including types 1, 2 and 3 NV). In certain embodiments, an anti-dyslipidemic agent (e.g., an apolipoprotein mimetic and / or a statin) is used alone to treat or slow the progression of atrophic AMD (including early AMD and intermediate AMD), and / or to prevent or delay the onset of AMD, advanced AMD and / or neovascular AMD. In further embodiments, two or more therapeutic agents (e.g., any combinations of an anti-dyslipidemic agent, an antioxidant, an anti-inflammatory agent, a complement inhibitor, a neuroprotector and an anti-angiogenic agent) that target multiple underlying factors of AMD (e.g., formation of lipid-rich deposits, oxidative stress, local inflammation, cell death and neovascularization) are used to treat or slow the progression of atrophic AMD (including non-central GA and central GA) or neovascular AMD (including types 1, 2 and 3 NV), and / or to prevent or delay the onset of AMD, advanced AMD and / or neovascular AMD.
Owner:MACREGEN INC
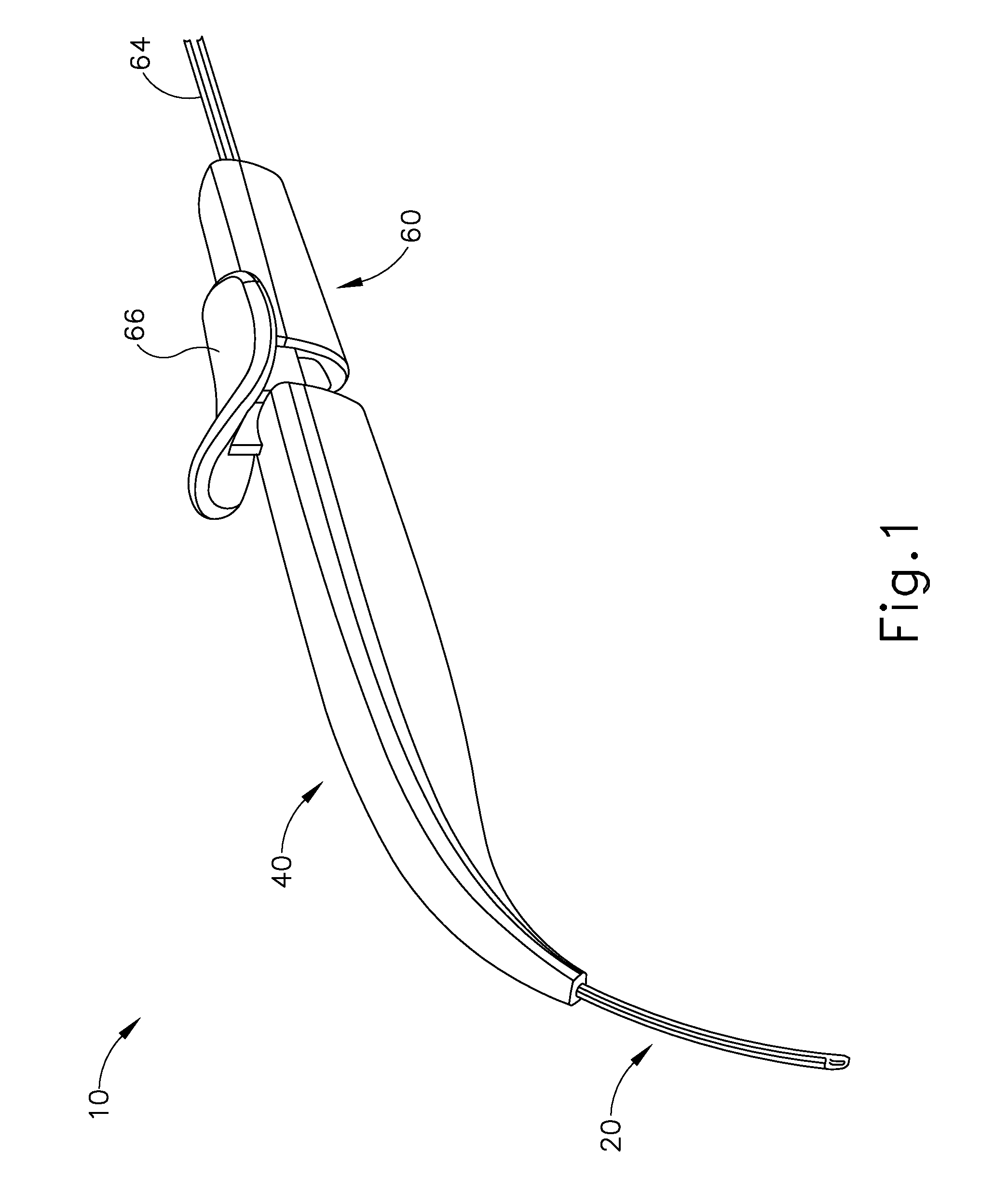
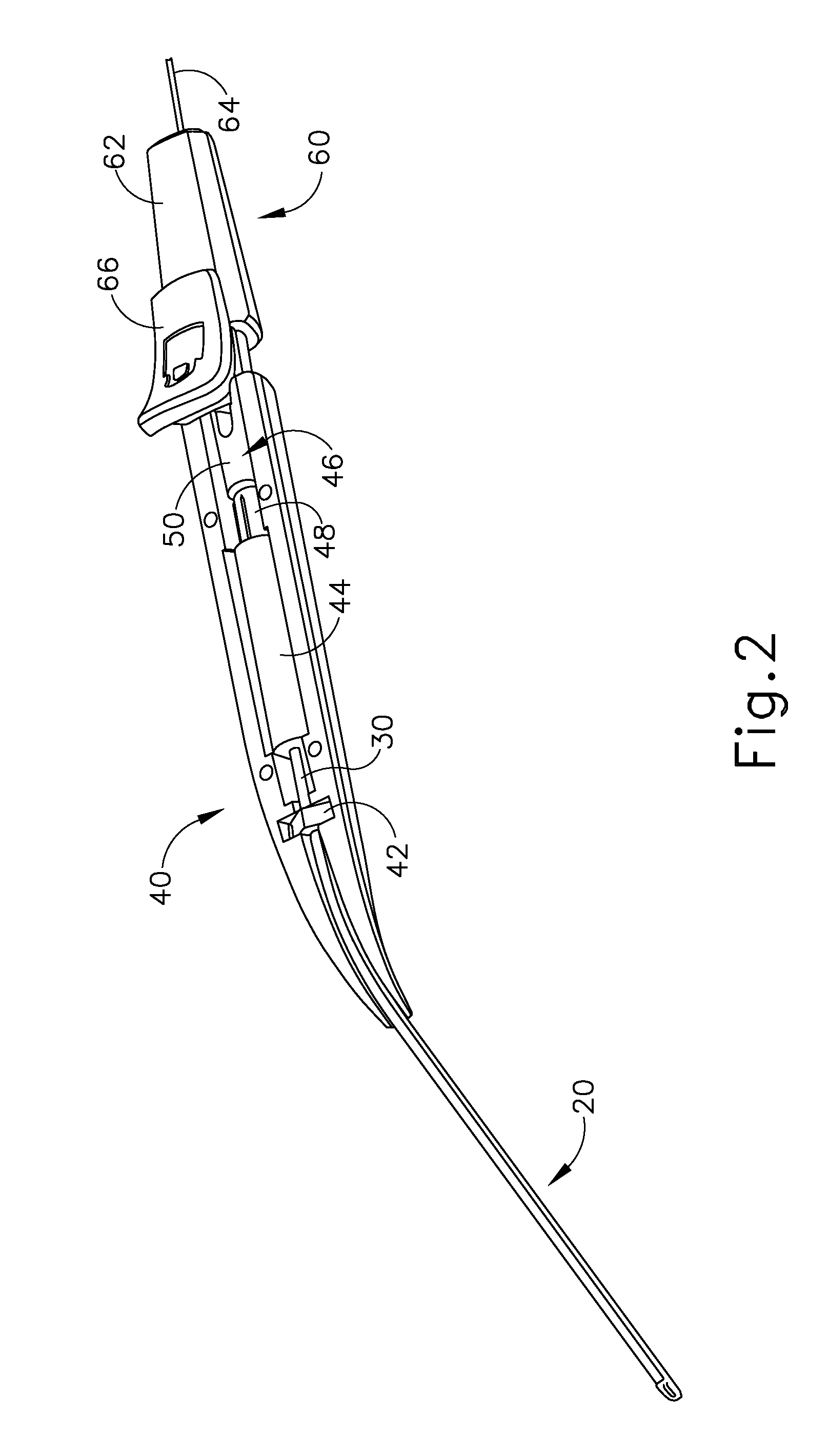
Method and apparatus for subretinal administration of therapeutic agent
Owner:GYROSCOPE THERAPEUTICS LTD +1
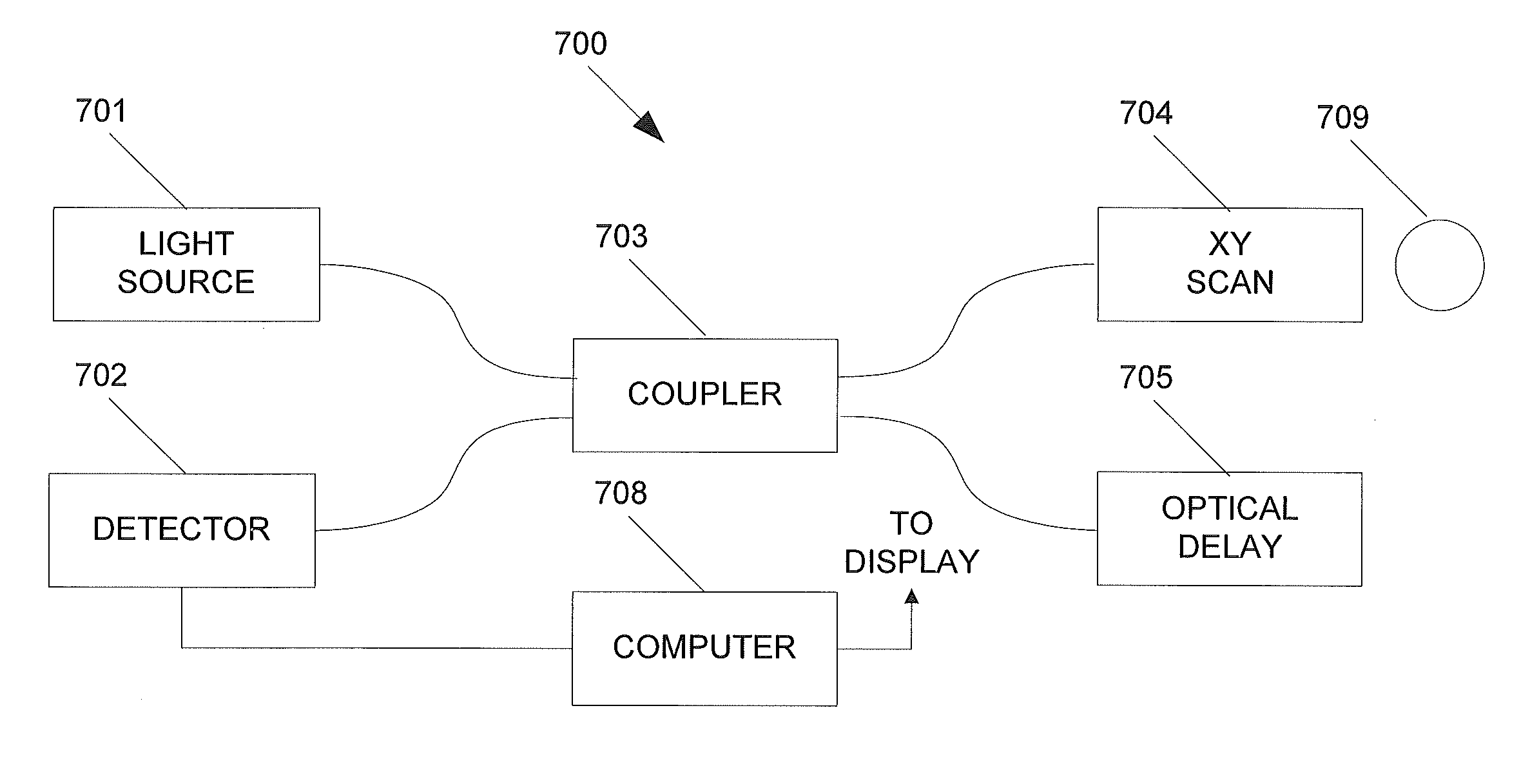
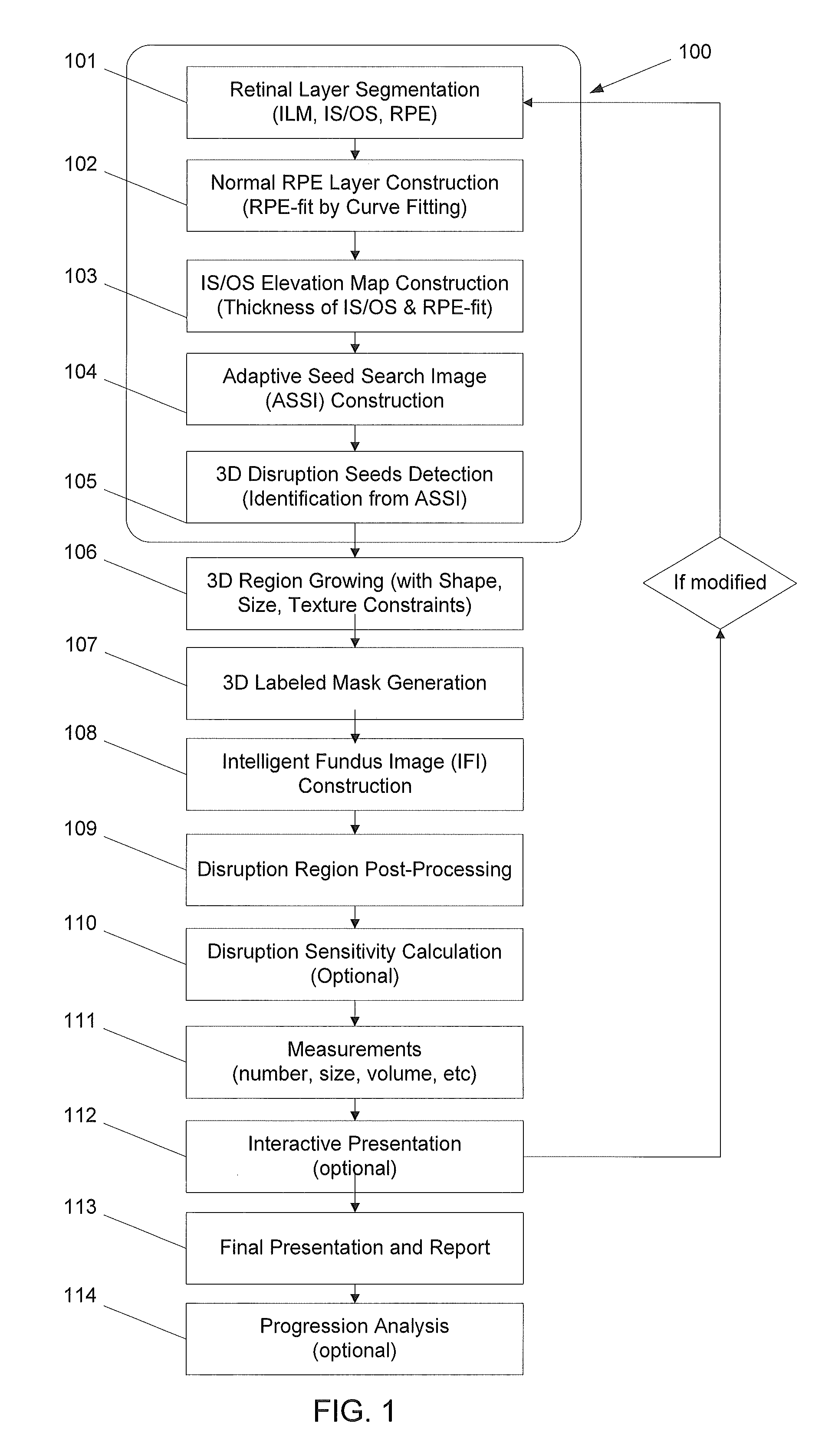
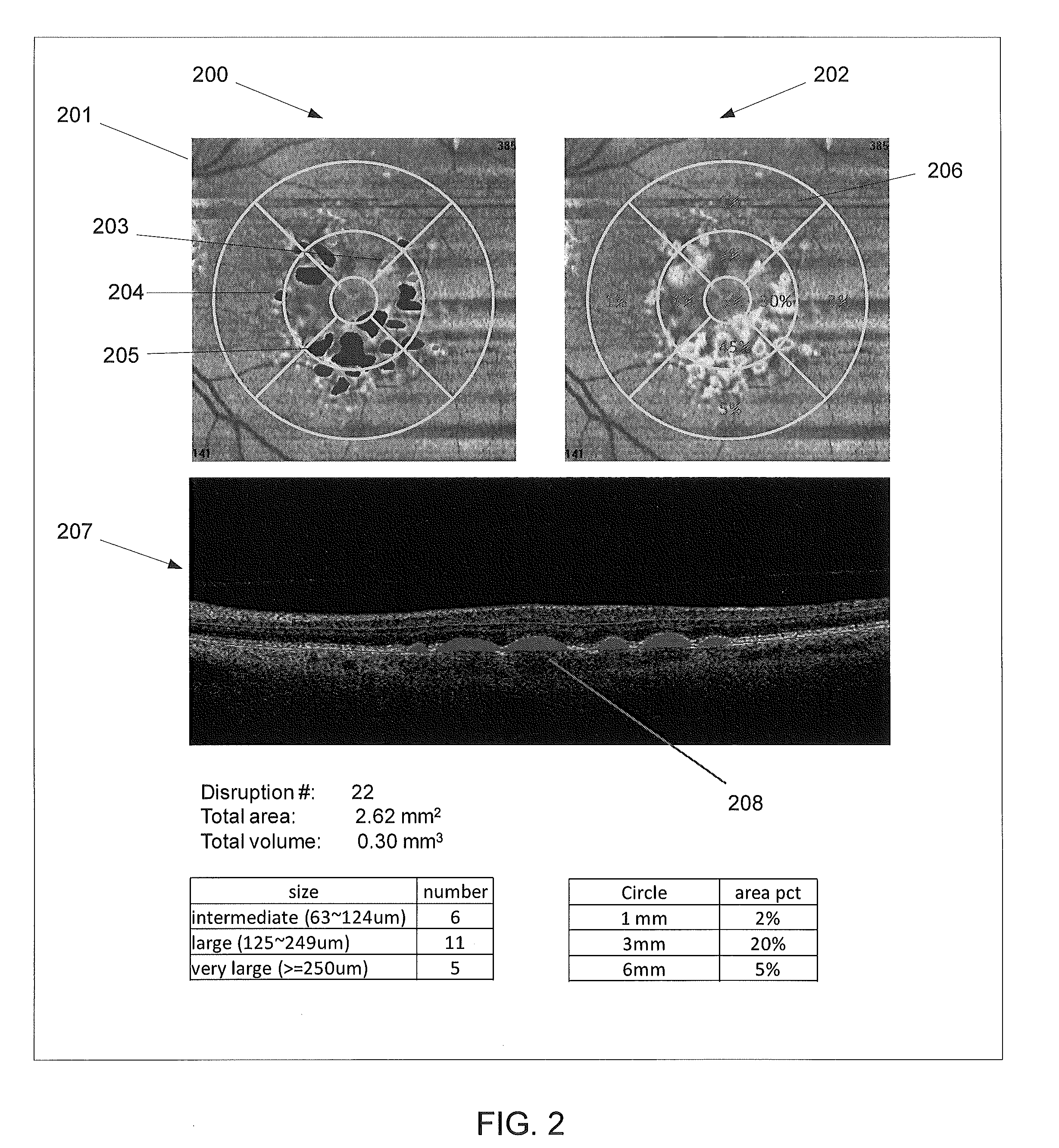
3D retinal disruptions detection using optical coherence tomography
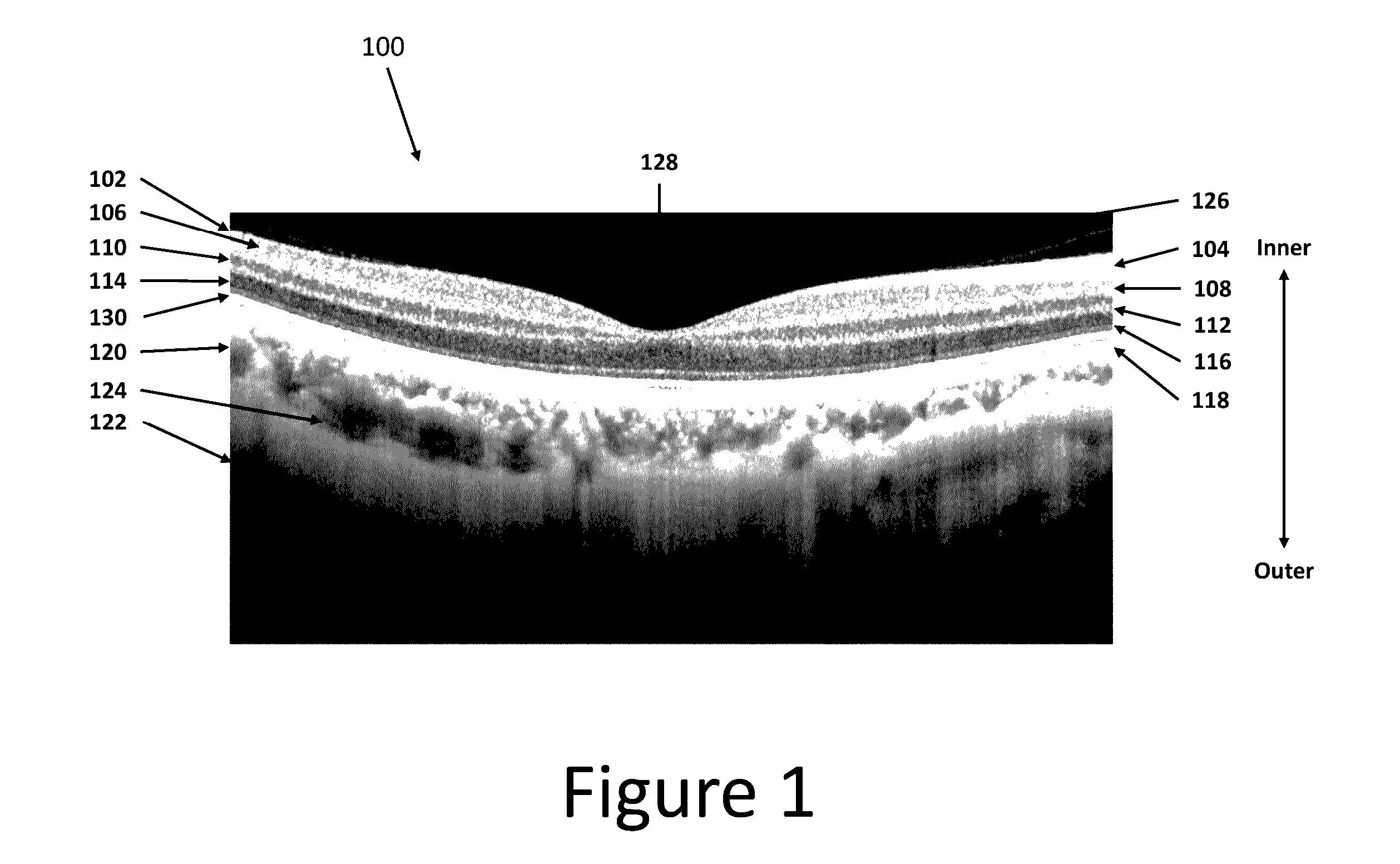
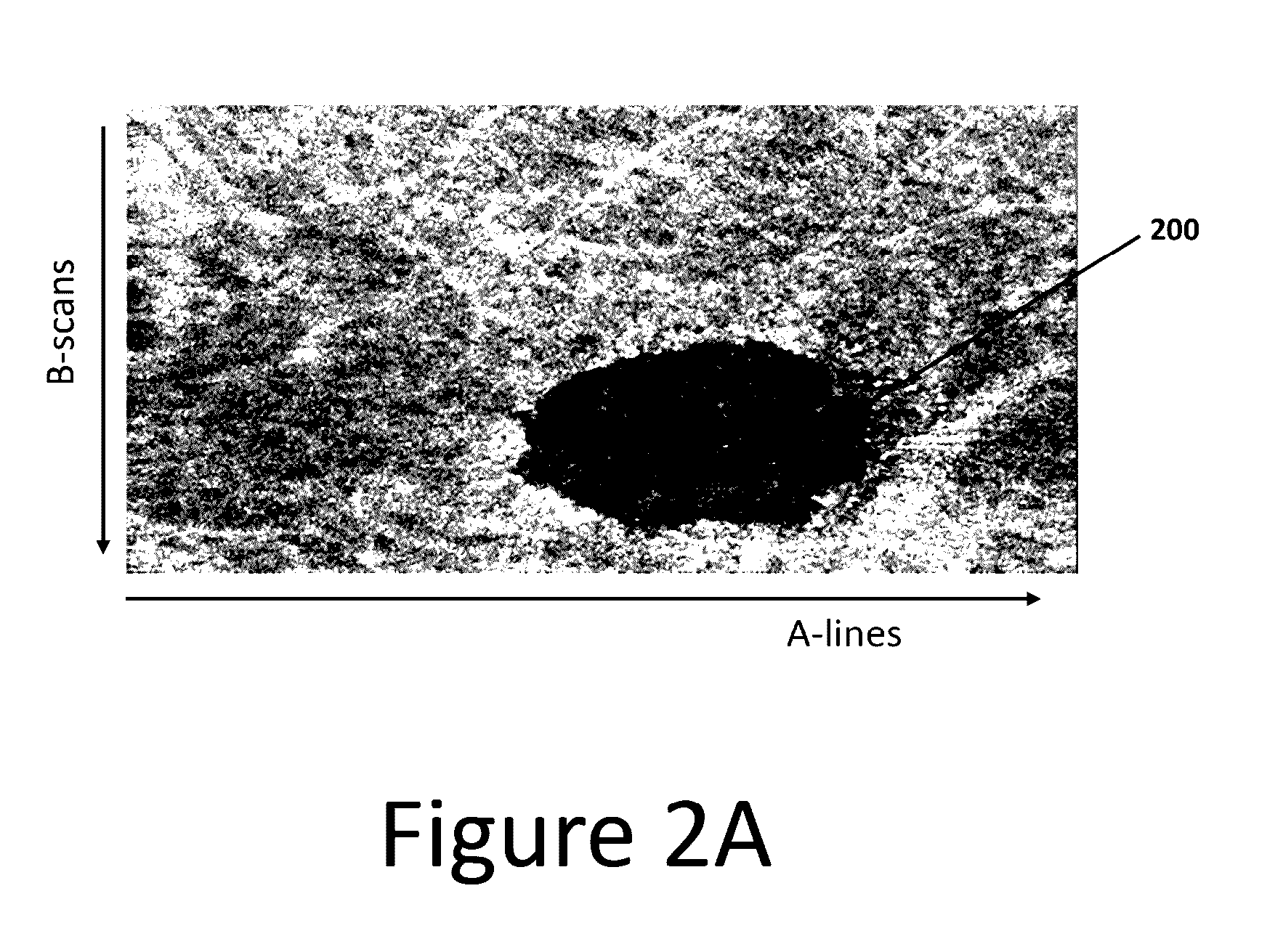
System and method for 3D retinal disruption / elevation detection, measurement and presentation using Optical Coherence Tomography (OCT) are provided. The present invention is capable of detecting and measuring the abnormal changes of retinal layers (retinal disruptions), caused by retinal diseases, such as hard drusen, soft drusen, Pigment Epithelium Detachment (PED), Choroidal Neovascularization (CNV), Geographic Atrophy (GA), intra retinal fluid space, and exudates etc. The presentations of the results are provided with quantitative measurements of disruptions in retina and can be used for diagnosis and treatment of retinal diseases.
Owner:OPTOVUE
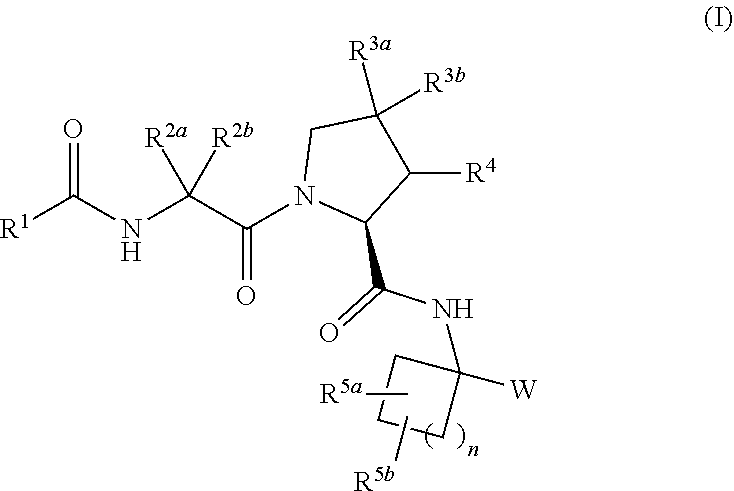
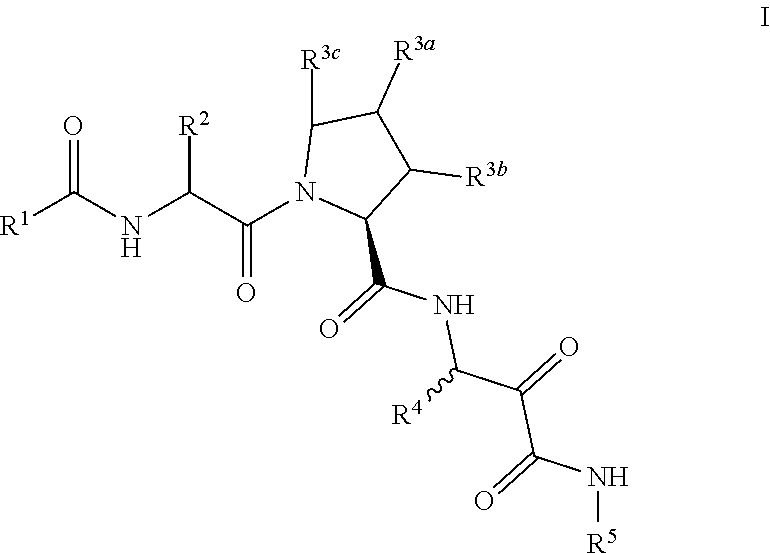
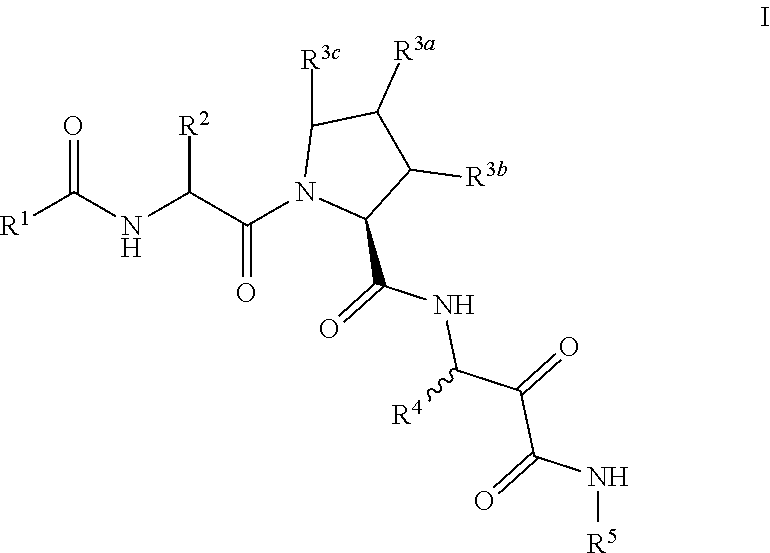
Carbocyclic prolinamide derivatives
This invention is directed to novel carbocyclic prolinamide derivatives of Formula (I), and pharmaceutically acceptable salts, solvates, solvates of the salt and prodrugs thereof, useful in the prevention (e.g., delaying the onset of or reducing the risk of developing) and treatment (e.g., controlling, alleviating, or slowing the progression of) of age-related macular degeneration (AMD) and related diseases of the eye. These diseases include dry-AMD, wet-AMD, geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells. The invention disclosed herein is further directed to methods of prevention, slowing the progress of, and treatment of dry-AMD, wet-AMD, and geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells, comprising: administration of a therapeutically effective amount of compound of the invention. The compounds of the invention are inhibitors of HTRA1. Thus, the compounds of the invention are useful in the prevention and treatment of a wide range of diseases mediated (in whole or in part) by HTRA1. The compounds of the invention are also useful for inhibiting HTRA1 protease activity in an eye or locus of an arthritis or related condition.
Owner:ORION OPHTHALMOLOGY LLC
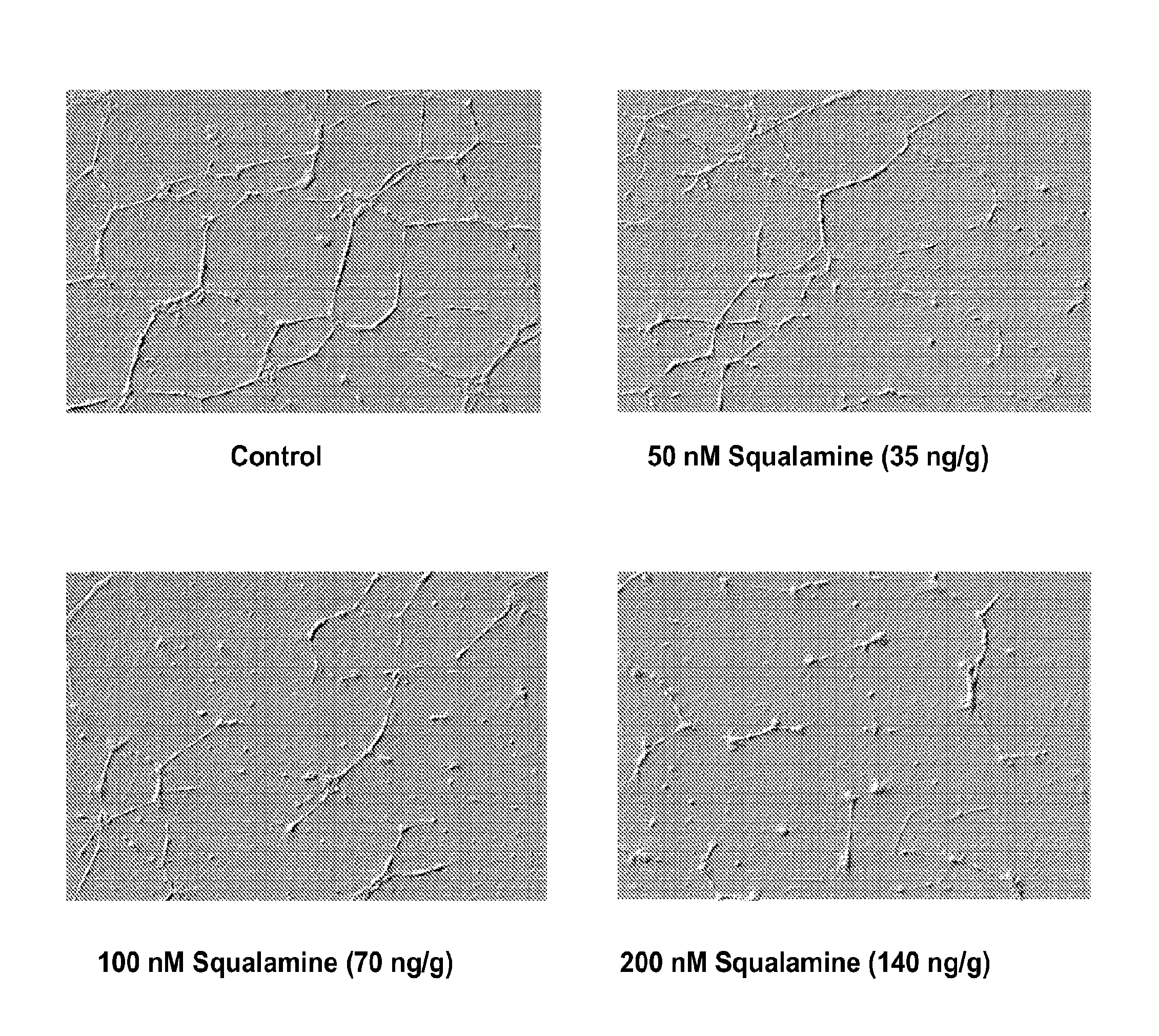
Ophthalmic Formulations of Squalamine
The invention relates to ophthalmic formulations of squalamine or its pharmaceutically acceptable salts for the treatment of conditions of the eye such as, for example, wet age-related macular degeneration (wet AMD), choroidal neovascularization, retinopathy, dry age-related macular degeneration (dry AMD), polypoidal choroidal vasculopathy, neovascularization following ocular surgery, macular edema, retinal venous occlusion, subchoroidal neovascularization, retinal epithelial detachment, pterygum or foveal geographic atrophy of the retinal pigment epithelium.
Owner:OXEA GMBH +1
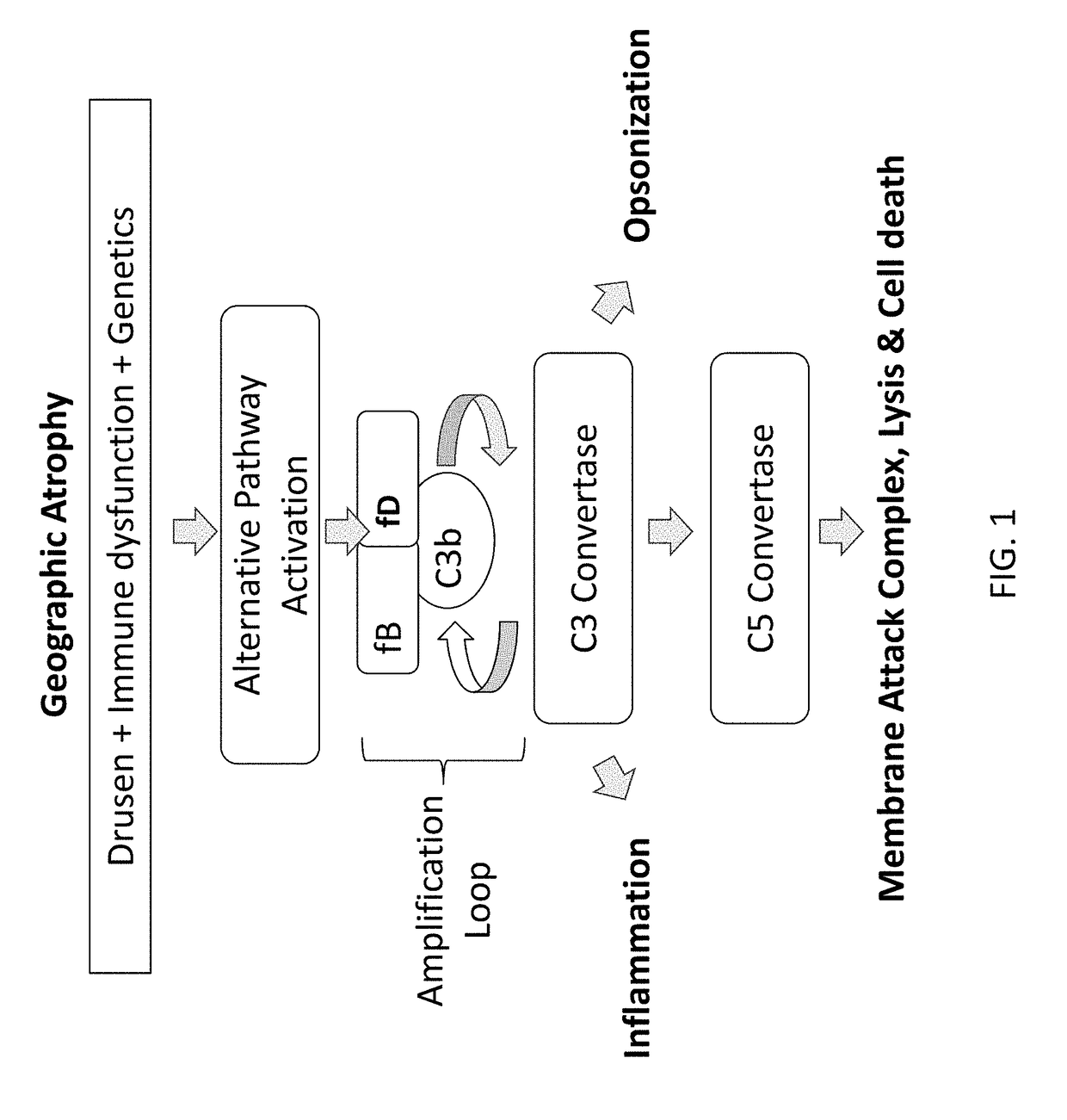
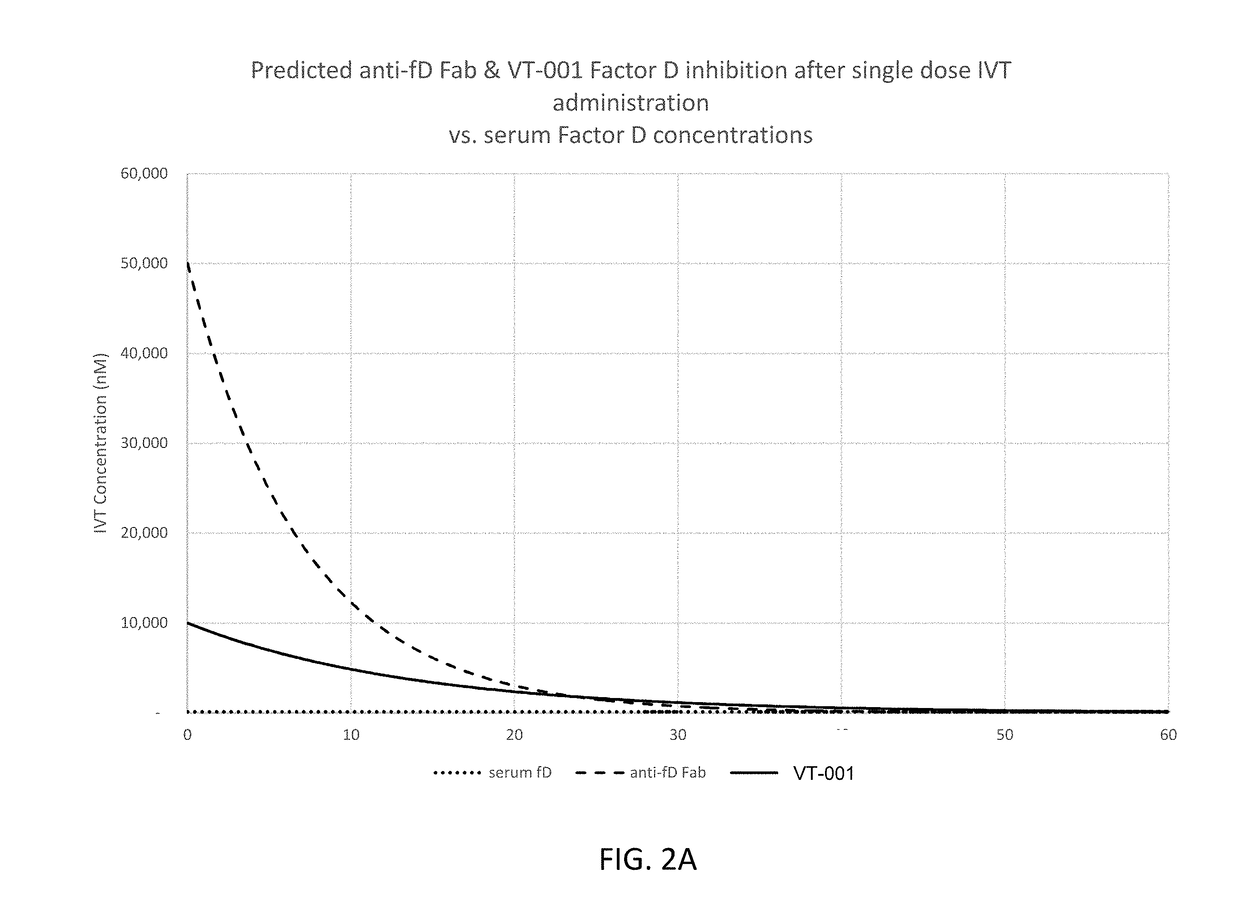
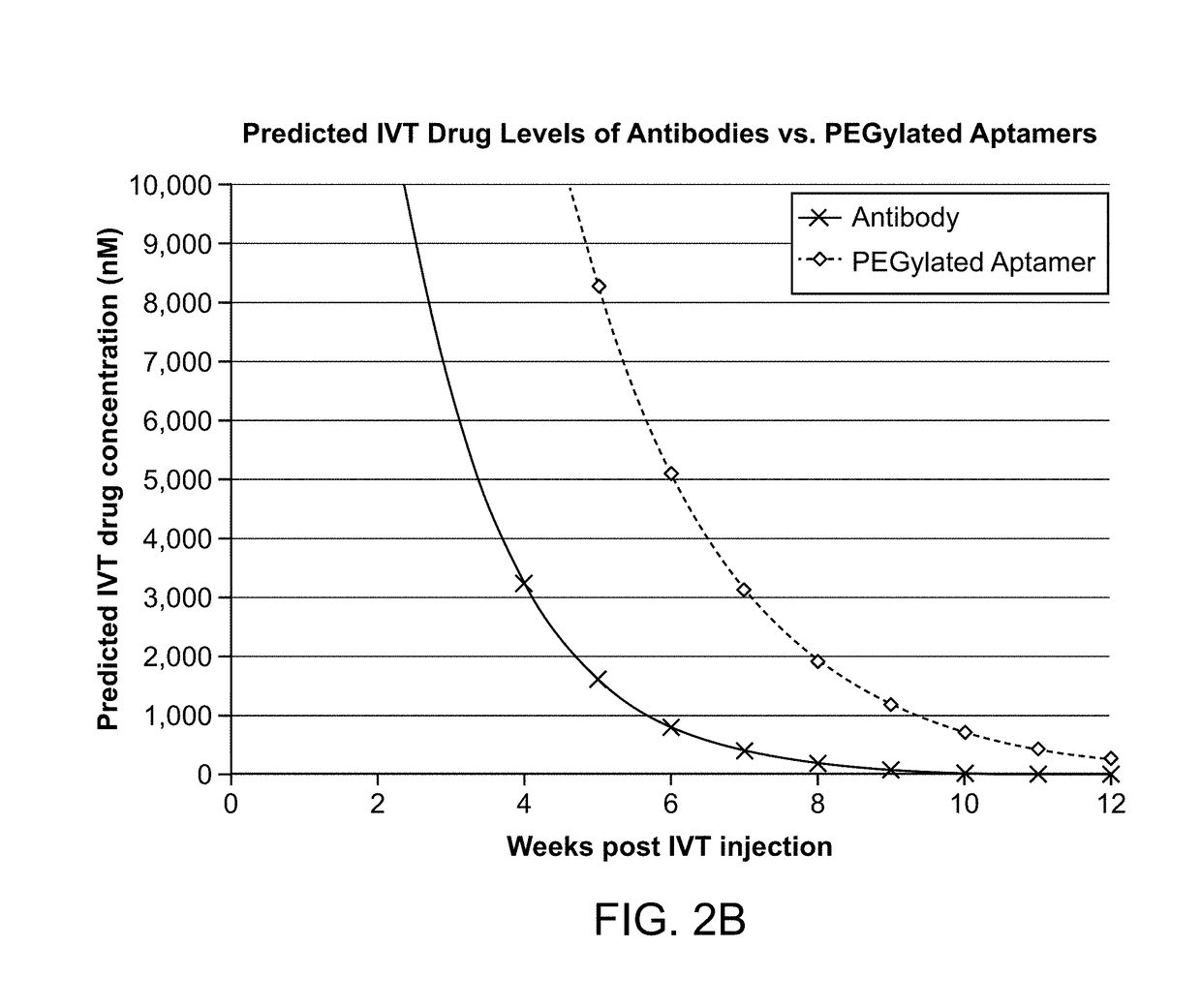
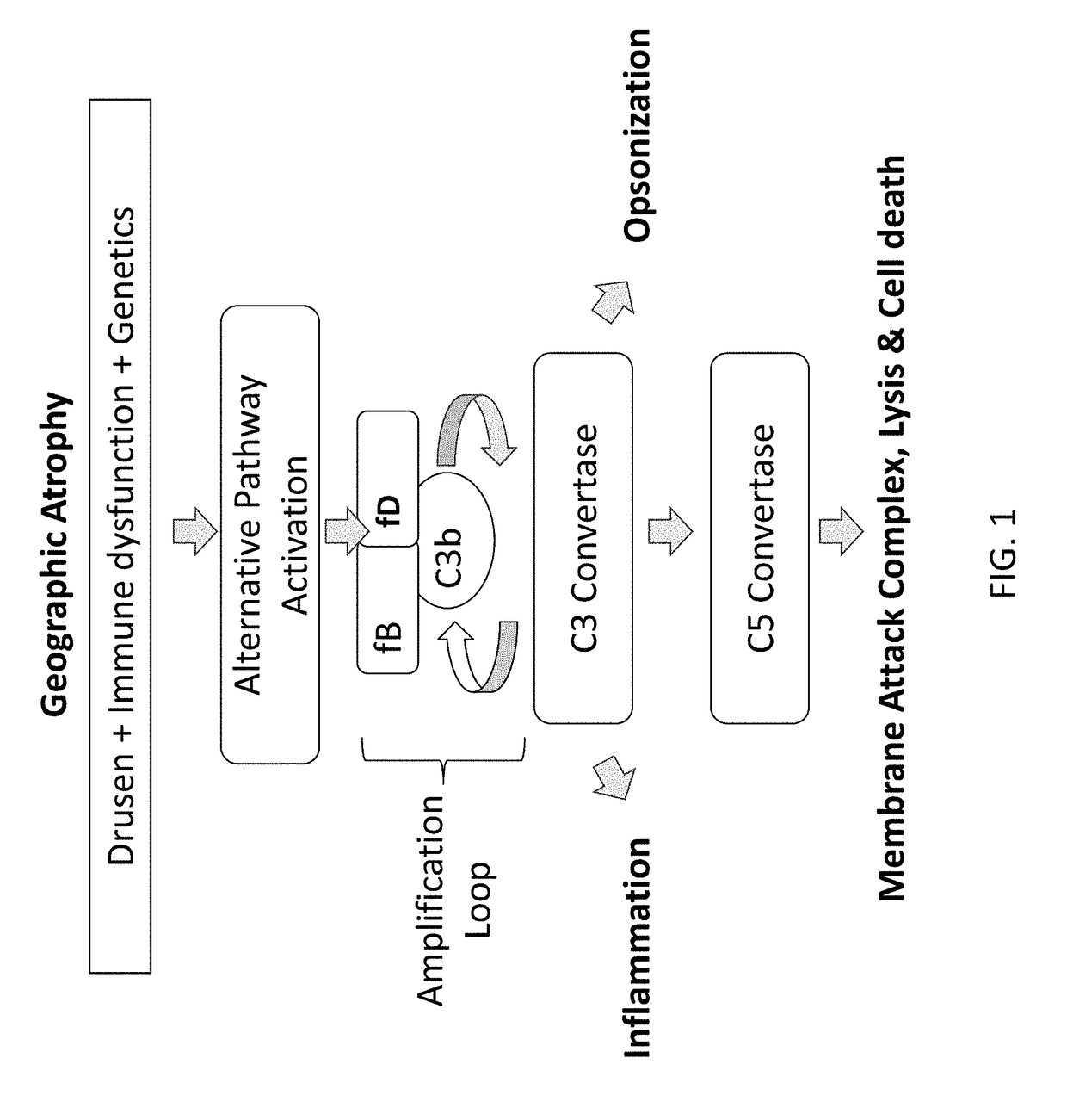
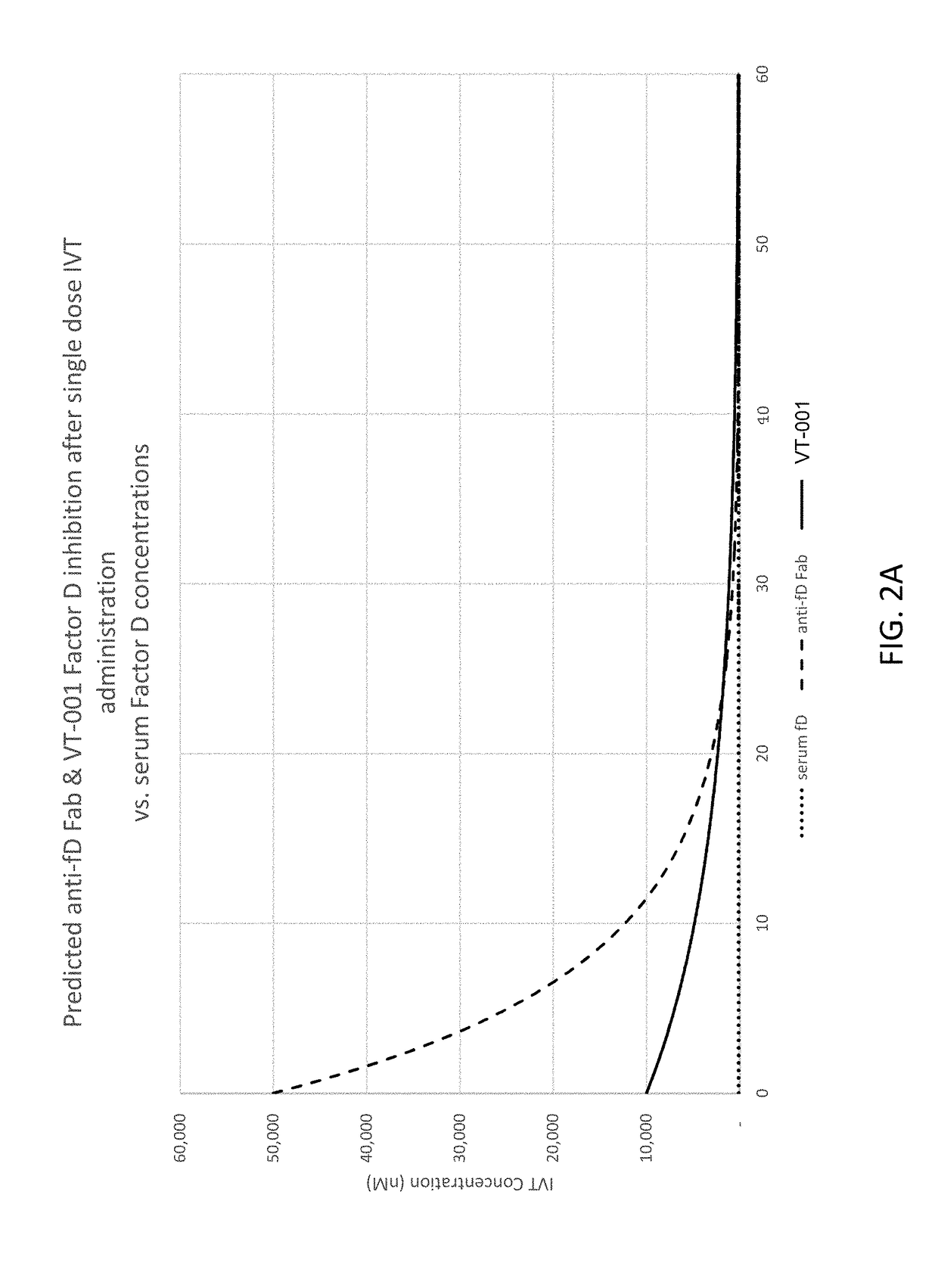
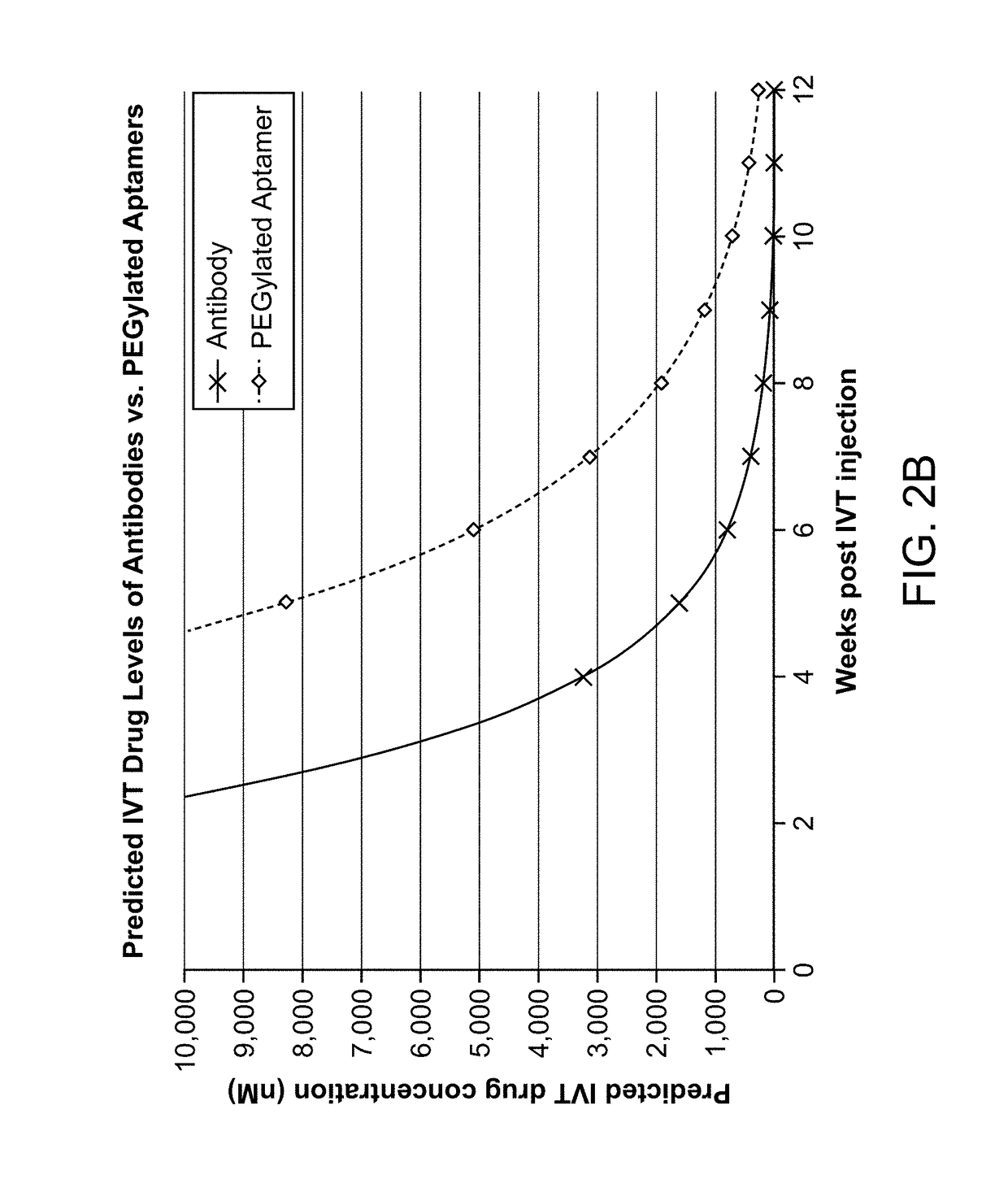
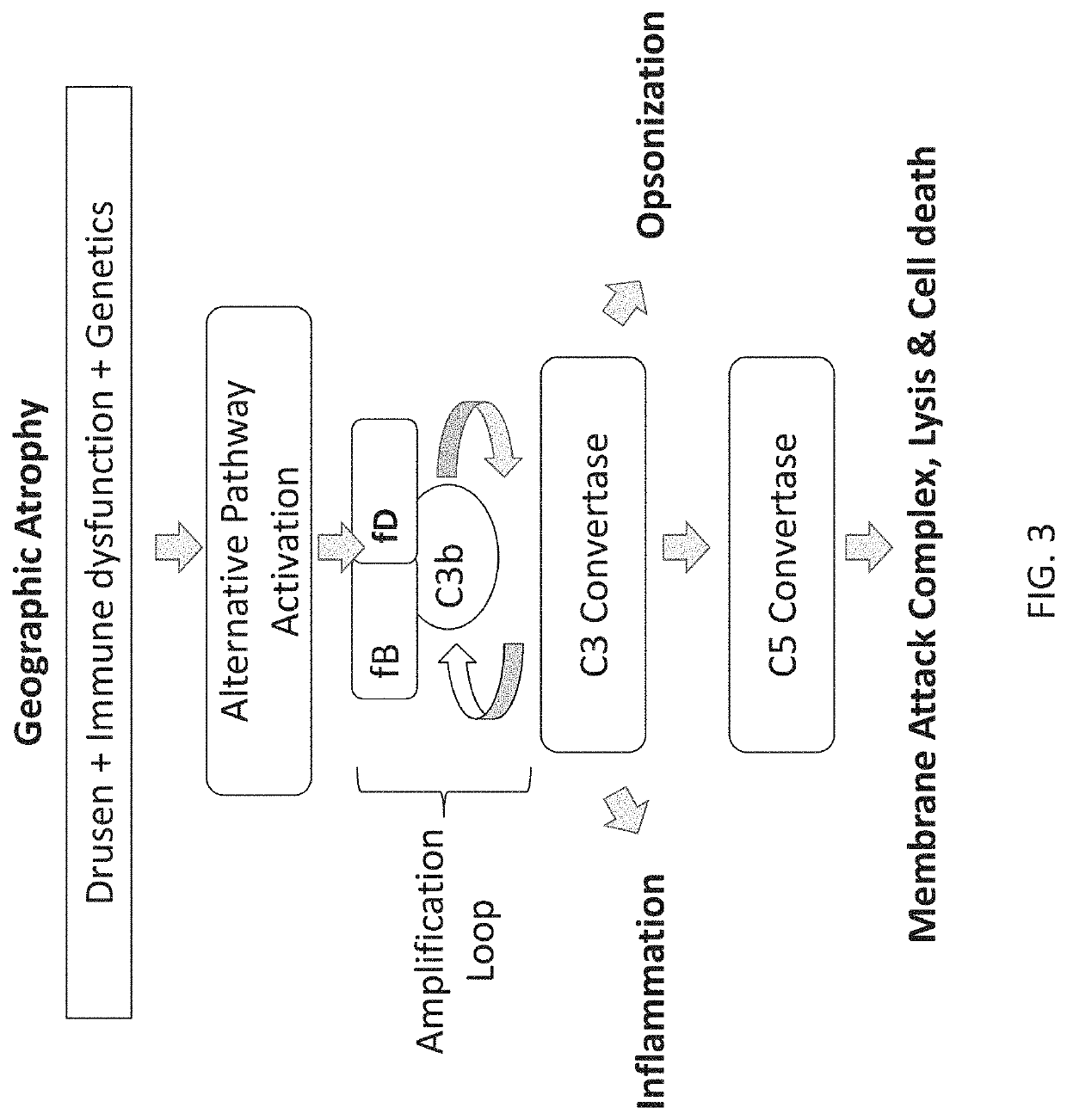
Compositions and methods for inhibiting factor d
ActiveUS20180051287A1Inhibit functioningOrganic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Biomarkers Useful in the Treatment of Subjects Having Disease of the Eye
ActiveUS20170370945A1Improve the level ofIncreasing and decreasing oxidative stressSenses disorderAntinoxious agentsRetinitis pigmentosaGeographic atrophy
The present invention provides biomarkers of oxidative stress in subjects with retinitis pigmentosa, age-related macular degeneration, diabetic retinopathy, Fuchs' dystrophy, diabetic macular edema (DME), geographic atrophy, Stargardt's disease, or retinal vein occlusion (RVO), and their use in identifying subjects in need of treatment and methods for staging the severity of the disease.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method of inhibiting alu RNA and therapeutic uses thereof
The presently-disclosed subject matter includes methods of identifying an Alu RNA inhibitor, and methods and compositions for inhibiting Alu RNA. Methods and compositions can be used for the treatment of geographic atrophy and other conditions of interest.
Owner:UNIV OF KENTUCKY RES FOUND
Compositions and methods for inhibiting Factor D
ActiveUS10174325B2Organic active ingredientsDrug compositionsGeographic atrophyDry age-related macular degeneration
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease.
Owner:396419 B C LTD
Use of Encapsulated Cell Therapy for Treatment of Ophthalmic Disorders
ActiveUS20180055766A1Promote regenerationImprove acuitySenses disorderPeptide/protein ingredientsDiseaseGlaucoma
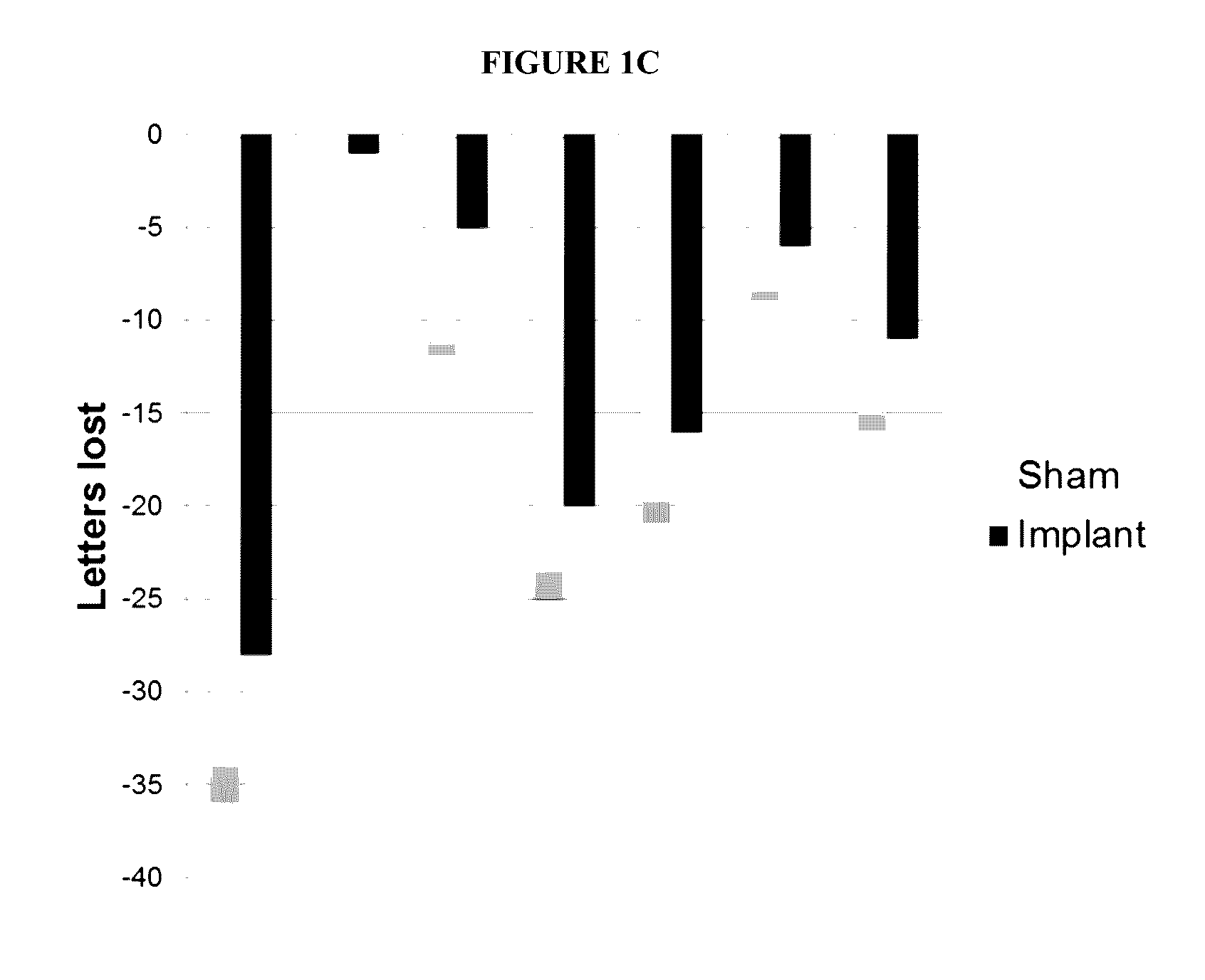
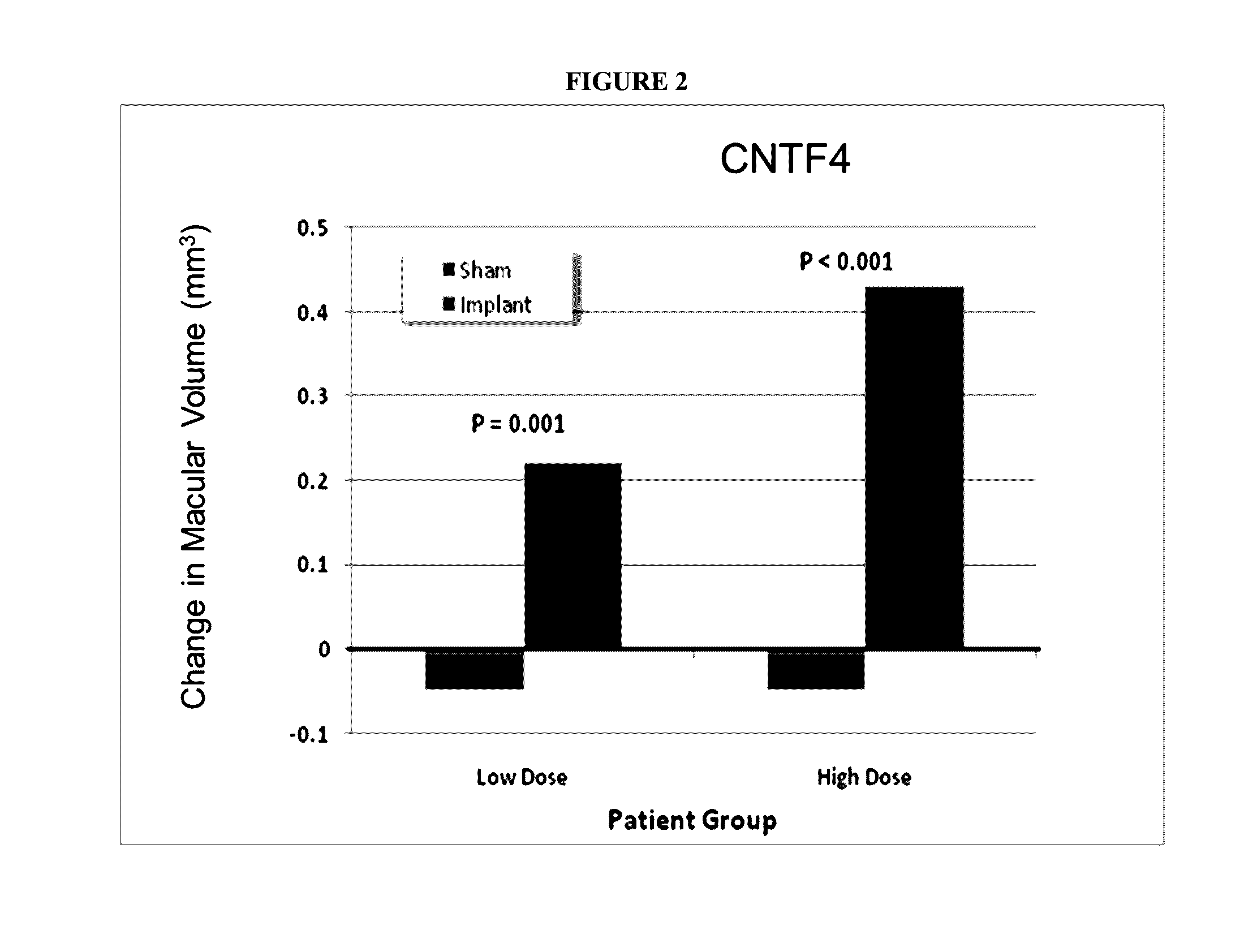
Described herein are methods and devices for the long term treatment of ophthalmic disorders. Also disclosed are encapsulated cell therapy (ECT) devices that secrete a biologically active molecule and methods for using the same for the treatment of various kinds of ophthalmic disorders, including retinitis pigmentosa, geographic atrophy (dry age-related macular degeneration), glaucoma and / or macular telangiectasia.
Owner:NEUROTECH USA
Topical delivery of therapeutic agents using cell-penetrating peptides for the treatment of age-related macular degeneration and other eye diseases
ActiveUS10905770B2Peptide/protein ingredientsHydroxy compound active ingredientsDyslipidemiaPrenylation
The present disclosure provides therapeutic agents for the treatment of age-related macular degeneration (AMD) and other eye disorders. One or more therapeutic agents can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (including non-central GA and central GA) and neovascularization (including types 1, 2 and 3 NV). In some embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent, an antioxidant, an anti-inflammatory agent, a complement inhibitor, a neuroprotector or an anti-angiogenic agent, or any combination thereof. In certain embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent (e.g., an apolipoprotein mimetic or / and a statin). In some embodiments, the one or more therapeutic agents are mixed with, non-covalently associated with or covalently bonded to a cell-penetrating peptide (CPP), encapsulated in CPP-conjugated nanoparticles, micelles or liposomes, or modified (e.g., stapled, prenylated, lipidated or coupled to a small-molecule α-helix mimic) to acquire membrane-translocating ability. In certain embodiments, the one or more therapeutic agents are administered by eye drop.
Owner:MACREGEN INC
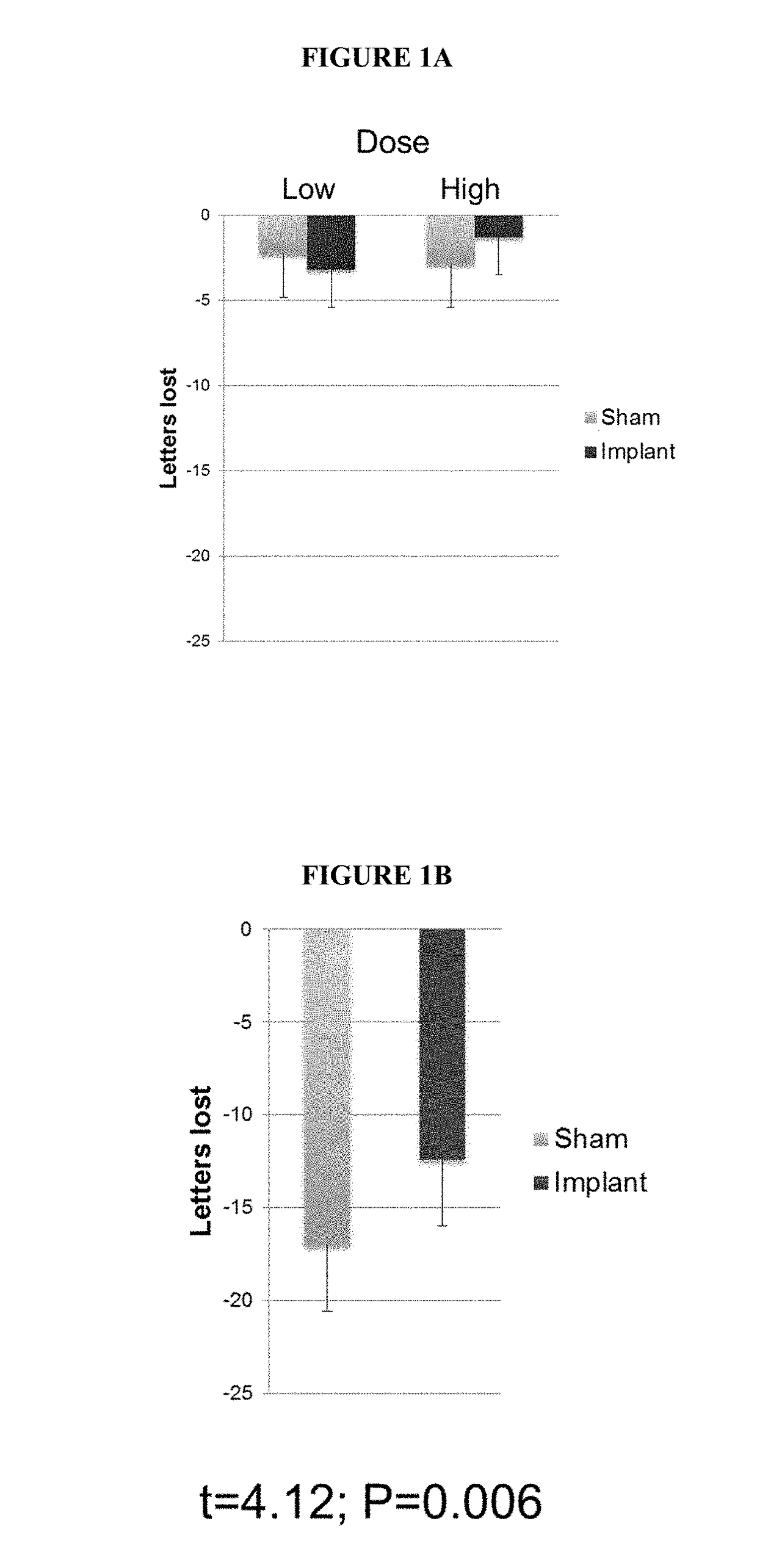
Impact of genetic factors on disease progression and response to Anti-c5 antibody in geographic atrophy
InactiveUS20170073754A1Subject is at riskMicrobiological testing/measurementImmunoglobulins against animals/humansRisk alleleGeographic atrophy
Pharmacogenetic analysis revealed an effect of risk alleles in ARMS2 and CFH genes on the response of subjects to anti-C5 antibodies in the treatment of the progression of geographic atrophy.
Owner:NOVARTIS AG
Treatment of age-related macular degeneration and other eye diseases with apolipoprotein mimetics
InactiveUS10426817B2Prevent and slow progressionApolipeptidesPeptide/protein ingredientsGeographic atrophyApolipoproteins E
The present disclosure provides apolipoprotein (apo) mimetics useful for the treatment of age-related macular degeneration (AMD) and other eye disorders. The apo mimetics can be peptides / polypeptides that mimic, e.g., the lipid-clearing action of apolipoproteins such as apoA-I and apoE. The apo mimetics can exert other beneficial effects, such as reduction of inflammation, oxidative stress and neovascularization. The apo mimetics can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (GA) (including non-central GA and central GA) and neovascularization (NV) (including types 1, 2 and 3 NV). The apo mimetics can be used alone or in conjunction with other therapeutic agents, such as a complement inhibitor and / or an anti-angiogenic agent, to treat AMD, including atrophic AMD and neovascular AMD, and other eye disorders.
Owner:MACREGEN INC
Use of Encapsulated Cell Therapy for Treatment of Ophthalmic Disorders
ActiveUS20160346197A1Increase nerve regenerationImprove acuitySenses disorderEye implantsDiseaseRetinitis pigmentosa
Described herein are methods and devices for the long term treatment of ophthalmic disorders. Also disclosed are encapsulated cell therapy (ECT) devices that secrete a biologically active molecule and methods for using the same for the treatment of various kinds of ophthalmic disorders, including retinitis pigmentosa, geographic atrophy (dry age-related macular degeneration), glaucoma and / or macular telangiectasia.
Owner:NEUROTECH USA
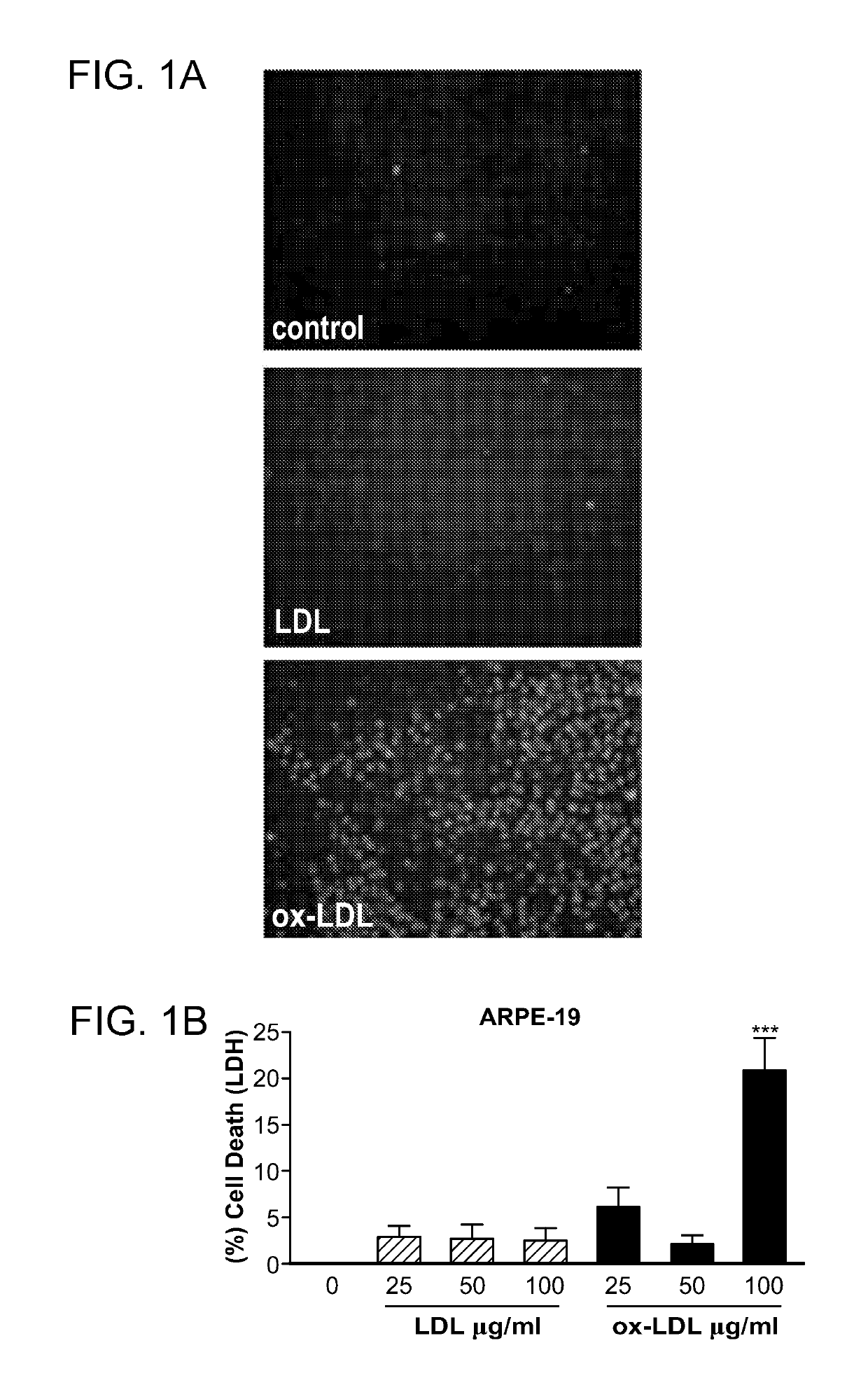
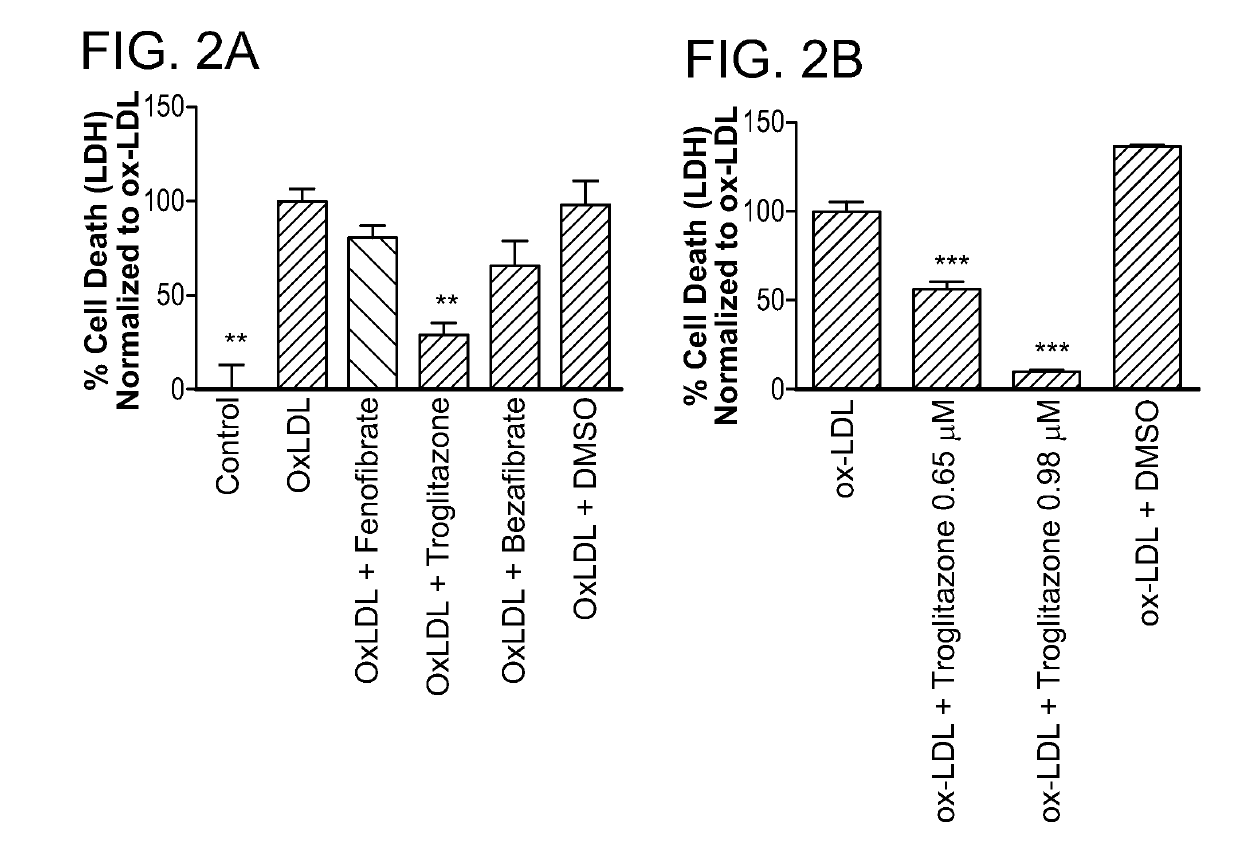
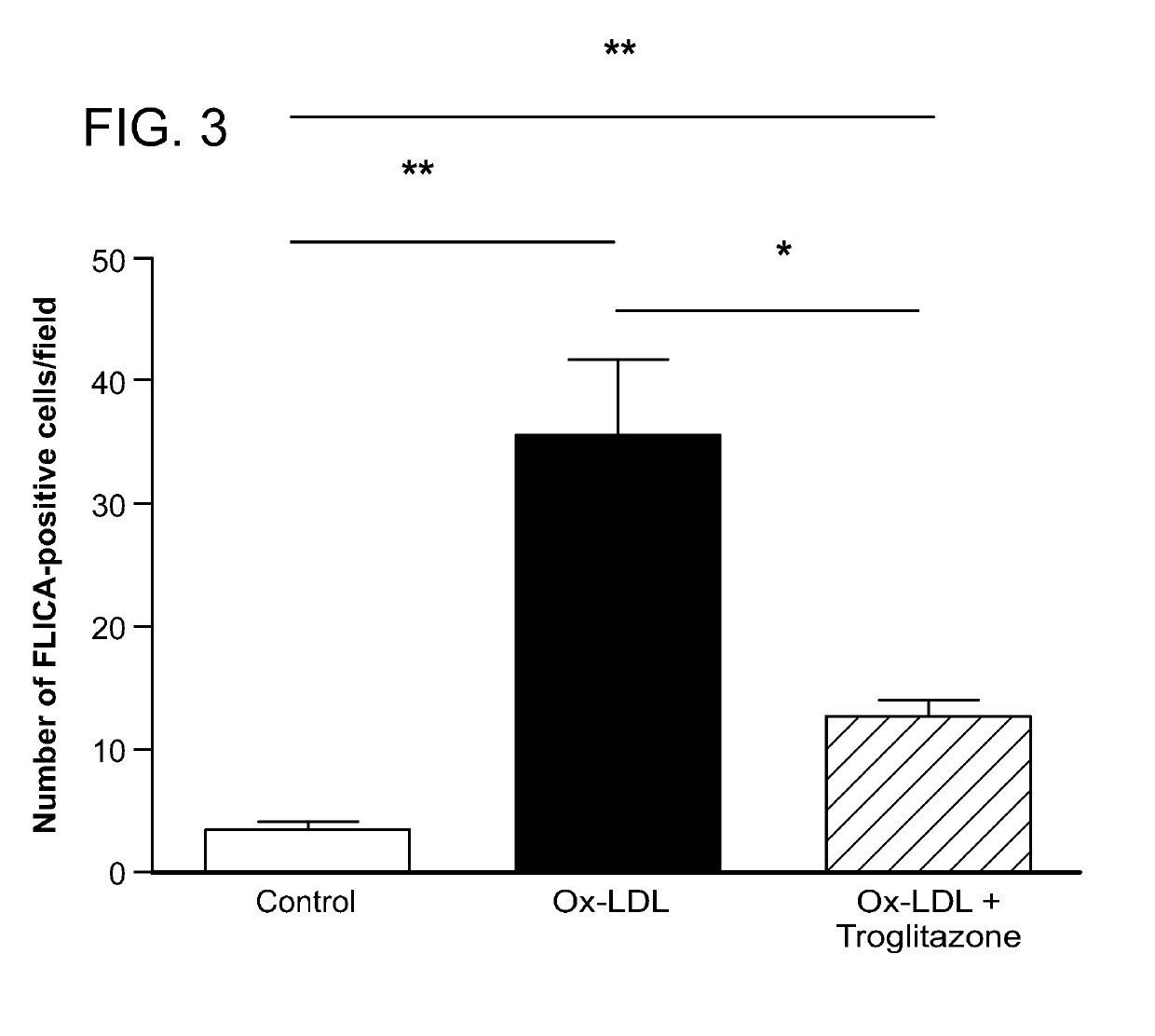
Peroxisome proliferator-activated receptor gamma selective agonists for inhibition of retinal pigment epithelium degeneration or geographic atrophy
InactiveUS20190152967A1Small sizeInhibit progressOrganic active ingredientsOrganic chemistryTroglitazoneGeographic atrophy
The invention provides a solution to the clinical problem of retinal pigment epithelium (RPE) degeneration or geographic atrophy (GA) associated with AMD. PPARΥ selective agonists, e.g., troglitazone and analogs thereof are used to reduce or inhibit RPE degeneration, GA, and / or the progression of dry AMD.
Owner:THE SCHEPENS EYE RES INST
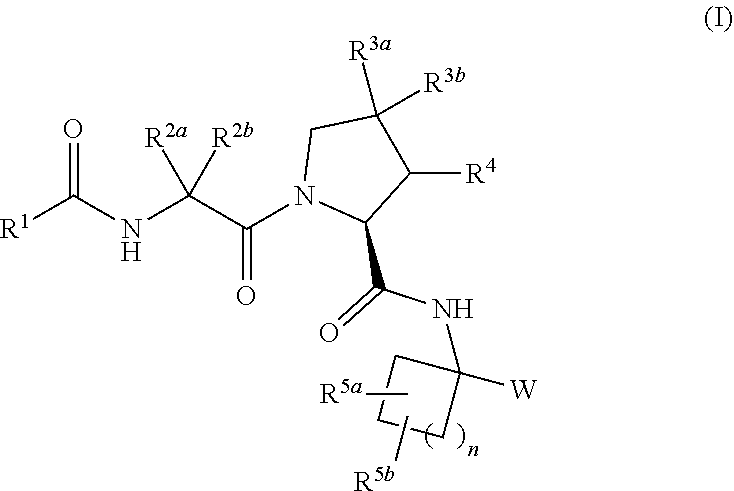
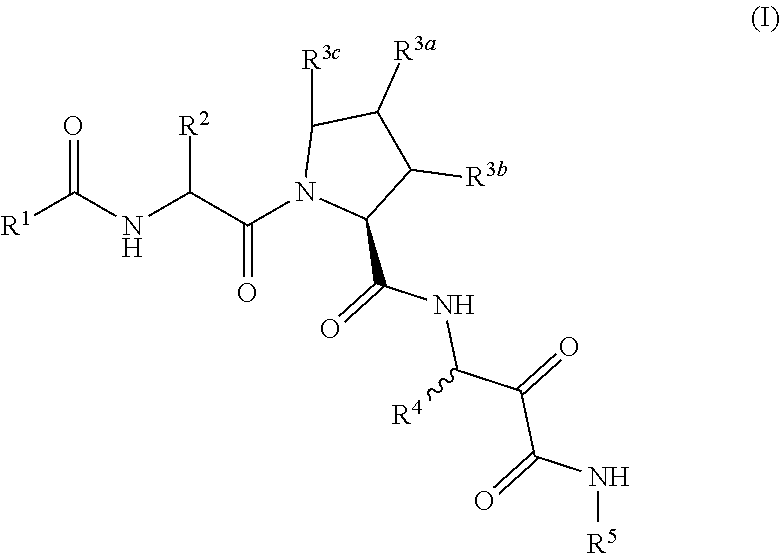
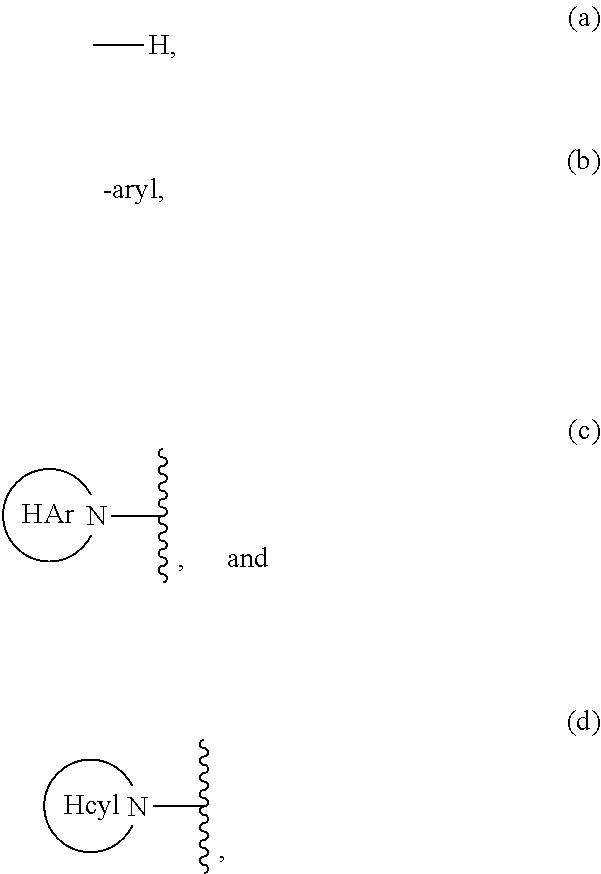
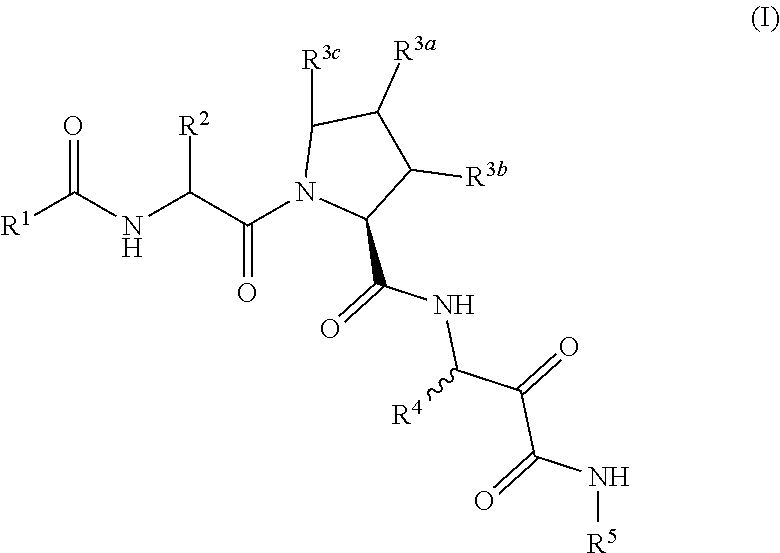
Aliphatic prolinamide derivatives
ActiveUS10730832B2Prevention and treatment of diseasesInhibitory activitySenses disorderOrganic chemistryDiabetic retinopathyDisease
This invention is directed to novel aliphatic prolinamide derivatives of Formula I, and pharmaceutically acceptable salts, solvates, solvates of the salt and prodrugs thereof, useful in the prevention (e.g., delaying the onset of or reducing the risk of developing) and treatment (e.g., controlling, alleviating, or slowing the progression of) of age-related macular degeneration (AMD) and related diseases of the eye. These diseases include dry-AMD, wet-AMD, geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells. The invention disclosed herein is further directed to methods of prevention, slowing the progress of, and treatment of dry-AMD, wet-AMD, and geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells, comprising: administration of a therapeutically effective amount of compound of the invention. The compounds of the invention are inhibitors of HTRA1. Thus, the compounds of the invention are useful in the prevention and treatment of a wide range of diseases mediated (in whole or in part) by HTRA1. The compounds of the invention are also useful for inhibiting HTRA1 protease activity in an eye or locus of an arthritis or related condition.
Owner:ORION OPHTHALMOLOGY LLC
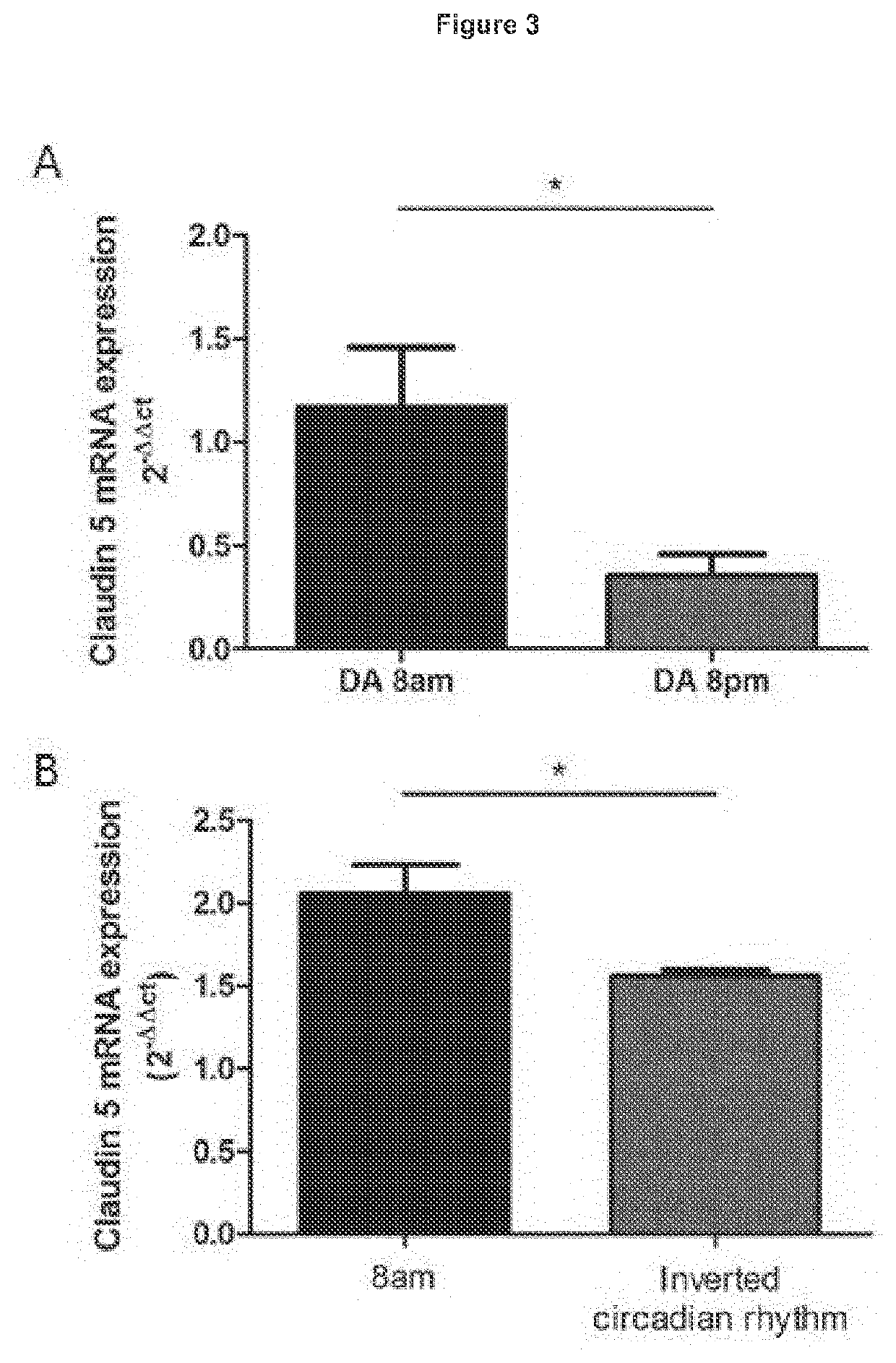
Treatment of age-related macular degeneration
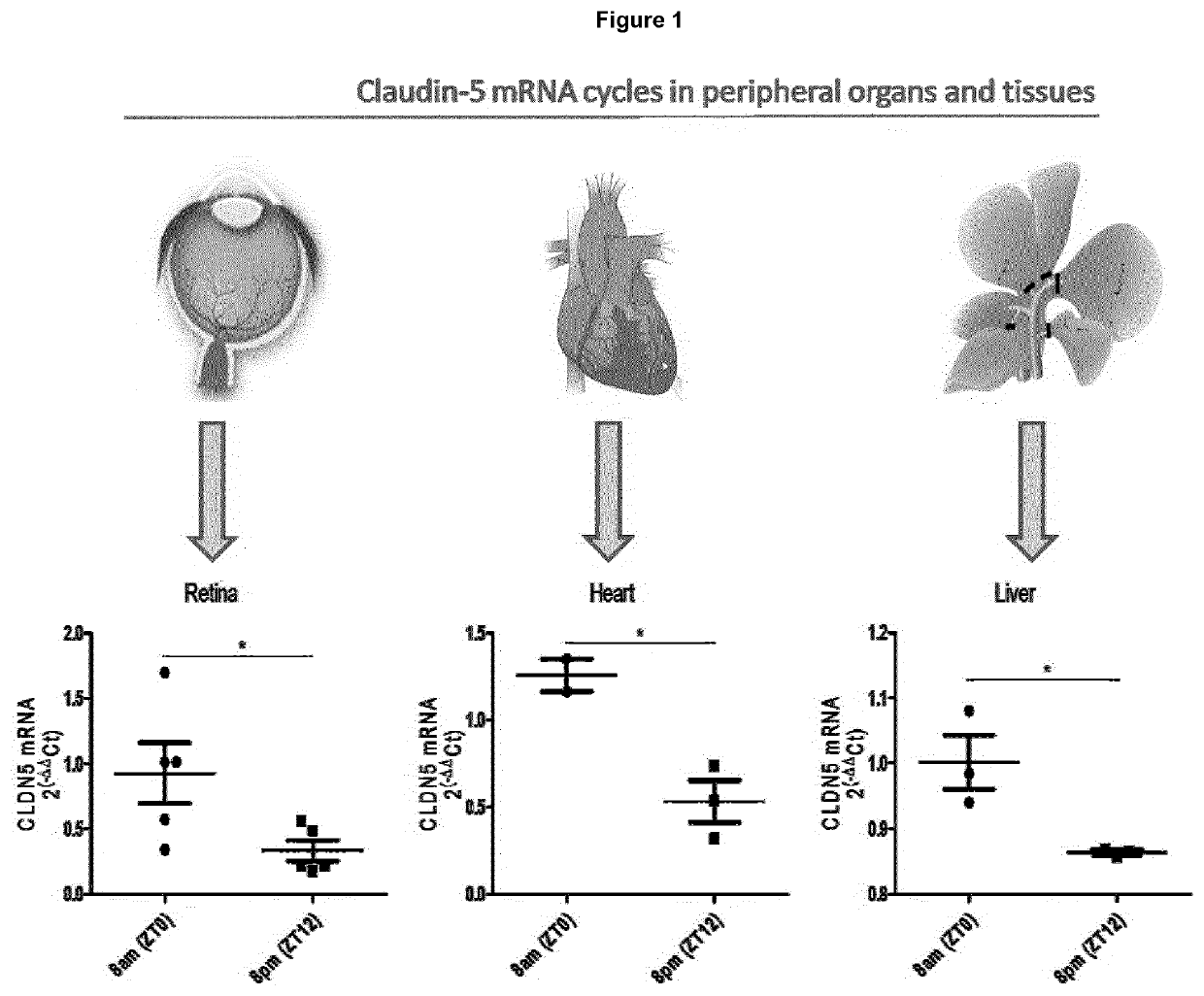
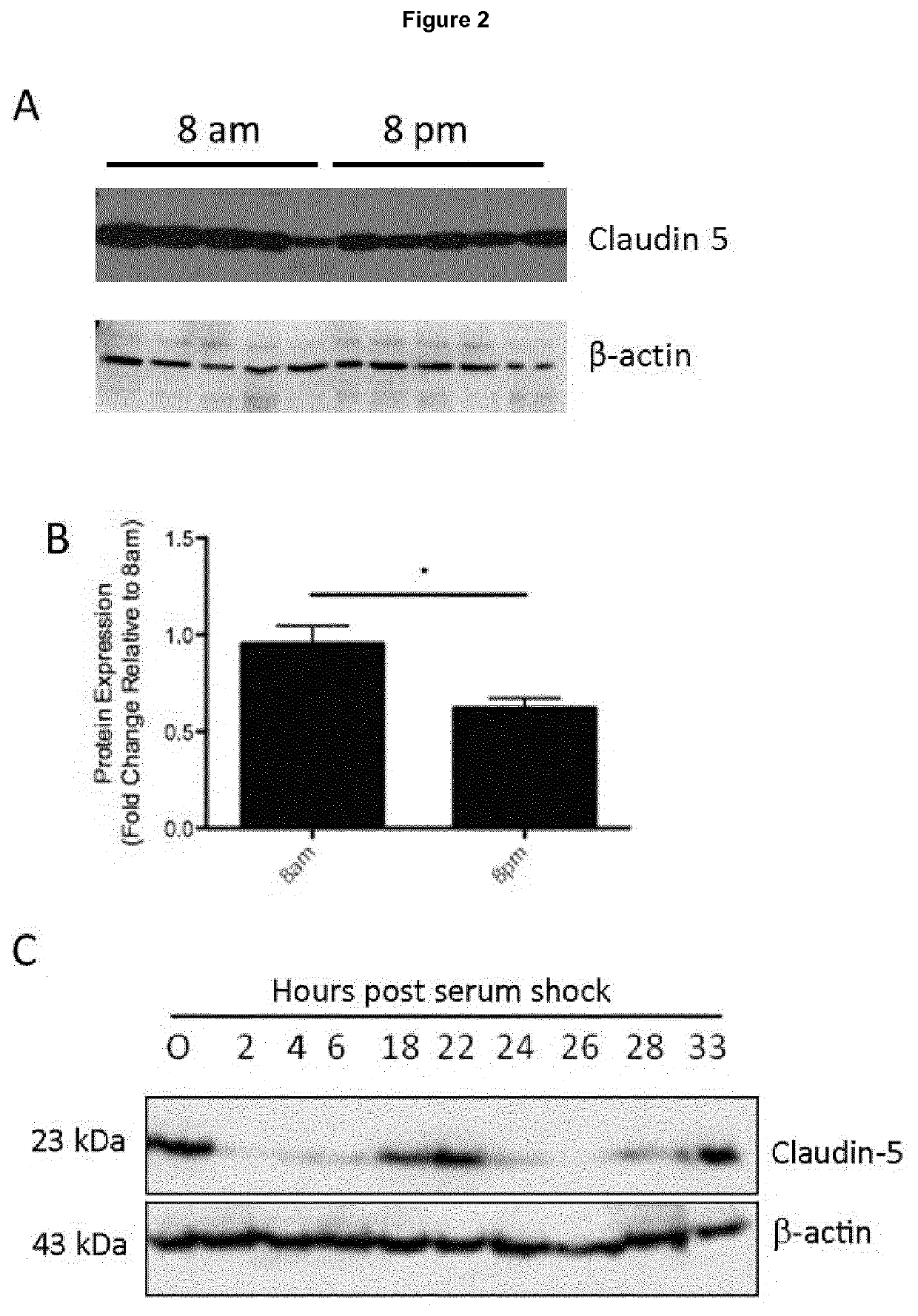
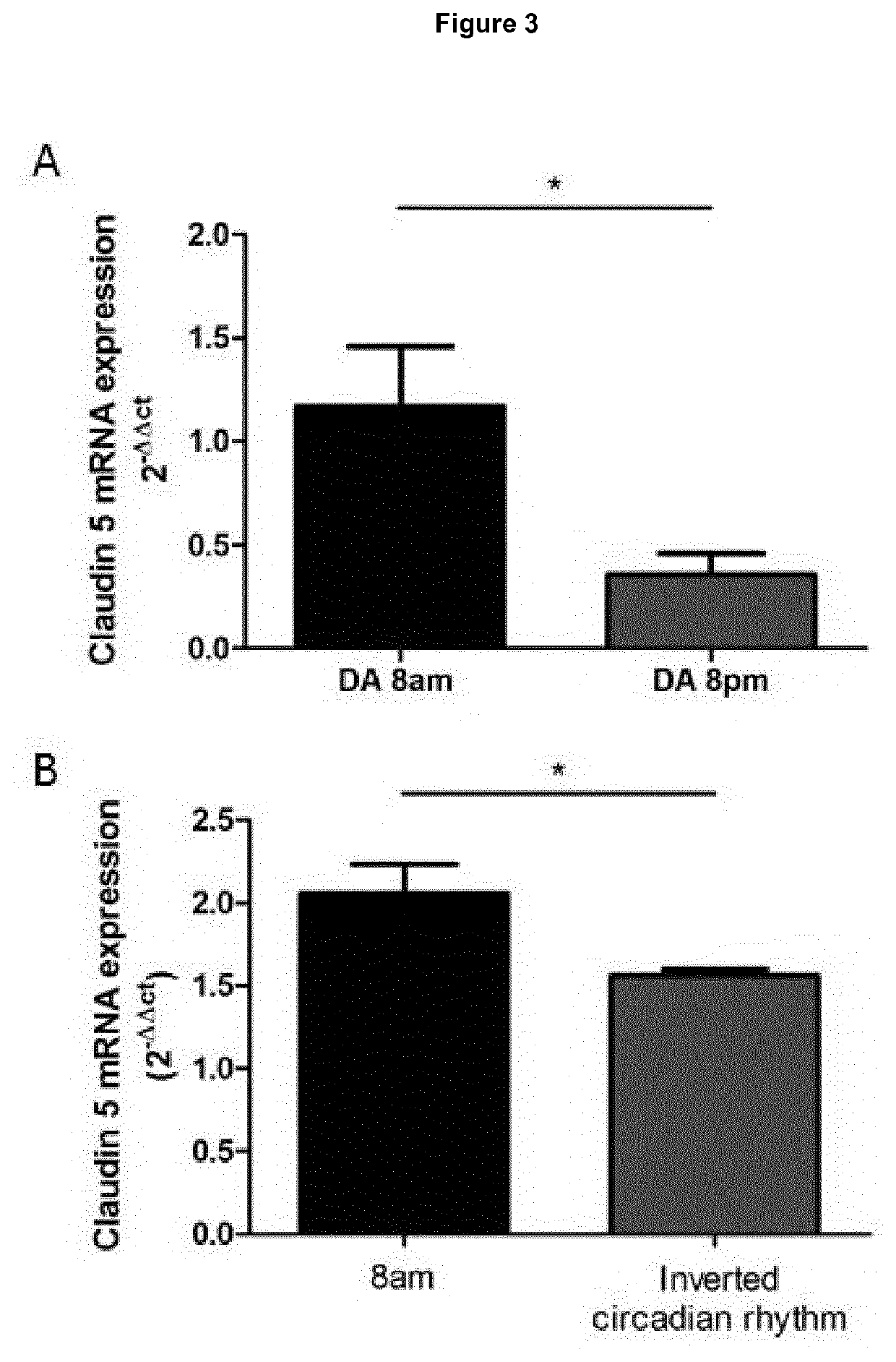
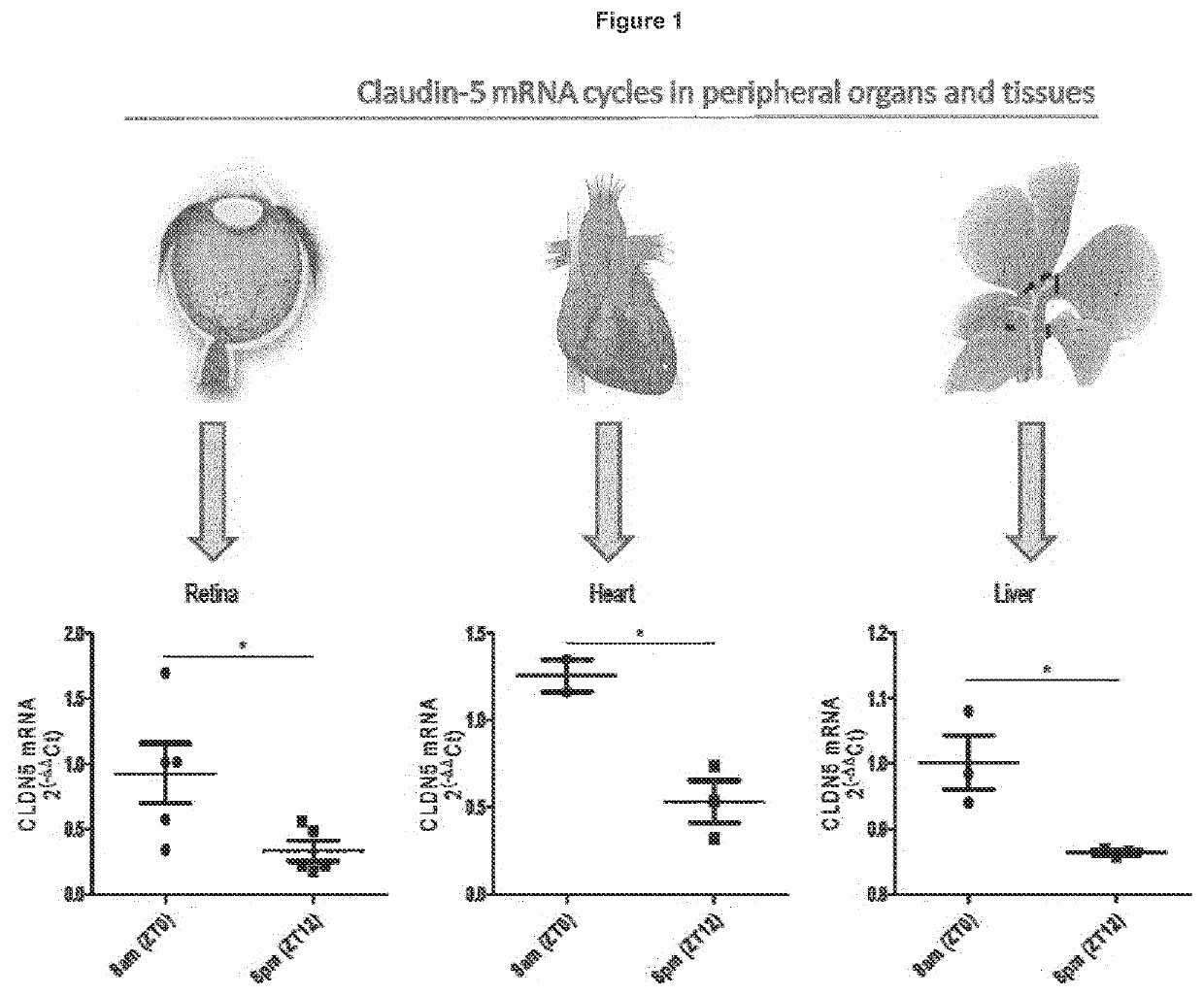
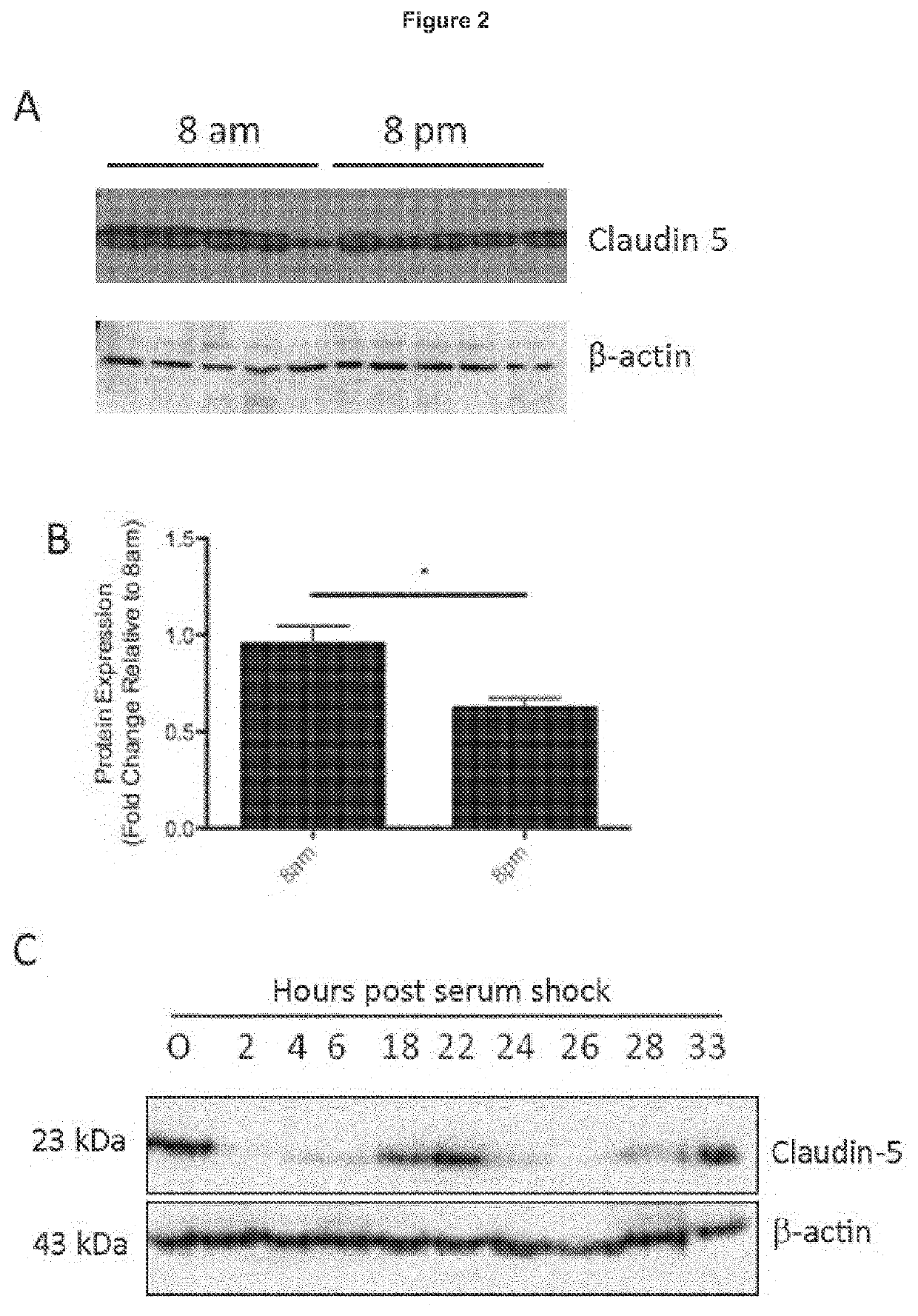
ActiveUS20200016236A1Restores natural circadian cyclingPeptide/protein ingredientsPharmaceutical delivery mechanismNectinGeographic atrophy
The present invention relates to a method and compositions for the treatment of age-related macular degeneration (AMD), in particular dry-AMD, specifically geographic atrophy (GA) or advanced dry-AMD. Specifically, the invention relates to an inner-blood-retinal-barrier (iBRB) or blood-brain-barrier (BBB) tight junction protein and / or a circadian clock protein for use in the prevention and / or treatment of age-related macular degeneration.
Owner:THE PROVOST FELLOWS FOUND SCHOLARS & THE OTHER MEMBERS OF BOARD OF THE COLLEGE OF THE HOLY & UNDIV TRINITY OF QUEEN EL
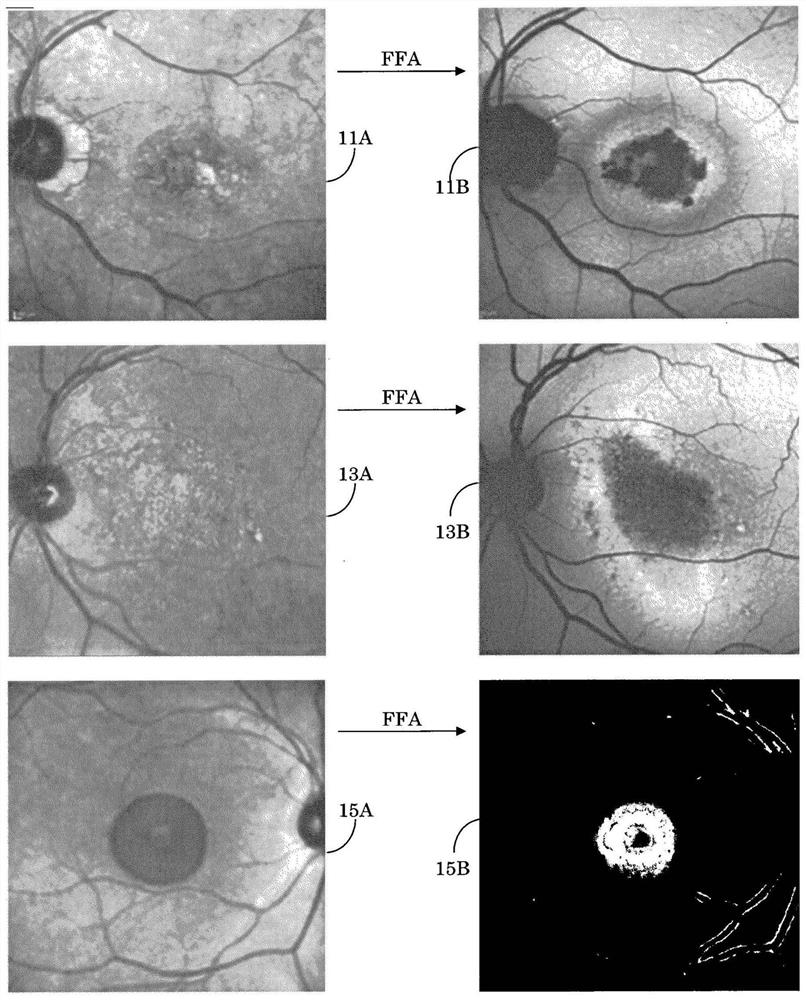
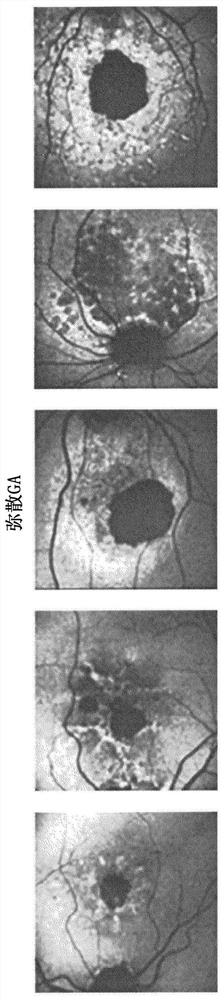
Segmentation and classification of geographic atrophy patterns in patients with age related macular degeneration in widefield autofluorescence images
An automated segmentation and identification system / method for identifying geographic atrophy (GA) phenotypic patterns in fundus autofluorescence images. A hybrid process combines a supervised pixel classifier with an active contour algorithm. A trained, machine learning model (e.g., SVM or U-Net) provides initial GA segmentation / classification, and this is followed by Chan-Vese active contour algorithm. The junctional zones of the GA segmented area are then analyzed for geometric regularity and light intensity regularity. A determination of GA phenotype is made, at least in part, from these parameters.
Owner:CARL ZEISS MEDITEC INC +1
Aliphatic prolinamide derivatives
ActiveUS20190330146A1Inhibit protease activityPrevention and treatment of diseasesSenses disorderOrganic chemistryDiabetic retinopathyGeographic atrophy
This invention is directed to novel aliphatic prolinamide derivatives of Formula I, and pharmaceutically acceptable salts, solvates, solvates of the salt and prodrugs thereof, useful in the prevention (e.g., delaying the onset of or reducing the risk of developing) and treatment (e.g., controlling, alleviating, or slowing the progression of) of age-related macular degeneration (AMD) and related diseases of the eye. These diseases include dry-AMD, wet-AMD, geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells. The invention disclosed herein is further directed to methods of prevention, slowing the progress of, and treatment of dry-AMD, wet-AMD, and geographic atrophy, diabetic retinopathy, retinopathy of prematurity, polypoidal choroidal vasculopathy, and degeneration of retinal or photoreceptor cells, comprising: administration of a therapeutically effective amount of compound of the invention. The compounds of the invention are inhibitors of HTRA1. Thus, the compounds of the invention are useful in the prevention and treatment of a wide range of diseases mediated (in whole or in part) by HTRA1. The compounds of the invention are also useful for inhibiting HTRA1 protease activity in an eye or locus of an arthritis or related condition.
Owner:ORION OPHTHALMOLOGY LLC
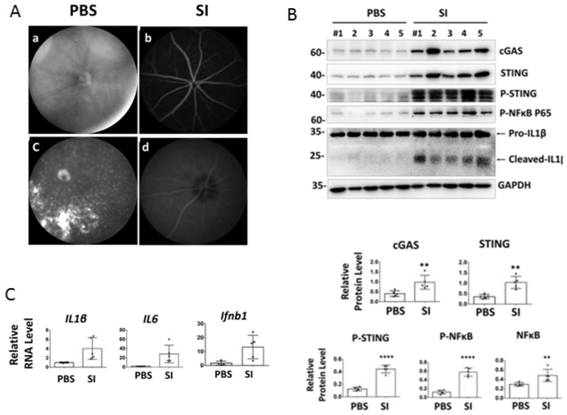
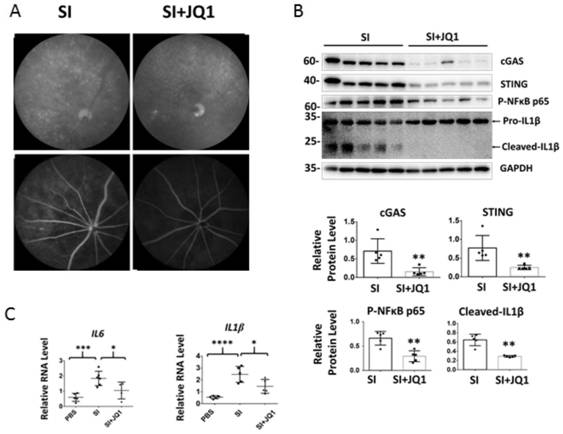
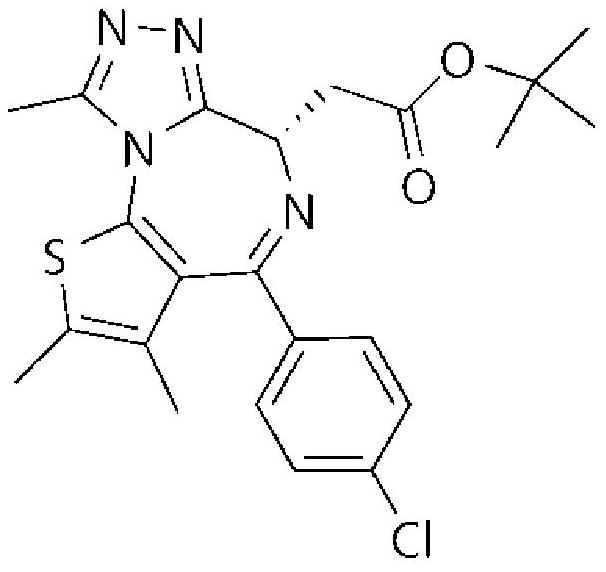
Applications of small molecule drug (+)-JQ1 in drugs for treating geographic atrophy type age-related macular degeneration
The invention discloses applications of a small molecule drug (+)-JQ1 in drugs for treating geographic atrophy type age-related macular degeneration, and specifically discloses the small molecule drug(+)-JQ1 having effects of inhibiting cGAS-STING signal pathways and regulatory and anti-inflammatory effects of the small molecule drug on autoimmunity. In a mouse disease model of sodium iodate injection induced geographic atrophy type age-related macular degeneration, the (+)-JQ1 is firstly provided that the (+)-JQ1 can inhibit the expression of cGAS and STING genes and achieve immunoregulationand anti-inflammatory effects by inhibiting the functions of the cGAS-STING signal pathways. The inhibition of the (+)-JQ1 on the cGAS-STING signal pathways and the anti-inflammatory effects of the(+)-JQ1 in the disease model of geographic atrophy type age-related macular degeneration are a promising clinical strategy of new use for old drugs, so that reference can be provided for researches on small molecular compounds for anti-autoimmune response and treatment of age-related macular degeneration diseases.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Protection of Cells from Degeneration and Treatment of Geographic Atrophy
PendingUS20200206236A1Organic active ingredientsAgainst vector-borne diseasesGeographic atrophyPharmacology
Therapeutic uses P2X7 inhibition and inhibition of IRAK1 and / or IRAK4, methods protecting a cell, and screening methods for identifying inhibitors are described herein.
Owner:UNIV OF KENTUCKY RES FOUND
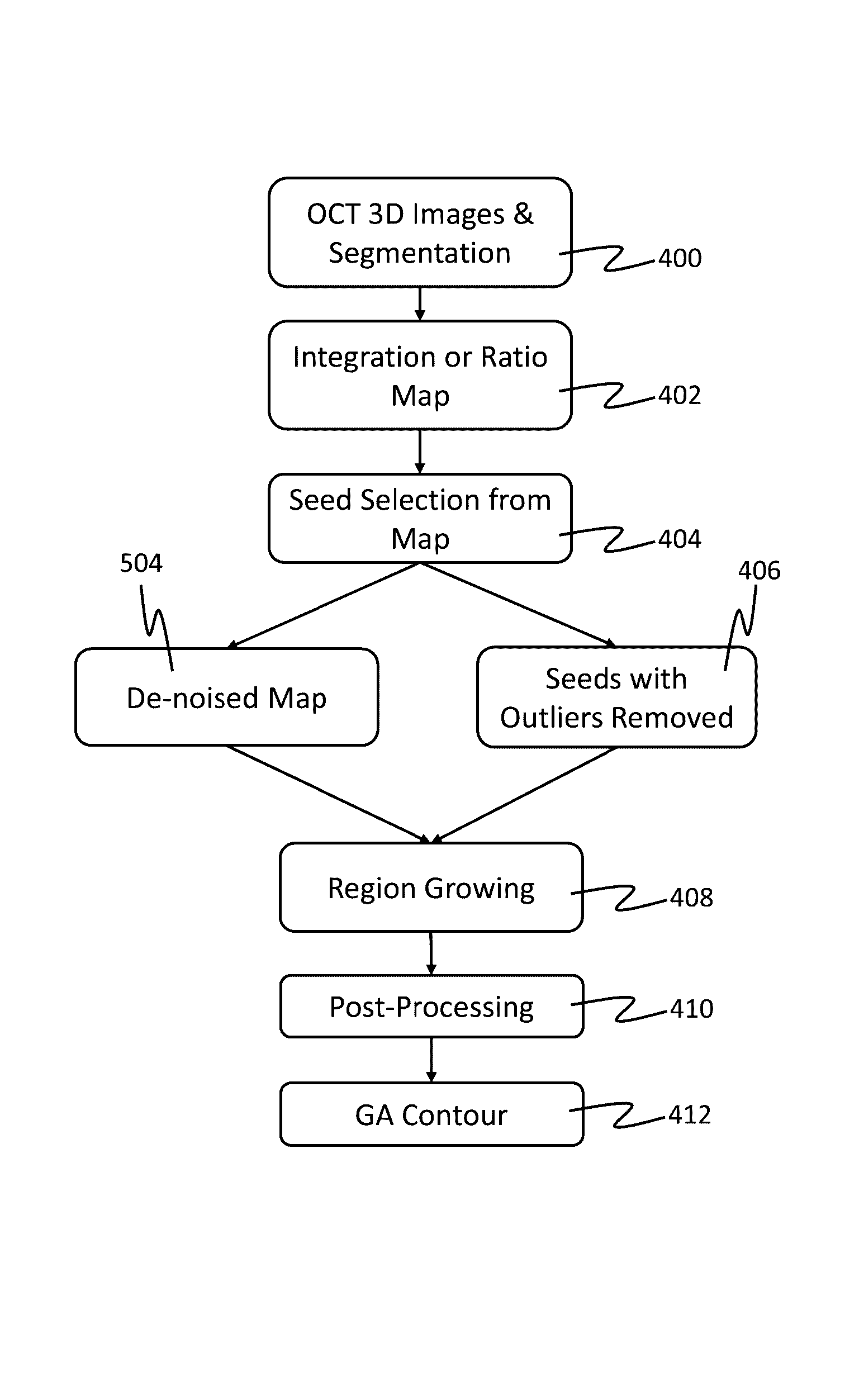
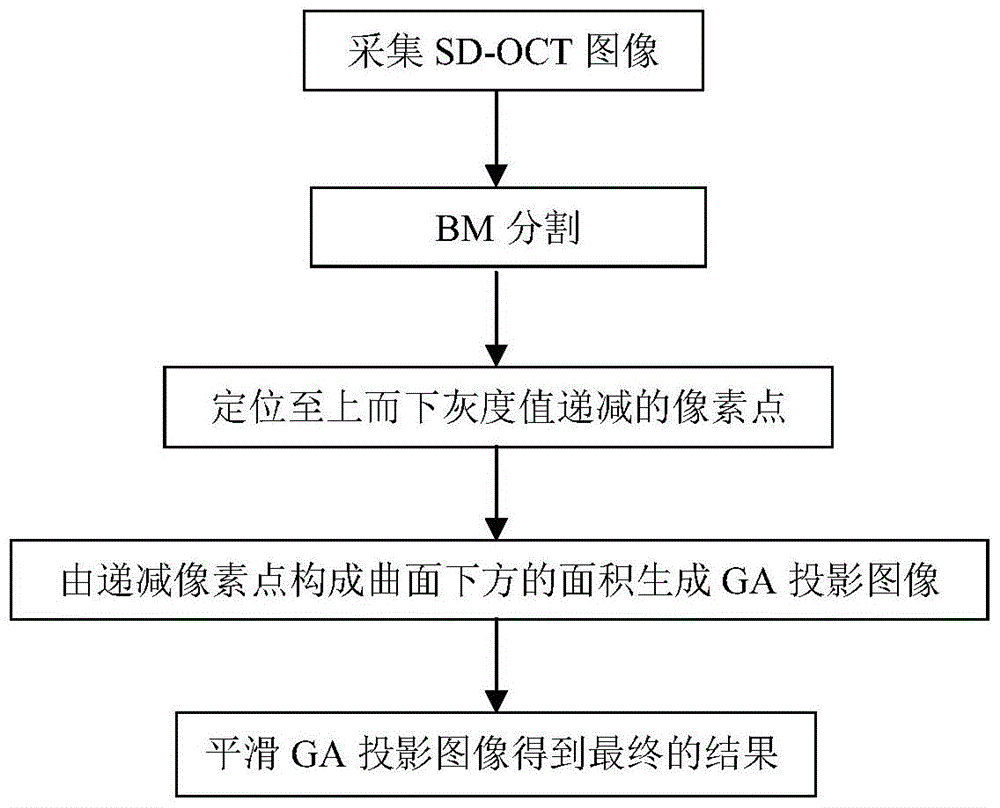
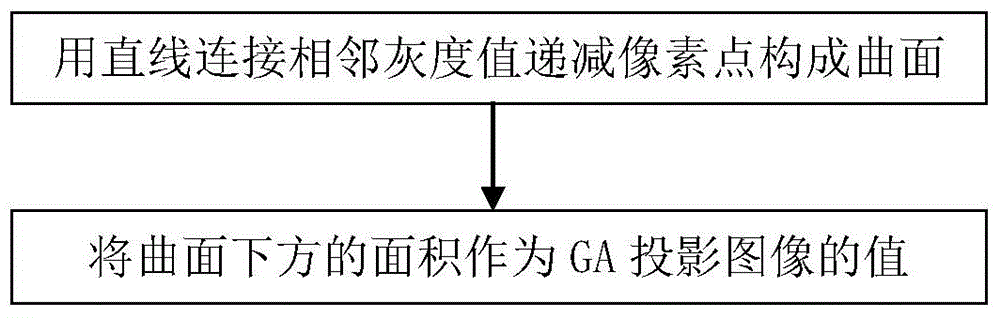
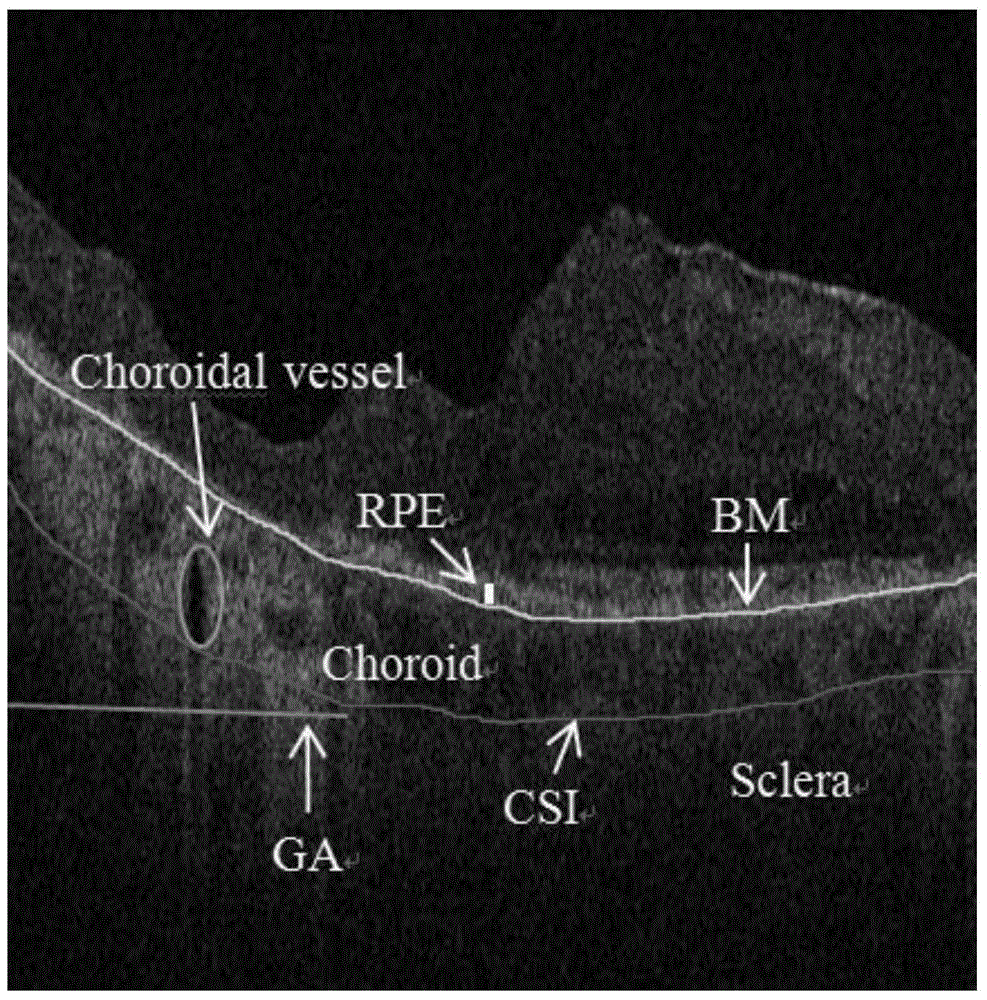
Map-like atrophy projection image generation method based on sd-oct retinal images
The present invention provides a method for generating a map-like atrophy projection image based on an SD-OCT retinal image, the realization of which comprises the following steps: Step 1, collecting an SD-OCT retinal image; Step 2, segmenting the Bruch's membrane boundary of the image obtained in Step 1 ; Step 3, in the 0.5 mm thick narrow band region below the boundary of Bruch's membrane, locate the pixels with decreasing gray values in each column from top to bottom; Step 4, form the gray value decreasing pixels obtained in step 3 A curved surface, and use the area between the curved surface and the abscissa as the value of the GA projection image to generate a projection image; and step 5, use median filtering to smooth the GA projection image generated in the aforementioned step 4 to obtain the final result. The SD-OCT retinal image-based geographic atrophy projection image generation method of the present invention considers and overcomes the influence of choroidal blood vessels on the GA projection image, and improves the contrast and gray consistency of the GA projection image.
Owner:NANJING UNIV OF SCI & TECH
Treatment of age-related macular degeneration
PendingUS20210401932A1Restores natural circadian cyclingPeptide/protein ingredientsPharmaceutical delivery mechanismNectinGeographic atrophy
The present invention relates to a method and compositions for the treatment of age-related macular degeneration (AMD), in particular dry-AMD, specifically geographic atrophy (GA) or advanced dry-AMD. Specifically, the invention relates to an inner-blood-retinal-barrier (iBRB) or blood-brain-barrier (BBB) tight junction protein and / or a circadian clock protein for use in the prevention and / or treatment of age-related macular degeneration.
Owner:TRINITY COLLEGE DUBLIN
Stem-loop compositions and methods for inhibiting factor D
ActiveUS11466276B2Organic active ingredientsPharmaceutical non-active ingredientsAptamerGeographic atrophy
The application discloses methods and compositions for the inhibition of the alternative complement pathway. The methods and compositions involve the use of aptamers for inhibiting complement Factor D. The application further provides anti-Factor D aptamers for the treatment of dry age-related macular degeneration, geographic atrophy, wet age-related macular degeneration or Stargardt disease. In some cases, stem-loop aptamers are provided for the inhibition of Factor D.
Owner:396419 B C LTD +1
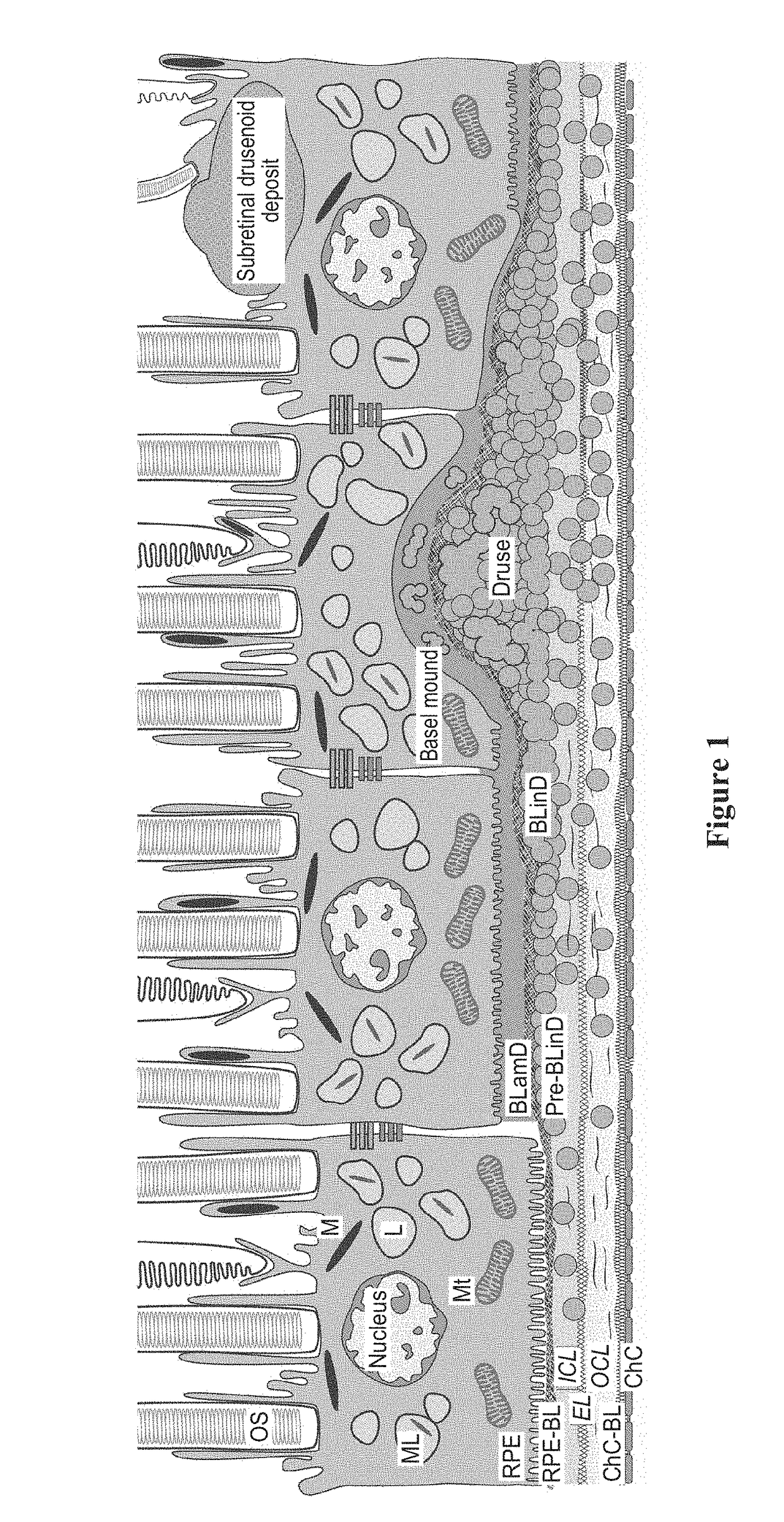
Compounds and compositions for inhibiting retinal pigment epithelium degeneration and methods using the same
ActiveUS20200316032A1Potent RPE protective activityPrevent degradationHydroxy compound active ingredientsPharmaceutical delivery mechanismPigmented retinal epitheliumAtrophy
There are provided inter alia compounds capable of inhibiting retinal pigment epithelium (RPE) degeneration or geography atrophy (GA) associated with age-related macular degeneration (AMD), and methods of using the same.
Owner:THE SCHEPENS EYE RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com