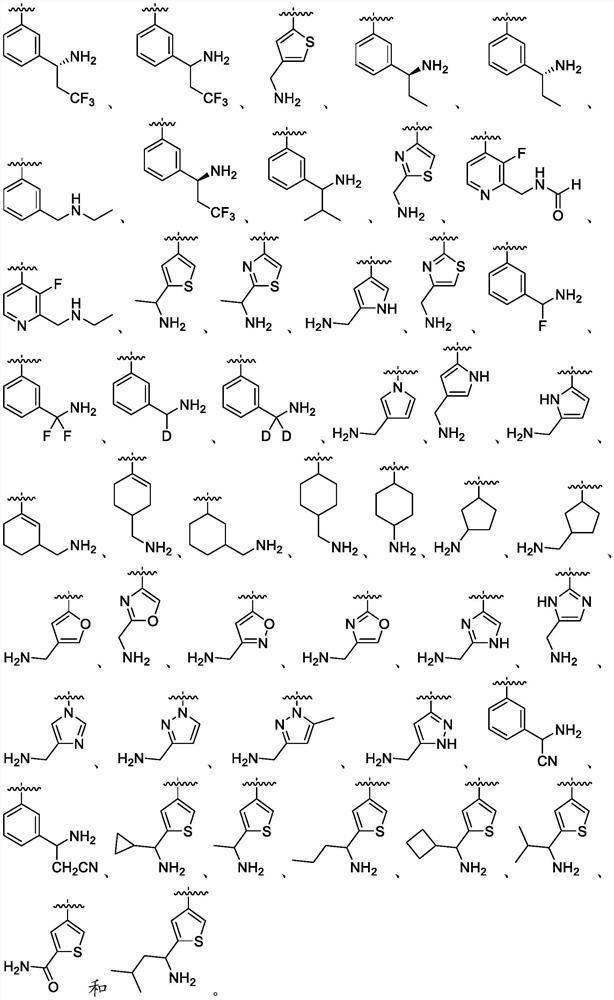
Oral complement factor D inhibitors
A free and compound technology, which is applied in the direction of medical preparations containing active ingredients, drug combinations, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] The preparation of such prodrug derivatives is discussed in various literature sources (examples are: Alexander et al, J. Med. Chem. 1988, 31, 318; Aligas-Martin et al, PCT WO0041531, page 30).
[0103] Prodrug forms of compounds with carboxyl groups include esters (-CO 2 R m ), where R m The group corresponds to any alcohol that will be released at pharmaceutically acceptable levels in vivo by enzymatic or hydrolytic methods. Another prodrug derived from the carboxylic acid form of the present disclosure can be the quaternary salt type structure described by Bodor et al., J. Med. Chem. 1980, 23,469.
[0104] As used herein, the terms "carrier" and "pharmaceutically acceptable carrier" refer to a diluent, adjuvant, excipient, or vehicle with which a compound is administered or formulated for administration. Non-limiting examples of such pharmaceutically acceptable carriers include liquids, such as water, saline, and oils; and solids, such as gum acacia, gelatin, star...
Embodiment
[0274] Now that the present invention has been described in detail, it will be more clearly understood by reference to the following examples, which are included for purposes of illustration only and are not intended to limit the invention.
[0275] plan 1
[0276]
[0277] Preparation of 2-(2-((7-(5-(1-aminopropyl)thiophen-3-yl)benzofuran-5-yl)methoxy)phenyl)acetic acid (1j)
[0278] Step-1: Preparation of (7-bromobenzofuran-5-yl)methanol (1b)
[0279] To 7-bromobenzofuran-5-carboxylic acid (1a) (10 g, 41.5 mmol; CAS# 286836-25-7) and N-methylmorpholine (5.47 mL, 49.8 mmol) in THF ( To the stirred solution in 200 mL) was added isobutyl chloroformate (6.54 mL, 49.8 mmol). The reaction mixture was stirred for 15 minutes, filtered through a pad of celite and the precipitate was washed with THF (3 x 20 mL). The filtrate was cooled to 0°C and NaBH was added carefully (fast release of gas) 4 (4.71 g, 124 mmol) in water (10 mL). The reaction mixture was stirred for 30 minute...
Embodiment 85
[1308] ICs of compounds are calculated according to the procedure reported in US Patent 6,653,340 B1, eg, at Column 74 (incorporated by reference) 50 value (ie, the concentration of compound that inhibits 50% of the enzymatic activity).
[1309] Specifically, compounds were dissolved in DMSO stock solutions at 10.0 or 100 mM. A portion of this stock solution was added to assay buffer in a final volume of 50 μL. Controls included buffer alone and the enzyme solution to which DMSO was added. Substrate is added to the wells immediately or after incubation at room temperature. The reaction rate was measured spectrophotometrically by producing product at 405 nm for 600 seconds. The background absorbance at 690 nm was measured for each well and subtracted from the absorbance at 405 nm.
[1310] The reaction rate of the enzyme alone was compared to the rate of the enzyme in the presence of the inhibitor, and percent inhibition was calculated as follows:
[1311] Percent inhibiti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com