Aryl, heteroaryl, and heterocyclic compounds for treatment of medical disorders
A technology of compounds and obstacles, applied in the directions of active ingredients of heterocyclic compounds, chemical instruments and methods, drug combinations, etc., can solve the problem of no small molecule factor D inhibitors and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0582] VII. Methods of Preparation of Active Compounds
[0583] acronym
[0584] (Boc) 2 O di-tert-butyl dicarbonate
[0585] CAN acetonitrile
[0586] AcOEt, EtOAc ethyl acetate
[0587] CH 3 OH, MeOH Methanol
[0588] CsF Cesium fluoride
[0589] CuI Cuprous iodide
[0590] DCM,CH 2 Cl 2 Dichloromethane
[0591] DIEA,DIPEA N,N-Diisopropylethylamine
[0592] DMA N,N-Dimethylacetamide
[0593] DMF N,N-Dimethylformamide
[0594] DMSO Dimethyl Sulfoxide
[0595] DPPA diphenylphosphoryl azide
[0596] Et 3 N,TEA Triethylamine
[0597] EtOAc ethyl acetate
[0598] EtOH ethanol
[0599] HATU 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridine 3-oxide hexafluorophosphate
[0600] HCl hydrochloric acid
[0601] i PR 2 Net N,N-Diisopropylethylamine
[0602] K 2 CO 3 potassium carbonate
[0604] MTBE methyl tert-butyl ether
[0605] Na 2 SO 4 sodium sulfate
[0606] NaCl sodium chloride
[0607] NaH sodium hy...
Embodiment 1
[0662] Embodiment 1. General synthetic route
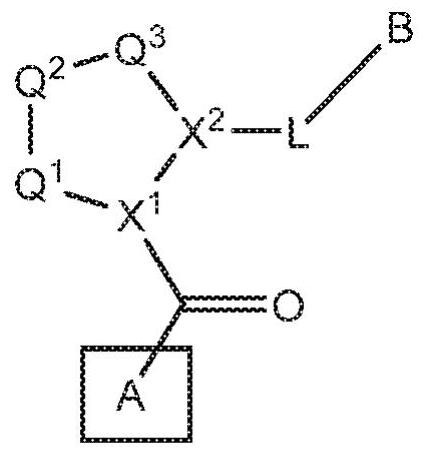
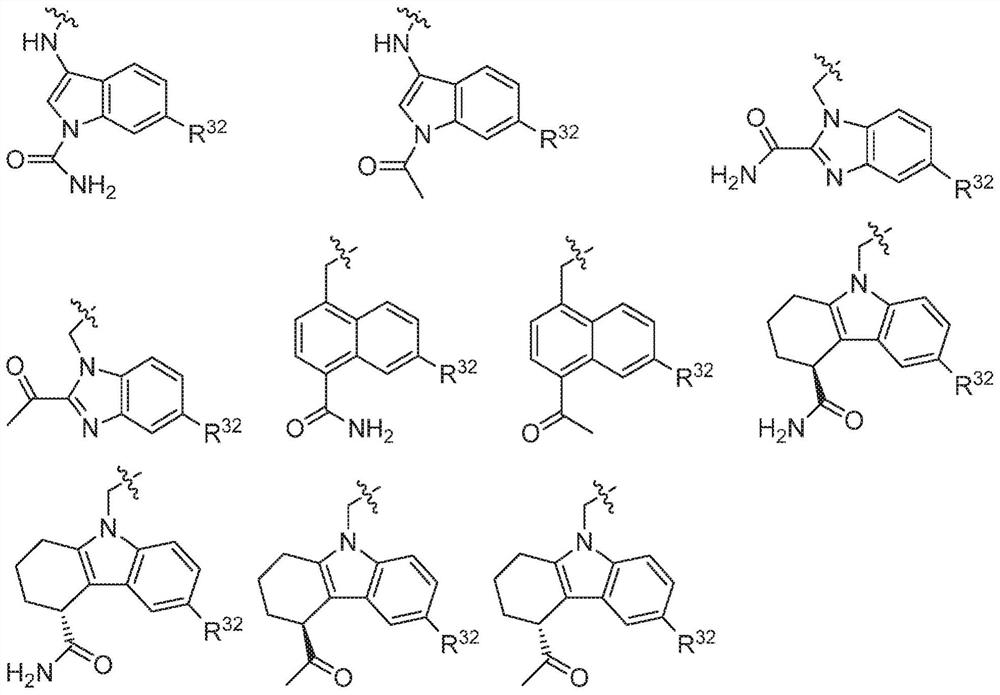
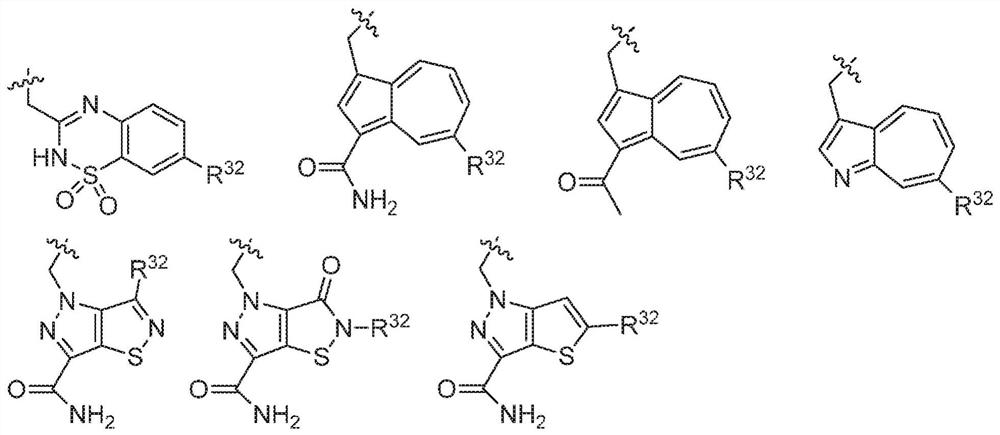
[0663] Compounds of the invention can be prepared, for example, from a central core. In one embodiment, the central core structure 1 is, for example, an N-protected amino acid, wherein X 1 is nitrogen and PG = protecting group. In one embodiment, the central core is coupled to an amine to generate an amide of structure 2 (where L-B includes a C(O)N moiety). Structure 2 can then be deprotected to generate Structure 3 . Structure 3 is coupled to structure 4 (A-COOH) to generate a second amide bond to form a compound within formula I. This chemical process is schematically shown in scheme 1.
[0664]
[0665] route 1
[0666] In an alternative embodiment, the central core structure 5 is reacted with a heterocyclic or heteroaryl compound to generate a compound of structure 6. In one embodiment, structure 6 is deprotected to generate the carboxylic acid (structure 7). In one embodiment, structure 7 is coupled to an amine to g...
Embodiment 2
[0695] Example 2. Examples of central synthons
[0696]
[0697] Z A for halogen.
[0698] In one embodiment, deuterated L-proline synthons are disclosed. Deuterated synthons include, but are not limited to, compounds such as:
[0699]
[0700] Structure A can be treated with deuterium oxide to generate structure B. See Barraclough, P. et al. Tetrahedron Lett. 2005, 46, 4653-4655; Barraclough, P. et al. Org. Biomol. Chem. 2006, 4, 1483-1491 and WO2014 / 037480 (p.103). Structure B can be reduced to generate structure C. See Barraclough, P. et al. Tetrahedron Lett. 2005, 46, 4653-4655; Barraclough, P. et al. Org. Biomol. Chem. 2006, 4, 1483-1491. Structure C can be treated with Mitsunobu reaction conditions to generate structure D. Structure B can be processed with DAST to generate structure E. See WO2014 / 037480. Structure A can be treated with sodium borodeuteride to generate structure F. See Dormoy, J.-R.; Castro, B. Synthesis 1986, 81-82. Structure F can be use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com