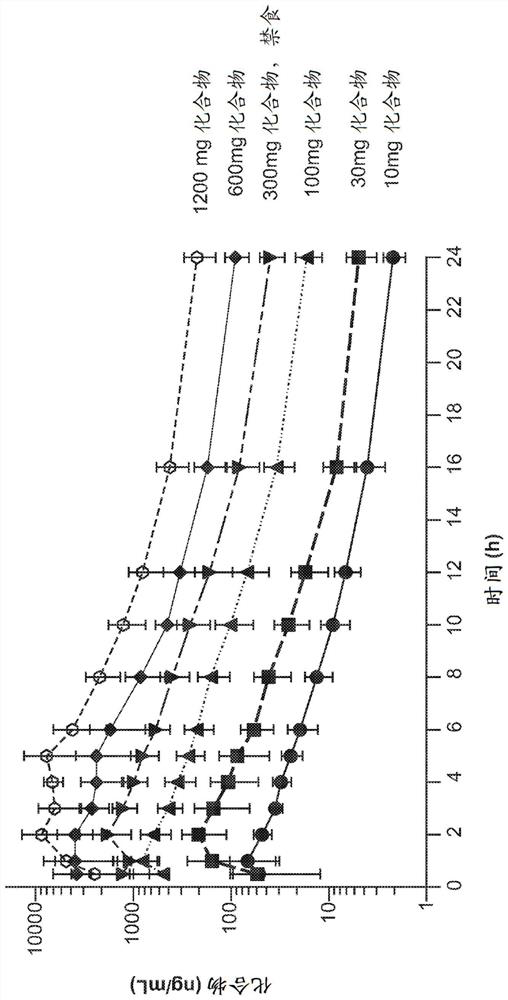
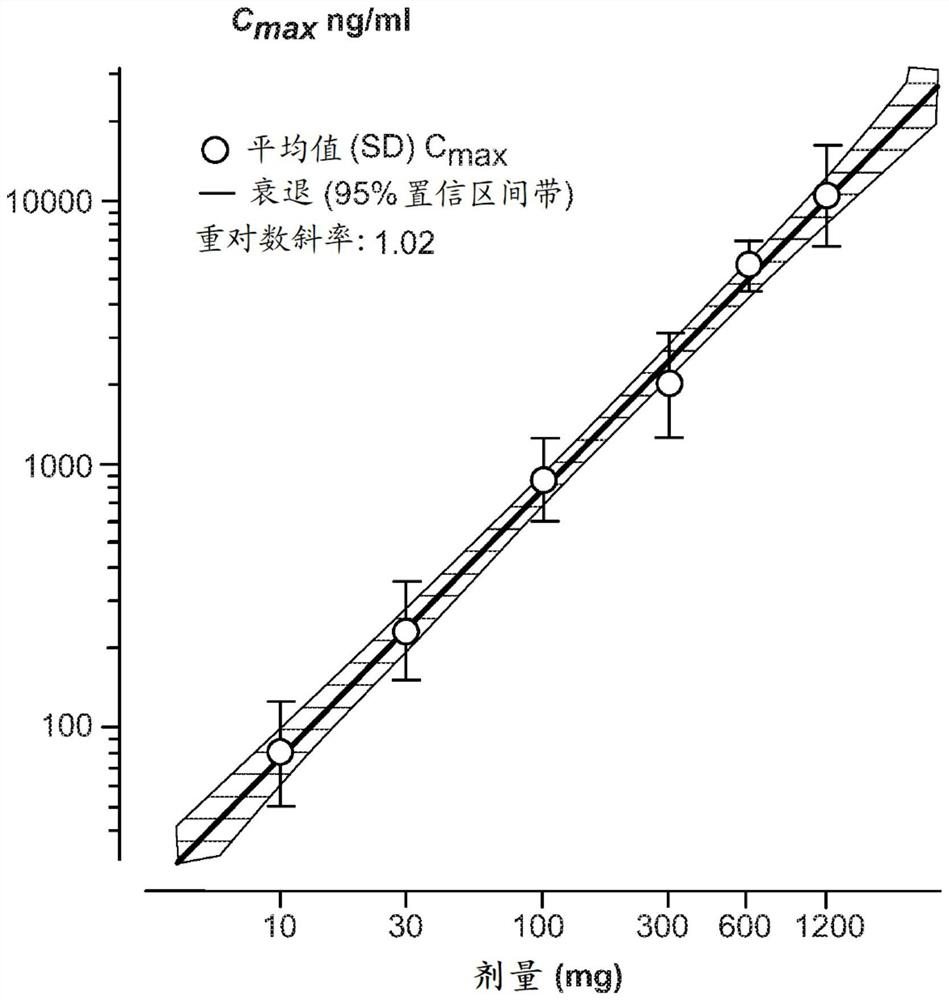
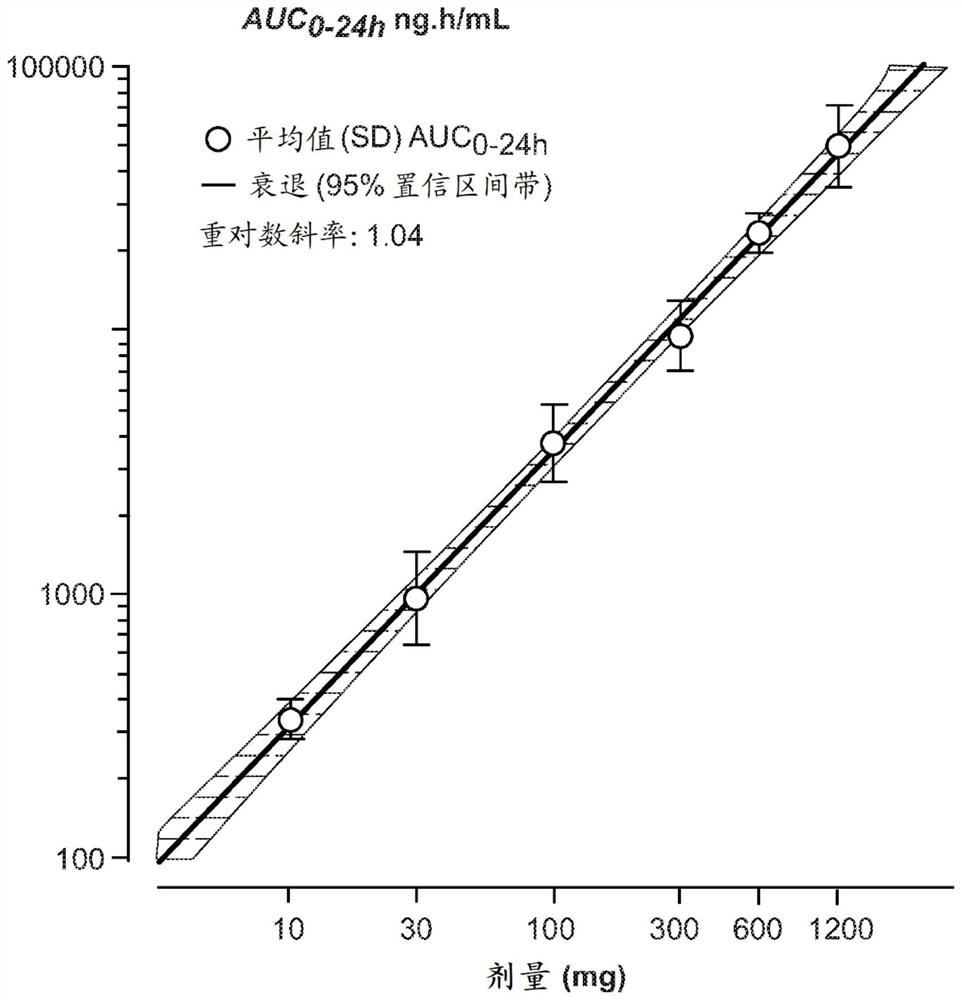
Dosing regimens for oral complement factor D inhibitors
An oral agent and pharmaceutical technology, applied in the field of oral administration of complement factor D inhibitors, capable of solving problems such as destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0149] introduction:
[0150] Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, life-threatening disorder characterized by hemolytic anemia, thrombosis, and impaired bone marrow function. Uncontrolled activity of the alternative pathway (AP) of complement leads to intravascular hemolysis triggered by membrane attack complex (C5b-9) formation in PNH patients. Terminal complement inhibition by IV administration of eculizumab or ravolizumab reduces intravascular hemolysis and is the standard of care in PNH. However, up to a third of eculizumab-treated patients remain transfusion dependent due to persistent AP activation and extravascular hemolysis mediated by C3 (Kelly RJ et al, Blood 2011;117(25): 6786-6792). The compounds of the present invention are novel, potent, selective and orally bioavailable small molecule inhibitors of human complement factor D. In preclinical in vitro studies, at low nanomolar drug concentrations, the compounds of the present invention inhibit AP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com