Morphic forms of complement factor d inhibitors
A Form, Dosage Technology Applied in the Field of Morphological Forms of Complement Factor D Inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0167] a) Isolated crystalline form I of compound 2, characterized in that it comprises XRPD pattern of at least three 2θ values of °, 17.4±0.2°, 18.0±0.2°, 18.4±0.2°, 21.1±0.2°, 22.0±0.2°, 22.6±0.2°, 23.8±0.2° and 27.4±0.2° ;
[0168] b) isolated crystalline form I of compound 2 of embodiment (a), characterized in that it comprises At least four of 0.2°, 16.2±0.2°, 17.4±0.2°, 18.0±0.2°, 18.4±0.2°, 21.1±0.2°, 22.0±0.2°, 22.6±0.2°, 23.8±0.2° and 27.4±0.2° XRPD patterns of 2θ values;
[0169] c) isolated crystalline form I of compound 2 of embodiment (a), characterized in that it comprises At least five of 0.2°, 16.2±0.2°, 17.4±0.2°, 18.0±0.2°, 18.4±0.2°, 21.1±0.2°, 22.0±0.2°, 22.6±0.2°, 23.8±0.2° and 27.4±0.2° XRPD patterns of 2θ values;
[0170] d) Isolated crystalline form I of compound 2 of embodiment (a), characterized in that it comprises At least six of 0.2°, 16.2±0.2°, 17.4±0.2°, 18.0±0.2°, 18.4±0.2°, 21.1±0.2°, 22.0±0.2°, 22.6±0.2°, 23.8±0.2° and 27.4±0.2° XRPD...
Embodiment
[0428] Scheme 1. Compound 1 ((2S,4R)-1-(2-(3-acetyl-5-(2-methylpyrimidin-5-yl)-1H-indazol-1-yl)acetyl)- Synthesis of N-(-6-bromopyridin-2-yl)-4-fluoropyrrolidine-2-carboxamide) Form II
[0429]
[0430] Step 1: Synthesis of tert-butyl (2S,4R)-2-((6-bromopyridin-2-yl)carbamoyl)-4-fluoropyrrolidine-1-carboxylate (3): under nitrogen atmosphere , N-Boc-trans-4-fluoro-L-proline (50.8 kg) was added to DCM (1000 L) in a glass-lined reactor. The reaction mixture was cooled to 0±5°C and N-methylimidazole (44.7 kg) was added while maintaining the temperature at 0±5°C. Methanesulfonyl chloride (29.97 kg) was slowly added to the reaction mixture followed by 2-amino-6-bromopyridine (2). The reaction temperature was warmed to room temperature and stirred for 12 h. The reaction was monitored by HPLC. After the reaction was complete, water (2,000 kg) was added, the reaction was stirred and the DCM layer was separated. The aqueous layer was extracted again with DCM (1000L). The combin...
Embodiment 2
[0436] Example 2. Solubility Evaluation of Amorphous Compound 1
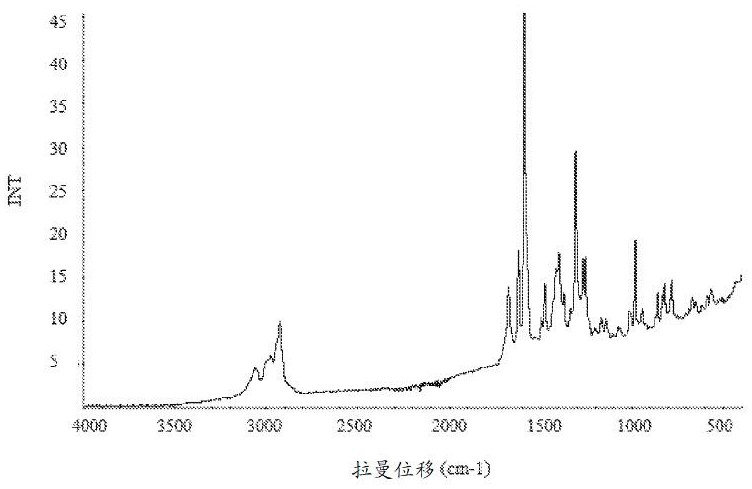
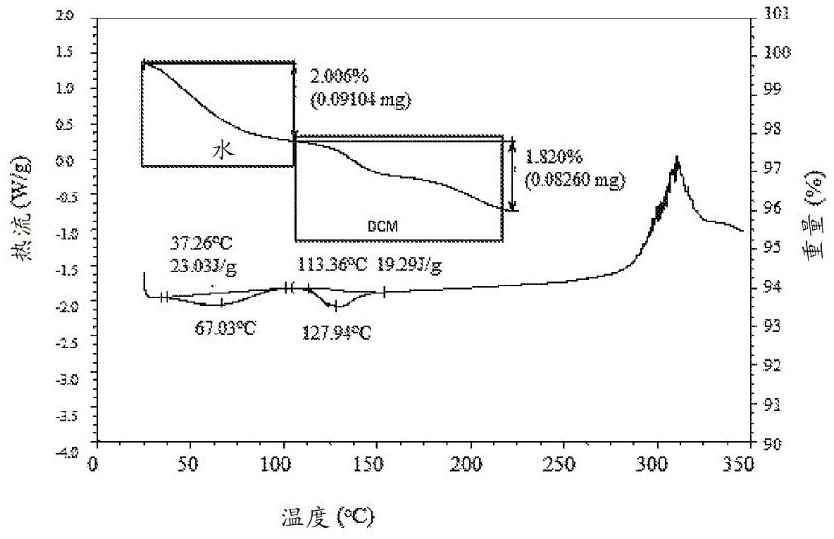
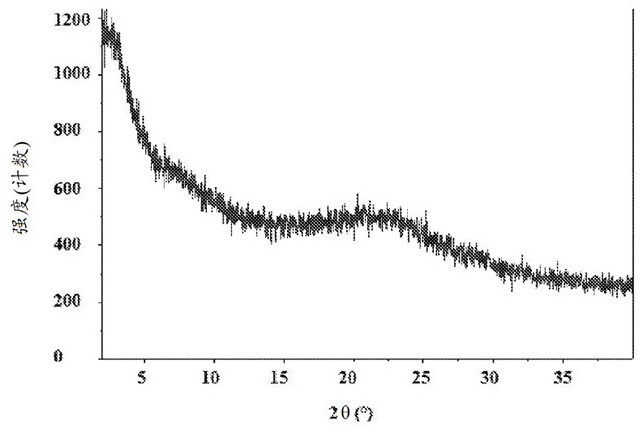
[0437] Through FT Raman spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), TGA combined with infrared exhaust gas detection (TGA-IR), polarized light microscopy (PLM), powder X-ray diffraction (PXRD) , Dynamic Vapor Sorption (DVS) and Modulated DSC Analysis of Amorphous Compound 1. Selected physicochemical data for amorphous compound 1 are shown in Figure 1A , Figure 1B , Figure 1C , Figure 1D , Figure 1E and Figure 1F middle. These figures indicate that the material is a light brown powder, which was tested by PXRD ( Figure 1C ) and PLM ( Figure 1D ) is determined to be amorphous. DSC data show low energy broad endotherms at 37.3°C and 113.4°C ( Figure 1B ). TGA-IR data ( Figure 1B ) indicates that the material contained residual water (2.0%) and DCM (1.8%). DVS analysis performed on the amorphous form indicated a water uptake of 4.0% from 0%-90% RH (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com