Anti-factor d antibodies and conjugates
An antibody and factor technology, applied in the direction of antibodies, anti-growth factor immunoglobulin, antibody medical components, etc., can solve problems such as prolonged time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0225] The term "pharmaceutical formulation" refers to a preparation which is in such a form as to allow the biological activity of the active ingredient contained therein to be effective and which does not contain additional components which are unacceptably toxic to the subject to which the formulation will be administered. point.
[0226] "Pharmaceutically acceptable carrier" refers to ingredients in pharmaceutical preparations other than the active ingredient that are non-toxic to the subject. Pharmaceutical carriers include, but are not limited to, buffers, excipients, stabilizers or preservatives.
[0227] As used herein, "treatment" (and its grammatical variants such as "treat / treating") refers to clinical intervention intended to alter the natural course of the individual being treated, and may be performed for prophylaxis or in clinical pathology during the process. Desired therapeutic effects include, but are not limited to, prevention of onset or recurrence of the...
Embodiment 1
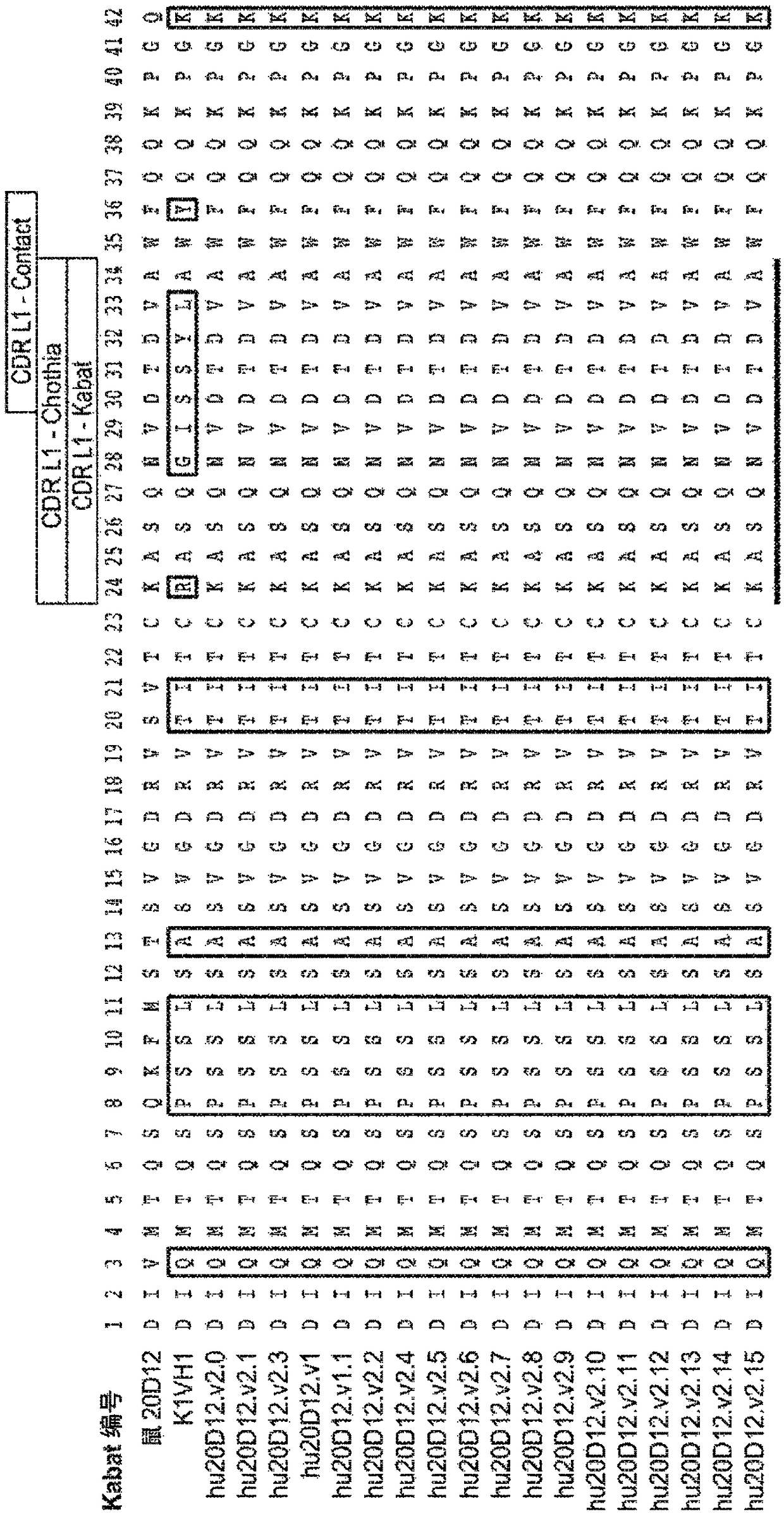
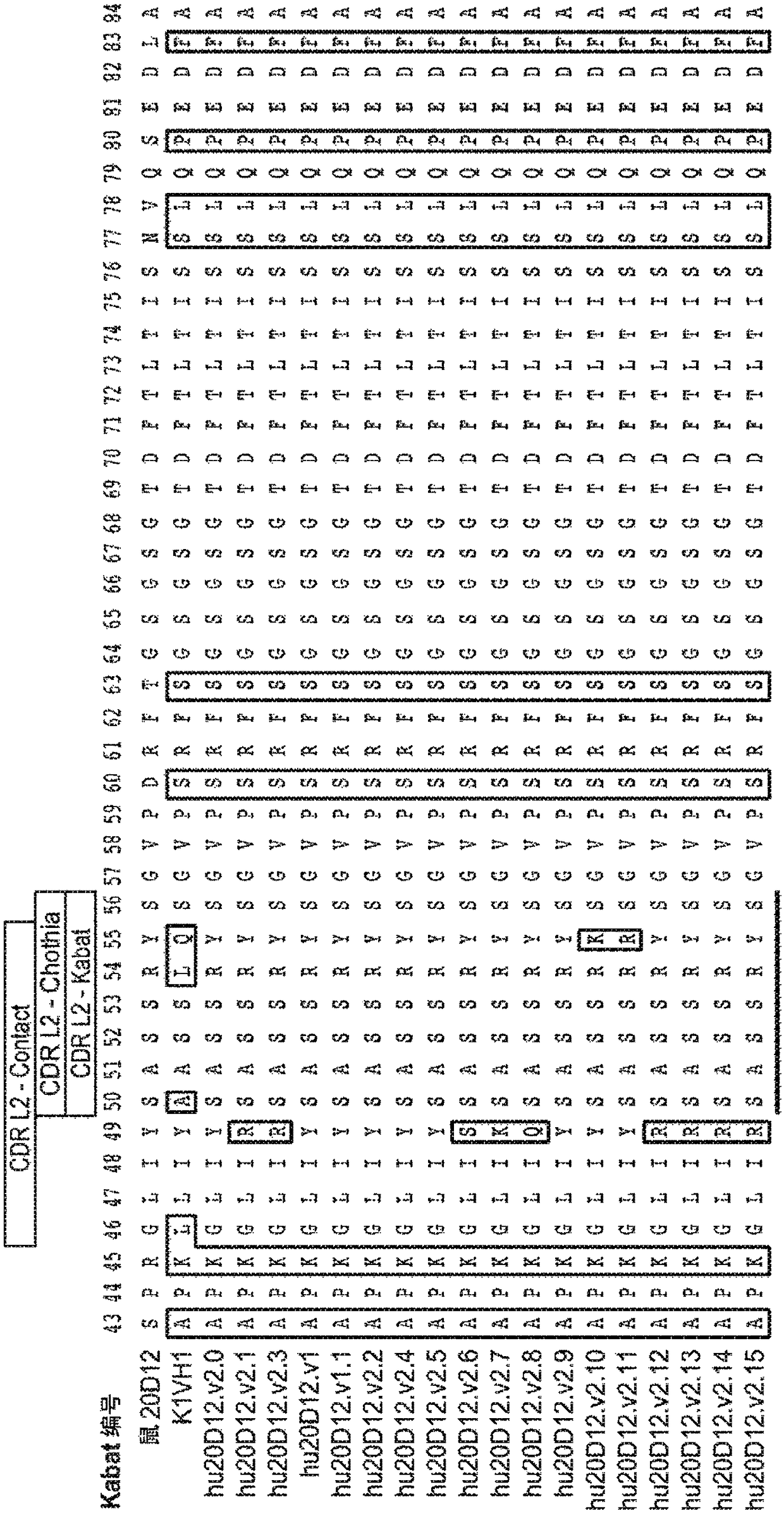
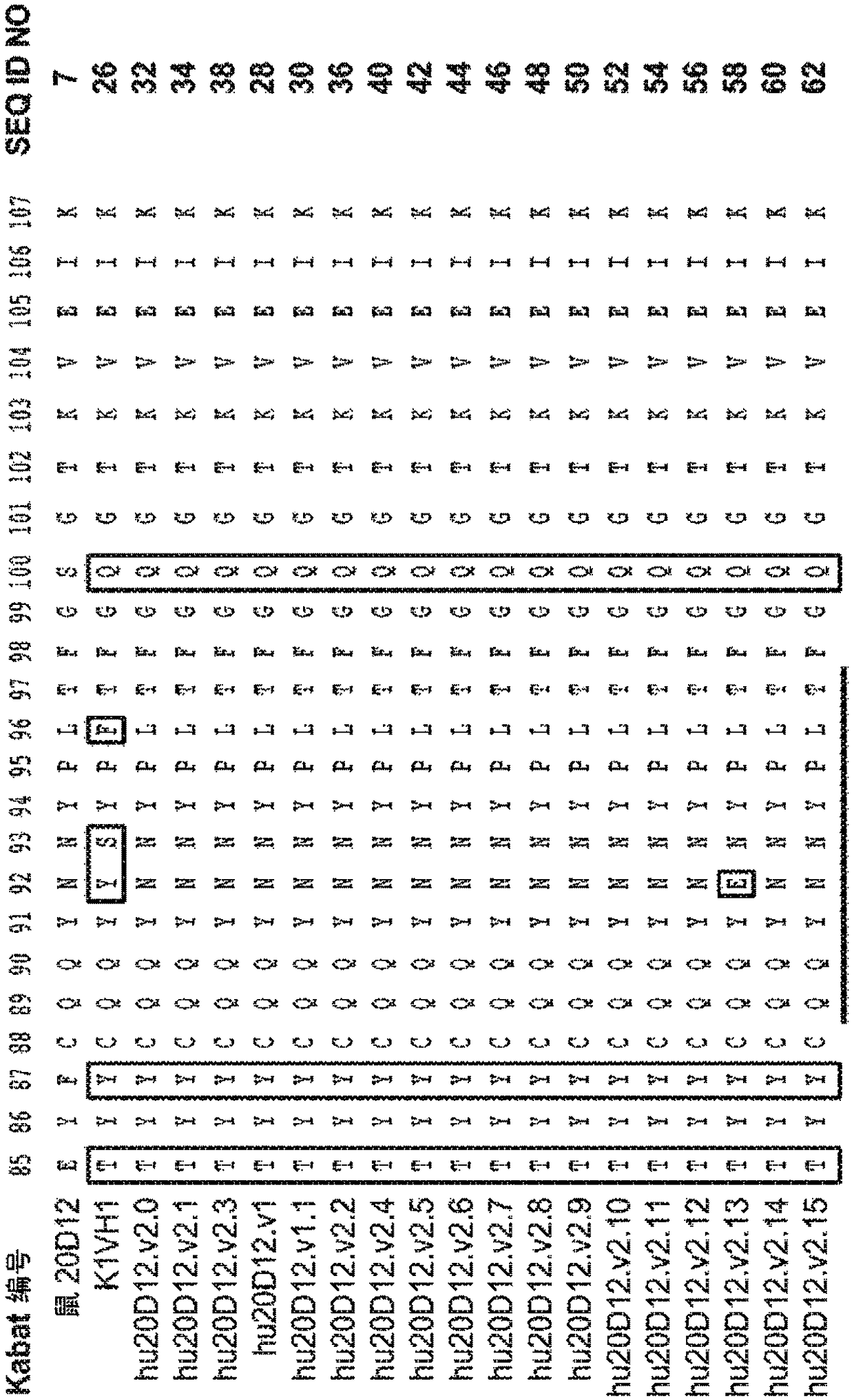
[0519]Example 1: Generation of Anti-Factor D Humanized Antibodies
[0520] Lampalizumab (also sometimes referred to as "aFD.WT" or "FCFD4515S") is a humanized anti-Factor D Fab fragment that potently inhibits Factor D and the alternative complement pathway by binding to an exosite on Factor D, It is currently in clinical development for the treatment of geographic atrophy (GA), an advanced form of dry AMD. Lampalizumab comprises a light chain of 214 residues (SEQ ID NO: 102) and a heavy chain of 223 residues (SEQ ID NO: 103).
[0521] Although the results of the human phase II clinical trial in GA indicated that a therapeutic effect was obtained with monthly intravitreal injections of aFD.WT, there is an incentive to use higher drug doses in order to achieve even better efficacy. At the same time, less frequent dosing would provide improved convenience to patients, have the potential benefit of reducing infection rates and increasing clinical efficacy, and could help treat pa...
Embodiment 2
[0529] Example 2: Production and characterization of hu20D12 Fab fragments
[0530] Part of the gene encoding the Fab fragment of hu20D12.v1 was subcloned into an E. coli expression vector similar to that previously described (Carter et al. (1992) BioTechnology 10:163). For small-scale expression and purification, the DNA was transformed into E. coli strain 64B4. Pick a single colony into 5 mL of LB medium (medium preparation code A2008) containing 50 μg / mL carbenecillin (medium preparation code A3232) and shake at 200 RPM in a 14 mL culture tube Incubate overnight at 37°C in an Innova incubator. These cultures were used to inoculate 250 mL of complete soy crap medium (media preparation code A4564) (50 μg / mL carbenicillin) in 1 L baffled shake flasks. Cultures were grown overnight at 30°C with shaking at 200 RPM, then harvested by centrifugation. Cell pellets were lysed with PopCulture medium (Invitrogen) and Fabs were purified on Gravitrap Protein G columns (GE Healthcare)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com